Abstract
While research in previous decades demonstrated a link between the pulvinar nucleus of the thalamus and visual selective attention, the pulvinar’s specific functional role has remained elusive. However, methodological advances in electrophysiological recordings in non-human primates, including simultaneous recordings in multiple brain regions, have recently begun to reveal the pulvinar’s functional contributions to selective attention. These new findings suggest that the pulvinar is critical for the efficient transmission of sensory information within and between cortical regions, both synchronizing cortical activity across brain regions and controlling cortical excitability. These new findings further suggest that the pulvinar’s influence on cortical processing is embedded in a dynamic selection process that balances sensory and motor functions within the large-scale network that directs selective attention.
Introduction
The thalamus consists of multiple nuclei that can be divided into first-order and second-(or higher-) order nuclei [1]. First-order nuclei connect the sensory periphery with the cortex and have been initially characterized as relay stations that convey sensory information without significant modification. Second-order nuclei, in contrast, receive information primarily from the cortex and their functional roles in perception and cognition have been for the most part mysterious. Indeed, they were known as a ‘graveyard for neuroscientists’, who sacrificed their careers in their attempts to shed light on their specific function [2]. These views have dramatically changed during the last decade due to a revival of interest in the role of the thalamus in perception and cognition in the primate field, after initial demonstrations of cognitive influences on thalamus in both humans and monkeys [3,4]. This revival of interest in the primate field has coincided with a methodological revolution to dissect local and large-scale circuits in the rodent field [5].
In this review, we will focus on thalamocortical function in the primate brain and on its dominant sensory domain, i.e. vision. Much of primate behavior is guided by information selected from complex visual environments. The resulting behavioral repertoire and its underlying neural basis is fundamentally different from that of rodents (see [6,7] with respect to thalamocortical organization). Particularly studies in non-human primates can guide models of thalamic function in humans, where the thalamus has been largely uncharted territory. In primates, the visual thalamus consists of two main nuclei, the lateral geniculate nucleus (LGN), a first-order nucleus, which relays the visual input from the retina primarily to primary visual cortex, and the pulvinar, a second-order nucleus, which is widely connected to the cortical visual system. While functions of the retino-geniculate pathway have been well characterized [8], functions of the pulvinar, its higher-order thalamic counterpart, have remained elusive. Here, we will focus on the functional role of pulvino-cortical interactions specifically in selective visual attention. This cognitive operation refers to the selection of visual information to guide behavior and is fundamental for other cognitive domains, such as memory and decision making.
Compelling evidence for a functional role of the pulvinar in selective attention has been provided by lesion studies in humans and monkeys. Functional or structural lesions of the pulvinar can lead to (i) thalamic neglect, a deficit in directing attention to contralesional space, as demonstrated in monkeys [9], (ii) spatial coding deficits, characterized by slowing of responses in the affected visual space [10,11], as demonstrated in humans, and (iii) errors in binding featural information [12,13]. A particularly striking deficit following pulvinar lesions is the inability to filter distracting stimuli that compete with a target stimulus for neural representation [14,15], thereby impairing visually-guided behavior more broadly. Similar attention deficits have been observed particularly after lesions of posterior parietal cortex [16], suggesting that the pulvinar is an integral part of a large-scale attention network. After a brief description of the anatomical organization of the pulvinar and its afferent and efferent projections, we will focus on the neural mechanisms underlying attention behaviors with particular emphasis on pulvino-cortical interactions (for cortical mechanisms, see [17–19]).
Pulvino-cortical pathways
The pulvinar is the largest nucleus in the primate thalamus. Its vast expansion during evolution scales with that of the primate neocortex [20–22], suggesting an important functional role in the increasing cognitive abilities of primates. The pulvinar is almost exclusively interconnected with the visual cortex, which forms the basis for characterizing its main subdivisions: the inferior pulvinar (PI) is interconnected with early visual areas, the lateral pulvinar (PL) with ventral and dorsal extrastriate areas, and the medio-dorsal pulvinar (PM) with higher-order frontal and parietal areas (Figure 1A; [23]). In non-human primates, PI and PL each contain a detailed retinotopic map informed by projections from early visual cortex and have accordingly a high proportion of visually responsive neurons. These characteristics have been utilized, using MRI, to establish a highly homologous functional organization of the human pulvinar (Figure 1C; [24]). A different subregion within PI contains the only region that receives input from the sensory periphery—either directly from the retina [26] or via the superior colliculus [27]—and projects to dorsal extrastriate cortex. In primates, this pathway appears to be particularly important during early development [28] and for visual plasticity after cortical lesions [29]. In the context of cognition, PM is a particularly critical part of the pulvinar. This subdivision appears to be primate-specific reflecting the vast expansion of parietal association and frontal cortex in primates relative to other species [30–32]. Since multiple areas in parietal and frontal cortex constitute an attention control network, it is of critical importance to understand the functional interactions of PM with cortex in the context of attention behaviors.
Figure 1: Anatomical and functional organization of pulvinar.
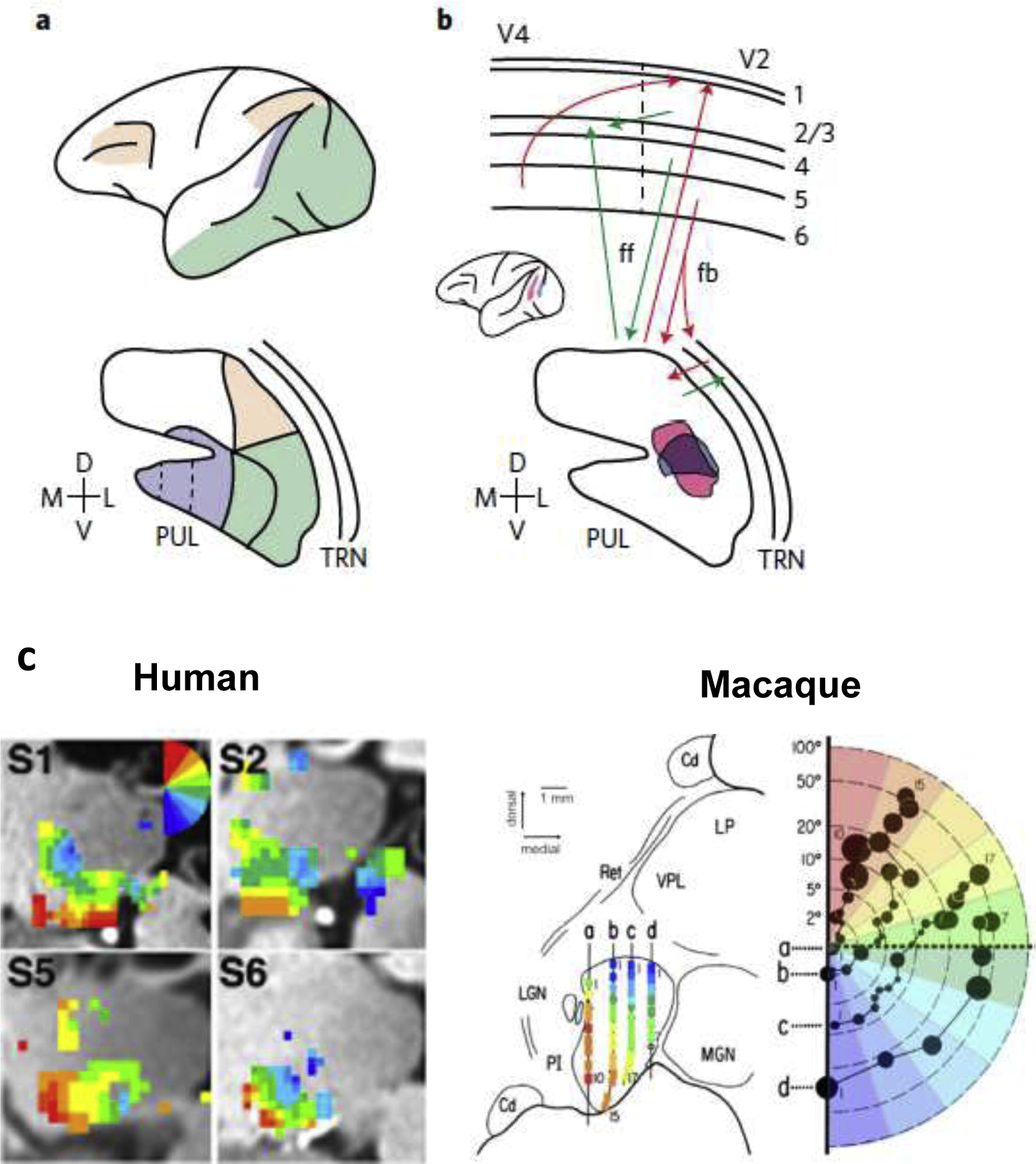
a. Fronto-parietal (pink), medio-temporal (violet) and infero-temporal and occipital cortex are interconnected with different subregions of the pulvinar (PUL). The ventro-medial part of inferior pulvinar is further subdivided (dotted lines; [21]) and is the only subregion that receives projections from the sensory periphery. b. Direct cortico-cortical connections and transthalamic feedforward and feedback pathways exemplified by V2-pulvino-V4 circuitry. Tracer injections into V2 (blue) and V4 (pink; inset) showed overlapping (purple) projection zones in the pulvinar, illustrating the replication principle of the indirect transthalamic pathways (adapted with permission from Figure 8 in [35]). c. Comparison of the topographic organization within the ventro-lateral pulvinar between humans and macaques. FMRI-defined polar angle maps (on left) from 4 human subjects (S1, S2, S5, S6, adapted from [24]) are compared to the electrophysiologically defined topographic organization of the macaque pulvinar (on right). Electrode penetrations a–d are color-coded relative to receptive field location (adapted with permission from [25]). Pulvinar, PUL; thalamic reticular nucleus, TRN; visual area 2, V2; visual area 4, V4; caudate, Cd; lateral geniculate nucleus, LGN; medial geniculate nucleus, MGN; inferior pulvinar, PI; lateral pulvinar, LP.
There are two well established cortico-pulvinar pathways that parallel the canonical input-output relationships constituting the visual processing hierarchy [33,34]: (i) a transthalamic feedforward pathway that originates from layer 5 of a ‘lower’ area (e.g. area V4 in Figure 1B), loops through a dedicated projection zone in the pulvinar, and feeds into layer 4 of a ‘higher’ area (e.g. area TEO in Figure 1B), and (ii) a feedback pathway that originates from layer 6 of a cortical area and projects to its pulvinar projection zone, also passing through the thalamic reticular nucleus (TRN) (Figure 1B). An important characteristic of the transthalamic pathway, known as the replication principle, is that it indirectly connects two cortical areas that share a direct cortico-cortical projection [35,36]. In this respect, the pulvinar can be thought of as a mosaic of projection zones that mirrors the cortico-cortical connectivity. The replication principle may be a fundamental feature of cortico-thalamic organization that extends to all higher-order thalamic nuclei [37]. It is important to note that the input-output loops of the ‘canonical transthalamic pathways’, as described above, certainly present a simplification of the rich thalamo-cortical connectivity that exists (see [38]). For example, pulvino-cortical projections can have spatially segregated targets in cortex [39,40], and cortico-pulvinar projections exhibit convergence from multiple sources particularly in PM [32]. However, this framework presents a starting point to explore functions of the transthalamic pathways, which we will discuss in the next sections.
Regulation of intra- and inter-areal cortical interactions
In a typical visual attention experiment, a stimulus presented to the periphery of the visual field serves as a cue to direct attention to a specific location. Attention is then to be sustained at that location until a target is presented and the animal responds with a manual response or an eye movement. Such a simple task engages a vast network of areas distributed across all major lobes and includes the thalamus [19]. The allocation of attention leads to an enhancement of the neural representation (e.g., of the spatial location in the above example) that can occur in several different ways: (i) by enhancing response rates (both on baseline and visually-evoked responses; [41]), (ii) by decreasing noise correlations [42,43], and (iii) by increasing synchronous firing in local neural populations [44]. These modulatory effects of attention have been demonstrated in many parts of the attention network, including the pulvinar. Indeed, the response modulation observed in the pulvinar appears to resemble that of the interconnected cortical areas (Figure 2A; [45–47]). This could be interpreted as evidence for a function of the transthalamic feedforward pathway in routing information through the thalamus, thereby indirectly relaying information from one cortical area to another [48,49]. However, such a relay function is not compatible with a number of observations. For example, effect sizes of response modulation in cortex and pulvinar can differ substantially [46,47]. Also, pulvinar neurons typically show less feature selectivity and larger receptive fields than their cortical inputs [50,51]. These differences between pulvinar neurons and their cortical inputs suggest convergent inputs from additional sources. It will be an important area of future inquiry to understand how the information that is carried through the transthalamic feedforward pathway is different from that of the corresponding cortico-cortical pathway (see Box 2).
Figure 2: Pulvino-cortical interactions during spatial attention.
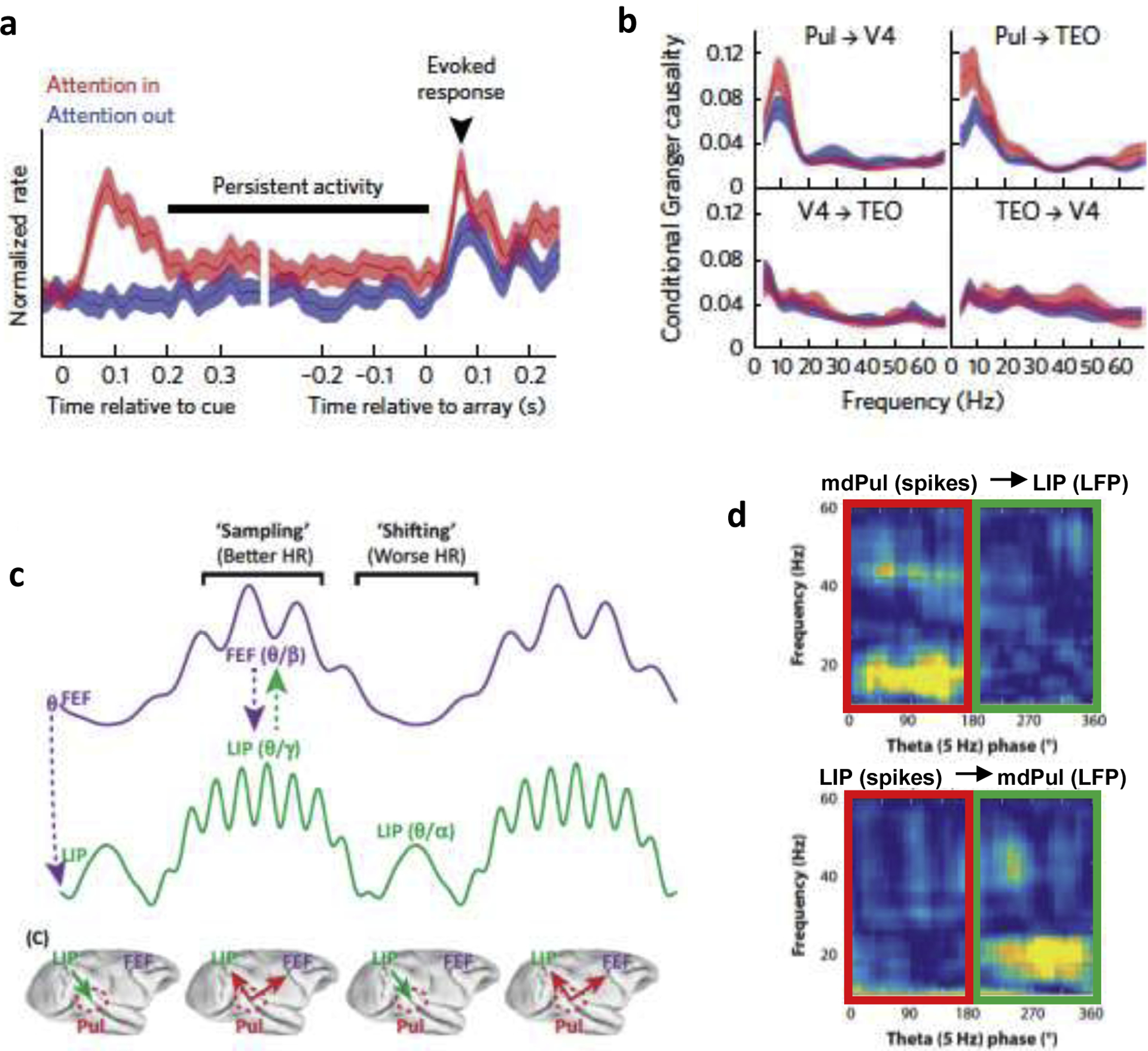
a. Attentional modulation of pulvinar responses when spatial attention is allocated at the receptive field that is recorded from (‘Attention in) versus elsewhere in the visual field (‘Attention out’). Elevated activity during the cue target interval (‘persistent activity’) and attention-related response enhancement of visually-evoked activity are apparent, similar to typical attention effects obtained in visual cortex (adapted with permission from [45]). b. Conditional granger causality analysis suggests that pulvinar increases coherence in alpha frequencies in V4 and TEO during the delay period. In contrast, V4 and TEO do not seem to exert functional influences onto each other or the pulvinar (adapted with permission from [45]). c. Spatial attention is characterized by alternating attentional states that promote either sampling at the attended location or a higher likelihood of shifting attentional resources to another location. These theta-rhythmic, alternating attentional states are associated with different oscillatory frequencies (e.g., beta and gamma oscillations) and different patterns of functional connectivity (c, adapted from [47]), with the pulvinar coordinating cortical activity specifically during periods of sensory sampling (d, red box denotes sampling phase, and green box shifting phase; adapted from [52]). Pulvinar, Pul; visual area 4, V4; frontal eye fields, FEF; lateral intraparietal are, LIP; mediodorsal pulvinar, mdPul.
Box 2 Open questions.
How are signals that are carried through transthalamic pathways transformed in the pulvinar, and how are these signals different from those conveyed through the corresponding cortico-cortical pathway? According to one proposal [55,80], the pulvinar may act as a ‘coincidence detector’ aligning external signals from the sensory environment, projected via cortex, with internal contextual signals. The pulvinar then produces a validation signal for cortex to further process the sensory signals. According to another mutually not exclusive proposal [81], the transthalamic feedforward pathway may not only carry sensory information, but also an efference copy for motor centers, projected via branched axons that target extrathalamic structures for motor control.
Do pulvino-cortical projections shape cortical computations and influence basic response properties such as spatial representation (e.g. receptive field size), or feature selectivity? For example, a recent study in rodents [82] demonstrated that neurons in the lateral posterior nucleus—the rodent homologue of the primate pulvinar—drive inhibitory neurons in layer 1 of V1, leading to non-selective inhibitory input to layer 2/3 neurons. This inhibitory input significantly enhances feature selectivity, including orientation, direction, spatial and size tuning, while not influencing feature preference (e.g. peak orientation of tuning curve). Corresponding evidence in non-human primates is needed to further understand the contribution of pulvino-cortical interactions to visual processing and perception (see [51]).
What controls the pulvinar? It is reasonable to assume that the pulvinar receives information about the internal contextual state and behavioral goals from prefrontal cortex (PFC). However, a substantial part of the pulvinar is not directly connected to PFC, and the pulvinar also lacks widespread internal connections across its different subparts (but see [85]). Given this lack of direct connectivity, the thalamic reticular nucleus (TRN) may be the missing part in a PFC-pulvinar control loop [84]. The TRN forms a thin shell of inhibitory neurons covering the lateral and anterior surface of the dorsal thalamus. The TRN receives input from both sensory cortices as well as widespread inputs from higher-order cortex, including PFC, and its output is sent exclusively to thalamus. The idea of a PFC-TRN-pulvinar control loop is intriguing but requires support from empirical evidence.
How are the transthalmic pathways dynamically recruited into specialized, large-scale cortical networks that support different cognitive functions? The pulvinar has not only been associated with attention function, but also with working memory [85], decision making [76,86] and conscious perception [87]. It is unclear which thalamo-cortical circuits are recruited for these different cognitive functions and whether the functional roles established for attention generalize to other cognitive domains.
How are human pulvinar and pulvino-cortical interactions transformed to reflect human-specific behaviors? Thalamocortical pathways and the visual processing hierarchy are shaped by a species-specific, visually-guided behavioral repertoire, meaning that their functional organization differs substantially across species. For example, PM, a primate-specific region of the pulvinar, reflects the vast expansion of frontal and parietal cortices in primates. In humans, there are further adaptations that support human-specific behaviors such as a specific functional organization in parietal cortex for sophisticated toolmaking [88]. The thalamic counterparts of these adaptations are largely unknown [89].
Given that the influence of cortex on pulvinar is quite unclear, what is known about the reverse influence of pulvinar on cortex? By recording simultaneously from two cortical areas in the monkey ventral extrastriate cortex (i.e. areas V4 and TEO) that share both direct cortico-cortical connectivity and a common pulvinar projection zone, Saalmann et al. ([45]) found evidence for a thalamic role in coordinating attention-related functional interactions across cortical areas. In addition to attentional modulation of spike rates (Figure 2A), attention enhanced coordinated spiking activity within the pulvinar and between the pulvinar and interconnected cortical areas. Such temporal coordination can be measured as a correlation of spiking activity relative to local population activity obtained from the field potential (i.e. spike-field coherence), either within or across areas. Saalmann et al. ([45]) measured these functional interactions during the task period following the cue and before target presentation (i.e. the delay period), when the wider cortical attention network is set up for target selection. During the delay period, neurons in all three areas showed enhanced coordinated spiking in the alpha and low beta ranges (<20 Hz). Strikingly, the pulvinar appeared to have the strongest influence on coordinated activity in V4 and TEO in a frequency band that matched that of the local attentional modulation (Figure 2B). In contrast, either cortical area had little influence on these interactions, suggesting that the pulvinar controlled the temporal coordination of functional interactions between cortical areas. These data are therefore consistent with the pulvinar having a primary role as a ‘timekeeper’, coordinating and optimizing functional interactions across cortical networks to enhance the efficiency of signal processing between nodes. Fiebelkorn et al. ([52]) has since provided evidence that this role of the pulvinar in coordinating cortical interactions is not limited to sensory cortical regions but also extends to functional interactions between higher-order cortical regions.
Studies using causal manipulations have further corroborated these ideas, showing that functional connectivity between cortical areas is weakened as a consequence of pulvinar inactivation [46,53]. These studies suggest that cortico-cortical information transmission is greatly compromised without pulvinar’s influences. During pulvinar inactivation, neural activity—spiking as well as gamma activity—was largely reduced in visual cortex [46,51], indicating a profound loss of responsiveness to visual stimuli. Pulvinar inputs to cortex may therefore be instrumental in controlling the excitability of neurons in superficial and/or granular layers, allowing them to respond normally to incoming visual information from downstream areas. Such control of excitability could occur via excitatory inputs on pyramidal neurons, or inhibitory control of interneurons, as suggested by increases in baseline firing during pulvinar inactivation [46]. Sensory gain control of cortical neurons by pulvinar appears to follow a response gain model, in which neural responses are scaled multiplicatively or additively [54]. Thus, another major functional role of the transthalamic pathways appears to be that of an ‘enabler’ of cortical function [55]. Computational studies will be needed to derive precise thalamo-cortical circuit models that may account for both pulvinar-driven temporal coordination and pulvinar-driven enabling of cortical function (see Box 1). In the next section, we will discuss the highly dynamic nature of these pulvino-cortical interactions and their associated functions.
Box 1 – Computational models of thalamo-cortical circuitry.
Computational models play an increasingly important role in capturing the complexity of local and long-distance circuits that characterize cognitive networks and their associated behaviors. Distributed circuit models of cortical large-scale networks emphasizing modules in frontal and parietal cortex have successfully captured behavioral and neural features of cognitive tasks, such as working memory and decision making, without considering thalamic contributions [74]. Recent models, however, have begun to include thalamic modules and examine their interactions with cortex. For example, Jaramillo and colleagues ([75]) built upon a model with two reciprocally connected cortical modules, showing that adding a pulvinar module provided critical flexibility during various cognitive behaviors. The pulvinar module was interconnected with the cortical modules through the “canonical” thalamo-cortical feedforward and feedback pathways, described in the section on “Pulvino-cortical pathways”. The overall connectivity in this large-scale circuit resulted from two sources: the direct cortico-cortical projection and the indirect transthalamic projections. Modulation of a single variable - pulvinar excitability, influenced by behavioral state - had a dramatic influence on cortical computation. For example, persistent neuronal spiking is a classic signature of both working memory and sustained attention tasks (see Figure 2A). According to Jaramillo and colleagues ([75]), when pulvinar excitability was low the pulvinar was not actively engaged and did not contribute to overall connectivity in the network. As a consequence, persistent activity across the network could not be established. That is, even though cortical area 1 generated task-related activity, it could not propagate to area 2 because of low overall connectivity across the network. Similarly, cortical area 2 was not able to generate recurrent feedback signals that would reinforce the representation of task-related activity in area 1, again contributing to a lack of persistent activity. When pulvinar excitability was sufficiently large, however, signals were able to propagate between cortical areas 1 and 2, setting up the recurrent network state that is necessary for sustaining activity over time (i.e., for persistent activity). Jaramillo and colleagues used their simple circuit model to reproduce several key physiological and behavioral findings from a wide variety of cognitive behaviors. That is, their model captured several proposed pulvinar functions, such as regulating effective interareal communication, as in the above example [45–47], controlling intra-areal excitability [46, 51], and generating signals reflecting decision confidence [76]. However, the mechanisms through which pulvinar excitability changes based on behavioral state remain largely unknown. A complete model of pulvinar function in cognition will require the inclusion of more detailed local circuits into such large-scale models. As we discussed in the main text, pulvino-cortical interactions are highly dynamic, and the temporal signature of these interactions is largely characterized by oscillatory activity in the alpha band [45–47]. However, the physiological mechanisms through which this pulvino-cortical alpha-band activity is generated remain unclear. Previous studies have linked two distinct mechanisms, based on either muscarinic acetylcholine receptor (mAChR) or metabotropic glutamate receptor 1 (mGluR1), to the generation of thalamic alpha-band activity [77,78]. A model by Vijayan and Kopell ([79]) suggested that these two mechanisms play specific functional roles, with mAChR-induced alpha-band activity as a mechanism for thalamic control of cortex, and mGluR1-induced alpha-band activity as a mechanism for cortical control of thalamus—through glutaminergic cortical projections to thalamus. It remains an open question, however, whether these previously described mechanisms for the generation of thalamic alpha-band activity apply to the pulvino-cortical interactions associated with cognition (e.g., spatial attention and working memory).
Rhythmic re-weighting of pulvino-cortical interactions
Classic views assume that attention-related modulation of neural activity locally and across the network, as described in the previous section, is continuous during attentional deployment. Recent work, however, has instead shown that attentional deployment is highly dynamic, characterized by alternating periods of relatively enhanced or diminished visual processing at the presently attended location [47]. Even though these alternations in the sensitivity of visual processing are not noted subjectively, they can be readily observed in behavioral data and occur about 4–6 times per second (i.e. at a frequency in the theta range) [56–59]. Importantly, these theta-dependent rhythmic alternations of visual processing, associated with better or worse behavioral performance during visual-target detection, have been observed in both humans [58,60] and non-human primates [61], thus constituting a fundamental property of attention function that has been preserved for more than 20 million years [47].
What is the function of these theta-dependent attentional rhythms? We recently proposed that attention-related, theta-rhythmic fluctuations in behavioral performance reflect the temporal coordination of potentially conflicting sensory and motor functions that occur in the fronto-parietal attention network [47]. The attention network directs both attention-related boosts in sensory processing, or ‘sampling’, and the orienting of attention to new targets, or ‘shifting’, for example by executing saccadic eye movements [19,62,63]. This functionality is reflected in the response properties of neurons in frontal and parietal cortex, which range from purely sensory (i.e. visually-driven) to purely motor (i.e. saccade-related) [64,65]. Sampling and shifting cannot occur at the same time and require temporal coordination of the associated neural populations and the wider circuits that they are embedded in. Theta-dependent attentional rhythms appear to coordinate two alternating states: a ‘sampling’ state that emphasizes the preferential processing of visual information from the attended location, and a ‘shifting’ state when there is an increased likelihood of attentional shifting through either covert attentional shifts (i.e., in the absence of eye movements) or through overt attentional shifts (i.e., with eye movements). These latter, ‘shifting periods,’ which are associated with relatively diminished visual processing, reflect windows of opportunity when it is easier to disengage from the presently attended location and shift to another location. Rhythmic sampling during attentional deployment thus provides critical flexibility, not only to prioritize visual processing at an attended location, but also to provide opportunities to re-allocate attentional resources, if needed, without locking them into one particular state for extended periods of time [47].
Both pulvino-cortical and cortico-cortical dynamics play pivotal roles in controlling these alternating attentional states. Sampling periods—associated with relatively better visual-target detection at the attended location—are characterized by greater synchronization of gamma (>35Hz) and beta (~15–30Hz) activity within and between frontal and parietal cortex (Figure 2C; [61]). Importantly, spiking activity of visual neurons is exclusively correlated with gamma activity, which has often been observed during enhanced visual processing [44], while spiking activity of visuo-motor neurons is exclusively correlated with beta activity, which has been linked to the suppression of motor actions [66,67]. The primate-specific mediodorsal pulvinar (or PM) coordinates these cortico-cortical interactions across the attention network [52], similar to what has been described in the previous section. However, it does so exclusively during sampling periods and not during shifting periods, further underlining the role of the transthalmic pathway in sensory gating. In contrast, shifting periods—associated with relatively worse visual-target detection at the attended location—are not only associated with a release of suppression in the motor system [61], but also with greater synchronization of alpha-band activity (~9–14 Hz), specifically within parietal cortex and between parietal cortex and the mediodorsal pulvinar [52].
Parieto-occipital alpha-band activity is frequently associated with the suppression of sensory processing in human studies [68,69], as well as the suppression of cortical neuronal firing [70]. We propose that parietal-driven synchronization in the pulvinar temporarily shuts down the transthalamic pathway and its sensory gating function, thereby providing a neural substrate for the frequently observed visual suppression following alpha synchronization in parietal cortex. As schematically summarized in Figure 2C, the neural evidence of rhythmic sampling during attentional deployment suggests a highly dynamic process across the attention network that results in alternating periods associated with either (i) enhanced visual processing and the suppression of attentional shifts (i.e., sampling periods) or (ii) suppression of visual processing and a release from the suppression of attentional shifts (i.e., shifting periods) [47]. This pattern of results is consistent with a previous proposal based on human lesion studies, which suggested that the pulvinar was associated with the engagement of attentional resources at a behaviorally relevant location, while parietal cortex was associated with a disengagement of attentional resources [71]. In summary, pulvino-cortical interactions rhythmically shift over time, reflecting the dynamic nature of spatial attention, even when the task at hand promotes sustained attention at a single location.
Conclusions
Cortico-centric views of cognition have begun to shift in recent years towards concepts that emphasize the critical importance of thalamo-cortical interactions in normal cognitive functioning and in mental disease [19,72,73]. The indirect transthalamic pathways that link cortex and pulvinar are associated with at least two major functions. First, these pathways serve to coordinate cortical networks recruited during cognition in time to enhance the efficiency of information transmission across the cortical network. Second, the pathway gates, or even enables sensory information processing by controlling local excitability of cortical neurons. Importantly, such temporal coordination and enabling of cortical activity only occur during network states that emphasize sensory processing and not during states that emphasize motor processing and are implemented through a pulvino-parietal control network. Many of these findings come from studies in non-human primates, which have laid the groundwork for developing biologically plausible models of the human thalamus to understand its functions in health and disease.
Highlights.
There is a revival of interest in the functional role of the pulvinar in cognition
The pulvinar temporally coordinates cortex to facilitate information transmission
The pulvinar enables sensory processing by controlling cortical excitability
Pulvino-cortical interactions are highly dynamic during selective attention
The pulvinar specifically influences cortex during periods of sensory processing
Acknowledgment
We gratefully acknowledge funding support from NIH (RO1MH64043, RO1EY017699, 21560-685 Silvio O. Conte Center), the James S. McDonnell Foundation and the Overdeck Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Sherman SM, Guillery RW: Functional connections of cortical areas: a new view from the thalamus. MIT Press; 2013. [Google Scholar]
- 2.Saalmann YB, Kastner S: Cognitive and Perceptual Functions of the Visual Thalamus. Neuron 2011, 71:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlonan K, Cavanaugh J, Wurtz RH: Guarding the gateway to cortex with attention in visual thalamus. Nature 2008, 456:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor DH, Fukui MM, Pinsk MA, Kastner S: Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 2002, 5:1203–1209. [DOI] [PubMed] [Google Scholar]
- 5.Kim CK, Adhikari A, Deisseroth K: Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 2017, 18:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.*.Harris JA, Mihalas S, Hirokawa KE, Whitesell JD, Choi H, Bernard A, Bohn P, Caldejon S, Casal L, Cho A, et al. : Hierarchical organization of cortical and thalamic connectivity. Nature 2019, 575:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]; Combining a large number of experiments using genetic viral tracing tools, this study provides a major expansion to the Allen Mouse Brain Connectivity Atlas resource. The authors labelled brain-wide connections in mice by layer and class of projection neurons and derived generalized anatomical principles to describe corticocortical, thalamocortical and corticothalamic projections. The connection patterns were modelled as either feedforward or feedback, and tested predictions of hierarchical positions for individual cortical and thalamic areas and for cortical network modules. The results show a shallow hierarchy within the corticothalamic network, suggesting the need of future computational models to capture the complexity of connections.
- 7.**.Bennett C, Gale SD, Garrett ME, Newton ML, Callaway EM, Murphy GJ, Olsen SR: Higher-Order Thalamic Circuits Channel Parallel Streams of Visual Information in Mice. Neuron 2019, 102:477–492.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Based on anatomical mapping, selective silencing and electrophysiological recordings in mouse lateral posterior nucleus (LP)- the rodent pulvinar homolog -, superior colliculus and visual cortex, two major pathways are established: one connecting the SC to ventral stream areas of visual cortex via posterior LP and another one connecting V1 to dorsal stream areas via anterior LP. The driving inputs, functional properties and details of downstream targets of LP provide circuit-specific mechanisms of higher order thalamic function and establish a functional roadmap for thalamo-cortical interactions in rodents.
- 8.Usrey WM, Alitto HJ: Visual Functions of the Thalamus. Annu Rev Vis Sci 2015, 1:351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke M, Turchi J, Smith K, Mishkin M, Leopold DA: Pulvinar Inactivation Disrupts Selection of Movement Plans. J Neurosci 2010, 30:8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danziger S, Ward R, Owen V, Rafal R: The Effects of Unilateral Pulvinar Damage in Humans on Reflexive Orienting and Filtering of Irrelevant Information. Behav Neurol 2002, 13:917570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafal RD, Posner MI: Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci 1987, 84:7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward R, Danziger S, Owen V, Rafal R: Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nat Neurosci 2002, 5:99–100. [DOI] [PubMed] [Google Scholar]
- 13.Arend I, Rafal R, Ward R: Spatial and temporal deficits are regionally dissociable in patients with pulvinar lesions. Brain 2008, 131:2140–2152. [DOI] [PubMed] [Google Scholar]
- 14.Danziger S, Ward R, Owen V, Rafal R: Contributions of the human pulvinar to linking vision and action. Cogn Affect Behav Neurosci 2004, 4:89–99. [DOI] [PubMed] [Google Scholar]
- 15.Snow JC, Allen HA, Rafal RD, Humphreys GW: Impaired attentional selection following lesions to human pulvinar: Evidence for homology between human and monkey. Proc Natl Acad Sci 2009, 106:4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG: Posterior parietal cortex and the filtering of distractors. Proc Natl Acad Sci 2003, 100:4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squire RF, Noudoost B, Schafer RJ, Moore T: Prefrontal Contributions to Visual Selective Attention. Annu Rev Neurosci 2013, 36:451–466. [DOI] [PubMed] [Google Scholar]
- 18.Buschman TJ, Kastner S: From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron 2015, 88:127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiebelkorn IC, Kastner S: Functional Specialization in the Attention Network. Annu Rev Psychol 2020, 71:221–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalfin BP, Cheung DT, Muniz JAPC, de Lima Silveira LC, Finlay BL: Scaling of neuron number and volume of the pulvinar complex in new world primates: Comparisons with humans, other primates, and mammals. J Comp Neurol 2007, 504:265–274. [DOI] [PubMed] [Google Scholar]
- 21.*.Baldwin MKL, Balaram P, Kaas JH: The evolution and functions of nuclei of the visual pulvinar in primates. J Comp Neurol 2017, 525:3207–3226. [DOI] [PubMed] [Google Scholar]; Comprehensive comparative review on the evolution of the pulvinar in mammals including discussion of pulvinar anatomy in new and old world monkeys, prosimian galagos, tree shrews and rodents. Functional principles are derived based on comparative anatomy.
- 22.Smaers JB, Gómez-Robles A, Parks AN, Sherwood CC: Exceptional Evolutionary Expansion of Prefrontal Cortex in Great Apes and Humans. Curr Biol 2017, 27:714–720. [DOI] [PubMed] [Google Scholar]
- 23.Kaas JH, Lyon DC: Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Century Neurosci Discov Reflecting Nobel Prize Golgi Cajal 1906 2007, 55:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arcaro MJ, Pinsk MA, Kastner S: The Anatomical and Functional Organization of the Human Visual Pulvinar. J Neurosci 2015, 35:9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender DB: Retinotopic organization of macaque pulvinar. J Neurophysiol 1981, 46:672–693. [DOI] [PubMed] [Google Scholar]
- 26.Warner CE, Kwan WC, Bourne JA: The Early Maturation of Visual Cortical Area MT is Dependent on Input from the Retinorecipient Medial Portion of the Inferior Pulvinar. J Neurosci 2012, 32:17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ZHOU N, MAIRE PS, MASTERSON SP, BICKFORD ME: The mouse pulvinar nucleus: Organization of the tectorecipient zones. Vis Neurosci 2017, 34:E011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridge H, Leopold DA, Bourne JA: Adaptive Pulvinar Circuitry Supports Visual Cognition. Trends Cogn Sci 2016, 20:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourne JA, Morrone MC: Plasticity of Visual Pathways and Function in the Developing Brain: Is the Pulvinar a Crucial Player? Front Syst Neurosci 2017, 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS: Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol 1997, 379:313–332. [PubMed] [Google Scholar]
- 31.Darian-Smith C, Tan A, Edwards S: Comparing thalamocortical and corticothalamic microstructure and spatial reciprocity in the macaque ventral posterolateral nucleus (VPLc) and medial pulvinar. J Comp Neurol 1999, 410:211–234. [PubMed] [Google Scholar]
- 32.Homman-Ludiye J, Mundinano IC, Kwan WC, Bourne JA: Extensive Connectivity Between the Medial Pulvinar and the Cortex Revealed in the Marmoset Monkey. Cereb Cortex 2019, 30:1797–1812. [DOI] [PubMed] [Google Scholar]
- 33.Marion R, Li K, Purushothaman G, Jiang Y, Casagrande VA: Morphological and neurochemical comparisons between pulvinar and V1 projections to V2. J Comp Neurol 2013, 521:813–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markov NT, Vezoli J, Chameau P, Falchier A, Quilodran R, Huissoud C, Lamy C, Misery P, Giroud P, Ullman S, et al. : Anatomy of hierarchy: Feedforward and feedback pathways in macaque visual cortex. J Comp Neurol 2014, 522:225–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams MM, Hof PR, Gattass R, Webster MJ, Ungerleider LG: Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J Comp Neurol 2000, 419:377–393. [DOI] [PubMed] [Google Scholar]
- 36.Shipp S: The functional logic of cortico–pulvinar connections. Philos Trans R Soc Lond B Biol Sci 2003, 358:1605–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips JM, Fish LR, Kambi NA, Redinbaugh MJ, Mohanta S, Kecskemeti SR, Saalmann YB: Topographic organization of connections between prefrontal cortex and mediodorsal thalamus: Evidence for a general principle of indirect thalamic pathways between directly connected cortical areas. NeuroImage 2019, 189:832–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rovó Z, Ulbert I, Acsády L: Drivers of the Primate Thalamus. J Neurosci 2012, 32:17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockland KS: Convergence and branching patterns of round, type 2 corticopulvinar axons. J Comp Neurol 1998, 390:515–536. [DOI] [PubMed] [Google Scholar]
- 40.Rockland KS, Andresen J, Cowie RJ, Robinson DL: Single axon analysis of pulvinocortical connections to several visual areas in the Macaque. J Comp Neurol 1999, 406:221–250. [DOI] [PubMed] [Google Scholar]
- 41.Luck SJ, Chelazzi L, Hillyard SA, Desimone R: Neural Mechanisms of Spatial Selective Attention in Areas V1, V2, and V4 of Macaque Visual Cortex. J Neurophysiol 1997, 77:24–42. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MR, Maunsell JHR: Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 2009, 12:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell JF, Sundberg KA, Reynolds JH: Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 2009, 63:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fries P, Reynolds JH, Rorie AE, Desimone R: Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science 2001, 291:1560. [DOI] [PubMed] [Google Scholar]
- 45.**.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S: The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science 2012, 337:753. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using simultaneous electrophysiological recordings from anatomically connected cortical areas and pulvinar in monkeys performing a visuospatial attention task, this study revealed a functional role for pulvinar in cortico-cortical information transmission. Alpha band coherence increased across areas V4, TEO and their interconnected pulvinar projection zone during sustained attention. Statistical analysis indicated that pulvinar had a greater influence on cortex than vice versa. This study was the first to establish a functional role for the pulvinar in cognition.
- 46.**.Zhou H, Schafer RJ, Desimone R: Pulvinar-cortex interactions in vision and attention. Neuron 2016, 89:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors combined reversible pu;vinar inactivation and simultaneous cortical electrophysiological recordings from macaque area V4 and infero-temporal cortex to probe the role of pulvinar in visuo-spatial attention causally. Following pulvinar inactivation, a reduction of visually-evoked responses and gamma coherence was observed in V4, along with severe behavioral deficits in the affected portion of the visual field. Pulvinar inactivation also caused an increase in low-frequency (0.5–20Hz) power of local population activity, often associated with inattention or sleep, suggesting that pulvinar keeps cortex in an active state.
- 47.**.Fiebelkorn IC, Kastner S: A Rhythmic Theory of Attention. Trends Cogn Sci 2019, 23:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review presents a novel attention theory based on recent findings of rhythmic properties that can be measured in behavior and their underlying neural substrates. The attention network alternates between two states associated with either sampling (at a behaviorally relevant location) or shifting (to another potential location). These alternating states serve to temporally coordinate the network in order to avoid functional conflicts between sensory and motor processing.
- 48.Sherman SM, Guillery RW: On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators .” Proc Natl Acad Sci 1998, 95:7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theyel BB, Llano DA, Sherman SM: The cortico-thalamo-cortical relay: a potent circuit for intercortical information flow. In Soc Neurosci Abstr. . 2008:17. [Google Scholar]
- 50.Chalupa L, Abramson B: Visual receptive fields in the striate-recipient zone of the lateral posterior-pulvinar complex. J Neurosci 1989, 9:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.*.Purushothaman G, Marion R, Li K, Casagrande VA: Gating and control of primary visual cortex by pulvinar. Nat Neurosci 2012, 15:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]; Combining reversible inactivation of lateral pulvinar and simultaneous electrophysiological recordings from primary visual cortex (V1) in anesthetized prosimian primates causal influences of pulvinar on cortex are explored. Pulvinar inactivation dramatically reduced visually-evoked responses of V1 neurons in supragranular layers. Focal excitation of pulvinar, on the other hand, increased visual responses in V1 within their receptive fields, but suppressed responses in the surrounding regions. First study in primates providing evidence that visual information cannot propagate to the next visual area without facilitating pulvinar influences, thus establishing a critical role for pulvinar in the regular functioning of visual cortex.
- 52.**.Fiebelkorn IC, Pinsk MA, Kastner S: The mediodorsal pulvinar coordinates the macaque fronto-parietal network during rhythmic spatial attention. Nat Commun 2019, 10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]; By recording simultaneously from macaque medio-dorsal pulvinar, frontal eye fields, and lateral intraparietal area, a pulvino-parietal control network is identified that rhythmically re-weighs functional connectivity between thalamus and cortex to set up states that emphasize sensory processing (e.g. associated with enhanced perceptual sensitivity) or motor processing (e.g. associated with diminished perceptual sensitivity at a given spatial location). This is the first study providing evidence for highly dynamic thalamo-cortical interactions during selective attention.
- 53.Eradath MK, Pinsk MA, Kastner S: A causal role for pulvinar in coordinating task independent cortico-cortical interactions. bioRxiv 2020, doi: 10.1101/2020.03.07.982215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Souza BOF, Cortes N, Casanova C: Pulvinar Modulates Contrast Responses in the Visual Cortex as a Function of Cortical Hierarchy. Cereb Cortex 2019, 30:1068–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakatos P, O’Connell MN, Barczak A: Pondering the Pulvinar. Neuron 2016, 89:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.VanRullen R, Carlson T, Cavanagh P: The blinking spotlight of attention. Proc Natl Acad Sci 2007, 104:19204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landau AN, Fries P: Attention Samples Stimuli Rhythmically. Curr Biol 2012, 22:1000–1004. [DOI] [PubMed] [Google Scholar]
- 58.Fiebelkorn IC, Saalmann YB, Kastner S: Rhythmic Sampling within and between Objects despite Sustained Attention at a Cued Location. Curr Biol 2013, 23:2553–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song K, Meng M, Chen L, Zhou K, Luo H: Behavioral Oscillations in Attention: Rhythmic α Pulses Mediated through θ Band. J Neurosci 2014, 34:4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helfrich RF, Fiebelkorn IC, Szczepanski SM, Lin JJ, Parvizi J, Knight RT, Kastner S: Neural Mechanisms of Sustained Attention Are Rhythmic. Neuron 2018, 99:854–865.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiebelkorn IC, Pinsk MA, Kastner S: A Dynamic Interplay within the Frontoparietal Network Underlies Rhythmic Spatial Attention. Neuron 2018, 99:842–853.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, et al. : A Common Network of Functional Areas for Attention and Eye Movements. Neuron 1998, 21:761–773. [DOI] [PubMed] [Google Scholar]
- 63.Moore T, Fallah M: Control of eye movements and spatial attention. Proc Natl Acad Sci 2001, 98:1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson KG, Biscoe KL, Sato TR: Neuronal Basis of Covert Spatial Attention in the Frontal Eye Field. J Neurosci 2005, 25:9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gregoriou GG, Gotts SJ, Desimone R: Cell-Type-Specific Synchronization of Neural Activity in FEF with V4 during Attention. Neuron 2012, 73:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pogosyan A, Gaynor LD, Eusebio A, Brown P: Boosting Cortical Activity at Beta-Band Frequencies Slows Movement in Humans. Curr Biol 2009, 19:1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Chen Y, Bressler SL, Ding M: Response preparation and inhibition: The role of the cortical sensorimotor beta rhythm. Neuroscience 2008, 156:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foxe J, Snyder A: The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol 2011, 2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jensen O, Mazaheri A: Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front Hum Neurosci 2010, 4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haegens S, Nácher V, Luna R, Romo R, Jensen O: α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci 2011, 108:19377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Posner MI, Petersen SE: The Attention System of the Human Brain. Annu Rev Neurosci 1990, 13:25–42. [DOI] [PubMed] [Google Scholar]
- 72.Parvizi J: Corticocentric myopia: old bias in new cognitive sciences. Trends Cogn Sci 2009, 13:354–359. [DOI] [PubMed] [Google Scholar]
- 73.Halassa MM, Kastner S: Thalamic functions in distributed cognitive control. Nat Neurosci 2017, 20:1669–1679. [DOI] [PubMed] [Google Scholar]
- 74.Murray JD, Jaramillo J, Wang X-J: Working Memory and Decision-Making in a Frontoparietal Circuit Model. J Neurosci 2017, 37:12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaramillo J, Mejias JF, Wang X-J: Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations. Neuron 2019, 101:321–336.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komura Y, Nikkuni A, Hirashima N, Uetake T, Miyamoto A: Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nat Neurosci 2013, 16:749–755. [DOI] [PubMed] [Google Scholar]
- 77.Hughes SW, Lörincz M, Cope DW, Blethyn KL, Kékesi KA, Parri HR, Juhász G, Crunelli V: Synchronized Oscillations at α and θ Frequencies in the Lateral Geniculate Nucleus. Neuron 2004, 42:253–268. [DOI] [PubMed] [Google Scholar]
- 78.Lőrincz ML, Kékesi KA, Juhász G, Crunelli V, Hughes SW: Temporal Framing of Thalamic Relay-Mode Firing by Phasic Inhibition during the Alpha Rhythm. Neuron 2009, 63:683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vijayan S, Kopell NJ: Thalamic model of awake alpha oscillations and implications for stimulus processing. Proc Natl Acad Sci 2012, 109:18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groh A, de Kock CPJ, Wimmer VC, Sakmann B, Kuner T: Driver or Coincidence Detector: Modal Switch of a Corticothalamic Giant Synapse Controlled by Spontaneous Activity and Short-Term Depression. J Neurosci 2008, 28:9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sherman SM: Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 2016, 19:533–541. [DOI] [PubMed] [Google Scholar]
- 82.Fang Q, Chou X, Peng B, Zhong W, Zhang LI, Tao HW: A Differential Circuit via Retino-Colliculo-Pulvinar Pathway Enhances Feature Selectivity in Visual Cortex through Surround Suppression. Neuron 2020, 105:355–369.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imura K, Rockland KS: Long-range interneurons within the medial pulvinar nucleus of macaque monkeys. J Comp Neurol 2006, 498:649–666. [DOI] [PubMed] [Google Scholar]
- 84.Zikopoulos B, Barbas H: Prefrontal Projections to the Thalamic Reticular Nucleus form a Unique Circuit for Attentional Mechanisms. J Neurosci 2006, 26:7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rotshtein P, Soto D, Grecucci A, Geng JJ, Humphreys GW: The role of the pulvinar in resolving competition between memory and visual selection: A functional connectivity study. Atten Short-Term Mem 2011, 49:1544–1552. [DOI] [PubMed] [Google Scholar]
- 86.Wilke M, Kagan I, Andersen RA: Effects of Pulvinar Inactivation on Spatial Decision-making between Equal and Asymmetric Reward Options. J Cogn Neurosci 2013, 25:1270–1283. [DOI] [PubMed] [Google Scholar]
- 87.Wilke M, Mueller K-M, Leopold DA: Neural activity in the visual thalamus reflects perceptual suppression. Proc Natl Acad Sci 2009, 106:9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kastner S, Chen Q, Jeong SK, Mruczek REB: A brief comparative review of primate posterior parietal cortex: A novel hypothesis on the human toolmaker. Neuropsychologia 2017, 105:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.**.Arcaro MJ, Pinsk MA, Chen J, Kastner S: Organizing principles of pulvino-cortical functional coupling in humans. Nat Commun 2018, 9:5382. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using fMRI in humans and a large variety of experimental tasks, this study comprehensively probed the functional organization of the human pulvinar and its coupling with cortical networks. The ventral pulvinar is coupled with occipito-temporal cortex and has specialized regions for faces and scenes. The dorsal pulvinar is coupled with frontal, parietal, and cingulate cortices, including the attention, default mode, and human-specific tool networks. As in other primates, the functional organization of pulvinar mirrors the principles governing cortical organization.


