Abstract
Background and purpose
Aneurysmal wall enhancement (AWE) on vessel wall MRI (VW-MRI) has been described as a new imaging biomarker of unstable unruptured intracranial aneurysms (UIAs). Previous studies of symptomatic UIAs are limited due to small sample sizes and lack of AWE quantification. Our study aims to investigate whether qualitative and quantitative assessment of AWE can differentiate symptomatic and asymptomatic UIAs.
Methods
Consecutive patients with UIAs were prospectively recruited for VW-MRI at 3T from October 2014 to October 2019. UIAs were categorized as symptomatic if presenting with sentinel headache or oculomotor nerve palsy directly related to the aneurysm. Evaluation of wall enhancement included enhancement pattern (0=none, 1=focal, 2=circumferential) and quantitative wall enhancement index (WEI). Univariate and multivariate analysis was used to identify the parameters associated with symptoms.
Results
267 patients with 341 unruptured intracranial aneurysms (93 symptomatic and 248 asymptomatic) were included in this study. Symptomatic UIAs more frequently showed circumferential AWE than asymptomatic UIAs (66.7% vs. 17.3%, P<0.001), as well as higher WEI (median (interquartile range), 1.3 (1.0-1.9) vs. 0.3 (0.1-0.9), P<0.001). In multivariate analysis, both AWE pattern and WEI were independent factors associated with symptoms (odds ratio=2.03 across AWE patterns [95% confidence interval (CI), 1.21 to 3.39], P=0.01; odds ratio=3.32 for WEI [95% confidence interval, 1.51 to 7.26], P=0.003). The combination of AWE pattern and WEI had an area under the curve of 0.91 to identify symptomatic UIAs, with a sensitivity of 95.7% and a specificity of 73.4%.
Conclusions
In a large cohort of UIAs with VW-MRI, both AWE pattern and WEI were independently associated with aneurysm-related symptoms. The qualitative and quantitative features of AWE can potentially be used to identify unstable intracranial aneurysms.
Keywords: Unruptured intracranial aneurysms, Magnetic resonance imaging, Symptoms, Inflammation, Wall enhancement index
Subject Terms: Biomarkers, inflammation, Magnetic Resonance Imaging (MRI), Cerebral Aneurysm
Introduction
Unruptured intracranial aneurysms (UIAs) affect approximately 3% to 5% of the adult population and carry a low risk (1-2% per year risk) of rupture and subarachnoid hemorrhage (SAH); aneurysm rupture, however, carries high risk of severe morbidity and mortality1. Evaluation and stratification of aneurysm rupture risk is a significant clinical challenge. It is suggested that aneurysm rupture may be predicted by certain clinical symptoms2-4. While it may be difficult to associate non-specific headache patterns with aneurysm risk, it is commonly accepted that any change in pattern or quality of headaches may be associated with UIA instability and should prompt rapid assessment and treatment2. More classically, sentinel headache (SH) characterized by severe, acute onset headache and oculomotor nerve palsy (ONP) caused by local mass effect are two well-known symptoms that strongly suggest aneurysm instability and precede aneurysm rupture5, 6.
Both structural fragility and aneurysm growth might be mediated by aneurysmal wall inflammation7, 8. Although conventional imaging is limited in its ability to detect inflammatory wall changes, aneurysm wall enhancement (AWE) on vessel wall magnetic resonance imaging (VW-MRI) has been explored as a novel marker of aneurysm wall inflammation9, 10. Clinically, AWE has also been reported as a marker of unstable UIAs, as AWE has been observed more frequently in symptomatic, growing or ruptured UIAs11-15. Most studies have evaluated AWE using non-standardized qualitative approaches; however, quantitative techniques exist16. These could improve comparison objectivity and reliability. In addition, previous studies of symptomatic UIAs have been limited by small sample size (number of symptomatic aneurysms <30), or less specific definitions of symptoms related to UIAs. So far there is little understanding of how qualitative AWE pattern and quantitative enhancement degree detected with VW-MRI of UIAs relate to specific symptoms such as SH and ONP.
This study aims to investigate the association between AWE characteristics (enhancement pattern and the quantitative enhancement degree) and symptomatic status (carefully defined SH or ONP) of UIA in a larger cohort of patients by a comprehensive multivariate analysis.
Materials and methods
The data that support the findings of this study are available from the first authors upon reasonable request (15803870813@163.com, wangyuting_330@163.com).
Study population
This study was approved by the local Ethical Committees, and informed consent was obtained from all participants for prospective evaluation. Consecutive patients with intracranial aneurysms who underwent VW-MRI examination from October 2014 to October 2019 were extracted from an institutional database at a tertiary center. Inclusion criteria were: (1) patients with intracranial aneurysms who were either asymptomatic or symptomatic with the following definition: a) with SH (development of a sudden and severe headache on the ipsilateral side of the aneurysms within 2 weeks of admission without prior history of headache within the previous 5 years)2, 17; or b) with ONP (a sudden headache with one or several symptoms of pupillary light reflex disappearing, ptosis or extraocular myo-paralysis on the ipsilateral side of the aneurysm within 1 month prior to admission)6, 17, 18; (2) patients underwent high resolution VW-MRI before any intervention. Exclusion criteria were: (1) presence of ruptured or dissecting aneurysms regardless of presence of UIAs, or presence of fusiform aneurysms or infundibulum in place of UIAs; (2) unsatisfactory image quality caused by motion artifacts; (3) lack of digital subtraction angiography (DSA) imaging or incomplete clinical records; (4) symptomatic patients with multiple UIAs (due to uncertainty of which UIA was definitely responsible for the patient’s specific symptoms).
Imaging protocol
The detailed DSA and MRI protocols were shown in Supplemental Methods. Cerebral angiography was performed on a fixed digital angiographic system with a single-plane flat panel detector. Three-dimensional time-of-flight MRA, then either 2D or 3D T1-weighted black blood fast-spin-echo vessel wall MRI were scanned on 3T MR scanners.
Imaging analysis
For DSA image analysis, two experienced neurointerventional radiologists (S.G. and X.G., 30 and 15 years of experience in neurointerventional radiology, respectively) analyzed DSA images independently to determine the location and size of the UIAs. Discordances between the two radiologists were resolved by consensus.
For qualitative analysis of VW-MRI images, two experienced neuroradiologists (J.C. and Y.Z., 30 and 20 years of experience in neuroradiology, respectively) who were blinded to the clinical data and DSA results of the patients reviewed the pre- and post-contrast T1-weighted images independently and determined the presence and patterns of AWE. The AWE patterns were qualitatively defined as adjusted from a previous publication12: pattern 0 (no wall enhancement or similar degree of enhancement to the normal arterial wall), pattern 1 (focal wall enhancement) or pattern 2 (circumferential wall enhancement). Focal wall enhancement involved the neck, the dome, the intermediate portion, or a bleb, whereas circumferential wall enhancement involved the entire aneurysm on all three slices12. We did not use the definition of “thick circumferential enhancement” (defined as >1mm enhancement) in the previous study12, because the big difference in resolution (0.9x0.9x1.0mm voxel size in the prior study as compared to 0.6 mm3 in the current study) might lead to inconsistent wall thickness measurements. Discordances between the two neuroradiologists were resolved by consensus.
For quantitative analysis of VW-MRI images, two experienced neuroradiologists (Q.F. and Y.Z.,10 years and 5 years of clinical experience in neuroradiology) who were blinded to the clinical data and DSA results evaluated the images of UIAs via Vessel-MASS software (MEDIS, Version: 2014-EXP) independently (Figure 1). Processing details were shown in the Supplemental Methods. The quantitative wall enhancement index (WEI) was calculated as follows according to previous publications (SI indicates signal intensity)15, 19:
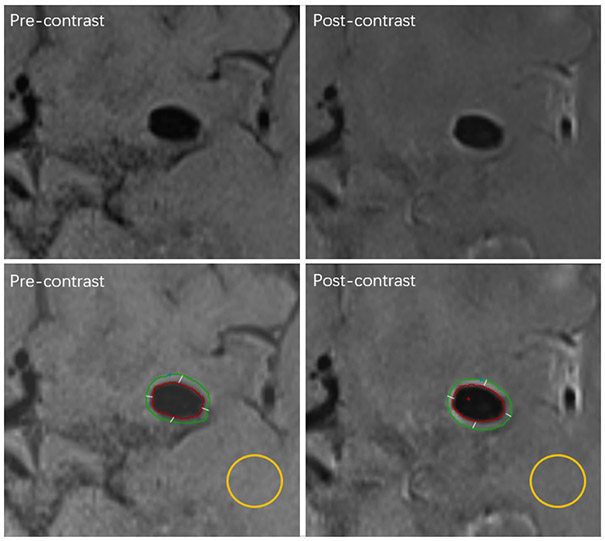
Figure 1.
Example case for image processing. First row, the original images. Second row, the contours of the aneurysm wall and the ROI (region of interests) of the white matter for normalization in calculation. The left column shows the pre-contrast vessel wall images on which the contour of ROI was traced manually, and the right column shows the post-contrast vessel wall images on which the contour of ROI was matched automatically, and the contour of ROI was segmented into quarters automatically.
Patient Management
All patients in this study underwent radiological studies after admission. Putative risk factors of aneurysm instability according to previous literature (age, female gender, hypertension, previous SAH, cigarette smoking, aspirin intake, familial history of intracranial aneurysm, aneurysm location, aneurysm size) were recorded7. Subsequently, multidisciplinary consultation (participants included neurosurgeons, neurologists, neurointerventional radiologists and neuroradiologists) was performed to determine if the patient’s symptom was clinically considered related to the intracranial aneurysm and if it was categorized as SH or ONP. Personalized management of patients with UIAs based on risk factors was also discussed. Symptomatic patients usually were recommended to undergo endovascular or surgical intervention if consented by the patients and permitted by their clinical conditions. VW-MRI scan was performed for the patient after the consultation. Therefore, the aforementioned clinical information and evaluation of the patients with UIAs was blinded to the VW-MRI analysis. Follow-up evaluation was conducted within 1 week after the intervention to record if the SH or ONP symptoms were relieved.
Statistical analysis
Data were assessed for normality by using the Shapiro-Wilk test. Continuous variables were summarized as means ± standard deviation if normally distributed, or median and interquartile ranges (IQRs) if otherwise. Categorical variables were presented as percentages. To assess interreader variability, intraclass correlation coefficient was applied for WEI measurements (two-way random effects, single rater/measurement, absolute agreement)20, and Cohen κ statistic was applied for interrater assessment of AWE patterns. Comparisons between groups were conducted using χ2 test for categorical variables (χ2 test for trend for ordinal categorical variables), and Mann–Whitney U test for continuous variables. Multi-collinearity testing was performed using variance inflation factor (VIF) as an evaluation standard. A VIF that equals 1 indicates no multi-collinearity among factors; a VIF between 1 and 5 indicates a moderate collinearity; a VIF between 5 to 10 indicates a high correlation that may be problematic; a VIF>10 indicates significant correlation leading to unreliability of the regression analysis21. Putative risk factors of aneurysm instability were tested in univariable binary analysis. The variables that achieved P<0.2 in the univariate analysis were further analyzed in the multivariate analysis using backward multiple logistic regression to determine factors independently associated with the symptoms. Logistic mixed regression method was used to adjust for individuals who contributed more than one aneurysm. The receiver operating characteristics curves were plotted for independent factors to differentiate symptomatic and asymptomatic aneurysms, and area under curves (AUC) were calculated. P<0.05 was regarded as significant, and all P values were two-sided.
Results
Patient demographics and aneurysmal wall enhancement characteristics
A total of the 397 patients with 511 aneurysms were initially identified, and 267 patients (mean age: 58.4±10.9 years old; female, 59.2%) with 341 aneurysms (median size: 5.4 mm, IQR: 3.5 -8.4 mm; 248 asymptomatic and 93 symptomatic) were finally included. The flow chart of patient selection is shown in Figure 2. Fifty-one patients received 2D T1-weighted sequences, and 216 patients received 3D T1-weighted sequence, corresponding to 59 aneurysms scanned by 2D T1-weighted sequences, and 282 aneurysms scanned by 3D T1-weighted sequence.
Figure 2.
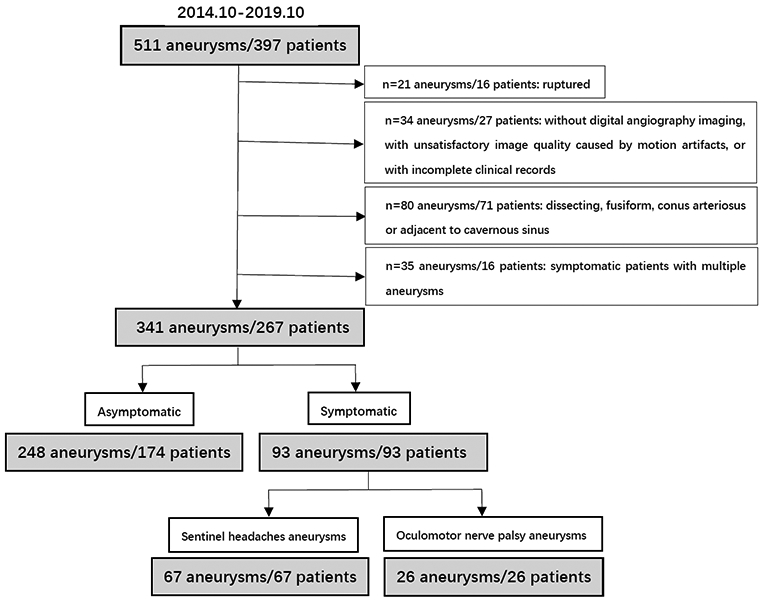
Flowchart of patient selection.
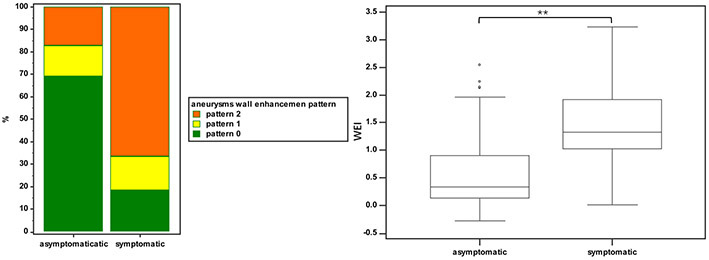
The demographic and imaging characteristics of the symptomatic and asymptomatic patients and aneurysms are listed in Table 1. Among the 341 UIAs, 248 UIAs (72.7%) demonstrated no symptoms, whereas 93 UIAs (27.3%) showed symptoms of either SH or ONP. Follow-up clinical evaluation confirmed that these symptoms improved or resolved in all the symptomatic patients after intervention. Asymptomatic UIAs more frequently did not show AWE as compared to symptomatic UIAs, and AWE pattern 2 was more frequently observed in symptomatic than asymptomatic UIAs (both P<0.001) (Table 1 and Figure 3A). Symptomatic UIAs had significantly higher WEI than asymptomatic aneurysms (median, 1.3 (1.0, 1.9) versus 0.3 (0.1, 0.9), P<0.001) (Table 1 and Figure 3B). Representative cases are shown in Figure 4.
Table 1.
Demographic and aneurysm characteristics
| Parameter | Total (n=341) |
Asymptomatic aneurysms (n=248) |
Symptomatic aneurysms (n=93) |
P Value |
|---|---|---|---|---|
| Age (years) | 58.4±10.9 | 60.4±9.5 | 53.3±12.6 | <0.001* |
| Female | 202 (59.2) | 141 (56.9) | 61 (65.6) | 0.14 |
| Hypertension | 222 (65.1) | 174 (70.2) | 48 (51.6) | 0.001* |
| Dyslipidemia | 166 (48.7) | 121 (48.8) | 45 (48.4) | 0.95 |
| Diabetes | 55 (16.1) | 40 (16.1) | 15 (16.1) | 1.00 |
| Previous SAH | 12 (3.5) | 10 (4.0) | 2 (2.2) | 0.40 |
| Current cigarette smoking | 72 (21.1) | 56 (22.6) | 16 (17.2) | 0.28 |
| Alcohol consumption# | 53 (15.5) | 43 (17.3) | 10 (10.8) | 0.14 |
| Aspirin intake | 43 (12.6) | 42 (16.9) | 1 (1.1) | 0.001* |
| Familial history of intracranial aneurysm | 23 (6.7) | 15 (6.0) | 8 (8.6) | 0.40 |
| Posterior circulation | 79 (23.2) | 50 (20.2) | 29 (31.2) | 0.03* |
| Aneurysm size(mm) | 5.4 (3.5, 8.4) | 4.9 (3.3, 7) | 7.1 (5, 12.3) | <0.001* |
| Thrombus | 34 (10.0) | 14 (5.6) | 20 (21.5) | <0.001* |
| AWE pattern | <0.001* | |||
| Pattern 0 | 188 (55.1) | 171 (69) | 17 (18.3) | |
| Pattern 1 | 48 (14.1) | 34 (13.7) | 14 (15.1) | |
| Pattern 2 | 105 (30.8) | 43 (17.3) | 62 (66.7) | |
| MR imaging quantitative measures | ||||
| WEI | 0.5 (0.2, 1.3) | 0.3 (0.1, 0.9) | 1.3 (1, 1.9) | <0.001* |
The data of continuous variables are mean ± standard deviation or median (interquartile range) as appropriate. The data of categorical variables are expressed as n (%).
Alcohol consumption, current alcohol consumption > 15g/ day.
Statistically significant.
SAH, subarachnoid hemorrhage; AWE, aneurysms wall enhancement; WEI, wall enhancement index.
Figure 3.
Comparison of the aneurysm wall enhancement between asymptomatic and symptomatic aneurysms. A, bar graphs comparing the aneurysm wall enhancement pattern for asymptomatic and symptomatic aneurysms. Pattern 0, no wall enhancement; pattern 1, focal wall enhancement; pattern 2, circumferential wall enhancement. B, plots comparing wall enhancement index (WEI) for asymptomatic and symptomatic aneurysms. Box-and-whisker plots represent medians (lines within boxes), interquartile ranges (upper and lower ends of boxes), greatest and least values (top and bottom lines), and outliers (data points beyond top and bottom lines) for WEI.
Figure 4.
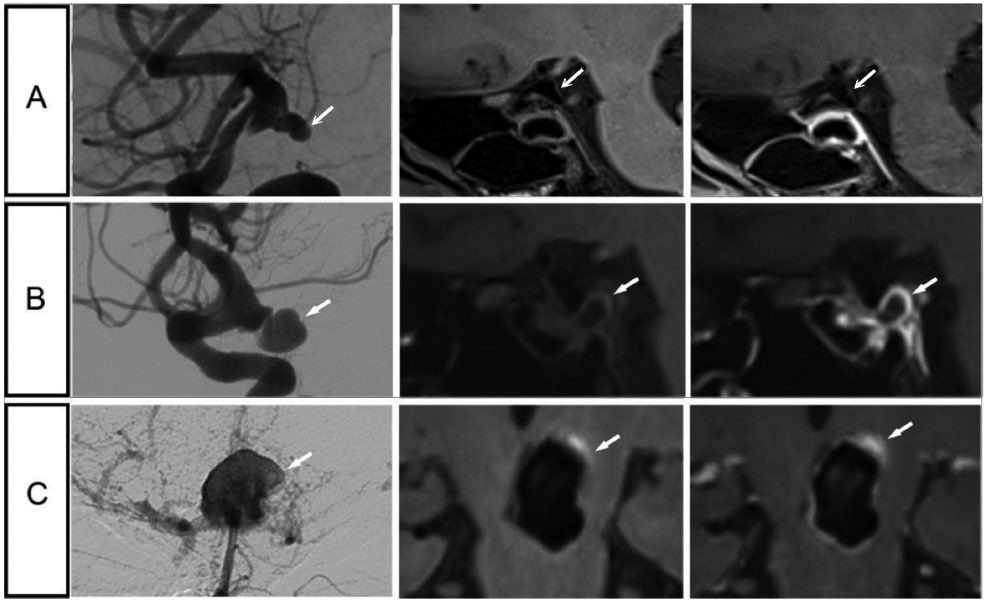
Representative cases of patients with symptomatic and asymptomatic intracranial aneurysms. A, Images of a 52-year-old man with an asymptomatic aneurysm at the right internal carotid artery terminal, measuring 3.8mm. B, Images of a 64-year-old woman with oculomotor nerve palsy and an aneurysm at the right internal carotid artery terminal, measuring 9.3mm. C, Images of a 51-year-old man with sentinel headache and an aneurysm at the top of basilar artery, measuring 16mm. Aneurysm wall hyper intensity is present prior to contrast administration, which is uncommon in the current cohort (9 aneurysms in the whole dataset). Potential explanation might be micro bleeding, which may be related to sentinel headache. For each aneurysm represented on a row, a digital subtraction angiography image is shown in the left column, a vessel wall image is in the middle column, and a post-contrast vessel wall image is shown in the right column.
The VIF value between AWE pattern and WEI was 2.91, indicating a moderate collinearity which is unlikely to substantially affect the following multivariate analysis significantly. Significantly different WEI was detected among different AWE patterns (P<0.001), and the box plots are shown in Supplemental Figure I. The WEI was significantly different between AWE pattern 0 and pattern 1 (P<0.01), and between AWE pattern 0 and pattern 2 (P<0.01). The WEI was not significantly different between AWE pattern 1 and pattern 2 (P=0.27). Aneurysm size had a moderate collinearity with parameters of AWE measurements (VIF<1.4), while aneurysm location had no collinearity with AWE pattern or WEI (VIF=1.0). Plots of aneurysmal wall enhancement measures (pattern and index) versus conventional features of aneurysms (size and location) are shown in Supplemental Figure II.
Factors associated with UIA symptoms
Multivariable regression analysis (Table 2) indicate that AWE pattern and WEI are independent risk factors associated with UIA symptoms (odds ratio=2.03 across AWE patterns [95% confidence interval (CI), 1.21 to 3.39], P=0.01; odds ratio=3.32 for WEI [95% CI, 1.51 to 7.26], P=0.003, respectively). Age and aspirin intake were independent protective factors for UIA symptoms.
Table 2.
Logistic mixed effect regression for factors associated with aneurysms in patients with symptoms (sentinel headache and oculomotor nerve palsy)
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age (year) | 0.94 | 0.91-0.96 | <0.001* | 0.94 | 0.91-0.97 | <0.001* |
| Female | 1.56 | 0.88-2.77 | 0.126 | 1.96 | 0.98-3.91 | 0.057 |
| Hypertension | 0.45 | 0.25-0.79 | 0.005* | 0.66 | 0.35-1.25 | 0.205 |
| Previous SAH | 0.50 | 0.09-2.80 | 0.431 | NA | ||
| Cigarette smoking | 0.65 | 0.32-1.31 | 0.227 | NA | ||
| Aspirin intake | 0.05 | 0.01-0.40 | 0.005* | 0.21 | 0.05-0.91 | 0.037* |
| Familial history of intracranial Aneurysm | 1.37 | 0.49-3.82 | 0.543 | NA | ||
| Posterior circulation | 1.83 | 0.99-3.39 | 0.054 | 2.01 | 0.92-4.40 | 0.079 |
| Aneurysm size (mm) | 1.10 | 1.05-1.16 | <0.001* | 0.99 | 0.94-1.05 | 0.639 |
| Thrombus | 4.51 | 1.96-10.4 | <0.001* | 0.93 | 0.34-2.57 | 0.883 |
| AWE pattern | 3.75 | 2.68-5.26 | <0.001* | 2.03 | 1.21-3.39 | 0.01* |
| WEI | 5.56 | 3.52-8.75 | <0.001* | 3.32 | 1.51-7.26 | 0.003* |
NA: not included in the model because of a p value of greater than 0.05
Statistically significant.
All binary variables were compared to no corresponding factor.
OR: odds ratio; CI: confidence intervals. SAH, subarachnoid hemorrhage; AWE, aneurysms wall enhancement; WEI, wall enhancement index.
The receiver operating characteristic curves (Supplemental Figure III) demonstrate that the AUC of age, aspirin intake, the AWE pattern, the WEI, and the AWE pattern+WEI to differentiate symptomatic from asymptomatic UIAs are 0.67, 0.58, 0.79, 0.78 and 0.91, respectively. The cutoff value of WEI to differentiate symptomatic UIAs was 0.56. The combination of AWE pattern and WEI achieved the highest AUC of 0.91, with optimized sensitivity of 95.7% and specificity of 73.4%.
Among the 93 patients with symptomatic UIAs (one aneurysm per patient), 67 patients (72%) presented with SH and 26 patients (28%) presented with ONP (as characteristics shown in Supplemental Table I). Aneurysms which were believed to cause ONP were found either with large sizes at nearby regions, or with various sizes at locations very adjacent to the optic nerve. Compared to aneurysms with SH, aneurysms with ONP were larger in size (16.0 mm vs. 6.3 mm, P<0.001). Patients with SH more frequently did not show AWE than those with ONP, whereas AWE pattern 2 was more frequently observed in patients with ONP (P= 0.02) (Supplemental Figure IV A). Aneurysms in patients with ONP had significantly higher WEI than those with SH (P<0.001) (Supplemental Figure IV B). Multivariable regression analysis indicated that aneurysm size and WEI were independent risk factors associated with specific symptoms (ONP vs. SH) (odds ratio=1.1 [95% CI, 1.0 to 1.2], P=0.03, odds ratio=18.6 [95% CI, 4.6 to 74.6], P<0.001) (Supplemental Table II).
Reproducibility
Interobserver agreement was excellent for the identification of AWE patterns (Cohen κ, 0.87; 95% CI: 0.83 to 0.92). The interobserver agreement was also excellent for the WEI measurements (intraclass correlation coefficient = 0.98; 95% CI, 0.97 to 0.98).
Discussion
In a large cohort of patients with UIAs (more than 300 UIAs and 93 symptomatic UIAs), our study demonstrates that both the qualitative and quantitative AWE characteristics were independent factors not only associated with the symptomatic status of UIAs, but also associated with specific symptoms (ONP or SH) of UIAs by using 3.0-T gadolinium-enhanced VW-MRI. Combining AWE pattern and quantitative WEI can achieve an AUC of 0.91 to differentiate symptomatic from asymptomatic UIAs. The current study is the largest study of UIAs evaluated by VW-MRI, and provides specific qualitative and quantitative aneurysm vessel wall characterization relating to particular symptom patterns.
SH has been proposed as resultant from microbleeds or “warning leaks” that may be generated by wall structural changes such as tearing in UIAs prior to rupture5, 22. ONP may be due to the rapid growth of UIAs and direct compression of the oculomotor nerve may be also generated by wall structural changes such as stretching6, 23. These symptoms were carefully evaluated and determined in clinical scenarios by multidisciplinary consultation in this study to represent the direct effects induced by the included UIAs as accurately as possible. In terms of imaging features, prior pathophysiological evidence has demonstrated the association of AWE on VW-MRI with inflammatory cells and prominent vasa vasorum in aneurysm walls corresponding with UIA instability and/or rupture risk24, 25. Although AWE has been observed in nearly all ruptured aneurysms, an increasing number of studies including this one indicated that AWE is an independent marker for aneurysm instability even prior to rupture12, 26. From a more comprehensive perspective, studying aneurysm symptomatology, AWE pattern and WEI could potentially help to better understand the underlying pathophysiological processes and their corresponding degree in pre-rupture UIAs, and therefore lead to better risk stratification and management of UIAs.
The findings of this study elucidate the association between AWE (pattern and quantitative WEI) and aneurysm vulnerability and could have the following clinical impacts. First, AWE can serve as new biomarkers for SH or ONP caused by UIAs, which is useful for patient screening. Second, AWE could provide objective evidence of instability to borderline cases, for instance, UIAs with borderline sizes and agnogenic headaches or other less specific symptoms, and thereby help decide whether to promote more aggressive treatment for these UIAs. Third, AWE could be used to identify aneurysms with higher rupture risk in patients with multiple UIAs (WEI could be particularly helpful when the patients have multiple enhanced UIAs), or which of these aneurysms is responsible for SAH in patients with aneurysmal rupture27, and therefore develop individual surgical plans. Fourth, the quantitative assessment of AWE compensates for the subjectivity of qualitative observation and could be potentially used for monitoring of response to anti-inflammatory treatment28, and the higher reproducibility and sensitivity will reduce the sample size needed in clinical trials. Finally, the combination of AWE pattern and WEI could improve the reliability of the identification of unstable aneurysms with a higher AUC than each factor in isolation.
Despite the accumulating evidence of the importance of AWE, the existing literature has employed a variety of enhancement assessment approaches without sufficient quantification. Some studies simply describe AWE as present or absent13, others defined circumferential enhancement12, 26, and a few studies graded the enhancement degree with reference to the pituitary infundibulum or normal vessel wall29, 30. To the best of our knowledge, this is the first report to use WEI to quantitatively evaluate AWE of symptomatic UIAs. Reproducibility of the measurement of WEI was demonstrated. We found that symptomatic UIAs exhibited significantly higher WEI. Similar to previous smaller-sampled qualitative studies of AWE in UIAs, this study reconfirmed that the AWE pattern is useful to distinguish symptomatic from asymptomatic UIAs12, 26. However, this study was able to account for possible confounding variables and demonstrate the independent association of AWE (pattern and quantitative WEI) with the symptomatic status of UIAs using a much larger sample. As shown in the Supplemental Figure II, the novel metrics of contrast uptake were likely related to conventional features such as aneurysm size and location, but the AWE pattern and WEI outperformed aneurysm size and location in the multivariable model and showed their independent association with symptomatic UIAs. Possible explanations include that WEI might serve as a marker of aneurysm wall inflammation, which directly links to aneurysm rupture risk; while aneurysm size and location are indirect markers of aneurysm rupture risk based on observational studies. It is known that many small aneurysms (<7mm) also rupture, 31 which could possibly be caused by strong inflammation of aneurysm walls. A moderate collinearity was found between AWE pattern and WEI, and there were no significant differences between WEI values in AWE pattern 1 and 2. This reflects that the specific AWE pattern (1 or 2) and WEI may provide complementary information of wall enhancement. In addition, this is the first study to determine the combination of qualitative and quantitative assessment of AWE (AWE pattern + WEI) had the largest AUC, relative to each independently, to identify symptomatic UIAs. We demonstrated that the use of quantitative analysis improved the ability of VW-MRI to identify symptomatic (or unstable) UIAs. Despite some overlap of AWE measurements between groups, there was relatively high AUC to differentiate the high-risk patient group from the low-risk group.
Our study also demonstrates both AWE patterns and WEI differ between UIAs in patients with SH and ONP. UIAs in patients with ONP showed more homogeneous AWE (84.6% of patients showed circumferential enhancement) and higher WEI (no overlap of IQR) than UIAs in patients with SH (no overlap of IQR). We speculate that the presumptive contained rupture of UIAs in patients with SH could be occluded by thrombosis prior to full-scale SAH, and the initial hemostatic process could also be accompanied by aneurysm wall inflammation. The two concomitant processes might be associated with more heterogeneous AWE patterns and lower WEI32. On the other hand, the rapid growth of UIAs in patients with ONP and the compression of the oculomotor nerve could be accompanied by aneurysm wall inflammation, and the usually-large diameters of such UIAs could lead to slow flow artifacts that mimic AWE33. These might be associated with more homogeneous AWE patterns and higher WEI. Such findings might reflect the complex pathophysiological scenarios of UIAs with different symptoms.
Finally, our data also revealed that age and aspirin intake were independent protective factors of the symptomatic status of the UIAs. The risk of aneurysm rupture has been shown to be higher in symptomatic UIAs but seems to decline over time, which may explain why age is an independent protective factor34. Hasan et al demonstrated that aspirin attenuated the inflammation in the UIA walls as shown by ferumoxytol-enhanced MRI35. A case report by Edjlali et al also suggested the anti-inflammatory effect of steroids on the walls of an UIA shown by gadolinium-enhanced MRI may have contributed to the resolution of ONP36. The protective role of anti-inflammatory medication also suggests the role of aneurysm wall inflammation in UIAs in patients with SH or ONP.
This study has several limitations. First, because of the cross-sectional design, we could not evaluate the causality between AWE characteristics and symptom status of UIAs in this study. Future studies with sufficient follow-up evaluations could provide further evidence to confirm our findings. Second, we did not confirm the relationship between WEI and the degree of aneurysmal wall inflammation with histopathologic confirmation. Further quantitative histopathologic analysis regarding the relationship between WEI and macrophage infiltration is needed for better understanding the pathophysiology of UIAs in patients with SH or ONP. Third, pre-contrast hyperintensity of the aneurysm wall might confound the ability to assess true contrast enhancement in that area. However, it was not common as only 9 aneurysms (2.6%) had pre-contrast hyperintensity in this cohort. In addition, WEI was calculated after normalization of the pre-contrast signal intensity. Finally, slow flow artifacts might mimic wall enhancement when using standard imaging sequences33, and the use of advanced blood suppression techniques including DANTE37 and MSDE38 may improve the confidence of characterizing AWE. We also used both 2D and 3D sequences, which may lead to heterogeneity in image analysis.
Conclusions
The current study shows both AWE patterns and quantitative WEI were independent factors associated with the symptomatic status (SH or ONP) of UIAs in a large cohort of patients. The use of quantitative assessment of AWE may improve the ability of VW-MRI to identify unstable UIAs.
Supplementary Material
Acknowledgments
Sources of Funding
This study is funded by National Natural Science Foundation of China (81871327).
Chengcheng Zhu receives grant support from National Institute of health (R00HL136883).
Haowen Xu receives grant support from National Key R&D Plan 2018 Targeted Project of China (2018YFC1311303).
Michael Levitt receives grants support from National Institute of health (R01NS105692, R01NS088072, U24NS100654, UL1TR002319, R25NS079200) and the American Heart Association (18CDA34110295).
Abbreviations
- UIA
unruptured intracranial aneurysm
- SAH
subarachnoid hemorrhage
- SH
sentinel headache
- ONP
oculomotor nerve palsy
- VW-MRI
vessel wall magnetic resonance imaging
- SI
signal intensity
- AWE
aneurysm wall enhancement
- WEI
wall enhancement index
- DSA
digital subtraction angiography
- IQR
interquartile ranges
- CI
confidence interval
- AUC
area under curves
- VIF
variance inflation factor
Footnotes
Disclosure
Michael Levitt: Unrestricted educational grants from Medtronic, Stryker and Philips Volcano. Consultant for Medtronic. Minor equity/ownership interest in Proprio, Cerebrotech, Synchron. Adviser to Metis Innovative.
References
- 1.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain mri in the general population. N Engl J Med. 2007;357:1821–1828 [DOI] [PubMed] [Google Scholar]
- 2.Gilard V, Grangeon L, Guegan-Massardier E, Sallansonnet-Froment M, Maltete D, Derrey S, Proust F. Headache changes prior to aneurysmal rupture: A symptom of unruptured aneurysm? Neurochirurgie. 2016;62:241–244 [DOI] [PubMed] [Google Scholar]
- 3.Yanaka K, Matsumaru Y, Mashiko R, Hyodo A, Sugimoto K, Nose T. Small unruptured cerebral aneurysms presenting with oculomotor nerve palsy. Neurosurgery. 2003;52:553–557; discussion 556-557 [DOI] [PubMed] [Google Scholar]
- 4.Gaberel T, Borha A, di Palma C, Emery E. Clipping versus coiling in the management of posterior communicating artery aneurysms with third nerve palsy: A systematic review and meta-analysis. World Neurosurg. 2016;87:498–506 e494 [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa D, Kudo K, Awe O, Zanaty M, Nagahama Y, Cushing C, Magnotta V, Hayakawa M, Allan L, Greenlee J, et al. Detection of microbleeds associated with sentinel headache using mri quantitative susceptibility mapping: Pilot study. J Neurosurg. 2018:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guresir E, Schuss P, Setzer M, Platz J, Seifert V, Vatter H. Posterior communicating artery aneurysm-related oculomotor nerve palsy: Influence of surgical and endovascular treatment on recovery: Single-center series and systematic review. Neurosurgery. 2011;68:1527–1533; discussion 1533-1524 [DOI] [PubMed] [Google Scholar]
- 7.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713 [DOI] [PubMed] [Google Scholar]
- 8.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–3622 [DOI] [PubMed] [Google Scholar]
- 9.Shimonaga K, Matsushige T, Ishii D, Sakamoto S, Hosogai M, Kawasumi T, Kaneko M, Ono C, Kurisu K. Clinicopathological insights from vessel wall imaging of unruptured intracranial aneurysms. Stroke. 2018;49:2516–2519 [DOI] [PubMed] [Google Scholar]
- 10.Hudson JS, Zanaty M, Nakagawa D, Kung DK, Jabbour P, Samaniego EA, Hasan D. Magnetic resonance vessel wall imaging in human intracranial aneurysms. Stroke. 2018:STROKEAHA118023701 [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Zhu C, Leng Y, Degnan AJ, Lu J. Intracranial aneurysm wall enhancement associated with aneurysm rupture: A systematic review and meta-analysis. Acad Radiol. 2019;26:664–673 [DOI] [PubMed] [Google Scholar]
- 12.Edjlali M, Guedon A, Ben Hassen W, Boulouis G, Benzakoun J, Rodriguez-Regent C, Trystram D, Nataf F, Meder JF, Turski P, et al. Circumferential thick enhancement at vessel wall mri has high specificity for intracranial aneurysm instability. Radiology. 2018;289:181–187 [DOI] [PubMed] [Google Scholar]
- 13.Vergouwen MDI, Backes D, van der Schaaf IC, Hendrikse J, Kleinloog R, Algra A, Rinkel GJE. Gadolinium enhancement of the aneurysm wall in unruptured intracranial aneurysms is associated with an increased risk of aneurysm instability: A follow-up study. AJNR Am J Neuroradiol. 2019;40:1112–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushige T, Shimonaga K, Ishii D, Sakamoto S, Hosogai M, Hashimoto Y, Kaneko M, Ono C, Mizoue T, Kurisu K. Vessel wall imaging of evolving unruptured intracranial aneurysms. Stroke. 2019;50:1891–1894 [DOI] [PubMed] [Google Scholar]
- 15.Omodaka S, Endo H, Niizuma K, Fujimura M, Inoue T, Sato K, Sugiyama SI, Tominaga T. Quantitative assessment of circumferential enhancement along the wall of cerebral aneurysms using mr imaging. AJNR Am J Neuroradiol. 2016;37:1262–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander MD, de Havenon A, Kim SE, Parker DL, McNally JS. Assessment of quantitative methods for enhancement measurement on vessel wall magnetic resonance imaging evaluation of intracranial atherosclerosis. Neuroradiology. 2019;61:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cianfoni A, Pravata E, De Blasi R, Tschuor CS, Bonaldi G. Clinical presentation of cerebral aneurysms. Eur J Radiol. 2013;82:1618–1622 [DOI] [PubMed] [Google Scholar]
- 18.Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257–268 [DOI] [PubMed] [Google Scholar]
- 19.Qi H, Liu X, Liu P, Yuan W, Liu A, Jiang Y, Li Y, Sun J, Chen H. Complementary roles of dynamic contrast-enhanced mr imaging and postcontrast vessel wall imaging in detecting high-risk intracranial aneurysms. AJNR Am J Neuroradiol. 2019;40:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Quality & Quantity. 2007;41:673–690 [Google Scholar]
- 22.Ostergaard JR. Warning leak in subarachnoid haemorrhage. BMJ. 1990;301:190–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjorkman J, Frosen J, Tahtinen O, Huttunen T, Huttunen J, Kurki MI, von Und Zu Fraunberg M, Koivisto T, Manninen H, Jaaskelainen JE, et al. Aneurysm size is the strongest risk factor for intracranial aneurysm growth in the eastern finnish population. Neurosurgery. 2019;84:1098–1103 [DOI] [PubMed] [Google Scholar]
- 24.Quan K, Song J, Yang Z, Wang D, An Q, Huang L, Liu P, Li P, Tian Y, Zhou L, et al. Validation of wall enhancement as a new imaging biomarker of unruptured cerebral aneurysm. Stroke. 2019;50:1570–1573 [DOI] [PubMed] [Google Scholar]
- 25.Larsen N, von der Brelie C, Trick D, Riedel CH, Lindner T, Madjidyar J, Jansen O, Synowitz M, Fluh C. Vessel wall enhancement in unruptured intracranial aneurysms: An indicator for higher risk of rupture? High-resolution mr imaging and correlated histologic findings. AJNR Am J Neuroradiol. 2018;39:1617–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Q, Guan S, Liu C, Wang K, Cheng J. Clinical significance of circumferential aneurysmal wall enhancement in symptomatic patients with unruptured intracranial aneurysms: A high-resolution mri study. Clin Neuroradiol. 2018;28:509–514 [DOI] [PubMed] [Google Scholar]
- 27.Omodaka S, Endo H, Niizuma K, Fujimura M, Endo T, Sato K, Sugiyama SI, Inoue T, Tominaga T. Circumferential wall enhancement on magnetic resonance imaging is useful to identify rupture site in patients with multiple cerebral aneurysms. Neurosurgery. 2018;82:638–644 [DOI] [PubMed] [Google Scholar]
- 28.Li W, Zhang Y, Tian Z, Zhu W, Liu J, Zhang Y, Yang X, Tian DC. Statin treatment for unruptured intracranial aneurysms study: A study protocol for a double-blind, placebo-controlled trial. Stroke Vasc Neurol. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Zhang Z, Zhu C, Feng J, Liu P, Kong Q, Zhang X, Zhang Q, Jin H, Ge H, et al. Wall enhancement of intracranial saccular and fusiform aneurysms may differ in intensity and extension: A pilot study using 7-t high-resolution black-blood mri. Eur Radiol. 2020;30:301–307 [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Wang X, Degnan AJ, Shi Z, Tian B, Liu Q, Hess C, Saloner D, Lu J. Wall enhancement of intracranial unruptured aneurysm is associated with increased rupture risk and traditional risk factors. Eur Radiol. 2018;28:5019–5026 [DOI] [PubMed] [Google Scholar]
- 31.Rutledge C, Jonzzon S, Winkler EA, Raper D, Lawton MT, Abla AA. Small aneurysms with low phases scores account for most subarachnoid hemorrhage cases. World Neurosurg. 2020 [DOI] [PubMed] [Google Scholar]
- 32.Meng H, Tutino VM, Xiang J, Siddiqui A. High wss or low wss? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35:1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalsoum E, Chabernaud Negrier A, Tuilier T, Benaissa A, Blanc R, Gallas S, Lefaucheur JP, Gaston A, Lopes R, Brugieres P, et al. Blood flow mimicking aneurysmal wall enhancement: A diagnostic pitfall of vessel wall mri using the postcontrast 3d turbo spin-echo mr imaging sequence. AJNR Am J Neuroradiol. 2018;39:1065–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. Stroke. 2013;44:2414–2421 [DOI] [PubMed] [Google Scholar]
- 35.Hasan DM, Chalouhi N, Jabbour P, Magnotta VA, Kung DK, Young WL. Imaging aspirin effect on macrophages in the wall of human cerebral aneurysms using ferumoxytol-enhanced mri: Preliminary results. J Neuroradiol. 2013;40:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edjlali M, Boulouis G, Derraz I, Ben Hassen W, Rodriguez-Regent C, Trystram D, Meder JF, Oppenheim C, Naggara O. Intracranial aneurysm wall enhancement decreases under anti-inflammatory treatment. Neurology. 2018;91:804–805 [DOI] [PubMed] [Google Scholar]
- 37.Jia S, Zhang L, Ren L, Qi Y, Ly J, Zhang N, Li Y, Liu X, Zheng H, Liang D, et al. Joint intracranial and carotid vessel wall imaging in 5 minutes using compressed sensing accelerated dante-space. Eur Radiol. 2020;30:119–127 [DOI] [PubMed] [Google Scholar]
- 38.Zhu C, Graves MJ, Yuan J, Sadat U, Gillard JH, Patterson AJ. Optimization of improved motion-sensitized driven-equilibrium (imsde) blood suppression for carotid artery wall imaging. J Cardiovasc Magn Reson. 2014;16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.