Abstract
Platelets are classically known as essential mediators of hemostasis and thrombosis. However, in recent years, platelets have gained recognition for their inflammatory functions which modulate the immune response during infectious diseases. Platelets contain various immunoreceptors that enable them to act as sentinels to recognize intravascular pathogens. Upon activation, platelets directly limit pathogen growth through the release of antimicrobial peptides and ensure pathogen clearance through activation of immune cells. However, aberrant platelet activation can lead to inflammation and thrombotic events.
Graphical Abstract
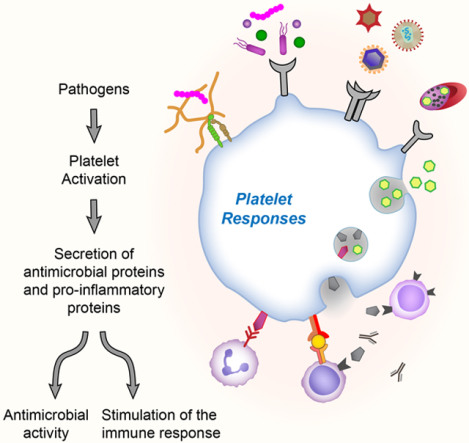
Introduction
Infectious diseases have a significant impact globally with high mortality and morbidity rates reported each year by the World Health Organization. Over the past few decades, new challenges associated with infectious diseases have placed additional burdens on health care due to the emergence of antimicrobial resistance, malaria, HIV/AIDS and the recent COVID-19 pandemic. As research continues to elucidate the pathogenesis of acute infections, one striking conclusion observed by many studies is the extensive contribution of platelets to host defense against pathogens. Platelets are small, anucleate cells derived from their mother cell the megakaryocyte, and considered critical in hemostasis and thrombosis. However, as platelets constantly scan the endothelium for vessel damage, they are well-positioned to act as first responders to detect invading pathogens resulting in their activation which triggers and contributes to the host immune response to fight the infection.1
On the other hand, pathological activation of platelets due to overwhelming pathogen invasion, damage to blood vessel walls or non-infectious inflammatory triggers can often lead to thromboinflammation, a process that intimately links inflammation and thrombosis, which can be detrimental to the host and can contribute to the pathophysiology of the disease.1 Thrombocytopenia, or low platelet counts, is common in acute infections and can correlate with disease severity.2 Increased vascular permeability is also common during thromboinflammation and infection. Both processes closely involve platelets and can lead to inflammatory vasculopathy and thrombosis.3
In this review, we discuss how platelets recognize and respond to a variety of pathogens beginning with an overview of platelet receptors involved in sensing invading pathogens. In addition, we examine how platelet activation contributes to host-defense through secretion of antimicrobial proteins and chemokines, which can directly interact with the pathogen, but also trigger the innate and adaptive immune system to combat against infections.
Platelets sense pathogens
Similar to innate immune cells, platelets contain pattern recognition receptors (PRRs) which recognize different components that increase during infection. These can be generically expressed microbial structures called pathogen-associated molecular patterns (PAMPs) or host-derived components known as damage-associated molecular patterns (DAMPs). Different families of PRRs are expressed on platelets, such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs)3, and are depicted in Figure 1. Engagement of these receptors by pathogens or antigens generally lead to platelet activation, differential granule release, and interaction with leukocytes, allowing platelets to act as both thrombotic and immune cells.1,3,4
Figure 1: During infectious diseases, platelets sense pathogens via receptors and respond through secretion and expression of antimicrobial proteins, cytokines and adhesion molecules.
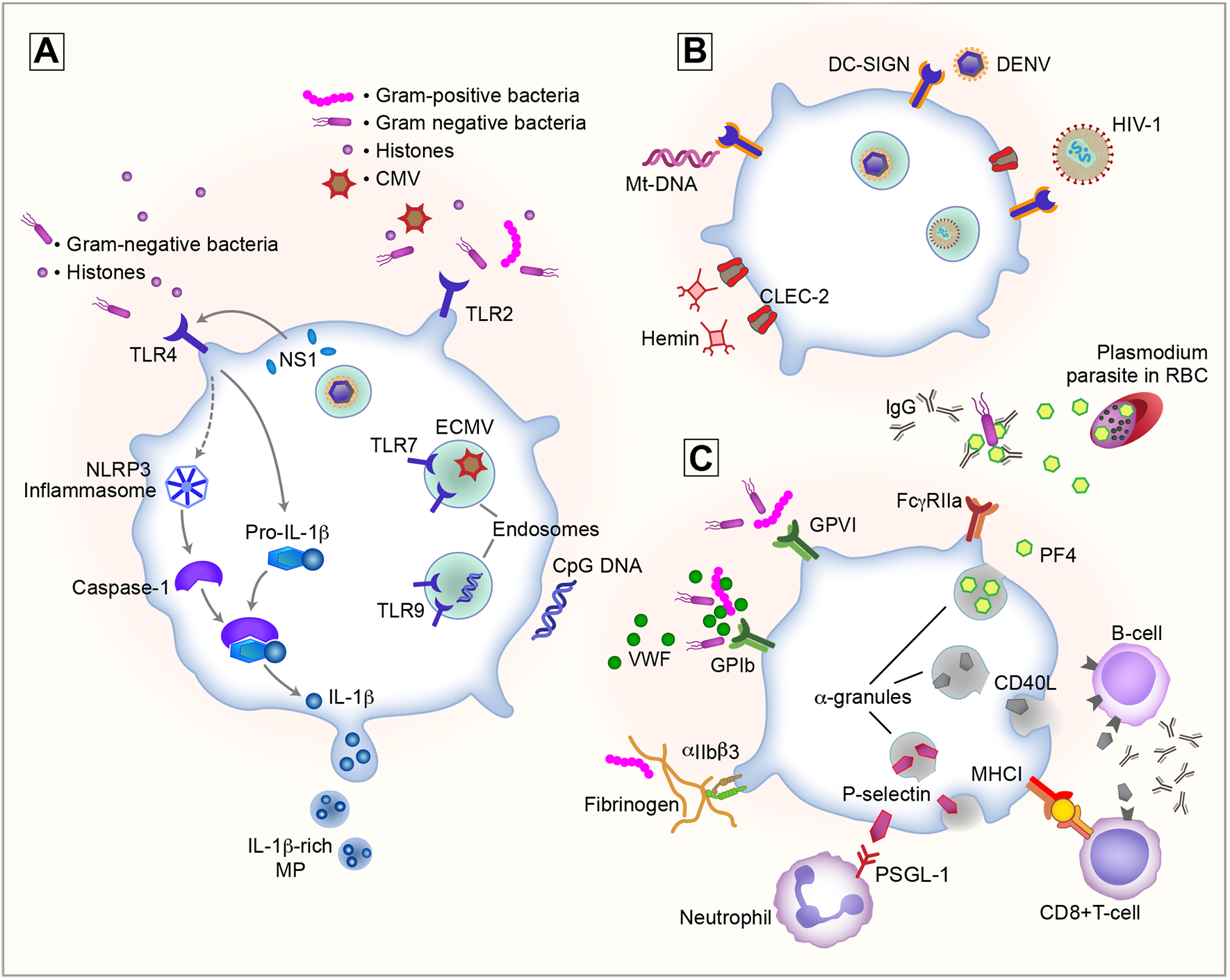
Platelets sense pathogens and host damage through recognition of pathogen- or damage-associated molecular patterns (PAMPs or DAMPs) using receptors. A) Toll-like receptors (TLRs) include surface receptors TLR2 and −4 with a broad tropism for bacterial pathogens as well as cytomegalovirus (CMV) and histones. Endosomal TLR7 and −9 recognize ssRNA viruses and unmethylated CpG DNA, respectively. TLR4 activation by NS1 or other TLR4 agonist induces splicing and synthesis of pro-IL-1ß, while caspase-1-mediated cleavage of pro-IL-1ß is activated by secondary signals. NLRP3 inflammasome activation results in the production IL-1ß-rich microparticles (MP). B) C-type lectin receptors DC-SIGN and CLEC-2 are involved in the binding of different viruses as well as the recognition of DAMPs such as hemin and mitochondrial DNA (Mt-DNA). C) Hemostatic platelet receptors GPIb, GPVI and αIIbß3 also interact with gram-positive and -negative bacteria either directly or indirectly using von Willebrand factor (VWF) or fibrinogen as bridging proteins. Engagement of the discussed receptors with their ligand results in the recognition of invading pathogens and triggers platelet activation and the release of α-granules. Released antimicrobial proteins such as platelet factor 4 (PF4) act as a first-line defense against the invading pathogens, killing Plasmodium parasites in infected red blood cells (RBCs) or coating gram-negative bacteria resulting in recognition of the opsonized bacterium with IgGs through FcγRIIa. Finally, surface receptors such as major histocompatibility complex class I (MHC-I) along with α-granules releasing proteins such as P-selectin and CD40L, result in recruitment and activation of both innate and adaptive immune responses.
Toll-like receptors –
Although all 10 human TLRs are detected at a transcriptional level in human platelets, only TLR1,−2,−3,−4,−6,−7 and −9 have reported functionality in platelets (Figure 1A).5–10 TLR4 and TLR2 are the best characterized platelet PRRs and both recognize structural components of pathogens, especially bacteria. TLR4 is specific for the Gram-negative bacterial cell wall component lipopolysaccharide (LPS) and activation of platelet TLR4 is linked to different aspects of the pathogenesis of sepsis.5,11–13 Many studies have attempted to examine the role of TLR4 agonists, including LPS, on platelet activation with conflicting results. In general studies have shown LPS directly induces cytokine release, including sCD40L and platelet activation factor.14 In addition, LPS has been shown to increase splicing of IL-1ß and tissue factor mRNA, which may contribute to both thrombosis and inflammation during sepsis.12,13 Others have reported LPS potentiates platelet activation when platelets are stimulated with low levels of platelet agonists, resulting in increased dense granule release, P-selectin expression, and platelet aggregation.15
TLR2 has a broader specificity, binding peptides from Gram-negative or Gram-positive bacteria, in conjunction with TLR1 or TLR6, respectively.6 Activation of TLR2 on platelets was first described through the use of a synthetic triacylated lipopeptide, that mimics the N-terminus of bacterial lipopeptides, called Pam3CSK4.7 Activation of TLR2 was also demonstrated by Gram-negative Porphyromonas gingivalis, which increased CD40L surface expression on platelets and induced platelet-neutrophil aggregate (PNA) formation.7,16 In addition, sepsis-inducing Gram-positive Streptococci strains through lipoteichoic acid and peptidoglycan interact with TLR2, resulting in platelet aggregation and αIIbß3 activation via phosphoinositide 3-kinase signaling.17,18
Interestingly, recent studies have demonstrated that platelet TLR engagement is not exclusive to bacteria. For example, platelet activation is induced by Dengue virus (DENV) nonstructural protein 1 (NS1) through TLR419 and the viral envelope glycoproteins from cytomegalovirus (CMV) via TLR220. Stimulation of platelet TLR2 and −4 by DAMPs, including histones in neutrophil extracellular traps (NETs), also result in platelet activation.21
Unlike TLR2 and −4, platelet TLR3, −7 and −9 are located intracellularly in endosomal compartments and recognize nucleotide derivatives.8 TLR3 traditionally recognizes dsRNA and is the least characterized of the three endosomal platelet TLRs. In vitro stimulation of platelets with TLR3 agonists have resulted in contradictory observations regarding platelet activation and further studies are necessary to elucidate the role of platelet TLR3.9,22 In contrast, platelet TLR7 plays an important role in the detection of ssRNA viruses including, influenza23, human immunodeficiency virus type-1 (HIV-1)24, hepatitis C virus25 and DENV26, as these viruses are known to be internalized by platelets. Previous studies have demonstrated activation of TLR7 by encephalomyocarditis virus (ECMV) resulting in mild thrombocytopenia and increased PNA formation, notably, without any pro-thrombotic effect.10 Activation of platelet TLR7 by influenza resulted in release of complement C3, triggers neutrophil-DNA release. This may enhance vascular occlusions and partially explain the increased risk of myocardial infarction observed in influenza patients.23 While TLR7 recognizes, ssRNA, platelet TLR9 typically recognizes dsDNA containing unmethylated CpG oligodeoxynucleotides from bacteria or viruses. TLR9 can also recognizes carboxy(alkylpyrrole) protein adducts, a class of DAMPs linked to oxidative stress that induce platelet activation and aggregation, enhancing in vivo thrombosis.27 Recently, activation of the TLR7 and −9 signaling pathways in platelets were further characterized. In these studies, both HIV-1 pseudovirions and synthetic TLR7 and −9 agonists induced platelet activation, including granule secretion and platelet leukocyte aggregate (PLA) formation, albeit to a lesser extent in comparison to thrombin stimulation. Importantly activation of the both TLR signaling pathways was dependent on endocytosis of the virion particles in a dynasore-sensitive, VAMP (vesicle-associated membrane protein)-3-and Arf6 (ADP-ribosylation factor 6)-dependent manner followed by endosomal acidification.8
C-type lectin receptors –
CLRs are a large family of surface receptors specialized in the recognition of glycans through their conserved carbohydrate-binding domains. Two platelet CLRs, involved in various infectious diseases, are dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and C-type lectin-like receptor 2 (CLEC-2) (Figure 1B). As a PRR, DC-SIGN recognizes N-linked high mannose glycans on viral envelope proteins, which enables viruses such as HIV-128 and DENV26 to bind platelets. Additionally, extracellular mitochondrial DNA (Mt-DNA), a DAMP released during inflammation, has been shown to induce platelet activation through DC-SIGN based on antibody blocking studies.29
Another platelet CLR with an important role during inflammation and infection is CLEC-2.28 Similar to DC-SIGN, HIV-1 and DENV bind CLEC-2 on platelets, which contributes to disease severity and the overall inflammatory response, especially during dengue infection.28,30 CLEC-2 is one of three platelet receptors, which contain immunoreceptor tyrosine-based activation motif (ITAM sequence) along with FcγR and FcγRIIa (see below). Besides viral binding to CLEC-2, podoplanin, the endogenous CLEC-2 ligand, plays an important role in bacterial infections. While the CLEC-2-podoplanin axis was shown to mediate inflammation-triggered thrombosis in the liver after Salmonella infection31, another study reported a thrombosis-independent inhibitory role towards systemic inflammation in sepsis32. In addition, to the role of CLEC-2 in thrombosis, the receptor is critical in regulating vascular integrity during inflammation. Loss of platelet CLEC-2 or podoplanin disrupts high endothelial venules, which are responsible for mediating lymphocyte trafficking to lymph nodes.33 Furthermore, loss of platelet CLEC-2 signaling leads to diminished immune response function of lymph nodes.34 Recently, hemin, a synthetic form of free heme, was also shown to activate platelets in a CLEC-2 dependent manner.35 Considering free heme is released during hemolysis, this interaction might be important in hemolytic infectious diseases including malaria and hemolytic-uremic syndrome.
NOD-like receptors –
NLRs are cytoplasmic PRRs that regulate inflammatory and apoptotic responses. The best characterized platelet NLR is nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing protein 3 (NLRP3), a sensor for inflammasome activation (Figure 1A). Platelet NLRP3 function was first described during DENV infection as DENV induces the assembly of the NLRP3 inflammasome, resulting in IL-1ß secretion through platelet-derived microparticles (MPs).36 Later, it was determined that NLRP3 activation was dependent on DENV NS1 binding to platelet TLR4. Activation of TLR4 induces splicing and synthesis of pro-IL-1ß, while cleavage of pro-IL-1ß to mature IL-1ß occurs in a caspase-1-dependent, which is activated by a secondary signal through ATP or other DAMPs.19 The increased synthesis of IL-1ß during Dengue infection is thought to enhance inflammation and endothelial cell permeability.
Platelet Fc receptor –
Besides PRRs human platelets also express Fcγ Receptor IIa (FcγRIIa), which is the only receptor on platelets to recognize the constant fragment (Fc) of immunoglobulin (Ig)G, which enables binding of IgG-immune complexes.37 IgG bound bacteria, including Escherichia coli and Streptococcus oralis, are recognized by FcγRIIa and trigger platelet activation and aggregation.38,39 In addition, FcγRIIa engagement renders platelets hypersensitive to other thrombotic stimuli as bacteria-induced platelet activation often occurs after simultaneous stimulation of FcγRIIa and additional platelet receptors.40–43 Similar to the role of FcγRIIa in bacterial infections, immunocomplexes of influenza A/H1N1 virus44 and fungal spores from Mucor circinelloides45 are also known to activate platelets through FcγRIIa and αIIbß3. Importantly, FcγRIIa is not expressed on mouse platelets, limiting relevant in vivo models.
Major Histocompatibility Complex (MHC) class I –
Platelets are also capable of processing pathogenic antigens through an MHC class I-dependent manner to activate the adaptive immune system. In an experimental cerebral malaria model (ECM), platelets were capable of directly presenting antigens to CD8+ T-cells (Figure 1C). Importantly, robust early antigen presentation by platelets resulted in activation of CD8+ T-cells and protected in ECM.46
Hemostatic platelet receptors –
Finally, platelets also express a wide range of receptors that are specific for platelets and megakaryocytes. In addition to their well-established roles in hemostasis and thrombosis, these receptors detect and interact with pathogens to regulate infection (Figure 1C).
Platelet activation through glycoprotein VI (GPVI), the major collagen receptor on platelets, was reported with recombinant staphylococcal superantigen-like protein 5 (SSL5).47 Furthermore, soluble GPVI was recently shown to predict the development of sepsis in ICU patients.48 Interestingly, platelet GPVI contributes to local host defense in a Klebsiella pneumoniae-induced mouse model of pneumonia-derived sepsis by regulating bacterial growth in the lungs.49 The involvement of GPVI in viral infections has been less studied with only hepatitis C virus reported to bind GPVI.50 However, the clinical significance of this interaction remains unclear.
Glycoprotein Ib (GPIb) is classically known to regulate platelet adhesion under high shear conditions by binding von Willebrand factor (VWF). However, several in vitro studies reported that GPIb also interacts directly with several bacterial proteins such as streptococcal protein B and streptococcal hemagglutinin51 of Streptococcus gordonii, SSL5 of Staphylococcus aureus47 and serine-rich protein A of S. sanguis52, inducing platelet activation. Several bacterial proteins also indirectly interact with GPIb through VWF.40 Importantly, platelet GPIb is also a key receptor in the surveillance mechanism consisting of platelets and Kupffer cells, the liver-resident macrophages, which detects and clears blood-borne bacterial infections. Upon infection the transient interactions of platelet GPIb with constitutively expressed VWF on the surface of Kupffer cells switches into a firm adhesion, followed by platelet aggregate formation around the bacterium.53 The role of GPIb during viral infections is less clear. A recent report suggests that DENV has a selective tropism for GPIb54, but further research is needed to investigate the clinical significance of this finding.
The most abundant glycoprotein on platelets is αIIbß3 integrin, which recognizes different ligands including fibrinogen. Direct interactions of bacterial proteins with αIIbß3 resulting in platelet adhesion have been previously described.42,55–57 In addition, bacteria can bind fibrinogen via bacterial surface proteins, including S. aureus clumping factor A41, the serine-aspartate repeat protein G of S. epidermidis42, and the M1 protein of S. pyogenes43 to interact with αIIbß3. This interaction, in combination with simultaneous stimulation of FcγRIIa, leads to platelet activation and aggregation.41–43 Integrin αIIbß3 is also critically involved in platelet migration. A recent study demonstrated platelets recruited to sites of injury become motile, enabling them to act as mechano-scavengers to collect bacteria-fibrin bundles within invaginations of the open canicular system. While these migrating platelets were unable to kill the engulfed bacteria (E. coli and S. aureus) directly, they facilitated bacterial removal by neutrophils.58
Activated platelets release α-granule content
Platelets act as specialized sentinels for pathogens invading the bloodstream. Recognition of PAMPs or DAMPs by different platelet receptors results in platelet activation and the release of platelet α-granules. One class of α-granule-stored proteins, particularly relevant to infectious diseases, are antimicrobial proteins (AMPs), subdivided in kinocidins, platelet microbicidal proteins (PMPs), and defensins.59 The most abundant kinocidin in platelets is platelet factor 4 (PF4; CXCL4) and is often used as the prototype to describe this family of AMPs. Its dual function is a direct consequence of its multidomain structure that consists of an anionic N-terminal chemokine domain with CXC-motif and a C-terminal AMP-like domain. The latter contains the typical amphipathic α-helix, in which cationic and hydrophilic side chains segregate on opposite sides of the molecule, allowing disruption of anionic microbial cell membranes.60 The antimicrobial activity of PF4 is particularly well-characterized for malaria parasites. Intraerythrocytic parasite killing by PF4 has been observed in vitro after binding to the erythrocytic Duffy-antigen receptor for chemokines.61 This interaction facilitates endocytosis and accumulation of PF4 into the infected erythrocyte resulting in lysis of the Plasmodium digestive vacuole (Figure 1C).62 This antiparasitic activity may be solely ascribed to its C-terminal region as a cyclic PF4 peptide dimer, containing only the last 14 amino acids, had a similar potency as full-size PF4.60 Importantly, the clinical relevance was recently demonstrated as PF4-mediated parasite killing occurred in samples of patients infected with all major human Plasmodium species.63 In addition, PF4 also works as an antimicrobial agent against bacteria and viruses. For example, platelets are able to kill bacteria in a PF4 and FcγRIIa-dependent manner. Secreted PF4 binds to bacterial polyanions on E. coli leading to the formation of anti-PF4/polyanion IgGs. These IgGs opsonize circulating E. coli leading to platelet FcγRIIa-mediated release of antimicrobial factors to destroy the opsonized bacteria (Figure 1C).64 PF4 can also inhibit HIV-1 entry into cells by blocking viral attachment.65
While PF4 has beneficial antimicrobial properties, the kinocidin is not completely protective during infection as PF4 also acts as a chemokine to recruit and activate leukocytes. Interestingly, elevated plasma levels of PF4 were observed in patients that died from severe cerebral malaria66 and PF4 deficiency improved outcomes after ECM67, demonstrating a detrimental role for PF4 besides its antimicrobial activity. Presumably, the immunomodulatory activity of the PF4 chemokine domain contributes to immune activation and T-cell recruitment to the brain, which aggravates ECM pathology.67 Interestingly, this role is consistent with other findings demonstrating that PF4 promotes leukocyte recruitment to the lungs of influenza infected mice to help in viral clearance.68
Other kinocidins include platelet basic protein (CXCL7) and RANTES (CCL5). The latter has proven anti-viral activity as it blocks HIV-1 entry to T-cells via CCR569 and assists PF4 in the recruitment of monocytes70. CXCL7 generates upon proteolytic cleavage neutrophil-activating peptide 2 (NAP-2), ß-thromboglobulin and connective tissue-activating peptide III (CTAP-III). Interestingly, C-terminal truncation of NAP-2 and CTAP-III results in PMPs called thrombocidins, which are bactericidal and fungicidal.71 Proteolytic cleavage of different PMPs is very common as PMPs are exposed to several proteases after secretion resulting in the formation of bactericidal, anti-viral and fugicidal peptides.72,73 Finally, defensins are a group of AMPs subdivided into α- and ß-defensins. While platelet ß-defensin 1 impairs the growth of S. aureus74, platelet α-defensin 1 has antibacterial activity against E. coli75. Interestingly, α-defensins are known to regulate human papillomaviruses infection.76 However, if platelet α-defensins have these anti-viral properties is unknown.
While many of the AMP and chemokines directly influence viral and bacterial pathogens, platelets also contain molecules, which help activate and regulate the innate and adaptive immune systems. P-selectin, an adhesion molecule exposed on the surface of activated platelets (Figure 1C), binds to endothelial cells and leukocytes through P-selectin glycoprotein ligand-1 (PSGL-1) and assists in the recruitment of circulating monocytes, neutrophils and lymphocytes to the inflamed endothelium.70 While, P-selectin and PSGL-1 initiate PLA formation, the interaction is further strengthened by leukocyte ß2-integrins interacting directly with GPIb and indirectly through fibrinogen binding to αIIbß3. PLA formation is a common feature in HIV77, influenza78, DENV79, severe sepsis80 and COVID-1981 and promotes thromboinflammation during infection. The recruitment and activation of innate immune cells (neutrophils and monocytes/macrophages) by activated platelets leading to thromboinflammation was just recently reviewed in70.
Platelets also play a major role in adaptive immunity, mainly through the release of CD40 ligand (CD40L) and tumor growth factor (TGF)-β, which are both located in α-granules. Both soluble and membrane-bound CD40L have immunomodulatory activities through binding to CD40 on immune cells. Activated platelets stimulate dendritic cells through soluble CD40L, resulting in increased phagocytosis and intracellular killing of bacteria.82 Platelet CD40L also regulates B-cell isotype switching and enhanced CD8 T-cell responses in mice infected with adenoviral vectors after prior immunization (Figure 1C).83
Platelet-derived TGF-β regulates differentiation of CD4+ T-cells into regulatory T-cells (Treg), which are immunosuppressive and help maintain tolerance towards self-antigens. Platelets contribute significantly to circulating levels of TGF-β which is required for the differentiation of Tregs. Interestingly, the importance of platelet TGF-β is underscored by the observation that Treg numbers and function are impaired in thrombocytopenic disorders.84,85
Conclusion
As part of the innate immune system, platelets recognize pathogens from all major classes of microorganisms. These interactions result in platelet activation and secretion of a broad range peptides to form a first-line defense against infection. Activated platelets also release chemokines and express ligands to activate leukocytes in order to trigger both the innate and adaptive immune response.
Highlights.
Platelets sense invading pathogens through their receptors, which results in platelet activation
Activated platelets release antimicrobial proteins and molecules that regulate the host response against infection
Antimicrobial proteins directly target the pathogen to limit the spread of the infection
Soluble and surface exposed molecules on activated platelets trigger both the innate and adaptive immune response
Acknowledgements
The authors want to thank Diana Lim for her excellent figure preparation
Sources of Funding
This work was supported by the NIA (K01AG059892).
Abbreviations
- AMP
Antimicrobial protein
- CD40L
CD40 ligand
- CLEC-2
C-type lectin-like receptor 2
- CLR
C-type lectin receptor
- CMV
Cytomegalovirus
- CTAP-III
Connective tissue-activating peptide III
- DAMP
Damage-associated molecular pattern
- DC-SIGN
Dendritic cell-specific intercellular adhesion Molecule-3-grabbing non-integrin
- DENV
Dengue virus
- ECM
Experimental cerebral malaria
- ECMV
Encephalomyocarditis virus
- E. coli
Escherichia coli
- FcγRIIa
Fcγ Receptor IIa
- GP
Glycoprotein
- HIV-1
Human immunodeficiency virus type-1
- Ig
Immunoglobulin
- LPS
Lipopolysaccharide
- MHC
Major histocompatibility complex
- Mt-DNA
Mitochondrial DNA
- MP
Platelet-derived microparticle
- NAP-2
Neutrophil-activating peptide 2
- NET
Neutrophil extracellular trap
- NLR
Nucleotide-binding oligomerization domain (NOD)-like receptor
- NLRP3
Nucleotide-binding oligomerization domain, Leucine-rich repeat and pyrin domain-containing protein 3
- NS1
Nonstructural protein 1 of Dengue virus
- PAMP
Pathogen-associated molecular pattern
- PF4
Platelet factor 4
- PLA
Platelet-leukocyte aggregate
- PMP
Platelet microbicidal protein
- PNA
Platelet-neutrophil aggregate
- PRR
Pattern recognition receptor
- PSGL-1
P-selectin glycoprotein ligand-1
- S. aureus
Staphylococcus aureus
- SSL5
Staphylococcal superantigen-like protein 5
- TGF-ß
Tumor growth factor-ß
- TLR
Toll-like receptors
- Treg
Regulatory T-cells
- VWF
von Willebrand factor
References
- 1.Guo L, Rondina MT. The era of thromboinflammation: Platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol. 2019;10:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claushuis TA, van Vught LA, Scicluna BP, Wiewel MA, Klein Klouwenberg PM, Hoogendijk AJ, Ong DS, Cremer OL, Horn J, Franitza M, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127:3062–3072 [DOI] [PubMed] [Google Scholar]
- 3.Middleton EA, Weyrich AS, Zimmerman GA. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev. 2016;96:1211–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers ME, Becker RE, Sailer A, Turner JR, Bubeck Wardenburg J. Synergistic action of staphylococcus aureus alpha-toxin on platelets and myeloid lineage cells contributes to lethal sepsis. Cell Host Microbe. 2015;17:775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional toll-like receptor-4. Blood. 2005;106:2417–2423 [DOI] [PubMed] [Google Scholar]
- 6.Hamzeh-Cognasse H, Berthelot P, Tardy B, Pozzetto B, Bourlet T, Laradi S, Garraud O, Cognasse F. Platelet toll-like receptors are crucial sensors of infectious danger moieties. Platelets. 2018;29:533–540 [DOI] [PubMed] [Google Scholar]
- 7.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee M, Huang Y, Joshi S, Popa GJ, Mendenhall MD, Wang QJ, Garvy BA, Myint T, Whiteheart SW. Platelets endocytose viral particles and are activated via tlr (toll-like receptor) signaling. Arterioscler Thromb Vasc Biol. 2020;40:1635–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anabel AS, Eduardo PC, Pedro Antonio HC, Carlos SM, Juana NM, Honorio TA, Nicolas VS, Sergio Roberto AR. Human platelets express toll-like receptor 3 and respond to poly i:C. Hum Immunol. 2014;75:1244–1251 [DOI] [PubMed] [Google Scholar]
- 10.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-tlr7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014;124:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet tlr4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469 [DOI] [PubMed] [Google Scholar]
- 12.Brown GT, Narayanan P, Li W, Silverstein RL, McIntyre TM. Lipopolysaccharide stimulates platelets through an il-1beta autocrine loop. J Immunol. 2013;191:5196–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rondina MT, Schwertz H, Harris ES, Kraemer BF, Campbell RA, Mackman N, Grissom CK, Weyrich AS, Zimmerman GA. The septic milieu triggers expression of spliced tissue factor mrna in human platelets. J Thromb Haemost. 2011;9:748–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, Garraud O. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91 [DOI] [PubMed] [Google Scholar]
- 15.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via tlr4/myd88 and the cgmp-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assinger A, Laky M, Badrnya S, Esfandeyari A, Volf I. Periodontopathogens induce expression of cd40l on human platelets via tlr2 and tlr4. Thromb Res. 2012;130:e73–78 [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Liu H, Luo X, Zhang P, Gao Y, Xie S, Xu K, Chang J, Ma L. Strains of group b streptococci from septic patients induce platelet activation via toll-like receptor 2. Clin Exp Pharmacol Physiol. 2017;44:335–343 [DOI] [PubMed] [Google Scholar]
- 18.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409 [DOI] [PubMed] [Google Scholar]
- 19.Quirino-Teixeira AC, Rozini SV, Barbosa-Lima G, Coelho DR, Carneiro PH, Mohana-Borges R, Bozza PT, Hottz ED. Inflammatory signaling in dengue-infected platelets requires translation and secretion of nonstructural protein 1. Blood Adv. 2020;4:2018–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assinger A, Kral JB, Yaiw KC, Schrottmaier WC, Kurzejamska E, Wang Y, Mohammad AA, Religa P, Rahbar A, Schabbauer G, et al. Human cytomegalovirus-platelet interaction triggers toll-like receptor 2-dependent proinflammatory and proangiogenic responses. Arterioscler Thromb Vasc Biol. 2014;34:801–809 [DOI] [PubMed] [Google Scholar]
- 21.Carestia A, Rivadeneyra L, Romaniuk MA, Fondevila C, Negrotto S, Schattner M. Functional responses and molecular mechanisms involved in histone-mediated platelet activation. Thromb Haemost. 2013;110:1035–1045 [DOI] [PubMed] [Google Scholar]
- 22.Blum P, Pircher J, Merkle M, Czermak T, Ribeiro A, Mannell H, Krotz F, Hennrich A, Spannagl M, Koppel S, et al. Arterial thrombosis in the context of hcv-associated vascular disease can be prevented by protein c. Cell Mol Immunol. 2017;14:986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koupenova M, Corkrey HA, Vitseva O, Manni G, Pang CJ, Clancy L, Yao C, Rade J, Levy D, Wang JP, et al. The role of platelets in mediating a response to human influenza infection. Nat Commun. 2019;10:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: Engulfment of hiv and staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029 [DOI] [PubMed] [Google Scholar]
- 25.de Almeida AJ, Campos-de-Magalhaes M, Brandao-Mello CE, de Oliveira RV, Yoshida CF, Lampe E. Detection of hepatitis c virus in platelets: Evaluating its relationship to viral and host factors. Hepatogastroenterology. 2007;54:964–968 [PubMed] [Google Scholar]
- 26.Simon AY, Sutherland MR, Pryzdial EL. Dengue virus binding and replication by platelets. Blood. 2015;126:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res. 2013;112:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaipan C, Soilleux EJ, Simpson P, Hofmann H, Gramberg T, Marzi A, Geier M, Stewart EA, Eisemann J, Steinkasserer A, et al. Dc-sign and clec-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin G, Wei Z, Ji C, Zheng H, Gu J, Ma L, Huang W, Morris-Natschke SL, Yeh JL, Zhang R, et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtdna release. Sci Rep. 2016;6:36222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung PS, Huang TF, Hsieh SL. Extracellular vesicles from clec2-activated platelets enhance dengue virus-induced lethality via clec5a/tlr2. Nat Commun. 2019;10:2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hitchcock JR, Cook CN, Bobat S, Ross EA, Flores-Langarica A, Lowe KL, Khan M, Dominguez-Medina CC, Lax S, Carvalho-Gaspar M, et al. Inflammation drives thrombosis after salmonella infection via clec-2 on platelets. J Clin Invest. 2015;125:4429–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayes J, Lax S, Wichaiyo S, Watson SK, Di Y, Lombard S, Grygielska B, Smith SW, Skordilis K, Watson SP. The podoplanin-clec-2 axis inhibits inflammation in sepsis. Nat Commun. 2017;8:2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, Pan Y, Sheng M, Yago T, Silasi-Mansat R, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet clec-2. Nature. 2013;502:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benezech C, Nayar S, Finney BA, Withers DR, Lowe K, Desanti GE, Marriott CL, Watson SP, Caamano JH, Buckley CD, et al. Clec-2 is required for development and maintenance of lymph nodes. Blood. 2014;123:3200–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourne JH, Colicchia M, Di Y, Martin E, Slater A, Roumenina LT, Dimitrov JD, Watson SP, Rayes J. Heme induces human and mouse platelet activation through c-type-lectin-like receptor-2. Haematologica. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA, et al. Platelets mediate increased endothelium permeability in dengue through nlrp3-inflammasome activation. Blood. 2013;122:3405–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao J, Al-Tamimi M, Baker RI, Andrews RK, Gardiner EE. The platelet fc receptor, fcgammariia. Immunol Rev. 2015;268:241–252 [DOI] [PubMed] [Google Scholar]
- 38.Moriarty RD, Cox A, McCall M, Smith SG, Cox D. Escherichia coli induces platelet aggregation in an fcgammariia-dependent manner. J Thromb Haemost. 2016;14:797–806 [DOI] [PubMed] [Google Scholar]
- 39.Tilley DO, Arman M, Smolenski A, Cox D, O’Donnell JS, Douglas CW, Watson SP, Kerrigan SW. Glycoprotein ibalpha and fcgammariia play key roles in platelet activation by the colonizing bacterium, streptococcus oralis. J Thromb Haemost. 2013;11:941–950 [DOI] [PubMed] [Google Scholar]
- 40.Byrne MF, Kerrigan SW, Corcoran PA, Atherton JC, Murray FE, Fitzgerald DJ, Cox DM. Helicobacter pylori binds von willebrand factor and interacts with gpib to induce platelet aggregation. Gastroenterology. 2003;124:1846–1854 [DOI] [PubMed] [Google Scholar]
- 41.Loughman A, Fitzgerald JR, Brennan MP, Higgins J, Downer R, Cox D, Foster TJ. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by staphylococcus aureus clumping factor a. Mol Microbiol. 2005;57:804–818 [DOI] [PubMed] [Google Scholar]
- 42.Brennan MP, Loughman A, Devocelle M, Arasu S, Chubb AJ, Foster TJ, Cox D. Elucidating the role of staphylococcus epidermidis serine-aspartate repeat protein g in platelet activation. J Thromb Haemost. 2009;7:1364–1372 [DOI] [PubMed] [Google Scholar]
- 43.Shannon O, Hertzen E, Norrby-Teglund A, Morgelin M, Sjobring U, Bjorck L. Severe streptococcal infection is associated with m protein-induced platelet activation and thrombus formation. Mol Microbiol. 2007;65:1147–1157 [DOI] [PubMed] [Google Scholar]
- 44.Boilard E, Pare G, Rousseau M, Cloutier N, Dubuc I, Levesque T, Borgeat P, Flamand L. Influenza virus h1n1 activates platelets through fcgammariia signaling and thrombin generation. Blood. 2014;123:2854–2863 [DOI] [PubMed] [Google Scholar]
- 45.Ghuman H, Shepherd-Roberts A, Watson S, Zuidscherwoude M, Watson SP, Voelz K. Mucor circinelloides induces platelet aggregation through integrin alphaiibbeta3 and fcgammariia. Platelets. 2019;30:256–263 [DOI] [PubMed] [Google Scholar]
- 46.Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, Yui K, Topham DJ, Baldwin WM 3rd, Morrell CN. Platelets present antigen in the context of mhc class i. J Immunol. 2012;189:916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu H, Armstrong PC, Khalil E, Chen YC, Straub A, Li M, Soosairajah J, Hagemeyer CE, Bassler N, Huang D, et al. Gpvi and gpibalpha mediate staphylococcal superantigen-like protein 5 (ssl5) induced platelet activation and direct toward glycans as potential inhibitors. PLoS One. 2011;6:e19190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montague SJ, Delierneux C, Lecut C, Layios N, Dinsdale RJ, Lee CS, Poulter NS, Andrews RK, Hampson P, Wearn CM, et al. Soluble gpvi is elevated in injured patients: Shedding is mediated by fibrin activation of gpvi. Blood Adv. 2018;2:240–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claushuis TAM, de Vos AF, Nieswandt B, Boon L, Roelofs J, de Boer OJ, van ‘t Veer C, van der Poll T. Platelet glycoprotein vi aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood. 2018;131:864–876 [DOI] [PubMed] [Google Scholar]
- 50.Zahn A, Jennings N, Ouwehand WH, Allain JP. Hepatitis c virus interacts with human platelet glycoprotein vi. J Gen Virol. 2006;87:2243–2251 [DOI] [PubMed] [Google Scholar]
- 51.Bensing BA, Lopez JA, Sullam PM. The streptococcus gordonii surface proteins gspb and hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein ibalpha. Infect Immun. 2004;72:6528–6537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plummer C, Wu H, Kerrigan SW, Meade G, Cox D, Ian Douglas CW. A serine-rich glycoprotein of streptococcus sanguis mediates adhesion to platelets via gpib. Br J Haematol. 2005;129:101–109 [DOI] [PubMed] [Google Scholar]
- 53.Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood-borne pathogens on kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol. 2013;14:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attatippaholkun N, Kosaisawe N, Y UP, Supraditaporn P, Lorthongpanich C, Pattanapanyasat K, Issaragrisil S. Selective tropism of dengue virus for human glycoprotein ib. Sci Rep. 2018;8:2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coburn J, Leong JM, Erban JK. Integrin alpha iib beta 3 mediates binding of the lyme disease agent borrelia burgdorferi to human platelets. Proc Natl Acad Sci U S A. 1993;90:7059–7063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miajlovic H, Zapotoczna M, Geoghegan JA, Kerrigan SW, Speziale P, Foster TJ. Direct interaction of iron-regulated surface determinant isdb of staphylococcus aureus with the gpiib/iiia receptor on platelets. Microbiology (Reading). 2010;156:920–928 [DOI] [PubMed] [Google Scholar]
- 57.Petersen HJ, Keane C, Jenkinson HF, Vickerman MM, Jesionowski A, Waterhouse JC, Cox D, Kerrigan SW. Human platelets recognize a novel surface protein, pada, on streptococcus gordonii through a unique interaction involving fibrinogen receptor gpiibiiia. Infect Immun. 2010;78:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B, Yavuz G, Luckner M, Ishikawa-Ankerhold H, Hennel R, et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell. 2017;171:1368–1382 e1323 [DOI] [PubMed] [Google Scholar]
- 59.Yeaman MR. Platelets: At the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12:426–437 [DOI] [PubMed] [Google Scholar]
- 60.Lawrence N, Dennis ASM, Lehane AM, Ehmann A, Harvey PJ, Benfield AH, Cheneval O, Henriques ST, Craik DJ, McMorran BJ. Defense peptides engineered from human platelet factor 4 kill plasmodium by selective membrane disruption. Cell Chem Biol. 2018;25:1140–1150 e1145 [DOI] [PubMed] [Google Scholar]
- 61.McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G, Foote SJ. Platelet factor 4 and duffy antigen required for platelet killing of plasmodium falciparum. Science. 2012;338:1348–1351 [DOI] [PubMed] [Google Scholar]
- 62.Love MS, Millholland MG, Mishra S, Kulkarni S, Freeman KB, Pan W, Kavash RW, Costanzo MJ, Jo H, Daly TM, et al. Platelet factor 4 activity against p. Falciparum and its translation to nonpeptidic mimics as antimalarials. Cell Host Microbe. 2012;12:815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kho S, Barber BE, Johar E, Andries B, Poespoprodjo JR, Kenangalem E, Piera KA, Ehmann A, Price RN, William T, et al. Platelets kill circulating parasites of all major plasmodium species in human malaria. Blood. 2018;132:1332–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palankar R, Kohler TP, Krauel K, Wesche J, Hammerschmidt S, Greinacher A. Platelets kill bacteria by bridging innate and adaptive immunity via platelet factor 4 and fcgammariia. J Thromb Haemost. 2018;16:1187–1197 [DOI] [PubMed] [Google Scholar]
- 65.Auerbach DJ, Lin Y, Miao H, Cimbro R, Difiore MJ, Gianolini ME, Furci L, Biswas P, Fauci AS, Lusso P. Identification of the platelet-derived chemokine cxcl4/pf-4 as a broad-spectrum hiv-1 inhibitor. Proc Natl Acad Sci U S A. 2012;109:9569–9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson NO, Jain V, Roberts CE, Lucchi N, Joel PK, Singh MP, Nagpal AC, Dash AP, Udhayakumar V, Singh N, et al. Cxcl4 and cxcl10 predict risk of fatal cerebral malaria. Dis Markers. 2011;30:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava K, Cockburn IA, Swaim A, Thompson LE, Tripathi A, Fletcher CA, Shirk EM, Sun H, Kowalska MA, Fox-Talbot K, et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo L, Feng K, Wang YC, Mei JJ, Ning RT, Zheng HW, Wang JJ, Worthen GS, Wang X, Song J, et al. Critical role of cxcl4 in the lung pathogenesis of influenza (h1n1) respiratory infection. Mucosal Immunol. 2017;10:1529–1541 [DOI] [PubMed] [Google Scholar]
- 69.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of rantes, mip-1 alpha, and mip-1 beta as the major hiv-suppressive factors produced by cd8+ t cells. Science. 1995;270:1811–1815 [DOI] [PubMed] [Google Scholar]
- 70.Martinod K, Deppermann C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets. 2020:1–11 [DOI] [PubMed] [Google Scholar]
- 71.Krijgsveld J, Zaat SA, Meeldijk J, van Veelen PA, Fang G, Poolman B, Brandt E, Ehlert JE, Kuijpers AJ, Engbers GH, et al. Thrombocidins, microbicidal proteins from human blood platelets, are c-terminal deletion products of cxc chemokines. J Biol Chem. 2000;275:20374–20381 [DOI] [PubMed] [Google Scholar]
- 72.Mohan KV, Rao SS, Atreya CD. Antiviral activity of selected antimicrobial peptides against vaccinia virus. Antiviral Res. 2010;86:306–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perkhofer S, Kehrel BE, Dierich MP, Donnelly JP, Nussbaumer W, Hofmann J, von Eiff C, Lass-Florl C. Human platelets attenuate aspergillus species via granule-dependent mechanisms. J Infect Dis. 2008;198:1243–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kraemer BF, Campbell RA, Schwertz H, Cody MJ, Franks Z, Tolley ND, Kahr WH, Lindemann S, Seizer P, Yost CC, et al. Novel anti-bacterial activities of beta-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7:e1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valle-Jimenez X, Ramirez-Cosmes A, Aquino-Dominguez AS, Sanchez-Pena F, Bustos-Arriaga J, Romero-Tlalolini MLA, Torres-Aguilar H, Serafin-Lopez J, Aguilar Ruiz SR. Human platelets and megakaryocytes express defensin alpha 1. Platelets. 2020;31:344–354 [DOI] [PubMed] [Google Scholar]
- 76.Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci U S A. 2006;103:1516–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Green SA, Smith M, Hasley RB, Stephany D, Harned A, Nagashima K, Abdullah S, Pittaluga S, Imamichi T, Qin J, et al. Activated platelet-t-cell conjugates in peripheral blood of patients with hiv infection: Coupling coagulation/inflammation and t cells. AIDS. 2015;29:1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rondina MT, Brewster B, Grissom CK, Zimmerman GA, Kastendieck DH, Harris ES, Weyrich AS. In vivo platelet activation in critically ill patients with primary 2009 influenza a(h1n1). Chest. 2012;141:1490–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hottz ED, Medeiros-de-Moraes IM, Vieira-de-Abreu A, de Assis EF, Vals-de-Souza R, Castro-Faria-Neto HC, Weyrich AS, Zimmerman GA, Bozza FA, Bozza PT. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J Immunol. 2014;193:1864–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rondina MT, Carlisle M, Fraughton T, Brown SM, Miller RR 3rd, Harris ES, Weyrich AS, Zimmerman GA, Supiano MA, Grissom CK. Platelet-monocyte aggregate formation and mortality risk in older patients with severe sepsis and septic shock. J Gerontol A Biol Sci Med Sci. 2015;70:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, et al. Platelet gene expression and function in patients with covid-19. Blood. 2020;136:1317–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishat S, Wuescher LM, Worth RG. Platelets enhance dendritic cell responses against staphylococcus aureus through cd40-cd40l. Infect Immun. 2018;86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Nieswandt B, Wang Y, Davidson BL, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19 [DOI] [PubMed] [Google Scholar]
- 84.Liu B, Zhao H, Poon MC, Han Z, Gu D, Xu M, Jia H, Yang R, Han ZC. Abnormality of cd4(+)cd25(+) regulatory t cells in idiopathic thrombocytopenic purpura. Eur J Haematol. 2007;78:139–143 [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Heck S, Patel V, Levan J, Yu Y, Bussel JB, Yazdanbakhsh K. Defective circulating cd25 regulatory t cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112:1325–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]


