Summary
Preparatory activity is observed across multiple interconnected brain regions prior to goal-directed movement. Preparatory activity reflects discrete activity states representing specific future actions. It is unclear how this activity is mediated by multi-regional interactions. Recent evidence suggests that the cerebellum, classically associated with fine motor control, contributes to preparatory activity in the neocortex. We review recent advances and offer perspective on the function of cortico-cerebellar interactions during goal-directed behavior. We propose that the cerebellum learns to facilitate transitions between neocortical activity states. Transitions between activity states enable flexible and appropriately timed behavioral responses.
Introduction
Neocortex is critical for adaptive, learned goal-directed behavior. Complex neural activity dynamics in the neocortex reflect the integration of external and internal information prior to selecting and executing specific actions or choices. Neocortex sends long range projections to all major subcortical motor areas, including the basal ganglia, midbrain, and the cerebellum [1–4]. In turn, the outputs of these subcortical structures primarily converge in the thalamic nuclei that target areas of the neocortex involved in movement planning and execution. What could be the function of these elaborate cortico-subcortical loops?
Here we offer a perspective on the interactions between neocortex and the cerebellum during goal-directed movements. The cerebellum is classically thought to be involved in motor control. Recent evidence suggests that the cerebellar function is more expansive [5–7], including reward prediction [8–12], motor planning [13–16], and navigation [17]. New advances in neurophysiological methods and behavioral paradigms in rodents now allow a closer examination of cortico-cerebellar interactions during complex sensorimotor tasks.
The same basic circuit structure is repeated across the cerebellum. This has led to the idea that the cerebellum may control activity in other parts of the brain in ways analogous to how the cerebellum controls motor effectors [18]. During motor control, the cerebellum uses efference copy of the motor commands to generate predictive signals that help steer movement toward desired endpoints [19,20]. In this review, we synthesise recent work on cerebellar and neocortical dynamics, and propose that goal-directed movement is prepared by steering neocortical dynamics into specific activity states representing specific future actions. The cerebellum learns to use one pattern of neocortical activity to enable the next, thus steering transitions between activity states (Fig 1). Transitions between activity states enable flexible and appropriate motor plans or response sequences. The same principles may broadly apply to cerebellar contribution towards neocortical computations during other non-motor cognitive behaviors.
Figure 1.
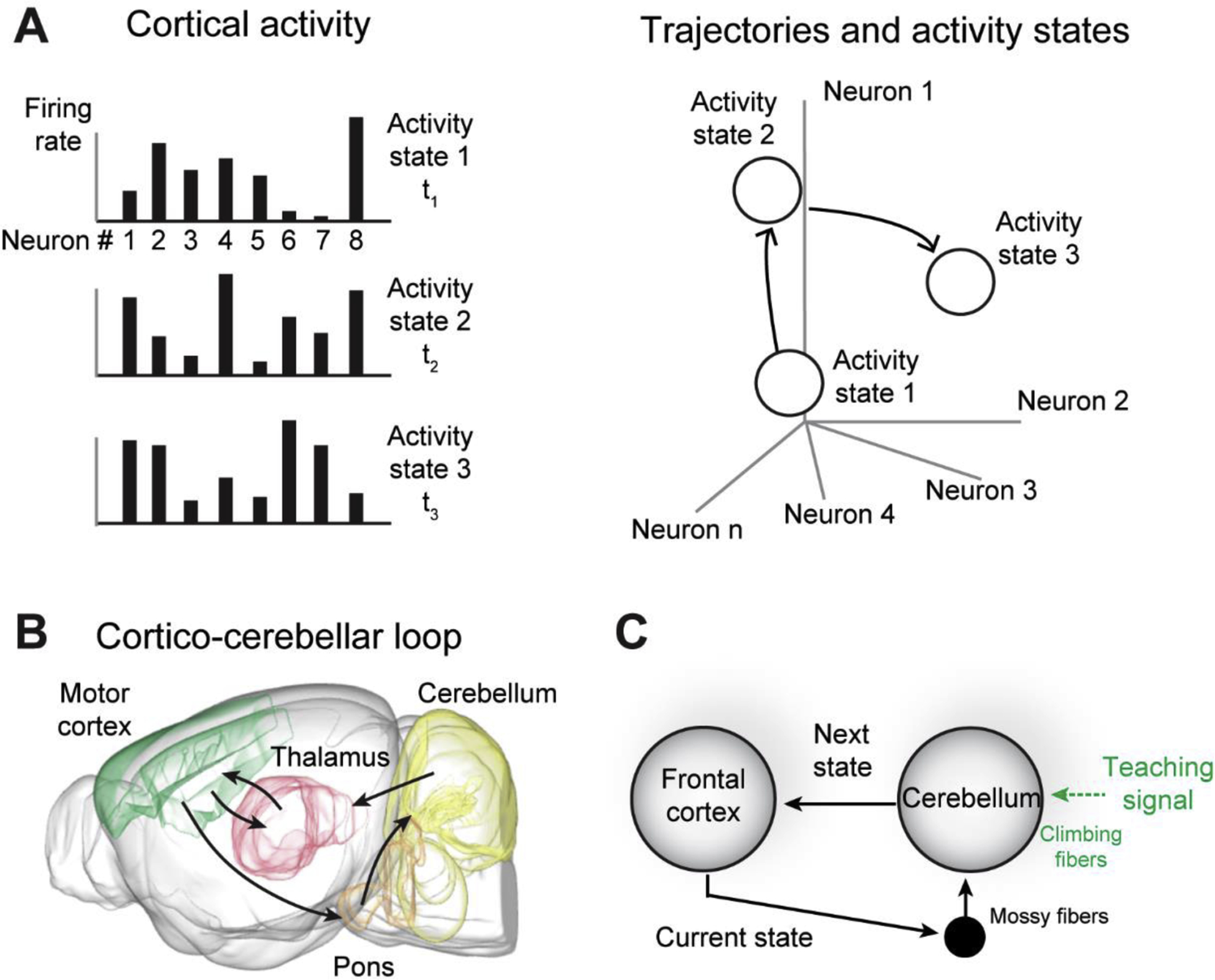
Neocortical activity states and cortico-cerebellar loop. A. Patterns of neocortical activity are specific states in activity space. Trajectories in activity space are transitions between activity states. B. Cortico-cerebellar loop. C. Proposed function of the cortico-cerebellar loop. The cerebellum steers transitions between neocortical activity states by using the current state to enable the next, guided by teaching signals.
Activity states in motor cortex represent upcoming actions
Frontal and motor areas of the neocortex are required for selecting appropriate goal-directed movements. For the purpose of this review, we define motor cortex as regions of the neocortex that have been implicated in motor planning and execution, encompassing areas elsewhere referred to as primary motor cortex, secondary motor cortex, premotor cortex, and anterior lateral motor cortex. Motor cortex neurons become active hundreds of milliseconds before movement onset [14,21–26]. Preparatory activity is best understood as a population response. In activity space, where individual dimensions are the firing rates of individual neurons, preparatory activity is represented as trajectories that converge onto discrete locations in activity space [27,28]. The process of motor planning can be described as bringing neocortical activity into specific endpoints in activity space. Trajectories describe transitions between neocortical activity states, with different trajectory endpoints corresponding to different future movements or movement plans (Fig 2A).
Figure 2.
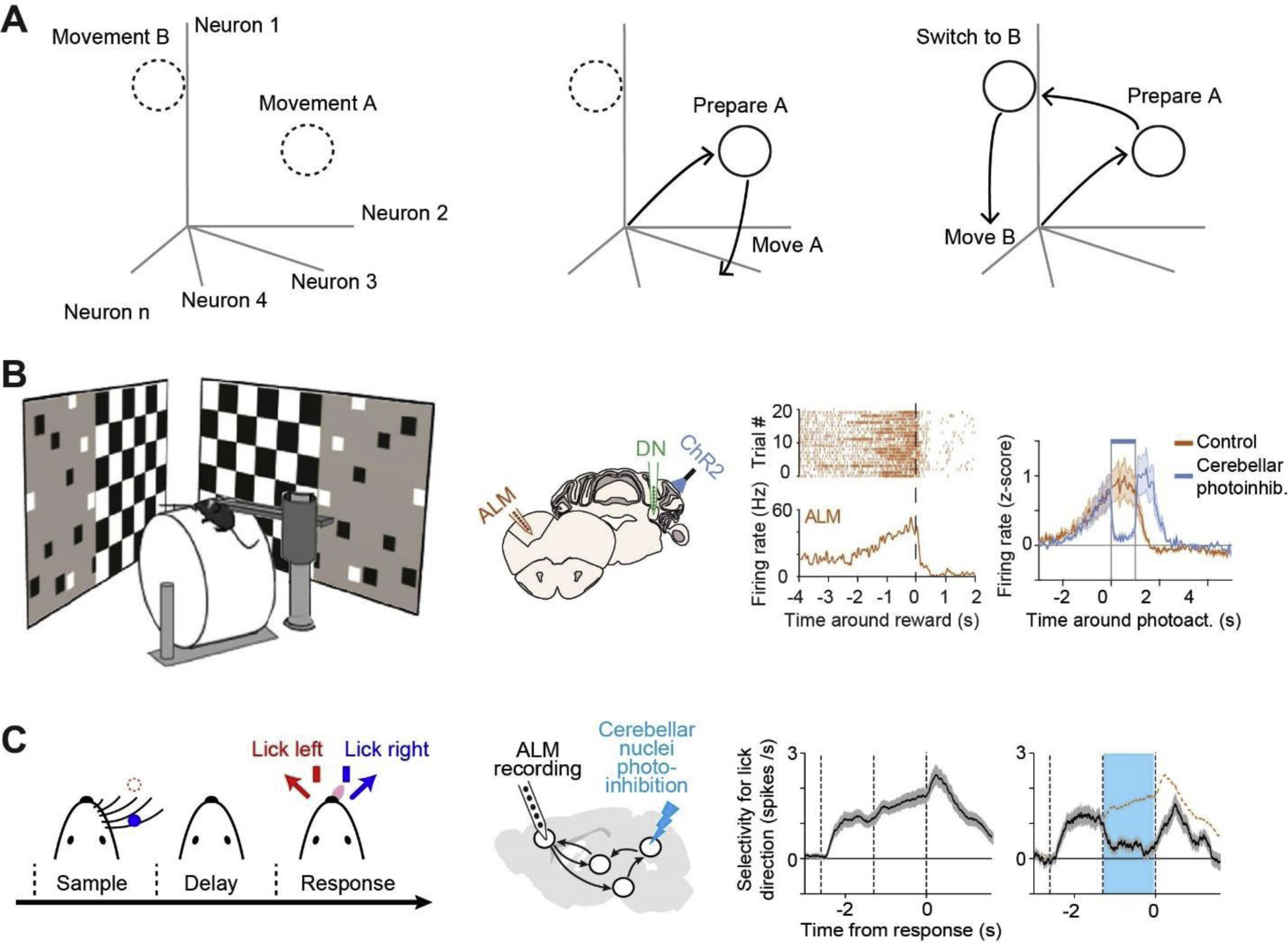
Preparatory activity mediated by the cortico-cerebellar loop. A. Discrete states in activity space correspond to specific movements. Preparatory activity trajectories converge to specific states during motor planning. Once the state is reached, the corresponding movement can be readily triggered. When the trajectory transitions to a different state, a different movement will result. B. Left, mouse navigating a virtual reality and anticipating reward at a particular location in the corridor indicated by visual landmarks. Middle, silicon probe recordings in ALM and the cerebellar nucleus while photoinhibiting the deep cerebellar nucleus. Right, preparatory activity in ALM neurons anticipates the stop which is abolished by silencing the cerebellar output [14]. C. Mouse performing a delayed response task. ALM preparatory activity anticipates directional licking which is abolished by silencing the cerebellar output [15].
Recent perturbation experiments have begun to establish a causal relationship between these endpoints in activity space and subsequent movements. For example, when activity trajectories are close to these endpoints, subsequent movement initiation is faster. When preparatory activity is pushed away from the endpoints by perturbations of neural activity, the reaction time is increased [29]. When activity is experimentally pushed to another endpoint, a different subsequent movement results, predicted by the perturbed dynamics [30,31] (Fig 2A). Thus, these endpoints represent internal activity states that specify upcoming movements, without triggering the movements [32,33]. Preparatory activity trajectories are thought to be limited to dimensions of activity space that are orthogonal to dimensions that drive downstream motor centers (output-potent) [32]. Once the endpoints are reached, the corresponding movement can be readily triggered by a separate movement initiation signal [34], which initiates the activity dynamics along the output-potent dimensions.
In theories of motor control, sensory feedback or unexpected perturbations do not trigger immediate motor response [35], but are integrated with the current motor state and intended movement goals to generate appropriate behavioral output [36–38]. The internal activity states that specify future movements without triggering the movement may serve intermediary roles that decouple incoming sensory input from premature motor output in order to enable flexible responses in a particular context. A recent study examined mice navigating a visual virtual reality in which the corridor sometimes changed direction unexpectedly. Motor cortex activity was necessary for corrective turns induced by the unexpected visual perturbation. In contrast, spontaneous turns were independent of motor cortex activity [39]. Conceptually similar experiments were also carried out in non-human primates during visually-guided arm movements [40]. Unexpected sensory perturbations induced activity changes in motor cortex, but the activity was initially orthogonal to dimensions of activity space that control movement. This created a brief ‘buffer’ decoupling the motor cortex output from the immediate sensory feedback activity, which later could reshape activity dimensions controlling movement to produce appropriate corrections [40,41]. These findings suggest that unexpected sensory feedback can rapidly reshape neocortical activity states representing upcoming movements and consequently generate appropriate motor responses. Consistent with this idea, lesions of the motor cortex in rats compromise their ability to respond to unexpected perturbations [42]. Optogenetic suppression of motor cortex impairs mice’s ability to make corrective movements [43].
Motor cortex dynamics transitioning between activity states representing different movements (prior to movement) likely mediate adaptive responses to sensory inputs and unexpected perturbations of sensory context.
Cortico-cerebellar loops in motor planning
How motor cortex dynamics map sensory input and perturbations onto appropriate activity states corresponding to specific actions remains unclear. In the dynamical systems view [27], motor cortex activity unfolds from current activity state into future activity states governed by intrinsic dynamics and external input. We propose that the cortico-cerebellar loop may be part of the same dynamic system that mediates the unfolding of activity dynamics during motor planning. We further propose that the cerebellar input could steer transitions between neocortical activity states, where it uses one pattern of activity to drive the next. The motor cortex sends a copy of its activity state to the cerebellum through the pontine nucleus and cerebellar feedback influence motor cortex activity through the thalamus (Fig 1B) [44]. Several recent studies show that the cortico-cerebellar loop propagates activity related to sensory, motor, and anticipatory signals before an upcoming movement [8,13–15,45].
The anterior lateral motor cortex (ALM) in the mouse is critical for motor planning and movement initiation [46–48]. Chabrol et al examined ALM and cerebellar activity in mice navigating a virtual reality. Mice received rewards at a particular location in the corridor indicated by visual landmarks. Mice learned to decelerate pre-emptively at the anticipated reward location and licked for reward. Long before mice stopped running, neurons in both ALM and cerebellar nuclei exhibited anticipatory activity that predicted the stop [14] (Fig 2B). In a conceptually similar experiment, Gao et al examined head-restrained mice performing a delayed response task in which they reported a tactile decision by directional licking. Preparatory activity predicting the upcoming licking direction was observed in both ALM and cerebellar nuclei [15] (Fig 2C). Furthermore, Wagner et al performed 2-photon calcium imaging in head-restrained mice performing a forelimb reaching task, correlated preparatory activity was observed in the rostral forelimb area (which overlaps with ALM) and cerebellar granule cells [8,13]. These results in rodents mirror those in non-human primates, where cerebellar output neurons exhibit ramping activity predictive of the timing and direction of self-initiated saccades [49–51]. Similar ramping before a saccade has also been observed in the motor thalamus [52] and motor cortex [53–55].
It is important to caution that some of the anticipatory activity might reflect reafferent signals from ongoing movements preceding a reward [56] or preparatory postural adjustments [57], which are not always measured in experiments. At the same time, cognitive signals within the motor system related to decision-making and motor planning could also be reflected in downstream motor effectors [58]. Within the tightly coupled sensorimotor loops, it remains to be determined which brain structures and specific activity dynamics causally contribute to preparatory activity that influences future behavior.
Recent studies have begun to use activity manipulation experiments to assess the causal contributions of motor cortex and the cerebellum to preparatory activity. These experiments show that preparatory activity in motor cortex and the cerebellum depends on each other. Preparatory activity observed across all three cerebellar nuclei [14,15] is abolished when inactivating ALM [15]. Consistent with motor cortex influencing cerebellar activity through the pontine nucleus during motor planning, inactivation of the pontine nucleus reduces correlated preparatory activity in motor cortex and cerebellar granule cells [13]. Conversely, optogenetic manipulation experiments show that the cerebellum influences the motor cortex by influencing the thalamus. Two studies found that preparatory activity in ALM was abolished by silencing the cerebellar nuclei [14,15] (Fig 2B–C). These findings suggest that the motor cortex and cerebellum work together to generate or maintain preparatory activity.
The cerebellum mediates transitions between neocortical activity states
Beyond demonstrating its necessity, these studies left unclear what specific function the cortico-cerebellar loop serves during motor planning. The theory of cerebellar function proposed by Marr and Albus [59–61] suggests that the cerebellum performs predictions guided by supervised learning [62,63]. The granule cells preprocess the mossy fiber input arriving from the pons into a basis set suitable for supervised learning [5,63,64]. More specifically, this preprocessing breaks the input stream into bits of features which are also dispersed over hundreds of milliseconds [65,66], where specific parts of the input can be associated to produce appropriate output patterns in Purkinje cells, the sole output neurons of the cerebellar cortex. Purkinje cell responses can be adjusted through a well-described plasticity mechanism, whereby a teaching signal (an error signal) provided by the climbing fibers instructs the association of Purkinje cell input [59–61]. The objective function of the cerebellum is therefore to minimize the error signal in climbing fibers by reweighting the inputs [63,65,67,68]. Once learned, the Purkinje cells can generate predictive signals that are triggered by inputs to minimize future errors. In this manner, the cerebellum can use one input activity pattern to enable the next [69]. Cerebellar inputs could be related to external sensory stimuli [65,68,70], internal signals such as efference copies of motor commands [20,71] or persistent activity associated with working memory [72,73].
This general function of the cerebellum may not only adjust movements, but could also influence activity in any region of the brain that is reciprocally connected with the cerebellum. The anatomical arrangement of the cortico-cerebellar loop provides a rich substrate for multiplexing of diverse representations and feedback control. The pyramidal tract-type neurons from the entire neocortex send collaterals into the pontine nucleus [4,74,75] that gives rise to diffuse mossy fiber input to the cerebellum [76–78]. The neocortical input activity therefore may represent sensory, motor, or internal signals, permitting the cerebellum to associate diverse contextual input with specific reward or teaching signals via the climbing fibers to shape output activity in the cerebellar nuclei. The cerebellar nuclei in turn can exert specific influences on the thalamus and motor cortex, and influence the neocortical activity into activity states corresponding to future actions appropriate for a given context. Transitions between activity states could be learned by plasticity mechanisms within the cerebellum (see below) and could be triggered by neocortical input activity related to sensory stimulus, current motor state, or unexpected perturbations. Thus, the cerebellum may steer the neocortical dynamics to enable an upcoming rewarding action, flexibly switch between different actions, or compensate for unexpected perturbations.
Dimension-specific interactions
Action-related information is coded in dynamics along specific dimensions in activity space [32,79]. In specific tasks, only activity dynamics along specific dimensions predict upcoming movements (“coding dimension”), while activity along other dimensions appears to have little influence on behavioral output [30,32,79,80]. Moreover, each task requires representation of a unique set of behavioural variables (e.g. choice, value, timing, etc.), which could be mapped onto different dimensions of population activity in the neocortex [81]. The cerebellar outputs are channeled through distinct cerebellar nuclei, which targets different but overlapping parts of the thalamus [15,82] (Fig 3), forming parallel cortico-cerebellar loops [44]. Distinct cortico-cerebellar loops may influence activity in overlapping swathes of motor cortex. We propose that distinct cortico-cerebellar loops may selectively influence motor cortex activity along subsets of dimensions (Fig 3). The influence of cerebellar feedback onto specific dimensions of neocortical activity may be established through learning. Each cortico-cerebellar loop may initially influence activity in multiple dimensions, and its influence along certain dimensions could be selectively reinforced by cerebellum-dependent supervised learning. Dimension-specific communication [83] may allow different cerebellar nuclei to differentially influence activity in motor cortex related to different actions.
Figure 3.
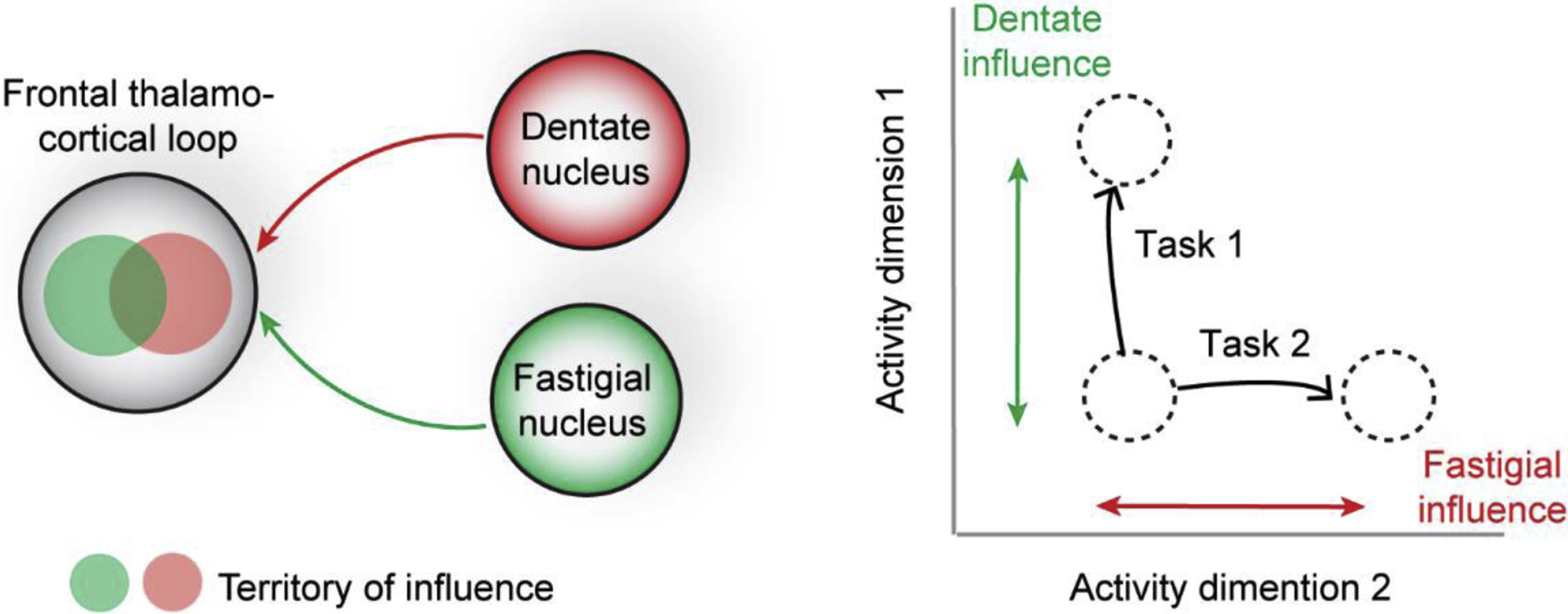
Dimension-specific cortico-cerebellar interactions. Left, distinct cerebellar nuclei influence activity in different but overlapping parts of the frontal thalamo-cortical loop. Right, distinct cerebellar nuclei influence the motor cortex activity along specific dimensions in activity space. Distinct cerebellar nuclei are differentially engaged by different tasks.
Consistent with this idea, a recent study by Gao et al examined the influence of fastigial and dentate activity on ALM dynamics related to motor planning of directional tongue movement [15]. Both fastigial and dentate perturbations produced activity changes in ALM. Interestingly, perturbation of the fastigial nucleus affected ALM activity that abolished selectivity for the direction of tongue movement along the coding dimension. In contrast, dentate nucleus perturbation changed ALM activity along dimensions orthogonal to the coding dimension, resulting in little effect on behavioural choice. A separate study by Chabrol et al. examined mice navigating a corridor while anticipating a reward and found that the dentate nucleus can drive ALM preparatory activity [14]. These differential engagements of cerebellar nuclei in different behaviors may arise from differential interactions along activity dimensions. The fastigial nucleus may influence the direction of certain types of movement [15,84] whereas the dentate nucleus signals the urgency/timing of upcoming actions [14,50,85]. It remains to be determined whether multiple parallel cortico-cerebellar loops influence activity along specific task-relevant dimensions during motor planning.
Learning transitions between neocortical activity states
The neocortex exhibits sequential activity representing sensory stimuli, behavioural choice or specific movements [86–89], essentially a series of activity states, which emerge as a result of learning [88,89]. Can the cerebellum influence transitions between activity states by learning to drive one pattern of neocortical activity from the previous? These transitions can be established through supervised learning in the cerebellum [59–61], whose mechanisms have been well established for eye blink conditioning [65,90–92] (Fig 4A). During eyeblink conditioning, Purkinje cells learn to associate the inputs from the pontine nucleus (signaling the conditioned stimulus, e.g. tone) with teaching signals from the inferior olive (signaling the unconditioned stimulus, e.g. air puff to eyelid). Once learned, Purkinje cells can produce appropriately timed activity that is triggered by a conditioned stimulus to anticipate a future air puff and drive the predictive eyelid closure [65] (Fig 4A).
Figure 4.
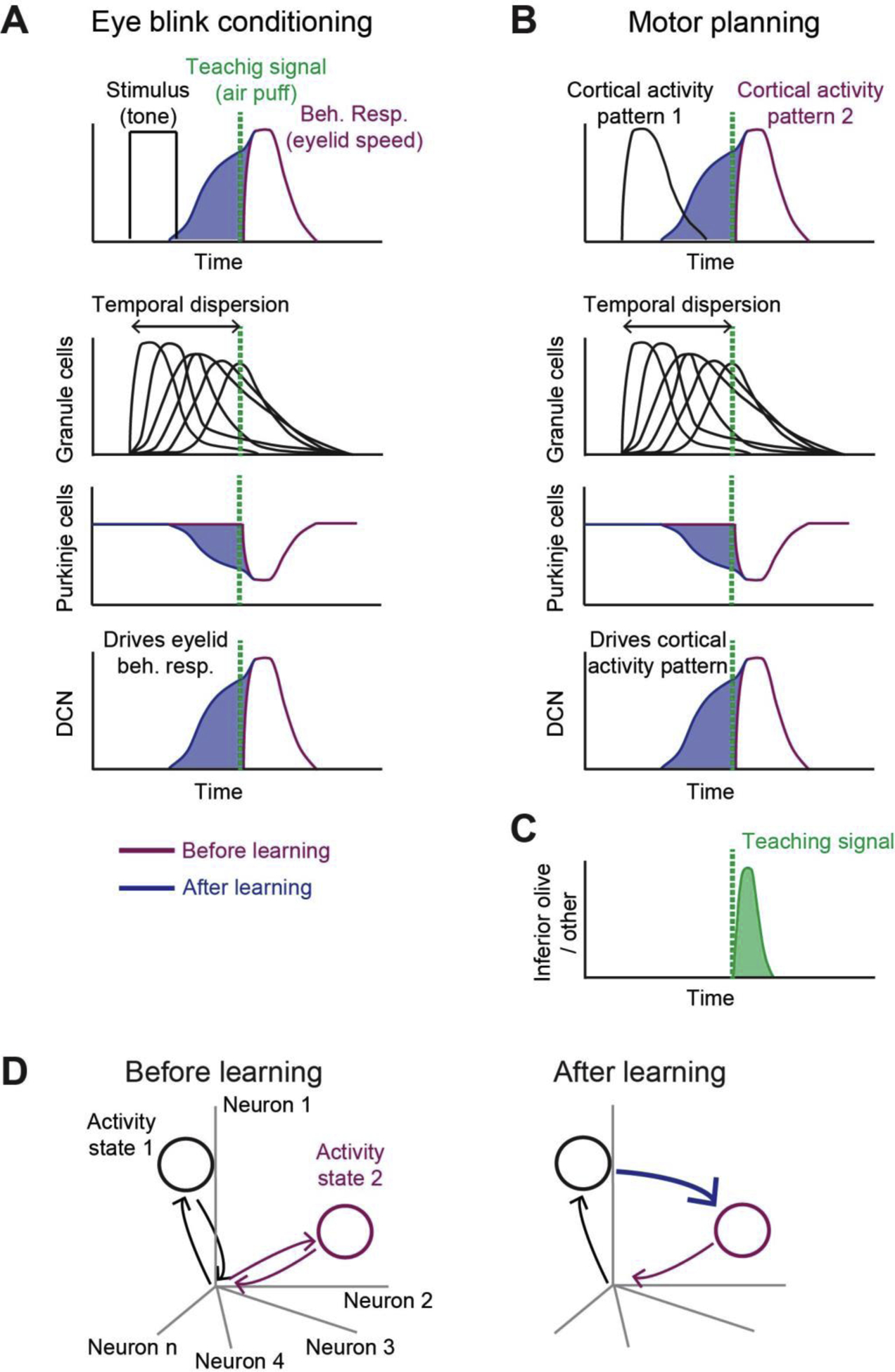
Hypothesis: the cerebellum learns to steer transitions between neocortical activity states. A. Top, during eye blink trace conditioning, a tone (black line) is followed by an aversive air puff (green dashed line), which triggers eyelid closure (purple line). If the tone consistently precedes the air puff, the cerebellum learns to produce anticipatory output (blue) that is triggered by the tone to drive predictive eyelid closure. Bottom, supervised learning mechanism within the cerebellum. The pontine input signaling the tone triggers diverse and temporally dispersed activity in the granule cells [65,66]. The Purkinje cells learn to reweigh the granule cell input via cerebellar LTD, guided by the teaching signal from the inferior olive (green), to produce timed suppression of Purkinje cells in anticipation of the air puff (blue). Once learned, the tone-triggered decrease in Purkinje cell responses disinhibits the deep cerebellar nucleus (DCN) neurons to drive eyelid closure before the air puff. B. Hypothetically, the same supervised learning mechanism in the cerebellum can learn to anticipate transitions between neocortical activity states. If one pattern of neocortical activity is consistently followed by another in conjunction with a teaching signal, the cerebellum may learn to generate predictive responses that drive the second neocortical activity pattern. C. Putative teaching signal that instructs association between neocortical activity patterns. The teaching signal may reflect an unpredicted occurrence of a neocortical activity pattern. A mismatch between the predicted neocortical activity pattern and the actual activity pattern may be represented in the inferior olive. D. Neocortical activity patterns correspond to specific states in activity space. The predictive responses learned by the cerebellum form new transitions between activity states.
We propose that the same supervised learning mechanism allows the cerebellum to learn transitions between relevant neocortical activity states (Fig 4B,D). If one neocortical activity pattern is consistently followed by the next concomitant with a teaching signal, the cerebellum could learn to increase the likelihood of transitions between these states. A possible teaching signal may be any mismatch between the prediction of the future state and the actual neocortical activity pattern [93] (Fig 4C), that may be represented in the inferior olive. Once the new transition is learned, the cerebellum could anticipate and steer the neocortical state transitions (Fig 4B, D). Copies of neocortical activity sent to the cerebellum and resultant cerebellar feedback may steer transitions of neocortical activity to the next predicted state. For example, cerebellar learning mechanism may form new associations between a cortical representation of sensory stimulus with a subsequent movement [94], effectively facilitating sensorimotor transformations. Furthermore, the same mechanism may associate representations of individual movement plans to form a future motor sequence [95], or compensate for unexpected sensory perturbations by learning appropriate transitions that convert output-null neocortical activity states (e.g. related to unexpected sensory perturbations) into output-potent ones (e.g. activity states representing specific actions) [39,40].
Similar cerebellar plasticity mechanisms that set up transitions between activity states may also support learning of timing of these transitions. It is well known that certain frontal and motor neocortical neurons exhibit activity that ramps to a fixed threshold before movement onset [53,96–100]. The speed of ramping predicts movement onset time [51,53,55,96,97], and the slope of ramping is flexibly adjusted depending on the timing of the task [31,101,102]. The ramping activity appears to originate from outside of motor cortex [30,31,51,52,103]. Similar ramps occur in the cerebellar cortical regions that reflect motor planning [14,104]. This ramping signal is consistent with a timing signal that anticipates an upcoming reward or rewarding action [8,11,12,14]. Thus, during goal-directed movement, the cerebellar cortex may compute a ramp in the same way it computes timing during eye blink conditioning [92,105], thereby appropriately adjusting the speed of transitions in neocortical activity states.
Interestingly, recent studies examining motor learning found that the motor cortex activity occupies similar dimensions in activity space before and after the learning but the same activity patterns are quickly re-associated with different motor outputs [106,107]. We suggest that a relatively limited repertoire of neocortical activity states serves as a substrate upon which the cerebellum carries out fast supervised learning to generate appropriate activity transitions, guided by specific teaching signals [94,108]. Recent imaging and electrophysiology recordings in the cerebellum revealed a multitude of teaching signals in climbing fibers that are related to behavioral errors [67,70,94], unexpected reward [11], and anticipatory signals that predict rewarding movement [11,12]. The cerebellum thus has access to sensory, motor, and internal signals, and in theory could guide association of relevant activity states and form transitions between them.
Funneling of trajectories toward activity states is described by attractor dynamics [27,31,109]. Cerebellar feedback connections may help establish specific attractors in motor cortex during motor planning, in addition to the thalamocortical and corticocortical connections. Additional work is needed to incorporate the learned dynamics in the cortico-cerebellar loop into the attractor network.
Closing thoughts
Building on a rich body of literature, we have proposed a conceptual framework for understanding the role of the cerebellum and motor cortex in predictive motor control and learning. In this framework, the cerebellum learns transitions between neocortical activity states, taking one pattern of activity to drive the next. What signals instruct the cerebellar learning of transitions between neocortical activity states? One possibility is that the teaching signals may be triggered by a mismatch between predictions of the internal model and current neocortical activity pattern (e.g. sensory input) [11,93,94].
To test this theory, it will be important to make use of closed-loop virtual reality and augmented reality behavioural assays within which require the animals to rapidly adjust their motor plans, while monitoring activity in the cerebellum and motor cortex. By introducing controlled sensory events that violate current internal model (e.g. unexpected sensory feedback) [39], one could test for the presence of error-induced activity in the cerebellum, and whether error signals facilitate new transitions in neocortical activity spaces that allow for subsequent behavioural adjustments.
The neocortex may exhibit a relatively fixed set of activity states upon which the cerebellum can learn new activity transitions by rapidly forming associations, guided by the teaching signal. The functional organization in the cerebellum may be related to reciprocal connectivity with neocortical regions, and with combinations of sensory and motor areas [45], potentially allowing the multiplexing of associable neocortical signals. The Purkinje cells in specific cerebellar regions may detect correlated patterns of neocortical activity distributed across multiple areas to steer transitions in neocortical activity sequences required for movement. Elucidating the functional organization of cortical-cerebellar loops and identification of relevant cerebellar regions involved in different tasks is therefore a major open question.
Finally, the cortico-cerebellar loop is part of a larger multi-regional circuit. Motor cortex also forms long-range loops with the basal ganglia and the superior colliculus. These loops also converge in the thalamus [110] and similarly play a role in action selection [111] and motor timing [51]. Resolving the relative contributions of distinct long-range loops during goal-directed behavior will require direct comparisons within the same behavioral task.
Highlight.
Preparatory activity reflects activity states representing specific actions
Preparatory activity is dependent on cortico-cerebellar interactions
The cerebellum learns to use one neocortical activity state to drive the next
Transitions between activity states enable flexible and appropriate actions
Acknowledgements
We thank Javier Medina, Tom Otis, Andrei Khilkevich, Shaul Druckmann, and Shogo Ohmae for insightful discussions, Karel Svoboda and anonymous reviewer for comments on the manuscript. This work is funded by the Robert and Janice McNair Foundation, Whitehall Foundation, Searle Scholars Program, the Pew Charitable Trusts, NIH (NS112312, NS113110), Simons Collaboration on the Global Brain, and Sainsbury Wellcome Centre Core grant (Wellcome Trust and Gatsby Foundation, 165296).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no actual or potential conflict of interest.
References
- 1.Shepherd GM: Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 2013, 14:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker A, Kalmbach B, Morishima M, Kim J, Juavinett A, Li N, Dembrow N: Specialized Subpopulations of Deep-Layer Pyramidal Neurons in the Neocortex: Bridging Cellular Properties to Functional Consequences. J Neurosci 2018, 38:5441–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Economo MN, Viswanathan S, Tasic B, Bas E, Winnubst J, Menon V, Graybuck LT, Nguyen TN, Smith KA, Yao Z, et al. : Distinct descending motor cortex pathways and their roles in movement. Nature 2018, 563:79–84. [DOI] [PubMed] [Google Scholar]
- 4.Winnubst J, Bas E, Ferreira TA, Wu Z, Economo MN, Edson P, Arthur BJ, Bruns C, Rokicki K, Schauder D, et al. : Reconstruction of 1,000 Projection Neurons Reveals New Cell Types and Organization of Long-Range Connectivity in the Mouse Brain. Cell 2019, 179:268–281 e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner MJ, Luo L: Neocortex-Cerebellum Circuits for Cognitive Processing. Trends Neurosci 2020, 43:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raymond JL: Research on the cerebellum yields rewards. Nature 2020, 579:202–203. [DOI] [PubMed] [Google Scholar]
- 7.Hull C: Prediction signals in the cerebellum: beyond supervised motor learning. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.**.Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L: Cerebellar granule cells encode the expectation of reward. Nature 2017, 544:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cerebellar granule cells signal reward and reward omission. A subset of granule cells exhibit activity that anticipates predictable reward, consistent with expectation of reward.
- 9.Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K: Cerebellar modulation of the reward circuitry and social behavior. Science 2019, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina JF: Teaching the cerebellum about reward. Nat Neurosci 2019, 22:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.**.Kostadinov D, Beau M, Pozo MB, Hausser M: Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Imaging of cerebellar climbing fibers during visuomotor behavior in mice shows a multitude of responses related to sensory, motor, reward, and predictions distributed across cerebellar lobules. The largest response is observed after a predicable rewarding action.
- 12.**.Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, Joshua M, Hull C: Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci 2018, 21:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]; In mice performing a visuomotor task, imaging of cerebellar climbing fibers shows the largest response after a movement that leads to a reward. The signal is consistent with predictions of rewarding actions and inconsistent with motor error signals.
- 13.**.Wagner MJ, Kim TH, Kadmon J, Nguyen ND, Ganguli S, Schnitzer MJ, Luo L: Shared Cortex-Cerebellum Dynamics in the Execution and Learning of a Motor Task. Cell 2019, 177:669–682 e624. [DOI] [PMC free article] [PubMed] [Google Scholar]; Motor cortex and cerebellar granule cells exhibit correlated preparatory activity predicting the direction of upcoming forelimb movement. Correlated activity emerges over learning. Silencing the pontine nucleus reduced cerebellar preparatory activity.
- 14.**.Chabrol FP, Blot A, Mrsic-Flogel TD: Cerebellar Contribution to Preparatory Activity in Motor Neocortex. Neuron 2019, 103:506–519 e504. [DOI] [PMC free article] [PubMed] [Google Scholar]; In mice navigating a virtual visual corridor and anticipating a reward, preparatory activity in motor cortex and dentate cerebellar nucleus predict the upcoming reward timing. Preparatory activity in motor cortex requires cerebellar input.
- 15.**.Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, Li N: A cortico-cerebellar loop for motor planning. Nature 2018, 563:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mouse ALM and cerebellar nuclei exhibit preparatory activity predicting the direction of upcoming licking movement. Silencing ALM abolishes cerebellar preparatory activity. The fastigial cerebellar nucleus drives ALM preparatory activity through the thalamus.
- 16.Deverett B, Koay SA, Oostland M, Wang SS: Cerebellar involvement in an evidence-accumulation decision-making task. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rochefort C, Arabo A, Andre M, Poucet B, Save E, Rondi-Reig L: Cerebellum shapes hippocampal spatial code. Science 2011, 334:385–389. [DOI] [PubMed] [Google Scholar]
- 18.Ito M: Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 2008, 9:304–313. [DOI] [PubMed] [Google Scholar]
- 19.Wolpert DM, Miall RC, Kawato M: Internal models in the cerebellum. Trends Cogn Sci 1998, 2:338–347. [DOI] [PubMed] [Google Scholar]
- 20.Shadmehr R, Smith MA, Krakauer JW: Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 2010, 33:89–108. [DOI] [PubMed] [Google Scholar]
- 21.Tanji J, Evarts EV: Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol 1976, 39:1062–1068. [DOI] [PubMed] [Google Scholar]
- 22.Wise SP: The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci 1985, 8:1–19. [DOI] [PubMed] [Google Scholar]
- 23.Riehle A, Requin J: Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol 1989, 61:534–549. [DOI] [PubMed] [Google Scholar]
- 24.Crutcher MD, Alexander GE: Movement-related neuronal activity selectively coding either direction or muscle pattern in three motor areas of the monkey. Journal of neurophysiology 1990, 64:151–163. [DOI] [PubMed] [Google Scholar]
- 25.Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV: Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron 2010, 68:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svoboda K, Li N: Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol 2018, 49:33–41. [DOI] [PubMed] [Google Scholar]
- 27.Shenoy KV, Sahani M, Churchland MM: Cortical control of arm movements: a dynamical systems perspective. Annual review of neuroscience 2013, 36:337–359. [DOI] [PubMed] [Google Scholar]
- 28.Afshar A, Santhanam G, Yu BM, Ryu SI, Sahani M, Shenoy KV: Single-trial neural correlates of arm movement preparation. Neuron 2011, 71:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churchland MM, Shenoy KV: Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol 2007, 97:348–359. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Daie K, Svoboda K, Druckmann S: Robust neuronal dynamics in premotor cortex during motor planning. Nature 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inagaki HK, Fontolan L, Romani S, Svoboda K: Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature 2019, 566:212–217. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman MT, Churchland MM, Ryu SI, Shenoy KV: Cortical activity in the null space: permitting preparation without movement. Nature neuroscience 2014, 17:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ames KC, Ryu SI, Shenoy KV: Neural dynamics of reaching following incorrect or absent motor preparation. Neuron 2014, 81:438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman MT, Seely JS, Sussillo D, Ryu SI, Shenoy KV, Churchland MM: The Largest Response Component in the Motor Cortex Reflects Movement Timing but Not Movement Type. eNeuro 2016, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cluff T, Crevecoeur F, Scott SH: A perspective on multisensory integration and rapid perturbation responses. Vision Res 2015, 110:215–222. [DOI] [PubMed] [Google Scholar]
- 36.Todorov E, Jordan MI: Optimal feedback control as a theory of motor coordination. Nat Neurosci 2002, 5:1226–1235. [DOI] [PubMed] [Google Scholar]
- 37.Scott SH: The computational and neural basis of voluntary motor control and planning. Trends Cogn Sci 2012, 16:541–549. [DOI] [PubMed] [Google Scholar]
- 38.Merel J, Botvinick M, Wayne G: Hierarchical motor control in mammals and machines. Nat Commun 2019, 10:5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.**.Heindorf M, Arber S, Keller GB: Mouse Motor Cortex Coordinates the Behavioral Response to Unpredicted Sensory Feedback. Neuron 2018, 99:1040–1054 e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]; In mice navigating a virtual visual corridor, the motor cortex is required for corrective turns in response to unexpected visual perturbations but not for spontaneous turns. Layer 5 neuron activities signal the corrective turns.
- 40.**.Stavisky SD, Kao JC, Ryu SI, Shenoy KV: Motor Cortical Visuomotor Feedback Activity Is Initially Isolated from Downstream Targets in Output-Null Neural State Space Dimensions. Neuron 2017, 95:195–208 e199. [DOI] [PMC free article] [PubMed] [Google Scholar]; In nonhuman primates controlling a computer cursor using premotor cortex activity, visuomotor perturbations induce activity that is initially limited to the output-null dimension and later aligns with the output-potent dimension to produce corrections.
- 41.Ames KC, Ryu SI, Shenoy KV: Simultaneous motor preparation and execution in a last-moment reach correction task. Nat Commun 2019, 10:2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopes G, Nogueira J, Dimitriadis G, Menedez JA, Paton JJ, Kampff AR: A robust role for motor cortex. BioRxiv 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bollu TP, Whitehead SC, Kardon B, Redd J, Liu MH, Goldberg JH: Tongue Kinematics. Cortex-dependent corrections as the mouse tongue reaches for, and misses, targets. BioRxiv 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strick PL, Dum RP, Fiez JA: Cerebellum and nonmotor function. Annu Rev Neurosci 2009, 32:413–434. [DOI] [PubMed] [Google Scholar]
- 45.**.Proville RD, Spolidoro M, Guyon N, Dugue GP, Selimi F, Isope P, Popa D, Lena C: Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat Neurosci 2014, 17:1233–1239. [DOI] [PubMed] [Google Scholar]; Inputs from the mouse vibrissa somatosensory cortex and vibrissa motor cortex converge in the crus 1 region of the cerebellum, and crus 1 output affects activity in the thalamus and vibrissa motor cortex. The cortico-cerebellar loop contributes to whisker sensorimotor behavior.
- 46.Guo ZV, Li N, Huber D, Ophir E, Gutnisky DA, Ting JT, Feng G, Svoboda K: Flow of cortical activity underlying a tactile decision in mice. Neuron 2014, 81:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N, Chen TW, Guo ZV, Gerfen CR, Svoboda K: A motor cortex circuit for motor planning and movement. Nature 2015, 519:51–56. [DOI] [PubMed] [Google Scholar]
- 48.Chen TW, Li N, Daie K, Svoboda K: A Map of Anticipatory Activity in Mouse Motor Cortex. Neuron 2017, 94:866–879 e864. [DOI] [PubMed] [Google Scholar]
- 49.Ashmore RC, Sommer MA: Delay activity of saccade-related neurons in the caudal dentate nucleus of the macaque cerebellum. J Neurophysiol 2013, 109:2129–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohmae S, Kunimatsu J, Tanaka M: Cerebellar Roles in Self-Timing for Sub- and Supra-Second Intervals. J Neurosci 2017, 37:3511–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.**.Kunimatsu J, Suzuki TW, Ohmae S, Tanaka M: Different contributions of preparatory activity in the basal ganglia and cerebellum for self-timing. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; The striatum and dentate cerebellar nucleus exhibit ramping activity before self-timed saccades in nonhuman primates. The slope of ramping activity in the striatum predicts the timing of action, whereas the dentate nucleus exhibits ramping before movement onset.
- 52.Tanaka M: Cognitive signals in the primate motor thalamus predict saccade timing. J Neurosci 2007, 27:12109–12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanes DP, Schall JD: Neural control of voluntary movement initiation. Science 1996, 274:427–430. [DOI] [PubMed] [Google Scholar]
- 54.Kim JN, Shadlen MN: Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci 1999, 2:176–185. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Narain D, Hosseini EA, Jazayeri M: Flexible timing by temporal scaling of cortical responses. Nat Neurosci 2018, 21:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK: Single-trial neural dynamics are dominated by richly varied movements. Nat Neurosci 2019, 22:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massion J: Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol 1992, 38:35–56. [DOI] [PubMed] [Google Scholar]
- 58.Selen LP, Shadlen MN, Wolpert DM: Deliberation in the motor system: reflex gains track evolving evidence leading to a decision. J Neurosci 2012, 32:2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marr D: A theory of cerebellar cortex. J Physiol 1969, 202:437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Albus JS: A theory of cerebellar function. Mathematical Biosciences 1971, 10:25–61. [Google Scholar]
- 61.Ito M: The cerebellum and neural control: Raven, San Diego; 1984. [Google Scholar]
- 62.Doya K: What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw 1999, 12:961–974. [DOI] [PubMed] [Google Scholar]
- 63.Raymond JL, Medina JF: Computational Principles of Supervised Learning in the Cerebellum. Annu Rev Neurosci 2018, 41:233–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cayco-Gajic NA, Silver RA: Re-evaluating Circuit Mechanisms Underlying Pattern Separation. Neuron 2019, 101:584–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Medina JF, Mauk MD: Computer simulation of cerebellar information processing. Nat Neurosci 2000, 3 Suppl:1205–1211. [DOI] [PubMed] [Google Scholar]
- 66.Chabrol FP, Arenz A, Wiechert MT, Margrie TW, DiGregorio DA: Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat Neurosci 2015, 18:718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medina JF, Lisberger SG: Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci 2008, 11:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suvrathan A, Payne HL, Raymond JL: Timing Rules for Synaptic Plasticity Matched to Behavioral Function. Neuron 2016, 92:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka H, Ishikawa T, Kakei S: Neural Evidence of the Cerebellum as a State Predictor. Cerebellum 2019, 18:349–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohmae S, Medina JF: Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci 2015, 18:1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R: Encoding of error and learning to correct that error by the Purkinje cells of the cerebellum. Nat Neurosci 2018, 21:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD: Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem 2009, 16:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegel JJ, Kalmbach B, Chitwood RA, Mauk MD: Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyelid conditioning. J Neurophysiol 2012, 107:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. : A mesoscale connectome of the mouse brain. Nature 2014, 508:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kita T, Kita H: The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci 2012, 32:5990–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biswas MS, Luo Y, Sarpong GA, Sugihara I: Divergent projections of single pontocerebellar axons to multiple cerebellar lobules in the mouse. J Comp Neurol 2019, 527:1966–1985. [DOI] [PubMed] [Google Scholar]
- 77.Serapide MF, Panto MR, Parenti R, Zappala A, Cicirata F: Multiple zonal projections of the basilar pontine nuclei to the cerebellar cortex of the rat. J Comp Neurol 2001, 430:471–484. [DOI] [PubMed] [Google Scholar]
- 78.Sillitoe RV, Joyner AL: Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol 2007, 23:549–577. [DOI] [PubMed] [Google Scholar]
- 79.Druckmann S, Chklovskii DB: Neuronal circuits underlying persistent representations despite time varying activity. Current biology : CB 2012, 22:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elsayed GF, Lara AH, Kaufman MT, Churchland MM, Cunningham JP: Reorganization between preparatory and movement population responses in motor cortex. Nat Commun 2016, 7:13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mante V, Sussillo D, Shenoy KV, Newsome WT: Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 2013, 503:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kebschull JM, Ringach N, Richman EB, Friedmann D, Kolluru SS, Jones RC, Allen WE, Wang Y, Zhou H, Cho SW, et al. : Cerebellar nuclei evolved by repeatedly duplicating a conserved cell type set. BioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Semedo JD, Zandvakili A, Machens CK, Yu BM, Kohn A: Cortical Areas Interact through a Communication Subspace. Neuron 2019, 102:249–259 e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robinson FR, Straube A, Fuchs AF: Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol 1993, 70:1741–1758. [DOI] [PubMed] [Google Scholar]
- 85.Ohmae S, Uematsu A, Tanaka M: Temporally specific sensory signals for the detection of stimulus omission in the primate deep cerebellar nuclei. J Neurosci 2013, 33:15432–15441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Runyan CA, Piasini E, Panzeri S, Harvey CD: Distinct timescales of population coding across cortex. Nature 2017, 548:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harvey CD, Coen P, Tank DW: Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 2012, 484:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peters AJ, Chen SX, Komiyama T: Emergence of reproducible spatiotemporal activity during motor learning. Nature 2014, 510:263–267. [DOI] [PubMed] [Google Scholar]
- 89.Peters AJ, Lee J, Hedrick NG, O’Neil K, Komiyama T: Reorganization of corticospinal output during motor learning. Nat Neurosci 2017, 20:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCormick DA, Thompson RF: Cerebellum: essential involvement in the classically conditioned eyelid response. Science 1984, 223:296–299. [DOI] [PubMed] [Google Scholar]
- 91.Jirenhed DA, Bengtsson F, Hesslow G: Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci 2007, 27:2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ten Brinke MM, Heiney SA, Wang X, Proietti-Onori M, Boele HJ, Bakermans J, Medina JF, Gao Z, De Zeeuw CI: Dynamic modulation of activity in cerebellar nuclei neurons during pavlovian eyeblink conditioning in mice. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keller GB, Mrsic-Flogel TD: Predictive Processing: A Canonical Cortical Computation. Neuron 2018, 100:424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.**.Sendhilnathan N, Ipata AE, Goldberg ME: Neural Correlates of Reinforcement Learning in Mid-lateral Cerebellum. Neuron 2020, 106:188–198 e185. [DOI] [PMC free article] [PubMed] [Google Scholar]; In nonhuman primates learning to associate visual stimuli with motor responses, Purkinje cells signal the outcome of decisions from the previous trial. The signal diminishes after the new association is learned, consistent with a reinforcement learning signal.
- 95.**.Khilkevich A, Zambrano J, Richards MM, Mauk MD: Cerebellar implementation of movement sequences through feedback. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; In eye blink conditioning, the cerebellum can learn to use the response from one eyeblink as input to trigger another eyeblink. The result suggests the cerebellum can learn a motor sequence by using feedbacks from one movement to drive the next.
- 96.Murakami M, Vicente MI, Costa GM, Mainen ZF: Neural antecedents of self-initiated actions in secondary motor cortex. Nature neuroscience 2014, 17:1574–1582. [DOI] [PubMed] [Google Scholar]
- 97.Maimon G, Assad JA: A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci 2006, 9:948–955. [DOI] [PubMed] [Google Scholar]
- 98.Roitman JD, Shadlen MN: Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 2002, 22:9475–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Churchland AK, Kiani R, Shadlen MN: Decision-making with multiple alternatives. Nat Neurosci 2008, 11:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thura D, Cisek P: Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron 2014, 81:1401–1416. [DOI] [PubMed] [Google Scholar]
- 101.Remington ED, Narain D, Hosseini EA, Jazayeri M: Flexible Sensorimotor Computations through Rapid Reconfiguration of Cortical Dynamics. Neuron 2018, 98:1005–1019 e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peixoto D, Kiani R, Chandrasekaran C, Ryu SI, Shenoy KV, Newsome WT: Population dynamics of choice representation in dorsal premotor and primary motor cortex. BioRxiv 2018. [Google Scholar]
- 103.Thura D, Cisek P: The Basal Ganglia Do Not Select Reach Targets but Control the Urgency of Commitment. Neuron 2017, 95:1160–1170 e1165. [DOI] [PubMed] [Google Scholar]
- 104.Lin Q, Manley J, Helmreich M, Schlumm F, Li JM, Robson DN, Engert F, Schier A, Nobauer T, Vaziri A: Cerebellar Neurodynamics Predict Decision Timing and Outcome on the Single-Trial Level. Cell 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halverson HE, Khilkevich A, Mauk MD: Relating cerebellar purkinje cell activity to the timing and amplitude of conditioned eyelid responses. J Neurosci 2015, 35:7813–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sadtler PT, Quick KM, Golub MD, Chase SM, Ryu SI, Tyler-Kabara EC, Yu BM, Batista AP: Neural constraints on learning. Nature 2014, 512:423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Golub MD, Sadtler PT, Oby ER, Quick KM, Ryu SI, Tyler-Kabara EC, Batista AP, Chase SM, Yu BM: Learning by neural reassociation. Nat Neurosci 2018, 21:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vyas S, O’Shea DJ, Ryu SI, Shenoy KV: Causal Role of Motor Preparation during Error-Driven Learning. Neuron 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang XJ: Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci 2001, 24:455–463. [DOI] [PubMed] [Google Scholar]
- 110.Guo ZV, Inagaki HK, Daie K, Druckmann S, Gerfen CR, Svoboda K: Maintenance of persistent activity in a frontal thalamocortical loop. Nature 2017, 545:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hikosaka O: Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci 2007, 1104:229–249. [DOI] [PubMed] [Google Scholar]


