Abstract
During the past several years, pre-clinical experiments have established that microRNAs (miRNAs), small non-coding RNAs, serve as key regulatory molecules of fracture healing. Their easy modulation with agonists and antagonists make them highly desirable targets for future therapeutic strategies, especially for pathophysiologic fractures that either do not heal (nonunions) or are delayed. It is now well documented that these problematic fractures lead to human suffering and impairment of life quality. Additionally, financial difficulties are also encountered as work productivity decreases and income is reduced. Moreover, targeting miRNAs may also be an avenue to enhancing normal physiological fracture healing. Herein we present the most current knowledge of the involvement of miRNAs during fracture healing in pre-clinical studies. Following a brief description on the nature of miRNAs and of the fracture healing process, we present data from studies focusing specifically, on miRNA regulation of osteoblast differentiation and osteogenesis (within the context of known signaling pathways), chondrocytes, angiogenesis, and apoptosis, all critical to successful bone repair. Further, we also discuss miRNAs and exosomes. We hope that this manuscript serves as a comprehensive review that will facilitate basic/translational scientists in the orthopaedic arena to realize and further decipher the biological and future therapeutic impact of these small regulatory RNA molecules, especially as they relate to the molecular events of each of the major phases of fracture healing.
Introduction
An estimated 12 to 15 million fractures occur annually in the United States, with approximately 4.3 million of these fractures classified as fragility fractures [1]. While the vast majority of these fractures heal properly, 5–10% can be expected to result in nonunion [2]. Fractures of the metatarsals, radius, and ankle carry the highest risk of nonunion, with additional risk factors including; multiple fractures, open fractures, operative treatment, higher energy, medications (e.g., opioids, anticonvulsants, insulin), osteoarthritis, and male gender [2]. Osteoporosis and other disorders resulting in low bone mass also contribute greatly to this high rate of nonunion and are risk factors for more than 50 million people in the US [3]. The high incidence of fractures in these patients impairs their economic, social, and functional activities, as well as their overall quality of life [4, 5]. As such, there is an overwhelming need to develop new therapeutics to alleviate the collective burden of these fractures. In order to develop such therapeutics, we must fully understand the molecular events associated with the processes of physiologic and pathophysiologic fracture repair, and as such, researchers around the globe have been working to molecularly and functionally characterize genes, signaling pathways, and regulatory RNAs in order to determine which are essential to the healing process [6–10]. The ultimate goal of this avenue of research is to target the individual molecules that are critical to a specific patient’s repair process and microRNAs are one class of molecules that may be able to achieve this ambitious goal.
The fracture repair process consists of several interdependent stages: hematoma formation, inflammation, osteogenesis, chondrogenesis, endochondral ossification, and remodeling (Figure 1) [11]. A hematoma develops immediately after a fracture, and subsequently serves as the source of a multitude of signaling molecules such as hormones, growth factors, and cytokines that direct cellular activity during the healing process [11]. This highly stimulatory chemical microenvironment induces periosteal osteoprogenitor cells, stem cells residing in the bone marrow [12], as well as in adjacent skeletal muscle [13], to differentiate into osteoblasts and chondrocytes via activation of thousands of individual genes and signaling pathways (e.g., bone morphogenetic protein [BMP] & Wnt) [10]. These cells then migrate to the fracture site and serve as the primary cells responsible for fracture callus (both soft and hard) formation [14]. The hard callus initially forms via intramembranous ossification that is initiated at sites adjacent to the fracture and proceeds towards the fracture site. In contrast, the soft callus forms via chondrogenesis at the fracture site and is subsequently converted into bone through endochondral ossification to bridge the fracture gap. Angiogenesis and apoptosis (of osteoblasts and chondrocytes) accompany intramembranous and endochondral ossification and are vital contributors to both of these processes. Lastly, the fracture callus is remodeled by active bone resorption in order to regain its original morphological and biomechanical structure.
Figure 1.
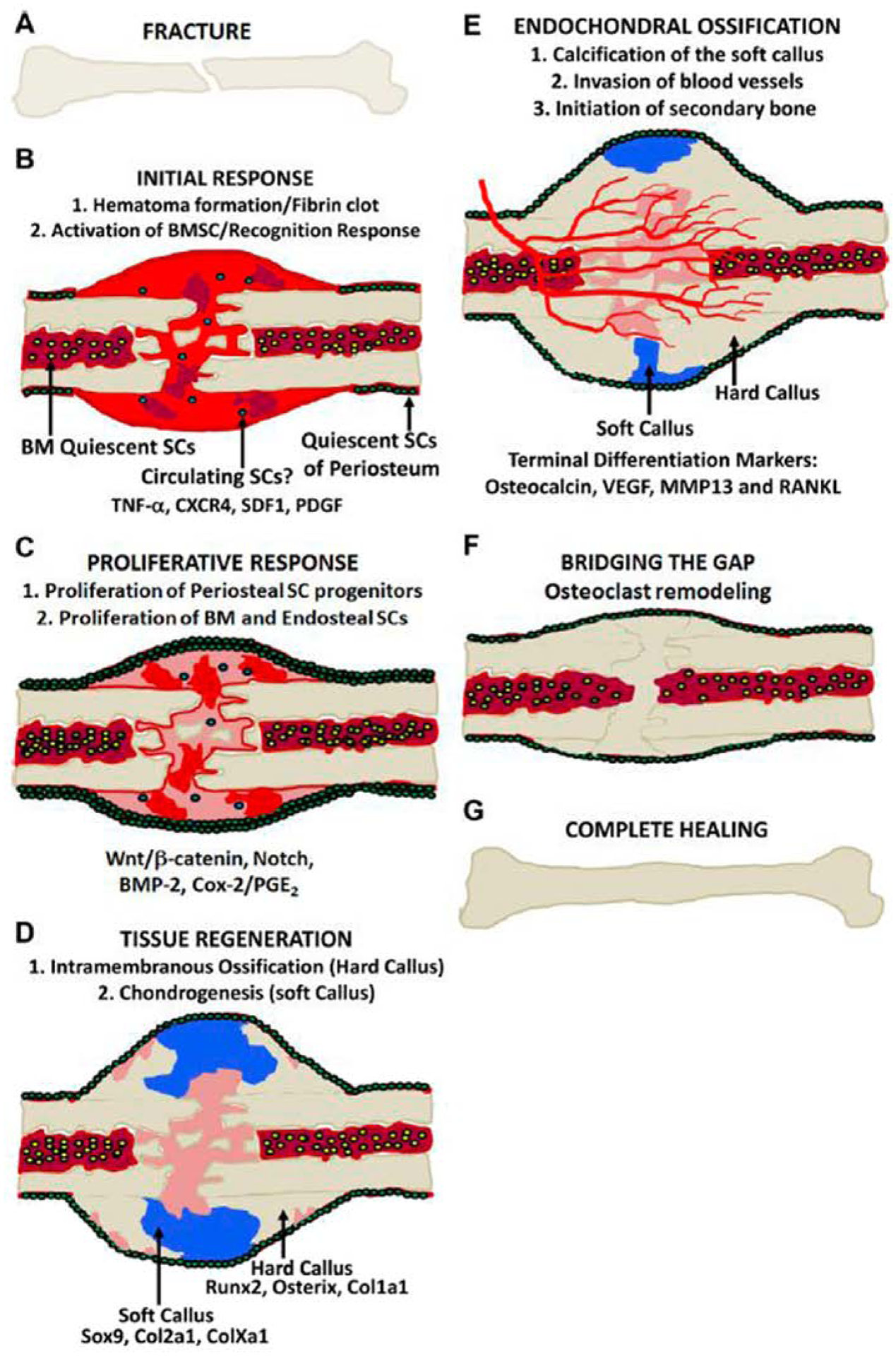
Fracture healing process. A) Fractured bone. B) Initial response showing the evelopment of a hematoma and formation of a fibrin clot at the fracture site. C) Proliferative esponse, indicated by the activation of periosteal and bone marrow stem cell progenitors and ctivation of various signaling pathways. D) Tissue regeneration, as a result of stem cell ifferentiation into osteoblasts and chondrocytes that lead to new bone directly through intramembranous ossification (hard callus) and cartilage (soft callus), respectively. E) ndochondral ossification, conversion of the soft callus into secondary bone accompanied by angiogenesis. F) Fractures healing occurs with bridging of the fracture with calcified tissue, but is completed with remodeling of the fracture which restores the bone to its normal size and shape. G) Complete healing. Adapted from [11].
Several research strategies have been advanced to accelerate fracture healing, generally based on chemical or physical interventions, and some have found their way into the clinic. To date, recombinant proteins such as BMPs and parathyroid hormone (PTH) analogs lead the chemical approach, whereas low intensity pulsed ultrasound (LIPUS) represents the most successful biophysical approach [15–17]. However, surgery still remains the most effective solution for the repair of recalcitrant nonunions. As such there is a need to develop new therapeutic modalities for enhancing fracture healing and one promising approach is the modulation of microRNAs, especially since it was previously demonstrated that hundreds of miRNAs are differentially expressed during the early phases of the repair process [18].
MicroRNAs (miRNAs) are a class of small (20 to 24 nucleotide) noncoding RNAs that bind to the 3’-untranslated region (UTR) of mRNA transcripts and regulate gene expression by blocking translation or directing the degradation of the target mRNA [19]. Primary miRNAs (primiRNAs) are first transcribed by an RNA polymerase and are then processed by RNase III endonuclease DROSHA and RNA binding protein DiGeorge Syndrome Critical Region 8 (DGCR8), which together form a microprocessor complex. The enzyme Dicer, along with transactivation response element binding protein (TRBP) further process the miRNA to generate mature, single-stranded miRNAs. Finally, the mature miRNA forms an RNA-induced silencing complex (RISC) with Dicer, TRBP, and other partner proteins with the target complementary mRNA to exert its inhibitory action. Importantly, miRNAs have been shown to regulate osteoblasts, chondrocytes, angiogenesis, and other processes crucial to bone formation and fracture healing [20]. miRNAs are found intracellularly, extracellularly, and in the circulatory system as free molecules and in exosomes; nano-sized extracellular vesicles that are released by a variety of cells and transport membrane and cytosolic proteins, mRNAs, and miRNAs to secondary cells. The activity of these secondary cells may be altered upon receiving this exosomic cargo [21, 22] and MSC-derived exosomes have been shown to be important in tissue repair [23, 24].
The goal of this review is to present a current view of what is known about the role of miRNAs during processes vital to fracture healing. A literature search was performed on PubMed in order to identify all research articles related to miRNAs and osteogenesis, chondrogenesis, angiogenesis, apoptosis and fracture healing that were published in the past five years. Only manuscripts that reported on mammalian bony or cartilage fractures or defects, or distraction osteogenesis models were included; therefore, manuscripts describing non-mammalian models, ectopic bone formation, bone homeostasis, or purely in vitro work were excluded. Also excluded were results dealing with circulating miRNAs in osteoporotic patients and other clinical studies of miRNA expression in fracture healing as these data was included in our previous review article [25]. The resulting manuscript has been organized into various sections dealing with the aforementioned processes.
miRNA Regulation of Osteoblastic Differentiation and Osteogenesis
Osteogenesis is the process of new bone formation and during fracture healing it proceeds as intramembranous ossification and endochondral ossification. The success of osteogenesis is obviously critical for physiological fracture healing and as such, regulation of osteogenesis is the most well-studied aspect of this process. Osteogenesis involves the initial osteogenic differentiation stem cells and their subsequent secretion of osteoid that finally undergoes mineralization resulting in mature bone. miRNA regulation of osteogenesis represents the largest number of studies related to miRNA regulation of fracture healing. All of the studies presented are listed in Table 1 and summarized schematically in Figure 2.
Table 1:
miRNA Regulation of Osteoblastic Differentiation and Osteogenesis
| miRNA Regulation of Osteoblastic Differentiation and Osteogenesis | ||
|---|---|---|
| Bone Morphogenetic Proteins | ||
| Authors | Design | Key Findings |
| Wang et al., 2019 [27] | Systemically injection of miR-186 mimic assessed in murine femoral fracture model. | miR-186 overexpression increased BMD, BV/TV, and biomechanics by activation of BMP-2 and BMP-7. |
| Deng et al., 2019 [28] | Intrathecal administration of miR-9 inhibitor assessed in rat femoral osteotomy model. | miR-9 inhibition enhanced fracture healing via attributed to modulation of BMP-7 signaling. |
| Lee et al., 2016 [29] | Microbubble-ultrasound miR-29b-3p delivery system evaluated in murine femoral fracture model. | miR-29b-3p enhanced fracture healing attributed to enhanced BMP/TGF-β signaling. |
| Sun et al., 2017 [33] | BMSCs overexpressing miR-503 evaluated in rat tibial distraction osteogenesis model. | miR-503 enhanced osteogenesis attributed to repression of SMURF1. |
| Li et al., 2017 [35] | ADSCs overexpressing miR-148b and BMP-2 assessed in murine calvarial defect model. | miR-148b and BMP-2 treatment enhanced defect healing via repression of Nog. |
| Xie et al., 2017 [36] | ADSCs expressing miR-146a or miR-146a inhibitor evaluated in rat calvarial defect model. | miR-146a inhibitor enhanced defect healing and expression of RUNX2 & OSX attributed to alleviation of SMAD4 repression. |
| Li et al., 2019 [37] | Rat femoral fracture model studied with Mg and SS implants. Effect of miR-138-5p and LOC103691336 on osteogenic differentiation of BMSCs evaluated in vitro. | Competition between LOC103691336 and BMP-R2 mRNA for binding to miR-138-5p regulates osteogenic differentiation of BMSCs. |
| Wnt/ϐ-catenin | ||
| Sun et al., 2019 [40] | Determined the effect of local injection of miR-26a mimics in rat nonunion fracture model. | miR-26a overexpression enhances fracture healing via repression of the Wnt antagonist, SOSTDC1. |
| Hu et al., 2018 [42] | BMSCs expressing miR-210-3p evaluated in canine mandibular defect model. | miR-210-3p enhanced defect healing attributed to repression of sclerostin. |
| Sui et al., 2018 [44] | Lipidoid-miRNA formulation of miR-335-5-p evaluated in murine calvarial defect. | miR-335-5-p enhanced defect healing and OCN expression attributed to decreased expression of DKK1. |
| Zhang et al., 2017 [45] | BMSCs expressing miR-355-5p evaluated in murine calvarial defect model. | miR-355-5p enhanced defect healing and expression of OCN attributed to repression of DKK1. |
| Teng et al., 2018 [46] | Local injection of agomiR-214-3p evaluated in murine tibial fracture model. | agomiR-214-3p reduced callus formation and led to disordered trabecular bone, confirming that miR-214-3p impairs fracture healing though inhibition of Wnt/β-catenin signaling. |
| Li et al., 2017 [47] | ADSCs expressing miR-214 sponge with and without BMP-2 implanted in femoral defects of Ovx rats. | Enhanced defect healing seen for treatment with miR-214 sponge and BMP-2 expressing ADSCs due to activation of Wnt/β-catenin and BMP-2 signaling. |
| Li et al., 2016 [49] | Compared miR-214 sponge to miR-140 sponge in regulating osteogenic differentiation of BMSCs and osteoclastic differentiation of Raw 264.7 cells. | miR-214 sponge was superior to miR-140S in enhancing osteogenic differentiation and miR-140S was superior in suppress osteoclastic differentiation. |
| Sun et al., 2015 [53] | Evaluated direct injection of miR-21 overexpressing BMSCs to rat femoral fractures | miR-21 accelerated endochondral ossification attributed to alleviation of Sox-2 repression of Wnt/β-catenin signaling. |
| Jia and Zhou, 2018 [54] | Measured miR-367 expression levels in murine tibial fracture calluses and determined effects of miR-367 on osteoblast activity. | Inhibition of miR-367 in vitro promoted osteoblast proliferation and migration and reduced apoptosis attributed to alleviation of Panx3 inhibition of Wnt/β-catenin signaling. |
| Shi et al., 2018 [58] | Measured effects of local delivery of BMSCs overexpressing miR-218 to murine femoral fractures. | miR-218 enhanced fracture healing attributed to activation of Wnt/β-catenin via repression of Wnt inhibitors Dkk-2, Sfrp2, Sost, and Tob1. |
| PTEN/PI3K/Akt | ||
| Yang et al., 2019 [62] | BMSCs overexpressing miR-21 evaluated in rat calvarial defect and canine mandibular defect models. | miR-374b is elevated in tibial fractures and overexpression of miR-374b enhances osteogenic differentiation via repression of PTEN. |
| Ge et al., 2018 [64] | Measured expression levels of miR-374b in BMSCs isolated from murine tibial fractures and determined the effects of miR-374b on osteogenic differentiation of BMSCs. | miR-374b is elevated in tibial fractures and overexpression of miR-374b enhances osteogenic differentiation via repression of PTEN. |
| Xiong et al., 2019 [64] | Assessed role of miR-26a-5p using murine femoral fracture model in conjunction with TBI. | miR-26a-5p enhances fracture healing via suppressing of PTEN and miR-26a-5p upregulation by TBI contributes to enhanced fracture healing seen with TBI. |
| Ou et al., 2019 [68] | Scaffolds containing mir-214 inhibitor evaluated in rat calvarial defect model. | miR-214 inhibitor enhanced defect healing and expression of OCN attributed to alleviation of ATF4 repression. |
| WWP1 | ||
| Tu et al., 2017 [71] | Injection of agomir-142-5p evaluated in murine femoral fracture model. | Enhanced fracture healing and expression of RUNX2, JUNB, & OCN attributed to repression of WWP1. |
| SATB2 | ||
| Tang et al., 2018 [73] | BMSCs expressing miR-383 inhibitor evaluated in rat calvarial defect model. | miR-383 inhibitor enhanced defect healing attributed to alleviation of SATB2 repression. |
| HOXA2 | ||
| Xie et al., 2016 [76] | ADSCs expressing mirR-135 evaluated in rat calvarial defect model. | miR-135 enhanced defect healing and expression of RUNX2 attributed to repression of HOXA2. |
| HMGA2, AQP1, and p38 MAPK | ||
| Tian et al., 2017 [78] | Assessed the function of miR-495 using a murine femoral drill-hole model. | Treatment with anit-miR-495 enhanced defect healing attributed to alleviated repression of HMGA2. |
| Zhu et al., 2019 [79] | Determined miR-495 expression in murine tibial fracture model and evaluated its effects of osteoblast differentiation in vitro. | mir-495 is downregulated during fracture healing and promotes osteoblast differentiation by binding to AQP to repress p38 MAPK signaling. |
Figure 2.
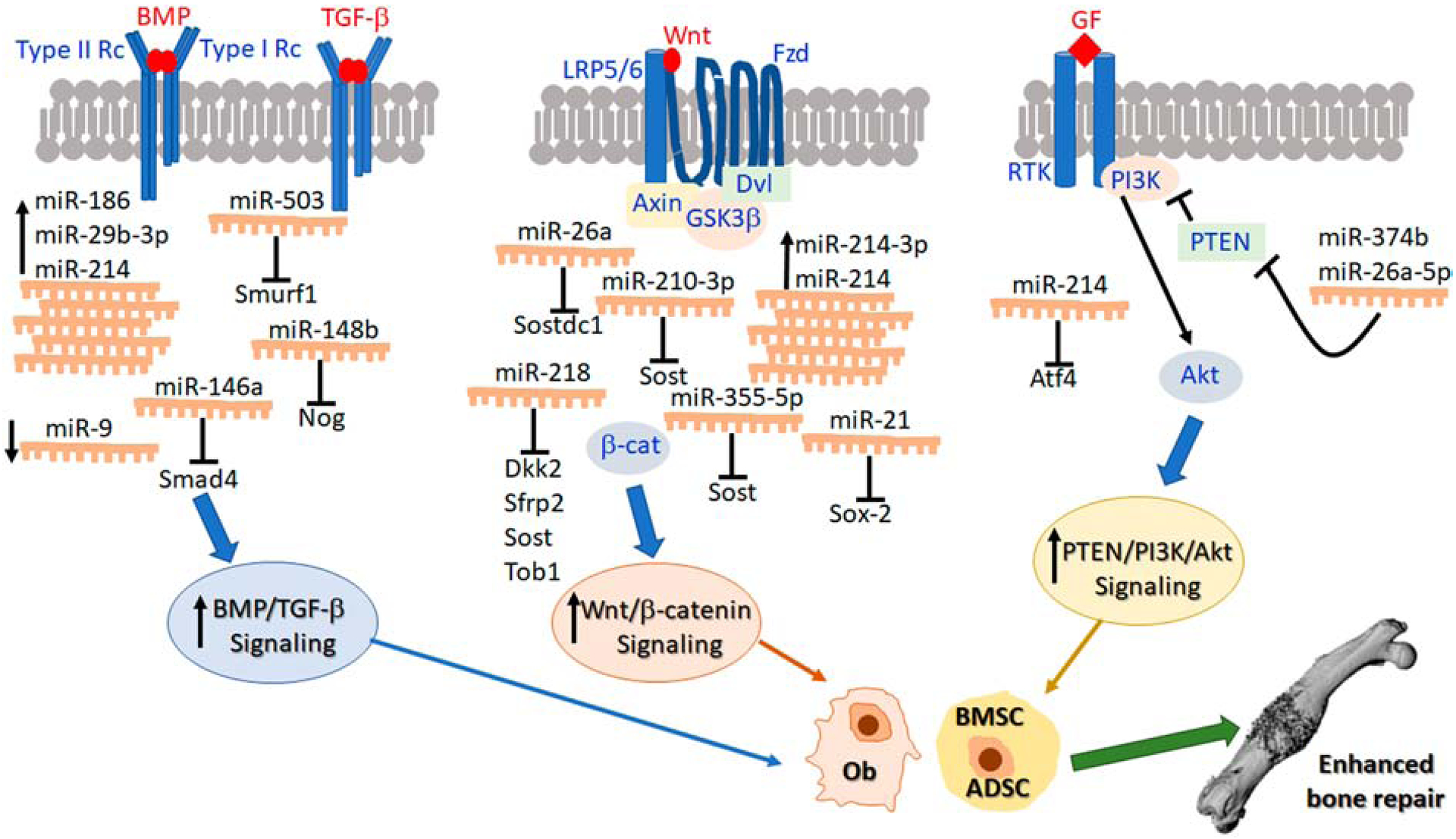
miRNA regulation of enhanced bone repair. This schematic represents the effects of modulating various miRNAs in osteoblasts and/or mesenchymal stem cells leading to activation of signaling pathways, particularly, BMP/TGF-β, Wnt/β-catenin and PTEN/PI3K/Akt that stimulate osteogenesis and ultimately enhance bone repair. The data represented in this schematic are also summarized in Table 1.
Bone Morphogenetic Proteins
Bone morphogenetic proteins (BMPs) are part of the transforming growth factor beta (TGF-β) superfamily of growth factors. BMP receptors are expressed by osteoblasts throughout fracture healing and the osteogenic activity of this pathway has long been used to enhance this process. Expectedly, several miRNAs have been reported to enhance BMP signaling. miR-186 has been shown to target Smad-6, an important feedback inhibitor in the BMP/Smad signaling pathway [26]. A study by Wang et al., investigated the effects of miR-186 on fracture healing through BMP regulation [27]. They over-expressed miR-186 in mice with femoral fractures and when compared to controls, miR-186 overexpression led to increased bone mineral density (BMD) and bone volume fraction (BV/TV), as assessed by micro-computed tomography (microCT). Similar improvement was seen in biomechanical properties, with maximum load, maximum radial degrees, elastic radial degrees, and rigidity all increased when compared to control groups. Direct silencing of Smad-6 resulted in similar outcomes. Finally, activation of BMP signaling was confirmed by increased mRNA and protein levels of BMP-2 and BMP-7, proving that miR-186 over-expression represents a potential therapeutic approach to enhance fracture healing. Similarly, Deng et al., 2019 evaluated the effect of miR-9 inhibition on the rat femur healing following osteotomy and showed increased BMD [28]. They attributed this improvement in healing to alterations in BMP-7 signaling.
Lee et al., tested the efficacy of miR-29b-3p in enhancing fracture healing [29]. miR-29 has been reported to target several negative regulators of osteogenic differentiation, including TGF-β3 [30], and miR-29b-3p has also been shown to bind to BMP-1 to regulate osteogenic differentiation of bone mesenchymal stem cells (BMSCs) [31]. In this study [29], transfection of BMSCs with miR-29b-3p significantly increased levels of Runt-related transcription factor 2 (Runx2), alkaline phosphatase (Alp), secreted phosphoprotein 1 (SPP1), and bone gammacarboxyglutamate protein (BGLAP). They then employed a microbubble-ultrasound system to deliver miR-29b-3p to the fracture sites of murine femoral fractures. The results showed that a single injection of miR-29b-3p significantly improved radiographic and histological measurements of callus bone area and biomechanical stiffness in comparison to repeated injections. Similarly, microCT analyses revealed increases in BV/TV and BMD for the mice treated with a single injection. These results show that miR-29b-3p may be useful in promoting fracture healing, but repeated administration may lead to impaired fracture healing.
Smad ubiquitination regulatory factor-1 (Smurf1) is a negative regulator of the TGFβ/BMP signaling pathway [32]. Sun et al., demonstrated that miR-503 binds to the 3’UTR of Smurf1, thereby enhancing BMP-2 expression and promoting osteogenic differentiation of BMSCs [33]. They then injected BMSCs overexpressing miR-503 into the distraction gap of rat tibiae undergoing distraction osteogenesis (DO). MicroCT analyses revealed increased BV/TV in the miR-503 group compared to controls. Biomechanical testing also supported enhanced osteogenesis in the miR-503 treated rats, with significant increases found for ultimate load and energy to failure.
Another regulator of BMP signaling is Noggin (Nog), which is upregulated by BMP-2 and acts as part of a negative feedback loop by blocking BMP binding to its receptors, thereby serving as a check to excessive BMP signaling [34]. Li et al., determined that miR-148b bound to the 3’UTR of Nog was able to ameliorate Nog repression of BMP-2 signaling during the osteogenic differentiation of adipose derived stem cells (ADSCs) [35]. Implantation of normal ADSCs, miR-148b expressing ADSCs, BMP-2 expressing ADSCs, and combined miR-148b and BMP-2 expressing ADSCs into calvarial defects in mice revealed no significant enhancement of defect healing in mice implanted with miR-148b or BMP-2 expressing ADSCs. However, the combined miR-148b or BMP-2 group induced significant increases in bone area (BA), bone volume (BV), and BMD as determined by microCT analyses (Figure 3). These results indicate that repression of Nog inhibition of BMP-2-induced osteogenesis via miR-148b overexpression can potentiate BMP-2 activity and enhance fracture healing.
Figure 3.
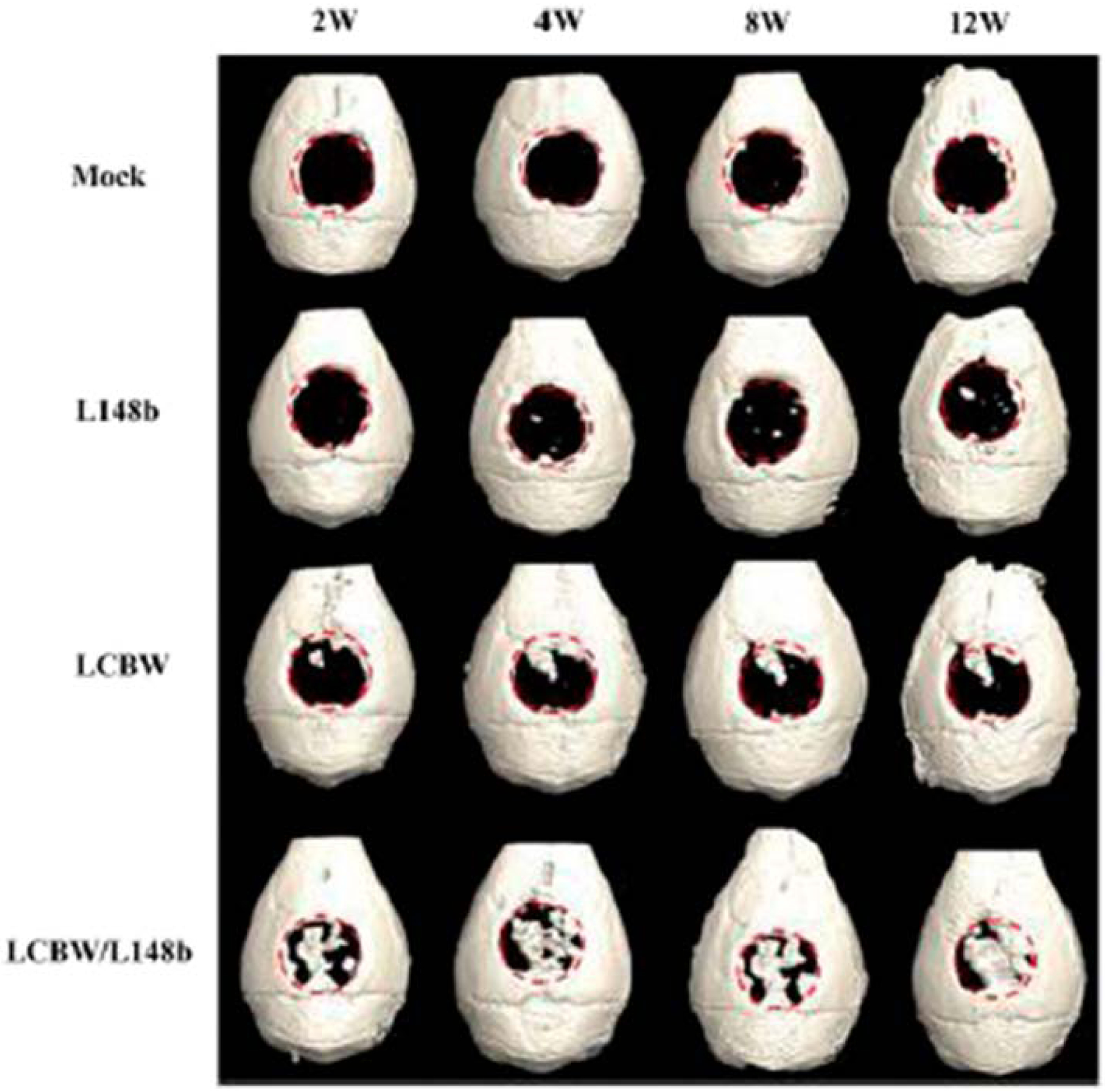
In vivo bone repair as monitored by microCT. Human ADSCs were mock-transduced (Mock group), co-transduced with Bac-Cre/Bac-L148b (L148b group) or with Bac-Cre/Bac-LCBW (LCBW group), or co-transduced with Bac-Cre/Bac-LCBW/Bac-L148b (LCBW/L148b group). The cells were seeded onto gelatin-coated poly(lactic-co-glycolic acid) (PLGA) scaffolds and implanted into critical-sized (4mm in diameter) calvarial defects in nude mice. The mice were scanned by micoCT at the indicated times. Adapted from [35].
Several miRNAs have also been shown to repress BMP signaling and the use of miR-inhibitors or antagonists represents another strategy to target the BMP signaling pathway in order to enhance fracture healing. Based on the importance of BMP-2 in fracture healing, Xie et al., performed expression profiling of miRNAs during BMP-2-induced osteogenic differentiation of ADSCs and found that the expression of miR-146a was robustly down-regulated during this process [36]. They then determined that miR-146a directly binds to the 3’UTR of Smad4, a BMP-2 co-activator. Finally, they transduced ADSCs with lentivirus encoding miR-146a, miR-146a inhibitor, and miR-negative control (miR-NC), loaded them onto porous poly (sebacoyl diglyceride) (PSeD) scaffolds and implanted them in rat calvarial defects. Defects treated with ADSCs expressing the miR-146a inhibitor displayed enhanced bone healing, as evidenced by higher BV/TV, trabecular number (Tb.N.), and BMD following microCT analysis. Furthermore, immunohistochemical (IHC) analyses validated that the miR-146a inhibitor increased the expression of Smad4, as well as Runx2 and osterix (Osx), critical osteoblast transcription factors.
BMP signaling can also be regulated by changes in its receptors. Li et al., analyzed the role of long noncoding RNAs (lncRNAs), which can inhibit miRNA activity, during fracture healing [37]. In this study, rats were implanted with femoral intramedullary rods made out of magnesium (Mg) or stainless steel (SS) and then subjected to fractures. Rats with the Mg implants demonstrated improved radiographic healing and higher expression osteogenic markers including Alp, Runx2, Osx, and osteocalcin (Ocn). In addition, BMP receptor II (BMP-R2) protein levels were also upregulated in fracture calluses of rats implanted with Mg rods. Subsequent microarray and cell culture analyses determined that Mg upregulates LOC103691336, a lncRNA that inhibits the binding of miR-138–5p to BMP-R2 mRNA. Transfection of BMSCs with miR-138–5p reduced their osteogenic differentiation while transfection with miR-138 inhibitors enhanced this process. Similarly, silencing of LOC103691336 reduced osteogenic differentiation with and without the addition of miR-138 inhibitors. Finally, competition between LOC103691336 and BMP-R2 mRNA for binding to miR-138–5p was shown to directly impact the expression levels of BMP-R2. These data suggest a novel mechanism by which Mg implants regulate osteogenic differentiation and demonstrate that miR138–5p inhibition by overexpression of LOC103691336 may be useful for promoting fracture healing.
The BMP signaling pathway is perhaps the most well-studied pathway related to fracture healing and it is no surprise that miRNA regulation of BMP signaling has also received much attention. Moreover, recombinant BMP-2 and BMP-7 are the only FDA approved proteins for fracture healing [38], making them attractive targets for miRNA-based therapeutics. Indeed, the studies by Wang et al., Sun et al., and Li et al., all demonstrate that miRNA modulation can increase BMP signaling to enhance fracture healing. Similarly, inhibition of BMP repressors showed similar efficacy in other studies. The fact that these studies utilized a wide variety of animal models supports the potential clinical translation of these findings. However, many of the studies used transfected cells as the means to deliver miRNAs/inhibitors and while this approach can validate targets, it presents a difficult path to clinical adoption. As such, these studies should be followed up by studies utilizing local delivery which would better pave the way to clinical trials for some of these promising targets.
Wnt/ϐ-catenin
The Wnt/β-catenin is another key signaling pathway, originally identified by our laboratory as activated during fracture repair [10], that is important for osteogenesis. It is also a major pathway that regulates chondrogenesis, and proper temporal activation of this pathway is vital to the success of the process [39]. A study by Sun et al., reported on the expression profiling of miRNAs from a rat nonunion osteotomy model and detected significantly decreased levels of miR-26a in both serum and bone [40]. Transfection of BMSCs with miR-26a mimics increased the expression levels of Alp and Ocn, suggesting that miR-26a enhances osteogenesis. They next identified Sclerostin domain-containing 1 (SOSTDC1), a BMP and Wnt antagonist secreted by osteoblasts, as a target of miR-26a and further experiments showed that injection of miR-26a mimics inactivated canonical Wnt/β-catenin signaling through the phosphorylation of glycogen synthase kinase 3β (GSK3β) and nuclear accumulation of β-catenin. These results support overexpression of miR-26a as a possible therapeutic agent to enhance healing in patients with nonunions.
Sclerostin, the SOST gene product, is a potent Wnt inhibitor and sclerostin neutralizing antibodies have shown tremendous promise in enhancing fracture healing [41]. Based on prior work showing that miR-210–3p had osteogenic activity, Hu et al., determined that miR-210–3P binds to the 3’UTR of SOST [42]. They then transduced BMSCs with lentivirus encoding miR-210-3p, loaded them onto beta tri-calcium phosphate (β-TCP) scaffolds and implanted them into canine mandibular defects. MicroCT evaluation revealed enhanced BMD, BV/TV, trabecular thickness (Tb.Th.), and trabecular number (Tb.N.) in the miR-210–3p group as compared to untransduced, scaffold only, and empty defect controls. Histologic and histomorphometric analyses complemented the microCT analyses and identified similar enhancement of defect healing in the miR-210–3p expressing BMSC group, with increased new bone and fluorochrome area, along with a decrease in the amount of scaffold remaining in the defects.
Dickkopf-1 (DKK-1) is another well-studied Wnt antagonist and miR-335–5-p was found to target the 3’UTR sequence of Dkk1 to promote osteogenesis [43]. In order to capitalize on this finding, Sui et al., developed a lipidoid-miRNA formulation of miR-335–5-p for local delivery and evaluated its efficacy in bone repair using the mouse calvarial defect model [44]. Results showed that delivery of the lipidoid miR-335–5-p formulation on a silk scaffold significantly increased microCT assessed BV/TV as compared to unloaded scaffolds and scrambled controls. Also, BMSCs transfected with miR-355–5p were able to enhance defect healing when compared to the untransfected cells.
Zhang et al., investigated the effects of miR-335–5-p on fracture healing [45]. They began by creating a transgenic mouse line that overexpressed miR-335–5-p driven the Osx promoter in osteoblast lineage cells. Expectedly, this resulted in a high bone mass phenotype that was accompanied by elevated expression of osteogenic genes. Similarly, osteogenic differentiation of BMSCs isolate from these mice demonstrated decreased Dkk1 expression and corresponding increases in osteogenic markers including Runx2, Osx, and Ocn. Finally, they loaded miR-335–5-p overexpressing or wild-type (WT) BMSCs on silk fibroin scaffolds and implanted them into calvarial defects in WT mice. Defects treated with miR-335–5-p overexpressing BMSCs displayed enhanced microCT-assessed BV/TV, as well as stronger IHC staining for Ocn when compared to WT BMSCs. Together, the data show that inhibition of Dkk1 repression of Wnt signaling via overexpression of miR-335–5-p has strong potential to enhance fracture healing
Not surprisingly, many miRNAs have also been identified as inhibitors of the Wnt/β- catenin signaling pathway. One example is miR-214–3p, which was evaluated by Teng et al., in a mouse tibial fracture model [46]. Following fracture induction, agomiR-214–3p was locally injected into the fracture site. Radiographic analyses demonstrated reduced callus formation and failure of cortical bridging in the agomiR-214–3p injected mice as compared to negative controls. Similarly, histological analyses found trabecular bone in the agomiR-214–3p group to be disordered and discontinuous. Immunoblots of callus protein extracts revealed significantly lower levels of β-catenin in the agomIR-214–3p injected mice, substantiating that miR-214–3p inhibits Wnt/β-catenin signaling and impairs fracture healing.
Li et al., further explored the role of miR-214 in a study of femoral defect healing in ovariectomized (Ovx) rats [47]. In this study, miR-214 was shown to directly target TGF-β Activated Kinase 1 (MAP3K7) Binding Protein 2 (TAB2) and catenin (cadherin-associated protein), beta 1 (CTNNBI), which regulate the noncanonical Wnt pathway [48] and encode β- catenin, respectively. Furthermore, ADSCs transfected with a Cre/loxP-based hybrid baculovirus (BV) vector that enabled prolonged expression of miR-214 based sponge (miR-214S), a miR-214 inhibitor, resulted in enhanced osteogenic and reduced adipogenic differentiation. Lastly, they generated femoral defects in Ovx rats and ADSCs expressing miR-214S, BMP-2, combined miR-214S and BMP-2, or mock transfected controls were loaded into gelatin scaffolds and implanted. Interestingly, the BMP-2 expressing ADSCs failed to enhance BMD, Tb.Th., or Tb.N in the defect, as measured by microCT. However, the miR-214S cells enhanced all of these parameters and the combined miR-214S and BMP-2 ADSCs resulted in the largest increases. A similar study from the same group used the same Cre/loxP-based BV vector to inhibit miR-140 by expressing miR-140 based sponge (miR-140S) [49], as miR-140 has been shown to impair osteogenic differentiation by targeting BMP-2 mRNA [50]. miR-214S was shown to be superior to miR-140S in enhancing osteogenic differentiation of BMSCs, but miR-140S was better able to suppress osteoclastic differentiation of Raw 264.7 cells. Unfortunately, they did not evaluate miR-140S expressing cells in the femoral defect model. However, together these data show that treatment with miR-214 or miR-140 based sponges represent a feasible way to enhance fracture healing.
SOX-2 is a negative regulator of Wnt/β-catenin signaling pathway and has been identified as a target for miR-21 [51]. Overexpression of miR-21 has also been shown to promote the osteogenic differentiation of BMSCs [52]. Sun et al., confirmed the osteogenic activity of miR-21, with overexpression in BMSCs enhancing mineralized nodule formation, as well as increasing mRNA levels of Osx, Alp, and osteopontin (Opn), that were accompanied equivalent decreases in SOX-2 mRNA [53]. They then performed femoral fractures in rats and injected miR-21 expressing or control BMSCs into the fracture site. MicroCT analysis revealed a significant increase in BV/TV in the miR-21 treated rats compared to controls and histologic analyses suggest this was due to accelerated endochondral ossification. In addition, ultimate load and energy to failure were found to be superior in the miR-21 group, indicating that miR-21 overexpression is a therapeutic target for fracture healing.
Another miRNA shown to repress Wnt/β-catenin signaling in the context of osteogenesis is miR-367. Jia and Zhou demonstrated that miR-367 binds to the 3’ UTR of pannexin 3 (Panx3) and that transfection of primary murine osteoblast with a miR-367 inhibitor upregulated mRNA and protein levels of Panx3, β-catenin, and Wnt5b [54]. Furthermore, mRNA and protein levels of miR-367 were elevated in the calluses of murine tibial fractures concomitant with reductions in Panx3, β-catenin, and Wnt5b when compared to intact tibiae. Furthermore, in vitro inhibition of miR-367 promoted osteoblast proliferation and migration while reducing apoptosis. This study is consistent with other work demonstrating that Panx3 inhibits Wnt/β-catenin signaling and proliferation in primary murine calvarial and C2C12 cells [55]. Thus, upregulation of Panx3 through miR-367 inhibition represents an additional approach to enhancing fracture healing via activation of Wnt/β-catenin signaling.
Finally, miR-218 has been shown to activate Wnt/β-catenin signaling by binding to the 3’UTR of the Wnt repressors Dickkopf-2 (Dkk-2), Secreted Frizzled Related Protein 2 (Sfrp2), Sost, and transducer of ERBB2, 1 (Tob1) to promote osteoblastic differentiation of BMSCs and ADSCs [56, 57]. Shi et al., verified that overexpression of miR-218 in BMSCs enhanced their osteogenic differentiation, as evidenced by increased mineralization and expression of Runx2, Osx, Alp, Ocn, and Opn, with overexpression of anti-miR-218 resulting in the opposite effect [58]. Subsequently, BMSCs overexpressing miR-218 locally administered to femoral fracture sites in mice enhanced BV/TV and significantly improved ultimate load and energy to failure (Figure 4). Finally, IHC analyses revealed elevated expression of Osx and Ocn. This study shows that miR-218 is another viable target that promotes Wnt/β-catenin signaling to enhance fracture healing.
Figure 4.
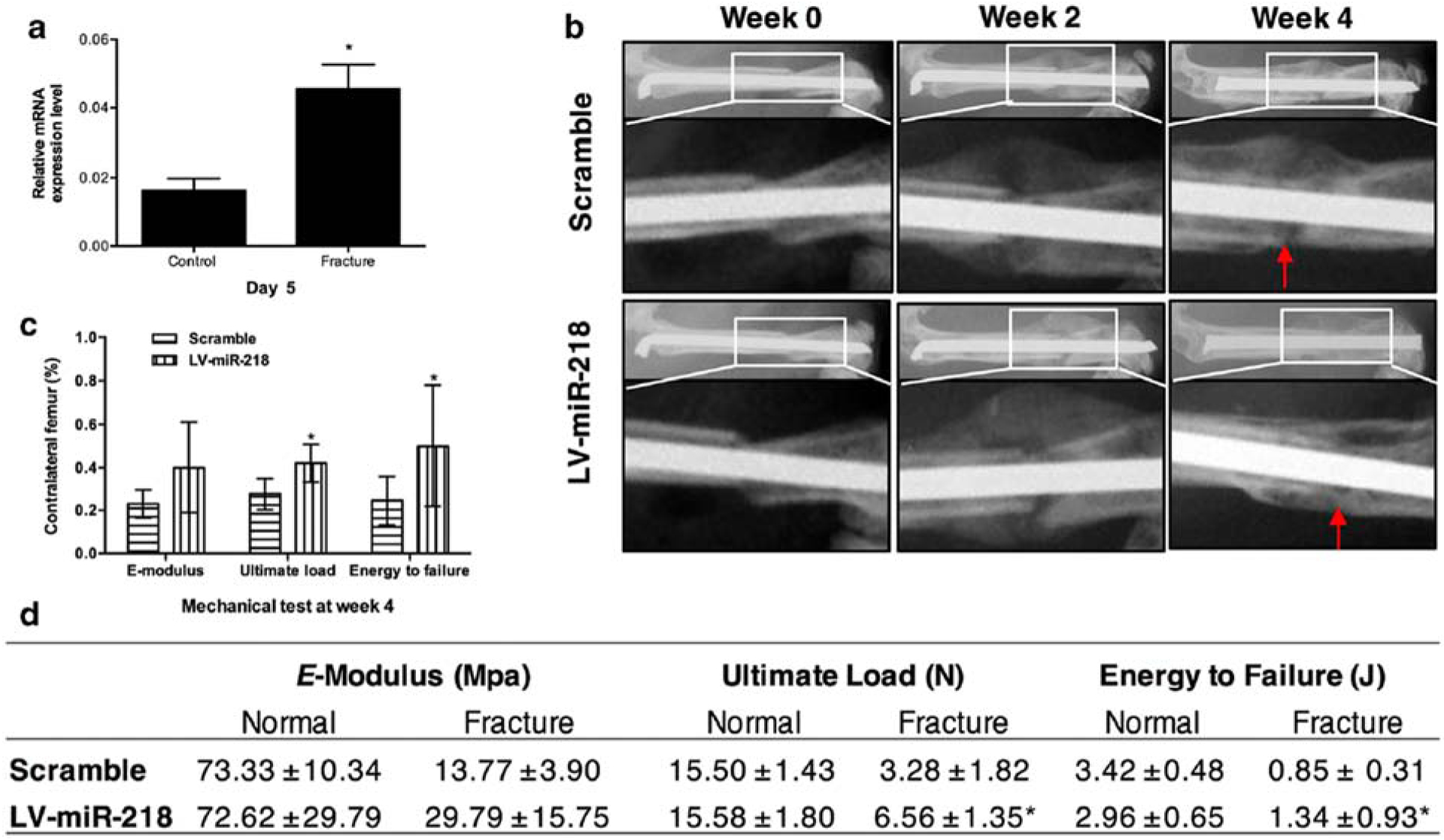
Newly formed bone was detected by X-ray and mechanical testing. a. miR-218 was significantly increased at day 5 post fracture. b. X-ray images at week 2 and week 4 post-fracture. c and d. Three-point bending mechanical test at week 4. *p < 0.05. Adopted from [58].
While Wnt signaling has a shorter history in the field of fracture healing than BMP signaling, it has rapidly garnered much attention and will likely result in the next class of therapeutics for fracture healing. The Wnt antagonists, sclerostin and DKK-1, are some of the most well-studied Wnt modulators and neutralizing antibodies to both of these proteins have been shown to be osteogenic and capable of enhancing fracture healing [59]. Consistent with this, the studies by Hu et al., [42] and Sui et al., [44] that used miRNAs to inhibit sclerostin and DKK-1, respectively, validated these targets for enhancing fracture healing. Similarly, the study by Shi et al., [58] used miR-218 to inhibit multiple Wnt antagonists and was also able to demonstrate enhanced fracture healing. As in the BMP studies, many of the studies in this section used exogenous cells as delivery vehicles and more direct approaches should be examined. Unfortunately, activation of Wnt signaling to enhance fracture healing carries a potential risk of promoting cancer as Wnt signaling is up-regulated in numerous types of cancers (e.g., colorectal, breast, leukemia, and melanoma) and has been shown to maintain cancer stem cells, promote metastases, and facilitate immune evasion [60]. Thus, Wnt-based therapeutics may only prove viable for short-term enhancement of fracture healing and will require rigorous studies to ensure that any such therapeutics are effective and safe.
PTEN/PI3K/Akt
The phosphatase and tensin homolog (PTEN)/ phosphoinositide 3-kinase (PI3K) /Akt pathway is also an important regulator of osteoblast differentiation [61]. Yang et al., observed that miR-21 regulated the PTEN/PI3K/Akt pathway in other systems and evaluated its function in osteogenesis [62]. Following lentiviral transduction of BMSCs with miR-21, they saw enhanced osteogenesis that was attributed to upregulation of PI3K, Akt, and hypoxia-inducible factor 1α (HIF-1α), concomitant with decreased PTEN expression. They consequently implanted miR-21-overexpressing BMSCs into rat calvarial defects using β-TCP scaffolds and identified increased BMD and Tb.Th. in the miR-21 group as compared to untransduced BMSCs and scaffold only controls. The study was then replicated in a canine mandibular defect model and, consistent with the rat study, microCT and histomorphometric analyses revealed enhanced defect healing in the miR-21 BMSC group compared to controls. Together, these data validate the efficacy of overexpression of miR-21 enhancing defect healing in two distinct species and strengthens its potential therapeutic potential.
miR-374b, known to play a role in tumorigenesis and cell growth and differentiation [63], was studied by Ge et al., to determine if it can regulate differentiation of BMSCs into osteoblasts [64]. First, PTEN was confirmed to be a direct target of miR-374b, which was then overexpressed in murine BMSCs where the authors observed enhanced expression of Alp, Runx2, Ocn, and Opn. In contrast, inhibition of miR-374b decreased the expression levels of these proteins, signifying that miR-374b promotes osteogenic differentiation of BMSCs via inhibition of PTEN. Finally, they showed that the expression of both miR-374b and PTEN was drastically elevated in BMSCs isolated from murine tibial fractures, confirming the activity of this signaling pathway during fracture healing.
PTEN inhibition in enhancing fracture healing was further supported by a study from Xiong et al., who evaluated the role of miR-26a-5p, a microRNA that was previously found essential for the regulation of bone growth in osteoporotic mice [65], during fracture healing [66]. PTEN was first confirmed to be a target of miR-26a-5p and transfection of MC3T3-E1 cells with agomiR-261–5p increase expression of Alp, collagen type I alpha 1 chain (Col1a1), Runx2, and Ocn mRNA, as well as enhanced matrix mineralization. Similarly, silencing of PTEN using siRNA also resulted in upregulation of the same osteoblast-related genes, confirming that osteoblast differentiation can be regulated by miR-26a-5p mediated repression of PTEN. Next, the expression of miR-26a-5p during femoral fracture healing in mice with and without concomitant with traumatic brain injuries (TBI) was evaluated and mice in the fracture + TBI group were characterized by significantly higher expression of miR-26a-5p and enhanced fracture healing, as evidenced by increases in BV, total volume (TV), BV/TV, and BMD. Finally, direct injection of agomiR-26a-5p into murine femoral fractures showed increased BV, TV, BV/TV, and BMD. These experiments prove that miR-26a-5p can accelerate osteoblast differentiation and thereby enhance fracture healing by suppressing PTEN and that miR-26a-5p upregulation contributes to enhanced fracture healing that often accompanies TBI.
In addition to its previously described role as a Wnt inhibitor, miR-214 has been shown to inhibit osteogenesis by binding to Activating Transcription Factor 4 (Atf4), which functions in the later stages of osteoblast differentiation and exerts its effects by activation of downstream Akt and ERK1/2 signaling [67]. Therefore, inhibition of miR-214 can also activate Akt signaling and potentially enhancing fracture healing. Ou et al., developed graphene oxide polyethyleneimine complexes to deliver miR-214 inhibitors, loaded them onto silk fibroin/hydroxyapatite scaffolds, and implanted them into rat calvarial defects [68]. Consistent with the earlier described miR-214 studies, microCT analyses found increased BV/TV and BMD in the defects of rats treated with the miR-214 inhibitor, as compared to unloaded scaffolds and empty controls. In addition, histological and IHC analyses displayed increased collagen and Ocn staining in the defects treated with the miR-214 inhibitor, confirming that miR-214 inhibition is a viable target for enhancing fracture healing, as well as presenting a novel approach to deliver miRNA inhibitors.
The PTEN/PI3K/Akt pathway has received less attention than the BMP and Wnt pathways during fracture healing. However, these miRNA-based studies confirm that PTEN inhibition is a valid target to enhance fracture healing. Of note, PTEN can be directly and indirectly targeted by multiple miRNAs (e.g., miR-374b, miR-26a-5p, and miR-21), creating several pathways to clinical adoption. As well, miR-21 also upregulated HIF-1α, a master regulator of several key processes in fracture healing [69], positioning miR-21 as a lead candidate for further evaluation.
WWP1
WW-domain-containing E3 ubiquitin protein ligase 1 (WWP1) is a member of the C2-WW-HECT subfamily of ubiquitin E3 ligases and has been shown to negatively impact osteoblastic differentiation [70]. Tu et al., determined that miR-142–5p targets the 3’UTR of WWP1 and that treatment of MC3T3-E1 cells with agomir-142–5p stimulated osteogenesis [71]. Next, direct injections in aged mice at the femoral fracture site with agomir-142–5p and negative controls resulted in higher radiographic scores and histological bone quality in the agomir 142–5p treated mice. In addition, microCT assessed BMD was increased, as were the protein expression levels of Runx2, JunB, and Ocn. Lastly, miR-142–5p levels were quantified in femoral fracture calluses from young and aged mice. The aged mice were found to express significantly lower levels of miR-142–5p, suggesting that age-related impairment of fracture healing may be mediated by diminution of miR-142–5p expression. These results will clearly need to be replicated in independent studies; however, identification of similar reductions in aged or, more importantly, osteoporotic human samples, would provide good evidence to support further studies of miR-142–5p in the context of fragility fractures.
SATB2
Special AT-rich-sequence–binding protein 2 (SATB2) belongs to a family of special AT-rich-sequence–binding proteins which bind to nuclear matrix attachment regions and serve as transcriptional regulators of numerous developmental processes, including osteoblastic differentiation [72]. Tang et al., established that miR-383 binds to the 3’UTR of STAB2 and inhibition of miR-383 is promotes osteogenic differentiation of BMSCs [73]. Implanting BMSCs expressing a miR-383 inhibitor into rat calvarial defects using a demineralized bone matrix scaffold revealed increased BV/TV and woven bone, in the miR-383 group compared to controls. These results indicate that miR-383 inhibition promotes STAB2-driven enhancement of fracture healing. More studies of miRNA regulation of SATB2 will be needed to determine if this represents a valid clinical target for enhancing fracture healing.
HOXA2
Previously, our lab established that Hox genes are expressed during fracture repair, including Homeobox A2 (Hoxa2) [74]. Hoxa2 is a negative regulator of the major osteoblast transcription factor, Runx2 [75]. In a study by Xie et al., miR-135 was found to repress Hoxa2 by binding to its 3’UTR [76]. Consistent with Hoxa2-induced inhibition of Runx2, lentiviral transduction of ADSCs with miR-135 drastically enhanced their osteogenic differentiation. miR-135-transduced ADSCs loaded on poly (sebacoyl diglyceride) (PSeD) polymeric scaffolds were then implanted in rat calvarial defects and microCT analyses showed the miR-135 group to have significantly enhanced BV/TV, BMD, and Tb.N, when compared to miR-135 inhibitor transduced cells, and controls. These findings were confirmed by histology, histomorphometry, and IHC staining showing increased Runx2 and decreased Hoxa2 expression in the miR-135 group. Therefore, miR-135 overexpression is another therapeutic option for enhancing fracture healing. However, as in prior sections, the use of exogenous cells transduced to express miR-135 does not represent an ideal path toward clinical adoption.
HMGA2, AQP1, and p38 MAPK
High Mobility Group AT-Hook 2 (HMGA2) is a nuclear binding protein that has been shown to promote the proliferation of BMSCs [77]. Tian et al., determined that HMGA2 is a direct target of miR-495, which negatively regulates osteoblast differentiation [78]. Specifically, overexpression of miR-495 in murine calvarial osteoblasts reduced mRNA and protein levels of Runx2, BMP-2, and Osx, while overexpression of anti-miR-495 had the opposite effect. Then, using a murine femoral drill-hole model, they demonstrated that treatment with anti-miR-495 enhanced bone regeneration, as evidenced by increased BV/TV, Tb.Th., and Tb.N.
HMGA2 is not the only target of miR-495, as Zhu et al., ascertained that miR-495 also binds to aquaporin-1 (AQP1) and thereby regulates the p38 mitogen-activated protein kinase (p38 MAPK) signaling pathway [79]. In contrast to the prior study, this study found that overexpression of miR-495 increased expression of Opn, Ocn, and ALP, as well as mineralized nodule formation in MC3T3-E1 cells. Similar effects were observed when p38 MAPK was chemically activated and the combination of miR-495 overexpression with p38 MAPK activation resulted in even greater enhancement of osteoblastic differentiation. Analysis of murine tibial fractures identified distinct upregulation of AQP1 and downregulation of miR-495 and p38 MAPK. These contrasting studies indicate that the precise role of miR-495 in fracture healing has yet to be clearly established and as such, further studies will be necessary to determine if miR-495 has utility in enhancing fracture healing.
miRNA Regulation of Chondrocytes
As opposed to the many studies on osteoblasts, very few reports are present in the literature dealing with chondrocytes, despite the fact that the formation of a cartilaginous soft callus and its subsequent conversion to bone via endochondral ossification is a vital component of fracture healing. All of the studies described in this section are listed in Table 2. A recent study reported that miR-9–5p regulates the development of osteoporosis by inhibiting osteogenesis via targeting of Wnt3a [80]. As Wnt3a has also been shown to regulate the activity of chondrocytes [81], Chen et al., investigated the function of miR-9–5p in cartilage remodeling [82]. They began by determining that mRNA expression levels of tenascin C (Tnc) were robustly elevated in chondrocytes isolated from mice with osteoarthritis (OA) and that Tnc is a binding target of miR-9–5p. Additionally, treatment of chondrocytes with a miR‐9‐5p mimic increased Alcian blue staining, promoted cell growth, migration, and expression of type II collagen, while decreasing apoptosis and type X collagen expression. Overexpression of miR-9–5p and downregulation of Tnc reduced chondrocyte apoptosis, as confirmed by TUNEL staining. Finally, the effects of miR-9–5p and Tnc on cartilage were evaluated following induction of tibial plateau fractures in mice with underlying knee OA. Improved cartilage quality, as evidenced by lower Mankin scores, was seen in knees overexpressing miR-9–5p compared to controls, with overexpression of Tnc demonstrating the reverse effect. While not a traditional fracture model, this study indicates that miR-9–5p inhibition can promote cartilage formation and maintenance after fracture.
Table 2:
miRNA Regulation of Chondrocytes
| miRNA Regulation of Chondrocytes | ||
|---|---|---|
| Authors | Design | Key Findings |
| Chen et al., 2019 [82] | Evaluated the effects of miR-9-5p on cartilage using a murine tibial plateau fracture model in mice with underlying knee OA | Overexpression of miR-9-5p inhibited chondrocytes apoptosis and improved cartilage remodeling attributed to repression of Tnc. |
| Baek et al., 2018 [85] | BMSCs expressing locked nucleic acid-anti-miR-449a evaluated in rat femoral osteochondral defect model. | miR-449a enhanced defect healing and expression of COL2A1 & ACAN attributed to repression of LEF1. |
Another cartilage regulatory protein is Sirtuin 1 (Sirt1), which has been shown to enhance interleukin-1b (IL-1β)-induced cartilage destruction [83], as well as promote the chondrogenic differentiation of BMSCs [84]. miR-449a binds to the 3’UTR of Sirt1 and silencing of miR-449a was shown to prevent IL-1 1β-induced expression of catabolic genes in cultured chondrocytes [83]. In an effort to determine if miR-449a inhibition can promote cartilage regeneration, Baek et al., transfected BMSCs with a locked nucleic acid (LNA) anti-miR-449a construct and implanted them into femoral osteochondral defects in mice using a fibrin gel carrier [85]. Histological and IHC analyses demonstrated improved GAG content, higher O-Driscoll scores, and enhanced expression of Col2A and aggrecan (Acan) in the anti-miR-449a treated defects relative to scrambled transfected BMSCs, untransfected BMSCs, and empty controls. This study intimates that repression of miR-449a can promote chondrogenesis and may prove useful in enhancing fracture healing.
Despite showing that modulation of both miR-9–5p and miR-449a can enhance cartilage regeneration, neither of these miRNAs have been evaluated in fracture healing models. Such evidence will be required before the clinical potential of these miRNAs can be assessed. In addition, given the importance of cartilage formation in fracture healing, additional work should be performed to identify miRNAs that regulate this process.
miRNA Regulation of Angiogenesis
The regeneration of functional blood vessels and capillary beds is integral to proper fracture healing [11]. As such, modulation of miRNAs that serve as regulators of angiogenesis has the potential to enhance fracture healing and a fair number of studies have been published (Table 3). miR-222 has been shown to inhibit angiogenesis by blocking endothelial cell proliferation and migration by targeting the stem cell factor receptor, c-Kit [86]. Moreover, miR-222 has also been reported to regulate the osteogenic differentiation of fibroblasts [87]. As such Yoshizuka et al., evaluated the effect of miR-222 modulation on fracture healing [88]. Initially, the authors analyzed osteogenic and chondrogenic differentiation of BMSCs after transfection with a miR-222 mimic or inhibitor. During osteogenesis, treatment with miR-222 mimic reduced mRNA expression levels of Runx2, Col1a1, and Ocn, as well as secretion of Alp and mineralized nodule formation, while transfection the miR-222 inhibitor reversed these activities. The effects on chondrogenesis were not as distinct, with the miR-222 mimic showing no effects on gene expression, but the miR-222 inhibitor upregulating Col2A1, Acan, and Sox9. Finally, they subjected rats to femoral fractures, in conjunction with periosteal cauterization, and delivered miR-222 mimic or inhibitor to the fracture site as part of an atelocollagen scaffold. Rats treated with miR-222 mimic displayed limited or no bony union radiographically and treatment with miR-222 inhibitor enhanced radiographic healing compared to both miR-222 mimic and negative controls. Most importantly, tissue sections from rats treated with miR-222 inhibitor displayed significantly higher capillary density than both miR-222 mimic and negative controls and a reduction in capillary density was found in miR-222 mimic compared to negative controls.
Table 3:
miRNA Regulation of Other Fracture Repair Processes
| Angiogenesis | ||
|---|---|---|
| Authors | Design | Key Findings |
| Yoshizuka et al., 2016 [88] | Assessed effects of miR-222 on angiogenesis and fracture healing using a rat femoral nonunion fracture model. | Treatment with miR-222 inhibitor enhanced healing and increased capillary density attributed to alleviation of c-Kit repression of angiogenesis. |
| Jiang et al., 2020 [89] | Measured miR-222 expression in BMSCs isolated from diabetic rats with femoral fractures and determined its effects on angiogenesis and osteogenesis. | Treatment of BMSCs with miR-222 mimic impaired osteogenic differentiation attributed to repression of TIMP-3. |
| Murata et al., 2014 [93] | Tested efficacy of systemic and local administration of antimiR-92a in murine femoral fracture model. | Systemic and local administration of antimir-92a increased angiogenesis and fracture healing, with cell culture studies suggesting this is solely through promotion of angiogenesis. |
| Janko et al., 2019 [94] | Treated rat femoral defects with BMCs expressing anti-miR-92a and anti-miR-335-5p on β-TCP scaffolds. | Inhibition of miR-92 increased blood vessel density, while combined inhibition of miR-92a and miR-335-5p had no effect, while anti-miR-335-5p and combined anti-miR-92a and anti-miR-335-5p enhanced fracture healing. |
| Apoptosis | ||
| Sun et al., 2019 [98] | Tested efficacy of direct injection of agomir-16-5p or antagomir-16-5p in murine femoral fracture model. | Treatment with antagomir-16-5p enhanced fracture healing via upregulation of BcI-2/Cylcin-D1 and decreased apoptosis. |
| Chen et al., 2020 [100] | Evaluated role of miR-701-3p in murine femoral fracture model. | Administration of antagomiR-701-3p enhanced fracture healing, as well as the number of PCNApositive cells, attributed to activation of FGFR-3. |
| Exosomes | ||
| Xu et al., 2020 [23] | Compared effects of BMSC exosomes isolated from young and old rats on osteogenic differentiation and the effects of miR-128-3p on rat femoral fractures. | Exosomes from young rats are more osteogenic and express lower levels of miR-128-3p, and |
| Chen et al., 2019 [103] | Generated exosomes overexpressing miR-375 and evaluated them in rat calvarial defect model. | miR-375 overexpressing exosomes enhanced defect healing attributed to repression of IGFBP-3. |
In separate study, Jiang et al., isolated BMSCs from rats with femoral fractures after induction of type 2 diabetes mellitus via injection of streptozotocin (Stz) [89]. Rats with diabetes and fractures displayed markedly increased levels of miR-222 that were accompanied by reductions Runx2, Alp, Col1A1, and Ocn mRNA. They then showed that miR-222 was able to target tissue inhibitor of metalloproteinases-3 (TIMP-3), a positive regulator of both osteogenesis and angiogenesis [90]. As expected, treatment of BMSCs with a miR-222 mimic reduced mRNA levels of Runx2, Alp, Col1A1, and Ocn, while the opposite effect was seen for miR-222 inhibition. Finally, treatment with TIMP-3 siRNA reversed the beneficial effects of miR-222 inhibition on osteogenic differentiation, indicating that the effects of miR-222 on osteogenesis are mediated by TIMP-3. Unfortunately, angiogenesis was not assessed in this study; however, these two studies support inhibition of miR-222 as a potential means to enhance fracture healing by promoting osteogenesis, chondrogenesis, and angiogenesis.
miR-92a is another inhibitor of angiogenesis that exerts its effects by targeting integrin subunit alpha 5 (ITGA5) and mitogen-activated protein kinase kinase 4 (MKK4) [91, 92]. Murata et al., identified miR-92a as downregulated in the plasma of patients with trochanteric fractures when compared to healthy controls [93]. Systemic and local administration of antimiR-92a to mice with femoral fractures resulted in increased BV, TV, BV/TV, and BMD. Furthermore, systemic administration of antimir-92a increased vessel volume, vessel number and area, CD31-positive vessels, and mRNA levels of VEGF-A, angiopoietin-1 (Ang1), and ITGA5 (Figure 5). They also assessed the impact of antimir-92a on osteoblastic and chondrocytic differentiation of primary murine calvarial osteoblasts and ATDC5 chondrogenic cells, respectively, and did not find any differences. As such, the authors concluded that inhibition of miR-92a enhances fracture healing solely through promotion of angiogenesis.
Figure 5.
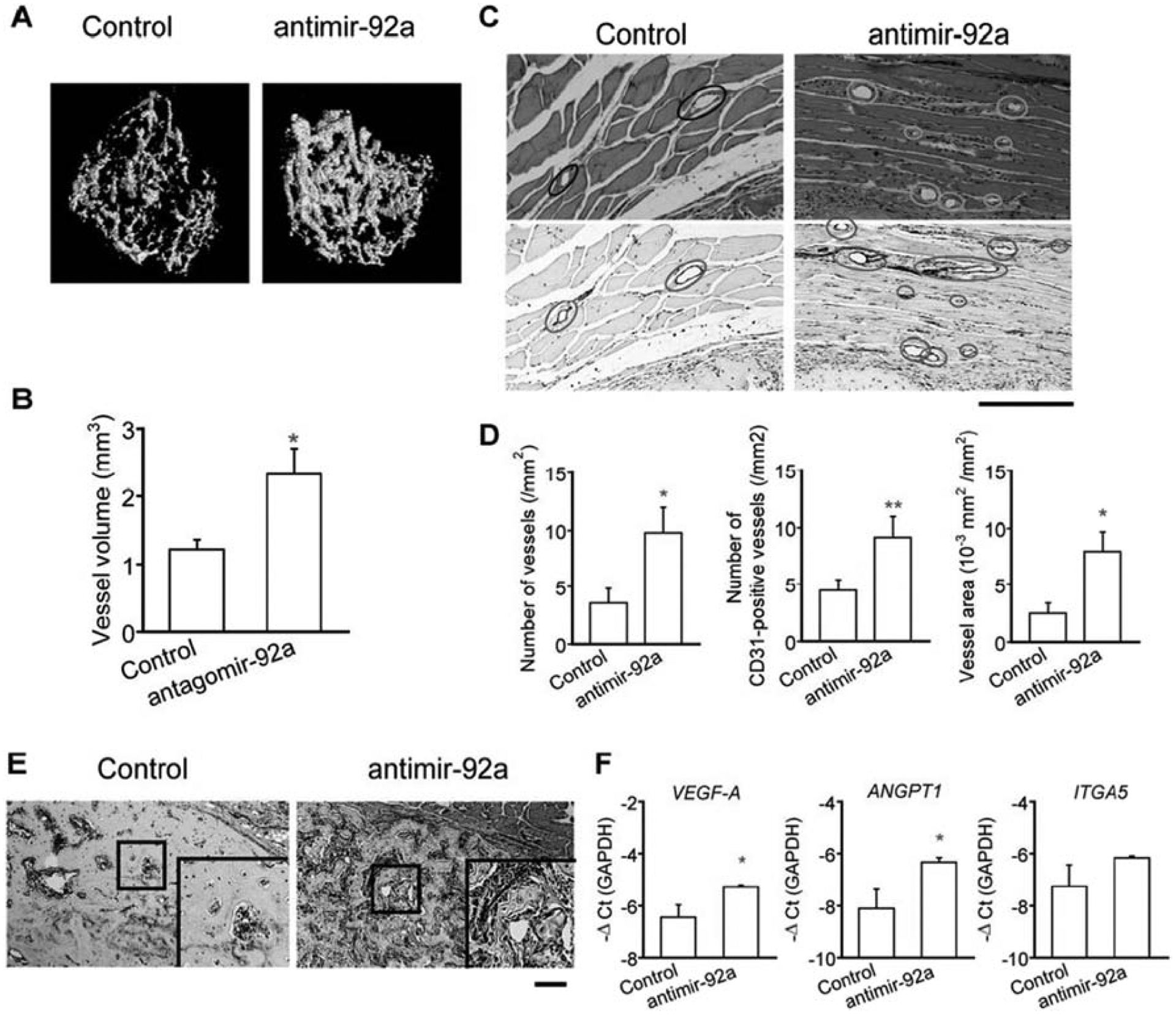
Suppression of miR-92a enhanced angiogenesis during fracture healing. A) Vascularity in the fractured femora of mice was visualized by microCT using a radioopaque silicon polymer medium on post fracture day 14. LNA was administered intravenously on days 0, 4, 7, 11, and 14 after the fracture. B) Vessel volume was quantified on 3D microCT images. n=5–6 mice per group. The data are shown as mean ± SEM. *p<0.05. C) Tissues surrounding the fracture callus on post fracture day 14 were stained with HE (upper panels) or immunohistochemically with anti-CD31 (lower panels). Scale bar=20μm. D) The number of vessels in HE-stained samples (left panel) and CD31-positive vessels (middle panel) were counted, and the ratio of vessel area was measured in HE-stained samples (right panel) (n=5–7 per group). E) Post fracture fay 14 calluses were stained with anti-CD31 (lower panels). Boxed areas are enlarged on right bottom. Scale bar for original images=200μm. F) The expression levels of VEGF-A, ANGPT1, and ITGA5 of callus from mice on post fracture day 14 were quantified by qRT-PCR (n=5, respectively). The data are shown as mean±SEM. *p<0.05; **p<0.01. Adopted from [93].
Another evaluation of the efficacy of miR-92a inhibition in enhancing fracture healing was performed by Janko et al., [94]. In addition to inhibiting miR-92a, this study also inhibited miR-335–5p, a modulator of osteogenic differentiation [95]. To do so, they created 5–6 mm long mid-shaft femoral defects, stabilized with locking plates, in rats. The defects were then filled with bone marrow mononuclear cells (BMC) transfected with scrambled control, anti-miR-92a, anti-miR-335–5p, or combination anti-miR-92a and anti-miR-335–5p that were loaded onto β-TCP scaffolds. As expected, inhibition of miR-92 significantly increased blood vessel density, as determined by IHC staining for alpha-smooth muscle actin (α-SMA); however, combined inhibition of miR-92a and miR-335–5p failed to enhance vessel density. In terms of bony healing, rats treated with anti-miR-335–5p and the combination of anti-miR-92a and anti-miR-335–5p showed increased bone area, but only the rats treated solely with anti-miR-335–5p had improved biomechanical outcomes, with these rats demonstrating a significant increase in ultimate force. Taken together, these results imply that local application of BMCs transfected with anti-miR-335–5p is superior to application of BMCs transfected with anti-miR-92a in enhancing the healing of critical-sized defects.
Taken together, these studies of miRNA modulation extend the broad base of literature that has supported facilitating angiogenesis as an effective means of enhancing fracture healing [96]. However, similar to the concerns raised for Wnt modulation, enhanced angiogenesis also carries a risk for promoting cancer progression, in particular solid tumor growth, survival, and metastases [96]. As such, any potential miRNA therapeutics focused on angiogenesis will need to be critically evaluated for safety and likely use for limited periods of time. Despite these concerns, inhibition of both miR-222 and miR-92a appears promising in promoting angiogenesis and enhancement of fracture healing, thereby justifying further studies to evaluate their potential clinical efficacy.
miRNA Regulation of Apoptosis
Apoptosis is yet another critical regulator of fracture healing and we have previously linked decreased osteoblast and chondrocyte apoptosis to the development of larger, stiffer, and stronger fracture calluses, hallmarks of enhanced fracture healing [97]. Not surprisingly, several miRNAs have been associated with regulation of apoptosis during fracture healing (Table 3) and miR-16–5p is one such example. Sun et al., measured serum levels of miR-16–5p in clinical samples and found that it was downregulated after fracture [98]. They then transfected MC3T3-E1 cells with agomir-16–5p or antagomir-16–5p and found that downregulation of miR-16–5p via antagomiR-16–5p-treatment enhanced proliferation and increased the percentage of cells in S phase, suggesting that antagomiR-16–5p may also stimulate cell cycle progression. Furthermore, flow cytometry analysis of annexin V staining revealed an increase in the percent of apoptotic cells after agomir-16–5p transfection, along with a corresponding decrease in the percent of apoptotic cells after antagomiR-16–5p transfection (Figure 6). They then determined at least one mechanism underlying miR-16–5p inhibition of osteoblast proliferation and promotion of osteoblast apoptosis; miR-16–5p binding to the 3’UTR of Bcl-2 and Cyclin-D1. Finally, femoral fractures were generated in mice and treated with direct injection of agomir-16–5p or antagomir-16–5p. MicroCT scans identified significant increases in BV, TV, and BV/TV in the mice treated with antagomir-16–5p along with concurrent decreases in these parameters in mice treated with agomir-16–5p. Collectively, these data show that miR-16–5p negatively regulates bone remodeling through the downregulation of BcI-2/Cylcin-D1 and increased apoptosis and inhibition of miR-16–5p can enhance fracture healing. Interestingly, miR-16–5p expression is elevated in women with osteoporosis and was also shown to target vascular endothelial growth factor A (VEGF-A), with agomiR-16–5p and siRNA against VEGF-A both inhibiting osteoblastic differentiation of BMSC to a similar extent [99]. Given the importance of angiogenesis in fracture healing it would be prudent to determine if miR-16–5p also affects this process.
Figure 6.
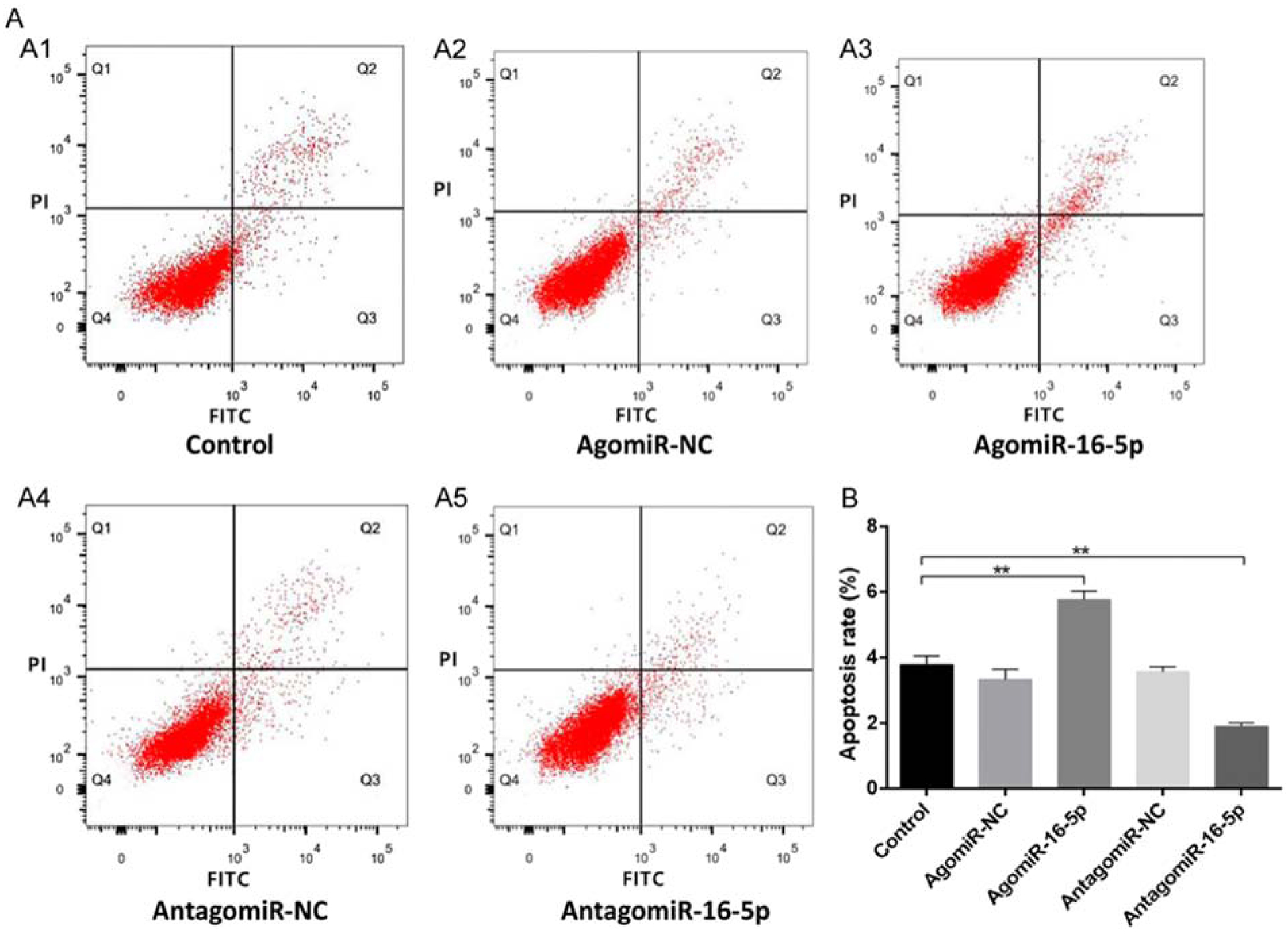
Downregulation of miR-16–5p inhibits osteoblast apoptosis. A. Apoptosis of osteoblasts in each group. B. Quantitative apoptosis rate in each group. All data were expressed as means ± SD. Significance is noted at these thresholds: *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA with a post hoc test was performed. Statistical differences between two groups were determined by Student’s t test. Adopted from [98].
Another mediator of apoptosis is miR-701–3p. Chen et al., performed a series of experiments focused on the role of lncRNA KCNQ1OT1 during fracture healing [100]. They first used RNA immunoprecipitation assays to verify that miR-701–3p is direct target of KCNQ10T1 and in turn, miR-701–3p targets fibroblast growth factor receptor 3 (FGFR-3). Silencing of KCNQ1OT1 in MC3T3-E1 cells induced cell cycle arrest at the G1 phase and promoted apoptosis in conjunction with decreases in protein expression of the anti-apoptotic gene, Bcl-2, and increases in the pro-apoptotic gene, Bax. Transfection with agomir-701–3p led to the same results confirming that KCNQ1OT1 acts as a sponge of miR-701–3p and a final series of transfection experiments validated that KCNQ1OT1-mediated removal of miR-701–3p promotes cell proliferation and inhibits apoptosis through the activation of FGFR-3. Femoral fractures were then generated in mice, followed by administration of agomiR-701–3p or antagomiR-701–3p via local injection. As expected, administration of agomiR-701–3p impaired fracture healing with microCT analyses showing reduced BV, TV, BV/TV, and BMD, while administration of antagomiR-701–3p increased these parameters. Surprisingly, the authors did not assess apoptosis, but did report a greater abundance of proliferating cell nuclear antigen (PCNA)-positive cells in the sections from the antagomiR-701–3p treated mice.
These data remain quite preliminary and more detailed studies into understanding how alterations in apoptosis and cell cycling can be modulated to enhance fracture healing must be carried out to validate apoptosis inhibition as a safe and effective clinical approach. Regardless, these studies support apoptosis inhibition as a viable method to enhance fracture healing and this approach may have particular importance in older populations where reduced cell numbers are associated with impaired fracture healing [101]. Similarly, strategies using miRNA modulation to promote cell proliferation and migration to fracture sites may also prove beneficial.
miRNA and Exosomes
Exosomes can be regarded as endogenous gene delivery vehicles and several exosomal-derived miRNAs have been shown to be upregulated or downregulated during osteogenic differentiation [102]. As such, delivery of exosomes represents an attractive option for enhancing fracture healing and these studies are listed in Table 3 and described below. A study by Xu et al., assessed the efficacy of exosomes derived from BMSCs in treating rat femoral fractures [23]. They first isolated exosomes from BMSCs collected from young (4 week) and old (72 week) rats and determined that there were no significant differences in their size distribution (50–150 nM) or expression of surface markers CD81 and CD63. They then evaluated the effects exposure to young exosomes (Young-Exos) and old exosomes (Aged-Exos) on osteogenic differentiation of young BMSCs. Compared to saline controls, Aged-Exos enhanced osteoblast differentiation, as seen by increases in Runx2, Alp, and Col1 mRNA, as well as Alp and Alizarin red staining. However, Young-Exos further enhanced all of these outcomes in comparison to both saline and Aged-Exos. Generating femoral fractures in rats and treated them with local injection of saline, Young-Exos, or Aged-Exos revealed that Aged-Exos were able to enhance callus volume and BT/TV compared to saline, with Young-Exos again proving superior to both saline and Ages-Exos. This pattern of enhancement was matched by similar increases in the expression levels of Runx2, Alp, and Col1 mRNA. Microarray analyses of the exosomes to identify differentially expressed miRNAs showed that Aged-Exos expressed higher levels of miR-99a-5p, miR-128–3p, and miR-26a-5p than Young-Exos, with the largest difference seen for miR-128–3p. Next, they established that miR-128–3p targets Smad5 and that osteogenic differentiation of BMSCs is similarly inhibited by miR-128–30 mimics and silencing of Smad5. Finally, they showed that treatment of rat femoral fractures with miR-128–3p antagomirs enhanced fracture healing, which was accompanied by robust upregulation of SMAD5. Results from these experiments indicate that Exos isolated from young BMSCs are superior to those isolated from aged BMSCs in enhancing fracture healing and this effect is due in part to the reduced levels of miR-128–3p in Young-Exos.
Another study that investigated exosomes and fracture healing was undertaken by Chen et al. [103]. This study involved enrichment of exosomes for miR-375, a regulator of BMP signaling and recently described biomarker for osteoporotic fractures [104, 105]. ADSCs were transfected to overexpress miR-375 and exosomes, termed Exo (miR-375), were collected from these cells and negative controls, Exo (NC). Subsequently, BMSCs were treated with the control Exo (NC) and Exo (miR-375) and assessed for osteogenic differentiation. Exposure to Exo (miR-375) enhanced Alp and Alizarin red staining, as well as mRNA expression of Runx2, ALP, Col1a1, and Ocn. They also determined that insulin-like growth factor binding protein 3 (IGFBP-3), a modulator of cell differentiation, is a direct target of miR-375. Furthermore, administration of recombinant IGFBP-3 in conjunction with Exo (miR-375) blocked osteogenic differentiation, indicating that the osteogenic effects of Exo (miR-375) are mediated by repression of IGFBP-3. Finally, they generated calvarial defects in rats and treated them with Exo (NC) and Exo (miR-375) loaded hydrogels, as well as unloaded hydrogels and nothing. MicroCT analyses revealed significant improvements in BV/TV and BMD for the Exo (NC) treated rats compared to empty defects, with Exo (miR-375) treatment further improving both of these parameters. These analyses were complemented by IHC analyses exhibiting increased Ocn and BMP-2 staining, concomitant with decreased IGFPB-3 staining in the Exo (miR-375) treated rats. Consistent with Xu et al. [23], this study shows that exosomes are capable of enhancing fracture healing and that enrichment of exosomes by age or overexpression of specific miRNAs can amplify their efficacy.
We are only beginning to understand the importance of endogenous exosomes in processes such as fracture healing, thus their development as therapeutics will take considerable time and effort. As such, enhanced or fully synthetic exosomes may provide a shorter path to clinical trials. Regardless of the source, considerable basic science studies need to be performed on exosomes before we can begin to evaluate their clinical potential to enhance fracture healing.
Summary and Conclusions
Fracture healing is a tremendously complex biological process and with every passing year we add hundreds of new studies to our knowledge. While miRNAs were first discovered in 1993 [106], their ubiquity in regulating biological processes was not appreciated until recently and our understanding of their role in the multitude of cellular activities requisite for successful fracture healing has only begun. miRNA regulation of osteoblast differentiation and function represents the aspect of fracture healing that has been most thoroughly studied. Not surprisingly, the BMP and Wnt/β-catenin signaling pathways are at the forefront of this avenue of research and activation of both of these pathways has shown efficacy in enhancing fracture healing in numerous small animal models. However, this review also shows that the lesser studied osteogenic signaling pathways of PTEN/PI3K/Akt, WWP1, SATB2, HOXA2, HMGA2, AQP1, and p38 MAPK are also valid targets for enhancing fracture healing by modulating miRNA activity. Reports on the effects of miRNAs on chondrocyte differentiation and function are much more limited and the majority of research in this area has focused on osteoarthritis rather than fracture healing. Though there is some overlap in these lines of research, it would be valuable to see additional studies focused on how miRNAs regulate chondrocytes and chondrogenesis explicitly during the fracture healing process. Similarly, we were unable to find any reports describing miRNA regulation of osteoclasts in the context of fracture healing and the contribution of these cells to fracture healing, especially endochondral ossification and remodeling, cannot be understated. The small number of identified studies of miRNA regulation of angiogenesis and apoptosis confirm that miRNAs regulate these processes during fracture healing and that, they too are valid targets for enhancing fracture healing.
The precise way to alter miRNA regulation of processes integral to fracture healing is beyond the scope of this review. The cited papers have utilized direct and systemic injection, delivery of cells expressing mimics or inhibitors on a variety of tissue engineering scaffolds, and implantation of various formulations of delivery matrices. While all approaches have shown efficacy, few studies reported on important issues such as the actual amount of inhibitor/activator that was delivered and how long it persisted systemically or locally. As such experiments comparable to the traditional pharmaceutical studies of bioavailability, pharmacokinetics/pharmacodynamics, and absorption, distribution, metabolism, and excretion (ADME) are warranted if we are to truly advance these potential therapeutics to clinical trials. Moreover, as clinical studies reporting on the expression and function of miRNAs during fracture healing continue to grow, it will be vital to correlate these clinical findings with existing preclinical data to facilitate their rapid translation to improving human fracture outcomes.
The ever-expanding number of publications on the effects of miRNAs on fracture healing gives us confidence that this avenue of research represents a rational and legitimate approach to clinically enhancing fracture healing and we look forward to the day that the first miRNA-based therapeutics are delivered to patients. Meanwhile, we must continue to press ahead with further exploration of the role of individual miRNA as regulators of the various phases of mammalian fracture healing.
Highlights.
Fractures are a major health care problem
MicroRNAs are becoming key players in understanding the fracture healing process
miRNAs regulate osteoblastic differentiation and osteogenesis via signaling pathways
miRNAs regular chondrogenesis
miRNAs regulate angiogenesis and apoptosis
Acknowledgements
The authors gratefully acknowledge the financial support by a grant, R15HD092931 (MH), from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Initiative: USBaJ The Burden of Musculoskeletal Diseases in the United States (BMUS), Third Edition 2014. [Google Scholar]
- [2].Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg 2016;151: e162775. [DOI] [PubMed] [Google Scholar]
- [3].Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. Journal of Bone and Mineral Research 2014;29: 2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Briggs A, Woolf A, Dreinhoefer K, Homb N, Hoy D, Kopansky-Giles D, Akesson K, March L. Reducing the global burden of musculoskeletal conditions. Bulletin of the World Health Organization 2018;96: 366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Force UPST. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA 2018;319: 2521–2531. [DOI] [PubMed] [Google Scholar]
- [6].Komatsu DE, Mary MN, Schroeder RJ, Robling AG, Turner CH, Warden SJ. Modulation of Wnt signaling influences fracture repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2010;28: 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin W, Xu L, Pan Q, Lin S, Feng L, Wang B, Chen S, Li Y, Wang H, Li Y, Wang Y, Lee WYW, Sun D, Li G. Lgr5-overexpressing mesenchymal stem cells augment fracture healing through regulation of Wnt/ERK signaling pathways and mitochondrial dynamics. Faseb j 2019;33: 8565–8577. [DOI] [PubMed] [Google Scholar]
- [8].Sun Z, Jin H, Zhou H, Yu L, Wan H, He Y. Guhong Injection promotes fracture healing by activating Wnt/beta-catenin signaling pathway in vivo and in vitro. Biomed Pharmacother 2019;120: 109436. [DOI] [PubMed] [Google Scholar]
- [9].Liu DB, Sui C, Wu TT, Wu LZ, Zhu YY, Ren ZH. Association of Bone Morphogenetic Protein (BMP)/Smad Signaling Pathway with Fracture Healing and Osteogenic Ability in Senile Osteoporotic Fracture in Humans and Rats. Med Sci Monit 2018;24: 4363–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem 2002;277: 30177–82. [DOI] [PubMed] [Google Scholar]
- [11].Hadjiargyrou M, O’Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res 2014;29: 2307–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Granero-Moltó F, Weis JA, Miga MI, Landis B, Myers TJ, O’Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009;27: 1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop 2013;37: 2491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clines GA. Prospects for osteoprogenitor stem cells in fracture repair and osteoporosis. Current opinion in organ transplantation 2010;15: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Y, Newman MR, Benoit DSW. Development of controlled drug delivery systems for bone fracture-targeted therapeutic delivery: A review. Eur J Pharm Biopharm 2018;127: 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou W, Yu L, Fan J, Wan B, Jiang T, Yin J, Huang Y, Li Q, Yin G, Hu Z. Endogenous Parathyroid Hormone Promotes Fracture Healing by Increasing Expression of BMPR2 through cAMP/PKA/CREB Pathway in Mice. Cell Physiol Biochem 2017;42: 551–563. [DOI] [PubMed] [Google Scholar]
- [17].Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am 2001;83: 259–70. [DOI] [PubMed] [Google Scholar]
- [18].Hadjiargyrou M, Zhi J, Komatsu DE. Identification of the microRNA transcriptome during the early phases of mammalian fracture repair. Bone 2016;87: 78–88. [DOI] [PubMed] [Google Scholar]
- [19].Hadjiargyrou M, Delihas N. The intertwining of transposable elements and non-coding RNAs. Int J Mol Sci 2013;14: 13307–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Waki T, Lee SY, Niikura T, Iwakura T, Dogaki Y, Okumachi E, Kuroda R, Kurosaka M. Profiling microRNA expression in fracture nonunions: Potential role of microRNAs in nonunion formation studied in a rat model. Bone Joint J 2015;97-b: 1144–51. [DOI] [PubMed] [Google Scholar]
- [21].Yu X, Odenthal M, Fries JWU. Exosomes as miRNA Carriers: Formation-Function-Future. International journal of molecular sciences 2016;17: 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Daly M, O’Driscoll L. MicroRNA Profiling of Exosomes. Methods Mol Biol 2017;1509: 37–46. [DOI] [PubMed] [Google Scholar]
- [23].Xu T, Luo Y, Wang J, Zhang N, Gu C, Li L, Qian D, Cai W, Fan J, Yin G. Exosomal miRNA-128–3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J Nanobiotechnology 2020;18: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med 2016;5: 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hadjiargyrou M, Komatsu DE. The Therapeutic Potential of MicroRNAs as Orthobiologics for Skeletal Fractures. J Bone Miner Res 2019;34: 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu R, Shen D, Sohun H, Ge D, Chen X, Wang X, Chen R, Wu Y, Zeng J, Rong X, Su X, Chu M. miR‑186, a serum microRNA, induces endothelial cell apoptosis by targeting SMAD6 in Kawasaki disease. Int J Mol Med 2018;41: 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang C, Zheng GF, Xu XF. MicroRNA-186 improves fracture healing through activating the bone morphogenetic protein signalling pathway by inhibiting SMAD6 in a mouse model of femoral fracture: An animal study. Bone Joint Res 2019;8: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deng J, Wu J, Zhu Y. Inhibition of MicroRNA-9 Improves Fracture Healing by Modulating the Bone Morphogenetic Protein-7 Pathway. Pharmacology 2019;104: 352–358. [DOI] [PubMed] [Google Scholar]
- [29].Lee WY, Li N, Lin S, Wang B, Lan HY, Li G. miRNA-29b improves bone healing in mouse fracture model. Mol Cell Endocrinol 2016;430: 97–107. [DOI] [PubMed] [Google Scholar]
- [30].Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 2009;284: 15676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang Y, Chen B, Li D, Zhou X, Chen Z. LncRNA NEAT1/miR-29b-3p/BMP1 axis promotes osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Pathol Res Pract 2019;215: 525–531. [DOI] [PubMed] [Google Scholar]
- [32].Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell 2003;14: 2809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun Y, Xu J, Xu L, Zhang J, Chan K, Pan X, Li G. MiR-503 Promotes Bone Formation in Distraction Osteogenesis through Suppressing Smurf1 Expression. Sci Rep 2017;7: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wan DC, Pomerantz JH, Brunet LJ, Kim JB, Chou YF, Wu BM, Harland R, Blau HM, Longaker MT. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem 2007;282: 26450–9. [DOI] [PubMed] [Google Scholar]
- [35].Li KC, Lo SC, Sung LY, Liao YH, Chang YH, Hu YC. Improved calvarial bone repair by hASCs engineered with Cre/loxP-based baculovirus conferring prolonged BMP-2 and MiR-148b co-expression. J Tissue Eng Regen Med 2017;11: 3068–3077. [DOI] [PubMed] [Google Scholar]
- [36].Xie Q, Wei W, Ruan J, Ding Y, Zhuang A, Bi X, Sun H, Gu P, Wang Z, Fan X. Effects of miR-146a on the osteogenesis of adipose-derived mesenchymal stem cells and bone regeneration. Sci Rep 2017;7: 42840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li D, Yu K, Xiao T, Dai Y, Liu L, Li H, Jiang D, Xiong L. LOC103691336/miR-138–5p/BMPR2 axis modulates Mg-mediated osteogenic differentiation in rat femoral fracture model and rat primary bone marrow stromal cells. J Cell Physiol 2019;234: 21316–21330. [DOI] [PubMed] [Google Scholar]
- [38].Hankenson KD, Gagne K, Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev 2015;94: 3–12. [DOI] [PubMed] [Google Scholar]
- [39].Zhong N, Gersch RP, Hadjiargyrou M. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone 2006;39: 5–16. [DOI] [PubMed] [Google Scholar]
- [40].Sun L, Li Z, Xue H, Ren C, Li M, Lu Y, Sun H, Zhang K. MiR-26a promotes fracture healing of nonunion rats possibly by targeting SOSTDC1 and further activating Wnt/β-catenin signaling pathway. Molecular and Cellular Biochemistry 2019;460. [DOI] [PubMed] [Google Scholar]
- [41].Choy MHV, Wong RMY, Chow SKH, Li MC, Chim YN, Li TK, Ho WT, Cheng JCY, Cheung WH. How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review. J Orthop Translat 2020;21: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hu B, Li Y, Wang M, Zhu Y, Zhou Y, Sui B, Tan Y, Ning Y, Wang J, He J, Yang C, Zou D. Functional reconstruction of critical-sized load-bearing bone defects using a Sclerostin-targeting miR-210–3p-based construct to enhance osteogenic activity. Acta Biomater 2018;76: 275–282. [DOI] [PubMed] [Google Scholar]
- [43].Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J. Effects of miR-335–5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res 2011;26: 1953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sui L, Wang M, Han Q, Yu L, Zhang L, Zheng L, Lian J, Zhang J, Valverde P, Xu Q, Tu Q, Chen J. A novel Lipidoid-MicroRNA formulation promotes calvarial bone regeneration. Biomaterials 2018;177: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang L, Tang Y, Zhu X, Tu T, Sui L, Han Q, Yu L, Meng S, Zheng L, Valverde P, Tang J, Murray D, Zhou X, Drissi H, Dard MM, Tu Q, Chen J. Overexpression of MiR-335–5p Promotes Bone Formation and Regeneration in Mice. J Bone Miner Res 2017;32: 2466–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Teng JW, Ji PF, Zhao ZG. MiR-214–3p inhibits β-catenin signaling pathway leading to delayed fracture healing. Eur Rev Med Pharmacol Sci 2018;22: 17–24. [DOI] [PubMed] [Google Scholar]
- [47].Li KC, Chang YH, Hsu MN, Lo SC, Li WH, Hu YC. Baculovirus-Mediated miR-214 Knockdown Shifts Osteoporotic ASCs Differentiation and Improves Osteoporotic Bone Defects Repair. Sci Rep 2017;7: 16225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li M, Wang H, Huang T, Wang J, Ding Y, Li Z, Zhang J, Li L. TAB2 scaffolds TAK1 and NLK in repressing canonical Wnt signaling. The Journal of biological chemistry 2010;285: 13397–13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li KC, Chang YH, Yeh CL, Hu YC. Healing of osteoporotic bone defects by baculovirus-engineered bone marrow-derived MSCs expressing MicroRNA sponges. Biomaterials 2016;74: 155–66. [DOI] [PubMed] [Google Scholar]
- [50].Hwang S, Park SK, Lee HY, Kim SW, Lee JS, Choi EK, You D, Kim CS, Suh N. miR-140–5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett 2014;588: 2957–63. [DOI] [PubMed] [Google Scholar]
- [51].Trohatou O, Zagoura D, Bitsika V, Pappa KI, Antsaklis A, Anagnou NP, Roubelakis MG. Sox2 suppression by miR-21 governs human mesenchymal stem cell properties. Stem Cells Transl Med 2014;3: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y, Tang L, Ding Y, Jin Y. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res 2013;28: 559–73. [DOI] [PubMed] [Google Scholar]
- [53].Sun Y, Xu L, Huang S, Hou Y, Liu Y, Chan KM, Pan XH, Li G. mir-21 overexpressing mesenchymal stem cells accelerate fracture healing in a rat closed femur fracture model. Biomed Res Int 2015;2015: 412327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jia HL, Zhou DS. Downregulation of microRNA-367 promotes osteoblasts growth and proliferation of mice during fracture by activating the PANX3-mediated Wnt/β-catenin pathway. J Cell Biochem 2018. [DOI] [PubMed]
- [55].Ishikawa M, Iwamoto T, Fukumoto S, Yamada Y. Pannexin 3 inhibits proliferation of osteoprogenitor cells by regulating Wnt and p21 signaling. J Biol Chem 2014;289: 2839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem 2012;287: 42084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang WB, Zhong WJ, Wang L. A signal-amplification circuit between miR-218 and Wnt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation. Bone 2014;58: 59–66. [DOI] [PubMed] [Google Scholar]
- [58].Shi L, Feng L, Liu Y, Duan JQ, Lin WP, Zhang JF, Li G. MicroRNA-218 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells and Accelerates Bone Fracture Healing. Calcif Tissue Int 2018;103: 227–236. [DOI] [PubMed] [Google Scholar]
- [59].Schupbach D, Comeau-Gauthier M, Harvey E, Merle G. Wnt modulation in bone healing. Bone 2020;138: 115491. [DOI] [PubMed] [Google Scholar]
- [60].Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017;36: 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Guntur AR, Rosen CJ. The skeleton: a multi-functional complex organ: new insights into osteoblasts and their role in bone formation: the central role of PI3Kinase. J Endocrinol 2011;211: 123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yang C, Liu X, Zhao K, Zhu Y, Hu B, Zhou Y, Wang M, Wu Y, Zhang C, Xu J, Ning Y, Zou D. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1alpha pathway and enhances bone regeneration in critical size defects. Stem Cell Res Ther 2019;10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bian H, Zhou Y, Zhou D, Zhang Y, Shang D, Qi J. The latest progress on miR-374 and its functional implications in physiological and pathological processes. Journal of Cellular and Molecular Medicine 2019;23: 3063–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ge JB, Lin JT, Hong HY, Sun YJ, Li Y, Zhang CM. MiR-374b promotes osteogenic differentiation of MSCs by degrading PTEN and promoting fracture healing. Eur Rev Med Pharmacol Sci 2018;22: 3303–3310. [DOI] [PubMed] [Google Scholar]
- [65].Li Y, Fan L, Hu J, Zhang L, Liao L, Liu S, Wu D, Yang P, Shen L, Chen J, Jin Y. MiR-26a Rescues Bone Regeneration Deficiency of Mesenchymal Stem Cells Derived From Osteoporotic Mice. Molecular Therapy 2015;23: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Xiong Y, Cao F, Hu L, Yan C, Chen L, Panayi AC, Sun Y, Zhou W, Zhang P, Wu Q, Xue H, Liu M, Liu Y, Liu J, Abududilibaier A, Mi B, Liu G. miRNA-26a-5p Accelerates Healing via Downregulation of PTEN in Fracture Patients with Traumatic Brain Injury. Mol Ther Nucleic Acids 2019;17: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A, Li D, Hou Z, Lv K, Kan G, Cao H, Wu H, Song J, Pan X, Sun Q, Ling S, Li Y, Zhu M, Zhang P, Peng S, Xie X, Tang T, Hong A, Bian Z, Bai Y, Lu A, Li Y, He F, Zhang G, Li Y. miR-214 targets ATF4 to inhibit bone formation. Nat Med 2013;19: 93–100. [DOI] [PubMed] [Google Scholar]
- [68].Ou L, Lan Y, Feng Z, Feng L, Yang J, Liu Y, Bian L, Tan J, Lai R, Guo R. Functionalization of SF/HAP Scaffold with GO-PEI-miRNA inhibitor Complexes to Enhance Bone Regeneration through Activating Transcription Factor 4. Theranostics 2019;9: 4525–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone 2004;34: 680–8. [DOI] [PubMed] [Google Scholar]
- [70].Zhao L, Huang J, Zhang H, Wang Y, Matesic LE, Takahata M, Awad H, Chen D, Xing L. Tumor necrosis factor inhibits mesenchymal stem cell differentiation into osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells 2011;29: 1601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tu M, Tang J, He H, Cheng P, Chen C. MiR-142–5p promotes bone repair by maintaining osteoblast activity. J Bone Miner Metab 2017;35: 255–264. [DOI] [PubMed] [Google Scholar]
- [72].Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Farinas I, Karsenty G, Grosschedl R. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell 2006;125: 971–86. [DOI] [PubMed] [Google Scholar]
- [73].Tang J, Zhang Z, Jin X, Shi H. miR-383 negatively regulates osteoblastic differentiation of bone marrow mesenchymal stem cells in rats by targeting Satb2. Bone 2018;114: 137–143. [DOI] [PubMed] [Google Scholar]
- [74].Gersch RP, Lombardo F, McGovern SC, Hadjiargyrou M. Reactivation of Hox gene expression during bone regeneration. J Orthop Res 2005;23: 882–90. [DOI] [PubMed] [Google Scholar]
- [75].Kanzler B, Kuschert SJ, Liu YH, Mallo M. Hoxa-2 restricts the chondrogenic domain and inhibits bone formation during development of the branchial area. Development 1998;125: 2587–97. [DOI] [PubMed] [Google Scholar]
- [76].Xie Q, Wang Z, Zhou H, Yu Z, Huang Y, Sun H, Bi X, Wang Y, Shi W, Gu P, Fan X. The role of miR-135-modified adipose-derived mesenchymal stem cells in bone regeneration. Biomaterials 2016;75: 279–294. [DOI] [PubMed] [Google Scholar]
- [77].Kalomoiris S, Cicchetto AC, Lakatos K, Nolta JA, Fierro FA. Fibroblast Growth Factor 2 Regulates High Mobility Group A2 Expression in Human Bone Marrow-Derived Mesenchymal Stem Cells. J Cell Biochem 2016;117: 2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tian Z, Zhou H, Xu Y, Bai J. MicroRNA-495 Inhibits New Bone Regeneration via Targeting High Mobility Group AT-Hook 2 (HMGA2). Medical science monitor : international medical journal of experimental and clinical research 2017;23: 4689–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhu L, Lin ZW, Wang G, Zhang H, Liu B, Xu QJ. MicroRNA-495 downregulates AQP1 and facilitates proliferation and differentiation of osteoblasts in mice with tibial fracture through activation of p38 MAPK signaling pathway. Sci Rep 2019;9: 16171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang H, Wang X, Zhao H, Zhou C. MicroRNA-9–5p promotes osteoporosis development through inhibiting osteogenesis and promoting adipogenesis via targeting Wnt3a. European review for medical and pharmacological sciences 2019;23: 456–463. [DOI] [PubMed] [Google Scholar]
- [81].Thomas RS, Clarke AR, Duance VC, Blain EJ. Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis. Arthritis Res Ther 2011;13: R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen H, Yang J, Tan Z. Upregulation of microRNA-9–5p inhibits apoptosis of chondrocytes through downregulating Tnc in mice with osteoarthritis following tibial plateau fracture. J Cell Physiol 2019;234: 23326–23336. [DOI] [PubMed] [Google Scholar]
- [83].Park KW, Lee KM, Yoon DS, Park KH, Choi WJ, Lee JW, Kim SH. Inhibition of microRNA-449a prevents IL-1beta-induced cartilage destruction via SIRT1. Osteoarthritis Cartilage 2016;24: 2153–2161. [DOI] [PubMed] [Google Scholar]
- [84].Buhrmann C, Busch F, Shayan P, Shakibaei M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J Biol Chem 2014;289: 22048–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Baek D, Lee KM, Park KW, Suh JW, Choi SM, Park KH, Lee JW, Kim SH. Inhibition of miR-449a Promotes Cartilage Regeneration and Prevents Progression of Osteoarthritis in In Vivo Rat Models. Mol Ther Nucleic Acids 2018;13: 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci 2008;29: 12–5. [DOI] [PubMed] [Google Scholar]
- [87].Yu F, Cui Y, Zhou X, Zhang X, Han J. Osteogenic differentiation of human ligament fibroblasts induced by conditioned medium of osteoclast-like cells. Biosci Trends 2011;5: 46–51. [DOI] [PubMed] [Google Scholar]
- [88].Yoshizuka M, Nakasa T, Kawanishi Y, Hachisuka S, Furuta T, Miyaki S, Adachi N, Ochi M. Inhibition of microRNA-222 expression accelerates bone healing with enhancement of osteogenesis, chondrogenesis, and angiogenesis in a rat refractory fracture model. J Orthop Sci 2016;21: 852–858. [DOI] [PubMed] [Google Scholar]
- [89].Jiang C, Xia W, Wu T, Pan C, Shan H, Wang F, Zhou Z, Yu X. Inhibition of microRNA-222 up-regulates TIMP3 to promotes osteogenic differentiation of MSCs from fracture rats with type 2 diabetes mellitus. J Cell Mol Med 2020;24: 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shen Y, Winkler IG, Barbier V, Sims NA, Hendy J, Lévesque J-P. Tissue Inhibitor of Metalloproteinase-3 (TIMP-3) Regulates Hematopoiesis and Bone Formation In Vivo. PLOS ONE 2010;5: e13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009;324: 1710–3. [DOI] [PubMed] [Google Scholar]
- [92].Lai L, Song Y, Liu Y, Chen Q, Han Q, Chen W, Pan T, Zhang Y, Cao X, Wang Q. MicroRNA-92a negatively regulates Toll-like receptor (TLR)-triggered inflammatory response in macrophages by targeting MKK4 kinase. J Biol Chem 2013;288: 7956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Murata K, Ito H, Yoshitomi H, Yamamoto K, Fukuda A, Yoshikawa J, Furu M, Ishikawa M, Shibuya H, Matsuda S. Inhibition of miR-92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J Bone Miner Res 2014;29: 316–26. [DOI] [PubMed] [Google Scholar]
- [94].Janko M, Dietz K, Rachor J, Sahm J, Schroder K, Schaible A, Nau C, Seebach C, Marzi I, Henrich D. Improvement of Bone Healing by Neutralization of microRNA-335–5p, but not by Neutralization of microRNA-92A in Bone Marrow Mononuclear Cells Transplanted into a Large Femur Defect of the Rat. Tissue Eng Part A 2019;25: 55–68. [DOI] [PubMed] [Google Scholar]
- [95].Tomé M, López-Romero P, Albo C, Sepúlveda JC, Fernández-Gutiérrez B, Dopazo A, Bernad A, González MA. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death & Differentiation 2011;18: 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bahney CS, Hu DP, Miclau T 3rd, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol (Lausanne) 2015;6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Komatsu DE, Bosch-Marce M, Semenza GL, Hadjiargyrou M. Enhanced bone egeneration associated with decreased apoptosis in mice with partial HIF-1alpha deficiency. J one Miner Res 2007;22: 366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sun Y, Xiong Y, Yan C, Chen L, Chen D, Mi B, Liu G. Downregulation of microRNA-16–5p ccelerates fracture healing by promoting proliferation and inhibiting apoptosis of osteoblasts n patients with traumatic brain injury. Am J Transl Res 2019;11: 4746–4760. [PMC free article] [PubMed] [Google Scholar]
- [99].Yu T, You X, Zhou H, He W, Li Z, Li B, Xia J, Zhu H, Zhao Y, Yu G, Xiong Y, Yang Y. MiR-16-p regulates postmenopausal osteoporosis by directly targeting VEGFA. Aging 2020;12: 9500–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen L, Xiong Y, Yan C, Zhou W, Endo Y, Xue H, Hu Y, Hu L, Leng X, Liu J, Lin Z, Mi B, Liu G. LncRNA KCNQ1OT1 accelerates fracture healing via modulating miR-701–3p/FGFR3 axis. Faseb j 2020;34: 5208–5222. [DOI] [PubMed] [Google Scholar]
- [101].Clark D, Nakamura M, Miclau T, Marcucio R. Effects of Aging on Fracture Healing. Curr steoporos Rep 2017;15: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xu J-F, Yang G-h, Pan X-H, Zhang S-J, Zhao C, Qiu B-S, Gu H-F, Hong J-F, Cao L, Chen Y, Xia B, Bi Q, Wang Y-P. Altered MicroRNA Expression Profile in Exosomes during Osteogenic ifferentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. PLOS ONE 2014;9: 114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, Jia L, Zhou Y. Exosomes derived from miR-75-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell rolif 2019;52: e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zarecki P, Hackl M, Grillari J, Debono M, Eastell R. Serum microRNAs as novel iomarkers for osteoporotic vertebral fractures. Bone 2020;130: 115105. [DOI] [PubMed] [Google Scholar]
- [105].Liu C, Yuan B, Chen H, Xu M, Sun X, Xu J, Gao Y, Chen C, Jiang H, Zhang J. Effects of MiR-75-BMPR2 as a Key Factor Downstream of BMP15/GDF9 on the Smad1/5/8 and Smad2/3 ignaling Pathways. Cell Physiol Biochem 2018;46: 213–225. [DOI] [PubMed] [Google Scholar]
- [106].Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75: 843–54. [DOI] [PubMed] [Google Scholar]


