Abstract
During the practice of meditation, the occurrence of self-generated and spontaneous thought and the tendency of the mind to wander away from the intended goal of meditation is ubiquitous, and comprises one of the fundamental teachings of meditation: a heightened awareness of where our attention is placed, and an awareness of the contents of our mind. Mind-wandering in the context of meditation provides individuals a unique and intimate opportunity to closely examine the nature of the wandering mind, cultivating an awareness of ongoing thought patterns while simultaneously cultivating equanimity (evenness of temper or disposition) and compassion towards the content of thoughts, interpretations, and bodily sensations. In this review we provide a theoretical framework highlighting the neurocognitive mechanisms by which contemplative practices influence the neural and phenomenological processes underlying spontaneous thought. Our theoretical model focuses on several converging mechanisms: the role of meta-awareness in facilitating an increased moment to moment awareness of spontaneous thought processes, the effects of meditation practice on key structures underlying both the top-down cognitive processes and bottom-up sensory processes implicated in attention and emotion regulation, and the influence of contemplative practice on the neural substrates underlying perception and perceptual decoupling.
Introduction
Over last few decades we have witnessed an exponential rise of scientific interest and research on the effects of meditation practices and mindfulness-based interventions on brain structure and function (Fox et al., 2014; Lazar et al., 2005), behavior (Flook, Goldberg, Pinger, & Davidson, 2015; Malouf, Youman, Stuewig, Witt, & Tangney, 2017; Singh, Lancioni, Wahler, Winton, & Singh, 2008), genetic expression (Epel et al., 2013; Ornish et al., 2013), medical outcomes (Morone, Greco, & Weiner, 2008; Rosenzweig et al., 2010), clinical outcomes (Goyal et al., 2014), military performance (Carter & Carter III, 2016), professional performance (McConville, McAleer, & Hahne, 2017) and more. Accumulating research findings provide compelling evidence that mind-body practices such as meditation are associated with improvements in cognitive and affective processes (Tang, Hölzel, & Posner, 2015). Meditation practices aim to generate a dispositional quality refered to as mindfulness, which is considered to be a self-regulated attentional state focused on present-moment experience, emphasizing the features of curiosity, openness, and acceptance (Dahl, Lutz, & Davidson, 2015; Kabat-Zinn, 2003). While a majority of meditation practices aim to generate mindfulness through techniques that cultivate attentional clarity and stability (Wallace, 1999), regulate emotional responses to the content of our thoughts and experiences (Teper, Segal, & Inzlicht, 2013), and cultivate compassion for oneself and others (Hofmann, Grossman, & Hinton, 2011), the precise definition and translation of the term mindfulness (Pali: sati or Sanskrit: smrti) is currently a heavily debated topic among Buddhist scholars and scientists (Kirk Warren Brown & Ryan, 2004; Fox et al., 2014). Given that 33.2% of U.S. adults report the use of mindfulness, meditation, or some form of complementary health approach or intervention (Okoro, Zhao, Li, & Balluz, 2012), there is an increasing need for scientific research that sets forth clear terminology and constructs, elucidates the specific mechanisms by which meditation and mindfulness based practices and interventions exert their influence, and provides context as to how they may be optimally tailored to meet individual needs.
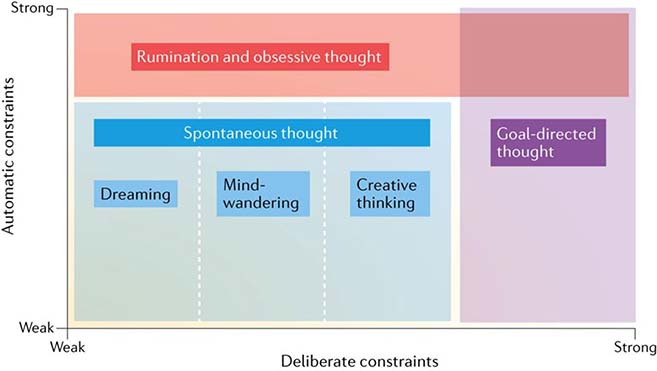
In parallel with the rise in popularity of contemplative science research (as well as the revival of methodologies that study the phenomenology of internal experience), the independent study of self-generated thought and the underlying psychological and neural mechanisms has become a key aim of cognitive neuroscientific research in recent years (Christoff, Irving, Fox, Spreng, & Andrews-Hanna, 2016; Fox et al., 2018). Self-generated thought constitutes a diverse and complex class of cognition which generally refers to mental content that occurs largely independent of the external environment, forming a stream of thoughts that can include memories, future plans, daydreams and fantasies, mental imagery, simulated social interactions, rumination, dreams, and more (Christoff et al., 2016). Like mindfulness, the scientific definition of self-generated thought and its corresponding phenomenology have been a recent subject of debate, with refinements in the categorical delineations between various forms of self-generated thought evolving alongside accumulating neuroimaging data. A recently proposed ‘state space’ framework developed by Christoff et al., 2016 highlights how both deliberate (i.e., intentional, top-down) and automatic (i.e., unconscious, bottom-up) constraints influence the content of self-generated thought (Fig. 1). According to this framework, self-generated thoughts include spontaneous (unconstrained and unintentional) forms of cognition like dreaming, mind-wandering, and creative thinking (Baird et al., 2012; Schooler et al., 2014), but also include forms of cognition such as rumination, obsessive thinking, and other habitual or automatically constrained patterns of thought (Mrazek, Smallwood, & Schooler, 2012; Unsworth & McMillan, 2013). Denoted by their independence from external stimuli, spontaneous thought, a term commonly interchanged with the broader term mind-wandering in the scientific literature and throughout this review (Andrews-Hanna, Irving, Fox, Spreng, & Christoff, 2017), can be understood as a mental state or sequence of mental states that arise relatively freely due to an absence of strong constraints on the contents of each state, and on the transitions from one mental state to another, lacking strong deliberate control (Christoff et al., 2016). General estimates suggest that individuals engage in some form of self-generated thought approximately 30–50% of waking hours (Killingsworth & Gilbert, 2010). Although cognitive psychologists and researchers have been investigating the specific mechanisms underlying spontaneous thought and mind-wandering for several decades now (Singer, 1966), advancements in research methodologies and scientific rigor over the last several years has allowed for a more nuanced neurophenomenological understanding of internal experience (Andrews-Hanna, Smallwood, & Spreng, 2014; Fox et al., 2014; Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015; J. Smallwood & Andrews-Hanna, 2013).
Figure 1.
The states space framework by Christoff et al., (2016) proposes a «conceptual space relating the concept of self-generated thought to deliberate and automatic constraints on cognition. Self-generated thought, by which we mean all those types of thought that are relatively independent of the external environment and immediate sensory inputs, spans a broad cognitive state space. Within this cognitive state space, both deliberate (intentional, top-down) and automatic (unconscious, bottom-up) constraints can influence the content of thought. “Spontaneous” thought is not only self-generated, but is also specifically characterized by relatively weak deliberate and automatic constraints. Rumination and obsessive thought are likewise self-generated and low in deliberate constraints, but are characterized by strong automatic constraints». Used with permission from Christoff et al., (2016).
These increasingly refined methodological approaches applied within the domains of self-generated thought and contemplative science (Bockelman, Reinerman-Jones, & Gallagher, 2013; Lutz & Thompson, 2003; Petitmengin & Lachaux, 2013; Thompson, 2008) have led to considerable progress in characterizing the first-person phenomenological experience and the associated neural correlates (Stawarczyk, 2018). Although the relevance of studying internal experience has garnered ample attention over the last decade, detailed phenomenological descriptions of the benefits of meditation, as well as the impact of mind-wandering on well-being, have been documented in scholarly Buddhists texts dating back over two thousand years (Santi-deva, 1961). Throughout these ancient writings an emphasis is placed on how attention, when it is not trained, becomes habitually prone to mind-wandering, agitation, and vapidity (Wallace, 1999), a mental state referred to as the ‘monkey mind’ (Gunaratana, 2010). During meditation practice the tendency of the mind to wander away from the intended object is ubiquitous (unless the meditation practice that does not include any specific object or target of attention), and its occurrence can be used as means of cultivating an awareness of its frequency, duration, and content, in order to gain insight into the nature of one’s thoughts and to facilitate the stability of attention. As meditation practice inherently cultivates an ability to monitor cognitive processes related to attention and distraction, meditation practitioners are particularly well suited to report on the phenomenological nature of these mental events, with accumulating evidence suggesting that meditation expertise increases the accuracy and reliability of first-person reporting and interoceptive acuity (Brandmeyer & Delorme, 2018; Fox et al., 2012).
Here we aim to further a dialogue regarding the convergence between historically separate lines of research (Mrazek et al., 2012) by providing a broad theoretical framework highlighting the key neurocognitive mechanisms by which contemplative practice may influence the underlying mechanisms mediating spontaneous thought processes. We start by exploring the wealth of literature supporting the relationship between contemplative practice and the manner by which the meditation training inherently cultivates a meta-awareness of the wandering mind. We specifically highlight the influence of contemplative practice on emotional reactivity and the reappraisal of spontaneous thoughts as key mechanisms by which meditation training influences the associated cognitive dynamics. We then delve into a more granular explication of spontaneous thought and mind-wandering, highlighting the intricacies of its phenomenology and its occurrence during contemplative practice. Subsequently we synthesize research findings to illustrate how spontaneous thought processes may be mediated by brain networks functionally and structurally linked to the practice of meditation. We then review the extensive literature on the role of a brain network known as the default network (DMN; Raichle et al., 2001) in both mind-wandering and meditation, and explore recent findings implicating distributed brain networks beyond the DMN. Lastly, we present a synthesis of findings highlighting the mechanisms by which meditation modulates attention and sensory perception, and the mechanisms underlying perceptual coupling and decoupling implicated in spontaneous thought processes. Throughout this manuscript we highlight the multidirectional relationship between these key mechanisms, and conclude with a section on perspectives, future directions, and the translational applications of this framework.
Meditation: cultivating meta-awareness and equanimity with the wandering mind
Scientific interest in the neurophysiological bases of meditation has in large part come from our understanding of neuroplasticity and various forms of experience-induced changes that occur in the brain (Lutz, Slagter, Dunne, & Davidson, 2008). The regular practice of meditation has been associated with increased functional connectivity (Farb et al., 2007; Froeliger et al., 2012a; Garrison, Scheinost, Constable, & Brewer, 2014; Garrison et al., 2014; Hasenkamp & Barsalou, 2012; Taren et al., 2015) as well as structural changes in the brain (Hofmann et al., 2011; Hölzel et al., 2011; Lazar et al., 2005). Lazar and colleagues (2005) were the first to show that the prefrontal cortex and right anterior insula, regions heavily implicated in attention monitoring and regulation (Lutz et al., 2008; Menon & Uddin, 2010; Tang & Posner, 2009), self-generated and spontaneous thought processes (Christoff, Ream, Geddes, & Gabrieli, 2003; Fox et al., 2018; Vanhaudenhuyse et al., 2011), interoception (Craig, 2002; Khalsa et al., 2008), and sensory processing (Haegens, Osipova, Oostenveld, & Jensen, 2010; Kerr et al., 2011; Kerr, Sacchet, Lazar, Moore, & Jones, 2013), were thicker in experienced meditation practitioners than in age and gender matched controls. They also found that the between-group differences in prefrontal cortical thickness were most pronounced in older practitioners, suggesting that meditation may slow age-related cortical thinning and that the thickness of these two specific areas also correlated with the degree of meditation experience. Lazar and colleagues provided some of the first structural evidence for experience-dependent cortical plasticity associated with meditation practice (Lazar et al., 2005). Additional research implicating the prefrontal cortex during focused attention meditation comes from the findings of Hasenkamp and colleagues (2012). The study highlights the naturalistic cognitive fluctuations between mind-wandering and attentional states derived from the practice of focused attention meditation. Their model proposes key intervals in the cognitive cycle of focused meditation: mind-wandering, awareness of mind-wandering, shifting of attention, and sustained attention and provides a foundation for the theoretical framework we discuss throughout this manuscript (Hasenkamp, Wilson-Mendenhall, Duncan, & Barsalou, 2012). Their findings support and extend theories regarding the central role of mind-wandering and its detection during focused meditation, as well as the cognitive correlates of distributed brain networks.
Despite the central role of mind-wandering and its occurrence during meditation (Banks, Welhaf, & Srour, 2015; Brandmeyer & Delorme, 2018; Evans & Segerstrom, 2011; Frewen, Evans, Maraj, Dozois, & Partridge, 2008; Hasenkamp & Barsalou, 2012; Hasenkamp et al., 2012; Jazaieri et al., 2014; Jha, Morrison, Parker, & Stanley, 2017; Morrison, Goolsarran, Rogers, & Jha, 2014; Mrazek, Franklin, Phillips, Baird, & Schooler, 2013; Stawarczyk & D’Argembeau, 2015; Vago & Zeidan, 2016; Zanesco et al., 2016), the explicit implications of long-term meditation practice on specific characteristics of self-generated thought and mind-wandering, such as the frequency, duration, content, and affect of spontaneous thoughts, with several exceptions (Banks et al., 2015; Evans & Segerstrom, 2011; Jazaieri et al., 2016; Sanger & Dorjee, 2016) have remained relatively unexplored. This is notable given that many of the benefits that come from meditation practice may be due to improved attention regulation and enhanced meta-awareness of ongoing thought processes (Lutz et al., 2008; Lutz et al., 2008; Menezes et al., 2013). Evidence for this comes from findings that focused attention meditation techniques have been shown to lead to an increased awareness of ongoing experience, emotions, and a decreased frequency with which mind-wandering occurs both during and outside of meditation practice (Baird, Mrazek, Phillips, & Schooler, 2014; Brandmeyer & Delorme, 2018; Dorjee, 2016; Sanger & Dorjee, 2016; Sze, Gyurak, Yuan, & Levenson, 2010). Mind-wandering in the context of meditation provides individuals a unique and intimate opportunity to examine the nature of mind-wandering and cultivate awareness of ongoing thought dynamics through the cyclical nature of the meditative process (Fig.2). Many meditation and mindfulness based practices emphasize the practice of non-judgmentally returning one’s attention to the breath or to the focal aim of the meditation practice (Baer, 2015; Davidson, 2010;Kabat-Zinn, 2003; Vago & Zeidan, 2016; Zeidan, Johnson, Diamond, David, & Goolkasian, 2010). Through this training, meditation practitioners learn to develop sustained attentional focus (Ainsworth, Eddershaw, Meron, Baldwin, & Garner, 2013; Lutz et al., 2008, 2009; Tang & Posner, 2009), metacognitive awareness of thoughts, feelings, and emotions (Baird, Mrazek, et al., 2014; Brandmeyer & Delorme, 2018; Dorjee, 2016; Sze et al., 2010), while simultaneously cultivating equanimity towards the content of thoughts, judgments, and experience (Hofmann et al., 2011; Jazaieri et al., 2014; Weng et al., 2013). It is important to emphasize that meditation practices are not intended to lead to a cessation of mind-wandering, but rather a mitigation of its potential deleterious effects and an improved awareness and openness to the passing nature of experience (Desbordes et al., 2015).
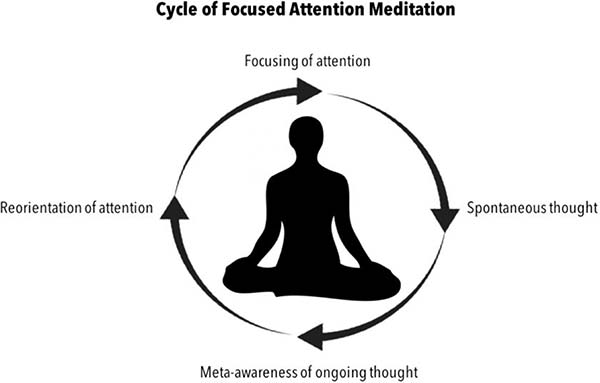
Figure 2.
The cycle of meditation and mind-wandering during a focused attention meditation practice. The neurocognitive model of how meditation cultivates awareness of mind-wandering by directly engaging the neural substrates implicated in attention regulation, perception, and meta-awareness. This cycle highlights the role of awareness of spontaneous thought and the cyclical observation and detection of involuntary shifts of attention as being at the core of focused meditation practice. Here a meditator begins meditating by 1) the focusing of attention, to then have their 2) attention shift to content of spontaneous thought, until the meditator 3) becomes aware the mind has been off focus, and 3) reorientes attention back to the focus of meditation.
The principal cycle of a focused attention meditation practice is to direct and maintain the focus of attention on a specified object (usually singular) of meditation. That is, one’s attention is completely occupied with focusing on the breath, a mental image, physical sensation, visual object, sound, or mantra (Travis & Shear, 2010; Wahbeh, Sagher, Back, Pundhir, & Travis, 2018). In open monitoring meditation practices (OM), the meditator focuses on cultivating awareness and acceptance of what is occurring in the present moment without any pre-determined focal object (Ainsworth et al., 2013; Colzato, Szapora, & Hommel, 2012a; Kabat-Zinn, 2003; Travis & Shear, 2010). Additional practices that help to facilitate increased awareness of the occurrence and content of mind-wandering are often added such as labeling emotions, thoughts, and the sensations in the body. These techniques aim to help prevent the meditator from harshly judging themselves once they become aware that the mind has wandered, while providing practitioners with a structured framework for effectively working with their thoughts (Tang et al., 2015).
Equanimity, which can be defined as an even-minded mental state or dispositional tendency toward all experiences or objects, regardless of their origin or their affective valence (pleasant, unpleasant, or neutral), is a key and central component of the meditation cycle and mindfulness, as with time, it transforms the way meditators respond and relate to their own internal thoughts and experience (Davidson, 2010; Desbordes et al., 2015). These techniques are also thought to facilitate the reappraisal of thoughts as passing phenomenon, while simultaneously associating the direct experience of the physical sensations associated with thoughts with a newly cultivated response of equanimity (Hölzel et al., 2011). Together, we suggest that these processes coalesce in creating a self-regulated closed-loop feedback system for the mind and body, eventually leading to enhanced detection of the early sensory signals associated with introspection and spontaneous thought processes during the meditation cycle (Fox et al., 2012; Kerr et al., 2011, 2013; Sze et al., 2010).
Evidence from our previous research implementing an experience sampling paradigm during an hour of focused meditation suggests that long-term meditation practice is associated with a reduced frequency of mind-wandering episodes (Brandmeyer & Delorme 2018). We additionally found a greater correspondence between the self-reports of meditation depth and the simultaneously measured electroencephalography (EEG) activity in long-term meditation practitioners, as compared to intermediate and novice practitioners. While this study demonstrated a direct effect of accrued meditation experience and reduced mind-wandering during meditation, it does not decipher whether this is due to a heightened meta-awareness of when the minds wander (e.g., detecting and redirecting their attention back to the focus of meditation more frequently), or improvements in sustained attention (engaged in longer periods of meditative absorption), or both. Additional research investigating the effects of meditation practice on mind-wandering have also found significant reductions in the number of mind-wandering events following a brief mindful breathing exercise (Mrazek et al., 2012), following one and three months of intensive meditation retreat practice (Zanesco et al., 2016), as well as following several weeks of meditation practice (Banks et al., 2015; Jazaieri et al., 2014; Jha et al., 2017; Morrison et al., 2014; Mrazek et al., 2013).
While further research should aim to garner a more granular understanding of these processes, we posit that this increased detection of mind-wandering observed in meditation practitioners most likely reflects a combination of enhanced attentional stability as well as improved meta-awareness, a position evidenced by a wealth of literature supporting the effects of both enhanced attention regulation (Ainsworth et al., 2013; Lutz et al., 2008; Menezes et al., 2013; Tang & Posner, 2009; van den Hurk, Giommi, Gielen, Speckens, & Barendregt, 2010) and enhanced metacognitive awareness (Baird, Mrazek, et al., 2014; Fox & Christoff, 2014; Sanger & Dorjee, 2016) in meditation and mindfulness practitioners.
Variations in tradition and technique are also likely to influence the degree of interplay between the focusing of attention, the aperture of awareness, and the processes underlying the evaluation of thought content and mind-wandering (Vago & Silbersweig, 2012; Vago & Zeidan, 2016). In the case of meditation practices in which the intentional generation of thoughts are explicitly employed - such as in loving kindness meditation (LKM) - these practices may require the individual to draw on ideas of the self and others, as well as past memories and conceptual notions of abstract concepts that often involve working with thought content explicitly and in ways that overlap with the processes involved in the generation of spontaneous thoughts. For example, in the case of focused attention, the target skill of focusing attention on the breath directly competes (opposing constructs) with mind-wandering for a limited set of cognitive resources. In the case of LKM, the thoughts specific to the practice become the spotlight of an internally directed self- or other-referential state of attention (Petersen & Posner, 2012). Theoretically, it may be impossible for meditators to practice LKM and have a dual awareness of mind-wandering at the same time because of the cognitive difficulty involved in holding two separate trains of thought simultaneously (although successive thoughts on the order of 1 second may be possible; Delorme & Brandmeyer, 2019). This is supported by a recent meta-analysis showing that during FA and OM meditation practices, with the only exception being LKM, large meta-analytic clusters were present in the posterior dorsolateral prefrontal cortex, as well as in the premotor and supplementary motor cortices, all frontal areas that are heavily implicated in higher order cognitive functions such as conflict detection, sustained attention, and emotional regulation (Amodio & Frith, 2006; Fox et al., 2016; Hanakawa et al., 2002).
A recent study by Fox and colleagues (2014) reported anatomical changes (e.g., increased cortical thickness; Lazar et al., 2005) in the frontopolar brain area across all three meditation practices (Fox et al., 2016, 2014). The frontopolar cortex is an area which has been functionally linked to meta-awareness and metacognitive capacity (Baird, Smallwood, Gorgolewski, & Margulies, 2013; Fleming & Dolan, 2012; Fleming, Huijgen, & Dolan, 2012; Fleming, Weil, Nagy, Dolan, & Rees, 2010; Fox et al., 2015; McCaig, Dixon, Keramatian, Liu, & Christoff, 2011), as well as in the evaluation of self-generated information (Christoff et al., 2003). As the frontopolar cortex serves as a hub for the frontoparietal control network (FPCN; (Dixon et al., 2018; Ptak, 2012; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2012; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008), it may also facilitate the alternation between endogenous and exogenous orientations of attention (Brandmeyer, 2017; Burgess, Dumontheil, & Gilbert, 2007; Dixon et al., 2018; Ptak, 2012; Spreng et al., 2012). Thus, while meta-awareness and metacognitive capacity appear to be cognitive features of all the aforementioned forms of contemplative practice, focused attention meditation practice appears to be specifically implicated in higher order cognitive functions, generally involving some focal point to be returned to upon the detection of spontaneous thought processes. For purposes of clarity we focus on research findings from studies investigating the effects of focused attention meditation practices (unless noted), as they are some of the most widely scientifically studied forms of meditation practice and best suited for exploring how contemplative practices may influence this phenomenology and the interplay between these constructs.
The phenomenology of spontaneous thought and the influence of contemplative practice
First-person accounts reveal spontaneous thought to be an incredibly complex phenomenon involving a multitude of time domains, and intellectual and creative content (Andrews-Hanna, Reidler, Huang, & Buckner, 2010; Fox & Christoff, 2014; Fox, Nijeboer, Solomonova, Domhoff, & Christoff, 2013; Klinger, 2009, 2013; McMillan, Kaufman, & Singer, 2013; Seli et al., 2018; J. Smallwood, 2013). It has been shown that mind-wandering episodes typically involve thinking about oneself, others, remembering the past, and planning for the future (Buckner, Andrews-Hanna, & Schacter, 2008; Gusnard, Raichle, & Raichle, 2001; Raichle et al., 2001). Across methodologies used to investigate self-generated forms of thought, research in healthy non-clinical populations shows that they are rated as mildly pleasant, positive, and enjoyable, and on average display a mild positive affect bias (Fox & Christoff, 2018). Research also suggests that mind-wandering serves as a mnemonic process, involving a variety of episodic forms of autobiographical memory facilitating an «autonoetic consciousness» unique to the human capacity of the awareness of self (Tulving & Craik, 2005; Vago & Zeidan, 2016). Tulving (2005) suggests that it is this autonoetic capacity which provides us the fundamental framework for the advancements in technology, society, our intelligence, and the intrinsic abilities necessary to navigate our complex internal and external environment. The ability to mentally move through time has been referred to as ‘mental time travel’ and has been directly linked to the episodic memory processes thought to generate mental content (Tulving, 2002).
Prospective bias, positive affect, goal-directed planning and creativity
Behavioral studies reveal dynamic interactions between spontaneous thought and executive function, that appear to depend on the difficulty of task the person might be doing (Smallwood, 2013; Smallwood & Andrews-Hanna, 2013). These findings support the context regulation hypothesis proposed by Smallwood and Andrews-Hanna (2013) which claims that the costs and benefits of mind-wandering partly depend on an individual’s ability to constrain task unrelated thought in situations that demand their attention (Andrews-Hanna, Smallwood, & Spreng, 2014; Smallwood & Andrews-Hanna, 2013). In healthy participants when demands on an individual are particularly low, evidence suggests that attentional resources are redirected towards supporting spontaneous thought processes that are predominately prospective in nature (Baird, Smallwood, & Schooler, 2011; D’Argembeau, Renaud, & Linden, 2011; J. Smallwood et al., 2011; Stawarczyk & D’Argembeau, 2015). In line with these findings, the prospective orientation of mind-wandering has been directly linked with the ‘current concerns’ of an individual (Andrews-Hanna et al., 2014; Klinger, 2013; McVay & Kane, 2010) suggesting that mind-wandering may facilitate autobiographical planning and the planning of future goals (Addis, Knapp, Roberts, & Schacter, 2012; Andrews-Hanna, Saxe, & Yarkoni, 2014; Baird et al., 2011).
These findings are supported by additional research showing that self-oriented thought increases the frequency of future thinking, and that prospective experiences mediate the memory advantage for self-referential information (Klein, Robertson, Delton, & Lax, 2012). Baird and colleagues (2011) implemented a choice reaction task to explore the temporal focus (i.e., past-, present-, or future-oriented) and cognitive orientation (i.e., self-related or goal-directed) of participants’ thoughts and found that participants’ thoughts were predominately future-oriented and self-related. They also found that when thoughts were both self-related and goal-directed they were more frequently future-focused than present or past-focused. These findings provide strong evidence that mind-wandering facilitates goal-directed planning in relation to personal concerns. Furthermore, individuals with improved executive control have been shown to limit their self-generated thought to nondemanding or unimportant contexts (Barron, Riby, Greer, & Smallwood, 2011). In line with these findings, social thoughts pertaining to one’s future have been shown to lead to subsequent positive thoughts, whereas social thoughts pertaining to one’s past precede negative mental content (Ruby, Smallwood, Engen, & Singer, 2013).
Retrospective bias, negative affect, rumination and clinical conditions
Research has shown that mind-wandering focused on the past is directly associated with increased negative affect in laboratory conditions (Ruby, Smallwood, Engen, & Singer, 2013; Smallwood et al., 2011; Stawarczyk, Majerus, & D’Argembeau, 2013) and in daily life (Poerio, Totterdell, & Miles, 2013). In a seminal study using experience sampling implemented with a mobile phone application, Killingsworth and Gilbert (2010) collected data on 2,250 participants and found that mind-wandering was associated with a reduced sense of well-being when mind-wandering events focused on the past. Psychopathological states such as anxiety and depression have also been linked to self-generated experiences that have past-oriented and perseverative features (Ottaviani & Couyoumdjian, 2013; Ottaviani, Shapiro, & Couyoumdjian, 2013), while an increased frequency of unaware mind-wandering was also associated with higher levels of depression (Deng, Li, & Tang, 2014). A majority of neurocognitive disorders can be characterized by dysfunctional regulation of both context and content, with alterations in both processes likely to yield devastating consequences on cognitive functioning and well-being (Andrews-Hanna et al., 2014). Furthermore, the neural mechanisms underlying spontaneous thought processes appear to play a direct role in clinical conditions such as post-traumatic stress disorder, depressive rumination, and a host of other mental health disorders where individuals have difficulty regulating the frequency of self-generated spontaneous thoughts (Andrews-Hanna, Saxe, & Yarkoni, 2014; Berman et al., 2011; Ehlers, Hackmann, & Michael, 2004; Nolen-Hoeksema, 2000; Poerio, Totterdell, & Miles, 2013; Smallwood, Fishman, & Schooler, 2007; Smallwood, McSpadden, & Schooler, 2007; Whitfield-Gabrieli & Ford, 2012).
These deficiencies often manifest as increased distractibility or elevated levels of mind-wandering (Smallwood, Fishman, et al., 2007; Watkins, 2008). Depressed individuals demonstrate an increased disposition for rumination and experience difficulties updating the contents of working memory and switching tasks (Deng et al., 2014). This leads to preexisting goal states exerting a stronger influence on ongoing mental processes and thought content in depressed individuals than for normal individuals (Andrews-Hanna et al., 2014; Fox et al., 2013, 2015). Individuals who score higher on constructs related to depression and trait negative affect on questionnaires also rate their self-generated thoughts as more negative in valence (Andrews-Hanna et al., 2013). Together these studies suggest that in the case of clinical and psychopathological conditions, an inability to monitor and disengage from the occurrence of excessive or distracting self-generated thoughts in a context-dependent manner is associated with impairments in wellbeing (Andrews-Hanna, Smallwood, & Spreng, 2014).
Opposing constructs?
When compared to mindfulness which aims to facilitate attentional focus, clarity, and stability, spontaneous thought and mind-wandering are characteristically defined as the disruption of focused attention or task focus (Smallwood & Schooler, 2006). Mind-wandering (spontaneous thought) has been shown to be particularly recurrent during nondemanding tasks and restful waking states (Kane et al., 2007; Singer, 1966), and appears to have a distinctly ‘mindless’ quality (Smallwood & Schooler, 2006), such as rapid and automatic responding during continuous performance tasks (Smallwood, Baracaia, Lowe, & Obonsawin, 2003), eye-movements during reading that demonstrate a reduced processing of the lexical or linguistic properties of what is being read (indicating perceptual decoupling; Reichle, Reineberg, & Schooler, 2010), as well as absent-minded forgetting (Smallwood et al., 2003; Smallwood & Schooler, 2006). The degree to which the constructs of mindfulness and mind-wandering oppose one another was explored in a set of studies by Mrazek and colleagues (2012) wherein they addressed the relationship between a dispositional measure of mindfulness using the Mindful Attention and Awareness Scale (MAAS; Brown & Ryan, 2003) and converging measures of both self-reported and indirect markers of mind-wandering. Negative correlations between dispositional mindfulness and four measures of mind-wandering suggest a relatively opposing relationship between the two constructs. They additionally found that eight minutes of mindful breathing reduces behavioral indicators of mind-wandering during a Sustained Attention to Response Task compared with both passive relaxation and reading. While these findings suggest a relatively opposing nature of the two constructs (Mrazek et al., 2012), the degree to which thoughts are constrained (or unconstrained) based on the task, or on the objectives of a given meditation practice is likely to influence the degree to with which these constructs oppose one another.
This is supported by findings from Colzato and colleagues (2012b) who found that open monitoring meditation (which promotes the ability to observe ongoing mental content and attend to various transient stimuli) increased creativity in an idea generation task, whereas focused attention meditation (training the ability to focus attention and awareness) did not. In an additional set of studies by Ostafin and Kassman (2012), the authors showed that a greater tendency toward mindfulness (which is generally taught as a style of open monitoring meditation), as assessed through the MAAS, was associated with an increased chance of solving the puzzles, and that a brief session of mindfulness meditation improved both situational mindfulness and problem solving. The authors take the position that mindfulness and the emphasis placed on the ‘present moment’ experience reduces the tendency toward habitual responses when searching for the solution to a creative problem. Zedelius and colleagues (2015) conducted a study to explore whether creative problems approached through an analytic meditation strategy or through an insight meditation (i.e., sudden awareness of a solution) would impact measures of creativity. They found impaired problem solving when approaching problems with insight meditation, whereas increased problem solving performance was associated with the use of the analytic (i.e., thought-based) meditation approach.
Baird and colleagues (2012) explored the hypothesis that mind-wandering would be associated with enhanced creativity through the use of incubation tasks that systematically varied in their levels of attentional demand and thus in their conduciveness to mind-wandering. Their findings suggest that performing an undemanding task during the incubation period improved creative performance to a greater extent than performing a demanding task, resting, or taking no break. They additionally found that scores on the daydreaming frequency subscale of the Imaginal Processes Inventory - a questionnaire measure that assesses individual’s tendency for mind-wandering in everyday life (Gold and Gold, 1982), correlated positively with scores on a classic creativity task both for repeated exposure and new exposure problems on the task. This last result suggests that specific types of spontaneous, unrelated thoughts facilitate creative problem solving, and that individuals who mind-wander more frequently in their daily lives may also be more creative in general. Although mind-wandering may be linked to compromised performance on a variety of experimenter-defined tasks (Barron et al., 2011; McVay & Kane, 2010), it also serves to facilitate creative ideas (Baird et al., 2012; Schooler et al., 2014) and memory consolidation (Addis et al., 2012).
Spontaneous thought content, affect, and well-being
Well-being is related to a complex variety of factors including culture, socioeconomic status, health, the quality of interpersonal relations, and specific psychological processes (Dinero, Conger, Shaver, Widaman, & Larsen-Rife, 2008). Research findings suggest that both the content of self-generated thought and the context under which it occurs significantly influence both the cognitive and affective impact of the experience, elucidating why the experience of mind-wandering can be detrimental for some individuals yet beneficial for others (Andrews-Hanna et al., 2014; Andrews-Hanna, Smallwood, & Spreng, 2014). Clinical research suggests that the ability to distance oneself and observe the ongoing internal train of thoughts plays a vital role in psychological wellbeing (Farb et al., 2007). Within the domain of cognitive psychology, latent conceptions of self are thought to underlie (to a significant degree) our thoughts and emotions and directly impact brain function (Hofmann, Schmeichel, & Baddeley, 2012). It has been proposed that one of the primary mechanisms by which contemplative practices affect well-being is by targeting and altering maladaptive self-referential thought patterns (Dahl et al., 2015). The content regulation hypothesis (Andrews-Hanna et al., 2014) suggests that a direct interaction between mood and content influence the relationship to affect (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Deng et al., 2014; Fox et al., 2018; Poerio et al., 2013; Ruby et al., 2013; Smallwood et al., 2011), with research directly linking the retrospective orientation of mind-wandering to premature aging (Epel et al., 2013) and negative affect.
Trait and state effects, emotion reappraisal, and the role of acceptance
Meditation training is thought to induce both state and trait effects. The term state effects refers to the distinction between ordinary mental states, and the specific mental states that are experienced during various styles of meditation practice. Changes that occur over months or years are referred to as trait effects. It is generally accepted that state effects translate to long-term trait effects with practice (Cahn & Polich, 2006). For example, thought reappraisal during meditation can be considered a state effect that occurs during the practice of meditation, however long-term training may lead to thought reappraisal that extends beyond formal meditation practice evolving into what could be considered a trait. Accumulative exposure to and awareness of the ongoing cognitive and affective dynamics involved in spontaneous thought processes plays a central role in the mechanisms underlying meditation practice (Hasenkamp et al., 2012), and the benefits observed in the context of emotion reappraisal (Hölzel et al., 2011). A growing body of literature now suggests that the key factors implicated in the regulation of emotion involve reappraisal, exposure, extinction, and reconsolidation (Hölzel et al., 2011). Hanley and Garland (2014) found that mindfulness practice led to an increase in positive reappraisal of thoughts. They argue that mindfulness practice facilitates positive reappraisal, with reappraisal functioning as an adaptive process through which stressful events are reconstructed as beneficial, meaningful, or benign.
Additional literature suggests that during mindfulness meditation meditators allow themselves to be affected by the experience of their thoughts while refraining from engaging in internal reactivity towards them by cultivating acceptance of bodily and affective responses (Hart, 2011; Hölzel et al., 2011). Thus, the accumulative impact of meditation training on the reappraisal of ongoing thoughts that occur during mind-wandering episodes (or even during more constrained forms of self-generated thought) may help to facilitate enhanced awareness and reduced reactivity to the content of spontaneous thoughts, and this may serve as one of the key mechanisms by which meditation practices shape the cognitive-affective lens through which we perceive the content of mind-wandering. Research investigating the effects of a brief attention-monitoring combined with an acceptance mindfulness training program found significantly reduced mind-wandering when compared with an attention-monitoring only mindfulness training program (Rahl, Lindsay, Pacilio, Brown, & Creswell, 2017). These findings directly support the key role of acceptance implicated in mind-wandering, and further elucidate the cognitive mechanisms underlying mindfulness training (Chiesa & Malinowski, 2011; Creswell & Lindsay, 2014; Franklin et al., 2013; Moore & Malinowski, 2009; Teper & Inzlicht, 2013). This research suggests that the aspect of ‘acceptance of present-moment experience’ cultivated in mindfulness training, which includes the acceptance of spontaneous thought and mind-wandering, leads to reduced emotional reactivity to adverse thought content allowing for an improved capacity for the reallocation of attention. These findings are supported by neuroimaging research which has shown notable similarities in the brain regions influenced by mindfulness meditation and those involved in mediating fear extinction, namely the hippocampus, amygdala, medial PFC, and the ventromedial PFC (Goldin & Gross, 2010; Hölzel et al., 2011; Lazar et al., 2000; Lou et al., 1999; Luders, Toga, Lepore, & Gaser, 2009; Newberg et al., 2001; Unsworth & McMillan, 2013). Furthermore, these regions (with the exception of the amygdala) coincide with the key hubs in the broader neural substrates of the default network (DMN), and as we will discuss in the following section, are heavily implicated during self-generated thought and mind-wandering.
Meditation, affect, and emotion regulation
Several recent studies have explored how the constructs of mindfulness and the valence of self-generated thought may interact. While these studies implement measures of mindfulness assessed via questionnaire (which are notoriously biased and have been the source of significant criticism; Van Dam et al., 2018), several recent studies provide evidence for the direct relationship between mindfulness and the valence of self-generated thought. Evans and colleagues (2011), as well as Andrews-Hannah and colleagues (2013), correlated participants scores on the Five Facets Mindfulness Questionnaire (FFMQ), which purports to measure trait levels of mindfulness (Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006), alongside the affective qualities of self-generated thoughts. They both found that higher trait mindfulness scores significantly predicted more emotionally positive thought content. In support of these findings, Frewen and colleagues (2008) found that dispositional mindfulness as measured by their Mindful Attention Awareness Scale (MAAS; Brown & Ryan, 2003) negatively correlated with the frequency of negative thoughts. These findings correspond with research suggesting that mindful individuals report heightened positive affect in general (Brown & Ryan, 2003; Hölzel et al., 2011; Jazaieri et al., 2014; Teper et al., 2013) and that meditation may facilitate positive affect. This is supported by findings by Jazaieri and colleauges (2016) who found that the number of hours of seated compassion meditation practice during nine weeks of compassion meditation training predicted the affective qualities of off-task thought. They found that the number of hours of practice predicted reduced off-task thoughts to both negative and neutral topics, and increased off-task thoughts to positive topics. Research studying the neural mechanisms underlying the regulation of emotion have been directly linked to brain regions associated with attention and cognitive control, including the dorsomedial, dorsolateral, and ventrolateral prefrontal cortex, as well as the posterior parietal cortex (Ochsner & Gross, 2005; Ochsner et al., 2004). Meditation and mindfulness practices may mediate emotion regulation and emotional reactivity to self-generated thought content by strengthening prefrontal cognitive control mechanisms via suppression of activity in the amygdala. Supporting this hypothesis, diminished activations in the amygdala in response to emotional stimuli have been found in experienced meditation practitioners (Tang et al., 2015).
Spontaneous thought is mediated by brain networks implicated in meditation
Significant progress over the last decade has been made in identifying the brain networks underlying mind-wandering and the generation and maintenance of self-referential thought processes. Research consistently shows default mode network (DMN) activations during both probe-caught and self-reported episodes of mind-wandering (Smallwood & Schooler, 2015), in addition to increased activation when individuals are at rest in an MRI scanner (Raichle et al., 2001). Interestingly, the emphasis on the flexible monitoring of self-referential thought processes and ongoing experience during meditation has been theoretically attributed to the increased functional connectivity within the DMN (Brewer et al., 2011; Garrison, Zeffiro, Scheinost, Constable, & Brewer, 2015; Jang et al., 2011; Tang et al., 2015; Wells et al., 2013), and between the DMN and the dorsolateral prefrontal cortex (dlPFC; Brewer et al., 2011) that is observed in experienced meditation practitioners. It is of importance to note here that the lateral PFC is a region associated with both exogenous and endogenous attentional states, meta-awareness, and executive functioning (Froeliger et al., 2012a; Hasenkamp & Barsalou, 2012; Teper et al., 2013).
Research in functional magnetic resonance imaging (fMRI) has identified the regions that comprise the DMN (Fig. 3; Raichle et al., 2001; Raichle, 2015) including the medial prefrontal cortex (specifically, the dorsal medial prefrontal cortex (dmPFC), the rostral anterior cingulate, and parts of the anterior and ventral mPFC), the lateral frontal cortex (the superior frontal cortex and the inferior frontal gyrus), the medial parietal cortex (the posterior cingulate and retrosplenial cortex), the medial temporal lobe (the hippocampus and parahippocampal cortices), the lateral parietal cortex (spanning the angular gyrus and the posterior supramarginal gyrus/TPJ), and the lateral temporal cortex (extending anteriorly to the temporal poles). The DMN also includes large areas of the cerebellum (including Crus I and Crus II subdivisions) and the striatum (the medial wall of the caudate and the posterior putamen; (Andrews-Hanna et al., 2010). While a set key regions originally identified through the repeated observation of patterns of deactivation during goal-directed tasks (as compared to passive control conditions) were thought to reflect the DMN (Raichle et al., 2001), recent findings have shown that during goal-directed tasks of an internal nature, significant task-dependent variability in DMN activations have been observed (Andrews-Hanna et al., 2010, 2014; Andrews-Hanna et al., 2014).
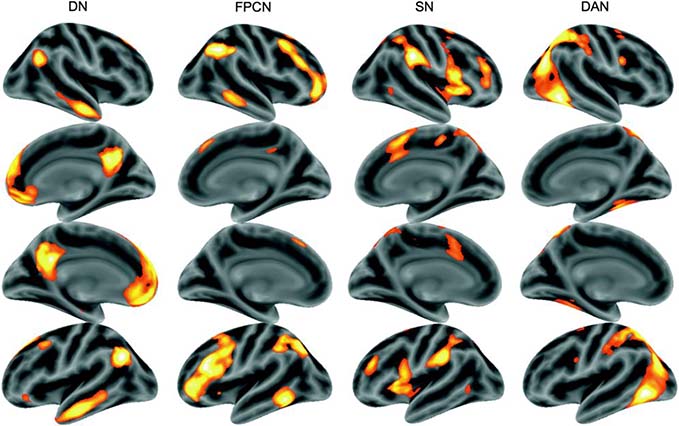
Figure 3.
Template maps for the cortical networks implicated in the cognitive processes underlying the meditative cycle: default network (DN), frontoparietal control network (FPCN), salience network (SN), and dorsal attention network (DAN). Color intensities indicate factor loading of each voxel with the network template in the reference dataset. Used with permission from Shaw et al., (2015).
Recent advances in resting-state functional connectivity (rsFC) analysis have now enabled a more comprehensive understanding of the functionally integrated relationship between spatially separated brain regions and the complexity of the DMN, revealing its distinct yet interacting subsystems (Andrews-Hanna et al., 2010, 2014; Fox et al., 2015; Gusnard et al., 2001). Andrews-Hanna and colleagues recently identified three key subsystems that constitute the DMN. A medial temporal subsystem comprised of the hippocampus, the parahippocampal cortex, the retrosplenial cortex (RSC), the posterior inferior parietal lobe, and the ventromedial prefrontal cortex (vmPFC). This subsystem significantly corresponds with meta-analytic maps pertaining to past and future autobiographical thought (i.e., autobiographical, past, future), episodic memory (i.e., episodic, memories, remember, recollection, recall) and contextual retrieval (Andrews-Hanna et al., 2014). A dorsal medial subsystem comprises the dorsal medial PFC (dmPFC), the temporoparietal junction (TPJ), the lateral temporal cortex, and the temporal pole, regions that correspond with meta-analytic maps pertaining to social cognition (i.e., mentalizing, social, person, theory of mind, mental, scenarios), as well as story comprehension and semantic and conceptual processing (i.e., sentence, story, meaning, knowledge, language, word, syntactic). A third subsystem is comprised of regions along the cortical midline, including the anterior medial PFC (amPFC) and the posterior cingulate cortex (PCC) exhibit strong functional coherence with both subsystems and are hypothesized to act as functional hubs, allowing information to transfer between subsystems. The PCC, angular gyrus, and amPFC are the most consistently engaged regions within the DMN, regions directly associated with self-related processes (i.e., self-referential, self, autobiographical, personal), emotion/evaluation (i.e., positive, negative, moral), and social and mnemonic processes, features shared by both the dorsal medial and medial temporal subsystem (i.e., social, person, mentalizing, recollection, retrieval, memories; Andrews-Hanna, Saxe, & Yarkoni, 2014). Additional findings suggest that the PCC hub is a heterogeneous brain structure, with subdivisions characterized by distinct patterns of structural and functional connectivity that echo the neural signals from several additional large-scale brain networks. These observations suggest that the broader PCC can be viewed as an important integration zone supporting bottom-up mechanisms of attention that enable behaviorally relevant sources of information to be drawn from memory and perceptual information. A recent paper by Andrews-Hanna and colleagues (2014) argues that together these neural systems support a majority of the mental content underlying self-generated thought.
Functional connectivity in regions of the DMN, a measure of the temporal correlation of the BOLD signal between these regions, has also been found to differ between meditators and controls, not only during meditation but also at rest (Brewer et al., 2011; Pagnoni, 2012; Taylor et al., 2013). This suggests that meditation training may alter the behavioral state that individuals enter when given the standard resting-state instructions. One interpretation of these findings would be that increased functional connectivity in the DMN may in turn reduce blood-oxygen-level-dependent imaging (BOLD) activity, reflecting an increased efficiency in the distribution of cognitive resources (Baars, 2005). Another possible explanation for the relationship between reduced BOLD activity and increased functional connectivity in advanced practitioners may be due to the increased connectivity between networks involved in monitoring and attention that overlap with the DMN, rather than specifically DMN. An additional alternative explanation may be that meditators engage in less spontaneous thought during rest and therefore show reduced BOLD signal in the DMN (although this does not account for the increased functional connectivity).
However, even if it were the case that the increased functional connectivity in the DMN reflects connections to regions overlapping with other networks, together these findings support the notion that both endogenous and exogenous forms of cognitive activity such as mind-wandering and meditation both recruit regions in both the DMN in addition to overlapping regions and networks implicated in the regulation of executive functions. While the relationship between the DMN and mind-wandering has been extensively studied within neuroscience (Dixon, Fox, & Christoff, 2014; Kucyi, Salomons, & Davis, 2013), activity in the DMN alone does not capture the broader neural landscape associated with the occurrence, duration, frequency, and maintenance of mind-wandering and spontaneous thought (Anticevic et al., 2012; Berman et al., 2011; Stawarczyk, Majerus, Maquet, & D’Argembeau, 2011; Vanhaudenhuyse et al., 2011; Whitfield-Gabrieli & Ford, 2012). A general picture of brain activity involved in mind-wandering and spontaneous thought is beginning to emerge, implicating brain networks beyond the DMN such as the frontoparietal control network (FPCN), the dorsal attention network (DAN), and the salience network (SN; Christoff et al., 2009; Dixon et al., 2018; Ellamil et al., 2016; Fox et al., 2016). The FPCN includes lateral prefrontal cortex, precuneus (PCu), the anterior extent of the inferior parietal lobule (aIPL), medial superior prefrontal cortex (msPFC) and the anterior insula (aINS; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2012). This network has been proposed to modulate top-down mechanisms involved in sustaining both endogenous and exogenous forms of attention allocation (Spreng et al., 2012). As discussed in Smallwood et al (2012) the ability to generate and sustain an internal train of thought is facilitated through cooperation between autobiographical information provided by the DMN and a frontal–parietal control network which helps sustain and buffer internal trains of thought against disruption by the external world. Spreng and colleagues (2012) suggest that the FPCN facilitates goal-directed cognition, which functions as a gatekeeping system by moderating the dynamic balance between activations in the DMN and the DAN (Fig. 3). It may also facilitate alternating or competing goal representations while maintaining directed attention to a given external task (i.e. driving, running; Spreng et al., 2012). Concurrent activations in both the DMN and core regions of the executive functioning network (dorsolateral PFC, mPFC, ACC), networks that were traditionally considered independent, anti-correlated, and thought to compete for cognitive resources, have been shown to co-activate during mind-wandering episodes, increasingly so when subjects reported being unaware of mind-wandering (Christoff et al., 2009; Fox et al., 2015).
Similar to the work of Andrews-Hanna and colleagues that fractionated the DMN, recent research by Dixon and colleagues (2018) examined patterns of FPCN functional connectivity across multiple conditions of varying cognitive demand, identifying two distinct subsystems within the FPCN. These two FPCN subsystems exhibited unique patterns of functional connectivity with the DMN and the DAN. The first subsystem FPCN(a) includes the rostrolateral prefrontal cortex, anterior inferior parietal lobule, presupplementary motor area, and middle temporal gyrus, and exhibited significantly stronger measures of connectivity with the DMN than the DAN. The FPCN(b) consists of the intraparietal sulcus, posterior inferior frontal sulcus/inferior frontal junction (IFS/IFJ), posterior superior frontal sulcus, and left posterior middle temporal gyrus, and exhibited the opposite pattern. These findings provide new evidence suggesting that the organization of the FPCN is both flexible and heterogeneous, and that it may emerge from separable DMN and DAN processing streams (Dixon et al., 2014, 2018). The authors propose that FPCN(a) may be preferentially involved in the regulation of introspective processes, whereas FPCN(b) may be preferentially involved in the regulation of visuospatial perceptual attention.
A number of additional structural and functional MRI studies on mindfulness training have investigated the neuroplasticity in brain regions supporting the regulation of attention. The anterior cingulate cortex (ACC), another key hub of the FPCN, is an area in the brain that has been consistently linked to improvements in attention regulation following training in mindfulness (Hölzel et al., 2011; Tang & Posner, 2009, 2014; Tang, Rothbart, & Posner, 2012). The ACC and the fronto-insular cortex are thought to enable executive attention and control by detecting the presence of conflicts emerging from incompatible streams of information processing, thus facilitating cognitive processing through long-range connections to other brain areas (van Veen & Carter, 2002). These mechanisms may work synergistically by establishing a process of enhanced meta-awareness and self-regulation following long-term meditation practice (Baird, Mrazek, et al., 2014; Baird et al., 2013, 2013; Dorjee, 2016; Fleming & Dolan, 2012; Sanger & Dorjee, 2016; Tang et al., 2015). Furthermore, the perigenual ACC is implicated in emotional feeling states, evidenced by patterns of activation that correspond with the valence of stimuli (Roy, Shohamy, & Wager, 2012). However, its role appears to be specifically related to the process of attributing conceptual meaning to bodily sensations and interweaving feeling states with self-referential thinking in addition to the capacity to identify and understand interoceptive feeling states (Fleming et al., 2010; Fox et al., 2018; Roy et al., 2012).
According to Hasenkamp and colleagues’ (2012) study, when attention was reoriented back to a focused attention meditation after an episode of mind-wandering, increased activations in the lateral PFC and inferior parietal cortex were observed, suggesting that executive resources were recruited to deactivate the DMN by decoupling the node shared by the FPCN and the DMN. Furthermore, increased activity in the dorsolateral PFC (dlPFC), a central hub of the FPCN that has been repeatedly implicated in studies of focused attention and executive control, was observed during focused attention meditation (Brewer et al., 2011). The work of Hasenkamp and Barslow (2012) investigated the brain networks directly implicated in the various phases of the meditation cycle. These findings highlighted the activity associated with the transitions between mind-wandering and a return to focused-attention, highlighting the key role of the salience network in signaling the detection of mind-wandering and the relaying of this information to the executive network. Metacognitive awareness of one’s thoughts, along with attention and performance, have also been directly linked the rostrolateral PFC (rlPFC; Fleming & Dolan, 2012; McCaig et al., 2011). Research investigating the neural correlates of lucid dreaming, wherein individuals become aware of and are engaged in the progression and content of their dreams, show that individuals who report a high degree of lucidity show increased grey matter volume in the medial and rlPFC, as well as enhanced rlPFC activity during tasks that require the subjects to monitor the contents of their thoughts (Filevich, Dresler, Brick, & Kühn, 2015).The FPCN has also been widely implicated in lucid rapid eye movement (REM) sleep when compared to non-lucid REM sleep (Dresler et al., 2012). Thus, accumulating research suggests that the FPCN plays a direct role in the control processes related to the meta-awareness of spontaneous thought and mind-wandering by monitoring and constraining the transitions between mental states by suppressing and directing the degree spontaneity (Fox & Christoff, 2014). Meditation practice may target these monitoring mechanisms through the active process of detecting mind-wandering and the redirecting of attention.
Neuroimaging results show that mindfulness meditation practitioners also exhibit significantly greater rsFC in the DAN when compared with meditation naive individuals, and that mindfulness meditation practice in the MRI scanner (msFC) was associated with increased functional connectivity when compared to resting state levels (i.e., msFC > rsFC) between the DAN and DMN and the right PFC node of the salience network (Froeliger et al., 2012a). These findings suggest that mindfulness practice enhances functional connectivity within attentional networks as well as across broadly distributed brain regions sub-serving the regulation of introspective, attentional, self-referential, and emotional processes (Brewer et al., 2011; Dixon et al., 2014; Froeliger et al., 2012b; Garrison et al., 2014). Additional neuroimaging evidence also indicates that sustained activity in the salience network has been observed during meditation in long-term practitioners (Fig. 3; Brefczynski-Lewis, Lutz, Schaefer, Levinson, & Davidson, 2007; Doll, Hölzel, Boucard, Wohlschläger, & Sorg, 2015; Hasenkamp et al., 2012). The salience network includes the bilateral anterior insula, lateral orbitofrontal cortex (OFC), anterior and mid-cingulate cortex, amygdala, and hypothalamus and is thought to facilitate the identification of relevant and salient stimuli and sustained cognitive focus (Doll et al., 2015; Menon & Uddin, 2010; Mooneyham & Schooler, 2013; Ptak, 2012; Seeley et al., 2007). Biases in affective and perceptual attention can be thought to reflect natural constraints, which serve to capture and sustain attention on a focal source (Christoff et al., 2016, 2016; Irving, 2016; Todd, Cunningham, Anderson, & Thompson, 2012). Although evidence for a relationship between mind-wandering and nature of these constraints is limited, recent studies on depression and anxiety suggests that the brain’s salience network may play a key role (McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014; Seeley et al., 2007; Young et al., 2017).
The salience network and activity in the dACC/pre-somatosensory motor area (pre-SMA) is thought to reflect the detection of conflict (e.g., mind-wandering when it occurs during an ongoing task), and may therefore be involved in determining the expected cost/benefit ratio tradeoffs of being either on- versus off-task. The insula has also been heavily implicated in the generation of self-generated thought and may be more heavily involved in viscero-somatic sensations (cardiac, respiratory, etc.) and feeling states (Fox 2018). The insula is also thought to play a role in detecting and signaling affective salience (Markovic, Anderson, & Todd, 2014; Menon & Uddin, 2010; Seeley et al., 2007), with the anterior insula having been shown to be directly related to the degree to which thoughts trigger, or are triggered by, physiological arousal and other concrete bodily feelings (Craig, 2002; Fox et al., 2018). Consistent with this hypothesis, awareness of visceral and internal psychological states, including heart rate and respiration is often referred to as interoception and has been consistently linked to activity in the insula (Craig and Craig, 2009; Critchley et al., 2004) in addition to metacognitive awareness (Fleming and Dolan, 2012) and emotional self-awareness (Craig, 2004). Additional research has found that viscero-somatic information is progressively refined from the posterior to anterior insula, the anterior insula contributing directly to interoceptive awareness (Craig, 2002). When comparing meditators during meditation versus non-meditation states, we find brain areas focused on self-regulation, focused problem-solving, adaptive behavior, interoception, monitoring body states, reorienting attention, and processing self-relevant information (Boccia et al., 2015). Given that approximately two-thirds of self-generated thoughts are emotional in nature, it is highly probable that self-generated thoughts regularly recruit brain areas and networks implicated in emotional processing (Andrews-Hanna et al., 2013; Fox et al., 2018; Ruby et al., 2013)
Meditation and modulations of attention regulation and sensory perception
Accumulating findings from contemplative neuroscience research suggest that meditation practice strengthens the top-down feedback mechanisms involved in the regulation of attention (Brefczynski-Lewis et al., 2007; Chan & Woollacott, 2007; Jha, Krompinger, & Baime, 2007; Lutz et al., 2009; MacLean et al., 2010; Moore & Malinowski, 2009; Slagter, Davidson, & Lutz, 2011; Slagter, Lutz, Greischar, Nieuwenhuis, & Davidson, 2008; Valentine & Sweet, 1999; van den Hurk et al., 2010). According to the neurocognitive model developed by Posner and Petersen, attention can be divided into three different anatomically and functionally distinct networks. These networks implement the functions of alerting (i.e., the anticipatory preparation for an incoming stimulus), orienting (i.e., the directing of attention to a specific stimulus), and conflict monitoring (i.e., executive attention: resolving conflict between competing neural activity; Petersen & Posner, 2012; Posner & Petersen, 1990). Additional distinctions between different forms of attention involve combinations of these three components (Posner & Rothbart, 2007). For example, sustained attention refers to the sense of vigilance during long continuous tasks and may involve both tonic alerting (i.e., intrinsic arousal that fluctuates on the order of minutes to hours) and orienting, whereas selective attention may involve either orienting (when a stimulus is present) and executive functions (when the processing of stored information is involved; Desimone & Duncan, 1995). Furthermore, regions of the dorsolateral prefrontal cortex that are heavily implicated in attentional processes that engage the executive network (Curtis & D’Esposito, 2003; D’Esposito, 2007; Miller & Cohen, 2001) have been directly implicated in the practice of focused attention meditation (Hofmann et al., 2012; Teper & Inzlicht, 2013).
These findings are consistent with the notion that meditation practices engage brain areas involved in inhibition (Brefczynski-Lewis et al., 2007), as well as in the detection of conflict between goal states (i.e., focused attention on the breath conflicting with the occurrence of spontaneous thought; Hasenkamp & Barsalou, 2012; Hasenkamp et al., 2012). This is further evidenced by research by Moore and Malinowski (2009) and Chan and Woollacott (2007) who found reduced effects of distracting and conflicting information in the Stroop task in mindfulness meditators. Van den Hurk and colleagues (2010) found reduced interference by distracting flankers in the attention network test in mindfulness meditators, validating previous findings that mindfulness meditation leads to an increased flexibility in the orientation of attention by reducing the time needed to shift attention from one location to another (Hodgins & Adair, 2010; Jha et al., 2007; van den Hurk et al., 2010). In a study comparing relaxation, open monitoring meditation, and focused attention meditation on performance on an emotional variant of the Attention Network Test (ANT), only focused attention and open monitoring practices were found to improve executive attention (Ainsworth et al., 2013). Together these findings contribute to a large and accumulating body of evidence that mindfulness meditation targets mechanisms implicated in executive attention and the detection of conflicting mental states.
Electroencephalography (EEG) findings from our previous work (Brandmeyer & Delorme, 2018) indicate that increased mid-frontal theta (4–6 Hz) and somatosensory alpha (8–12 Hz), cortical oscillations that have been observed during tasks assessing measures of executive function (Bollimunta, Mo, Schroeder, & Ding, 2011; Cavanagh & Frank, 2014; Cavanagh & Shackman, 2015; Enriquez-Geppert, Huster, Figge, & Herrmann, 2014), were also present during internally guided states of focus such as meditation, a result consistent with previous findings in the literature (Aftanas & Golocheikine, 2001; Kerr et al., 2013). These findings may also suggest a functional relationship between the sources contributing to cortical mid-frontal theta activity and the broader FPCN, a network involved in maintaining top-down representations of goal states, learning, directed attention, and the regulation of spontaneous thought (Cavanagh & Frank, 2014; deBettencourt, Cohen, Lee, Norman, & Turk-Browne, 2015). The role of cortical theta in meditation practice and the cultivation of top-down control via the enhancement of monitoring and conflict detection falls in line with the established literature regarding its specific role in learning (Haegens et al., 2010; Swick & Turken, 2002). Cavanagh & Frank (2014) have suggested that cortical theta (4–6 Hz) oscillations may serve as a candidate mechanism by which neurons communicate top-down control over long range and broad networks. Mid-frontal theta has been proposed to function as a temporal template for organizing mid-frontal neuronal processes (Cavanagh & Frank, 2014), with theta-band phase dynamics thought to entrain disparate neural systems when cognitive control is needed (e.g., through entrainment of cortical and subcortical areas via the cingulate cortex; Bollimunta, Chen, Schroeder, & Ding, 2009; Morecraft & Tanji, 2009). Our previous findings provide evidence in support of the claims posited by Spreng and colleagues (2012) that the maintenance of both internal and external orientations of focus may be maintained by similar cortical theta synchronization mechanisms and suggest that meditation training may target the neural substrates underlying these oscillations.
Spontaneous fluctuations between two distinct and supposedly opposite modes during resting-state brain activity have been observed (Fransson, 2005). One of these modes is characterized by the presence of slow theta oscillations, a cortical activity associated with reduced levels of vigilance. The other mode is characterized by the presence of fast oscillations of 12–30 Hz, which are usually associated with high vigilance levels (Laufs et al., 2003). These spontaneous patterns of increased and decreased theta activity have been associated with periods of mind-wandering and periods of concentration, as shown in a study by Braboszcz and Delorme (2011). During a breath awareness counting task in which subjects used the self-report method to indicate mind-wandering events, they showed an increase in occipitoparietal theta and frontocentral delta (1–3 Hz) during mind-wandering, and suggest that these findings may reflect the increased BOLD activity observed in fMRI studies investigating the DMN (Braboszcz & Delorme, 2011). A functional relationship between cortical phase-locking and fluctuations in endogenous attentional states has been suggested by investigations examining the impact of training in focused attention meditation on the degree of cortical phase-locking to stimuli presentations in sustained attention tasks (Lutz et al., 2009; Slagter et al., 2008). Lutz and colleagues (2009) found that three months of focused meditation training resulted in a smaller attentional blink and reduced brain-resource allocation to the first target (T1), demonstrated by a significantly smaller T1-elicited P3b (i.e. a neural index of resource allocation after training). Subjects with the largest decrease in cognitive resource allocation to T1 showed the largest reduction in the measured attentional-blink size, suggesting that an ability to accurately identify T2 depends upon the efficient deployment of cognitive resources to T1.
The authors hypothesized that the mental training induced increases in phase-locking were related to the capacity to sustain task-related attentional focus and a reduced tendency to engage in task-unrelated thoughts. It may be that long term meditation practice engages both top-down mechanisms underlying sustained attention as well as bottom-up processing of distracting sensory or thought related information (Lutz et al., 2008). In a separate study, long-term Tibetan Nyingmapa and Kagyupa Buddhist practitioners were able to self-induce sustained high-amplitude gamma-band (25–42 Hz) oscillations and phase-synchrony, most notably over the lateral frontoparietal electrodes (Lutz, Greischar, Rawlings, Ricard, & Davidson, 2004). Interestingly, in a study by Baird and colleagues (2014), they explored the sensory decoupling that occurs during mind-wandering, and whether it was mediated by the phase of ongoing cortical oscillations across one or more frequencies (Baird, Smallwood, Lutz, & Schooler, 2014). This was done by analyzing the impact of task-unrelated thought on phase of cortical activity to sensory stimuli during a vigilance task, wherein a time-frequency analysis of the oscillatory neural response revealed a decrease in theta-band cortical phase-locking, which peaked over parietal scalp regions.
Recent findings published by Braboszcz and colleagues (2017) compared practitioners of three different meditation traditions (Vipassana, Himalayan Yoga, and Isha Shoonya) with a control group during a meditative and instructed mind-wandering condition, and found that all meditators showed higher parieto-occipital 60–110 Hz gamma amplitude than control subjects as a trait effect observed during meditation and when considering meditation and instructed mind-wandering periods together. Moreover, this gamma power was positively correlated with participants’ meditation experience. Additionally, they controlled for the potential contamination of muscle artifact and studied artifact activity in different experimental conditions using independent component analysis (Braboszcz et al., 2017; Delorme and Makeig, 2004; >Delorme et al., 2007). Cahn and colleagues (2010) found that the cross-experimental session occipital gamma power was significantly larger in meditators with more than 10 years of daily practice, and that the meditation-related gamma power increase was similarly the strongest in such advanced practitioners (Cahn, Delorme, & Polich, 2010). These findings suggest that long-term Vipassana meditation contributes to increased parieto-occipital gamma power related to long-term meditational expertise, and lend support to the link between meditation practice and increased EEG coherence (thought to facilitate the central executive functions of cognitive control and working memory; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005). This in turn may result in the self-regulation of lower level elements of neurogenesis (Vago & Silbersweig, 2012), increased cognitive flexibility (Slagter et al., 2011, 2008), and efficient distribution of limited brain resources (Baars, 2005; Lutz et al., 2008, 2009).
Another intriguing finding emerging from the field of contemplative neuroscience involves the mediating role of contemplative and meditative practices on the neural mechanisms underlying sensory perception. In a study using magnetoencephalography (MEG) recording of the somatosensory cortex finger representation, Kerr and colleagues (2011) found that experienced meditators showed an enhanced alpha power modulation in response to a cue, potentially reflecting an enhanced filtering of inputs to primary sensory cortex. They also found that experienced meditators demonstrated modified alpha rhythm properties and an increase in non-localized tonic alpha power when compared to controls. An electroencephalography (EEG) study by Braboszcz and Delorme (2011) showed enhanced cortical processing of sensory stimuli during a sustained breath-focus task when compared to periods of time in which subjects reported mind-wandering. Known as the perceptual decoupling hypothesis, behavioral and neurocognitive evidence indicates that when mental events arise that are unrelated to perception they are frequently associated with a decoupling of attention from perception (Schooler et al., 2011), and that changes in spontaneous thought and mind-wandering can be either coupled or decoupled from exogenous and external perceptual events in the surrounding environment (reflecting the extent to which an individual can constrain mind-wandering; Smallwood & Schooler, 2015). Interestingly, Whitmarsh and colleagues (2014) investigated participant’s metacognitive ability to report on their attentional focus, and found that a contralateral somatosensory alpha power decrease was correlated with higher reported attentional focus to either their left or right hand respectively (Whitmarsh, Barendregt, Schoffelen, & Jensen, 2014). Enhanced body awareness was also found to be associated with greater subjective emotional experience and awareness of heart beats during exposure to emotionally provocative stimuli in Vipassana meditators, when compared to expert dancers, and controls (Sze et al., 2010). These findings can most likely be attributed to the emphasis on somatic attention training in mindfulness meditation techniques in which individuals train to develop both interoceptive awareness and metacognition; a process in which one cultivates an awareness and understanding of one’s own thought processes, and to an overall somatosensory awareness of physical sensations, feelings, and thought content (Bishop et al., 2004; Farb et al., 2007; Segal, Teasdale, & Williams, 2004). Interestingly, Baird and colleagues (2014) found that a 2-week meditation program led to significantly enhanced metacognitive judgments of cognition on a trial-by-trial basis in the domain of memory, but not for perceptual decisions, suggesting that while only 2 weeks of meditation training can enhance certain elements of introspective acuity, such improvements may not apply equally to all cognitive domains, or at least may require more than 2 weeks of meditation practice.
Perspectives
Throughout this manuscript we provide a comprehensive neurocognitive framework outlining the central role of spontaneous thought processes in the meditative cycle, however many outstanding questions remain. While research findings suggest that accumulative meditation practice reduces the frequency of spontaneous thought episodes (Brandmeyer & Delorme, 2018; Wenk-Sormaz, 2005; Zanesco et al., 2016), it has yet to be established whether reductions in mind wandering reflect the meta-cognitive accuracy cultivated during practice, or an increased allocation of attentional resources (improved sustained attention), or both. It may also be the case that meditation practice facilitates the unification of various attentional mechanisms so as to further mitigate mind-wandering. It is our perspective that it is most likely the case that meditative experience enhances metacognitive skills from the onset increasing an individual’s propensity to detect spontaneous thought. This would occur through the initial and repetitious «flexing» of the cognitive activity associated with the meditative cycle, with an emphasis placed on detecting when attention has drifted away from the meditative focus and bringing it back to the object (see Fig. 2). With accumulative practice this leads to earlier detection and sensitivity to the occurrence of mind wandering, eventually facilitating lower level neurogenesis and neuroplasticity that translate to faculties such as a the capacity for longer periods of sustained attention.
One way of deciphering the differences between this meta-cognition vs. improved attention regulation question would be to use both self- (assessing the meta-awareness of mind wandering) and probe-caught (reflecting a more direct measure of on- or off-task measure of sustained attention) experiential sampling methods. Several studies have effectively implemented this methodology in a normal population to illuminate the relationship between mind-wandering and meta-awareness. This approach was initially used to examine mind-wandering while reading and revealed that whereas participants caught themselves mind-wandering roughly 4 times in a 45 min period, they were regularly caught mind-wandering in about 15% of experience sampling probes (Franklin, Smallwood, & Schooler, 2011; Unsworth & McMillan, 2013). This type of paradigm could be applied to meditators, and a regression analysis could be used to investigate whether the impact of years of experience influence changes in meta awareness and measures of sustained attention.
It is also important to address how using experiential sampling methods may influence the depth and quality of meditation practice in experimental settings. Interviews of meditators in experimental contexts indicate that the majority of participants are unable to experience particularly ‘deep’ meditative states, however advanced practitioners report reduced disruption from the experience sampling probes compared to novices (Brandmeyer & Delorme, 2018). However, all meditators in the study experienced a progressive ‘increase in the depth’ of their meditation over time suggesting they were progressively better at engaging their meditation practice and performing the task simultaneously (there was a 10 min period of training prior to the beginning of the task). It has also been argued that all of the aforementioned methods of measurement predominantly address the contents of thought at a specific point in time, but elucidate very little regarding the neural dynamics leading up to the measurement of a given mental state (i.e., mind wandering or meditation; Smallwood & Schooler, 2015). Thus, nuanced methodologies such as machine learning and online classification are necessary to understand how mental states are related and evolve over time, without having to rely on subjective first person reporting (Girn et al., 2017).
Another open research question is to determine to what degree different types of meditation practice influence the awareness, duration, and frequency of mind-wandering episodes. Although meditation research is a relatively established scientific domain within both academic and clinical contexts, as mentioned, much debate regarding incompatible definitions and conceptions of mindfulness and meditation remain within both academic and traditional Buddhist contexts (Brandmeyer, Delorme, & Wahbeh, 2019; Dunne, 2015; Sharf, 2015; Van Dam et al., 2018). Similarly, the manner in which mind-wandering is defined directly influences the corresponding neuroimaging data, thus future research should aim to differentiate the distinctive forms of spontaneous cognition at both a behavioral, phenomenological, and neural level (Fox & Christoff, 2018). Lastly, refined phenomenological research has yet to explore whether meditation experience exerts a preferential influence on the real time dynamics of thought content, such as the orientation (past, present, future) and the valence (pleasant, neutral, or unpleasant) of spontaneous thought processes during meditation, as well as during daily life. These findings would be particularly beneficial in therapeutic intervention trajectories, as well as in informing the broader translational and clinical applications.
One translational application of this intersection of meditation and mind wandering research includes the development of closed-loop neurofeedback paradigms. Furthermore, protocols that implement novel features including the covert detection of mind-wandering during meditation and alternative modalities of sensory feedback such as haptic feedback (as opposed to auditory or visual feedback) should be explored, as they may enable practitioners to remain in a relatively natural meditative state. Methodological approaches implementing closed-loop neurofeedback and machine learning for training meditation and detecting mind wandering have already emerged (Bixler & D’Mello, 2016; Faber, Bixler, & D’Mello, 2018; Hutt et al., 2019; Krasich et al., 2018). While no definitive scientific consensus has been reached in terms of identifying generalizable and reliable neural markers that can serve to index meditative states and depth for neurofeedback training purposes, our previous research (Brandmeyer, 2017) found behavioral improvements on a working memory task after only 8 sessions of FM theta closed-loop neurofeedback wherein subjects applied focused attention meditation instructions while upregulating frontal midline theta. Additional research by Lutterveld and Brewer (2017) found a high moment-to-moment correspondence between neurofeedback source-localized gamma activity from the PCC and subjective experience of effortless awareness. Another recent study found that when patients engaged in the Muse meditation neurofeedback (a wearable EEG head-band device and application that implements proprietary algorithms) for 20 minutes daily during the period ranging from breast cancer diagnosis until 3 months after surgical treatment, they observed reduced fatigue and reduced stress as well as an improved quality of life (Millstine et al., 2019; Bhayee et al., 2016). Other methodological approaches such as the use of pupillometry and eye tracking during sustained visual attention tasks, the use of respiration and heart-rate variability (HRV) for assessing the down regulation of the sympathetic nervous system. New and novel methods that examine the causal role of brain regions, such as the lateral PFC, MCC, dorsal ACC, and PCC should all be considered potential candidates of feedback based on the literature presented throughout this manuscript, as these regions are heavily implicated in the meditative cycle and hold promise as candidates for future closed-loop neuro- and biofeedback protocols and applications.
Conclusions
Individual differences in executive functions and cognitive control have been shown to predict well-being across a broad array of personal, academic, and professional domains (Hirsh & Inzlicht, 2010), with impaired cognitive control (i.e., impaired attention and emotion regulation) considered to be the hallmark predictor of clinical disorders such as ADHD, obsessive compulsive disorder, schizophrenia, depression, and anxiety (Cho, Konecky, & Carter, 2006; Friedman et al., 2007; LeMoult & Gotlib, 2019; Mazaheri et al., 2014; Snyder, Kaiser, Warren, & Heller, 2015; Yordanova, Kolev, & Rothenberger, 2013). The findings presented throughout this manuscript provide a comprehensive theoretical framework showcasing the processes underlying self-generated thought and the key role that metacognitive awareness plays in enhancing cognitive regulation and which may serve to potentially buffer individuals against a wide array of cognitive deficiencies that arise from the inability to regulate self-generated thought content (i.e. rumination, habitual thinking). Many of the benefits resulting from contemplative practice are likely the result of an increased engagement with the neural circuity underlying the regulation of attention, emotions, cognition, somatosensory processing, and metacognition. Here, we highlighted how spontaneous thought processes are likely mediated by brain networks functionally and structurally linked to the practice of meditation, having focused on broadly distributed networks including the DMN, fPCN, DAN, and the salience network. We explored the key mechanisms by which meditation appears to modulate attention and sensory perception, meta-awareness, perceptual coupling, and decoupling, all of which are directly implicated in the regulation, maintenance, content, valence, and overall generation of spontaneous thought processes, broader cognitive functioning, and well-being.
Akwnowledgments
This work was supported by grants from the Agence Nationale pour la Recherche (ANR-12-JSH2-0009), the BIAL foundation (BIAL-08-162), and a T32 award (T32 AT00399) granted at the University of California San Francisco, Osher Center for Integrative Medicine from the National Center for Complementary and Integrative Health of the National Institutes of Health. The authors wish to thank Cédric Cannard and Phillippe Goldin for their contributions towards the manuscript.
References
- Addis DR, Knapp K, Roberts RP, & Schacter DL (2012). Routes to the past: Neural substrates of direct and generative autobiographical memory retrieval. NeuroImage, 59(3), 2908–2922. 10.1016/j.neuroimage.2011.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aftanas LI, & Golocheikine SA (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neuroscience Letters, 310(1), 57–60. 10.1016/S0304-3940(01)02094-8 [DOI] [PubMed] [Google Scholar]
- Ainsworth B, Eddershaw R, Meron D, Baldwin DS, & Garner M (2013). The effect of focused attention and open monitoring meditation on attention network function in healthy volunteers. Psychiatry Research, 210(3), 1226–1231. 10.1016/j.psychres.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Amodio DM, & Frith CD (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews. Neuroscience, 7(4), 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Irving ZC, Fox KCR, Spreng RN, & Christoff K (2017). The Neuroscience of Spontaneous Thought: An Evolving, Interdisciplinary Field. ArXiv:1704.02533 [q-Bio]. Retrieved from http://arxiv.org/abs/1704.02533 [Google Scholar]
- Andrews-Hanna JR, Kaiser RH, Turner AEJ, Reineberg A, Godinez D, Dimidjian S, & Banich M (2013). A penny for your thoughts: Dimensions of self-generated thought content and relationships with individual differences in emotional wellbeing. Frontiers in Psychology, 4 10.3389/fpsyg.2013.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, & Buckner RL (2010). Evidence for the Default Network’s Role in Spontaneous Cognition. Journal of Neurophysiology, 104(1), 322–335. 10.1152/jn.00830.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Saxe R, & Yarkoni T (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage, 91, 324–335. 10.1016/j.neuroimage.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, & Krystal JH (2012). The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences, 16(12), 584–592. 10.1016/j.tics.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars BJ (2005). Global workspace theory of consciousness: Toward a cognitive neuroscience of human experience. Progress in Brain Research, 150, 45–53. 10.1016/S0079-6123(05)50004-9 [DOI] [PubMed] [Google Scholar]
- Baer RA (2015). Mindfulness-Based Treatment Approaches: Clinician’s Guide to Evidence Base and Applications. Elsevier. [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, & Toney L (2006). Using Self-Report Assessment Methods to Explore Facets of Mindfulness. Assessment, 13(1), 27–45. 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- Baird B, Mrazek MD, Phillips DT, & Schooler JW (2014). Domain-specific enhancement of metacognitive ability following meditation training. Journal of Experimental Psychology: General, 143(5), 1972–1979. 10.1037/a0036882 [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, & Margulies DS (2013). Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(42), 16657–16665. 10.1523/JNEUROSCI.0786-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Lutz A, & Schooler JW (2014). The Decoupled Mind: Mind-wandering Disrupts Cortical Phase-locking to Perceptual Events. Journal of Cognitive Neuroscience, 26, 2596–2607. 10.1162/jocn_a_00656 [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Mrazek MD, Kam JWY, Franklin MS, & Schooler JW (2012). Inspired by Distraction: Mind Wandering Facilitates Creative Incubation. Psychological Science, 23(10), 1117–1122. 10.1177/0956797612446024 [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, & Schooler JW (2011). Back to the future: Autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition, 20(4), 1604–1611. 10.1016/j.concog.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Banks JB, Welhaf MS, & Srour A (2015). The protective effects of brief mindfulness meditation training. Consciousness and Cognition, 33, 277–285. 10.1016/j.concog.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Barron E, Riby LM, Greer J, & Smallwood J (2011). Absorbed in Thought: The Effect of Mind Wandering on the Processing of Relevant and Irrelevant Events. Psychological Science, 22(5), 596–601. 10.1177/0956797611404083 [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, & Jonides J (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–555. 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhayee S, Tomaszewski P, Lee DH, Moffat G, Pino L, Moreno S, & Farb NAS (2016). Attentional and affective consequences of technology supported mindfulness training: A randomised, active control, efficacy trial. BMC Psychology, 4(1), 60 10.1186/s40359-016-0168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, … Devins G (2004). Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science and Practice, 11(3), 230–241. 10.1093/clipsy.bph077 [DOI] [Google Scholar]
- Bixler R, & D’Mello S (2016). Automatic gaze-based user-independent detection of mind wandering during computerized reading. User Modeling and User-Adapted Interaction, 26(1), 33–68. 10.1007/s11257-015-9167-1 [DOI] [Google Scholar]
- Bockelman P, Reinerman-Jones L, & Gallagher S (2013). Methodological lessons in neurophenomenology: Review of a baseline study and recommendations for research approaches. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, & Ding M (2009). Characterizing Oscillatory Cortical Networks with Granger Causality. In Josic K, Rubin J, Matias M, & Romo R (Eds.), Coherent Behavior in Neuronal Networks (pp. 169–189). 10.1007/978-1-4419-0389-1_9 [DOI] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, & Ding M (2011). Neuronal Mechanisms and Attentional Modulation of Corticothalamic Alpha Oscillations. Journal of Neuroscience, 31(13), 4935–4943. 10.1523/JNEUROSCI.5580-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braboszcz C, Cahn BR, Levy J, Fernandez M, & Delorme A (2017). Increased Gamma Brainwave Amplitude Compared to Control in Three Different Meditation Traditions. PLOS ONE, 12(1), e0170647 10.1371/journal.pone.0170647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braboszcz C, & Delorme A (2011). Lost in thoughts: Neural markers of low alertness during mind wandering. NeuroImage, 54(4), 3040–3047. 10.1016/j.neuroimage.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Brandmeyer T (2017). Investigating the role of oscillations in endogenous and exogenous attentional states: Novel methods in neurophenomenology (Phdthesis, Université Paul Sabatier - Toulouse III). Retrieved from https://tel.archives-ouvertes.fr/tel-01772802/document [Google Scholar]
- Brandmeyer T, & Delorme A (2018). Reduced mind wandering in experienced meditators and associated EEG correlates. Experimental Brain Research, 236(9), 2519–2528. 10.1007/s00221-016-4811-5 [DOI] [PubMed] [Google Scholar]
- Brandmeyer T, Delorme A, & Wahbeh H (2019). The neuroscience of meditation: Classification, phenomenology, correlates, and mechanisms. Progress in Brain Research, 244, 1–29. 10.1016/bs.pbr.2018.10.020 [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, & Davidson RJ (2007). Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences, 104(27), 11483–11488. 10.1073/pnas.0606552104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y-Y, Weber J, & Kober H (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America, 108(50), 20254–20259. 10.1073/pnas.1112029108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, & Ryan RM (2003). The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology, 84(4), 822–848. 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- Brown Kirk Warren, & Ryan RM (2004). Perils and Promise in Defining and Measuring Mindfulness: Observations From Experience. Clinical Psychology: Science and Practice, 11(3), 242–248. 10.1093/clipsy.bph078 [DOI] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, & Gilbert SJ (2007). The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences, 11(7), 290–298. 10.1016/j.tics.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Cahn BR, Delorme A, & Polich J (2010). Occipital gamma activation during Vipassana meditation. Cognitive Processing, 11(1), 39–56. 10.1007/s10339-009-0352-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, & Polich J (2006). Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin, 132(2), 180–211. 10.1037/0033-2909.132.2.180 [DOI] [PubMed] [Google Scholar]
- Carter KS, & Carter III R (2016). Breath-based meditation: A mechanism to restore the physiological and cognitive reserves for optimal human performance. World Journal of Clinical Cases, 4(4), 99–102. 10.12998/wjcc.v4.i4.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–421. 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Shackman AJ (2015). Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology-Paris, 109(1), 3–15. 10.1016/j.jphysparis.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, & Woollacott M (2007). Effects of Level of Meditation Experience on Attentional Focus: Is the Efficiency of Executive or Orientation Networks Improved? The Journal of Alternative and Complementary Medicine, 13(6), 651–658. 10.1089/acm.2007.7022 [DOI] [PubMed] [Google Scholar]
- Chiesa A, & Malinowski P (2011). Mindfulness-based approaches: Are they all the same? Journal of Clinical Psychology, 67(4), 404–424. 10.1002/jclp.20776 [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, & Carter CS (2006). Impairments in frontal cortical γ synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences, 103(52), 19878–19883. 10.1073/pnas.0609440103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KCR, Spreng RN, & Andrews-Hanna JR (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews. Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LPT, & Gabrieli JDE (2003). Evaluating Self-Generated Information: Anterior Prefrontal Contributions to Human Cognition. Behavioral Neuroscience, 117(6), 1161–1168. 10.1037/0735-7044.117.6.1161 [DOI] [PubMed] [Google Scholar]
- Colzato LS, Szapora A, & Hommel B (2012a). Meditate to Create: The Impact of Focused-Attention and Open-Monitoring Training on Convergent and Divergent Thinking. Frontiers in Psychology, 3 10.3389/fpsyg.2012.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Szapora A, & Hommel B (2012b). Meditate to Create: The Impact of Focused-Attention and Open-Monitoring Training on Convergent and Divergent Thinking. Frontiers in Psychology, 3 10.3389/fpsyg.2012.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Creswell JD, & Lindsay EK (2014). How Does Mindfulness Training Affect Health? A Mindfulness Stress Buffering Account. Current Directions in Psychological Science, 23(6), 401–407. 10.1177/0963721414547415 [DOI] [Google Scholar]
- Curtis CE, & D’Esposito M (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7(9), 415–423. 10.1016/S1364-6613(03)00197-9 [DOI] [PubMed] [Google Scholar]
- Dahl CJ, Lutz A, & Davidson RJ (2015). Reconstructing and deconstructing the self: Cognitive mechanisms in meditation practice. Trends in Cognitive Sciences, 19(9), 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Renaud O, & Linden MV der. (2011). Frequency, characteristics and functions of future-oriented thoughts in daily life. Applied Cognitive Psychology, 25(1), 96–103. 10.1002/acp.1647 [DOI] [Google Scholar]
- Davidson RJ (2010). Empirical explorations of mindfulness: Conceptual and methodological conundrums. Emotion, 10(1), 8–11. 10.1037/a0018480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBettencourt MT, Cohen JD, Lee RF, Norman KA, & Turk-Browne NB (2015). Closed-loop training of attention with real-time brain imaging. Nature Neuroscience, 18(3), 470–475. 10.1038/nn.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Brandmeyer T (2019). When the meditating mind wanders. Current Opinion in Psychology, 28, 133–137. 10.1016/j.copsyc.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Deng Y-Q, Li S, & Tang Y-Y (2014). The Relationship Between Wandering Mind, Depression and Mindfulness. Mindfulness, 5(2), 124–128. 10.1007/s12671-012-0157-7 [DOI] [Google Scholar]
- Desbordes G, Gard T, Hoge EA, Hölzel B, Kerr C, Lazar SW, … Vago DR (2015). Moving Beyond Mindfulness: Defining Equanimity as an Outcome Measure in Meditation and Contemplative Research. Mindfulness, 6(2), 356–372. 10.1007/s12671-013-0269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, & Duncan J (1995). Neural Mechanisms of Selective Visual Attention. Annual Review of Neuroscience, 18(1), 193–222. 10.1146/annurev.ne.18.030195.001205 [DOI] [PubMed] [Google Scholar]
- D’Esposito M (2007). From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1481), 761–772. 10.1098/rstb.2007.2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Fox KCR, & Christoff K (2014). A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia, 62, 321–330. 10.1016/j.neuropsychologia.2014.05.024 [DOI] [PubMed] [Google Scholar]
- Dixon ML, Vega ADL, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, & Christoff K (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences, 115(7), E1598–E1607. 10.1073/pnas.1715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll A, Hölzel BK, Boucard CC, Wohlschläger AM, & Sorg C (2015). Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Frontiers in Human Neuroscience, 9 10.3389/fnhum.2015.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorjee D (2016). Defining Contemplative Science: The Metacognitive Self-Regulatory Capacity of the Mind, Context of Meditation Practice and Modes of Existential Awareness. Frontiers in Psychology, 7 10.3389/fpsyg.2016.01788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler M, Wehrle R, Spoormaker VI, Koch SP, Holsboer F, Steiger A, … Czisch M (2012). Neural correlates of dream lucidity obtained from contrasting lucid versus non-lucid REM sleep: A combined EEG/fMRI case study. Sleep, 35(7), 1017–1020. 10.5665/sleep.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne JD (2015). Buddhist Styles of Mindfulness: A Heuristic Approach. In Ostafin BD, Robinson MD, & Meier BP (Eds.), Handbook of Mindfulness and Self-Regulation (pp. 251–270). 10.1007/978-1-4939-2263-5_18 [DOI] [Google Scholar]
- Ehlers A, Hackmann A, & Michael T (2004). Intrusive re-experiencing in post-traumatic stress disorder: Phenomenology, theory, and therapy. Memory, 12(4), 403–415. 10.1080/09658210444000025 [DOI] [PubMed] [Google Scholar]
- Ellamil M, Fox KCR, Dixon ML, Pritchard S, Todd RM, Thompson E, & Christoff K (2016). Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. NeuroImage, 136, 186–196. 10.1016/j.neuroimage.2016.04.034 [DOI] [PubMed] [Google Scholar]
- Enriquez-Geppert S, Huster RJ, Figge C, & Herrmann CS (2014). Self-regulation of frontal-midline theta facilitates memory updating and mental set shifting. Frontiers in Behavioral Neuroscience, 8 10.3389/fnbeh.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Puterman E, Lin J, Blackburn E, Lazaro A, & Mendes WB (2013). Wandering Minds and Aging Cells. Clinical Psychological Science, 1(1), 75–83. 10.1177/2167702612460234 [DOI] [Google Scholar]
- Evans DR, & Segerstrom SC (2011). Why do Mindful People Worry Less? Cognitive Therapy and Research, 35(6), 505–510. 10.1007/s10608-010-9340-0 [DOI] [Google Scholar]
- Faber M, Bixler R, & D’Mello SK (2018). An automated behavioral measure of mind wandering during computerized reading. Behavior Research Methods, 50(1), 134–150. 10.3758/s13428-017-0857-y [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, & Anderson AK (2007). Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience, 2(4), 313–322. 10.1093/scan/nsm030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filevich E, Dresler M, Brick TR, & Kühn S (2015). Metacognitive mechanisms underlying lucid dreaming. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(3), 1082–1088. 10.1523/JNEUROSCI.3342-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, & Dolan RJ (2012). The neural basis of metacognitive ability. Phil. Trans. R. Soc. B, 367(1594), 1338–1349. 10.1098/rstb.2011.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Huijgen J, & Dolan RJ (2012). Prefrontal Contributions to Metacognition in Perceptual Decision Making. Journal of Neuroscience, 32(18), 6117–6125. 10.1523/JNEUROSCI.6489-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, & Rees G (2010). Relating Introspective Accuracy to Individual Differences in Brain Structure. Science, 329(5998), 1541–1543. 10.1126/science.1191883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flook L, Goldberg SB, Pinger L, & Davidson RJ (2015). Promoting prosocial behavior and self-regulatory skills in preschool children through a mindfulness-based kindness curriculum. Developmental Psychology, 51(1), 44–51. 10.1037/a0038256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Andrews-Hanna JR, Mills C, Dixon ML, Markovic J, Thompson E, & Christoff K (2018). Affective neuroscience of self-generated thought. Annals of the New York Academy of Sciences, 1426(1), 25–51. 10.1111/nyas.13740 [DOI] [PubMed] [Google Scholar]
- Fox KCR, & Christoff K (2014). Metacognitive Facilitation of Spontaneous Thought Processes: When Metacognition Helps the Wandering Mind Find Its Way. In Fleming SM & Frith CD (Eds.), The Cognitive Neuroscience of Metacognition (pp. 293–319). 10.1007/978-3-642-45190-4_13 [DOI] [Google Scholar]
- Fox KCR, & Christoff K (2018). The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming. Oxford University Press. [Google Scholar]
- Fox KCR, Dixon ML, Nijeboer S, Girn M, Floman JL, Lifshitz M, … Christoff K (2016). Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neuroscience & Biobehavioral Reviews, 65, 208–228. 10.1016/j.neubiorev.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Fox KCR, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, … Christoff K (2014). Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience & Biobehavioral Reviews, 43, 48–73. 10.1016/j.neubiorev.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Fox KCR, Nijeboer S, Solomonova E, Domhoff GW, & Christoff K (2013). Dreaming as mind wandering: Evidence from functional neuroimaging and first-person content reports. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, & Christoff K (2015). The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage, 111, 611–621. 10.1016/j.neuroimage.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Fox KCR, Zakarauskas P, Dixon M, Ellamil M, Thompson E, & Christoff K (2012). Meditation Experience Predicts Introspective Accuracy. PLOS ONE, 7(9), e45370 10.1371/journal.pone.0045370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MS, Mrazek MD, Anderson CL, Smallwood J, Kingstone A, & Schooler J (2013). The silver lining of a mind in the clouds: Interesting musings are associated with positive mood while mind-wandering. Frontiers in Psychology, 4 10.3389/fpsyg.2013.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MS, Smallwood J, & Schooler JW (2011). Catching the mind in flight: Using behavioral indices to detect mindless reading in real time. Psychonomic Bulletin & Review, 18(5), 992–997. 10.3758/s13423-011-0109-6 [DOI] [PubMed] [Google Scholar]
- Fransson P (2005). Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping, 26(1), 15–29. 10.1002/hbm.20113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Evans EM, Maraj N, Dozois DJA, & Partridge K (2008). Letting Go: Mindfulness and Negative Automatic Thinking. Cognitive Therapy and Research, 32(6), 758–774. 10.1007/s10608-007-9142-1 [DOI] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, & Hewitt JK (2007). Greater Attention Problems During Childhood Predict Poorer Executive Functioning in Late Adolescence. Psychological Science, 18(10), 893–900. 10.1111/j.1467-9280.2007.01997.x [DOI] [PubMed] [Google Scholar]
- Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, … Sobin P (2012a). Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond. Evidence-Based Complementary and Alternative Medicine: ECAM, 2012, 680407 10.1155/2012/680407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, … Sobin P (2012b). Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond [Research article]. 10.1155/2012/680407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Constable RT, & Brewer JA (2014). BOLD signal and functional connectivity associated with loving kindness meditation. Brain and Behavior, 4(3), 337–347. 10.1002/brb3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Zeffiro TA, Scheinost D, Constable RT, & Brewer JA (2015). Meditation leads to reduced default mode network activity beyond an active task. Cognitive, Affective, & Behavioral Neuroscience, 15(3), 712–720. 10.3758/s13415-015-0358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girn M, Mills C, Laycock E, Ellamil M, Ward L, & Christoff K (2017). Neural Dynamics of Spontaneous Thought: An Electroencephalographic Study. In Schmorrow DD & Fidopiastis CM (Eds.), Augmented Cognition. Neurocognition and Machine Learning (Vol. 10284, pp. 28–44). 10.1007/978-3-319-58628-1_3 [DOI] [Google Scholar]
- Gold SR, & Gold RG (1982). Actual daydream content and the Imaginal Processes Inventory. Journal of Mental Imagery, 6(1), 169–173. [Google Scholar]
- Goldin PR, & Gross JJ (2010). Effects of Mindfulness-Based Stress Reduction (MBSR) on Emotion Regulation in Social Anxiety Disorder. Emotion (Washington, D.C.), 10(1), 83–91. 10.1037/a0018441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EMS, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA (2014). Meditation Programs for Psychological Stress and Well-being: A Systematic Review and Meta-analysis. JAMA Internal Medicine, 174(3), 357–368. 10.1001/jamainternmed.2013.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, & Raichle ME (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews. Neuroscience, 2(10), 685–694. 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Haegens S, Osipova D, Oostenveld R, & Jensen O (2010). Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Human Brain Mapping, 31(1), 26–35. 10.1002/hbm.20842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, & Shibasaki H (2002). The Role of Rostral Brodmann Area 6 in Mental-operation Tasks: An Integrative Neuroimaging Approach. Cerebral Cortex, 12(11), 1157–1170. 10.1093/cercor/12.11.1157 [DOI] [PubMed] [Google Scholar]
- Hanley AW, & Garland EL (2014). Dispositional Mindfulness Co-varies with Self-Reported Positive Reappraisal. Personality and Individual Differences, 66, 146–152. 10.1016/j.paid.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart W (2011). The Art of Living: Vipassana Meditation as Taught by S.N. Goenka. Pariyatti.
- Hasenkamp W, & Barsalou LW (2012). Effects of meditation experience on functional connectivity of distributed brain networks. Frontiers in Human Neuroscience, 6, 38 10.3389/fnhum.2012.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, & Barsalou LW (2012). Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. NeuroImage, 59(1), 750–760. 10.1016/j.neuroimage.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Hirsh JB, & Inzlicht M (2010). Error-related negativity predicts academic performance. Psychophysiology, 47(1), 192–196. 10.1111/j.1469-8986.2009.00877.x [DOI] [PubMed] [Google Scholar]
- Hodgins HS, & Adair KC (2010). Attentional processes and meditation. Consciousness and Cognition, 19(4), 872–878. 10.1016/j.concog.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Grossman P, & Hinton DE (2011). Loving-kindness and compassion meditation: Potential for psychological interventions. Clinical Psychology Review, 31(7), 1126–1132. 10.1016/j.cpr.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, & Baddeley AD (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180. 10.1016/j.tics.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, & Ott U (2011). How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 6(6), 537–559. 10.1177/1745691611419671 [DOI] [PubMed] [Google Scholar]
- Hutt S, Krasich K, Mills C, Bosch N, White S, Brockmole JR, & D’Mello SK (2019). Automated gaze-based mind wandering detection during computerized learning in classrooms. User Modeling and User-Adapted Interaction. 10.1007/s11257-019-09228-5 [DOI] [Google Scholar]
- Irving ZC (2016). Mind-wandering is unguided attention: Accounting for the “purposeful” wanderer. Philosophical Studies, 173(2), 547–571. 10.1007/s11098-015-0506-1 [DOI] [Google Scholar]
- Jang JH, Jung WH, Kang D-H, Byun MS, Kwon SJ, Choi C-H, & Kwon JS (2011). Increased default mode network connectivity associated with meditation. Neuroscience Letters, 487(3), 358–362. 10.1016/j.neulet.2010.10.056 [DOI] [PubMed] [Google Scholar]
- Jazaieri H, Lee IA, McGonigal K, Jinpa T, Doty JR, Gross JJ, & Goldin PR (2016). A wandering mind is a less caring mind: Daily experience sampling during compassion meditation training. The Journal of Positive Psychology, 11(1), 37–50. 10.1080/17439760.2015.1025418 [DOI] [Google Scholar]
- Jazaieri H, McGonigal K, Jinpa T, Doty JR, Gross JJ, & Goldin PR (2014). A randomized controlled trial of compassion cultivation training: Effects on mindfulness, affect, and emotion regulation. Motivation and Emotion, 38(1), 23–35. 10.1007/s11031-013-9368-z [DOI] [Google Scholar]
- Jha AP, Krompinger J, & Baime MJ (2007). Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience, 7(2), 109–119. 10.3758/CABN.7.2.109 [DOI] [PubMed] [Google Scholar]
- Jha AP, Morrison AB, Parker SC, & Stanley EA (2017). Practice Is Protective: Mindfulness Training Promotes Cognitive Resilience in High-Stress Cohorts. Mindfulness, 8(1), 46–58. 10.1007/s12671-015-0465-9 [DOI] [Google Scholar]
- Kabat-Zinn J (2003). Mindfulness-Based Interventions in Context: Past, Present, and Future. Clinical Psychology: Science and Practice, 10(2), 144–156. 10.1093/clipsy.bpg016 [DOI] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, & Kwapil TR (2007). For Whom the Mind Wanders, and When: An Experience-Sampling Study of Working Memory and Executive Control in Daily Life. Psychological Science, 18(7), 614–621. 10.1111/j.1467-9280.2007.01948.x [DOI] [PubMed] [Google Scholar]
- Kerr CE, Jones SR, Wan Q, Pritchett DL, Wasserman RH, Wexler A, … Moore CI (2011). Effects of mindfulness meditation training on anticipatory alpha modulation in primary somatosensory cortex. Brain Research Bulletin, 85(3), 96–103. 10.1016/j.brainresbull.2011.03.026 [DOI] [PubMed] [Google Scholar]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, & Jones SR (2013). Mindfulness starts with the body: Somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, & Tranel D (2008). Interoceptive awareness in experienced meditators. Psychophysiology, 45(4), 671–677. 10.1111/j.1469-8986.2008.00666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MA, & Gilbert DT (2010). A Wandering Mind Is an Unhappy Mind. Science, 330(6006), 932–932. 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Klein SB, Robertson TE, Delton AW, & Lax ML (2012). Familiarity and Personal Experience as Mediators of Recall When Planning for Future Contingencies. Journal of Experimental Psychology. Learning, Memory, and Cognition, 38(1), 240–245. 10.1037/a0025200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger E (2009). Daydreaming and fantasizing: Thought flow and motivation In Handbook of imagination and mental simulation (pp. 225–239). New York, NY, US: Psychology Press. [Google Scholar]
- Klinger E (2013). Goal Commitments and the content of thoughts and dreams: Basic principles. Frontiers in Psychology, 4 10.3389/fpsyg.2013.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasich K, McManus R, Hutt S, Faber M, D’Mello SK, & Brockmole JR (2018). Gaze-based signatures of mind wandering during real-world scene processing. Journal of Experimental Psychology: General, 147(8), 1111–1124. 10.1037/xge0000411 [DOI] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, & Davis KD (2013). Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18692–18697. 10.1073/pnas.1312902110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, & Kleinschmidt A (2003). Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences, 100(19), 11053–11058. 10.1073/pnas.1831638100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, & Benson H (2000). Functional brain mapping of the relaxation response and meditation. NeuroReport, 11(7), 1581 Retrieved from https://journals.lww.com/neuroreport/Fulltext/2000/05150/Functional_brain_mapping_of_the_relaxation.42.aspx [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, … Fischl B (2005). Meditation experience is associated with increased cortical thickness. Neuroreport, 16(17), 1893–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, & Gotlib IH (2019). Depression: A cognitive perspective. Clinical Psychology Review, 69, 51–66. 10.1016/j.cpr.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, & Nowak M (1999). A 15O-H2O PET study of meditation and the resting state of normal consciousness. Human Brain Mapping, 7(2), 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, & Gaser C (2009). The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. NeuroImage, 45(3), 672–678. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3184843/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, & Davidson RJ (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences, 101(46), 16369–16373. 10.1073/pnas.0407401101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, & Davidson RJ (2008). Attention regulation and monitoring in meditation. Trends in Cognitive Sciences, 12(4), 163–169. 10.1016/j.tics.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, & Davidson RJ (2009). Mental Training Enhances Attentional Stability: Neural and Behavioral Evidence. Journal of Neuroscience, 29(42), 13418–13427. 10.1523/JNEUROSCI.1614-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, & Thompson E (2003). Neurophenomenology Integrating Subjective Experience and Brain Dynamics in the Neuroscience of Consciousness [Text]. Retrieved October 18, 2018, from https://www.ingentaconnect.com/content/imp/jcs/2003/00000010/F0020009/art00004
- MacLean KA, Ferrer E, Aichele SR, Bridwell DA, Zanesco AP, Jacobs TL, … Saron CD (2010). Intensive Meditation Training Improves Perceptual Discrimination and Sustained Attention. Psychological Science, 21(6), 829–839. 10.1177/0956797610371339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf ET, Youman K, Stuewig J, Witt EA, & Tangney JP (2017). A Pilot RCT of a Values-Based Mindfulness Group Intervention with Jail Inmates: Evidence for Reduction in Post-Release Risk Behavior. Mindfulness, 8(3), 603–614. 10.1007/s12671-016-0636-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, & Todd RM (2014). Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research, 259, 229–241. 10.1016/j.bbr.2013.11.018 [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Fassbender C, Coffey-Corina S, Hartanto TA, Schweitzer JB, & Mangun GR (2014). Differential Oscillatory Electroencephalogram Between Attention-Deficit/Hyperactivity Disorder Subtypes and Typically Developing Adolescents. Biological Psychiatry, 76(5), 422–429. 10.1016/j.biopsych.2013.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig RG, Dixon M, Keramatian K, Liu I, & Christoff K (2011). Improved modulation of rostrolateral prefrontal cortex using real-time fMRI training and meta-cognitive awareness. NeuroImage, 55(3), 1298–1305. 10.1016/j.neuroimage.2010.12.016 [DOI] [PubMed] [Google Scholar]
- McConville J, McAleer R, & Hahne A (2017). Mindfulness Training for Health Profession Students—The Effect of Mindfulness Training on Psychological Well-Being, Learning and Clinical Performance of Health Professional Students: A Systematic Review of Randomized and Non-randomized Controlled Trials. EXPLORE, 13(1), 26–45. 10.1016/j.explore.2016.10.002 [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, & Pessoa L (2014). Network Organization Unfolds over Time during Periods of Anxious Anticipation. Journal of Neuroscience, 34(34), 11261–11273. 10.1523/JNEUROSCI.1579-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan R, Kaufman SB, & Singer JL (2013). Ode to positive constructive daydreaming. Frontiers in Psychology, 4 10.3389/fpsyg.2013.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2010). Adrift in the Stream of Thought: The Effects of Mind Wandering on Executive Control and Working Memory Capacity. In Gruszka A, Matthews G, & Szymura B (Eds.), Handbook of Individual Differences in Cognition: Attention, Memory, and Executive Control (pp. 321–334). 10.1007/978-1-4419-1210-7_19 [DOI] [Google Scholar]
- Menezes CB, de Paula Couto MC, Buratto LG, Erthal F, Pereira MG, & Bizarro L (2013). The Improvement of Emotion and Attention Regulation after a 6-Week Training of Focused Meditation: A Randomized Controlled Trial [Research article]. 10.1155/2013/984678 [DOI] [PMC free article] [PubMed]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An Integrative Theory of Prefrontal Cortex Function. Annual Review of Neuroscience, 24(1), 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Millstine DM, Bhagra A, Jenkins SM, Croghan IT, Stan DL, Boughey JC, … Pruthi S (2019). Use of a Wearable EEG Headband as a Meditation Device for Women With Newly Diagnosed Breast Cancer: A Randomized Controlled Trial. Integrative Cancer Therapies, 18, 1534735419878770 10.1177/1534735419878770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham BW, & Schooler JW (2013). The costs and benefits of mind-wandering: A review. Canadian Journal of Experimental Psychology = Revue Canadienne De Psychologie Experimentale, 67(1), 11–18. 10.1037/a0031569 [DOI] [PubMed] [Google Scholar]
- Moore A, & Malinowski P (2009). Meditation, mindfulness and cognitive flexibility. Consciousness and Cognition, 18(1), 176–186. 10.1016/j.concog.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, & Tanji J (n.d.). Cingulofrontal Interactions and the Cingulate Motor Areas. 32. [Google Scholar]
- Morone NE, Greco CM, & Weiner DK (2008). Mindfulness meditation for the treatment of chronic low back pain in older adults: A randomized controlled pilot study. PAIN, 134(3), 310–319. 10.1016/j.pain.2007.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AB, Goolsarran M, Rogers SL, & Jha AP (2014). Taming a wandering attention: Short-form mindfulness training in student cohorts. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek MD, Franklin MS, Phillips DT, Baird B, & Schooler JW (2013). Mindfulness Training Improves Working Memory Capacity and GRE Performance While Reducing Mind Wandering. Psychological Science, 24(5), 776–781. 10.1177/0956797612459659 [DOI] [PubMed] [Google Scholar]
- Mrazek MD, Smallwood J, & Schooler JW (2012). Mindfulness and mind-wandering: Finding convergence through opposing constructs. Emotion, 12(3), 442–448. 10.1037/a0026678 [DOI] [PubMed] [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, & d’Aquili E (2001). The measurement of regional cerebral blood flow during the complex cognitive task of meditation: A preliminary SPECT study. Psychiatry Research: Neuroimaging, 106(2), 113–122. 10.1016/S0925-4927(01)00074-9 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology, 109(3), 504–511. [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, & Gross JJ (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23(2), 483–499. 10.1016/j.neuroimage.2004.06.030 [DOI] [PubMed] [Google Scholar]
- Okoro CA, Zhao G, Li C, & Balluz LS (2012). Use of complementary and alternative medicine among US adults with and without functional limitations. Disability and Rehabilitation, 34(2), 128–135. 10.3109/09638288.2011.591887 [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, … Blackburn EH (2013). Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. The Lancet Oncology, 14(11), 1112–1120. 10.1016/S1470-2045(13)70366-8 [DOI] [PubMed] [Google Scholar]
- Ostafin BD, & Kassman KT (2012). Stepping out of history: Mindfulness improves insight problem solving. Consciousness and Cognition, 21(2), 1031–1036. 10.1016/j.concog.2012.02.014 [DOI] [PubMed] [Google Scholar]
- Ottaviani C, & Couyoumdjian A (2013). Pros and cons of a wandering mind: A prospective study. Frontiers in Psychology, 4, 524 10.3389/fpsyg.2013.00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Shapiro D, & Couyoumdjian A (2013). Flexibility as the key for somatic health: From mind wandering to perseverative cognition. Biological Psychology, 94(1), 38–43. 10.1016/j.biopsycho.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Pagnoni G (2012). Dynamical Properties of BOLD Activity from the Ventral Posteromedial Cortex Associated with Meditation and Attentional Skills. Journal of Neuroscience, 32(15), 5242–5249. 10.1523/JNEUROSCI.4135-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience, 35(1), 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitmengin C, & Lachaux J-P (2013). Microcognitive science: Bridging experiential and neuronal microdynamics. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poerio GL, Totterdell P, & Miles E (2013). Mind-wandering and negative mood: Does one thing really lead to another? Consciousness and Cognition, 22(4), 1412–1421. 10.1016/j.concog.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Posner MI, & Petersen SE (1990). The Attention System of the Human Brain. Annual Review of Neuroscience, 13(1), 25–42. 10.1146/annurev.ne.13.030190.000325 [DOI] [PubMed] [Google Scholar]
- Posner MI, & Rothbart MK (2007). Research on Attention Networks as a Model for the Integration of Psychological Science. Annual Review of Psychology, 58(1), 1–23. 10.1146/annurev.psych.58.110405.085516 [DOI] [PubMed] [Google Scholar]
- Ptak R (2012). The Frontoparietal Attention Network of the Human Brain: Action, Saliency, and a Priority Map of the Environment. The Neuroscientist, 18(5), 502–515. 10.1177/1073858411409051 [DOI] [PubMed] [Google Scholar]
- Rahl HA, Lindsay EK, Pacilio LE, Brown KW, & Creswell JD (2017). Brief Mindfulness Meditation Training Reduces Mind-Wandering: The Critical Role of Acceptance. Emotion (Washington, D.C.), 17(2), 224–230. 10.1037/emo0000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle Marcus E. (2015). The Brain’s Default Mode Network. Annual Review of Neuroscience, 38(1), 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Reichle ED, Reineberg AE, & Schooler JW (2010). Eye Movements During Mindless Reading. Psychological Science, 21(9), 1300–1310. 10.1177/0956797610378686 [DOI] [PubMed] [Google Scholar]
- Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, & Beasley D (2010). Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. Journal of Psychosomatic Research, 68(1), 29–36. 10.1016/j.jpsychores.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. 10.1016/j.tics.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby FJM, Smallwood J, Engen H, & Singer T (2013). How self-generated thought shapes mood—The relation between mind-wandering and mood depends on the socio-temporal content of thoughts. PloS One, 8(10), e77554 10.1371/journal.pone.0077554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger KL, & Dorjee D (2016). Mindfulness training with adolescents enhances metacognition and the inhibition of irrelevant stimuli: Evidence from event-related brain potentials. Trends in Neuroscience and Education, 5(1), 1–11. 10.1016/j.tine.2016.01.001 [DOI] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, & Doppelmayr M (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology, 57(2), 97–103. 10.1016/j.ijpsycho.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Schooler JW, Mrazek MD, Franklin MS, Baird B, Mooneyham BW, Zedelius C, & Broadway JM (2014). Chapter One - The Middle Way: Finding the Balance between Mindfulness and Mind-Wandering. In Ross BH (Ed.), Psychology of Learning and Motivation (Vol. 60, pp. 1–33). 10.1016/B978-0-12-800090-8.00001-9 [DOI] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Teasdale JD, & Williams JMG (2004). Mindfulness-Based Cognitive Therapy: Theoretical Rationale and Empirical Status In Mindfulness and acceptance: Expanding the cognitive-behavioral tradition (pp. 45–65). New York, NY, US: Guilford Press. [Google Scholar]
- Seli P, Kane MJ, Smallwood J, Schacter DL, Maillet D, Schooler JW, & Smilek D (2018). Mind-Wandering as a Natural Kind: A Family-Resemblances View. Trends in Cognitive Sciences, 22(6), 479–490. 10.1016/j.tics.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf RH (2015). Is mindfulness Buddhist? (And why it matters). Transcultural Psychiatry, 52(4), 470–484. 10.1177/1363461514557561 [DOI] [PubMed] [Google Scholar]
- Shaw EE, Schultz AP, Sperling RA, & Hedden T (2015). Functional Connectivity in Multiple Cortical Networks Is Associated with Performance Across Cognitive Domains in Older Adults. Brain Connectivity, 5(8), 505–516. 10.1089/brain.2014.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JL (1966). Daydreaming: An introduction to the experimental study of inner experience. New York, NY, US: Crown Publishing Group/Random House. [Google Scholar]
- Singh NN, Lancioni GE, Wahler RG, Winton ASW, & Singh J (2008). Mindfulness approaches in cognitive behavior therapy. Behavioural and Cognitive Psychotherapy, 36(6), 659–666. 10.1017/S1352465808004827 [DOI] [Google Scholar]
- Slagter HA, Davidson RJ, & Lutz A (2011). Mental Training as a Tool in the Neuroscientific Study of Brain and Cognitive Plasticity. Frontiers in Human Neuroscience, 5 10.3389/fnhum.2011.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Nieuwenhuis S, & Davidson RJ (2008). Theta Phase Synchrony and Conscious Target Perception: Impact of Intensive Mental Training. Journal of Cognitive Neuroscience, 21(8), 1536–1549. 10.1162/jocn.2009.21125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J (2013). Distinguishing how from why the mind wanders: A process–occurrence framework for self-generated mental activity. Psychological Bulletin, 139(3), 519–535. 10.1037/a0030010 [DOI] [PubMed] [Google Scholar]
- Smallwood J, & Andrews-Hanna J (2013). Not all minds that wander are lost: The importance of a balanced perspective on the mind-wandering state. Frontiers in Psychology, 4, 441 10.3389/fpsyg.2013.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Fishman DJ, & Schooler JW (2007). Counting the cost of an absent mind: Mind wandering as an underrecognized influence on educational performance. Psychonomic Bulletin & Review, 14(2), 230–236. [DOI] [PubMed] [Google Scholar]
- Smallwood JM, Baracaia SF, Lowe M, & Obonsawin M (2003). Task unrelated thought whilst encoding information. Consciousness and Cognition, 12(3), 452–484. 10.1016/S1053-8100(03)00018-7 [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, & Schooler JW (2007). The lights are on but no one’s home: Meta-awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin & Review, 14(3), 527–533. [DOI] [PubMed] [Google Scholar]
- Smallwood J, & Schooler JW (2006). The restless mind. Psychological Bulletin, 132(6), 946–958. 10.1037/0033-2909.132.6.946 [DOI] [PubMed] [Google Scholar]
- Smallwood J, & Schooler JW (2015). The Science of Mind Wandering: Empirically Navigating the Stream of Consciousness. Annual Review of Psychology, 66(1), 487–518. 10.1146/annurev-psych-010814-015331 [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW, Turk DJ, Cunningham SJ, Burns P, & Macrae CN (2011). Self-reflection and the temporal focus of the wandering mind. Consciousness and Cognition, 20(4), 1120–1126. 10.1016/j.concog.2010.12.017 [DOI] [PubMed] [Google Scholar]
- Snyder HR, Kaiser RH, Warren SL, & Heller W (2015). Obsessive-Compulsive Disorder Is Associated With Broad Impairments in Executive Function: A Meta-Analysis. Clinical Psychological Science, 3(2), 301–330. 10.1177/2167702614534210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2012). Intrinsic Architecture Underlying the Relations among the Default, Dorsal Attention, and Frontoparietal Control Networks of the Human Brain. Journal of Cognitive Neuroscience, 25(1), 74–86. 10.1162/jocn_a_00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, & D’Argembeau A (2015). Neural correlates of personal goal processing during episodic future thinking and mind-wandering: An ALE meta-analysis. Human Brain Mapping, 36(8), 2928–2947. 10.1002/hbm.22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, & D’Argembeau A (2013). Concern-induced negative affect is associated with the occurrence and content of mind-wandering. Consciousness and Cognition, 22(2), 442–448. 10.1016/j.concog.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, & D’Argembeau A (2011). Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity. PloS One, 6(2), e16997 10.1371/journal.pone.0016997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, & Turken AU (2002). Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences, 99(25), 16354–16359. 10.1073/pnas.252521499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze JA, Gyurak A, Yuan JW, & Levenson RW (2010). Coherence between emotional experience and physiology: Does body awareness training have an impact? Emotion, 10(6), 803–814. 10.1037/a0020146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-Y, Hölzel BK, & Posner MI (2015). The neuroscience of mindfulness meditation. Nature Reviews Neuroscience, 16(4), 213–225. 10.1038/nrn3916 [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, & Posner MI (2009). Attention training and attention state training. Trends in Cognitive Sciences, 13(5), 222–227. 10.1016/j.tics.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, & Posner MI (2014). Training brain networks and states. Trends in Cognitive Sciences, 18(7), 345–350. 10.1016/j.tics.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Rothbart MK, & Posner MI (2012). Neural correlates of establishing, maintaining, and switching brain states. Trends in Cognitive Sciences, 16(6), 330–337. 10.1016/j.tics.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taren AA, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown KW, … Creswell JD (2015). Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: A randomized controlled trial. Social Cognitive and Affective Neuroscience, 10(12), 1758–1768. 10.1093/scan/nsv066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, Scavone G, Breton E, Roffe-Vidal S, … Beauregard M (2013). Impact of meditation training on the default mode network during a restful state. Social Cognitive and Affective Neuroscience, 8(1), 4–14. 10.1093/scan/nsr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper R, & Inzlicht M (2013). Meditation, mindfulness and executive control: The importance of emotional acceptance and brain-based performance monitoring. Social Cognitive and Affective Neuroscience, 8(1), 85–92. 10.1093/scan/nss045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper R, Segal ZV, & Inzlicht M (2013). Inside the Mindful Mind: How Mindfulness Enhances Emotion Regulation Through Improvements in Executive Control. Current Directions in Psychological Science, 22(6), 449–454. 10.1177/0963721413495869 [DOI] [Google Scholar]
- Thompson E (2008). Neurophenomenology and Contemplative Experience. The Oxford Handbook of Religion and Science 10.1093/oxfordhb/9780199543656.003.0015 [DOI] [Google Scholar]
- Todd RM, Cunningham WA, Anderson AK, & Thompson E (2012). Affect-biased attention as emotion regulation. Trends in Cognitive Sciences, 16(7), 365–372. 10.1016/j.tics.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Travis F, & Shear J (2010). Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Consciousness and Cognition, 19(4), 1110–1118. 10.1016/j.concog.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Tulving E (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53, 1–25. 10.1146/annurev.psych.53.100901.135114 [DOI] [PubMed] [Google Scholar]
- Tulving E, & Craik FIM (2005). The Oxford Handbook of Memory. Oxford University Press. [Google Scholar]
- Unsworth N, & McMillan B (2013). Mind Wandering and Reading Comprehension: Examining the Roles of Working Memory Capacity, Interest, Motivation, and Topic Experience. Journal of Experimental Psychology: Learning, Memory, and Cognition, 39(3), 832–842. 10.1037/a0029669 [DOI] [PubMed] [Google Scholar]
- Vago D, & Silbersweig D (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience, 6 10.3389/fnhum.2012.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago D, & Zeidan F (2016). The brain on silent: Mind wandering, mindful awareness, and states of mental tranquility. Annals of the New York Academy of Sciences, 1373(1), 96–113. 10.1111/nyas.13171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine ER, & Sweet PLG (1999). Meditation and attention: A comparison of the effects of concentrative and mindfulness meditation on sustained attention. Mental Health, Religion & Culture, 2(1), 59–70. 10.1080/13674679908406332 [DOI] [Google Scholar]
- Van Dam NT, van Vugt MK, Vago DR, Schmalzl L, Saron CD, Olendzki A, … Meyer DE (2018). Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation. Perspectives on Psychological Science, 13(1), 36–61. 10.1177/1745691617709589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk PAM, Giommi F, Gielen SC, Speckens AEM, & Barendregt HP (2010). Greater efficiency in attentional processing related to mindfulness meditation. Quarterly Journal of Experimental Psychology, 63(6), 1168–1180. 10.1080/17470210903249365 [DOI] [PubMed] [Google Scholar]
- van Lutterveld R, Houlihan SD, Pal P, Sacchet MD, McFarlane-Blake C, Patel PR, … Brewer JA (2017). Source-space EEG neurofeedback links subjective experience with brain activity during effortless awareness meditation. NeuroImage, 151, 117–127. 10.1016/j.neuroimage.2016.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, & Carter CS (2002). The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiology & Behavior, 77(4–5), 477–482. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, … Laureys S (2011). Two distinct neuronal networks mediate the awareness of environment and of self. Journal of Cognitive Neuroscience, 23(3), 570–578. 10.1162/jocn.2010.21488 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. Journal of Neurophysiology, 100(6), 3328–3342. 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Sagher A, Back W, Pundhir P, & Travis F (2018). A Systematic Review of Transcendent States Across Meditation and Contemplative Traditions. EXPLORE, 14(1), 19–35. 10.1016/j.explore.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Wallace BA (1999, February 1). The Buddhist tradition of Samatha: Methods for refining and examining consciousness [Text] Retrieved October 8, 2018, from https://www.ingentaconnect.com/content/imp/jcs/1999/00000006/F0020002/932
- Watkins ER (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134(2), 163–206. 10.1037/0033-2909.134.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RE, Yeh GY, Kerr CE, Wolkin J, Davis RB, Tan Y, … Kong J (2013). Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: A pilot study. Neuroscience Letters, 556, 15–19. 10.1016/j.neulet.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HY, Fox AS, Shackman AJ, Stodola DE, Caldwell JZK, Olson MC, … Davidson RJ (2013). Compassion Training Alters Altruism and Neural Responses to Suffering. Psychological Science, 24(7), 1171–1180. 10.1177/0956797612469537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk-Sormaz H (2005). Meditation can reduce habitual responding. Alternative Therapies in Health and Medicine, 11(2), 42–58. [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Ford JM (2012). Default Mode Network Activity and Connectivity in Psychopathology. Annual Review of Clinical Psychology, 8(1), 49–76. 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- Whitmarsh S, Barendregt H, Schoffelen J-M, & Jensen O (2014). Metacognitive awareness of covert somatosensory attention corresponds to contralateral alpha power. NeuroImage, 85, 803–809. 10.1016/j.neuroimage.2013.07.031 [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, & Rothenberger A (2013). Chapter 18—Event-related oscillations reflect functional asymmetry in children with attention deficit/hyperactivity disorder. In Başar E, Başar-Eroĝlu C, Özerdem A, Rossini PM, & Yener GG (Eds.), Supplements to Clinical Neurophysiology (pp. 289–301). 10.1016/B978-0-7020-5307-8.00018-1 [DOI] [PubMed] [Google Scholar]
- Young CB, Raz G, Everaerd D, Beckmann CF, Tendolkar I, Hendler T, … Hermans EJ (2017). Dynamic Shifts in Large-Scale Brain Network Balance As a Function of Arousal. Journal of Neuroscience, 37(2), 281–290. 10.1523/JNEUROSCI.1759-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanesco AP, King BG, MacLean KA, Jacobs TL, Aichele SR, Wallace BA, … Saron CD (2016). Meditation training influences mind wandering and mindless reading. Psychology of Consciousness: Theory, Research, and Practice, 3(1), 12–33. 10.1037/cns0000082 [DOI] [Google Scholar]
- Zedelius CM, & Schooler JW (2015). Mind wandering “Ahas” versus mindful reasoning: Alternative routes to creative solutions. Frontiers in Psychology, 6 10.3389/fpsyg.2015.00834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Johnson SK, Diamond BJ, David Z, & Goolkasian P (2010). Mindfulness meditation improves cognition: Evidence of brief mental training. Consciousness and Cognition, 19(2), 597–605. 10.1016/j.concog.2010.03.014 [DOI] [PubMed] [Google Scholar]