Abstract
QS-21 is a triterpene glycoside saponin found in the bark of the Chilean soap bark tree Quillaja saponaria. It is a highly potent vaccine adjuvant that is included in two approved vaccines and has shown promise in numerous other vaccine candidates in the research and clinical pipelines. One major hurdle to the widespread use of this adjuvant is the difficulty of obtaining it in high yield and purity. Previously re- ported purification approaches either showed suboptimal purity and/or yield, lacked efficiency, or had strict requirement on the composition of the starting material. Here, we report the development of a new two-step orthogonal chromatographic process, consisting of a polar reversed-phase (RP) chromatography step followed by a hydrophilic interaction chromatography (HILIC) step, for purifying QS-21 from a commercially available Quillaja saponaria bark extract with high yield and > 97% purity. This process makes available a simple and efficient method for obtaining highly pure QS-21 from saponin-enriched bark extract.
Keywords: QS-21, Adjuvant, Saponin, Hydrophilic Interaction Chromatography, Orthogonal chromatographic process, Quillaja saponaria
1. Introduction
Adjuvants are compounds which help enhance immune response to vaccine antigens. The molecular adjuvant QS-21 is a triterpenoid that belongs to a class of amphiphilic molecules called saponins. It is found in the bark of the Chilean soap bark tree Quillaja saponaria [1]. The name “QS-21” initially referred to the 21st chromatography fraction of an early attempt by Kensil et. al. to separate a Quillaja saponaria bark extract by reversed-phase (RP) chromatography, which displayed strong adjuvant activity and a favorable toxicity profile relative to other studied fractions [2]. Subsequent characterizations identified the principle constituent of this particular fraction as an acylated triterpene glycoside with a molecular formula of C92 O46 H148 and a molecular weight of 1,990 Da, now commonly known as “QS-21” [3, 4]. In recent years, QS-21 has attracted much attention as a vaccine adjuvant due to its high potency and unique advantage of being able to elicit both humoral and cellular immune responses toward a wide range of antigens, which is instrumental to the success of vaccines against many pathogens [5, 6]. The success of QS-21 as a vaccine adjuvant is demonstrated by two currently approved vaccines: Shingrix®, a shingles vaccine first approved by the US Food and Drug Adminstration (FDA) in 2017 and now available in many other countries around the world that shows > 90% efficacy, even in subjects over 80 years old; and Mosquirix®, the world’s first malaria vaccine approved by the European Medicines Agency (EMA) in 2015 for infants and young children in endemic regions [6]. The adjuvant formulation in both vaccines, named AS01, is a liposomal system containing the adjuvant components QS-21 and Monophosphoryl Lipid A (MPL®) [7, 8]. In addition, numerous other vaccine candidates covering a wide range of diseases in the research and clinical pipelines contain QS-21 as a critical adjuvant component, including a promising tuberculosis vaccine candidate which showed 50% protection in a Phase 2 clinical trial three years after the second dose [9–12].
Despite the clinical success of QS-21-containing vaccines, a major hindrance to future vaccine development and production is the challenge of obtaining the saponin in high purity and yield [13]. Indeed, despite blockbuster sales of Shingrix® in 2019, production was unable to meet demand [14], although it is unclear whether the vaccine supply issues are related specifically to QS-21. Of greater concern is that adjuvant-related supply issues would also impact production of the malaria and tuberculosis vaccine candidates that rely on the same components, including QS-21. While some emerging work is now focusing on producing QS-21 and its variants synthetically [15–17], currently the molecule is still primarily sourced from nature. The QS-21 content in the Quillaja saponaria tree bark is not only limited but also variable in response to climate and environmental factors [2, 13]. Additionally, purification is performed from a complex natural mixture containing a large number of impurities that are chemically very similar to the molecule of interest [18–21]. Therefore, it is of high importance to develop a robust purification process for QS-21 that maximizes both yield and purity.
QS-21 is an amphiphilic molecule consisting of a central quillaic acid triterpene core, flanked on either side by a branched trisaccharide and a linear tetrasaccharide, which is in turn connected to a fatty acyl chain. The trisaccharide attached at the C3-position of the quillaic acid comprises D-glucuronic acid, D-galactose, and D-xylose. The tetrasaccharide connected via the C28 carboxylate on the triterpene is a linear chain of D-fucose, L-rhamnose and D-xylose linked to one of two isomeric sugars, D-apiose or D-xylose, giving rise to two compositional isomers denoted as QS-21api (65%) and QS-21xyl (35%), respectively [22, 23]. Additionally, an L-arabinose-terminated acyl chain is attached to the fucose residue via a hydrolytically labile ester bond. Intramolecular trans- esterification of this bond between the 4- and 3-hydroxyl groups on the fucose ring occurs naturally in solution, resulting in two regioisomers, QS-21A and QS-21B at a ratio of 20:1 [3, 24, 25]. There- fore, QS-21 is not a single molecular entity but rather consists of four isomeric forms, all of which have previously been isolated and were found to possess comparable adjuvant activity [22–24].
Commercially available saponin-enriched extracts of Quillaja saponaria tree bark, which often serve as the starting materials for purifying QS-21, are complex mixtures of polyphenolics and a large number of saponins that are structurally and chemically similar to QS-21, with differences primarily in the hydrophilic sugar residues, making purification a challenge [1, 10, 18–21, 26]. Previously reported purification approaches either showed suboptimal purity and/or yield, lacked efficiency, or had strict requirement on the composition of the starting material [10, 27–29].
We previously employed a first generation process for purifying QS-21 for use in preclinical and clinical studies [30, 31], which consisted of running a commercially available saponin-enriched extract of Quillaja saponaria bark, such as Quil-A® or VET-SAP®, through an RP C18 step followed by an RP C8 step. Due to the lack of orthogonality between the two steps, the second step did not provide substantial additional separation. As a result, obtaining high purity was only possible by sacrificing yield. Even with careful selection of collected fractions, an overall yield of only ~ 0.5% (out of total starting material) was typically achieved at a purity level of ~85% (as assessed on an analytical C4 or C8 column). Given the amphiphilic nature of Quillaja saponaria saponins, RP alone does not offer the all-around retention and selectivity required to achieve the high level of yield and purity needed for this particular application. An increase in yield could reduce the production cost of QS-21, while a higher purity level is crucial from both a regulatory standpoint and known toxicity from other saponin components typically contained in Quillaja Saponaria bark extracts. We thus set out to develop a new purification process for QS-21 with improved yield and purity.
2. Experimental
2.1. Materials
VET-SAP®, a saponin-enriched Quillaja Saponaria extract in the form of a beige-colored dry powder, was purchased from Desert King International (San Diego, CA, USA). Acetonitrile (MeCN, HPLC grade), formic acid (99.0+% Optima™ LC/MS grade), ammonium acetate (Crystalline/HPLC grade), ammonium formate (Crystalline/ACS grade) and glacial acetic acid (HPLC grade) were purchased from Fisher Scientific (Hampton, NH, USA). Acetonitrile/formic acid 0.1% (20 L NOWPak®) used in the preparative engineering run of the first step was purchased from MilliporeSigma (Burlington, MA, USA). Disposable filter units (250 mL, 0.2 μm Polyethersulfone membrane) used for filtering dissolved starting extract and disposable syringe filters (25 mm, 0.2 μm PTFE membrane) used for filtering dissolved intermediate from the first chromatography step were purchased from Corning Inc. (Corning, NJ, USA). All purchased reagents and supplies were used as is. Milli-Q water (H2O) was obtained from an in-house Milli-Q® Ultrapure Lab Water System (MilliporeSigma, Burlington, MA, USA).
Analytical Gemini® C18 (250 × 4.6 mm, 5 μm, 110 Å), preparative Gemini® C18 (250 × 21.2 mm, 5 μm, 110 Å), Synergi™ Hydro RP (250 × 4.6 mm, 4 μm, 80 Å), analytical Luna® Omega Polar C18 (250 × 4.6 mm, 5 μm, 100 Å), preparative Luna® Omega Polar C18 (250 × 50 mm, 5 μm, 100 Å), Kinetex® HILIC (250 × 4.6 mm, 5 μm, 100 Å), Luna® NH2 (250 × 4.6 mm, 5 μm, 100 Å) and Luna® Omega SUGAR (250 × 4.6 mm, 3 μm, 100 Å) were purchased from Phenomenex (Torrance, CA, USA). Preparative Luna® Omega Polar C18 was fitted with a 15 × 30.0 mm guard column. TSKgel®-Amide 80 (250 × 4.6 mm, 5 μm, 100 Å) was purchased from TOSOH Bioscience (Tokyo, Japan). Zorbax® NH2 (250 × 4.6 mm, 5 μm, 70 Å) and Polaris™ C8 (250 × 21.2 mm, 5 um, 110 Å) were purchased from Agilent Technologies (Santa Clara, CA, USA). Analytical Syncronis™ HILIC (250 × 4.6 mm, 5 μm, 100 Å) and preparative Syncronis™ HILIC (250 × 21.2 mm, 5 μm, 100 Å) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Preparative Syncronis™ HILIC was fitted with a 10 × 21.2 mm guard column. Sequant® ZIC®-HILIC (250 × 4.6 mm, 5 μm, 200 Å) was purchased from MilliporeSigma (Burlington, MA, USA). Vydac® C4 (250 × 4.6 mm, 5 μm, 300 Å) was purchased from Avantor (Radnor Township, PA, USA).
2.2. Instrumentation
All analytical development was performed on a Shimadzu Prominence HPLC system (Shimadzu, Kyoto, Japan). All preparative development and engineering runs were performed on a Gilson GX-281 preparative HPLC system (Gilson, Middleton, WI, USA). All chromatography runs were performed at room temperature (~22 °C). Sample lyophilization after each chromatography step was done on a VirTis FreezeMobile 25 EL (SP Scientific, Warminster, PA, USA). LC/MS analyses were done on either an Agilent Technologies 6460 Triple Quadrupole (QqQ) LC/MS system or an Agilent 6520 Quadrupole Time-of-Flight (Q-TOF) LC/MS system as indicated in the Results Section. Each of the two systems was equipped with an Agilent 1290 Infinity LC system.
2.3. Methods
All chromatography conditions are detailed in figure captions in the Results and Discussion section or the Supplementary Information.
LC/QqQ-MS analyses on the Agilent 6460 Triple Quadrupole system were run as follows: 10 μL of 1 mg/mL samples dissolved in 50:50 MeCN: H2O with 0.05 wt% ammonium acetate were injected into the LC module without a column and run isocratically in 50:50 MeCN: H2O with 0.05 wt% ammonium acetate at 0.2 mL/min. UV absorption was detected at 210 nm. MS was run in negative ion mode with electrospray ionization (ESI), 100–2,500 m/z, scan time 500, fragmentor 80, accelerator voltage 7 V, step size 0.1 amu, delta EMV(−) 50–200, gas temperature 350°C, gas flowrate 9 L/min, nebulizer 45 psig, and capillary voltage 4,500 V.
LC/Q-TOF MS analyses on the Agilent 6520 Quadrupole Time-of-Flight system were run as follows: Samples in the form of lyophilized powders were resuspended at 1 mg/mL in 50:50 MeCN: Milli-Q water. 20 μL of each sample was injected into the LC module without a column and run isocratically in 50:50 MeCN: H2O with 0.05 wt% ammonium acetate at 0.1 mL/min. Elucidation of the composition of major and minor peaks in the final purified QS-21 was carried out by first separating the sample on the analytical C4 column. Mobile phase A: H2O with 0.05 wt% ammonium acetate, mobile phase B: MeCN with 0.05 wt% ammonium acetate. Gradient condition: 0.0 min-35 % B, 20.0 min-40 % B, 21.0 min-40 % B, 26.0 min-90 % B, 34.0 min-90 % B, 35.0 min-35 % B.UV absorption was detected at 210 nm. MS was run in dual ESI negative ion mode, 20 0–2,50 0 m/z, scan rate 3 spectra/sec, gas temperature 350°C, gas flowrate 12 L/min, nebulizer 50 psig, capillary voltage 2,500 V, and fragmentor 145.
3. Results and Discussion
3.1. Analytical development of the first step
The development objective was to improve upon our first generation two-step RP chromatographic process. Given the amphiphilic nature of saponins, hybrid polar C18 phases were investigated as better options for the first step than the traditional C18 phase, in an effort to achieve retention and selectivity toward both the non-polar and polar moieties on these molecules. Table 1 summarizes the columns and conditions tested in this study. Two columns, Synergi Hydro RP (250 × 4.6 mm, 4 μm, 80 Å) and Luna Omega Polar C18 (250 × 4.6 mm, 5 μm, 100 Å), were chosen to be compared to Gemini C18 (250 × 4.6 mm, 5 μm, 110 Å), a preparative version of which was used in the first step of our first generation process. As shown in Fig. 1A, VET-SAP® dissolved in H2O without any pre-treatment was separated by a 20–80% B gradient (mobile phase A: H2O with 0.1% formic acid, mobile phase B: MeCN with 0.1% formic acid) on all three columns. Both Synergi Hydro RP and Luna Omega Polar C18 outperformed Gemini C18, with Luna Omega Polar C18 showing the highest resolution. Progressively narrowing down the gradient on Luna Omega Polar C18 from 20–80% to 30–70%, then to 40–50% mobile phase B, resulted in additional peak separation (Fig. 1B).
Table 1.
Columns and corresponding conditions used for the development of the new chromatographic purification process for QS-21. Listed gradients are the main separation gradients of each method. For full gradient profiles, refer to figure captions. FA: formic acid, AA: ammonium acetate, AF: ammonium formate.
| Column Name | Column Specifications | Column Chemistry | Flowrate (mL/min) | Injection Volume | Sample Concentration (mg/mL) | Condition(s) | Figure(s) |
|---|---|---|---|---|---|---|---|
| First Chromatographic Step | |||||||
| Gemini C18 | 250 × 4.6 mm, 5 μm, 110 Å | C18 | 1 | 50 μL | 1 | Gradient: 20–80% MeCN with 0.1% FA | Fig. 1A |
| Synergi Hydro RP | 250 × 4.6 mm, 4 μm, 80 Å | Polar C18 | 1 | 50 μL | 1 | Gradient: 20–80% MeCN with 0.1% FA | Fig. 1A |
| Luna Omega Polar C18 | 250 × 4.6 mm, 5 μm, 100 Å | Polar C18 | 1 | 50 μL | 1 | Gradients: 20–80%, 30–70%, 40–50% MeCN with 0.1% FA | Fig. 1 |
| Luna Omega Polar C18 | 250 × 50 mm, 5 μm, 100 Å | Polar C18 | 85 | 1, 3, 5, 10 mL | 100 | Gradients: 35–55%, 37–50%, 38–49%, 40–47%, 40–45% MeCN with 0.1% FA |
Fig. 2, SI Fig. 1B |
| Second Chromatographic Step | |||||||
| Kinetex HILIC | 250 × 4.6 mm, 5 μm, 100 Å | Unbonded silica | 1 | 20 μL | 1 | Isocratic: 80:20 MeCN:H 2 O with 5 mM AA (pH 5.8) or AF (pH3.2) |
SI Fig. 3 |
| TSKgel-Amide 80 | 250 × 4.6 mm, 5 μm, 100 Å | Amido | 1 | 20 μL | 1 | Isocratic: 80:20 MeCN:H 2 O with 5 mM AA (pH 5.8) |
Fig. 3 |
| Zorbax NH 2 | 250 × 4.6 mm, 5 μm, 70 Å | Amino | 1 | 20 μL | 1 | Isocratic: 50:50, 60:40, 65:35 MeCN:H 2 O with 5 mM AA (pH 5.8) |
Fig. 4 |
| Luna NH 2 | 250 × 4.6 mm, 5 μm, 100 Å | Amino | 1 | 20 μL | 1 | Isocratic: 50:50 MeCN:H 2 O with 5 mM AA (pH 5.8) |
SI Fig. 4 |
| Luna Omega SUGAR | 250 × 4.6 mm, 3 μm, 100 Å | Amido/amino | 1 | 20 μL | 1 | Isocratic: 70:30, 75:25 MeCN:H 2 O with 5 mM AA (pH 5.8) |
SI Fig. 5 |
| Syncronis HILIC* | 250 × 4.6 mm, 5 μm, 100 Å | Sulfobetaine | 1 | 20| 2, 5, 30, 60 μL | 1| 40 | Isocratic: 80:20 MeCN:H 2 O with 5 mM AA (pH 5.8) or AF (pH3.2) |
Fig. 5, SI Fig. 6 |
| Syncronis HILIC | 250 × 21.2 mm, 5 μm, 100 Å | Sulfobetaine | 20 | 250, 600 μL | 40 | Isocratic: 80:20 MeCN:H 2 O with 5 mM AA (pH 5.8) |
Fig. 6, SI Fig. 8A |
| Sequant ZIC-HILIC | 250 × 4.6 mm, 5 μm, 200 Å | Sulfobetaine | 1 | 5, 30 μL | 40 | Isocratic: 80:20 MeCN:H 2 O with 5 mM AA (pH 5.8) |
SI Fig. 7 |
On the analytical Syncronis HILIC column, testing of run conditions was performed with 20 μL sample injection at 1 mg/mL, while sample loading study was performed with 2, 5, 30 and 60 μL injection volumes at 40 mg/mL.
Fig. 1.
Analytical development of the first chromatographic step. A) UV210nm traces of VET-SAP® separated on analytical Gemini C18 (250 × 4.6 mm, 5 μm, 110 Å, black), Synergi Hydro RP(250 × 4.6 mm, 4 μm, 80 Å, red) and Luna Omega Polar C18 (250 × 4.6 mm, 5 μm, 110 Å, blue). Gradient condition: 0.0 min-20% B, 5.0 min-20% B, 25.0 min-80% B, 30.0 min-20% B. B) UV210nm traces of VET-SAP®separated on analytical Luna Omega Polar C18 with progressively narrowed gradients. Black trace gradient: 0.0 min-20% B, 5.0 min-20% B, 25.0 min-80% B, 30.0 min-20% B. Red trace gradient: 0.0 min-30% B, 5.0 min-30% B, 25.0 min-70% B, 30.0 min-30% B. Blue trace gradient: 0.0 min-40% B, 5.0 min-40% B, 25.0 min-50% B, 27.5 min-80% B, 30.0 min-40% B. In both figures, 50 μL of VET-SAP® dissolved in H2O at 1 mg/mL was injected. Mobile phase A: H2O with 0.1% formic acid, mobile phase B: MeCN with 0.1% formic acid, flowrate: 1 mL/min. Peak containing QS-21 is indicated with an arrow.
3.2. Preparative development of the first step
Next, scale-up development was carried out on a preparative Luna Omega Polar C18 column (250 × 50 mm, 5 μm, 100 Å). As shown in Fig. 2A, the separation gradient was progressively narrowed from 35–55%, to 37–50%, 38–49%, and 40–47% mobile phase B, in order to move the QS-21-containing peak (indicated by an arrow) to an earlier elution time to reduce run time and thereby solvent consumption. Sample loading was subsequently increased from 5 mL to 10 mL at 100 mg/mL with the 40–47% B gradient ( Fig. 2B black and red traces) to maximize throughput while main-taining sufficient resolution for fraction collection. Eluate of the 10 mL injection run was fractionated and the fractions were analyzed by LC/Q-TOF MS to pinpoint the area of interest to be collected as the semi-pure intermediate, herein dubbed “second generation semi-pure”. SI Fig. 1 shows the fraction collection windows of both the first generation and second generation first steps and corresponding LC/MS characterizations of the collected intermediates, where singly charged negative ion of QS-21 was detected at an m/z value of 1,989. Comparing the MS spectra between the two reveals that the semi-pure intermediate produced by the second generation first step is considerably more pure than that of the first generation first step, evident in the reduced number of impurity ion peaks, particularly at m/z greater than 1,200, where singly charged saponins are found [32]. This improvement can be attributed to the increased peak resolution seen on the polar C18 column, which is expected to translate into higher purity and yield in the final product as less tradeoff would be needed between the two parameters. The 40–47% B gradient was further truncated after elution of the QS-21-containing peak to minimize run time and solvent consumption (Fig. 2B blue trace). The final selected condition, as summarized in Table 2, consisted of a 10 mL sample loading at 100 mg/mL, a 40–45% mobile phase B separation gradient, and a fractionation window as shown in Fig. 2 B. Each run takes 53 minutes and uses 2.05 L MeCN and 2.28 L H 2 O. The fractionated semi-pure intermediate was evaporated under a steady stream of nitrogen to remove the MeCN and was subsequently lyophilized to dryness in preparation for the second step.
Fig. 2.
Preparative development of the first chromatographic step. A) UV210nm traces of VET-SAP® separated on a preparative Luna Omega Polar C18 column (250 × 50 mm, 5 μm, 100 Å) with progressively narrowed gradients. Black trace: 0.0 min-35% B, 15.0 min-35% B, 75.0 min-55% B, 80.0 min-55% B, 85.0 min-90% B, 100.0 min-90% B, 105.0 min-35% B, 125.0 min-35% B, 1 mL injection. Red trace: 0.0 min-37% B, 15.0 min-37% B, 60.0 min-50% B, 63.0 min-50% B, 68.0 min-90% B, 78.0 min- 90% B, 83.0 min-37% B, 95.0 min-37% B, 3 mL injection. Blue trace: 0.0 min-38% B, 15.0 min-38% B, 60.0 min-49% B, 62.0 min-49% B, 67.0 min-90% B, 72.0 min-90% B, 77.0 min-38% B, 85.0 min-38% B, 3mL injection. Yellow trace: 0.0 min-40% B, 15.0 min-40% B, 40.0 min-47% B, 43.0 min-90% B, 46.0 min-90% B, 50.0 min- 40% B, 60.0 min-40% B, 5.0 mL injection. VET-SAP® was dissolved in H2O at 100 mg/mL. B) UV 210nm traces of 5 mL (black) and 10 mL (red) of 100 mg/mL VET-SAP® in H2O separated on preparative Luna Omega Polar C18 with gradient condition: 0.0 min-40% B, 15.0 min-40% B, 40.0 min-47% B, 43.0 min-90% B, 46.0 min-90% B, 50.0 min-40% B, 60.0 min-40% B. UV 210nm trace (blue) of 10 mL of 100 mg/mL VET-SAP® in H2O separated on the same column with gradient condition: 0.0 min-40% B, 15.0 min-40% B, 33.0 min-45% B, 36.0 min-90% B, 39.0 min-90% B, 43.0 min-40% B, 53.0 min-40% B. Dotted lines bracket the fractionation window collected as “second generation semi-pure” intermediate. In both figures, mobile phase A: H2O with 0.1% formic acid, mobile phase B: MeCN with 0.1% formic acid, flowrate: 85 mL/min. Peak containing QS-21 is indicated with an arrow.
Table 2.
Final optimized conditions of the new two-step orthogonal chromatographic purification process for QS-21 developed in this study. FA: formic acid, AA: ammonium acetate.
| Column | Flowrate (mL/min) | Injection Volume | Sample Concentration (mg/mL) | Condition | Purity | Yield | Solvent Consumption/Injection | Time /Injection (min) | |
|---|---|---|---|---|---|---|---|---|---|
| First Step | Luna Omega Polar C18 (250 × 50 mm, 5 μm, 100 Å) | 85 | mL | 100 | Gradient: 40–45% MeCN with 0.1% FA | – | ~ 4% | 2.05 L MeCN 2.28 H 2 O | 53 |
| Second Step | Syncronis HILIC (250 × 21.2 mm, 5 μm, 100 Å) | 20 | μL | 40 | Isocratic: 80:20 MeCN:H 2 O with 5 mM AA (pH 5.8) | ˃ 97% | ˃ 2% | 0.50 L MeCN 0.15 H 2 O | 30 |
3.3. Analytical development of the second step
The first generation process consisted of two sequential RP chromatography steps. Due to the lack of orthogonality between the two steps, the second step provided very little additional separation (SI Fig. 2). Recognizing the fact that the subtle differences among these saponins lie in their hydrophilic glycosyl groups, analytical development of the second generation second step therefore mainly focused on screening various stationary phases in the hydrophilic interaction chromatography (HILIC) mode, taking advantage of its well established orthogonality to the RP mode in retention and selectivity [33, 34]. Guo et. al. previously demonstrated excellent orthogonality between the two techniques for the separation of Panax notoginseng saponins, which are structurally similar to Quillaja saponaria saponins [35]. Seven columns were screened, spanning a range of stationary phase chemistries including bare silica, amide, amino, zwitterionic and combined amide/amino. The columns and conditions tested are summarized in Table 1. Because development of the two steps were carried out in parallel, the sample used for analytical development of the second generation second step was semi-purified material from the first step of our first generation process, herein dubbed “first generation semi-pure”.
The column tested in the bare silica category was Kinetex HILIC (250 × 4.6 mm, 5 μm, 100 Å). As can be seen from SI Fig. 3A, when running “first generation semi-pure” isocratically in 80:20 MeCN: H2O buffered by 5 mM ammonium acetate (pH 5.8) and superimposing on QS-21 purified using our first generation process (“first generation purified”) as reference, this column shows slightly improved separation of impurities in the semi-pure intermediate as shoulder peaks compared to the first generation C8 step (SI Fig. 2), though no baseline separation was achieved. pH 5.8 buffered by ammonium acetate was the primary condition tested with all HILIC columns in this study based on a previous report that hydrolysis of the ester bond linking the fatty acyl chain to the fucose, causing de-acylation of QS-21, is minimized around pH 5.5 [24]. Changing the buffer to 5 mM ammonium formate (pH 3.2) resulted in a later elution time and slightly broadened peaks without significant change in the separation profile (SI Fig. 3B).
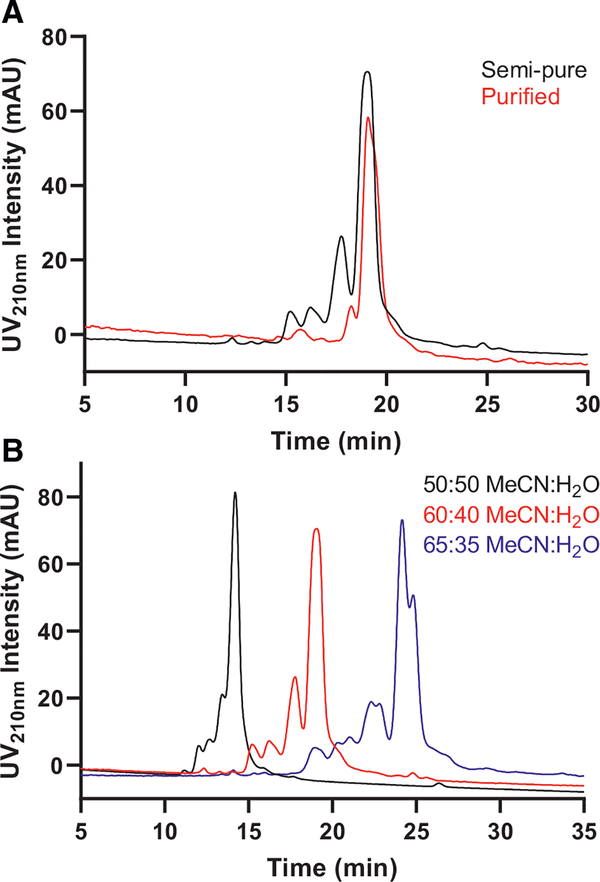
The column tested in the amide category was TSKgel-Amide 80 (250 × 4.6 mm, 5 μm, 100 Å). A flat baseline was not as easily achieved with the amide- and amino-based columns tested in this study. Fig. 3 shows superimposed “first generation semi-pure” and “first generation purified” run on TSKgel-Amide 80 isocratically in 80:20 MeCN:H2O, 5 mM ammonium acetate (pH 5.8). As evident from the figure, a cluster of impurity peaks centered around 16.0 min could be baseline separated from the main peak on this column, an improvement over C8 and Kinetex HILIC.
Fig. 3.
Analytical development of second step on a TSKgel-Amide 80 column. UV210nm traces of “first generation semi-pure” (black) and “first generation purified” (red) on an analytical TSKgel-Amide 80 column (250 × 4.6 mm, 5 μm, 100 Å). 20 μL of each sample dissolved in 70:30 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) at 1 mg/mL was injected. Column was run isocratically in 80:20 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) at 1 mL/min flowrate.
Next, two amino-based columns, Zorbax NH2 (250 × 4.6 mm, 5 μm, 70 Å) and Luna NH2 (250 × 4.6 mm, 5 μm, 100 Å), were screened. As can be seen from Fig. 4A, “first generation semi-pure” run on Zorbax NH2 isocratically in 60:40 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) eluted with several impurity shoulder peaks in front of the main peak. Varying the isocratic mobile phase condition by testing 50:50 and 65:35 MeCN:H2O, 5 mM ammonium acetate (pH 5.8), showed that further decreasing water content in the mobile phase results in more prominent impurity separation along with an overall longer elution time ( Fig. 4 B). Luna NH2 tested in 50:50 MeCN:H2O, 5 mM ammonium acetate (pH 5.8, SI Fig. 4) showed a similar separation profile as Zorbax NH2 tested under the same condition.
Fig. 4.
Analytical development of second step on a Zorbax NH2 column. A) UV210nm traces of “first generation semi-pure” (black) and “first generation purified” (red) separated on an analytical Zorbax NH2 column (250 × 4.6 mm, 5 μm, 70 Å). Column was run isocratically in 60:40 MeCNH2O, 5 mM ammonium acetate (pH 5.8) at 1 mL/min flowrate. 20 μL of each sample dissolved in the mobile phase at 1 mg/mL was injected. B) Comparison of mobile phase conditions on the separation of semi-pure intermediate on analytical Zorbax NH2. Column was run isocratically in 50:50 (black), 60:40 (red) or 65:35 (blue) MeCN:H2O, 5 mM ammonium acetate (pH 5.8) at 1 mL/min flowrate. 20 μL of “first generation semi-pure” dissolved in the corresponding mobile phases at 1 mg/mL were injected.
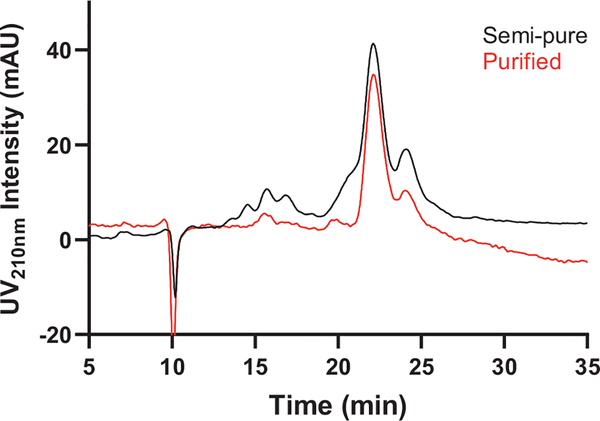
The two columns tested in the zwitterionic category were Syncronis HILIC (250 × 4.6 mm, 5 μm, 100 Å) and SeQuant® ZIC®-HILIC (250 × 4.6 mm, 5 μm, 200 Å), both of which are surface modified with zwitterionic sulfobetaine groups. The usefulness of sulfobetaine-functionalized stationary phases for the separation of saponins has previously been demonstrated with saponin standards from ginseng [36]. Given the similarity of surface chemistry between the two columns tested, run conditions were primarily tested on the Syncronis HILIC, while SeQuant® ZIC®-HILIC was compared later in terms of sample loading. Fig. 5A shows superimposed “first generation semi-pure” and “first generation purified” separated on Syncronis HILIC in 80:20 MeCN:H2O, 5 mM ammonium formate (pH 3.2). Several impurity peaks are visible, with a baseline-separated cluster centered around 12.5 min and others manifested as shoulder peaks. Running the same samples in 80:20 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) on this column resulted in even higher peak resolution ( Fig. 5B).
Fig. 5.
Analytical development of second step on a Syncronis HILIC column. UV210nm traces of “first generation semi-pure” (black) and “first generation purified” (red) separated on an analytical Syncronis HILIC column (250 × 4.6 mm, 5 μm, 100 Å). Column was run isocratically in A) 80:20 MeCN:H2O, 5 mM ammonium formate (pH 3.2) and B) 80:20 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) at 1 mL/min flowrate. 20 μL of each sample dissolved in 70:30 MeCN:H2O, buffered by corresponding salt in the mobile phase at 1 mg/mL was injected.
One additional column screened was a hybrid amide/amino column, Luna Omega SUGAR (250 × 4.6 mm, 3 μm, 100 Å). SI Fig. 5 shows “first generation semi-pure” run isocratically on this column with 70:30 or 75:25 MeCN:H2O, 5 mM ammonium acetate (pH 5.8). As evident from the chromatograms, this hybrid column chemistry does not appear to offer better separation than either of the two insdividual surface chemistries alone in this particular application.
Among all of the column and condition combinations tested (Table 1), Syncronis HILIC run isocratically in 80:20 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) showed the best resolution and peak shape. It is theorized that the pH-independent presence of both positive and negative charges on this column play an important role in its superior separation of the type of sample in this application. This column also offered one of the shortest run times, with all major peaks eluting before 16 min, which is expected to translate to the additional benefits of higher throughput and lower mobile phase consumption. Additionally, pH 5.8 is in close range with pH 5.5 suggested by literature for optimal QS-21 stability [24]. Before developing on the preparative scale, a preliminary loading study was done on the analytical column. As shown in SI Fig. 6, four different injection volumes, 2, 5, 30 and 60 uL of 40 mg/mL “first generation semi-pure” were tested. Significant resolution loss occurred between 5 and 30 uL. As an additional comparison, another zwitterionic sulfobetain-based column, SeQuant ZIC-HILIC, was tested at the same condition and scales. As can be seen from SI Fig. 7, 5 and 30 uL injection volumes at 40 mg/mL both resulted in slightly lower resolution on the SeQuant ZIC-HILIC compared to the Syncronis HILIC column (SI Fig. 6). Thus, Syncronis HILIC was chosen for further scale-up development.
3.4. Preparative development of the second step
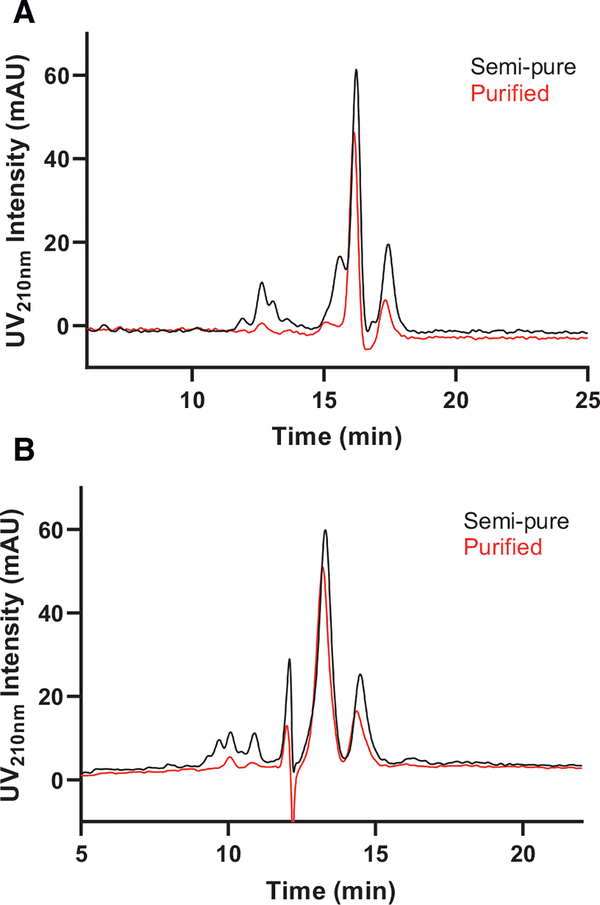
A 250 × 21.2 cm Syncronis HILIC column (5 μm, 100 Å) was selected for preparative development of the new second step. Using the analytical loading study results (SI Fig. 6) as reference, two injection volumes, 250 μL and 600 μL at 40 mg/mL were tested on the preparative column, equivalent to 12.5 μL and 30 μL at 40 mg/mL on the analytical counterpart, respectively. As can be seen from Fig. 6A, the preparative results are consistent with the analytical results, where 600 μL injection volume resulted in notice- able loss in resolution compared to 250 μL. The eluate of the 600 μL injection run was fractionated and the fractions were analyzed by LC/QqQ-MS to determine the area of interest for fraction collection. SI Fig. 8 shows MS spectra of the three key fractions analyzed, namely the left shoulder centered around 15 min and the two tallest peaks eluting around 16 and 17 min (SI Fig. 8A, fractions 1, 2 and 3). MS revealed that while the left shoulder was rich in impurities, the two later-eluting peaks both contained singly deprotonated QS-21 with an m/z value of 1,989 as the most abundant ion. The two peaks are likely compositional isomers of QS-21, where the terminal glycosyl group in the linear tetrasaccharide chain is either a D-apiose or D-xylose, which have been previously separated by HILIC chromatography and were found to have comparable adjuvant activity [22, 23]. This speculation is further supported by calculating the ratio of the AUCs of the two peaks, giving roughly 68:32, which is in close agreement with the 65:35 ratio established in the literature [24]. Therefore, it was decided that these two peaks do not need to be baseline separated for purification purposes and can be collected and combined as the final purified product. The de-acylated version of QS-21 with an m/z value of 1,512 was also detected in both peaks (SI Fig. 8 C and D). De-acylation of QS-21 occurs naturally in aqueous solution via hydrolysis of the ester linkage connecting the fatty acyl chain to the fucose ring [24] and can likely be reduced by minimizing sample handling in aqueous solution. In-source fragmentation in MS is also a possible cause for de-acylation.
Fig. 6.
Preparative development of the second step. UV210nm traces of A) “first generation semi-pure” and B) “second generation semi-pure” intermediates on a preparative Syncronis HILIC column (21.2 × 250 mm, 5 μm, 100 Å) with two sample loading volumes. 250 μL (red) and 600 μL (blue) of each sample dissolved in 65:35 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) at 40 mg/mL were injected. Column was run isocratically in 80:20 MeCN:H2O, 5 mM ammonium acetate (pH 5.8) at 20 mL/min flowrate. Dotted lines bracket the fraction collection window on the higher loading trace in B).
After preparative development of the new first step was completed, the “second generation semi-pure” intermediate obtained from preparative Luna Omega Polar C18 was available to be run on the preparative Syncronis HILIC column. Again, 250 μL and 600 μL at 40 mg/mL were tested (Fig. 6B). Although 600 μL showed reduced resolution between the two isomer peaks, it was selected as the final injection volume since the two isomers were to be combined as the final purified product. Comparing the preparative chromatograms of the semi-pure intermediates purified by the first and second generation first steps (Fig. 6) provides additional support for the enhanced separation of the second generation first step. Eluate of the 600 μL injection was fractionated and the fractions were analyzed by LC/Q-TOF MS to identify the area of interest to be collected as final purified material (area bracketed by dotted lines in Fig. 6B), herein dubbed “second generation purified”. The final selected condition for this step, as summarized in Table 2, consisted of a 600 μL sample loading at 40 mg/mL, isocratic elution at 80:20 MeCN:H2O with 5 mM ammonium acetate (pH 5.8), and a fractionation window as shown in Fig. 6B. Each run takes 30 minutes and uses 0.50 L MeCN and 0.15 L H2O. The collected final product was evaporated under a steady stream of nitrogen to remove the MeCN and was subsequently lyophilized to dryness for final characterizations or storage.
3.5. Characterization of final purified QS-21
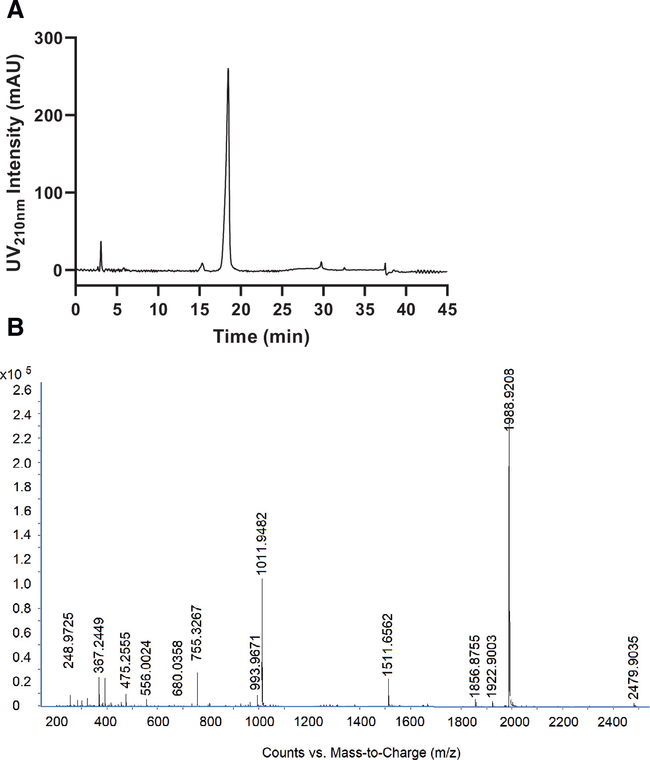
Characterization of the “second generation purified” material on an analytical C4 column showed a minor peak ~15.5 min and a major peak ~ 18.0 min ( Fig. 7 A). Further analysis by LC/Q-TOF MS revealed that QS-21 appears as the most abundant ion in both of these peaks (SI Fig. 9). Therefore, it is speculated that the major and minor peaks correspond to A- and B-regioisomers of QS-21, respectively, which naturally occur in solution from the intramolecular trans-esterification of the acyl chain between the 3- and 4-hydroxyl groups on the fucose ring [3, 19, 24, 25]. This interconversion can likely be reduced by minimizing sample processing in aqueous solution, though a previous study has similarly separated the two regioisomers on a C4 column and confirmed that both have adjuvant activity [24], thus the minor peak does not need to be removed as impurity. AUC of the major peak was calculated to be 97.8%, suggesting that the final purified product is at least 97.8% pure. This marks a significant increase from the ~85% purity obtained from our first generation process. LC/Q-TOF MS characterization (Fig. 7B) further confirmed the high purity of the final purified product, where besides singly charged QS-21 which was detected as the most abundant ion, three other impurity ions at m/z 1,856.9, 1,922.9 and 2,480.0 were detected at much lower intensities. Remaining lower mass ions include doubly charged QS-21 at m/z 994.0, ammonium adduct of doubly charged QS-21 at m/z 1,011.9, singly charged de-acylated QS-21 at m/z 1,511.7 and doubly charged de-acylated QS-21 at m/z 755.3.
Fig. 7.
Characterization of QS-21 purified by the second generation method. A) UV 210nm trace of “second generation purified” QS-21 run on an analytical Vydac C4 column (250 × 4.6 mm, 5 μm, 300 Å). Mobile phase A: H2O with 0.1% formic acid, mobile phase B: MeCN with 0.1% formic acid, flowrate: 1mL/min. 50 μL of sample dissolved in 35% B at 1 mg/mL was injected. Gradient condition: 0.0 min-35% B, 20.0 min-40% B, 21.0 min-40% B, 26.0 min-90% B, 34.0 min-90% B, 35.0 min-35% B. B) “Second generation purified” QS-21 analyzed by LC/Q-TOF MS. QS-21 [M-H]−1 : 1,989.9.
3.6. Implementation of the newly developed process
An engineering batch was subsequently produced to implement the newly developed second generation process and assess final yield and purity. A total of 16.7 g of VET-SAP® was processed, resulting in production of 648 mg of “second generation semi-pure” intermediate and 339 mg of “second generation purified” final product at 97.2% pure, excluding the B-regioisomer. The new process thus gives an overall yield of 2.1% out of total starting material, which quadruples that of our first generation process while also resulting in ~12% higher purity. The % recovery is difficult to determine since the exact QS-21 content in commercially available saponin-enriched extracts of Quillaja saponaria such as VET-SAP® is unknown and has been found to have batch-to-batch variation. Nevertheless, based on the fractionation areas of the two steps in the new process, it is speculated that the majority of the QS-21 content can be recovered by this method. Such a high yield should significantly increase throughput, thereby cutting production cost and time. SI Table 1 summarizes the solvent and time consumption of this engineering run. A total of 48.35 L of MeCN, 42.81 L of H2O and 28.5 h of chromatographic time were used.
3.7. Comparison with previously reported methods
Our results compare favorably to those previously reported. In a 1991 patent, Kensil et. al. described a process for purifying QS-21, where methanol extracted Quil-A® was subjected to silica chromatography followed by RP chromatography on a C4 column. Although the collected QS-21 fraction appeared as a single band in thin layer chromatography (TLC), Fast Atom Bombardment Mass Spectroscopy (FAB-MS) analysis showed that it was still a rather complex mixture. [29]. In a similar protocol described by Brunner et. al., Quil-A® was subjected to silica chromatography followed by RP chromatography on a C18 column. The purified QS-21 appeared as a single peak with a leading shoulder on an analytical C18 column, though ESI-QTOF-MS analysis still revealed a considerable number of impurity ions. The yield of purified QS-21 with respect to the weight of Quil-A® was reported to be ~2% [10]. In a 2001 patent by Kensil et. al., two example processes were described for the purification of QS-21. In the first approach, a dialyzed and lyophilized aqueous Quillaja saponaria bark extract was separated on a silica column, followed by RP chromatography on a C18 column and a final capture and release step on a larger par- ticle size C18 column. Final yield was 2.95% with respect to the starting extract [28], though it is worth noting that the starting extract prepared by the inventors is likely ~50% more enriched in QS-21 than Quil-A® as compared by the group previously [29]. The second approach used polyvinylpolypyrrolidone (PVPP) to first remove the polyphenolics from an aqueous bark extract of Quillaja saponaria, followed by two sequential RP chromatography steps on phenyl columns and a final capture and release step on a C8 column. Yield for the second approach was not reported. Purity from both methods were reported to be ≥98% by RP HPLC though no chromatogram was shown. FAB-MS characterization of the final purified product showing a limited m/z range of 1,50 0–2,20 0 revealed three primary impurity ions besides the predominant QS-21 ion [28]. A 2019 patent application by GlaxoSmithKline Biologicals (GSK) describes a process for purifying QS-21 (a component of their licensed Shingrix® and Mosquirix® vaccines). A crude aqueous extract of Quillaja saponaria is first subjected to PVPP adsorption followed by a first RP chromatography step on a polystyrene column, a second RP chromatography step on a phenyl column, and a final capture and release step on a C8 column to concentrate the purified material before lyophilization. No yield information was given. UPLC-UV/MS characterization shows that the purified QS-21 contains at least 93% triterpenoid glycosides having negative ion m/z of 1,855.9, 1,987.9, or 2,001.9, excluding the B-regioisomer, and at least 98% triterpenoid glycosides having negative ion m/z of 1,517.7, 1,711.8, 1,855.9, 1,987.9, 2,001.9, 2,017.9 or 2,118, including the B-regioisomer. The m/z 2,017.9 species appears as the most dominant impurity peak in LC characterization. Because this impurity cannot be removed by this particular process and its amount has been found to vary in Quillaja saponaria crude extracts, a primary focus of the patent is therefore to define an acceptable range of the % amount of this component in the starting extract, which significantly limits the applicability of the process [27]. Additionally, the more recently described processes involve laborious procedures which can limit both efficiency and yield. In contrast to these previous technologies, our process uses only two sequential chromatographic steps to achieve the high purity and yield reported herein. A commercially available saponin-enriched Quillaja saponaria bark extract can be used as the starting material without the need for any pre-treatment. The final product does not contain any impurity ion with m/z 2,017.9, thus no strict requirement on the composition of the starting extract is needed.
4. Conclusions
In conclusion, we have developed a new chromatographic process for the purification of the QS-21 saponin with high yield and > 97% purity. This marks a significant improvement over our first generation process as well as previously reported technology from other groups [10, 27–29]. Our process uses a commercially available saponin-enriched Quillaja saponaria bark extract as the starting material, without the need for any pre-treatment or strict requirement on the composition of the extract. Accounting for the amphiphilic nature of Quillaja saponaria saponins, the newly developed process comprises two orthogonal chromatographic steps in series, a polar RP step followed by a HILIC step, providing the all-around retention and selectivity that is crucial to achieving both high yield and purity for this application. Both columns utilized in this process are commercially available at various prepaative scales. We herein demonstrate a pilot scale production batch with VET-SAP® as the starting material. The method can be easily adapted for using other Quillaja saponaria bark extracts and further scale up can be achieved with larger column sizes and increased number of injections. This process makes available a simple and efficient method for obtaining highly pure QS-21 from saponin-enriched bark extract.
Supplementary Material
Acknowledgements
The project described has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201300029C. LC/MS analyses were performed at the University of Washington Department of Medicinal Chemistry Mass Spectrometry Center. The authors thank Dale Whittington and Tauri Senn at the center for MS technical support.
Footnotes
Declaration of Competing Interest
Yizhi Qi and Christopher B. Fox are inventors on a patent application describing the purification of QS-21, and are employees of IDRI which has product assets associated with QS-21.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.chroma.2020.461705.
References
- [1].Kensil CR, QS-21 adjuvant, in: O’Hagan DT(Ed.), Vaccine Adjuvants, Ed., Humana Press, Totowa, NJ, 2000, pp. 259–271, doi: 10.1385/1-59259-083-7:259. [DOI] [Google Scholar]
- [2].Kensil CR, Patel U, Lennick M, Marciani D, Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex, J. Immunol 146 (1991) 431–437 PMID: 1987271. [PubMed] [Google Scholar]
- [3].Jacobsen NE, Fairbrother WJ, Kensil CR, Lim A, Wheeler DA, Powell MF, Structure of the saponin adjuvant QS-21 and its base-catalyzed isomerization product by 1H and natural abundance 13C NMR spectroscopy, Carbohydr. Res. 280 (1996) 1–14, doi: 10.1016/0008-6215(95)00278-2. [DOI] [PubMed] [Google Scholar]
- [4].Kensil CR, Soltysik S, Patel U, Marciani DJ, Structure/function relationship in adjuvants from Quillaja saponaria Molina, in: Brown F, Chanok RM, Ginsberg HS, Lenner RA (Eds.), Vaccines 92, Cold Spring Harbor, Eds., Cold Spring Harbor, NY, 1992, pp. 35–40. [Google Scholar]
- [5].Kensil CR, Liu G, Anderson C, Storey J, Effects of QS-21 on innate and adaptive immune responses, in: Hackett CJ, Harn DA (Eds.), Vaccine Adjuvants, Eds., Humana Press, Totowa, NJ, 2006, pp. 221–234, doi: 10.1007/978-1-59259-970-7_11. [DOI] [Google Scholar]
- [6].Lacaille-Dubois M-A, Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review, Phytomedicine 60 (2019), doi: 10.1016/j.phymed.2019.152905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Didierlaurent AM, Laupèze B, Pasquale AD, Hergli N, Collignon C, Garçon N, Adjuvant system AS01: helping to overcome the challenges of modern vaccines, Expert Rev. Vaccines 16 (2017) 55–63, doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- [8].Didierlaurent AM, Berger A, Heineman TC, Henderickx V, Silva FTD, Vekemans J, Voss G, Garçon N, The development of the adjuvant system AS01: a combination of two immunostimulants MPL and QS-21 in liposomes, in: Immunopotentiators in Modern Vaccines, Academic Press, 2017, pp. 265–285, doi: 10.1016/B978-0-12-804019-500014-1. [DOI] [Google Scholar]
- [9].Zhu D, Tuo W, QS-21: a potent vaccine adjuvant, Nat. Prod. Chem. Res. 3 (2016) e113, doi: 10.4172/2329-6836.1000e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brunner L, Barnier-Quer C, Collin N, QS-21 Adjuvant, Laboratory-Scale Purification Method and Formulation Into Liposomes, in: Fox CB (Ed.), Vaccine Adjuvants, Ed., Springer Nature, New York, NY, 2017, pp. 73–86, doi 10.1007/978-1-4939-6445-1_5. [DOI] [PubMed] [Google Scholar]
- [11].Kensil CR, Kammer R, QS-21: a water-soluble triterpene glycoside adjuvant, Expert Opin. Investig. Drugs 7 (1998) 1475–1482, doi: 10.1517/13543784.7.9.1475. [DOI] [PubMed] [Google Scholar]
- [12].Tait DR, Hatherill M, Meeren OVD, Ginsberg AM, Brakel EV, Salaun B, Scriba TJ, Akite EJ, Ayles HM, Bollaerts A, Demoitié M-A, Diacon A, Evans TG, Gillard P, Hellström E, Innes JC, Lempicki M, Malahleha M, Martinson N, Vela DM, Muyoyeta M, Nduba V, Pascal TG, Tameris M, Thienemann F, Wilkinson RJ, Roman F, Final Analysis of a Trial of M72/AS01 E Vaccine to Prevent Tuberculosis, N, Engl. J. Med. 25 (2019) 2429–2439, doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- [13].Ragupathi G, Gardner JR, Livingston PO, Gin DY, Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer, Expert Rev. Vaccines 10 (2013) 463–470, doi: 10.1586/erv.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Centers for Disease Control and Prevention, Current Vaccine Shortages & Delays, https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html#note3, June 3, 2020.
- [15].Fernández-Tejada A, Chea EK, George C, Pillarsetty N, Gardner JR, Livingston PO, Ragupathi G, Lewis JS, Tan DS, Gin DY, Development of a minimal saponin vaccine adjuvant based on QS-21, Nat. Chem. 6 (2014) 635–643, doi: 10.1038/nchem.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fernández-Tejada A, Walkowicz WE, Tan DS, Gin DY, Semisynthesis of analogues of the saponin immunoadjuvant QS-21, in: Fox C (Ed.), Vaccine Adjuvants, Ed., Springer Nature, New York, NY, 2017, pp. 45–71, doi: 10.1007/978-1-4939-6445-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang P, Dai Q, Thogaripally P, Zhang P, Michalek SM, Synthesis of QS-21-based immunoadjuvants, J. Org. Chem. 78 (2013) 11525–11534, doi: 10.1021/jo402118j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kite GC, Howes M-JR, Simmonds MSJ, Metabolomic analysis of saponins in crude extracts of Quillaja saponaria by liquid chromatography/mass spectrometry for product authentication, Rapid Commun. Mass Spectrom. 18 (2004) 2859–2870, doi: 10.1002/rcm.1698. [DOI] [PubMed] [Google Scholar]
- [19].Wang Y, Lu X, Xu G, Development of a comprehensive two-dimensional hydrophilic interaction chromatography/quadrupole time-of-flight mass spectrometry system and its application in separation and identification of saponins from Quillaja saponaria, J. Chromatogr. A 1181 (2008) 51–59, doi: 10.1016/j.chroma.2007.12.034. [DOI] [PubMed] [Google Scholar]
- [20].van Setton D, van de Werken G, Zomer G, Kersten GFA, Glycosyl Compositions and Structural Characteristics of the Potential Immuno-adjuvant Active Saponins in the Quillaja saponaria Molina Extract Quil A, Rapid Commun. Mass Spectrom. 9 (1995) 660–666, doi: 10.1002/rcm.1290090808. [DOI] [PubMed] [Google Scholar]
- [21].Nyberg NT, Baumann H, Kenne L, Solid-Phase Extraction NMR Studies of Chromatographic Fractions of Saponins from Quillaja saponaria, Analytical Chemistry 75 (2003) 268–274. [DOI] [PubMed] [Google Scholar]
- [22].Kensil CA, Soltysik S, Marciani DJ, Saponin-antigen conjugates and the use thereof, US 5,583,112, December 10, 1996.
- [23].Soltysik S, Bedore DA, Kensil CR, Adjuvant Activity of QS-21 Isomers, Ann. N. Y. Acad. Sci. (1993) 392–395, doi: 10.1111/j.1749-6632.1993.tb44041.x. [DOI] [PubMed] [Google Scholar]
- [24].Cleland JL, Kensil CR, Lim A, Jacobsen NE, Basa MSL, Wheeler DA, Wu JY, Powell MF, Isomerization and Formulation Stability of the Vaccine Adjuvant QS-21, J, Pharm. Sci. 85 (1996) 22–28, doi: 10.1021/js9503136. [DOI] [PubMed] [Google Scholar]
- [25].Pedebos C, Pol-Fachin L, Pons R, Teixeira CV, Verli H , Atomic model and micelle dynamics of QS-21 saponin, Molecules 19 (2014) 3744–3760, doi: 10.3390/molecules19033744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dalsgaard K III. Saponin adjuvants, Isolation of a substance from Quillaja saponaria Molina with adjuvant activity in foot-and-mouth disease vaccines, in: Archiv fur die gesamte virusforschung, Springer-Verlag, New York, 1974, pp. 243–254. PMID: 4365900. [PubMed] [Google Scholar]
- [27].Baig AT, Denet FGC, Garcia JJD, Farrenburg CA, Lawrence LL, Meyers KR, Sandvick JK, Vandenburg JY, Saponin Purification, WO 2019/106192 A1, June 06, 2019.
- [28].Kensil CA, Saponin Adjuvant Compositions, US 6,231,859 B1, May 15, 2001.
- [29].Kensil CA, Marciani DJ, Saponin Adjuvant, US 5,057,540, October 15, 1991.
- [30].Mordmüller B, Sulyok M, Egger-Adam D, Resende M, Jongh W.A.d., Jensen MH, Smedegaard HH, Ditlev SB, Soegaard M, Poulsen L, Dyring C, Calle CL, Knoblich A, Ibáñez J, Esen M, Deloron P, Ndam N, Issifou S, Houard S, Howard RF, Reed SG, Leroy O, Luty AJF, Theander TG, Krem- sner PG, Salanti A, Nielsen MA, First-in-human, Randomized, Double-blind Clini- cal Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria, Clin. Infect. Dis. 69 (2019) 1509–1516, doi: 10.1093/cid/ciy1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chu J-HJ, Chiang C-CS, Ng M-L, Immunization of Flavivirus West Nile Recombinant Envelope Domain III Protein Induced Specific Immune Response and Protection Against West Nile Virus Infection, J. Immunol 178 (2007) 2699–2705, doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- [32].Pham HL, Ross BP, mcGeary RP, Shaw PN, Hewavitharana AK, Davies NM, Saponins from Quillaja saponaria Molina: isolation, characterization and ability to form immuno stimulatory complexes (ISCOMs), Curr. Drug Deliv. 3 (2006) 389–397, doi: 10.2174/156720106778559092. [DOI] [PubMed] [Google Scholar]
- [33].Buszewski B, Noga S, Hydrophilic interaction liquid chromatography (HILIC)— a powerful separation technique, Anal. Bioanal. Chem. 402 (2012), doi: 10.1007/s00216-011-5308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jandera P, Stationary phases for hydrophilic interaction chromatography, their characterization and implementation into multidimensional chromatography concepts, J. Sep. Sci. 31 (2008) 1421–1437. [DOI] [PubMed] [Google Scholar]
- [35].Guo X, Zhang X, Feng J, Guo Z, Xiao Y, Liang X, Purification of Saponins from Leaves of Panax Notoginseng Using Preparative Two-Dimensional Reversed- Phase Liquid Chromatography/Hydrophilic Interaction Chromatograph, Anal. Bioanal. Chem. 405 (2013) 3413–3421, doi: 10.1007/s00216-013-6721-8. [DOI] [PubMed] [Google Scholar]
- [36].Yu D, Guo Z, Shen A, Yan J, Dong X, Jin G, Long Z, Liang L, Liang X, Syn- thesis and Evaluation of Sulfobetaine Zwitterionic Polymer Bonded Stationary Phase, Talanta (2016) 161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.