Abstract
Calcification, fibrosis and chronic inflammation are the predominant features of calcific aortic valve disease, a life-threatening condition. Drugs that induce serotonin (5-HT) are known to damage valves, and activated platelets, which carry peripheral serotonin, are known to promote calcific aortic valve stenosis. However, the role of 5-HT in valve leaflet pathology is not known. We tested whether serotonin mediates inflammation-induced matrix mineralization in valve cells. Realtime RT-PCR analysis showed that murine aortic valve interstitial cells (VICs) expressed both serotonin receptor types 2A and 2B (Htr2a and Htr2b). Although Htr2a expression was greater at baseline, Htr2b expression was induced several-fold more than Htr2a in response to the pro-calcific TNF-α treatment. 5-HT also augmented TNF-α-induced osteoblastic differentiation and matrix mineralization of VIC, but 5-HT alone had no effects. Inhibition of serotonin receptor type 2B, using specific inhibitors or lentiviral knockdown in VIC, attenuated 5-HT effects on TNF-α-induced osteoblastic differentiation and mineralization. 5-HT treatment also augmented TNF-α-induced matrix metalloproteinase-3 expression, which was also attenuated by Htr2b knockdown. Htr2b expression in aortic roots and serum levels of peripheral 5-HT were also greater in the hyperlipidemic Apoe−/− mice than in control normolipemic mice. These findings suggest a new role for serotonin signaling in inflammation–induced calcific valvulopathy.
Keywords: Inflammation, valve, serotonin, calcification, HTR2B
INTRODUCTION
Calcific aortic valve disease (CAVD), once considered a result of simple “wear-and-tear,” is now recognized as a regulated process (Zipes, 2019), driven, in part, by inflammatory, metabolic, and/or mechanical factors (Al-Aly et al., 2007; Aikawa et al., 2007; Lai et al., 2012; Li et al., 2015; Lim et al., 2016b; Tintut et al., 2000). Its two prominent pathological features, calcification and fibrosis, also cause its prominent pathophysiological features, stiffening and narrowing, which restrict flow and reduce cardiac output leading to heart failure and death.
Several lines of evidence link serotonin to valve structure and integrity. Premature valve disease occurs, even in young healthy individuals, in response to receptor-specific serotonergic agents. CAVD risk is also increased by selective serotonin reuptake inhibitor (SSRI) agents used for depression (Lin et al., 2016). Selective serotonin receptor type 2B (HTR2B) agonists, such as the appetite suppressants, benfluorex and fenfluramine, which are no longer used, and pergolide (Rothman et al., 2000), a treatment for Parkinson’s Disease, introduce a several-fold increase in risk of aortic and mitral valvulopathy, in a dose- and duration-dependent manner in patients (Andrejak et al., 2014; Dahl et al., 2008; Frachon et al., 2010; Tribouilloy et al., 2012; Van Camp et al., 2003; Van Camp et al., 2004). Others who have endogenously high serotonin (5-hydroxytryptamine; 5-HT) levels, as occurs in carcinoid syndrome, also have a high risk of similar valve disease (Robiolio et al., 1995; Sandmann et al., 2009). Notably, the valve disease in these reports is described as regurgitant, which may invoke an image of floppy and prolapsed leaflets. However, it is a form of “restrictive” valve regurgitation (Zanettini et al., 2007) in which fibrosis causes thickening, retraction, and stiffening resulting in leakage due to incomplete leaflet coaptation (Ferrans and Roberts, 1976; Le Ven et al., 2011). Thus, while the physiology involves flow regurgitation, the pathology more closely resembles stenotic than typical regurgitant disease. Structural and mechanical changes in the valves of these patients include fibrosis leading to thickening and, in some cases, dense nodules (Ferrans and Roberts, 1976; Le Ven et al., 2011), setting the stage for CAVD when superimposed on inflammation.
In cardiovascular tissues, serotonin type-2 receptors are the most highly expressed type, and of those, the subtype, HTR2A, is the one most highly expressed in valve interstitial cells (VIC) (Xu et al., 2002). In valve myofibroblasts, HTR2B antagonism has been shown to inhibit transforming growth factor-beta (TGF-β)-induced myofibroblastic differentiation of porcine aortic VIC (Hutcheson et al., 2012). As evidence of the importance of serotonin receptor type 2 in fibrosis, in a mouse model of bleomycin-induced pulmonary fibrosis, Htr2a and Htr2b mRNA expression increased significantly, and antagonists to the HTR2B reduced fibrotic changes including collagen formation and myofibroblastic differentiation (Fabre et al., 2008).
Although a small percentage of 5-HT is synthesized in the brain by tryptophan hydroxylase 2 (Gershon, 1999), it generally does not enter the peripheral circulation due to the blood-brain barrier. The majority (95%) of peripheral 5-HT is synthesized by tryptophan hydroxylase 1 in the gastrointestinal tract and released into plasma, where most is taken up by platelets and stored in dense granule vesicles until they are released upon platelet activation (Gershon, 1999). Recently Bouchareb and colleagues demonstrated the presence of platelet aggregates on diseased human and mouse valves by immune-gold scanning electron microscopy and provided evidence that platelet granule release contributes to calcific aortic valve stenosis in an inflammatory mouse model (Bouchareb et al., 2019). One process that activates platelet release of serotonin-containing granules is disturbed flow (Jackson et al., 2009), and, as the valve area progressively narrows, flow disturbance and Reynold’s number increase to the point of turbulence. Thus, valve leaflets are expected to be bathed in activated platelets releasing 5-HT. In this report, we investigated the effects of 5-HT signaling on inflammation-induced calcific valvulopathy using murine primary VIC, which we have previously shown to have robust matrix mineralization in response to pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) (Li et al., 2015).
MATERIALS AND METHODS
Human TNF-α was purchased from R&D Systems (Minneapolis, MN), rat-tail type I collagen was from Corning (Corning, NY), 5-HT was from Tocris (Minneapolis, MN), and ketanserin tartrate and SB-204741 were from Abcam (Cambridge, MA). Antibodies specific to mouse HTR2A and HTR2B were purchased from Biobryt (San Francisco, CA).
VIC isolation and characterization
Experimental protocols were reviewed and approved by the UCLA Institutional Animal Care and Use Committee. Aortic valve leaflets were carefully excised from C57BL/6 mice and cultured via an explant technique, as described previously (Lim et al., 2016a). Cells were grown in DMEM supplemented with 20% FBS, penicillin, and streptomycin. For osteoblastic differentiation, cells were cultured in alpha-MEM supplemented with 10% FBS (charcoal stripped FBS), 5 mmol/L beta-glycerophosphate and 50 μg/ml of ascorbic acid, and indicated agents. Where indicated, VIC were pretreated with the indicated antagonists 30 min prior to addition of TNF-α (25 ng/mL) and/or 5-HT (10 μM).
Lentiviral transduction
VIC were transduced per the manufacturer’s protocol with lentiviral particles carrying constructs (scrambled shRNA, Smad6 shRNA, Htr2a shRNA, or Htr2B shRNA, all from Santa Cruz Biotechnology, Dallas, TX). Briefly, cells were seeded at 120K/plate (to reach ~ 50% confluency on day 2) in 60-mm culture dishes in growth medium (DMEM containing 20% FBS). On day 2, the medium was replaced with 5 mL of growth medium supplemented with polybrene (5 μg/mL, Santa Cruz Biotechnology), and lentiviral particles (~2.5 x 105 units/dish in 50 μL) were added drop-wise. On day 3, medium containing the viral particles were removed, the growth medium (without polybrene) was added, and the cells were incubated overnight. On day 4, the cells were trypsinized and re-seeded in the growth medium supplemented with puromycin (2 μg/ml; Santa Cruz Biotechnology) for selection. The cells were used 3-5 passages after the transduction. The knockdown of target genes was confirmed by real-time quantitative RT-qPCR (Supplementary Fig.).
RNA isolation and real-time quantitative RT-qPCR
Total RNA was isolated using TRIzol reagent. Real-time RT-qPCR (One-Step RT-qPCR SuperMix Kit, BioChain Inc, Newark, CA) was performed using mouse gene specific primers and MX3005P (Stratagene, La Jolla, CA). As an internal control, values were normalized to the expression of Actb, the gene for beta-actin. The sequences of the primers are shown in the Table.
Table 1.
Primer sequences for real-time PCR analysis
| Gene Name | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| Htr2a | TTC CTT GTC ATG CCC GTG TC | AGT TGA AGC GGC TAT GGT GA |
| Htr2b | AGA AAA GGC TGC AGT ACG CT | GTC CAG GGA AAT GGC ACA GA |
| Htr2c | GCT GGA CCG GTA TGT AGC AA | GCT TTC GTC CCT CAG TCC AA |
| Bmp2 | TGT CCA ATC CGT GAG AAC | TGC CAT CAT CAC TTC CTG |
| Mmp3 | TTA AAG ACA GGC ACT TTT GGC | CCC TCG TAT AGC CCA GAA CT |
| Actb (beta-actin) | GGC TGT ATT CCC CTC CAT CG | CCA GTT GGT AAC AAT GCC ATG T |
Matrix calcification
After the indicated periods of culture, cells were incubated overnight in 0.6 N HCl, and matrix calcium levels (normalized to total protein) were analyzed by the o-cresolphthalein complexone method (Teco Diagnostics, Anaheim, CA) in quintuplicate.
ELISA
Following the manufacturer’s protocol, serum levels of 5-HT were determined by ELISA (Eagle Biosciences, Amherst, NH) from Apoe−/− mice (5-months old) fed a high-fat diet (TD.94059, Envigo, Madison, WI) for 3 months and C57BL/6 mice on the standard chow diet. Apoe−/− mice (C57BL/6-congenic Apoetm1Unc strain), purchased from the Jackson Laboratory, were produced by backcrossing at least 10 generations to C57BL/6 inbred mice. C57BL/6 are the recommended controls by the Jackson Laboratory.
Histology
Mouse hearts from C57BL/6 and Apoe−/− mice were isolated, embedded in optimal cutting temperature (OCT) compound, and 10-μm cryosections were obtained. Immunohistochemistry using antibodies specific to serotonin receptors (HTR2A and HTR2B) and hematoxylin was performed on the aortic root sections and imaged by light microscopy (magnification, 10X).
Western blotting
VIC were plated in the growth media for 3 days. The media was switched to serum free media overnight prior to treatment with 5-HT in serum-free media for 5, 8 or 10 min. The protein extracts (equal concentration) were run on SDS PAGE and the blots probed with antibodies (Cell Signaling Technology, Beverly, MA) specific to phospho-p44/42 MAPK and total p44/42 MAPK, a loading control.
Statistical analysis
Experiments were performed with ≥ 3 replicates in each experimental condition, and data were expressed as mean ± SD. Results were compared using a two-tailed t-test or one-way analysis of variance, followed by Tukey post-hoc analysis for comparisons across more than two groups. p < 0.05 was considered statistically significant.
RESULTS
Expression of 5-HT receptor subtypes in valve interstitial cells at baseline and in response to TNF-α
We assessed the expression of type 2 serotonin receptors in murine aortic VICs, by isolating RNA and performing realtime RT-PCR. Results showed that, at baseline, Htr2a expression dominated over that of Htr2b (Fig. 1A). Htr2c expression was not detected (data not shown). A similar pattern of expression was found in vascular smooth muscle cell cultures (Fig. 1A). Treatment of VIC with the inflammatory cytokine, TNF-α induced expression of both receptor subtypes, but interestingly, the induction in response to TNF-α was over 20-fold greater in Htr2b than in Htr2a (Fig. 1B).
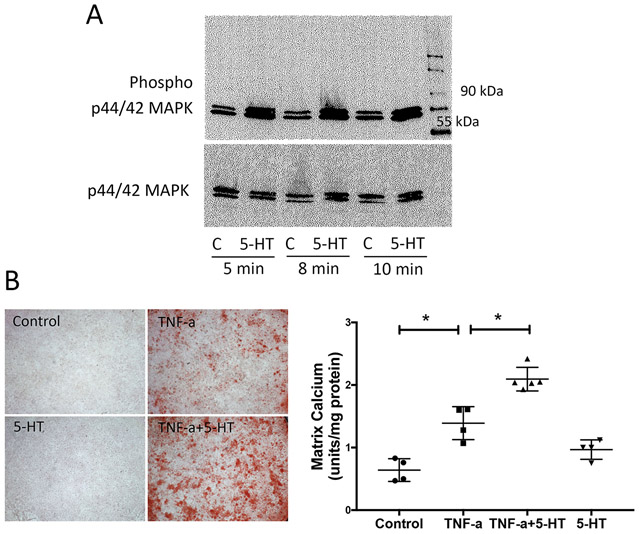
Figure 1. Expression of 5-HT receptor subtypes in VIC at baseline and in response to TNF-α.
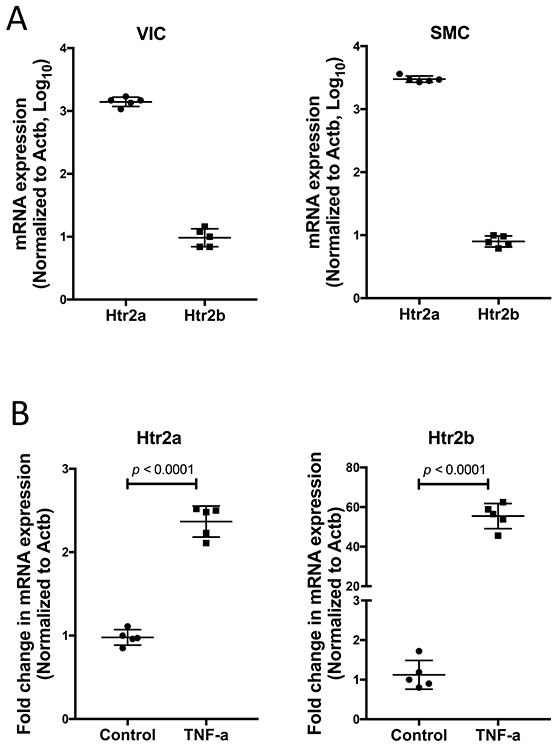
(A) Realtime RT-qPCR analyses of VIC and vascular smooth muscle cells (SMC) for baseline expression of type-2 serotonin receptors (presented in log10 scale). (B) Realtime RT-qPCR analyses for Htr2a and Htr2b expression in VIC treated with TNF-α (25 ng/mL) for 11 days. *p < 0.0001.
Effects of 5-HT on TNF-α induction of matrix mineralization in VICs
We next determined the effects of 5-HT on VICs. Results showed that 5-HT at 10 μM activated the phosphorylation of ERK (Fig. 2A), while 5-HT treatment alone (1, 5 or 10 μM) did not affect matrix mineralization (data not shown). We next tested the effects on inflammation-induced matrix mineralization of VICs, using the pro-inflammatory cytokine, TNF-α that we found to induce matrix mineralization of VIC (Lim et al., 2016a). VICs were treated with 5-HT and/or TNF-α, and matrix calcification was assessed qualitatively by alizarin red staining and quantitatively by o-cresolphthalein complexone. Results showed that TNF-α-induced matrix mineralization of VIC was augmented by 5-HT (Fig. 2B).
Figure 2. Effects of 5-HT on TNF-α induction of matrix mineralization in VIC.
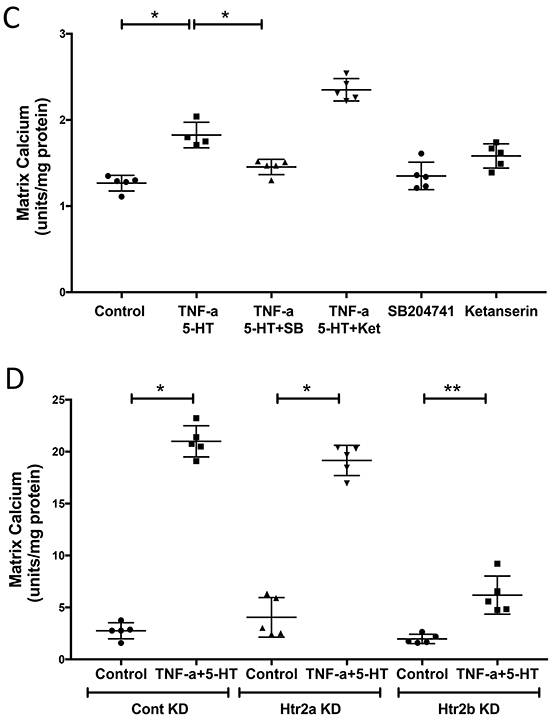
(A) 5-HT effects on phosphorylation of p44/42 MAPK, assessed by Western blot, in VIC treated with vehicle (control) or 5-HT for 5, 8 or 10 min. , and p44/42 MAPK was used as a loading control. (B, left) Qualitative analysis of matrix mineralization, assessed by alizarin red staining, in VIC treated with vehicle (control), TNF-α and/or 5-HT, as indicated, for 11 days. Magnification 2X. (B, right) Quantitative analysis of matrix mineralization, assessed by o-cresolphthalein complexone, in VIC treated with control, TNF-α (25 ng/mL) and/or 5-HT (10 μM), as indicated, for 14 days. *p < 0.001. (C) Quantitative analysis of matrix mineralization, in VIC treated with vehicle, TNF-α+5-HT, SB (25 μM; SB204741), or ketanserin (1 μM), as indicated. *p < 0.0001, **p = 0.003. (D) Quantitative analysis of matrix mineralization, in control VIC and in Htr2a or Htr2b knocked down VIC treated with vehicle or TNF-α+5-HT, as indicated. *p < 0.0001.
Effect of HTR2B inhibition on TNF-α-induced matrix calcification in VIC
We further assessed which receptor sub-type (HTR2A and/or HTR2B) mediates the effects of 5-HT on mineralization. We used ketanserin tartrate (HTR2A/2C antagonist) as a selective HTR2A inhibitor since these cells lack Htr2c expression and SB-204741, a selective HTR2B inhibitor. Pre-treatment of VIC with ketanserin tartrate or SB-204741 showed that HTR2B inhibition, but not HTR2A, attenuated the effects of 5-HT on TNF-α-induced matrix mineralization (Fig. 2C). We next knocked down the expression of these receptors in VIC using lentiviral transduction with Htr2a shRNA or Htr2b shRNA. Results confirmed that matrix mineralization was attenuated in Htr2b-knockdown VIC but not in Htr2a-knockdown VIC (Fig. 2D).
We assessed the effects of 5-HT and its receptors on BMP-2, a master regulator of osteoblastic differentiation. Treatment with 5-HT augmented the induction of Bmp2 expression by TNF-α (Fig. 3A). This effect of 5-HT was attenuated in Htr2b-knockdown VIC but not in Htr2a-knockdown VIC (Fig. 3B). Altogether these findings suggest that HTR2B is the primary receptor mediating the effects of 5-HT on matrix mineralization.
Figure 3. Effects of 5-HT on TNF-α-induced Bmp2 expression in VIC.
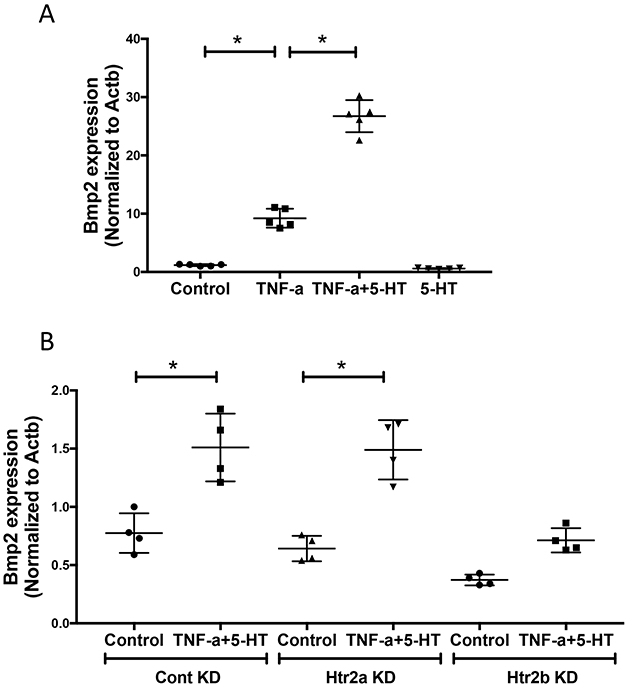
(A) Realtime RT-qPCR analyses for Bmp2 expression in VIC treated with vehicle, TNF-α (25 ng/mL) and/or 5-HT (10 μM) for 8 days. *p < 0.0001. (B) Realtime RT-qPCR analyses for Bmp2 expression in control VIC and in Htr2a-knockdown VIC, or in Htr2b-knockdown VIC treated with vehicle or TNF-α+5-HT, as indicated for 9 days. *p = 0.0003.
We tested whether 5-HT also affects other TNF-α-induced genes, such as matrix metalloproteinases (MMPs). Co-treatment of VIC with 5-HT and/or TNF-α showed that TNF-α-induced Mmp3 expression was also augmented by co-treatment with 5-HT in VIC (Fig. 4A). Inhibition of HTR2A as well as HTR2B attenuated these effects of 5-HT on Mmp3 expression (Fig. 4B), suggesting that both receptor subtypes may play a role.
Figure 4. Effects of 5-HT on TNF-α-induced Mmp3 expression in VIC.
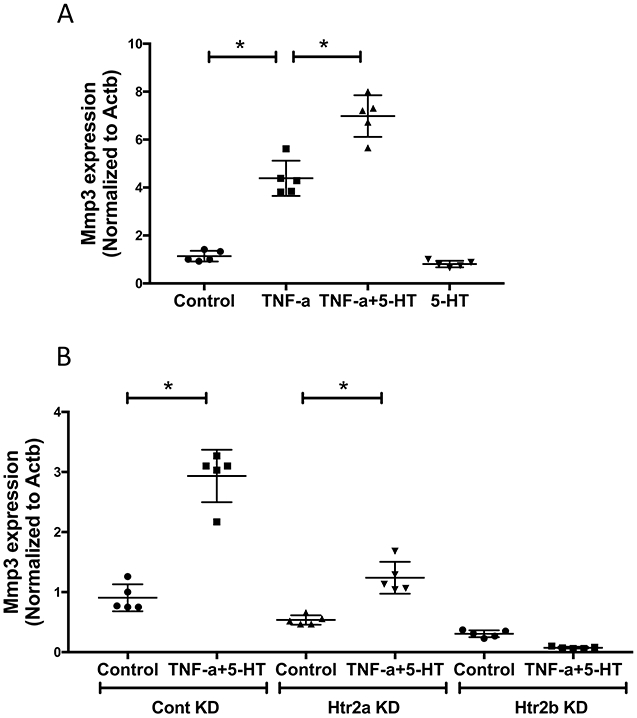
(A) Realtime RT-qPCR analyses for Mmp3 expression in VIC treated with vehicle, TNF-α (25 ng/mL) and/or 5-HT (10 μM) for 4 days. *p < 0.0001. (B) Realtime RT-qPCR analyses for Mmp3 expression in control VIC and in Htr2a-knockdown VIC or in Htr2b-knockdown VIC, treated with vehicle, TNF-α+5-HT, as indicated. *p = 0.0001.
Peripheral 5-HT levels and receptor subtype expression in hyperlipidemic mice
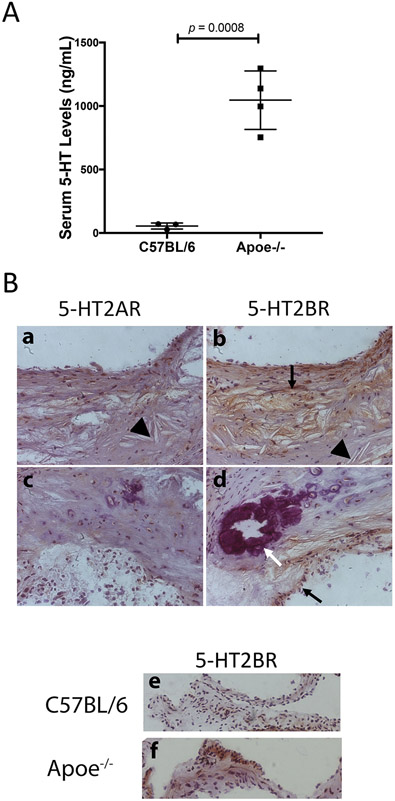
To determine the role of 5-HT in an inflammatory CAVD mouse model, we determined 5-HT levels in serum from wild type C57BL/6 (n = 3) and hyperlipidemic Apoe−/− mice (n = 4). As shown in Fig. 5A, hyperlipidemic mice had markedly higher serum 5-HT levels than the normolipemic mice. Since type 2 serotonin receptors are known to be expressed with functional signaling in sheep valve cells (Xu et al., 2002), we tested their expression in mouse aortic roots. Immunohistochemical staining with specific antibodies to serotonin receptors showed that both HTR2A and HTR2B were detected in the aortic roots of Apoe−/− mice. However, HTR2B immunoreactivity was more prominent in the areas of calcium and cholesterol clefts (Fig. 5B a-d). It was also greater in aortic valve leaflets of Apoe−/− mice compared with those of wild type mice (Fig. 5B e-f).
Figure 5. Serum 5-HT levels and expression of HTR2A and HTR2B in mouse atherosclerotic lesions.
(A) Peripheral 5-HT levels, assessed by ELISA, in WT (C57BL/6) and hyperlipidemic (Apoe−/−) mice. (B) Immunohistochemical analysis of HTR2A (a and c) and HTR2B (b, d, e and f) in mouse aortic roots (counterstained with hematoxylin). Calcium mineral is stained purple (white arrow), serotonin receptor immunopositivity is indicated in brown (black arrows), and cholesterol clefts are identified by arrowheads.
DISCUSSION
It has been shown that an inflammatory cytokine, TNF-α, is associated with greater progression of aortic valve stenosis (Swierszcz et al., 2011). It is also a potent osteoinductive factor in both valvular and vascular cells (Al-Aly et al., 2007; Buendia et al., 2015; Cheng et al., 2010; Cola et al., 2004; Ikeda et al., 2012; Kaden et al., 2005; Lai et al., 2012; Li et al., 2015; Tintut et al., 2000; Yu et al., 2011; Zhao et al., 2012) and induces Mmp3 (Shindo et al., 2014). In the present study, using VIC, which contribute to the mechanical characteristics vital for maintaining the unique dynamic behavior of the valve leaflets (Taylor et al., 2003), we demonstrated that VIC express mRNA for serotonin receptor subtypes, Htr2a and Htr2b, but not Htr2c. Interestingly, although, at baseline, Htr2b expression was lower than that of Htr2a, it was upregulated highly in response to TNF-α, than Htr2a. Results also showed that, while 5-HT alone did not affect matrix mineralization and expression of Bmp2 in VIC, it markedly increased pro-calcific effects of TNF-α. However, if the matrix stiffens, we cannot exclude the possibility that cytoskeletal and metabolic effects could alter expression of the normalization control gene used in our study or any other reference genes, such as glyceraldehyde 3-phosphate dehydrogenase. We also found that, in lentiviral knockdown experiments using shRNA, Htr2b, but not Htr2a or control, attenuated the effects of 5-HT. Although the protein and mRNA levels may not necessarily correlate, based on the findings of a positive effect limited to one gene and receptor subtype-specific inhibitors, the effects of 5-HT appear to be mainly through the receptor type 2B (HTR2B).
5-HT signaling also affects skeletal tissue. Patients receiving SSRIs for depression have, not only increased risk of valve disease, but also lower bone mass (Feuer et al., 2015; Rauma et al., 2016; Richter et al., 2014). The most commonly prescribed SSRI, fluoxetine, was also found to inhibit osteoblast differentiation and mineralization as well as to inhibit fracture healing in mice (Bradaschia-Correa et al., 2017). Using inducible osteoblastic/osteocytic cells, Kellermann’s group has shown that HTR2B was upregulated prior to matrix mineralization (Locker et al., 2006), and Htr2b knockdown prevented post-translational modification of the differentiation marker, alkaline phosphatase (Baudry et al., 2010). Consistent with these findings, HTR2B-deficient mice have lower bone density due to reduced bone formation (Collet et al., 2008).
Platelet activation and dysfunction are prevalent in aortic valve disease (Prohaska et al., 2008), and release of their granule contents, including peripheral 5-HT, is evident in the plasma levels of patients with aortic stenosis (Rouzaud-Laborde et al., 2015). Our findings also show that mice with atherosclerosis (Apoe−/− mice on a high-fat diet) had higher serum 5-HT levels than the control mice (C57BL/6 mice on a chow diet). Although unlikely, we cannot rule out the possibility that lack of APOE may affect serum 5-HT levels independent of hyperlipidemia. Apoe−/− mice also expressed higher levels of HTR2B than HTR2A, and their expression of HTR2B co-localized with calcium deposits and cholesterol clefts in the aortic valve leaflets.
Matrix metalloproteinase expression is upregulated in response to inflammation. In Apoe−/− mice with diet-induced CAVD, single-photon emission computed tomographic (SPECT) imaging showed increased levels of activated MMPs in the valve ring (Jung et al., 2015). Mmp3 (stromelysin-1) expression, in particular, is increased in a dose and time-dependent manner in response to inflammatory factors (Galis et al., 1994; Henney et al., 1991; Uzui et al., 2002). MMPs are also known to be associated with calcification in the vasculature (Freise et al., 2016; Zhao et al., 2016). MMP-3 is linked to clinical coronary (Pollanen et al., 2002) and aortic arch calcification (Lee et al., 2010). In patients with calcific mitral valve disease, the degree of calcification correlates directly with the level of MMP-3 (Aloui et al., 2018). In the present study, we found that 5-HT augmented TNF-α induction of Mmp3 expression. Interestingly, this effect appears to be mediated by Htr2a as well as by Htr2b.
In summary, our findings suggest that inflammation induces the serotonin receptor subtype HTR2B and that its activation in the context of inflammation has a novel role in calcific aortic valvulopathy.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Jinxiu Lu for technical assistance. This work was supported by funding from the National Institutes of Health (AG061586, HL137647).
Footnotes
CONFLICT OF INTEREST
None
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, and Weissleder R. 2007. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 116:2841–2850. [DOI] [PubMed] [Google Scholar]
- Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, and Towler DA. 2007. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 27:2589–2596. [DOI] [PubMed] [Google Scholar]
- Aloui S, Zidi W, Ouali S, Guizani I, Hadj-Taieb S, Mourali MS, Feki M, and Allal-Elasmi M. 2018. Association of matrix metalloproteinase 3 and endogenous inhibitors with inflammatory markers in mitral valve disease and calcification. Mol Biol Rep. 45:2135–2143. [DOI] [PubMed] [Google Scholar]
- Andrejak M, Szymanski C, Marechaux S, Arnalsteen E, Gras V, Remadi JP, and Tribouilloy C. 2014. Valvular heart disease associated with long-term treatment by methysergide: a case report. Therapie. 69:255–257. [DOI] [PubMed] [Google Scholar]
- Baudry A, Bitard J, Mouillet-Richard S, Locker M, Poliard A, Launay JM, and Kellermann O. 2010. Serotonergic 5-HT(2B) receptor controls tissue-nonspecific alkaline phosphatase activity in osteoblasts via eicosanoids and phosphatidylinositol-specific phospholipase C. J Biol Chem. 285:26066–26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchareb R, Boulanger MC, Tastet L, Mkannez G, Nsaibia MJ, Hadji F, Dahou A, Messadeq Y, Arsenault BJ, Pibarot P, Bosse Y, Marette A, and Mathieu P. 2019. Activated platelets promote an osteogenic programme and the progression of calcific aortic valve stenosis. Eur Heart J. 40:1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradaschia-Correa V, Josephson AM, Mehta D, Mizrahi M, Neibart SS, Liu C, Kennedy OD, Castillo AB, Egol KA, and Leucht P. 2017. The Selective Serotonin Reuptake Inhibitor Fluoxetine Directly Inhibits Osteoblast Differentiation and Mineralization During Fracture Healing in Mice. J Bone Miner Res. 32:821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia P, Montes de Oca A, Madueno JA, Merino A, Martin-Malo A, Aljama P, Ramirez R, Rodriguez M, and Carracedo J. 2015. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 29:173–181. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, and Towler DA. 2010. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 107:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cola C, Almeida M, Li D, Romeo F, and Mehta JL. 2004. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun. 320:424–427. [DOI] [PubMed] [Google Scholar]
- Collet C, Schiltz C, Geoffroy V, Maroteaux L, Launay JM, and de Vernejoul MC. 2008. The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J. 22:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl CF, Allen MR, Urie PM, and Hopkins PN. 2008. Valvular regurgitation and surgery associated with fenfluramine use: an analysis of 5743 individuals. BMC Med. 6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre A, Marchal-Somme J, Marchand-Adam S, Quesnel C, Borie R, Dehoux M, Ruffie C, Callebert J, Launay JM, Henin D, Soler P, and Crestani B. 2008. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur Respir J. 32:426–436. [DOI] [PubMed] [Google Scholar]
- Ferrans VJ, and Roberts WC. 1976. The carcinoid endocardial plaque; an ultrastructural study. Hum Pathol. 7:387–409. [DOI] [PubMed] [Google Scholar]
- Feuer AJ, Demmer RT, Thai A, and Vogiatzi MG. 2015. Use of selective serotonin reuptake inhibitors and bone mass in adolescents: An NHANES study. Bone. 78:28–33. [DOI] [PubMed] [Google Scholar]
- Frachon I, Etienne Y, Jobic Y, Le Gal G, Humbert M, and Leroyer C. 2010. Benfluorex and unexplained valvular heart disease: a case-control study. PLoS One. 5:e10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freise C, Kretzschmar N, and Querfeld U. 2016. Wnt signaling contributes to vascular calcification by induction of matrix metalloproteinases. BMC Cardiovasc Disord. 16:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, and Libby P. 1994. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 94:2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD 1999. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 13 Suppl 2:15–30. [PubMed] [Google Scholar]
- Henney AM, Wakeley PR, Davies MJ, Foster K, Hembry R, Murphy G, and Humphries S. 1991. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 88:8154–8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson JD, Ryzhova LM, Setola V, and Merryman WD. 2012. 5-HT(2B) antagonism arrests non-canonical TGF-beta1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol. 53:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Souma Y, Akakabe Y, Kitamura Y, Matsuo K, Shimoda Y, Ueyama T, Matoba S, Yamada H, Okigaki M, and Matsubara H. 2012. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem Biophys Res Commun. 425:39–44. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Nesbitt WS, and Westein E. 2009. Dynamics of platelet thrombus formation. J Thromb Haemost. 7 Suppl 1:17–20. [DOI] [PubMed] [Google Scholar]
- Jung JJ, Razavian M, Challa AA, Nie L, Golestani R, Zhang J, Ye Y, Russell KS, Robinson SP, Heistad DD, and Sadeghi MM. 2015. Multimodality and molecular imaging of matrix metalloproteinase activation in calcific aortic valve disease. J Nucl Med. 56:933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden JJ, Kilic R, Sarikoc A, Hagl S, Lang S, Hoffmann U, Brueckmann M, and Borggrefe M. 2005. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med. 16:869–872. [PubMed] [Google Scholar]
- Lai CF, Shao JS, Behrmann A, Krchma K, Cheng SL, and Towler DA. 2012. TNFR1-activated reactive oxidative species signals up-regulate osteogenic Msx2 programs in aortic myofibroblasts. Endocrinology. 153:3897–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ven F, Tribouilloy C, Habib G, Gueffet JP, Marechaux S, Eicher JC, Blanchard-Lemoine B, Rousseau J, Henon P, Jobic Y, and Etienne Y. 2011. Valvular heart disease associated with benfluorex therapy: results from the French multicentre registry. Eur J Echocardiogr. 12:265–271. [DOI] [PubMed] [Google Scholar]
- Lee JE, Choi YK, Seo HA, Jeon JH, Jeong JY, Moon SS, Kim JG, Kim BW, Kim SW, Min Y, Kim JY, and Lee IK. 2010. Impact of ENPP1 and MMP3 gene polymorphisms on aortic calcification in patients with type 2 diabetes in a Korean population. Diabetes Res Clin Pract. 88:87–96. [DOI] [PubMed] [Google Scholar]
- Li X, Lim J, Lu J, Pedego TM, Demer L, and Tintut Y. 2015. Protective Role of Smad6 in Inflammation-Induced Valvular Cell Calcification. J Cell Biochem. 116:2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Ehsanipour A, Hsu JJ, Lu J, Pedego T, Wu A, Walthers CM, Demer LL, Seidlits SK, and Tintut Y. 2016a. Inflammation Drives Retraction, Stiffening, and Nodule Formation via Cytoskeletal Machinery in a Three-Dimensional Culture Model of Aortic Stenosis. Am J Pathol. 186:2378–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Ehsanipour A, Hsu JJ, Lu J, Pedego T, Wu A, Walthers CM, Demer LL, Seidlits SK, and Tintut Y. 2016b. Inflammation Drives Retraction, Stiffening, and Nodule Formation via Cytoskeletal Machinery in a Three-Dimensional Culture Model of Aortic Stenosis. Am J Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Hsiao FY, Liu YB, Gau SS, Wang CC, and Shen LJ. 2016. Antidepressants and Valvular Heart Disease: A Nested Case-Control Study in Taiwan. Medicine (Baltimore). 95:e3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker M, Bitard J, Collet C, Poliard A, Mutel V, Launay JM, and Kellermann O. 2006. Stepwise control of osteogenic differentiation by 5-HT(2B) receptor signaling: nitric oxide production and phospholipase A2 activation. Cell Signal. 18:628–639. [DOI] [PubMed] [Google Scholar]
- Pollanen PJ, Lehtimaki T, Ilveskoski E, Mikkelsson J, Kajander OA, Laippala P, Perola M, Goebeler S, Penttila A, Mattila KM, Syrjakoski K, Koivula T, Nikkari ST, and Karhunen PJ. 2002. Coronary artery calcification is related to functional polymorphism of matrix metalloproteinase 3: the Helsinki Sudden Death Study. Atherosclerosis. 164:329–335. [DOI] [PubMed] [Google Scholar]
- Prohaska W, Zittermann A, Luth JU, Inoue K, Koster-Eiserfunke W, Baller D, Korfer R, and Kleesiek K. 2008. Prevalent platelet dysfunction in patients with aortic valve disease. J Heart Valve Dis. 17:542–547. [PubMed] [Google Scholar]
- Rauma PH, Honkanen RJ, Williams LJ, Tuppurainen MT, Kroger HP, and Koivumaa-Honkanen H. 2016. Effects of antidepressants on postmenopausal bone loss - A 5-year longitudinal study from the OSTPRE cohort. Bone. 89:25–31. [DOI] [PubMed] [Google Scholar]
- Richter T, Paluch Z, and Alusik S. 2014. The non-antidepressant effects of citalopram: a clinician's perspective. Neuro Endocrinol Lett. 35:7–12. [PubMed] [Google Scholar]
- Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, and Feldman JM. 1995. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 92:790–795. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, and Roth BL. 2000. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 102:2836–2841. [DOI] [PubMed] [Google Scholar]
- Rouzaud-Laborde C, Delmas C, Pizzinat N, Tortosa F, Garcia C, Mialet-Perez J, Payrastre B, Sie P, Spreux-Varoquaux O, Sallerin B, Carrie D, Galinier M, Parini A, and Lairez O. 2015. Platelet activation and arterial peripheral serotonin turnover in cardiac remodeling associated to aortic stenosis. Am J Hematol. 90:15–19. [DOI] [PubMed] [Google Scholar]
- Sandmann H, Pakkal M, and Steeds R. 2009. Cardiovascular magnetic resonance imaging in the assessment of carcinoid heart disease. Clin Radiol. 64:761–766. [DOI] [PubMed] [Google Scholar]
- Shindo S, Hosokawa Y, Hosokawa I, Ozaki K, and Matsuo T. 2014. Genipin inhibits MMP-1 and MMP-3 release from TNF-a-stimulated human periodontal ligament cells. Biochimie. 107 Pt B:391–395. [DOI] [PubMed] [Google Scholar]
- Swierszcz J, Dubiel JS, Krzysiek J, and Sztefko K. 2011. One-year observation of inflammatory markers in patients with aortic valve stenosis. J Heart Valve Dis. 20:639–649. [PubMed] [Google Scholar]
- Taylor PM, Batten P, Brand NJ, Thomas PS, and Yacoub MH. 2003. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 35:113–118. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Patel J, Parhami F, and Demer LL. 2000. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 102:2636–2642. [DOI] [PubMed] [Google Scholar]
- Tribouilloy C, Rusinaru D, Marechaux S, Jeu A, Ederhy S, Donal E, Reant P, Arnalsteen E, Boulanger J, Ennezat PV, Garban T, and Jobic Y. 2012. Increased risk of left heart valve regurgitation associated with benfluorex use in patients with diabetes mellitus: a multicenter study. Circulation. 126:2852–2858. [DOI] [PubMed] [Google Scholar]
- Uzui H, Harpf A, Liu M, Doherty TM, Shukla A, Chai NN, Tripathi PV, Jovinge S, Wilkin DJ, Asotra K, Shah PK, and Rajavashisth TB. 2002. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation. 106:3024–3030. [DOI] [PubMed] [Google Scholar]
- Van Camp G, Flamez A, Cosyns B, Goldstein J, Perdaens C, and Schoors D. 2003. Heart valvular disease in patients with Parkinson's disease treated with high-dose pergolide. Neurology. 61:859–861. [DOI] [PubMed] [Google Scholar]
- Van Camp G, Flamez A, Cosyns B, Weytjens C, Muyldermans L, Van Zandijcke M, De Sutter J, Santens P, Decoodt P, Moerman C, and Schoors D. 2004. Treatment of Parkinson's disease with pergolide and relation to restrictive valvular heart disease. Lancet. 363:1179–1183. [DOI] [PubMed] [Google Scholar]
- Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, and Liang B. 2002. Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells. Am J Pathol. 161:2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, and Furukawa K. 2011. Tumor necrosis factor-alpha accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther. 337:16–23. [DOI] [PubMed] [Google Scholar]
- Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, and Pezzoli G. 2007. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med. 356:39–46. [DOI] [PubMed] [Google Scholar]
- Zhao G, Xu MJ, Zhao MM, Dai XY, Kong W, Wilson GM, Guan Y, Wang CY, and Wang X. 2012. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int. 82:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YG, Meng FX, Li BW, Sheng YM, Liu MM, Wang B, Li HW, and Xiu RJ. 2016. Gelatinases promote calcification of vascular smooth muscle cells by up-regulating bone morphogenetic protein-2. Biochem Biophys Res Commun. 470:287–293. [DOI] [PubMed] [Google Scholar]
- Zipes DP, Libby P, Bonow RO, Mann DL, Tomaselli GF . 2019. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Saunders, Philadelphia. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.