Abstract
Necrotizing enterocolitis (NEC) is an inflammatory bowel necrosis of premature infants, and is a leading cause of morbidity and mortality in infants born between 23–28 weeks of gestation. Fifty to 95% of all infants with NEC develop thrombocytopenia (platelet counts <150 × 109/L) within 24–72 hours of receiving this diagnosis. In many patients, thrombocytopenia is severe and is treated with one or more platelet transfusions. However, the underlying mechanism(s) and biological implications of NEC-related thrombocytopenia remain unclear. This review presents current evidence from human and animal studies on the clinical features and mechanisms of platelet depletion in NEC. Anecdotal clinical experience is combined with evidence from laboratory studies and from an extensive literature search in databases PubMed, EMBASE, Scopus, and the electronic archives of abstracts presented at the annual meetings of the Pediatric Academic Societies. To avoid bias in identification of existing studies, key words were short-listed prior to the actual search both from anecdotal experience and from PubMed’s Medical Subject Heading (MeSH) thesaurus.
1. Introduction
Necrotizing enterocolitis (NEC) is an inflammatory bowel necrosis of premature infants, and a leading cause of mortality in infants born between 22–28 weeks’ gestation (1, 2). Histopathologically, NEC is characterized by coagulative necrosis, inflammation, interstitial hemorrhages, and reparative changes. These changes typically begin in the mucosa and progress outward towards the serosa (3). The etiology of NEC remains uncertain; there are associations with hypoxia and hypothermia, inadequate anti-microbial defenses due to immature Paneth cells, mucosal inflammation in severely anemic infants that may worsen with red blood cell (RBC) transfusions, or following enteral exposure to immunological stimulants (4–7). Premature infants who develop enteric dysbiosis with a predominance of Gram-negative bacteria and have bacterial overgrowth may also be at enhanced risk of NEC (8–11)(8–11)(8–11)(8–11).
Fifty to 95% of all infants with NEC develop thrombocytopenia (platelet counts <150 × 109/L) within 24–72 hours of receiving this diagnosis (11). This review focuses on thrombocytopenia in acute NEC, summing up anecdotal clinical experience, laboratory studies, and mechanistic information from an extensive literature search using the databases PubMed, EMBASE, and Scopus, and the electronic archive of abstracts submitted for the annual meetings of the Pediatric Academic Societies. To avoid bias, key words were short-listed from personal experience and from PubMed’s Medical Subject Heading (MeSH) thesaurus before the actual search.
2. Low platelet counts in NEC
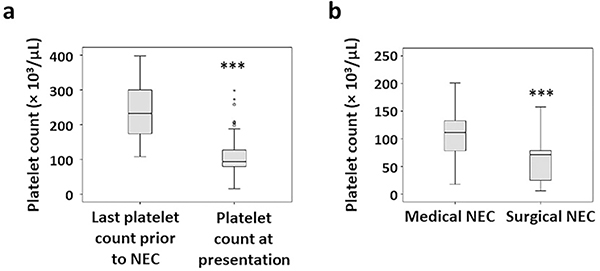
Thrombocytopenia (platelet counts <150 × 109/L) is a frequently encountered hematological abnormality in infants with NEC (11). Patients with confirmed NEC typically show decreased platelet counts within 24 hours of disease onset (Figure 1a). This thrombocytopenia may worsen until 72 hours, and the depth of the nadir correlates with the severity and extent of bowel injury. In an occasional infant, thrombocytopenia may precede abdominal signs of NEC by up to 24 hours (12, 13). In our institutional reviews, we have noted these drops in platelet counts to correlate with Bell’s clinical stage of NEC (Figure 1b). Infants with advanced NEC needing surgery may show platelet counts as low as 30–60 × 109/L (11).
Figure 1. Diagnosis of NEC is associated with thrombocytopenia.
(a) Box-whisker plots depict the last available platelet counts prior to NEC and the platelet counts at presentation. N=76 cases of NEC, who were born at a gestational age (average ± SEM) of 26.6±0.3 weeks, had a birth weight of 845±63 grams, and age at onset of NEC was 21.2±4 days; (b) Box-whisker plots show lowest platelet counts in infants treated medically vs. those who needed surgery for NEC. *** p<0.001
NEC is a leading cause of acute and subacute thrombocytopenia in premature and critically ill infants admitted to tertiary referral centers (11). Several reports describe a 50–90% drop in platelet counts in acute NEC (14–17). Some of the variability in the observed fall in platelet counts at diagnosis could be rooted in the proportion of infants admitted with Bell’s stage 1/feeding intolerance, who may have some delay or uncertainty in the diagnosis of NEC (11). In patients who have a confirmed diagnosis, thrombocytopenia seems to correlates better with the severity and extent of bowel injury. A rapid drop in platelet counts below 100 × 109/L within 12–24 hours of disease onset may indicate a high likelihood of bowel necrosis and need for surgical intervention (14). In a review of 58 infants with NEC, Ververidis et al. (14) reported 54 (93%) infants with platelet counts <100 × 109/L within 24 hours of disease onset. Kenton et al. (15) detected thrombocytopenia in 47/91 (52%) of their patients with NEC. Nearly a third of enrolled patients in these two studies who developed severe thrombocytopenia (<50 × 109/L) within 24 hours of diagnosis, needed surgery. Ragazzi et al. (16) noted thrombocytopenia in 129/232 (56%) patients and the magnitude of drop in platelet counts reflected the extent of disease. Similar observations were reported by Hutter (18), Patel (17), and O’Neill et al. (19).
During acute NEC, thrombocytopenia predicts adverse clinical outcomes. Hutter et al. (18) noted significantly lower platelet nadirs in infants with fatal NEC than in survivors (46.5 × 109/L vs. 69.3 × 109/L). In the study described above, Ververidis et al. (14) reported 16/58 (28%) deaths; these patients had lower nadirs in platelet counts than the survivors. Infants who survived maintained platelet counts >100 × 109/L during the course of the disease. Ragazzi et al. (16) recorded thrombocytopenia in 86% infants with fatal disease. Similar results were reported by Kenton et al. (15), who observed higher platelet counts in survivors (median 203 × 109/L vs. 33 × 109/L in non-survivors; p<0.001). Severe thrombocytopenia was a significant predictor of mortality (adjusted odds ratio, OR 6.39) and of complications such as cholestasis and short bowel syndrome (adjusted OR 5.47). Amongst survivors, platelet counts recovered to >150 × 109/L within 7 to 10 days (14, 15, 17). In another study, Baer et al. (20) reviewed 11,281 neonates treated in level III NICUs, and identified 273 (2.4%) with platelet counts <50 × 109/L. NEC was noted in 52 (14%) of these infants. The same group identified NEC as the leading diagnosis in very high users of platelet transfusions (≥20 platelet transfusions). Interestingly, infants who develop NEC may show altered thrombopoiesis with a lower platelet mass index (platelet count × mean platelet volume) than controls before the onset of NEC, even within the first postnatal week (21).
3. Pathophysiology of thrombocytopenia in NEC
The exact mechanism of NEC-related thrombocytopenia remains unclear. The following sections provide an overview of evidence from human and murine studies.
(a). Human studies
Patients with confirmed NEC may show a rapid drop in platelet counts and only brief, limited corrections in platelet counts following transfusions. The site of platelet consumption are unclear, although microthrombotic events in the diseased intestine remain an important possibility (22, 23). Conceptually, platelet activators such as bacterial products, platelet-activating factor, arachidonic acid metabolites, and coagulation factors may be important in NEC pathogenesis (11, 24). These mediators can stimulate endothelial cells and macrophages in concert with thromboplastin released from the gangrenous bowel, to induce secondary mediators such as inflammatory cytokines and nitric oxide. Together, these stimuli can promote platelet activation and aggregation in the microvasculature (11, 19).
The kinetic basis of thrombocytopenia in human NEC remains a subject of investigation. Brown et al. (25, 26) evaluated thrombopoiesis in 20 critically ill infants with sepsis or NEC by measuring serial platelet counts, blood thrombopoietin (Tpo) levels, reticulated platelets (RPs), and megakaryocyte progenitors. Elevated Tpo levels were associated with increased megakaryopoiesis and platelet release. The authors speculated that the severity of illness and specific platelet and/or bacterial products could downregulate thrombopoiesis. In another study, Cremer et al. (27) followed platelet counts and immature platelet fractions (IPFs) in 21 infants with surgical NEC or late-onset sepsis. Platelet counts correlated with IPFs; infants with fatal NEC had lower platelet counts and IPFs than survivors. The reasons for this dampened thrombopoiesis during severe NEC were unclear, but could include known inhibitors of megakaryopoiesis such as platelet factor (PF)-4, released from activated platelets (28). Kampanatkosol et al. (29) also found fewer circulating reticulated platelets in infants with late-onset NEC than controls, possibly due to decreased or failing thrombopoiesis. However, Cekmez et al. (30) have noted that infants who went to develop NEC had higher mean platelet volumes, possibly reflecting increased thrombopoiesis, even in their early neonatal period.
We can appreciate that human data on the mechanisms of NEC-related thrombocytopenia are relatively limited, and sometimes conflicting. In the following sections, we describe our ongoing efforts to develop a murine model to understand the changes in circulating platelets and bone marrow megakaryocytes during NEC-like intestinal injury.
(b). Murine model of NEC-related thrombocytopenia
We have developed a murine model of NEC-related thrombocytopenia where we administer an immunological stimulant, trinitrobenzene sulfonate (TNBS) in two doses of 50 mg/kg in 30% ethanol (w/v) by gavage and rectal instillation, on postnatal day (P) 10. These pups develop an acute necrotizing ileocolitis resembling human NEC within 15–18 hours (Figure 2) (12). These intestinal changes recapitulated the intestinal necrosis, the regional predilection for distal ileum and proximal colon (31), characteristic features of the mucosal inflammatory response and induction of specific cytokines (31, 32), monocyte/macrophage infiltration (31, 33), and specific changes in genome-wide transcription/inflammatory signaling in the intestine (7, 32). TNBS did not cause intestinal damage in germ-free pups (31), indicating that its inflammatory effects (a) required the presence of intestinal microflora; and (b) were not secondary to a direct chemical/corrosive action. TNBS-induced ileocolitis in pups was clearly distinct from its effects in adult mice, which were comprised of subacute/chronic inflammatory mucosal changes such as basal cryptitis and were most prominent in the distal colon (31).
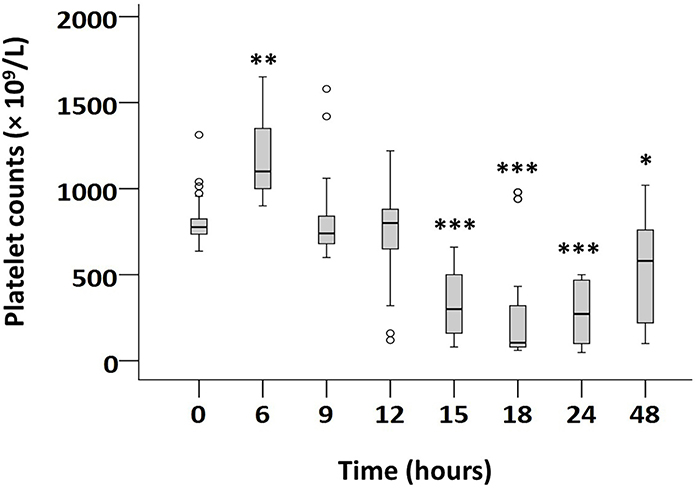
Figure 2. Blood platelet concentrations in severe murine NEC-like injury.
Boxplots show serial platelet concentrations in P10 mouse pups in control and severe NEC-like injury groups. Severe intestinal injury seen in 63 pups; 45 euthanized at earlier time-points for physical distress. Control mice (N=65) received vehicle (ethanol) alone and showed no distress or significant changes in platelet counts. Jonckheere-Terpstra test for ordered alternatives. * P<0.05, ** P<0.01, *** P<0.001 vs. control.
To investigate the kinetic basis of NEC-related thrombocytopenia, we compared mice in control and NEC-like injury groups for serial platelet counts, platelet volume indices, and immature platelet fractions in blood, and the number/ploidy of megakaryocytes in the bone marrow (12). Pups with intestinal injury showed decreased platelet counts at 15–24 hours. The severity of thrombocytopenia correlated with the severity of intestinal injury and resembled the drop in platelet counts seen in human NEC (14, 16–19). We also noted increased platelet volume and IPF, platelet distribution width, platelet-large cell ratio, and the number/ploidy of bone marrow megakaryocytes, indicating increased platelet production (12). Pups with mild intestinal injury showed a reduction in platelet counts at 18 hours with a nadir at 24 hours. In moderate-severe injury, platelet counts were lower than controls at 15 hours and reached a minimum at 18 hours. These platelet counts then showed a time-dependent recovery between 24–48h (hazard ratio = 0.996, 95% confidence interval 0.995–0.997). The commitment of megakaryocytes and thrombopoiesis seen in this model resembled human NEC and favored increased peripheral consumption of platelets, not impaired production, as the likely mechanism of thrombocytopenia.
Our investigations of platelet indices in murine NEC-like intestinal injury show that thrombocytopenia develops despite increased platelet production (12):
Mean platelet volume (MPV): quotient of the plateletcrit (ratio of platelet volume to whole blood volume, expressed as percentage) and the platelet count (× 109/L) (34). At 18 hours, the median (range) MPV in mild, moderate, and severe intestinal injury at 7.5 (6.6–7.7), 7.2 (6.5–9.6), and 7.6 (6.4–9.9) fL, respectively, was significantly higher than 6.6 fL (range 6.1–8.3) in controls.
Platelet distribution width (PDW): range of platelet volumes at 20% frequency (peak of the frequency histogram = 100%) (35). PDW was increased in pups with intestinal injury.
Platelet-large cell ratio (P-LCR): proportion of large platelets (>12 fL) in the total platelet population (35). P-LCR was increased in murine NEC-like injury, indicating increased number of larger, presumably younger platelets in the circulation (29).
Immature platelet fraction (IPF): Pups with NEC-like injury showed more immature platelets, which are larger (forward light scatter) and carry more nucleic acid (side fluorescence intensity) (27, 34, 36). At 18 hours, pups with NEC-like injury had a larger IPF (more immature platelets in total platelet population; 13.4%, range 3.1–33.5% vs. controls, 7.4%; range 2.9–11.9%; p<0.001) (27).
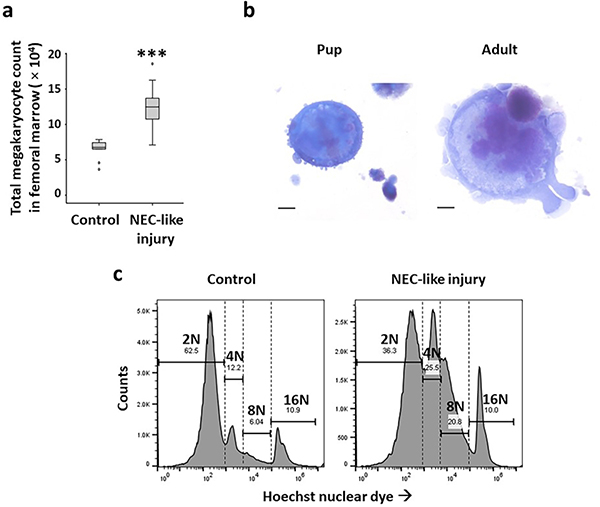
Bone marrow megakaryocytes: Mouse pups with NEC-like injury carried more megakaryocytes (5.34±0.2 × 104 vs. 13.45±0.36 × 104/mL of marrow preparation; Figure 3a) at the 18-hour time-point (12). Similar to human infants (37), pups had smaller megakaryocytes than adult mice (median area = 4749, range 2876–5107 μm2 vs. 8906, range 6345–1345 μm2; Figure 3b). In NEC-like injury, megakaryocytes showed increased nuclear ploidy (cells≥8N: median 14.8, range 12.4–18.2 in control vs. 30.9, range 18.1–36.4 in NEC-like injury), indicating increased megakaryocyte differentiation Figure 3c.
Figure 3. Megakaryocytes in murine NEC-like injury.
(a) Box-whisker plots show CD41+ megakaryocyte counts in control and NEC-like injury; N=8 pups/group; *** p<0.001; (b) Mouse pups have smaller megakaryocytes (median area = 4749 μm2) than adult mice (8906 μm2). Data represent 5 mice/group; p<0.001 (c) Histograms show nuclear ploidy of megakaryocytes stained with the Hoechst nuclear dye, in control and NEC-like injury. Data represent N=8 pups/group.
(c). Platelet activation in murine NEC-like injury
Platelet activation was an early event during NEC-like injury, seen within 3 hours before any changes in platelet counts or histopathological evidence of mucosal injury (13). These activated platelets carried an activated conformation of the integrin GPIIb/IIIa (38, 39) and increased platelet endothelial cell adhesion molecule (PECAM)-1/CD31 (40). Neonatal platelets expressed P-selectin at low levels and upregulated it at 24 hours after induction of NEC-like injury (41).
Platelets collected 3 hours after induction of NEC-like injury showed increased aggregability with collagen. Pups in the NEC-like injury group showed evidence of platelet dense granule discharge as early as 3 hours. These platelets contained fewer dense granules, and released less ATP upon collagen stimulation. The release of α-granule contents such as platelet factor (PF)-4/CXC motif ligand (CXCL) was relatively delayed; plasma PF4/CXCL4 levels rose transiently at 6 hours but consistently only after 12 hours. These platelets showed consistent α-granule depletion beyond 18 hours after induction of NEC.
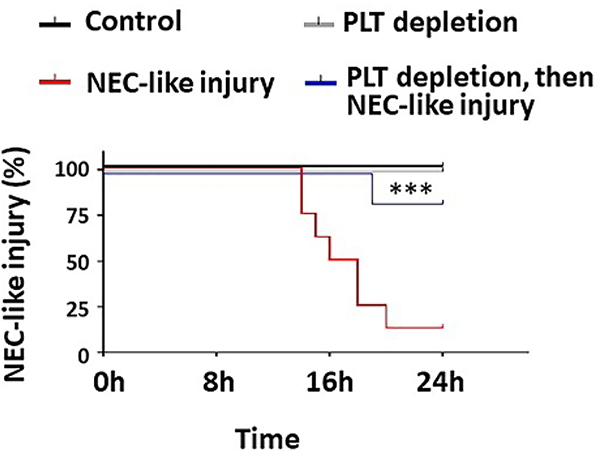
In murine NEC-like injury, the negative effects of early platelet activation are evident from the protective effects of platelet depletion to levels around 50–100 × 109/L (13). In our murine model, platelet depletion reduced the severity of bowel injury and improved survival (Figure 4), without increasing the severity of hemorrhages in the injured intestine (13). These studies support the possibility emerging from human studies that transfused, activated platelets could cause harm by releasing pre-formed vasoconstrictors and inflammatory mediators. (42) (43).
Figure 4. Platelet depletion protects against murine neonatal NEC-like injury.
Kaplan-Meier curves summarize survival data from animals in control (N = 3), intestinal injury (N = 8), platelet depletion (N = 5), and platelet depletion followed by intestinal injury (N = 6) groups. Mantel-Cox log-rank test, *** P<0.001.
(d). Thrombin activates platelets in murine NEC-like injury
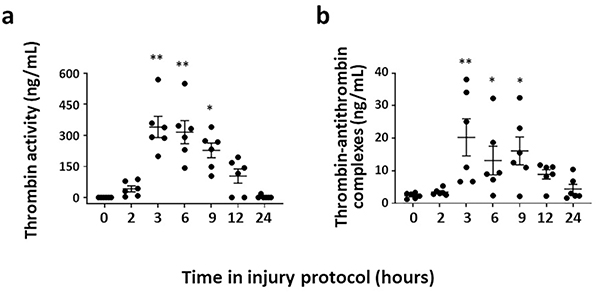
We investigated the role of platelet activators such as thrombin, thromboxane A2 (TxA2), endotoxin, and platelet activating factor (PAF) in NEC-like injury (13). Plasma thrombin activity and thrombin-antithrombin complexes were elevated at 3 hours into NEC-like injury (Figure 5). Endotoxin and PAF rose later, while no changes were seen in TxA2 (13). The role of thrombin was confirmed ex vivo when platelets from control pups were resuspended in plasma from pups with intestinal injury, which activated platelet GPIIb/IIIa. These changes were blocked by thrombin inhibitors such as bivalirudin or D-phenylalanyl-prolyl-arginyl chloromethyl ketone (44, 45). These thrombin inhibitors also blocked CD31 expression and dense granule discharge (35).
Figure 5. Thrombin activates platelets during murine NEC-like injury.
Scatterplots (means ± SEM; top to bottom) show (a) plasma thrombin activity and (b) thrombin-antithrombin complexes measured at various time-points between 0–24h after initiation of intestinal injury. N = 6 pups. Friedman’s test for repeated measures, * P<0.05, ** P<0.01, and *** P<0.001 vs. control
(e). Murine neonatal platelets are highly responsive to thrombin
Murine neonatal platelets responded to thrombin and release dense granule contents within 15 minutes. In contrast, adult platelets may take ≥30 minutes. Due to developmental differences, neonatal platelets express several mediators of thrombin-induced signaling at higher levels than in adults (46). In proteomic analysis, neonatal platelets express higher levels of platelet glycoprotein 1b; vasodilator stimulated phosphoprotein; guanine nucleotide-binding protein, alpha 13; guanine nucleotide-binding protein, g(q)-α; cytosolic phospholipase A-2 (PLA2); phospholipase A-2 activating protein; synaptosomal-associated protein 23; ras homolog gene family, member A (RhoA); Rho GDP-dissociation inhibitor 1, and Rho GDP-dissociation inhibitor 2.
Neonatal plasma showed less thrombin antagonistic activity than adults (47–50). These differences could be explained by lower expression of antithrombin and α2-macroglobulin in neonatal plasma, but not by the differences in the levels of α1-antitrypsin, tissue factor-pathway inhibitor, or heparin cofactor-II.
(f). Tissue factor activates thrombin and promotes platelet consumption during NEC
In the adult intestine, TF is expressed in the perivascular smooth muscle, epithelia, leukocytes, and platelets (51). In contrast, the neonatal intestine expressed TF in resident macrophages but not in perivascular or epithelial cells. These macrophages showed TF expression in well-defined cytoplasmic compartments. Ex vivo, studies confirmed constitutive and LPS-induced expression of TF and its release in microvesicles. LPS-inducible macrophage TF expression was particularly interesting when we considered the emerging link between NEC and enteric dysbiosis with enrichment of Gram-negative bacteria.
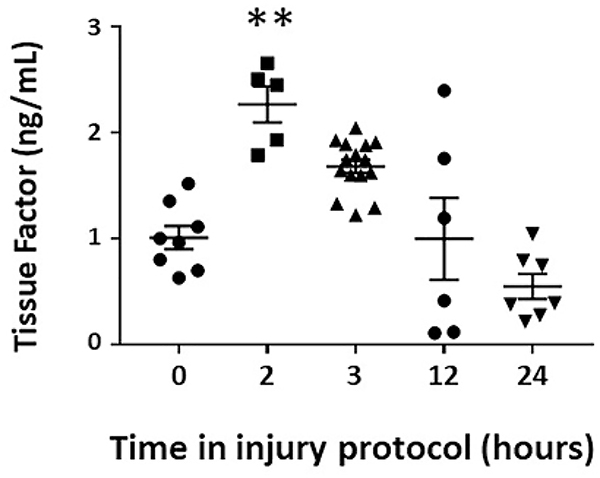
Mouse pups with NEC-like injury showed increased plasma TF levels beginning at the 2-hour time-point, preceding the rise in thrombin activity at 3 hours (Figure 6). We first confirmed these findings in human infants; premature human infants with NEC had significantly higher plasma TF concentrations than controls (mean ± standard deviation 51.11±17.23 pg/mL compared to 4.48±1.26 pg/mL in controls). We then checked TF levels in murine intestine; there was a transient drop in TF at these time-points, suggesting that pre-formed TF stores may have been released from the injured intestine. This TF presumably formed TF-VIIa complexes to activate circulating prothrombin during the pathogenesis of NEC-like injury. Inhibition of factor VII activation with PCI-27483 (N-aminoiminomethyl benzimidazol aminosulfonyl dihydroxy biphenyl acetyl aspartic acid) blocked this rise in plasma thrombin in NEC-like injury (52).
Figure 6. Plasma tissue factor (TF) concentrations during murine NEC-like injury.
Scatterplot (means ± SEM) show serial TF concentrations. N = 6 mice/group; Kruskal Wallis H test with Dunn’s post-test, * P<0.05, ** P<0.01 vs. control
Mouse studies implicated thrombin-mediated platelet activation in a pathogenetic role in NEC-like injury. Unfortunately, systemic inhibition of thrombin is unpredictable in premature infants and may be either impaired or increase the risk of hemorrhagic complications (53, 54). Therefore, we evaluated antithrombin bivalirudin-tagged perfluorocarbon-core nanoparticles (NPs), which can bind thrombin in nascent blood clots and prevent progressive activation of the coagulation cascades without increasing the risk of systemic hemorrhagic complications (55–57). Considering the in-vivo half-life of about 4 hours for these NPs, we administered two doses, the first given 1 hour prior to induction of NEC-like injury and a second dose 4 hours later (55–57). Bivalirudin NPs prevented thrombocytopenia, improved survival, and reduced the severity of intestinal injury, without increasing hemorrhages into the injured intestine. There was some leukocyte infiltration and submucosal edema but no major mucosal damage (13).
4. Platelet transfusions in human infants
Current management protocols in human NEC emphasize careful monitoring of thrombocytopenia and aggressive treatment with platelet transfusions if needed, because of the risk of life-threatening hemorrhagic complications in these patients (58–70). Most platelet transfusions in the NICU are administered prophylactically to correct platelet counts, not actual bleeding, and data on the efficacy and safety of these transfusions remain scant. In the United States, transfusion thresholds may be relatively liberal compared to those in Europe, and nearly 50% of ELBW infants may receive ≥1 platelet transfusions during their hospital stay (65). An automatized audit system showed that 60% of all platelet transfusions were given outside of established guidelines, mostly at platelet counts higher than recommended (71).
There is some evidence linking platelet transfusions with adverse outcomes in NEC. Baer et al. (61, 72, 73) and Garcia et al. (74) showed that the number of platelet transfusions predicted mortality in infants with diagnosis of NEC. Kenton et al. (15, 75) noted that platelet transfusions predicted complications of NEC such as short bowel syndrome and cholestasis. Del Vecchio et al. (66) found an association between platelet transfusions and mortality in critically ill infants. Compared to infants who did not receive platelet transfusions, 1 platelet transfusion increased the relative risk of death by 10.4-fold, and >4 platelet transfusions increased it 29.9 times. The mechanism of these inferior outcomes, whether it represents direct harm from transfused platelets, or because these infants were sicker in the first place, is unclear (20, 61, 73). Sensitivity analysis on regression data suggested important effects of platelet transfusions on adverse outcomes (76). The evaluation of platelet transfusions remains difficult because of ethical concerns in withholding a potentially life-saving therapy from a critically ill infant. A few attempts sought to compare platelet transfusion practices with relatively conservative thresholds aiming to reduce platelet transfusions, but these efforts were only modestly ambitious with minor reductions in platelet transfusion thresholds (61). These studies were not adequately powered to answer these questions and showed no difference in outcomes (20, 73).
5. Recombinant peptides
Interleukin-11, Tpo, and Tpo-mimetic agents such as romiplostim and eltrombopag may not be useful in acute NEC (65, 77). Some of these peptides/growth factors could be used in selected infants with persistent thrombocytopenia in short bowel syndrome and cholestasis (77).
6. Summary
Thrombocytopenia is a common clinical finding in NEC, typically seen within 24–72 hours after developing NEC. The reduction in platelet counts is usually proportional to the severity and stage of NEC. The likely mechanism of this thrombocytopenia is the consumption of platelets in microthrombi formed in the intestinal microvasculature. Some neonates with severe NEC may also have concomitant suppression of the thrombopoietic response, resulting in prolonged thrombocytopenia. There is no consensus yet regarding the threshold for platelet transfusions in these infants with thrombocytopenia. There is a need for carefully designed studies to evaluate the safety and efficacy of platelet transfusions in NEC.
Article impact.
Fifty to 95% of infants with necrotizing enterocolitis (NEC) develop idiopathic thrombocytopenia (platelet counts <150 × 109/L) within 24–72 hours of disease onset
Early clinical trials suggest that moderate thrombocytopenia may be protective in human NEC, although further work is needed to fully understand this relationship
We have developed a neonatal murine model of NEC-related thrombocytopenia, where enteral administration of an immunological stimulant, trinitrobenzene sulfonate, on postnatal day 10 induces an acute necrotizing ileocolitis resembling human NEC
In this murine model, thrombocytopenia is seen at 15–18 hours due to platelet consumption, and mildmoderate thrombocytopenia is protective
Funding:
NIH awards HL124078 and HL133022 (to AM)
Footnotes
Disclosure: No financial conflicts to declare.
Category of study: Review
Patient consent: Not needed
References
- 1.Neu J, Walker WA 2011. Necrotizing enterocolitis. N Engl J Med 364:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, et al. , Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N 2010. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remon JI, et al. 2015. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J Perinatol 35:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasivajjula H, Maheshwari A 2014. Pathophysiology and current management of necrotizing enterocolitis. Indian J Pediatr 81:489–497. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, et al. 2012. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech 5:522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MohanKumar K, et al. 2019. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun 10:3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MohanKumar K, et al. 2016. Trinitrobenzene Sulfonic Acid-induced Intestinal Injury in Neonatal Mice Activates Transcriptional Networks Similar to those seen in Human Necrotizing Enterocolitis. Pediatr Res 81:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho TBT, et al. 2018. Dichotomous Development of the Gut Microbiome in Preterm Infants. Microbiome 6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho TBT, et al. 2019. Enteric Dysbiosis and Fecal Calprotectin Expression in Premature Infants. Pediatr Res 85:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fundora JB, Guha P, Shores DR, Pammi M, Maheshwari A 2019. Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria. Pediatr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maheshwari A 2015. Immunologic and Hematological Abnormalities in Necrotizing Enterocolitis. Clin Perinatol 42:567–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namachivayam K, MohanKumar K, Garg L, Torres BA, Maheshwari A 2017. Neonatal Mice with Necrotizing Enterocolitis-like Injury Develop Thrombocytopenia despite Increased Megakaryopoiesis. Pediatr Res 81:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namachivayam K, et al. 2020. Targeted Inhibition of Thrombin Attenuates Murine Neonatal Necrotizing Enterocolitis. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ververidis M, et al. 2001. The clinical significance of thrombocytopenia in neonates with necrotizing enterocolitis. J Pediatr Surg 36:799–803. [DOI] [PubMed] [Google Scholar]
- 15.Kenton AB, et al. 2005. Severe thrombocytopenia predicts outcome in neonates with necrotizing enterocolitis. J Perinatol 25:14–20. [DOI] [PubMed] [Google Scholar]
- 16.Ragazzi S, Pierro A, Peters M, Fasoli L, Eaton S 2003. Early full blood count and severity of disease in neonates with necrotizing enterocolitis. Pediatr Surg Int 19:376–379. [DOI] [PubMed] [Google Scholar]
- 17.Patel CC 1977. Hematologic abnormalities in acute necrotizing enterocolitis. Pediatr Clin North Am 24:579–584. [DOI] [PubMed] [Google Scholar]
- 18.Hutter JJ Jr., Hathaway WE, Wayne ER 1976. Hematologic abnormalities in severe neonatal necrotizing enterocolitis. J Pediatr 88:1026–1031. [DOI] [PubMed] [Google Scholar]
- 19.O’Neill JA Jr. 1981. Neonatal necrotizing enterocolitis. Surg Clin North Am 61:1013–1022. [PubMed] [Google Scholar]
- 20.Baer VL, Lambert DK, Henry E, Christensen RD 2009. Severe Thrombocytopenia in the NICU. Pediatrics 124:e1095–1100. [DOI] [PubMed] [Google Scholar]
- 21.Okur N, et al. 2016. Platelet mass index in very preterm infants: can it be used as a parameter for neonatal morbidities? J Matern Fetal Neonatal Med 29:3218–3222. [DOI] [PubMed] [Google Scholar]
- 22.Sola MC, Del Vecchio A, Rimsza LM 2000. Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit. Clin Perinatol 27:655–679. [DOI] [PubMed] [Google Scholar]
- 23.Hyman PE, Abrams CE, Zipser RD 1987. Enhanced urinary immunoreactive thromboxane in neonatal necrotizing enterocolitis. A diagnostic indicator of thrombotic activity. Am J Dis Child 141:686–689. [DOI] [PubMed] [Google Scholar]
- 24.Hsueh W, et al. 2003. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 6:6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown EG, Sweet AY 1982. Neonatal necrotizing enterocolitis. Pediatr Clin North Am 29:1149–1170. [DOI] [PubMed] [Google Scholar]
- 26.Brown RE, et al. 2008. Effects of sepsis on neonatal thrombopoiesis. Pediatr Res 64:399–404. [DOI] [PubMed] [Google Scholar]
- 27.Cremer M, et al. 2013. Low immature platelet fraction suggests decreased megakaryopoiesis in neonates with sepsis or necrotizing enterocolitis. J Perinatol 33:622–626. [DOI] [PubMed] [Google Scholar]
- 28.Lambert MP, et al. 2007. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood 110:1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kampanatkosol R, et al. 2014. The relationship between reticulated platelets, intestinal alkaline phosphatase, and necrotizing enterocolitis. J Pediatr Surg 49:273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cekmez F, et al. 2013. Mean platelet volume in very preterm infants: a predictor of morbidities? Eur Rev Med Pharmacol Sci 17:134–137. [PubMed] [Google Scholar]
- 31.MohanKumar K, et al. 2012. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol 303:G93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MohanKumar K, et al. 2016. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin Perinatol 41:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MohanKumar K, et al. 2016. Smad7 Interrupts TGF-β Signaling in Intestinal Macrophages and Promotes Inflammatory Activation of these Cells during Necrotizing Enterocolitis. Pediatr Res 79:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe Y, et al. 2006. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF). Thromb Res 118:463–469. [DOI] [PubMed] [Google Scholar]
- 35.Patrick CH, Lazarchick J, Stubbs T, Pittard WB 1987. Mean platelet volume and platelet distribution width in the neonate. Am J Pediatr Hematol Oncol 9:130–132. [DOI] [PubMed] [Google Scholar]
- 36.Briggs C, Kunka S, Hart D, Oguni S, Machin SJ 2004. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol 126:93–99. [DOI] [PubMed] [Google Scholar]
- 37.Sola-Visner MC, Christensen RD, Hutson AD, Rimsza LM 2007. Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatr Res 61:479–484. [DOI] [PubMed] [Google Scholar]
- 38.Allen DL, Abrahamsson S, Murphy MF, Roberts DJ 2012. Human platelet antigen 1a epitopes are dependent on the cation-regulated conformation of integrin alpha(IIb)beta(3) (GPIIb/IIIa). J Immunol Methods 375:166–175. [DOI] [PubMed] [Google Scholar]
- 39.Bergmeier W, et al. 2002. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry 48:80–86. [DOI] [PubMed] [Google Scholar]
- 40.Baker-Groberg SM, Lattimore S, Recht M, McCarty OJ, Haley KM 2016. Assessment of neonatal platelet adhesion, activation, and aggregation. J Thromb Haemost 14:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhne T, et al. 1996. Platelet-surface glycoproteins in healthy and preeclamptic mothers and their newborn infants. Pediatr Res 40:876–880. [DOI] [PubMed] [Google Scholar]
- 42.Curley A, et al. 2018. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- 43.Stanworth SJ, et al. 2009. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics 124:e826–834. [DOI] [PubMed] [Google Scholar]
- 44.Schmaier AH, Meloni FJ, Nawarawong W, Jiang YP 1992. PPACK-thrombin is a noncompetitive inhibitor of alpha-thrombin binding to human platelets. Thromb Res 67:479–489. [DOI] [PubMed] [Google Scholar]
- 45.Gladwell TD 2002. Bivalirudin: a direct thrombin inhibitor. Clin Ther 24:38–58. [DOI] [PubMed] [Google Scholar]
- 46.Mull MM, Hathaway WE 1970. Altered platelet function in newborns. Pediatr Res 4:229–237. [DOI] [PubMed] [Google Scholar]
- 47.Manco-Johnson MJ 1989. Neonatal antithrombin III deficiency. Am J Med 87:49S–52S. [DOI] [PubMed] [Google Scholar]
- 48.O’Keeffe D, et al. 2004. The heparin binding properties of heparin cofactor II suggest an antithrombin-like activation mechanism. J Biol Chem 279:50267–50273. [DOI] [PubMed] [Google Scholar]
- 49.Tollefsen DM 2002. Heparin cofactor II deficiency. Arch Pathol Lab Med 126:1394–1400. [DOI] [PubMed] [Google Scholar]
- 50.Golino P 2002. The inhibitors of the tissue factor:factor VII pathway. Thromb Res 106:V257–265. [DOI] [PubMed] [Google Scholar]
- 51.Drake TA, Morrissey JH, Edgington TS 1989. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol 134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 52.Ramanathan RK, et al. 2019. A Phase 2 Study of PCI-27483, a Factor VIIa Inhibitor in Combination with Gemcitabine for Advanced Pancreatic Cancer. Oncology 96:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vieira A, Berry L, Ofosu F, Andrew M 1991. Heparin sensitivity and resistance in the neonate: an explanation. Thromb Res 63:85–98. [DOI] [PubMed] [Google Scholar]
- 54.Shah JK, et al. 1992. Thrombin inhibition is impaired in plasma of sick neonates. Pediatr Res 31:391–395. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, et al. 2015. Antithrombin nanoparticles improve kidney reperfusion and protect kidney function after ischemia-reperfusion injury. Am J Physiol Renal Physiol 308:F765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou KK, Pan H, Ratner L, Schlesinger PH, Wickline SA 2013. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano 7:8605–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou HF, et al. 2014. Peptide-siRNA nanocomplexes targeting NF-kappaB subunit p65 suppress nascent experimental arthritis. J Clin Invest 124:4363–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Josephson CD, et al. 2009. Platelet transfusion practices among neonatologists in the United States and Canada: results of a survey. Pediatrics 123:278–285. [DOI] [PubMed] [Google Scholar]
- 59.Sparger KA, et al. 2016. Platelet Transfusion Practices Among Very-Low-Birth-Weight Infants. JAMA Pediatr 170:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cremer M, et al. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion 51:2634–2641. [DOI] [PubMed] [Google Scholar]
- 61.Baer VL, et al. 2007. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. J Perinatol 27:790–796. [DOI] [PubMed] [Google Scholar]
- 62.Borges JP, et al. 2013. Restrictive guideline reduces platelet count thresholds for transfusions in very low birth weight preterm infants. Vox Sang 104:207–213. [DOI] [PubMed] [Google Scholar]
- 63.Christensen RD, Carroll PD, Josephson CD 2014. Evidence-based advances in transfusion practice in neonatal intensive care units. Neonatology 106:245–253. [DOI] [PubMed] [Google Scholar]
- 64.Christensen RD, et al. 2006. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol 26:348–353. [DOI] [PubMed] [Google Scholar]
- 65.Cremer M, Sallmon H, Kling PJ, Buhrer C, Dame C 2016. Thrombocytopenia and platelet transfusion in the neonate. Semin Fetal Neonatal Med 21:10–18. [DOI] [PubMed] [Google Scholar]
- 66.Del Vecchio A, et al. 2001. Platelet transfusions in the neonatal intensive care unit:factors predicting which patients will require multiple transfusions. Transfusion 41:803–808. [DOI] [PubMed] [Google Scholar]
- 67.Fernandes CJ, O’Donovan DJ 2006. Platelet transfusions in infants with necrotizing enterocolitis. Curr Hematol Rep 5:76–81. [PubMed] [Google Scholar]
- 68.Goel R, et al. 2019. Platelet transfusion practices in immune thrombocytopenia related hospitalizations. Transfusion 59:169–176. [DOI] [PubMed] [Google Scholar]
- 69.Murray NA, Howarth LJ, McCloy MP, Letsky EA, Roberts IA 2002. Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients. Transfus Med 12:35–41. [DOI] [PubMed] [Google Scholar]
- 70.Stanworth SJ 2012. Thrombocytopenia, bleeding, and use of platelet transfusions in sick neonates. Hematology Am Soc Hematol Educ Program 2012:512–516. [DOI] [PubMed] [Google Scholar]
- 71.Petaja J, Andersson S, Syrjala M 2004. A simple automatized audit system for following and managing practices of platelet and plasma transfusions in a neonatal intensive care unit. Transfus Med 14:281–288. [DOI] [PubMed] [Google Scholar]
- 72.Baer VL, et al. 2011. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion 51:264–269. [DOI] [PubMed] [Google Scholar]
- 73.Baer VL, et al. 2008. Adherence to NICU transfusion guidelines: data from a multihospital healthcare system. J Perinatol 28:492–497. [DOI] [PubMed] [Google Scholar]
- 74.Garcia MG, et al. 2001. Epidemiologic and outcome studies of patients who received platelet transfusions in the neonatal intensive care unit. J Perinatol 21:415–420. [DOI] [PubMed] [Google Scholar]
- 75.Kenton AB, et al. 2005. Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. J Perinatol 25:173–177. [DOI] [PubMed] [Google Scholar]
- 76.Lin DY, Psaty BM, Kronmal RA 1998. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 54:948–963. [PubMed] [Google Scholar]
- 77.Sparger KA, Li N, Liu Z, Ramsey H, Sola-Visner MC 2013. Developmental Differences Between Newborn and Adult Mice In Response To Romiplostim. Blood 122:3542. [DOI] [PMC free article] [PubMed] [Google Scholar]