Abstract
Objectives
Cancer survivorship status among patients evaluated for chest pain at the emergency department (ED) warrants high degree of suspicion. However, it remains unclear whether cancer survivorship is associated with different risk of major adverse cardiac events (MACE) compared to those with no history of cancer. Furthermore, while HEART score is widely used in ED evaluation, it is unclear whether it can adequately triage chest pain events in cancer survivors. We sought to compare the rate of MACE in patients with a recent history of cancer in remission evaluated for acute chest pain at the ED to those with no history of cancer; and compare the performance of a common chest pain risk stratification score (HEART) between the two groups.
Methods
We performed a secondary analysis of a prospective observational cohort study of chest pain patients presenting to the EDs of three tertiary care hospitals in the U.S. Cancer survivorship status, HEART scores, and the presence of MACE within 30 days of admission were retrospectively adjudicated from the charts. We defined patients with recent history of cancer in remission as those with a past history of cancer of less than 10 years, and currently cured or in remission.
Results
The sample included 750 patients (age 59±17; 42% females, 40% Black), while 69 patients (9.1%) had recent history of cancer in remission. A cancer in remission status was associated with a higher comorbidity burden, older age, and female sex. There was no difference in risk of MACE between those with a cancer in remission and their counterparts in both univariate (17.4% vs. 19.5%, OR= 0.87 [95% CI 0.45–1.66], p = 0.67) and multivariable analysis adjusting for demographics and comorbidities (OR= 0.62 [95% CI 0.31–1.25], p = 0.18). Patients with cancer in remission had higher HEART score (4.6±1.8 vs. 3.9±2.0, p=0.006), and a higher proportion triaged as intermediate risk (68% vs. 56%, OR = 1.67 [95% CI 1.00–2.84], p = 0.05), however, no difference in the performance of HEART score existed between the groups (AUC=0.86 vs. 0.84, p=0.76).
Conclusions
There was no difference in rate of MACE between those with recent history of cancer in remission compared to their counterparts. A higher proportion of patients with cancer in remission were triaged as intermediate risk by the HEART score, but we found no difference in the performance of the HEART score between the groups.
Keywords: Cancer, Chest Pain, HEART score, Major Adverse Cardiac Events
INTRODUCTION
The link between cancer and excess risk of heart disease has received special attention in recent years. The mechanism for this link is complex, but it is speculated that both conditions share similar pathophysiologic pathways in terms of chronic inflammation and oxidative stress.1 In addition, it is known that cancer treatments are associated with a sharp peak in cardiovascular events (e.g., myocardial infarction) that can last for weeks, or even years after completing treatment2–5. With an estimated 16.9 million cancer survivors in the US today,6 they represent a population frequently evaluated at the emergency department (ED), with chest pain being one of the most common chief complaints leading to ED visits among this population7. Moreover, nearly two thirds of these patients are aged 65 years or older and suffer from a higher prevalence of comorbidities compared to the general population, making their evaluation a distinctive challenge.8,9
Chest pain is the second leading cause for ED visits among US adults.10 The underlying etiology for chest pain varies widely from case to case. Identifying cases that require further evaluation or immediate intervention, thus, remains a challenging task. There are currently numerous risk assessment scores to evaluate chest pain at the ED setting. A key component in all of these risk assessment tools is the patient’s past medical history of cardiovascular risk factors11. Despite the known excess risk of cardiovascular events among cancer survivors, these risk assessment scores, including the widely used HEART score, do not account for cancer survivorship status in risk estimation calculations. The HEART score (Table 1) is a commonly used tool to risk stratify patients presenting to the ED with chest pain,12 and has been previously validated for predicting major adverse cardiac events (MACE). In fact, HEART is currently the decision tool that most accurately identifies patients who are eligible for early discharge.11,13,14 Although ED clinicians usually evaluate cancer survivors presenting with chest pain with a high degree of clinical suspicion, it is unknown if these patients are under or over triaged compared to their counterparts. The primary aim of this study was to examine the association between a recent history of cancer in remission among chest pain patients arriving to the ED by ambulance with the occurrence of 30-day major adverse cardiac events (MACE); and the secondary aim of the study was to compare the performance of the HEART score between these patients with recent history of cancer in remission and their counterparts.
Table 1.
Calculation of the HEART Score for Risk Stratification of Chest Pain Patients in the ED
| History | Slightly Suspicious | 0 |
| Moderately Suspicious | 1 | |
| Highly Suspicious | 2 | |
| EKG | Normal | 0 |
| Non-specific Repolarization Disturbance | 1 | |
| Significant Deviation | 2 | |
| Age | < 45 | 0 |
| 45–64 | 1 | |
| ≥65 | 2 | |
| Risk Factors | No Known Risk Factors | 0 |
| 1–2 Risk Factors | 1 | |
| ≥3 Risk Factors OR Atherosclerotic Disease | 2 | |
| Troponin | Normal Limit | 0 |
| 1–3x Normal Limit | 1 | |
| >3x Normal Limit | 2 |
0 to 3 points: Low Risk; 4 to 6 points: Intermediate Risk; 7 to 10 points: High Risk
METHODS
Design, Sample, and Setting
This was a secondary analysis of the data collected from the EMPIRE study (ECG Methods for the Prompt Identification of Coronary Events). Study design and methods were previously described in detail15. Briefly, EMPIRE was an observational cohort study that enrolled consecutive patients 18 years of age or older who had chief complaint of non-traumatic chest pain and were transported via ambulance by Pittsburgh Emergency Medical Services (EMS) to one of three participating UPMC-affiliated tertiary care centers. For this analysis we used available data from the first study cohort (2013–2014, n=750). This study was approved by the Institutional Review Board of the University of Pittsburgh.
Data Collection
Clinical data were obtained for each patient from the in-hospital (Powerchart, Cerner Inc., North Kansas City, MO) and the prehospital (emsCcharts Inc., Warrendale, PA) electronic health record by independent reviewers per an a priori defined data coding scheme. Clinical data elements followed the American College of Cardiology recommendations for measuring the management and outcomes of patients with acute coronary syndrome16 and included demographics, anthropometrics, past medical history, home medications, clinical presentation and course of hospitalization, laboratory tests, imaging studies, cardiac catheterization, treatments, and in-hospital complications. In addition, we further audited the etiology of chest pain and categorized each patient into one of these four categories as we previously described17 (1) Acute Coronary Syndrome (ACS) defined as elevation of cardiac troponin (i.e., > 99th percentile) and/or the presence of focal myocardial ischemia on cardiac imaging (i.e., echocardiogram, nuclear imaging, or angiographic evidence), (2) non-ischemic cardiopulmonary disease (e.g., stable coronary disease, pulmonary embolism, pericarditis, heart failure, etc.), (3) other non-cardiac disease (e.g., GI-related, substance-related, etc.), and (4) non-specific chest pain (e.g., musculoskeletal pain, unknown, etc.). Finally, the initial ED evaluation was documented using (1) Emergency Severity Index (ESI) score (range 1–5; 1 = critical condition, 5 = stable condition), and (2) HEART score (History, ECG, Age, Risk factors, Troponin) (range 0–10: 0 = lowest risk, 10 = highest risk). ESI score was abstracted from electronic health records as per triage nurse documentations during the indexed encounter. The HEART scores were retrospectively computed by independent reviewers blinded to outcome data based on ED admission documentation. The HEART score calculation followed the original derivation method,12 as previously described in our prior work.11 In short, the calculation was based on the following components (Table 1): (1) history of present illness, (2) electrocardiogram, (3) age, (4) risk factors (diabetes, smoking, hypercholesterolemia, family history of coronary artery disease, obesity, history of coronary revascularization, myocardial infarction, stroke or peripheral arterial disease), and (5) troponin assay. Each component of the risk score is assigned 0–2 points, and subsequently all components are summed up to a total score ranging from 0–10. A total score of 0–3 is considered low-risk, and subjects are eligible for early discharge without further evaluation; a total score of 4–6 is deemed intermediate-risk and requires further observation for evaluation or admission; a score of 7 or more is deemed high risk and suggests a need for immediate intervention.
Objectives and Endpoints
The main independent variable was the status of recent history of cancer in remission. We defined patients with recent history of cancer in remission as those with a past history of cancer of less than 10 years, and are currently cured or in remission stage of cancer of any type. We did not enroll any patients with ongoing active cancer.
The primary study outcome was the incidence of major adverse cardiac events (MACE) within 30 days of initial presentation, including events occurring during ED evaluation and hospitalization. MACE included ACS, all-cause death, cardiac arrest, ventricular tachyarrhythmia; coronary re-vascularization, and 30-day re-infarction. The incidence of MACE was adjudicated by two independent reviewers after reviewing all available medical records, including any ED visits, hospital admissions, and outpatient visits within 30 days of the indexed encounter. In case of disagreement, agreement was reached by a third reviewer as previously described11.
Statistical Analysis
Continuous variables were described with mean and standard deviation. Categorical variables were described using frequency counts and percentages. We assessed for the presence of normal distribution for continuous variables using quantile-quantile plots. Variables not normally distributed were reported as median [IQR]. Independent samples t-test (or Mann–Whitney test for skewed data) and chi-square statistics were used to compare the differences of continuous and categorical variables between groups, respectively. Area under the receiver operative curve (AU-ROC) was used to compare the predictive value of HEART score in patients with or without cancer history. Chi-square test statistic was used to compare the AU-ROC between the two groups. The significance level was set at 0.05 for two-sided hypothesis testing. SPSS® version 25 (IBM, Armonk, NY) were used for all the analysis.
RESULTS
This analysis included 750 patients aged 59±17 years; with 42% females and 40% black. Overall, there were 69 patients (9.1%) with a recent history of cancer in remission (breast cancer, n=17; lung cancer, n=12; skin cancer, n=10; prostate cancer, n=9, and other cancers, n=21). Table 2 compares the demographic and clinical characteristics between cancer patients in remission and those with no history of cancer. In general; the status of cancer in remission was associated with older age; female sex; and having a history of hypertension, dyslipidemia, known coronary artery disease, known heart failure, and prior percutaneous coronary intervention.
Table 2.
Demographic and Clinical Characteristics of Study Sample
| Characteristic | History of Cancer | |
|---|---|---|
| Yes (n=69, 9%) | No (n=681, 91%) | |
| Demographics | ||
| Age (years) | 71 ± 13 | 58 ± 16 |
| Female Sex | 45 (65.2%) | 272 (39.9%) |
| Black Race | 28 (40.6%) | 273 (40.1%) |
| Past Medical History | ||
| Hypertension | 57 (82.6%) | 462 (68.5%) |
| Dyslipidemia | 38 (55.1%) | 221 (32.8%) |
| Diabetes Mellitus | 23 (33.3%) | 173 (25.7%) |
| Known CAD | 33 (47.8%) | 215 (31.9%) |
| Known Heart Failure | 19 (27.5%) | 111 (16.5%) |
| Prior MI | 22 (31.9%) | 183 (27.3%) |
| Prior PCI / CABG | 31 (44.9%) | 176 (26.1%) |
| Initial ED Triage | ||
| ESI Triage Score | 1.9 ± 0.7 | 2.0 ± 0.8 |
| HEART Score | 4.6 ± 1.8 | 3.9 ± 2.0 |
| Admitted to Hospital | 55 (79.7%) | 507 (74.4%) |
| Course of Hospitalization | ||
| Length of Stay (days) | 2.0 [1.0–3.5] | 1.0 [0.5–3.0] |
| Confirmed ACS | 9 (13.0%) | 106 (15.6%) |
| 30-Day MACE | 12 (17.4%) | 133 (19.5% |
| Medications | ||
| Beta Blockers | 32 (46.4%) | 276 (40.5%) |
| Ace Inhibitors | 21 (30.4%) | 151 (22.2%) |
| Aspirin | 47 (68.1%) | 380 (55.8%) |
Values are n (%), mean ± SD, or median [IQR].
Abbreviation: CAD: coronary artery disease; PCI: percutaneous coronary intervention; MI: myocardial infarction; ESI: Emergency Severity Index; MACE: major adverse cardiac events.
Bold indicates significant differences between groups.
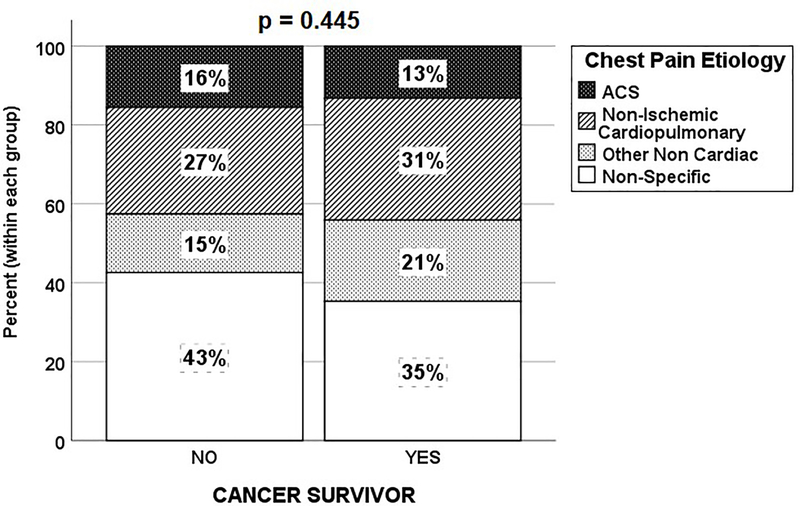
Upon initial ED triage, patients with cancer in remission received higher HEART scores compared to their counterparts (Table 2). Patients with cancer in remission had higher odds to be triaged as intermediate-to-high risk (i.e., HEART score ≥4) compared to their counterparts (68% vs. 56%, OR = 1.67 [95% CI 1.00–2.84], p = 0.05); and experienced a higher length of stay (3.3±5.6 vs. 2.2±3.3, p<0.01). However, although patients with cancer in remission received on average a high acuity ESI score of 2 and had 80% chance of being admitted to the hospital, these numbers were not different compared to their counterparts. In addition, during hospitalization, the distribution of chest pain etiology was not different between those with cancer in remission and their counterparts (Figure 1).
Figure 1.
Etiology of Chest Pain in Patients with Cancer in Remission and those with no Past History of Cancer
There was a total of 259 MACE events occurring in 145 patients (19%), including ACS (n=115), death (n=9), cardiac arrest (n=12), ventricular tachyarrhythmia (n=13), coronary revascularization (n=74), post-admission pulmonary embolism (n=2), post-admission acute heart failure (n=11), and 30-day re-infarction (n=23). There were 9 (13%) patients with ACS among cancer in remission group, with 22% of these patients presenting as unstable angina. On the other hand, 106 (16%) patients with ACS presented in those with no history of cancers, with 12% of these classified as unstable angina. There was no difference in the subsequent experience of MACE between patients with cancer in remission and their counterparts in both a univariate analysis, (17.4% vs. 19.5%, mean proportion difference 0.02% [95% CI −5.4% – 0.75%], OR = 0.87 [95% CI 0.45–1.66], p = 0.67, Table 2). History of cancer in remission was not associated with MACE after adjusting for age, sex, race, and other baseline comorbidities in multivariable analysis (Table 3).
Table 3.
Univariate and Multivariable analysis examining the association between recent history of cancer in remission and 30-day major adverse cardiac events
| Variable | Univariate OR (95% CI) | Multivariate OR (95% CI) |
|---|---|---|
| Cancer in Remission Status | 0.87 (0.45–1.66) | 0.62 (0.31–1.25) |
| Age | 1.02 (1.01–1.03) | 1.02 (1.01–1.04) |
| Male Sex | 1.08 (0.75–1.57) | 1.02 (0.68–1.53) |
| Black Race | 0.37 (0.24–0.56) | 0.40 (0.26–0.63) |
| Hypertension | 0.95 (0.64–1.41) | 0.64 (0.39–1.04) |
| Diabetes Mellitus | 1.51 (1.02–2.23) | 1.58 (1.00–2.48) |
| Current Smoker | 0.99 (0.68–1.43) | 1.21 (0.80–1.83) |
| Dyslipidemia | 1.10 (0.75–1.60) | 0.78 (0.50–1.21) |
| CAD | 1.38 (0.95–2.01) | 0.63 (0.36–1.11) |
| Prior Myocardial Infarction | 1.69 (1.15–2.48) | 1.44 (0.86–2.42) |
| Congestive Heart Failure | 1.45 (0.92–2.26) | 1.21 (0.71–2.04) |
| Prior PCI or CABG | 2.09 (1.43–3.05) | 2.14 (1.22–3.38) |
Abbreviations: CAD: coronary artery disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting.
Bold Indicates significance at p<0.05.
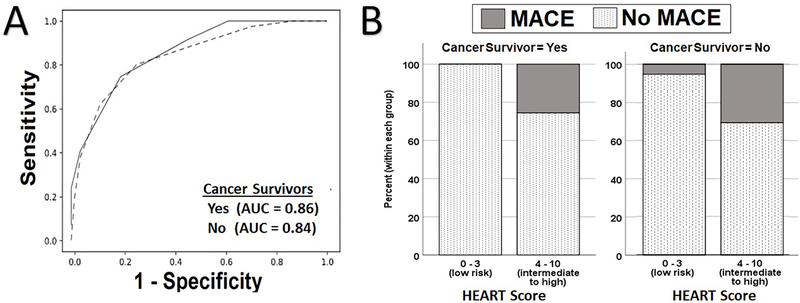
Finally, we did not find a difference in the performance of the HEART score in predicting MACE in both groups. As seen in Figure 2A, there were no differences in the area under the ROC curve between patients with cancer in remission (0.86 [95% CI 0.75–0.97]) and their counterparts (0.84 [95% CI 0.80–0.88], c2=0.093, df=2, p=0.76). Notably, no events occurred in the low risk group in patients with cancer in remission, while 5% of patients with no history of cancer classified as low risk had an event (Figure 2B). The sensitivity and specificity of the HEART score for cancer in remission status patients were 100% [95% CI 74%−100%] and 39% [95% CI 26%−52%], respectively. Meanwhile, the sensitivity and specificity were 88% [95% CI 83%−94%] and 52% [95% CI 47%−55%], respectively, in those with no history of cancer.
Figure 2.
A) Performance of HEART score in Predicting 30-Day MACE in patients with Cancer in Remission and their counterparts; B) Rate of 30-Day MACE in low risk vs intermediate to high risk HEART classes in Study Groups
DISCUSSION
In this study, we sought to examine the association between cancer in remission status with the occurrence of MACE in patients evaluated at the ED for chest pain, and compare the performance of the HEART score in patients with cancer in remission and their counterparts. We found that while cancer in remission patients do receive a higher risk assessment score, their risk of MACE and the distribution of their chest pain etiology are similar to the general chest pain population. We did not find a difference in the performance of HEART score in risk stratifying patients with cancer in remission compared to their counterparts. To our knowledge, this is the first study that specifically considers recent cancer remission status in the initial triage of patients with chest pain.
Prevalence & Baseline Characteristics
The prevalence of cancer survivors among chest pain population is approximately similar to the general US population. Our data show that patients with cancer in remission compromise 9% of our entire sample; and 17% of those 65 years of age or older. In 2016, there were an estimated 15.5 million cancer survivors in the United States8, including around 10 million patients 65 years of age or older. These latter numbers correspond to 5% and 18% of the general US population, respectively, which are roughly close to the prevalence seen in our data. Similarly, the older age of cancer survivors compared to general chest pain population is a mere reflection of the fact that two thirds of cancer survivors are older than 65 years of age6. And this consequently explains the higher rate of cardiovascular risk factors observed in this study (Table 2).
On the other hand, our data show that 65% of patients with cancer in remission in our sample were females; slightly higher compared to the expected distribution of 52% among cancer survivors in the US. The reason for this contrast is undetermined and is not explained by the prevalence of breast cancer survivors in our data since it approximates the expected distribution in the US (25% vs. 23%)6.
Impact on Outcomes
There were no differences in the distribution of chest pain etiology between cancer in remission group and their counterparts. However, although not significant, a larger proportion of patients with cancer in remission had chest pain related to either cardiac or non-cardiac disease rather than a non-specific or unknown cause when compared to general chest pain patients (Figure 1). Prior studies report that cardiovascular toxicity is a common consequence of cancer treatment which may trigger several mechanisms including, but not limited to, direct injury to cardiac muscle and provoking ischemic disease through prompting the formation of blood clots or vasospasm of coronary arteries18,19. Moreover, baseline differences between the groups, particularly patients with cancer in remission being older with more preexisting comorbidities, could be the principal reason behind patients with cancer in remission having more cardiac or non-cardiac disease as the underlying etiology, rather than a non-specific or unknown cause, as observed in non-cancer patients. More importantly, our 30-day follow up data does not show any difference in the subsequent rate of MACE when comparing the two groups (Table 2).
Finally, our data show that there was no difference in the performance of HEART when triaging chest pain patients with cancer in remission compared to those with no history of cancer, despite not considering the past history of cancer in risk estimation. This could be explained by the fact that patients with cancer in remission are older and present with a higher comorbidity burden, which results in higher HEART scores in this group. As such, cancer in remission patients in our study had higher odds to be triaged as intermediate-to-high risk (HEART score ≥ 4) compared to their counterparts. In sum, it seems to be that patients with cancer in remission presenting with chest pain mostly resemble the intermediate risk population of HEART score with both their stratification and their subsequent rate of events. However, it is worth noting that these clinical findings do not reflect the direct impact of cancer survivorship status in patients in isolation, but are rather an assessment of the clinical course of patients with cancer in remission presenting with chest pain in our cohort.
Clinical Implications
This study has some immediate clinical implications. Patients with a history of recent cancer in remission need to be approached with a high degree of clinical suspicion due to their complex cardiovascular burden, which was exhibited in this study by the high likelihood of patients with cancer in remission to be stratified as intermediate risk by HEART score. Moreover, this potential risk was emphasized by the overall rate of events in those with cancer in remission status (17.4%), which mirrors that of intermediate risk population of HEART score and is far higher than the 2% of events occurring in the low risk population12. Results of this study suggest that HEART score can be used as an appropriate tool to triage those with a recent history of cancer in remission presenting with chest pain. Adequate prediction of MACE can help clinicians make quick, reliable decisions and avoid using unnecessary radioactive diagnostics or invasive procedures.
Limitations
Our sample size of patients with cancer in remission (n=69) in this study was small. Further research is needed to expand the study in larger population. Moreover, the HEART score was computed retrospectively, which is especially important for scoring the history component of the HEART score. To address this issue, we used a systematic coding scheme by a reviewer blinded to clinical outcomes and reviewed all available ED records for complete and accurate assessment. In addition, we assessed for the occurrence of outcomes within a 30-day period, rather than the 6-week follow-up period originally described in the HEART score development. Furthermore, our study has limitations in terms of generalizability. The population of chest pain patients consisted of patients who arrived to the ED by means of EMS transport. This limits the generalizability of our findings considering that patients at the general ED include walk in patients, which potentially possess different characteristics than those arriving via EMS. Moreover, due to our enrollment criteria for patients with recent history of cancer in remission, this study cannot infer on either patients going through current active cancer treatment or those who had active cancer more than 10 years ago. Moreover, it cannot infer on the impact of being a cancer survivor in isolation since cancer survivors in our population were older and had more comorbidities. Finally, we do not know the type and duration of previous or ongoing cancer treatment. Different cancer treatments may induce different cardiac toxicities. Future research is needed to collect more information about cancer treatment and identify the relationship between types of cancer treatment and the presentation of chest pain among cancer survivors.
CONCLUSIONS
We did not observe a difference in the rate of MACE between those with a history of cancer remission within 10 years compared to their counterparts. Although a higher proportion of patients with cancer in remission were triaged as intermediate risk according to the HEART score, we found no difference in the performance of HEART score between the two groups.
Acknowledgments
Funding: National Institute of Health R01-HL-137761
Footnotes
Conflict of Interest: None
References
- 1.Johnson CB, Davis MK, Law A, Sulpher J. Shared Risk Factors for Cardiovascular Disease and Cancer: Implications for Preventive Health and Clinical Care in Oncology Patients. Can J Cardiol. 2016;32(7):900–907. [DOI] [PubMed] [Google Scholar]
- 2.Geiger S, Lange V, Suhl P, Heinemann V, Stemmler HJ. Anticancer therapy induced cardiotoxicity: review of the literature. Anticancer Drugs. 2010;21(6):578–590. [DOI] [PubMed] [Google Scholar]
- 3.Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30(2):181–191. [DOI] [PubMed] [Google Scholar]
- 4.Naaktgeboren WR, Linschoten M, de Graeff A, et al. Long-term cardiovascular health in adult cancer survivors. Maturitas. 2017;105:37–45. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–2754. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 7.Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(19):2683–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 9.Edgington A, Morgan MA. Looking beyond recurrence: comorbidities in cancer survivors. Clin J Oncol Nurs. 2011;15(1):E3–12. [DOI] [PubMed] [Google Scholar]
- 10.Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008(7):1–38. [PubMed] [Google Scholar]
- 11.Al-Zaiti SS, Faramand Z, Alrawashdeh MO, Sereika SM, Martin-Gill C, Callaway C. Comparison of clinical risk scores for triaging high-risk chest pain patients at the emergency department. Am J Emerg Med. 2019;37(3):461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153–2158. [DOI] [PubMed] [Google Scholar]
- 13.Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168(2):795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poldervaart JM, Langedijk M, Backus BE, et al. Comparison of the GRACE, HEART and TIMI score to predict major adverse cardiac events in chest pain patients at the emergency department. Int J Cardiol. 2017;227:656–661. [DOI] [PubMed] [Google Scholar]
- 15.Al-Zaiti SS, Martin-Gill C, Sejdic E, Alrawashdeh M, Callaway C. Rationale, development, and implementation of the Electrocardiographic Methods for the Prehospital Identification of Non-ST Elevation Myocardial Infarction Events (EMPIRE). J Electrocardiol. 2015;48(6):921–926. [DOI] [PubMed] [Google Scholar]
- 16.Cannon CP, Brindis RG, Chaitman BR, et al. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease. J Am Coll Cardiol. 2013;61(9):992–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivero D, Alhamaydeh M, Faramand Z, et al. Nonspecific electrocardiographic abnormalities are associated with increased length of stay and adverse cardiac outcomes in prehospital chest pain. Heart Lung. 2019;48(2):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–3131. [DOI] [PubMed] [Google Scholar]
- 19.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]