Abstract
Background:
One mechanism by which acute psychosocial stress effects health-related cognitions and behaviors is through changes in the level of mental abstraction when processing information. However, it is unclear whether levels of mental abstraction would be higher or lower after an acute psychosocial stressor.
Objectives:
This research examined acute psychosocial stress’s impact on levels of mental abstraction.
Design:
Randomized between-subjects experimental design.
Methods:
A diverse sample of 164 undergraduate students aged 18–24 years old were randomly assigned to an acute psychosocial stressor or non-stressful control condition. Blood pressure (BP), heart rate (HR), and negative affect were monitored throughout the study and mental abstraction was measured at the end of each condition.
Results:
Mental abstraction was statistically significantly higher (i.e., more abstract) after the stress condition than after the control condition (p = 0.005, d = 0.44). This association was partially explained by negative affect (p = 0.017), but not BP or HR (ps > 0.60).
Conclusions:
Acute psychosocial stress is associated with higher levels of mental abstraction after the stressor. These findings may have implications for stress-relevant interventions as accounting for the level of mental abstraction may enhance the efficacy of the intervention.
Keywords: Acute psychosocial stress, Psychological outlook, Mental abstraction, Experimental design, Behavioral Identification Form
Psychosocial stress is a ubiquitous process that people experience throughout their daily lives. The deleterious impact that chronic and repeatedly experienced psychosocial stressors have on health and well-being has been well established (see for e.g., Epel et al., 2004; Glaser & Kiecolt-Glaser, 2005; Juster, S, McEwen, Lupien, McEwen, & Lupien, 2010; Robinson, 2018; Seib et al., 2014). Acute and short-lived psychosocial stressors, despite being a normal part of daily lives, can also impact health and well-being by increasing the frequency of negative health-related cognitions and behaviors (Cloutier et al., 2019; B. Kudielka et al., 2004; Schoofs et al., 2008; Shields et al., 2016; Steptoe et al., 2007). More frequent engagement in negative health-related cognitions and behaviors can accumulate over time and manifest as health ailments later in life (DeLongis et al., 1988; Epel et al., 2018; Handzon & Chen, 2010), highlighting a need to understand the underlying mechanisms. One potential mechanism by which acute psychosocial stress may exert its influence on health-related cognitions and behaviors is through changes in how people process information from their environments (i.e., how people see their world).
There are several different styles people can draw upon when processing information from their environment. One of the most studied styles of information processing is mental abstraction, which is the process of reducing information to “allow for efficient storage and retrieval of central knowledge (e.g., categorization)” (Burgoon, Henderson, & Markman, 2013, p. 502). Levels of mental abstraction range from high (i.e., more abstract) to low (i.e., more concrete), where higher levels are associated with a broadened perspective and lower levels are associated with a narrowed perspective (Gray & Tall, 2007; Reed, 2016). People tend to rely on prior knowledge of a stimuli when under higher levels of mental abstraction and external cues from the environment when under lower levels of mental abstraction (Burgoon, Henderson, & Markman, 2013; Gray & Tall, 2007; Reed, 2016). Individual differences in levels of mental abstraction have been associated with several health-related cognitions and behaviors.
In any given moment, people can process information under varying levels of mental abstraction, which can affect how people think and behave. Lower levels of mental abstraction have been associated with poorer performance on judgment and decision-making tasks, worse self-control and impulsivity, and life satisfaction (Burger & Bless, 2015; Burgoon, Henderson, & Wakslak, 2013; Fujita & Han, 2009; Fujita & Roberts, 2010; Liberman & Trope, 2014; Updegraff & Suh, 2007). The level of mental abstraction is largely determined by mood, with negative moods associated with lower levels of mental abstraction (Bless & Burger, 2017). As acute psychosocial stress reliably results in negative mood states (Het & Wolf, 2007; B. Kudielka et al., 2004; McRae et al., 2006), lower levels of mental abstraction may partially explain associations with negative health-related cognitions and behaviors.
Acute psychosocial stress also effects several types of executive functions, which may result in different levels of mental abstraction than from mood. For instance, acute psychosocial stress is associated with executive functioning failures involving impaired working memory, slower reaction times, and problems with inhibitions (Shields et al., 2016). One consequence of executive functioning failures after a stressor is mind wandering. Mind wandering is a process where one’s thoughts wander freely away from the current activity, especially when it is unpleasant (McVay & Kane, 2010; Smallwood & Schooler, 2015). Mind wandering thus serves to distance the person from the unpleasant event, and increased distance facilitates higher levels of mental abstraction (Liberman & Trope, 2014; Trope & Liberman, 2010; Yoav et al., 2006). After a stressor, people are also more likely to engage in spontaneous self-distancing, which is a related process where a person attempts to psychologically distance themselves from a stressful situation (Ayduk & Kross, 2010; Kross & Ayduk, 2011). This body of work suggest that acute psychosocial stress may result in higher levels of mental abstraction as a coping mechanism.
The extant literature suggest that acute psychosocial stress may be linked with differences in levels of mental abstraction, although the direction of this association remains unclear. On one hand, increased negative mood after a stressor may result in lower levels in mental abstraction. On the other hand, failed executive functioning after a stressor may result in higher levels in mental abstraction. Addressing this gap in the literature may have important implications for health interventions. The purpose of this paper is to investigate the levels of mental abstraction after the stressor and examine one of the possible explanatory variables, namely stress-induced mood. Some recent work has found that delivering health messages congruent with the level of mental abstraction of the patient increased the effectiveness of how the intervention was received (Landau et al., 2019). Understanding how stress affects levels of mental abstraction could enhance health interventions delivered to populations that may be experiencing stress. Additionally, mental abstraction is a modifiable factor that may serve as an intervention target to disrupt the link between stress and negative health-related cognitions and behaviors.
Despite the apparent link between acute psychosocial stress and mental abstraction, and the potential relevance for interventions, we are unaware of any study directly investigating this link. Therefore, the primary purpose of this study was to examine how acute psychosocial stress affects levels of mental abstraction using an experimental design. We addressed the following research questions and hypotheses:
Does acute psychosocial stress affect levels of mental abstraction? We tested between group differences in mental abstraction measured after the stress or control conditions.
Do stress processes explain the association between acute psychosocial stress and levels of mental abstraction? We tested whether negative affect, blood pressure, or heart rate explained the association between acute psychosocial stress and mental abstraction.
Methods
Power Analysis
A power analysis was conducted in R v. 3.6.0 (R Core Team, 2018) with the pwr package v. 1.2–2 (Champely, 2018) in two steps. For the exploratory hypotheses, we wanted to oversample the stress condition to facilitate these analyses. As this was for exploratory analyses, we powered this condition to detect small effect sizes. With power = .80, alpha = .05, and a small effect size (f2 = 0.11), a sample size of at least 71 participants was required to detect a statistically significant effect using a simple regression analysis. We aimed to achieve at least 90 participants in the stress condition to make sure we had ample power to detect small effects. For the primary hypothesis, we powered our sample to detect a medium effect size (d = 0.475) that was observed in a meta-analysis on mental abstraction (Soderberg et al., 2014). Assuming at least 90 participants in the stress condition, with a medium effect size, alpha = .05, and power = .80, a sample size of at least 64 people was needed to detect a statistically significant effect with a two-tailed t-test. We aimed to achieve at least 70 participants in the control condition to make sure we had ample power to detect the effect.
Participants
A diverse sample of undergraduate students between the ages of 18 and 24 (M = 20.07, SD = 1.33) were recruited from the University’s SONA student subject pool between January and May 2018 (N = 168). Participant were randomly assigned to the stress (n = 99) or control (n = 67) conditions. The unbalanced design between the groups was intentional to facilitate future subgroup analyses within the stress condition. We created a sheet with 99 indicators for the stress condition and 67 indicators for the control condition and randomized their order. Each time slot assigned to a participant was than assigned the next unused condition from the randomized list. Two participants dropped out the study early because the stress condition was too distressful, resulting in a final sample size of 166 (nStress = 97; nControl = 67). Participants were primarily women (77%) and reflected a diverse range of ethnic and racial backgrounds (53% Latino/Hispanic, 22% Asian/Pacific Islander, 10% White/Caucasian, and 4% Black/African American). See Table 1 for full participant characteristics. Informed consent was collected from all participants prior to the beginning of the study.
Table 1:
Descriptive Statistics
| TSST (n = 97) | c-TSST (n = 67) | |
|---|---|---|
| Age (M±SD) | 20.10 (1.43) | 20.05 (1.26) |
| Gender (%) | ||
| Male | 29.9 | 19.4 |
| Female | 70.1 | 80.6 |
| Ethnicity (%) | ||
| African-American | 4.9 | 2.9 |
| Asian/Pacific Islander | 21.6 | 19.4 |
| Caucasian/White | 11.9 | 8.2 |
| Hispanic/Latino | 59.8 | 55.2 |
| First Generation College (%) | 72.2 | 73.1 |
| Academic Standing (%) | ||
| Freshman | 44.3 | 40.3 |
| Sophomore | 20.6 | 23.9 |
| Junior | 13.4 | 17.9 |
| Senior | 21.6 | 19.4 |
| BMI (M±SD) | 25.05 (5.22) | 25.12 (4.99) |
| BDI (M±SD) | 28.01 (5.79) | 29.19 (7.17) |
| Anxiety Disorder (%) | 8.2 | 13.4 |
| Hormonal Contraceptive (%) | 17.5 | 23.9 |
| Medication (%) | ||
| Cortisol | 1.0 | 1.5 |
| Anti-Depressants | 3.1 | 4.5 |
| Anti-Anxiety | 1.0 | 10.4 |
| Recreational Drug Use (%) | 11.3 | 7.5 |
| NA at T1 (M±SD) | 13.81 (2.67) | 14.06 (2.91) |
| NA at T2 (M±SD) | 20.3 (6.76) | 14.06 (3.07) |
| MAP at T1 (M±SD) | 82.88 (9.18) | 83.20 (7.70) |
| MAP at T2 (M±SD) | 80.47 (7.65) | 79.87 (6.01) |
| MAP at T3 (M±SD) | 87.02 (9.71) | 79.90 (6.22) |
| MAP at T4 (M±SD) | 95.86 (14.76) | 81.34 (8.93) |
| MAP at T5 (M±SD) | 85.94 (9.56) | 80.36 (7.43) |
| HR at T1 | 70.65 (10.41) | 73.10(10.19) |
| HR at T2 | 70.87 (10.38) | 72.33 (9.88) |
| HR at T3 | 76.32 (12.61) | 72.15 (9.46) |
| HR at T4 | 78.70 (18.57) | 70.79 (12.68) |
| HR at T5 | 70.67 (11.86) | 70.61 (9.77) |
| BIF (M±SD) | 15.98 (4.77) | 14.05 (3.85) |
Note: TSST = Trier Social Stress Test group; c-TSST = Control Trier Social Stress Test group; M = mean, SD = Standard deviation; BMI = Body mass index; BDI = Beck depression inventory; MAP at T# = Mean arterial pressure at time point; HR at T# = Heart rate at time point; BIF = Behavioral identification form.
Procedure
Prior to the beginning of the experiment, timeslots were randomly assigned to either the stressor or control conditions. Participants signed up for available time slots via the university’s online SONA system but were unaware of which condition they were assigned to. We asked participants to refrain from consuming caffeine or engaging in physical activity for one hour prior to their scheduled appointment. Upon arrival to the lab, participants completed the informed consent, several psychosocial questionnaires, and their height and weight were measured. Heart rate (HR) and blood pressure (BP) were then collected followed by a measure of perceived stress (Time 1; 16 minutes before the onset of the stressor or control condition). Participants than began an acclimation period where they were provided a selection of neutral magazines to read while they sat quietly for 15 minutes. After the acclimation period ended, HR, BP, affect, and perceived stress were collected to establish a baseline (Time 2: 2 minutes before the onset of the stressor or control condition). Next, participants began the protocol for the stress or control conditions. After the research leader explained the task to the participant, HR, BP, and perceived stress were collected for measures of anticipatory stress (Time 3; marking the onset of the stressor or control condition). Participants were then given 5 minutes to prepare for the task by taking notes before the primary stress or control task began. The primary tasks for the stressor and control conditions lasted 5 minutes. Upon completion of the protocol, HR, BP, affect, and perceived stress were collected for measures of stress reactivity (Time 4: 10 minutes after onset of the stressor or control condition). Next, participants completed the measure of mental abstraction (primary outcome). HR, BP, and perceived stress were then collected a final time for a measure of return to baseline (Time 5; 15 minutes after the onset of the stressor or control condition). Finally, participants completed a demographic questionnaire and were debriefed for the purpose of the study. The study protocol was approved by the campus Internal Review Board. The full study protocol, including detailed scripts, can be found at https://osf.io/fbx4h/.
Manipulation
Stress Condition.
Acute psychosocial stress was induced in the laboratory using the public speech portion of the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993; B. Kudielka et al., 2007). The math portion was excluded because mathematics are an inherently abstract process that could impact our outcome (Schley & Fujita, 2014). We felt that this was an acceptable omission as previous work has found the anticipation of the speech task is enough to elicit a stress response (Dickerson & Kemeny, 2004; Starcke et al., 2008).
This stressor was conducted in an experimental room that consisted of a table with four chairs, three facing the door and the other with the back to the door. Participants entered the room and were directed to sit in the chair with the back to the door. After the acclimation period, participants were told that they had to prepare a five-minute speech about their ideal job for a committee of experts in nonverbal communication. The participants were then directed to look behind them at the door where three research assistants, dressed in white laboratory coats and holding clipboards, were standing. Participants were then provided with a pen and a piece of paper and left in the room alone for five minutes to prepare for their speech. After the preparation period had ended, the stress committee reentered the room. One of the committee members directed the participant to stand on an “X” marked on the floor and collected any notes created during the preparation phase. A second committee member turned on a video camera and tape recorder and stated a participant number and the date. Participants were not actually recorded but believed that they were until the end of the study. The committee did not provide a cue to begin the speech. Committee members were instructed to look and act displeased with the speech, with only the lead committee member (i.e., the “president”) occasionally interrupting with discouraging remarks (e.g., please make eye contact with the committee). After five minutes, the “president” informed participants that the task was now over and the lead researcher reentered the room.
Control Condition.
Although several control conditions have been developed for the TSST, there is no standardized control condition that can address all research inquiries. The friendly-TSST (Wiemers et al., 2012) and the placebo-TSST (Het et al., 2009), for instance, were designed to isolate the effects of cortisol and hypothalamus-pituitary-adrenal axis activation. However, both of these control condition still elicit a sympathetic nervous system (i.e., HR, BP, salivary alpha-amylase), leaving ambiguity about the effects of fast acting stress responses (Het et al., 2009; Wiemers et al., 2012). As the primary aim in this study involved acute psychosocial stress in general, we developed a new control condition that was not associated with cardiovascular reactivity, a marker of sympathetic nervous system activation. Full details on the development of the control condition can be found at https://osf.io/fbx4h/.
This control condition was conducted in the same room as the experimental condition and the procedures only differed in the following ways. After the acclimation period, participants were told that they would need to prepare an essay describing their ideal job while standing at a desk. Participants were directed to look behind them at a standing desk that was placed in front an “X” marked on the floor. Participants were then told that they would have five minutes to prepare for their essay (while still sitting at table) in any way they thought would be helpful, such as an outline. They were provided with a pen and paper and told that they could use whatever materials they made during this time when writing the final essay. To help reduce stress, the lead researcher told participants that we were only interested in them writing and that nobody would actually read what they wrote. However, participants were told that somebody would skim their writing to make sure that they wrote about their ideal job. When the preparation period ended, the lead researcher reentered the room and directed the participants to the standing desk. After five minutes, the researcher reentered the experiment room.
Measures
Mental Abstraction.
Mental Abstraction was measured using the Behavioral Identification Form (BIF) (Vallacher & Wegner, 1989). The BIF was originally developed to evaluate individual differences in how people think about behaviors, but has since been repurposed to measure mental abstraction (Fujita & Roberts, 2010; Lammers, 2012). The BIF contains 25 statements of behaviors that require participants to choose between two explanations; one explanation reflecting abstract construal with focus on WHY someone would complete this behavior and the other explanation reflecting concrete construal with focus on HOW someone would complete this behavior. For example, participants were presented with the behavior “Making a list” and would have to select between the more abstract explanation “Getting organized” and the more concrete explanation “Writing things down.” The BIF is scored by counting the total number of abstract explanations that were selected, with higher scores reflecting higher levels of mental abstraction. In this sample, the BIF exhibited adequate internal consistency (α = 0.76).
Mood.
Mood was measured using the Positive Affect and Negative Affect Schedule (PANAS) at two time points; once before the stressor (at Time 2) and once immediately after the stressor (at Time 4). The PANAS is a 20-item measure of positive and negative affective states (Watson et al., 1988). Participants were asked to rate the degree to which they were experiencing each emotion on a 1 (very slightly or not at all) to 5 (extremely) point Likert-type rating scale. Higher scores on a subscale indicate a greater degree of feeling the emotion. Consistent with previous work, we focused on the negative affect subscale of the PANAS in this study. The negative affect subscale exhibited adequate internal consistency both before (α = 0.73) and after (α = 0.91) the stressor or control conditions.
Perceived Stress.
Perceived stress was evaluated via a single item visual analogue scale at each time point. Participants were asked to write an X on a line that was anchored by “Not stressed at all” on the left side and “Completely stressed” on the right side. Research assistants measured the distance the X was placed on the line from the left anchor in centimeters so that a greater distance reflected greater perceived stress.
Cardiovascular Measures.
HR and BP collected through a pressure cuff on the non-dominant arm (Omron BP742N 5 Series) at each time point. BP measurements were transformed into mean arterial pressure (MAP). MAP is a weighted average of systolic blood pressure (SBP) and diastolic blood pressure (DBP) and is calculated according to previous literature (Henry et al., 2002).
Control Variables.
Models estimated to address research question 2 controlled for the following variables associated with stress processes: Gender, depression using the Beck Depression Inventory (BDI), body mass index, (BMI), and whether participants were taking anti-anxiety medication and/or hormonal contraceptives.
Analytic Approach
All data were analyzed in the R programming language (R Core Team, 2018) and alpha levels for all analyses were set to 0.05. For a manipulation check, mixed-effects models were estimated to evaluate how changes in mood, perceived stress, HR, and BP differed between experimental and control conditions using the lme4 package (Bates et al., 2015). Welch’s t-test was estimated using the t.test function to evaluate mean differences in mental abstraction between the stressor and control conditions (Research Question 1). Welch’s t-test has recently been recommended as the default when comparing mean differences between groups as it corrects for violations of homogeneity of variance and is better equipped to handle unbalanced designs than the student’s t-test (Delacre et al., 2017). Cohen’s d and a corresponding 95% confidence interval (C.I.95%) were estimated as a measure of effect size using the cohen.d function from the effsize package in R. Indirect effects were estimated using a structural equation modeling (SEM) approach in Lavaan with bootstrapped standard errors. Separate models were estimated to examine whether negative affect, MAP, or HR after the procedure explained the associations between experimental condition and mental abstraction. In each model, we controlled for the previous levels of negative affect, MAP, or HR. Separate models were estimated for reactivity of negative affect, perceived stressed, HR, and MAP. All results for SEM models were presented as unstandardized slopes (b), standard errors (se), p-values, and 95% confidence intervals (C.I.95%). See our OSF page documenting how our thinking about this study has changed over time (https://osf.io/fbx4h/).
Results
Manipulation Check
Statistically significant interaction terms between Condition (stressor vs. control) and negative affect (F[1,161] = 64.11, p < 0.001), perceived stress (F[4,653] = 24.04, p < 0.001), HR (F[4,653] = 16.37, p < 0.001), and MAP (F[4,653] = 39.52, p < 0.001 were observed. These findings indicated that there was a subjective and cardiovascular response in the stress condition and not the control condition, suggesting that these conditions had the intended effects. See Figures 1 and 2 for a graphical depiction of negative affect and MAP. See our OSF page for graphical depictions of perceived stress and HR.
Figure 1:
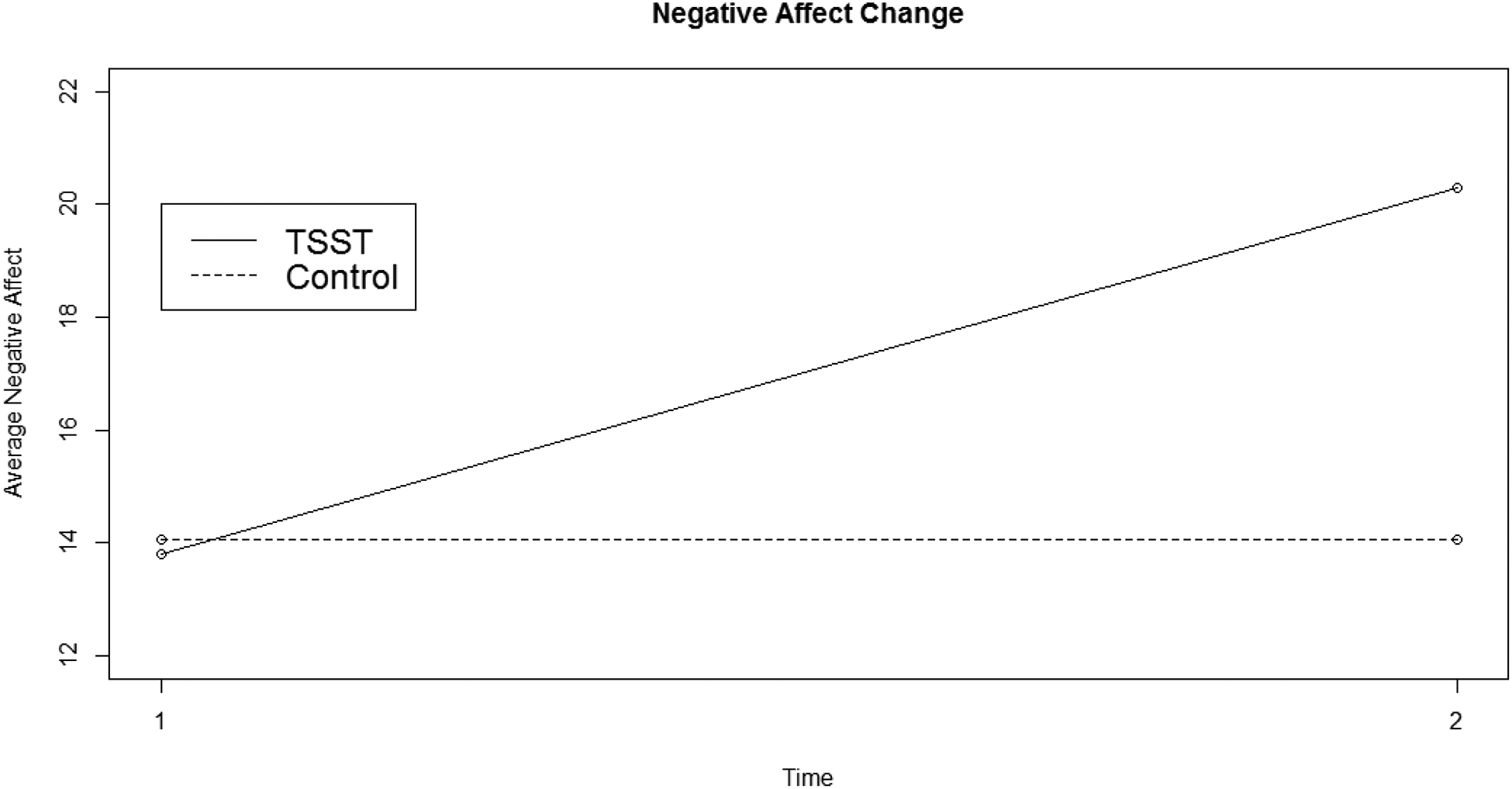
Negative Affect Reactivity
Note: TSST = Trier Social Stress Test
Figure 2:
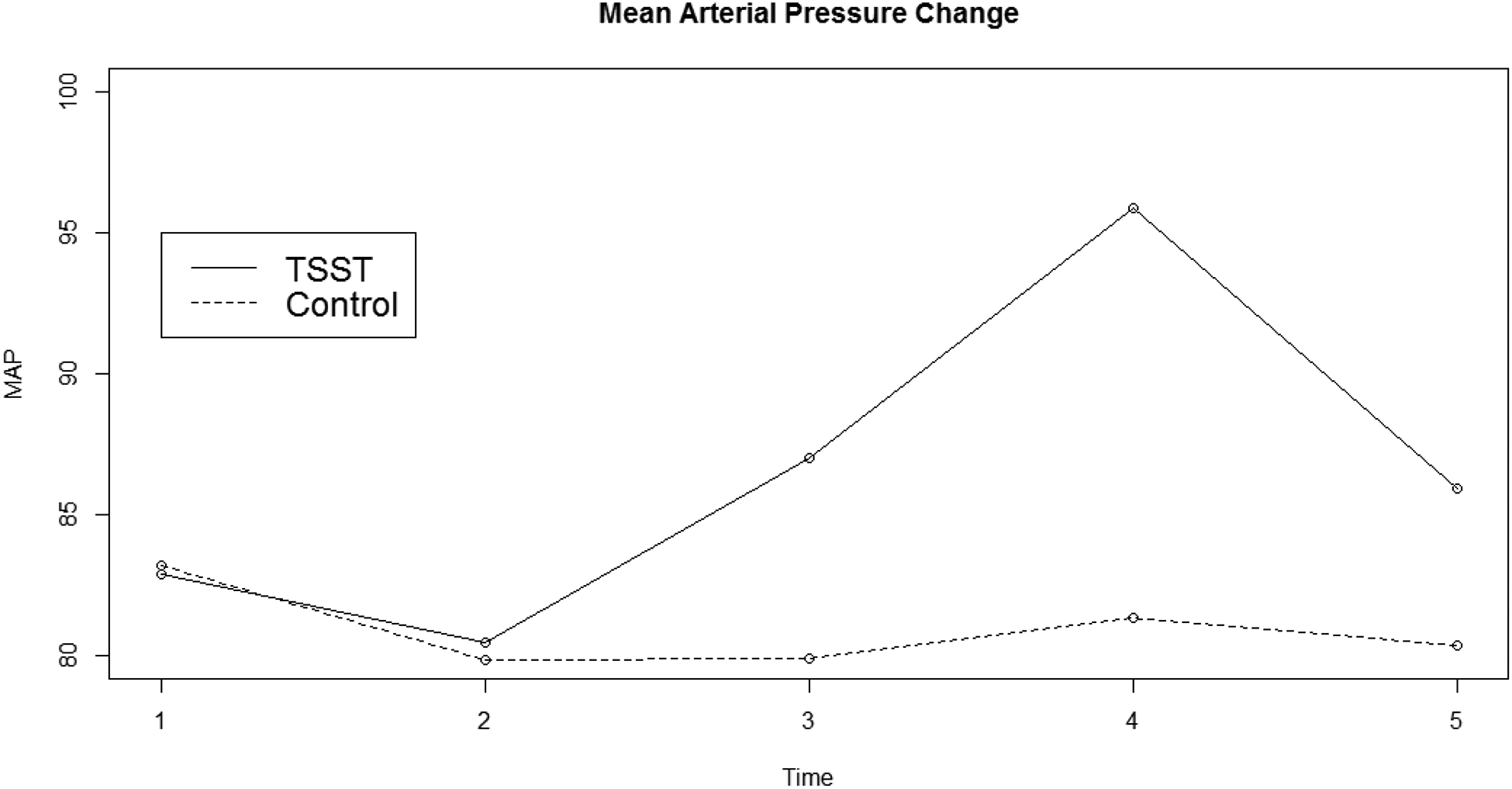
Mean Arterial Pressure Reactivity
Note: TSST = Trier Social Stress Test
Research Question 1
Average levels of the BIF were statistically significantly higher after the stress condition (M = 15.98, SD = 4.77) than after the control condition (M = 14.05, SD = 3.85), t(154.85) = 2.83, p = 0.005, d = 0.438, C.I.95% d:[0.12, 0.76]. This suggests that exposure to a psychosocial stressor was associated with higher levels of mental abstraction. As a robustness check, we estimated an analysis of covariance controlling for BMI, BDI, oral contraceptive use, and prescription of anti-anxiety medication. The average differences in BIF remained statistically significantly higher after the stress condition than the control condition (F[1, 158.504] = 7.97, p = 0.024). None of the covariates were statistically significantly associated with the outcome (all ps > 0.52).
Research Question 2
The association between condition and BIF was significantly explained by negative affect after the experimental protocol (stress or control conditions), bindirect = 1.07, se = 0.45, p = 0.017. Condition was associated with higher levels of negative affect (bdirect = 6.73, se = 0.71, p <0.001), which was associated with higher levels of BIF (bdirect = 0.16, se = 0.07, p = 0.020). Full results are displayed in Table 2. The association between condition and BIF was not significantly explained by MAP (b = −0.22, se = 0.43, p = 0.61) or HR (b = 0.08, se = 0.19, p = 0.66). Full results for these models are displayed in Table 3 and Table 4, respectively.
Table 2:
Indirect Effect Results for Negative Affect
| b | se | p | C.I. | R2 | |||
|---|---|---|---|---|---|---|---|
| Mental Abstraction ON | 0.09 | ||||||
| Condition | 1.02 | 0.77 | 0.18 | [−0.43, 2.56] | |||
| NAt4 | 0.16 | 0.07 | 0.02 | [0.03, 0.29] | |||
| NAt4 ON | 0.43 | ||||||
| Condition | 6.73 | 0.71 | <0.001 | [5.32, 8.13] | |||
| NAt2 | 0.70 | 0.14 | <0.001 | [0.39, 0.93] | |||
| BMI | −0.09 | 0.08 | 0.24 | [−0.23, 0.07] | |||
| BDI | 0.21 | 0.07 | 0.002 | [0.08, 0.35] | |||
| Gender | −0.99 | 1.03 | 0.34 | [−3.07, 1.07] | |||
| Anti-Anx | −1.43 | 1.60 | 0.37 | [−4.85, 1.60] | |||
| Contraceptives | −0.18 | 1.06 | 0.87 | [−2.20, 2.04] | |||
| Indirect Effect | 1.07 | 0.45 | 0.017 | [0.18, 1.99] | |||
| Total Effect | 2.09 | 0.69 | 0.002 | [0.67, 3.38] | |||
Note: Bold indicates statistically significant effect (p <0.05). NAt4 = Negative affect after the stressor; NAt2 = Negative affect before the stressor; BMI = Body mass index; BDI = Beck depression inventory; Anti-anx = Anti-Anxiety Medication use; Contraceptives = contraceptive use; b = unstandardized slope; se = standard error; C.I. = 95% confidence interval
Table 3:
Indirect Effect Results for Mean Arterial Pressure
| b | se | p | C.I. | R2 | |||
|---|---|---|---|---|---|---|---|
| Mental Abstraction ON | 0.05 | ||||||
| Condition | 2.25 | 0.78 | 0.004 | [0.65, 3.70] | |||
| MAPt4 | −0.02 | 0.03 | 0.60 | [−0.08, 0.06] | |||
| MAPt4 ON | 0.49 | ||||||
| Condition | 13.73 | 1.38 | <0.001 | [10.82, 16.41] | |||
| MAPt2 | 1.02 | 0.11 | <0.001 | [0.80, 1.23] | |||
| BMI | 0.10 | 0.15 | 0.49 | [−0.15, 0.45] | |||
| BDI | −0.21 | 0.19 | 0.26 | [−0.55, 0.13] | |||
| Gender | 1.48 | 2.68 | 0.55 | [−2.89, 7.11] | |||
| Anti-Anx | −1.13 | 3.05 | 0.71 | [−7.39, 4.38] | |||
| Contraceptives | 1.67 | 1.82 | 0.36 | [−1.76, 5.52] | |||
| Indirect Effect | −0.22 | 0.43 | 0.61 | [−1.08, 0.75] | |||
| Total Effect | 2.03 | 0.67 | 0.002 | [0.64, 3.35] | |||
Note: Bold indicates statistically significant effect (p <0.05). MAPt4 = Mean Arterial Pressure after the stressor; MAPt2 = Mean Arterial Pressure before the stressor; BMI = Body mass index; BDI = Beck depression inventory; Anti-anx = Anti-anxiety medication use; Contraceptives = contraceptive use; b = unstandardized slope; se = standard error; C.I. = 95% confidence interval
Table 4:
Mediation Results for Heart Rate
| b | se | p | C.I. | R2 | |||
|---|---|---|---|---|---|---|---|
| Mental Abstraction ON | 0.08 | ||||||
| Condition | 1.95 | 0.74 | 0.008 | [0.53, 3.36] | |||
| HRt4 | 0.01 | 0.02 | 0.67 | [−0.03, 0.05] | |||
| HRt4 ON | 0.44 | ||||||
| Condition | 9.12 | 1.96 | <0.001 | [5.48, 13.37] | |||
| HRt2 | 1.00 | 0.14 | <0.001 | [0.69, 1.27] | |||
| BMI | −0.07 | 0.17 | 0.67 | [−0.47, 0.25] | |||
| BDI | −0.07 | 0.17 | 0.69 | [−0.36, 0.30] | |||
| Gender | 0.54 | 2.92 | 0.85 | [−4.94, 6.61] | |||
| Anti-Anx | −1.41 | 2.71 | 0.60 | [−6.59, 4.12] | |||
| Contraceptives | −0.41 | 2.36 | 0.86 | [−5.32, 3.74] | |||
| Indirect Effect | 0.08 | 0.19 | 0.66 | [−0.25, 0.48] | |||
| Total Effect | 2.04 | 0.71 | 0.004 | [0.66, 3.43] | |||
Note: Bold indicates statistically significant effect (p <0.05). HRt4 = Heart Rate after the stressor; HRt2 = Heart Rate before the stressor; BMI = Body mass index; BDI = Beck depression inventory; Anti-anx = Anti-anxiety medication use; Contraceptive = Contraceptive use; b = unstandardized slope; se = standard error; C.I. = 95% confidence interval
Discussion
Acute psychosocial stress increases the frequency of negative health-related cognitions and behaviors, which may accumulate over time and manifest in health ailments later in life. One mechanism whereby these associations manifest is how people process information from their environments (i.e., how they see the world) after a stressor. In this study, we developed an experimental design to evaluate how acute psychosocial stress affected levels of mental abstraction, a style of information processing that involves reducing processed information into a few broad categories (at higher levels; Burgoon, Henderson, & Markman, 2013; Gray & Tall, 2007). The findings in this study indicated that average levels of mental abstraction were higher after the stress condition than the control condition. The observed effect size was also consistent with previous meta-analyses in the field (Soderberg et al., 2014), which suggests a moderate sized effect between stress and mental abstraction. These associations were explained by levels of negative affect after the procedure, but not MAP or HR.
Our findings diverge from an alternative prediction that levels of mental abstraction would be lower after a stressor due to negative affect. A large body of work has found that negative affect is associated with a narrowed perspective (i.e., lower levels of mental abstraction) and lower levels of mental abstraction (Bless & Burger, 2017; Fredrickson, 2001). We did find that the association between acute psychosocial stress and levels of mental abstraction was explained by negative affect after the stressor, however, the association was in the opposite direction. Namely, we found a positive association between negative affect and levels of mental abstraction. One reason for these discrepant findings may be with how negative affect was induced in this study compared to the extant literature. Negative mood is typically induced by viewing negatively valanced videos and images, or through priming paradigms (Bless & Burger, 2017; Maryam Fakhrhosseini & Jeon, 2017). Negative mood in our study was induced through a psychosocial stressor, which also involves other cognitive and physiological processes that may differentially affect mental abstraction. We attempted to address this by testing how cardiovascular markers of stress reactivity (i.e., MAP and HR) explained the association, but did not detect an effect. Further research is required to unpack these associations and understand the underlying mechanisms.
The findings from this study were consistent with predictions that levels of mental abstraction would be higher after a stressor due to changes in executive functions. Previous work has found that mind wandering may increase after a stressor because of failed executive functioning. Mind wandering is an abstract meta-cognitive process where thoughts become disengaged from the immediate task and wander freely, often in response to a task that is too demanding or distressing (Smallwood et al., 2009; Smallwood & Schooler, 2015). Mind wandering occurs when executive functions fail and serves to distance a person from a distressing event, and increasing distance is associated with higher levels of mental abstraction (Liberman & Trope, 2014; Trope & Liberman, 2010). Relatedly, people may also engage in spontaneous self-distancing after a stressor to psychologically remove themselves from the distressing environment (Ayduk & Kross, 2010). When under acute stress, the elevated levels of mental abstraction may then facilitate effective coping strategies such as reframing the stressor as a challenge in the moment and reducing emotional distress (Smallwood et al., 2009; Streamer et al., 2017). Though, levels of mental abstraction that remain elevated long after the stressor could interfere with concrete processing that may be necessary to better understand and adapt to the stressful experience (Carver & Vargas, 2011). However, mind wandering and spontaneous self-distancing were not measured in this study and their role in this process remains unclear. Additionally, it is not clear how levels of mental abstraction would be affected under conditions of chronic stress.
It is important to note that these findings reflect what the average level of mental abstraction was after a stressor. As the stress response is a dynamic process, its effects on levels of mental abstraction may differ depending on when during the process it is measured. For instance, measuring mental abstraction during a stressor, rather than after, may yield lower levels of mental abstraction because activation of the sympathetic nervous system may encourage focus on the immediate environment to facilitate survival (Epel et al., 2018; Granger et al., 2007; Robinson, 2018). Additionally, mental abstraction may be higher when measured later in the process, when cortisol has peaked, because the effects on executive functions such as working memory will be more prominent (Epel et al., 2018; B. M. Kudielka et al., 2004; Tsigos & Chrousos, 2002). Adopting a repeated measures design would facilitate these inquiries. However, most of the commonly used measures of mental abstraction (i.e., the BIF) have not been validated for repeated measures. Future work may consider adapted these measures and adopting intensive repeated measures designs, such as ecological momentary assessment (see for e.g., Smyth & Stone, 2003), which can shed insight into whether the associations between acute stress and mental abstraction vary throughout the process in natural environments.
Strengths and Future Directions
This study has several strengths that should be considered. First, we developed an experimental procedure to isolate the association between stress and levels of mental abstraction, increasing the internal validity of our claims. The control condition was carefully designed to approximate the stress condition as closely as possible, including having participants standing in the same spot and completing a task using the same topic (speaking or writing about an ideal job). Second, our findings extend the previous literature, that has typically used predominantly white college students, by establishing a link between acute psychosocial stress and mental abstraction in a racially and ethnically diverse sample. However, the sample was still primarily college students self-identifying as women and exploring these associations in non-college students with a balanced distribution of gender may increase external validity and facilitate moderator analyses.
Conclusion
Exposure to an acute psychosocial stressor may alter how people process information from their environments and subsequently how they see the world. Using a laboratory experimental manipulation of stress, we observed higher levels of mental abstraction after the stress group than the control group. Participants may have engaged in mind wandering or spontaneous self-distancing to cope with the stressor. As many health interventions are delivered to stressed populations or during moments of stress, researchers may consider matching their interventions to the level of mental abstraction patients are processing information within a given moment. Future research is necessary to better understand the mechanisms underlying acute psychosocial stress and levels of mental abstraction.
Source of funding:
The first author was partially supported by grant number T32DA017629 from the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), during the preparation of this manuscript.
Footnotes
Disclosure of interest: The authors report no conflicts of interest.
References
- Ayduk Ö, & Kross E (2010). From a distance: Implications of spontaneous self-distancing for adaptive self-reflection. Journal of Personality and Social Psychology, 98, 809–829. 10.1037/a0019205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software2, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bless H, & Burger AM (2017). Mood and the Regulation of Mental Abstraction. Current Directions in Psychological Science, 26(2), 159–164. 10.1177/0963721417690456 [DOI] [Google Scholar]
- Burger AM, & Bless H (2015). Affect and the weight of idealistic versus pragmatic concerns in decision situations. European Journal of Social Psychology, 46(3), 323–340. 10.1002/ejsp.2164 [DOI] [Google Scholar]
- Burgoon EM, Henderson MD, & Markman AB (2013). There Are Many Ways to See the Forest for the Trees: A Tour Guide for Abstraction. Perspectives in Psychological Science, 8(5), 501–520. 10.1177/1745691613497964 [DOI] [PubMed] [Google Scholar]
- Burgoon EM, Henderson MD, & Wakslak CJ (2013). How Do We Want Others to Decide? Pers Soc Psychol B, 39(6), 826–838. 10.1177/0146167213481247 [DOI] [PubMed] [Google Scholar]
- Carver C, & Vargas S (2011). Stress, Coping, and Health In Friedman HS (Ed.), The Oxford Handbook of Health Psychology (pp. 162–188). Oxford University Press, USA: https://books.google.com/books?hl=en&lr=&id=J3-78PdF83kC&pgis=1 [Google Scholar]
- Champely S (2018). pwr: Basic Functions for Power Analysis. R Package Version 1.2–2. https://cran.r-project.org/package=pwr [Google Scholar]
- Cloutier RM, Blumenthal H, Trim RS, Douglas ME, & Anderson KG (2019). Real-Time social stress response and subsequent alcohol use initiation among female adolescents. Psychology of Addictive Behaviors, 33(3), 254–265. 10.1037/adb0000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacre M, Lakens D, & Leys C (2017). Why Psychologists Should by Default Use Welch’s t-test Instead of Student’s t-test. International Review of Social Psychology, 30(1), 92–101. 10.5334/irsp.82 [DOI] [Google Scholar]
- DeLongis A, Folkman S, & Lazarus RS (1988). The Impact of Daily Stress on Health and Mood: Psychological and Social Resources as Mediators. Journal of Personality and Social Psychology, 54(3), 486–495. 10.1037/0022-3514.54.3.486 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America, 101(49), 17312–17315. 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, & Mendes WB (2018). More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology, 49, 146–169. 10.1016/j.yfrne.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The Role of Positive Emotions in Positive Psychology: The Broaden-and-Build Theory of Positive Emotions. American Psychologists, 56(3), 218–226. 10.1037//0003-066x.56.3.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, & Han HA (2009). Moving beyond deliberative control of impulses: The effect of construal levels on evaluative associations in self-control conflicts. Psychological Science, 20(7), 799–804. 10.1111/j.1467-9280.2009.02372.x [DOI] [PubMed] [Google Scholar]
- Fujita K, & Roberts JC (2010). Promoting Prospective Self-Control Through Abstraction. Journal of Experimental Social Psychology, 46(6), 1049–1054. 10.1016/j.jesp.2010.05.013 [DOI] [Google Scholar]
- Glaser R, & Kiecolt-Glaser J (2005). Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology, 5, 243–251. 10.1038/nri1571 [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, & Whembolua G-L (2007). Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiology & Behavior, 92(4), 583–90. 10.1016/j.physbeh.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Gray E, & Tall D (2007). Abstraction as a natural process of mental compression. Mathematics Education Research Journal, 19(2), 23–40. 10.1007/BF03217454 [DOI] [Google Scholar]
- Handzon MD, & Chen E (2010). Daily stress, cortisol, and sleep: The moderating role of childhood psychosocial environments. Health Psychology, 29(4), 394–402. 10.1037/a0019879 [DOI] [PubMed] [Google Scholar]
- Henry JB, Miller MC, Kelly KC, & Champney D (2002). Mean arterial pressure (MAP): An alternative and preferable measurement to systolic blood pressure (SBP) in patients for hypotension detection during hemapheresis. Journal of Clinical Apheresis, 17(2), 55–64. 10.1002/jca.10022 [DOI] [PubMed] [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, & Wolf OT (2009). Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology, 34(7), 1075–1086. 10.1016/j.psyneuen.2009.02.008 [DOI] [PubMed] [Google Scholar]
- Het S, & Wolf OT (2007). Mood changes in response to psychosocial stress in healthy young women: effects of pretreatment with cortisol. Behavioral Neuroscience, 121(1), 11–20. 10.1037/0735-7044.121.1.11 [DOI] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Kross E, & Ayduk Ö (2011). Making meaning out of negative experiences by self-distancing. Current Directions in Psychological Science2, 20(3), 187–191. 10.1177/0963721411408883 [DOI] [Google Scholar]
- Kudielka B, Hellhammer D, & Kirschbaum C (2007). Ten years of research with the Trier Social Stress Test—revisited In Harmon-Jones E & Winkielman P (Eds.), Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior (Issue October 2015, pp. 56–83). Guilford Press. [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer D, & Kirschbaum C (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology, 29(1), 83–98. 10.1016/S0306-4530(02)00146-4 [DOI] [PubMed] [Google Scholar]
- Kudielka B, Schommer N, & Hellhammer D (2004). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology, 29(8), 983–992. 10.1016/j.psyneuen.2003.08.009 [DOI] [PubMed] [Google Scholar]
- Lammers J (2012). Abstraction Increases Hypocrisy. Journal of Experimental Social Psychology, 48(2), 475–480. 10.1016/j.jesp.2011.07.006 [DOI] [Google Scholar]
- Landau MJ, Cameron LD, Arndt J, Hamilton WK, Swanson TJ, & Bultmann M (2019). Beneath the Surface: Abstract Construal Mindset Increases Receptivity to Metaphors in Health Communications. Social Cognition, 37(3), 314–340. 10.1521/soco.2019.37.3.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman N, & Trope Y (2014). Traversing psychological distance. Trends in Cognitive Sciences, 18(7), 364–369. 10.1016/j.tics.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Maryam Fakhrhosseini S, & Jeon M (2017). Affect/Emotion Induction Methods In Emotions and Affect in Human Factors and Human-Computer Interaction (pp. 235–253). Academic Press. [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, & Timmerman M (2006). Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol Clin Exp, 21(6), 377–385. 10.1002/hup.778 [DOI] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2010). Does Mind Wandering Reflect Executive Function or Executive Failure? Comment on Smallwood and Schooler (2006) and Watkins (2008). Psychological Bulletin, 136(2), 188–197. 10.1037/a0018298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statsitical Computing; https://www.r-project.org/ [Google Scholar]
- Reed SK (2016). A Taxonomic Analysis of Abstraction. Perspect Psychol Sci, 11(6), 817–837. 10.1177/1745691616646304 [DOI] [PubMed] [Google Scholar]
- Robinson AM (2018). Let’s talk about stress: History of stress research. Review of General Psychology, 22(3), 334–342. 10.1037/gpr0000137 [DOI] [Google Scholar]
- Schley DR, & Fujita K (2014). Seeing the Math in the Story: On How Abstraction Promotes Performance on Mathematical Word Problems. Social Psychological and Personality Science, 5(8), 953–961. 10.1177/1948550614539519 [DOI] [Google Scholar]
- Schoofs D, Preuß D, & Wolf OT (2008). Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology, 33(5), 643–653. 10.1016/j.psyneuen.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Seib C, Whiteside E, Humphreys J, Lee K, Thomas P, Chopin L, Crisp G, O’Keeffe A, Kimlin M, Stacey A, Anderson D, Angela O, Kimlin M, Stacey A, & Anderson D (2014). A longitudinal study of the impact of chronic psychological stress on health-related quality of life and clinical biomarkers: protocol for the Australian Healthy Aging of Women Study. {BMC} Public Health, 14(9), 1–8. 10.1186/1471-2458-14-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, & Yonelinas AP (2016). The effects of acute stress on core executive funcitons: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668. 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Fitzgerald A, Miles LK, & Phillips LH (2009). Shifting Moods, Wandering Minds: Negative Moods Lead the Mind to Wander. Emotion, 9(2), 271–276. 10.1037/a0014855 [DOI] [PubMed] [Google Scholar]
- Smallwood J, & Schooler JW (2015). The Science of Mind Wandering: Empirically Navigating the Stream of Consciousness. Annual Review of Psychology, 66(1), 487–518. 10.1146/annurev-psych-010814-015331 [DOI] [PubMed] [Google Scholar]
- Smyth JM, & Stone AA (2003). Ecological momentary assessment research in behavioral medicine. Journal of Happiness Studies, 4(1), 35–52. 10.1023/A:1023657221954 [DOI] [Google Scholar]
- Soderberg CK, Callahan SP, Kochersberger AO, Amit E, & Ledgerwood A (2014). The Effects of Psychological Distance on Abstraction: Two Meta-analyses. Psychol Bull, 141(3), 525–548. 10.1037/bul0000005 [DOI] [PubMed] [Google Scholar]
- Starcke K, Wolf OT, Markowitsch HJ, & Brand M (2008). Anticipatory stress influences decision making under explicit risk conditions. Behavioral Neuroscience, 122(6), 1352–1360. 10.1037/a0013281. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, & Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–912. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Streamer L, Seery MD, Kondrak CL, Lamarche VM, & Saltsman TL (2017). Not I, but she: The beneficial effects of self-distancing on challenge/threat cardiovascular responses. J Exp Soc Psychol, 70, 235–241. 10.1016/j.jesp.2016.11.008 [DOI] [Google Scholar]
- Trope Y, & Liberman N (2010). Construal-level theory of psychological distance. Psychol Rev, 117(2), 440 10.1037/a0018963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, & Chrousos GP (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. 10.1016/s0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- Updegraff J. a., & Suh EM (2007). Happiness is a warm abstract thought: Self-construal abstractness and subjective well-being. The Journal of Positive Psychology, 2(1), 18–28. 10.1080/17439760601069150 [DOI] [Google Scholar]
- Vallacher RR, & Wegner DM (1989). Levels of personal agency: Individual variation in action identification. J Pers Soc Psychol, 57(4), 660 10.1037/0022-3514.57.4.660 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Wiemers US, Schoofs D, & Wolf OT (2012). A friendly version of the Trier Social Stress Test does not activate the HPA axis in healthy men and women. Stress, 16(2), 254–260. 10.3109/10253890.2012.714427 [DOI] [PubMed] [Google Scholar]
- Yoav B-A, Liberman N, & Trope Y (2006). The association between psychological distance and construal level: Evidence from an implicit association test. J Exp Psychology Gen, 135(4), 609 10.1037/0096-3445.135.4.609 [DOI] [PubMed] [Google Scholar]


