Abstract
Objective:
The platelet phenotype in certain patients and clinical contexts may differ from healthy conditions. We evaluated platelet activation through specific receptors in healthy men and women, comparing this to patients presenting with ST-segment-elevation Myocardial Infarction (STEMI) and non-ST-segment-elevation Myocardial Infarction (NSTEMI).
Approach and Results:
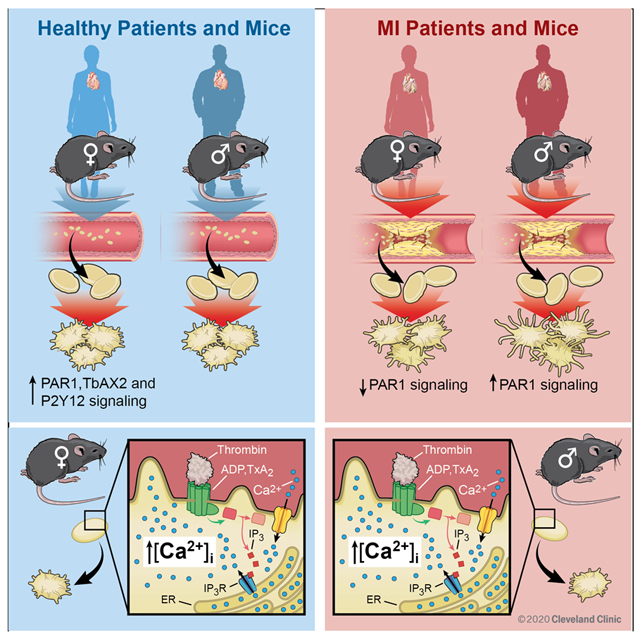
We identified independent predictors of platelet activation through certain receptors and a murine MI model further explored these findings. Platelets from healthy women and female mice are more reactive through protease-activated receptors (PARs) compared to platelets from men and male mice. Multivariate regression analyses revealed male sex and NSTEMI as independent predictors of enhanced PAR1 activation in human platelets. Platelet protease-receptor activated 1 (PAR1) signaling decreased in women and increased in men during MI which was the opposite of what was observed during healthy conditions. This trend was also observed in male and female mice in which thrombin-mediated platelet calcium mobilization was augmented coincident with platelet activation in males and attenuated in females at the time of MI.
Conclusions:
Sex-specific signaling in platelets appears to be a cross-species phenomenon. The divergent platelet phenotype in males and females at the time of MI suggests a sex-specific antiplatelet drug regimen should be prospectively evaluated.
Keywords: Platelet, PAR, Female, Myocardial Infarction, NSTEMI
Graphical Abstract
Introduction
Anti-platelet therapy administered to patients with MI consists of aspirin and a P2Y12 receptor antagonist. In humans and in relevant murine models of vascular disease, platelet signaling and responsiveness to anti-platelet medications may differ from healthy conditions in which anti-platelet medications are first characterized 1-4. Patients with established vascular disease have a heightened risk of recurrent atherothrombotic events from excessive platelet activation. Recurrent atherothrombotic events in patients often manifest as NSTEMI 5. Long-term mortality for NSTEMI is greater than for STEMI even when adjustments are made for appropriate use of interventional coronary procedures. This observation raises the possibility that medical therapy prescribed according to established guidelines for NSTEMI could be refined 6, 7.
Women who sustain an acute MI are treated less aggressively. For example, women with NSTEMI are less likely to undergo early diagnostic angiography and receive treatment. This observation may account for poorer post-MI outcomes in women 8. A recent meta-analysis suggested that low dose aspirin may be less efficacious in males than females 9. Past preclinical studies evaluating antiplatelet and antithrombotic agents rarely accounted for sex as a biological variable 10, 11. To test the hypothesis that platelet activation and post-receptor signal transduction pathways in males and females may differ, we examined the behavior of platelets in healthy humans and mice, comparing this to the time of myocardial infarction.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request to S. J. Cameron.
Human Studies
This study was approved by the Institutional Review Board at the University of Rochester (# 61784 for patients and #1644 for healthy volunteers) and Cleveland Clinic (#19-1451 for patients and 20-413 for healthy volunteers). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Healthy volunteer subjects were recruited by answering a posted document, and all were free from anti-platelet agent use or vasoactive substances. The post-MI patient cohort included patients with both STEMI and NSTEMI. Delayed consent was granted for patients presenting with STEMI in order to avoid interfering with the door-to-balloon time. This permitted the collection of platelets before patients received a loading does of a P2Y12 receptor antagonists, and before coronary angiography or arterial instrumentation. STEMI patients were then consented within 24 hrs of blood draw after recovery from moderate sedation following coronary angiography. The sample was discarded if the patient declined to enroll and sign the consent form. For patients with NSTEMI, subjects were already in the emergency department or hospital for 1-10 hours and identified by elevated plasma cardiac troponin (plasma value greater than the 99th percentile of a healthy population with a 10% assay CV) with concomitant symptoms consistent with cardiac chest pain. Each patient was treated with 325 mg aspirin in the emergency department or the ambulance at least 30 minutes prior to venous blood draw. We enrolled 155 subjects: 38 healthy volunteers, and 117 subjects with acute MI. Basic patient demographics were recorded in a confidential manner and stored in a secure fashion with the principle investigator.
Flow cytometry was utilized with measurement of platelet surface P-selectin to assess platelet function in a dose-dependent manner, as we described previously 12. Platelet surface P-selectin is a marker of platelet alpha granule exocytosis in response to pharmacologic doses of platelet receptor agonists: ADP (P2Y12 receptor), TRAP-6 (PAR1), GYPGKF (PAR4), Thrombin (PAR1/4 for human platelets and PAR3/4 for murine platelets), and U46619 (thromboxane receptor). For patents with STEMI and NSTEMI, a fixed agonist concentration of 10 μM for ADP, U46619, and TRAP-6 was used based on our previous publication. For the purposes of interrogating platelet activity, this technique shows similar data to light transmission aggregometry 6. Blood was collected by a trained medical professional into citrate plasma tubes. Analysis occurred within 60 minutes of blood collection. Platelet rich plasma (PRP) was isolated, with each concentration of agonist stimulation performed in quadruplicate. Following 15 minutes of agonist stimulation, 1 μL of labeled CD62P (P-selectin) antibody was incubated in the dark for 30 minutes. Samples were fixed in 2% formalin, then platelet surface P-selectin was quantified on an Accuri Flow Cytometer (BD Biosciences). Data was then processed through FloJo (Ashland, Oregon).
Platelet Spreading
Glass slides pre-coated the prior day with fibrinogen at a final concentration of 2.5 mg/mL in PBS. The fibrinogen-coated slides were washed thrice with PBS and then incubated for 1 hour at 37°C. Platelets were then fixed in 4% formaldehyde in 0.25% Triton X-100 for 20 minutes and then incubated with 0.3 μM rhodamine phalloidin for 30 minutes. The samples were then gently washed thrice with PBS, and then imaged with a confocal microscope. The investigator was blinded to the identity of the platelets until the analysis was complete and decoding occurred. Random fields of platelets were imaged, and the average platelet area was calculated using ImageJ software (NIH).
Immunoassay
Sandwich Enzyme-linked immunosorbant assays (ELISAs) were conducted according to the manufacturer’s instructions. The ELISA for cAMP was purchased from R & D Systems.
Experimental Animals
All mouse protocols were approved by the University Committee on Animal Resources (UCAR). Eight-week-old male and female wild-type C57BL/6J mice were used in this study. A mild MI was induced by left anterior descending coronary artery (LAD) cryo-ablation in which a small caliber, liquid nitrogen cooled probe was applied for 10 seconds to induce coronary artery closure as described 13. The integrity of the model for causing myocardial ischemia was verified by retro-orbital infusion of 2% methylene as we describe elsewhere, and then Image J software used to calculate the ischemic area at risk 2. Retro-orbital blood collected into heparinized Tyrodes solution as described by us previously was used to isolate mouse PRP and keep platelets in a silent state prior to agonist stimulation1, 2, 12, 14. Prior to stimulation, the platelets were washed with fresh Tyrode’s solution and centrifuged again which may remove heparin. Echocardiographic analysis of LV function by LVEF was made as the mean value from measurements in M-mode and B-mode with volumes in parasternal long axis, both performed using the Vevo2100 echocardiography system (VisualSonics, Toronto, Canada) and a linear-array 40 MHz transducer (MS-550D) as we reported previously 2.
Platelet Calcium Imaging
For murine calcium imaging, animals sedated under isofluorane anesthesia were subjected to MI as described, followed by retro-orbital removal of blood (500 μL) into heparinized Tyrodes solution. Washed platelets were then incubated with Fura2-AM (5 μM, 1 hour at 37°C) followed by benchtop centrifugation for 5 minutes at 2700 rpm with 10 μM PgI2. Excess Fura2 was removed and the platelet pellet was resuspended in fresh Tyrode’s solution in a 96-well plate, and platelet calcium release over 10 minutes was assessed by Abs340 nm /Abs380nm following automatic injection of 0.6 U/mL thrombin on a FexStation 3 (Molecular Devices, San Jose, CA). Data were expressed as change in fluorescence divided baseline fluorescence (F/F0).
Reagents
TRAP-6 (Cayman Chemicals # 3497), 2-methyl-ADP (Tocris, Bristol, UK # 475193-31-8), U46619 (Cayman Chemical # 56985-40-1), Thrombin (Sigma # 9002-04-4), PAR4 (Anaspec #AS-60778), CD62P-PE antibody Clone AK4 # 12-0628-62 (Thermo Fisher Waltham, MA # F7496), CD62P-FITC (BD Pharmigen # Bdb553744) and FITC-Fibrinogen antibody (BD Pharimigen # F7496), Fura2-AM (Thermo Fisher #F1221). GYPGKF (Anaspec # AS-60778). AYPGKF (Anaspec # AS-60218-1), FURA-2 (Thermo Fisher #F1221). Please refer to the online supplemental file for precise details on major resources utilized.
Statistics
Normalcy of data were firstly evaluated by the Shapiro–Wilk test. Gaussian-distributed data were analyzed using an unpaired, 2-tailed Student’s t test for comparison between two groups. For non-Gaussian-distributed data, the Mann-Whitney U test was used. Dichotomous clinical variables are presented as frequencies and evaluated by χ2. For Gaussian-distributed data in 3 or more groups, 1-way ANOVA with a Bonferroni correction to the p-value to account for multiple comparisons, otherwise the Kruskal–Wallis test followed by Dunn post-test was used. Multivariate regression analyses were conducted in the post-MI cohort to identify clinical variables independently associated with platelet activation. Three separate models were performed to test for male/female and NSTEMI/STEMI specific differences in platelet reactivity through PAR1, the thromboxane receptor, and the P2Y12 receptor. In each model, we included the following variables: sex, STEMI or NSTEMI (MI), age (rounded to nearest decade), hyperlipidemia (cholesterol), chronic kidney disease (renal function), chronic venous insufficiency (venous disease) , hypertension (blood pressure), presently on platelet inhibitors (anti-platelet therapy), and peripheral artery disease (arterial disease.). Results include the parameter estimate (95% confidence interval, C.I.) unless otherwise stated. Regression analyses were conducted using SAS version 9.4 (SAS Institute, North Carolina). Graphical data and simple group comparisons were illustrated using GraphPad Prism 8.1.1. (GraphPad Software, Inc., La Jolla, CA). Significance was accepted as a P value <0.05.
Results
Sex differences in platelet signaling in healthy humans.
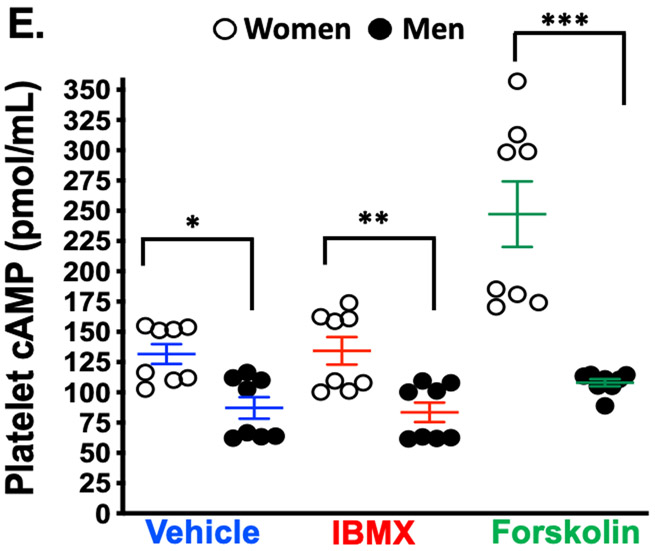
Pharmacologic interrogation of the three platelet signaling pathways for which orally-available antagonists exist revealed striking differences in platelets isolated from healthy women compared to men, with more reactivity observed in women (Figure. 1A-C). Consistent with a previous report, platelet spreading on a fibrinogen matrix which activates glycoprotein IIb/IIIa (GPIIb/IIIa) receptor was greater in healthy women than men 11 (Figure 1D). Certain platelet post-receptor signal transduction pathways converge to decrease adenyl cyclase-mediated cyclic AMP (cAMP) hydrolysis from ATP when platelets are activated. Conversely, increasing platelet cAMP suppresses platelet reactivity and thrombosis 15. Phosphodiesterase (PDE) which hydrolyses and inactivates cAMP can be pharmacologically inactivated by 3-isobutyl 3-methylxanthine (IBMX) while forskolin can be used to directedly augment adenyl cyclase activity and increase platelet cAMP 16. Evaluating these enzymes involved in the balance of platelet cAMP production and inactivation revealed basal cAMP and adenyl cyclase-stimulated cAMP production were both greater in platelets from healthy men compared with healthy women, while inhibiting PDE activity was similar to vehicle treatment (Figure. 1E).
Figure 1. Platelet activation and signaling in healthy subjects:
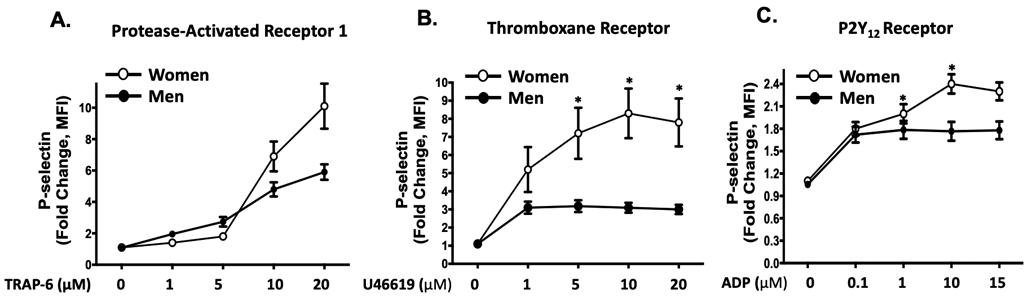
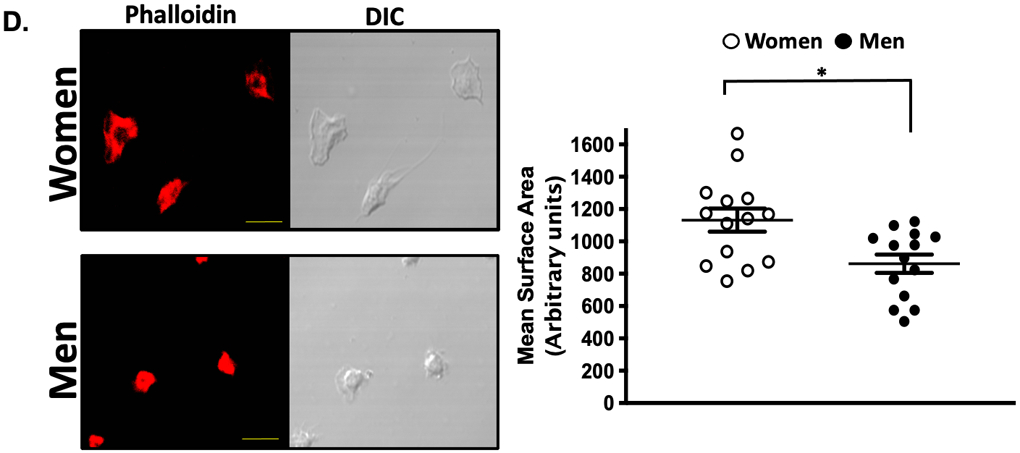
Platelets from healthy women and men were isolated and stimulated ex vivo with agonists for (A) PAR1 (TRAP-6), (B) the thromboxane receptor (U46619), and (C) the P2Y12 receptor (ADP) at the indicated concentrations. Platelet activation was assessed by FACS for alpha granule secretion (surface p-selectin) as mean fold increase from baseline ± SEM. * P <0.05 between groups at the indicated time point by the Kruskal-Wallis test followed by Dunn's post-test correction. Data are representative of n=11 men and n=17 women. (D) Male and female platelets were assessed for adhesion to fibrinogen after 30 mins at 37 °C by confocal microscopy. Data are represented as mean platelet surface area ± SEM. (n=3 in each group, 4-5 random fields per subject, 10-20 platelets per field). *P=0.007 between groups, t-test. Phalloidin-PE stains actin cytoskeleton red. Yellow scale bar= 5μM. (E) Platelets were isolated from healthy male and female subjects, and incubated for 3 hours with either IBMX to inhibit phosphodiesterase or forskolin to stimulate adenylyl cyclase. Platelet lysates were flash frozen, and an ELISA was conducted to determine GαS activity by cAMP production. Data are presented as mean ± SEM, n=8 men and n=8 women. * P=0.0099 **P=0.009 and ***P=0.0002 between male and female groups, by Mann-Whitney U test. IBMX=3-isobutyl-1-methylxanthine (100 μM, 30 minutes) inhibits platelet phosphodiesterase. Forskolin (1 μM, 30 minutes) activates platelet adenylyl cyclase.
Sex differences in platelet signaling in patients at the time of MI.
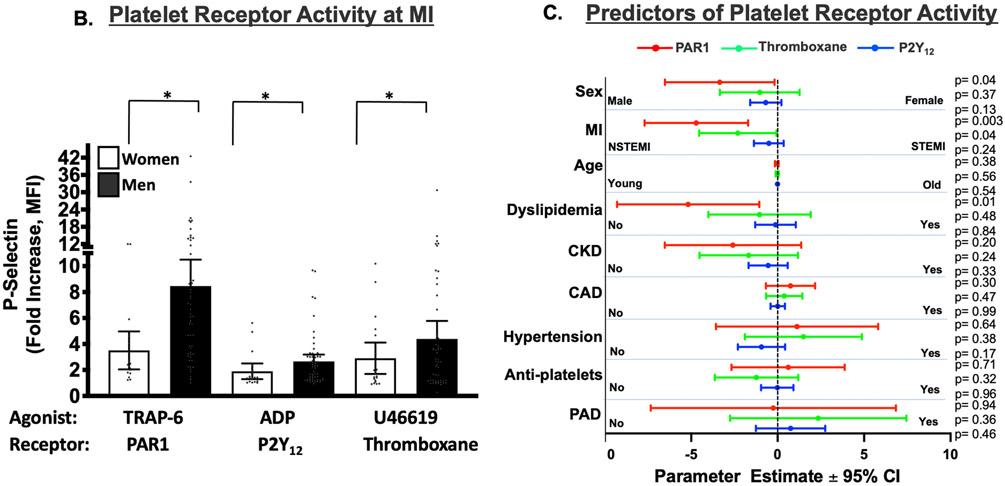
Using the agonist concentration which best evoked platelet responses in healthy subjects we examined platelet activation in women and men presenting acutely with MI prior to a loading dose of a P2Y12 receptor antagonist. Fig. 2A shows the clinical characteristics of the study population. With the exception of HDL, which is slightly greater in women, there were no statistical differences in demographic or clinical variables between women and men. Contrary to our observation in healthy individuals, platelets evaluated at the time of MI were 2.7-fold more reactive through PAR1 in men than in women (P=0.0001), and 1.6-fold more reactive in men than women through the platelet P2Y12 receptor (P=0.047). There was no difference in platelet activation between men and women through the platelet thromboxane receptor at the time of MI (Figure. 2B).
Figure 2. (A). Predictors of Platelet Reactivity at the Time of MI:
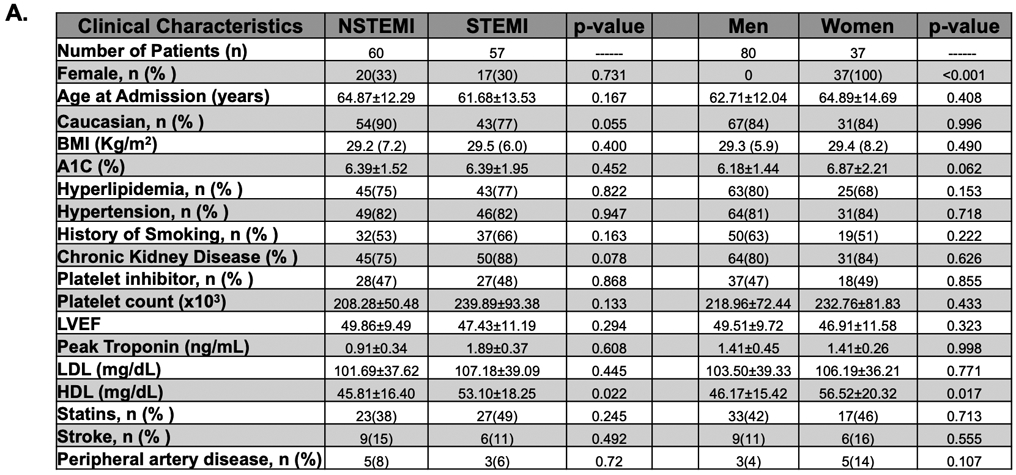
(A) Demographic of the post-MI study population. When stratifying for sex and NSTEMI/STEMI, except for HDL, the study populations were similar. There were 60 NSTEMI and 57 STEMI patients, and males comprised a similar proportion of each of these cohorts (30-33%.) Data are shown as mean ± SD. Level of significance is noted. HDL=high density lipoprotein. LDL=low density lipoprotein. LVEF=left ventricular ejection fraction. BMI=Body Mass Index. All data presented reflect variables at the time of patient evaluation in the emergency department. (B) Blood was drawn from women and men at the time of STEMI or NSTEMI diagnosis, prior to coronary angiography and prior to loading with a P2Y12 receptor antagonist. Isolated platelets were stimulated for 15 mins with an agonist for: PAR1 (TRAP-6, 10 μM), the thromboxane receptor (U46619, 10 μM), or the P2Y12 receptor (ADP, 10 μM). Platelet activation was assessed by FACS for alpha granule secretion (surface p-selectin) as mean fold increase from baseline ± 95% C. I. Differences between women and men for each agonist was assessed by the Mann-Whitney U test. *P=0.0001. **P=0.0473. NS=Not significant. n=80 males, n=37 females. (C) Independent predictors of platelet reactivity as forest plots illustrating multivariate-adjusted mean parameter estimates ± 95% C.I. following regression analyses for platelet function in response to surface receptors agonists at 10 μM. Red=PAR1, Green=Thromboxane, Blue=P2Y12. Level of significance is noted. Data are representative of 60 NSTEMI and 57 STEMI patients. CKD=chronic kidney disease. CAD=existing coronary artery disease. PAD=peripheral artery disease.
Using multivariate regression analysis, we determined that NSTEMI is the strongest clinical predictor in the model for platelet PAR1 signaling (−4.71, 95% CI −7.7 to −1.7, P=0.003) which confirmed our prior observation of highly reactive platelets through surface PAR1 in patients with NSTEMI but not STEMI 12. Contrary to what we observed in platelets from healthy women, less platelet activation was observed through PAR1 in the post-MI environment in women than in men (−3.35, 95% CI −6.52 to −0.2, P=0.04). Multivariate regression analysis also revealed dyslipidemia as an independent predictor of platelet PAR1 activation (−5.18, 95% CI −9.29 to −1.06, P=0.01), and NSTEMI to be an independent predictor of platelet activation through the thromboxane receptor (−2.31, 95% CI −4.54 to −0.07, P=0.04) (Figure. 2C).
Given the apparent sex-dependence observed for platelet PAR1 signaling in patients with NSTEMI, platelets from healthy individuals and platelets from patients with NSTEMI were pharmacologically interrogated in a dose-dependent manner using the non-specific PAR agonist thrombin and the PAR4 agonist peptide GYPGKF. While healthy female platelets are more reactive at lower doses of thrombin, male platelets are more reactive at higher doses of thrombin (Fig. 3A). Conversely, at the time of NSTEMI, platelets from men were 3-fold more reactive to the highest concentration of thrombin compared with platelets from women (Fig. 3B). Irrespective of healthy conditions or NSTEMI, platelets from men and women were similarly reactive to the PAR4 peptide GYPGKF, though showing attenuated activation profiles during NSTEMI (2.8-fold less in men and 8.0-fold less in women at the maximum agonist dose compared to healthy conditions, Fig. 3 C-D).” Comparing platelets isolated from patients immediately when MI is diagnosed to platelets from healthy individuals, platelet PAR1 signaling appears to decrease in women and increase in men at the time of MI. Similarly, platelets isolated from women at the time of MI diagnosis were less active through the platelet P2Y12 receptor than platelets in healthy women, and non-significantly more active through the platelet P2Y12 receptor compared to platelets from healthy men (Figure SI).
Figure 3. PAR-mediated platelet activation in healthy individuals and following MI:
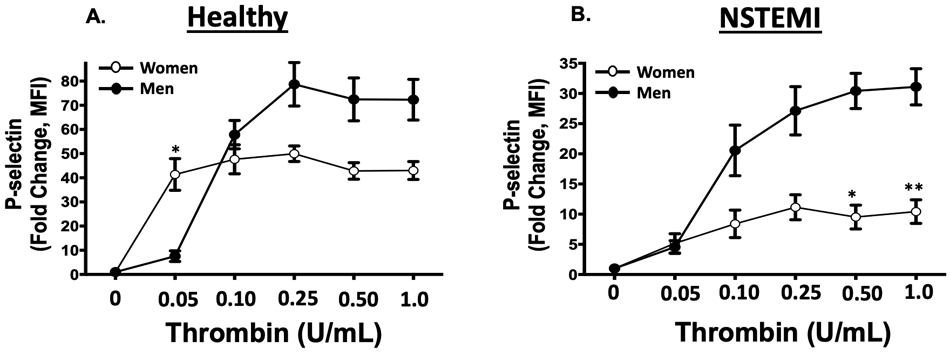
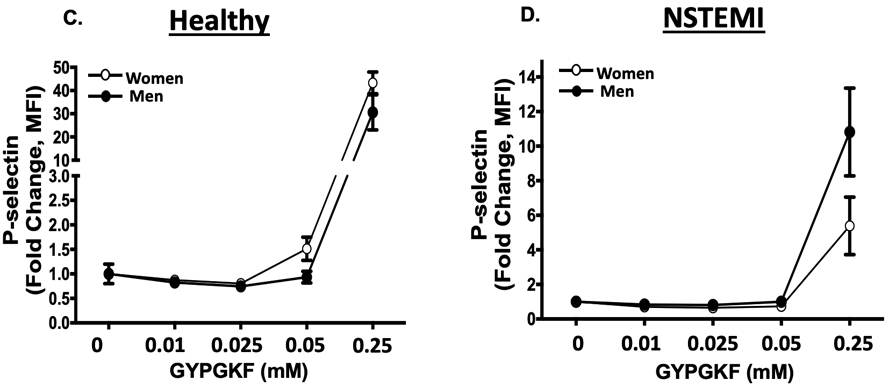
(A) Platelets were isolated from healthy women and men (n=10; n=5 in each group) then stimulated with human thrombin for 15 minutes (0=1.0 u/mL). * <0.05 between groups, assessed one-way ANOVA followed by Bonferroni's multiple comparisons test. (B) Platelet were isolated from women and men at the time of NSTEMI (n=10; n=5 in each group) then stimulated with human thrombin (0=1.0 u/mL) for 15 minutes. *P=0.028 and **P=0.054 assessed one-way ANOVA followed by Bonferroni's multiple comparisons test. Platelet activation was assessed by FACS for alpha granule secretion (surface p-selectin) as mean fold increase from baseline ± SEM. (C) Platelets were isolated from healthy women and men (n=10; n=5 in each group) then stimulated with the PAR4 peptide GYPGKF. (D) Platelets were isolated from women and men at the time of NSTEMI (n=10; n=5 in each group) then stimulated with the PAR4 peptide GYPGKF. Platelet activation was assessed by FACS for alpha granule secretion (surface p-selectin) as mean fold increase from baseline ± SEM. P=NS between heathy males and females and between NSTEMI males and females at each concentration assessed by one-way ANOVA followed by Bonferroni's multiple comparisons test.
Reasons for changes in platelet signaling patterns in patients could include alterations in platelet surface receptor density or post-receptor signal transduction pathways in health and during MI. While we did observe some sex-dependent alterations in platelet surface receptor expression in health and at the time of NSTEMI by flow cytometry (Figs. SII-SV), the magnitude and direction of changes were not concordant with the observed changes in platelet reactivity. Given that PAR1 couples to the GPCR Gαq subunit 17, protein immunoblotting was conducted and found to be similar in health for male and female platelets but disproportionately elevated in men compared with women at the time of NSTEMI (Fig. SVI). The GPCR Gαq subunit couples to enzymes liberating Ca2+ from the endoplasmic reticulum (ER) including phospholipase C (PLC) and, similarly, PLC isoforms including PLCγ1 are activated when platelets are stimulated with thrombin and by mobilized Ca2+ 18, 19. The expression of PLCγ1 and activation of PLCγ1 (determined by phosphorylation) were similar in men and women at the time of NSTEMI (Fig. SVII).
Sex differences in platelet signaling in mice:
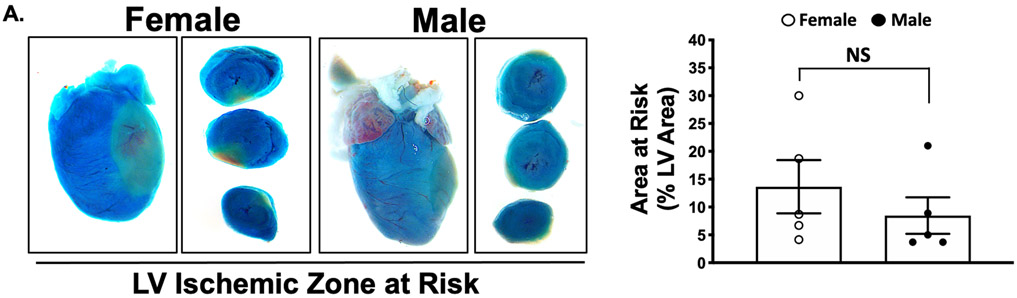
The platelet receptor proteomic profile, surface receptor agonist sensitivity, and the response to anti-platelet agents differs in ischemic and hypoxic conditions 1. Platelet PAR signaling in healthy humans is reciprocally associated with activation at the time of MI. We explored this further in mice using the mild MI model of left-anterior descending (LAD) coronary artery cryoablation. This model better simulates platelet activation in NSTEMI. A similar ischemic area at risk was observed at the time of MI in male and female mice (Fig. 4A). Consistent with what we observed in humans, healthy female platelets were more activated by thrombin ex vivo compared to males in mice (P< 0.001). Peak platelet activation by PAR was both greater and also augmented at the time of MI in male mice compared with female mice (5.2-fold for females and 11-fold for males, P < 0.05 between groups) (Figure 4B). Using the PAR4-activating peptide GYPGKF to stimulate platelets, peak platelet activation was only slightly greater in healthy male than female mice. Conversely, three days following MI, platelet activation through PAR4 was attenuated in both groups (by 1.9-fold in males and 2.5-fold in females) (Figure 4C). Since PAR signaling in platelets mobilizes downstream calcium (Ca2+) to induce platelet degranulation, we re-evaluated the platelet response to thrombin immediately at the time of MI following LAD cryoablation. Platelet Ca2+ mobilization at the time of MI increased in males and decreased in females (Fig. 4D). Myocardial systolic performance determined by left ventricular ejection fraction (LVEF) and fractional shortening (FS) three days following MI was higher in female mice than in male mice. Increased platelet reactivity observed in males at the time of MI, therefore, is associated with poorer post-MI myocardial function (Figure SVIII-SIX).
Figure 4: Platelet Reactivity in a mild murine model of MI.
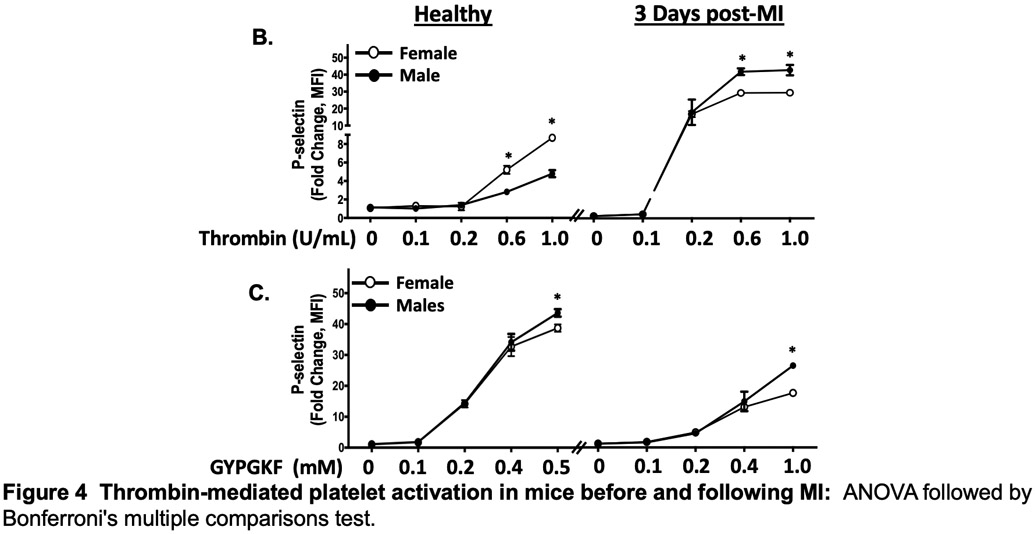
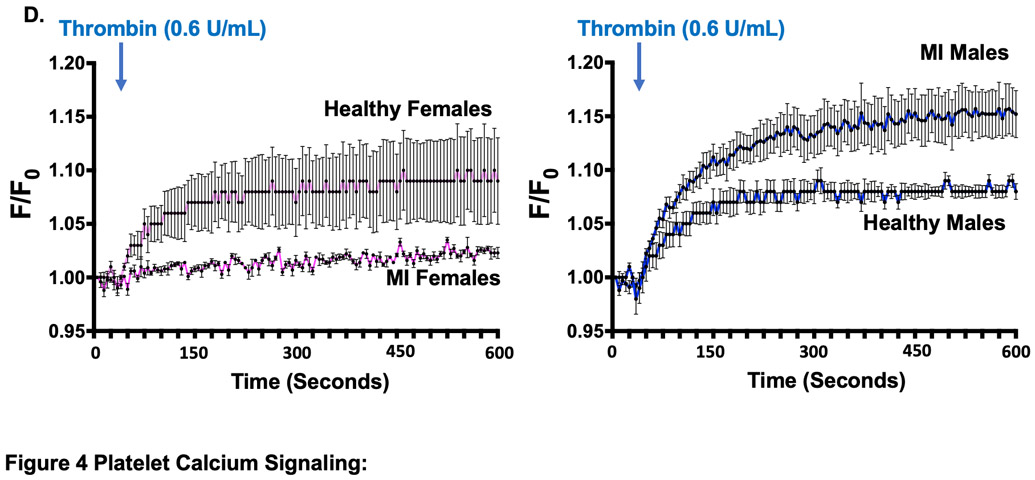
(A) Male and female C57BL/6J mice underwent sham surgery LAD infarction by cryoablation, followed immediately by retro-orbital infusion of 2% methylene blue to indicate areas of LV perfusion. Perfused tissue is blue and ischemic tissue is pink. LV region at risk quantified as % LV area (mean % of LV area ± SEM for n=5 mice). Representative images are shown for male and female mice. LAD=left anterior descending coronary artery. LV=left ventricle. Differences between groups was assessed by t-test, and not found to be significant 3 days following MI. Platelets were stimulated ex vivo with (B) thrombin or (C) PAR4 specific agonist GYPGKF. Platelet activation was assessed by FACS (surface p-selectin). Data are represented as mean fold increase over baseline (0) ± SEM for n=5 mice, each performed in quadruplicate. * P <0.05 between groups, assessed by one-way ANOVA followed by Bonferroni's multiple comparisons test. (D) Platelet calcium mobilization in males and female mice at the time of MI. 5 minutes after LAD occlusion, platelet Ca2+ mobilization was assessed by FURA-2 as Abs340 nm /Abs380nm and the ratio expressed as fluorescence, F/baseline Fluorescence, F0. Data are presented as mean ± SEM for n=5 mice in each group.
Discussion
To our knowledge, the present study is the first to directly compare platelet signaling in women and men in healthy conditions and in the post-MI environment. We purposefully investigated only platelet signaling pathways for which anti-platelet agents exist to maximize the translational impact of the investigation. Comparing platelet signaling in health to the immediate post-MI environment, a reciprocal switch in platelet activation through PAR1 occurs, with attenuated signaling in females and augmented signaling in males noted at the time of MI. A very recent study investigating the significantly increased risk of MI manifesting mostly as NSTEMI in younger women compared to men did not identify a clinical variable or mechanism to account for their observation20. The authors, however, do suggest microvascular obstruction as a cause which is a vascular territory in which platelets are most reactive21.
Platelet activation by thrombin in both males and females was accentuated at higher agonist concentrations. In addition, platelet activation in men and women through PAR4 was more pronounced at higher agonist concentrations. This sex-specific observation was also noted in mice suggesting a conserved rather than a species-specific physiologic phenomenon.
A sex difference in platelet PAR4 signaling in mice and humans was mild and absent, respectively, in health and at the time of MI. Comparing humans and mice, PAR4-mediated platelet activation during MI was significantly attenuated. These observations are consistent with previous reports on platelet PAR1 and PAR4 activation kinetics that demonstrated initial platelet activation by thrombin is PAR1-mediated22, 23, while sustained or chronic platelet activation depends more on PAR4. The requirement for a higher PAR4 concentration to see the latter effect may explain diminished platelet PAR4 sensitivity during MI.
Receptor-mediated platelet activation depends on signal amplification through GPCRs and second messengers including cAMP and Ca2+. Our study failed to demonstrate that changes in surface receptor expression account for substantial observed sex-specific differences in platelet activation in health and at the time of MI. However, adenylyl cyclase-mediated ATP hydrolysis and (inhibitory) cAMP production in male platelets appears to be higher in healthy conditions while thrombin-induced (stimulatory) Ca2+ mobilization in male platelets was greater than female platelets at the time of MI. In other areas of cardiovascular biology, differences in Ca2+-mediated signaling and effector function have been reported24-26. These observations are consistent with the concept that distal components of the signal transduction cascade account for sex-specific alterations in platelet activation and should be investigated and refined as potential targets for sex-specific antiplatelet therapy in the context of acute MI.
Augmented platelet activation through PAR1 following MI was pronounced in NSTEMI and not STEMI which supports prior reports that platelet signaling in both conditions are dissimilar 12, 27, 28. It is potentially revealing that both platelet PAR1 and thromboxane receptor signaling but not P2Y12 receptor signaling were augmented in NSTEMI following adjustment in the multivariate model. This suggests that blocking platelet thromboxane production with aspirin and platelet PAR1 directly with a PAR1 antagonist or indirectly with a thrombin inhibitor or a Factor Xa inhibitor to prevent pro-thrombin to thrombin conversion may be beneficial, and especially so for males. A promising recent Phase II clinical study that included patients with angina and NSTEMI indicates the PAR1 antagonist PZ-128 – even when used with aspirin and clopidogrel – does not result in major bleeding, and reduces myocardial necrosis coincident with reduced PAR1-mediated platelet activation 29. While this promising result supports the relevance of platelet PAR1 at the time of MI, it is very important to note that the patients in this investigation were mostly male (73% - 94% for placebo and three concentrations of PZ-128 utilized). The inclusion of females in early clinical studies is critical since platelet signaling as noted in our study is not the same.
Women have historically been under-studied in scientific investigations. We confirmed the data by Becker et al. by showing thromboxane-mediated platelet activation in healthy conditions is significantly greater in women than men 10. Well-designed population-based studies failed to show that aspirin protects from adverse cardiovascular events in men when used for the purposes of primary prevention 30, 31. However, aspirin confers some protection against thrombotic stroke in women 32. A meta-analysis of five studies by Rothwell et al. suggested low dose aspirin may lack a protective effect in the majority of treated men due to possible ‘under-dosing’9. Body weight and height both tend to be higher in men. The conclusion of this study is reasonable since adverse cardiovascular outcomes were predicted by, but not necessarily caused by, differences in body weight and height.
Based on the current data, healthy women have platelets that are particularly sensitive to activation through thromboxane-mediated pathways. Platelet thromboxane production is blocked when aspirin irreversibly acetylates cyclooxygenase 32. A recent study confirmed that lower aspirin doses (81-100 mg) satisfactorily inhibit the thrombotic but not the anti-inflammatory properties of platelets while higher doses of aspirin, through platelet prostacyclin inhibition, promote bleeding in a murine model of arterial thrombosis. It is noteworthy that the investigators examined only male mice 33. We therefore caution that there is neither clear evidence to suggest empirically increasing the dose of aspirin in men confers additional protection from thrombosis, nor is there evidence to suggest that high dose aspirin necessarily promotes bleeding in women. Conducting a study in healthy women and men with low (81 mg), intermediate (162 mg), and high (325 mg) dose aspirin, assessing thromboxane B2 for adherence and efficacy, then evaluating several methods of platelet activation through the thromboxane receptor will be required to determine whether a divergent platelet phenotype in women and men is responsible for the differences in cardiovascular outcomes observed with aspirin use.
Off-target effects of anti-platelet agents and unexpected lack of efficacy in patients with certain demographic profiles or in certain clinical scenarios has been extensively reported. Single nucleotide polymorphisms for genes encoding enzymes for P2Y12 antagonist metabolisms, opiate-mediated alterations in P2Y12 antagonist absorption, and metabolic disease-mediated alterations in the P2Y12 receptor conformation are proposed mechanism for failure of clopidogrel and ticagrelor to exert their anti-platelet affect 3, 34, 35. Enteric-coated aspirin which may delay absorption, a change in the activity of drug transporters, and possible under-dosing are proposed explanations for the alteration in the efficacy of aspirin. 9, 36. Determination of a sex-specific difference in these clinical situations is a logical extension of previous studies.
We previously reported in a small patient cohort that platelets from patients with NSTEMI preferentially activate through PAR1 compared to platelets from patients with STEMI or from healthy control subjects 12. The present study confirms this observation in a larger patient cohort and identifies male sex as an independent predictor of platelet PAR1 signaling in NSTEMI. Focusing more on inhibiting platelet Protease Activated Receptors (PARs) or circulating thrombin is a logical mechanistic intervention based on previous investigations. The Acute Coronary Syndromes (TRACER) study evaluated a PAR1 antagonist added to dual anti-platelet therapy (DAPT), showing some benefit in reducing adverse cardiovascular events with an increased risk of major bleeding 37. A PAR1 antagonist alone in patients with established vascular disease significantly prevented arterial thrombosis and limb ischemia 38. Similarly, the MANAGE study showed that directly inhibiting thrombin with dabigatran in patients with established vascular disease and myocardial injury at the time of surgery conferred protection from thrombotic emergencies and vascular death 39. We previously reported that proteomic profiles, agonist sensitivity, and anti-platelet medication efficacy are altered in humans and in murine models of MI and peripheral artery disease 1, 12. We now suggest, based on the present data, that platelets in patients with acute MI are primed toward augmented PAR signaling in men more than women. We observed differences in PAR-mediated calcium mobilization in male and female platelets at the time of MI. This suggests differences in post-receptor signal transduction cascades between male and female platelets that may be capitalized upon as sex-specific targets for new anti-platelet medications.
Using the Factor Xa inhibitor rivaroxaban alone in the ATLAS ACS2 study 40 or in combination with aspirin in the COMPASS study, there was a dramatic reduction in atheroembolic events and improved mortality in patients with known vascular disease. It is noteworthy that these studies also showed more outcome data in men than women, raising the possibility that indirectly blocking thrombin with a Xa inhibitor intuitively may have more survival benefit in males based on the data we present here 41, 42. Since Xa inhibitors indirectly inhibit circulating Factor IIa (thrombin) activity downstream 43, it is revealing that our present study confirmed in both humans and in mice that thrombin-mediated signaling in platelets is an especially important signaling pathway in males in the post-MI environment. Inhibiting both platelet thrombin and thromboxane signaling in multiple vascular beds could be one mechanism for improving outcomes in stable vascular disease and in patients transitioning off dual anti-platelet therapy between 6 to 24 months post-MI. We suggest that greater attention should be directed to consider whether changes in the platelet phenotype in women and men are responsible for differences in outcomes and responsiveness to anti-platelet medications.
Supplementary Material
Highlights.
Platelet activation in men and women differs in health and at the time of myocardial infarction
Male sex and non-ST Segment Myocardial Infarction were independent predictors of platelet PAR1 activation.
In both mice and humans, thrombin-mediated platelet activation is augmented in males and attenuated in females at the time of myocardial infarction.
Sex-specific platelet signaling and the performance of antiplatelet medications – especially through platelet PARs – should be prospectively evaluated.
Acknowledgements:
We would like to Dr. Johns Gassler for advice on timing of blood collection, and Ms. Rachel Schmidt for technical assistance.
Sources of Funding: Grants: from NHLBI 4-K08HL128856 and LRP 120200, as well as a University of Rochester Department of Medicine Pilot Grant to SJC.
Non-Standard Abbreviations and Acronyms
- GPCR
G-Protein-Coupled Receptor (GPCR)
- STEMI
ST-Segment Elevation Myocardial Infarction
- NSTEMI
Non-ST-Segment Elevation Myocardial Infarction
- PAR
Protease-activated receptor (PAR)
Footnotes
Disclosures: None of the listed authors have any relevant disclosures.
References
- 1.Cameron SJ, Mix DS, Ture SK, Schmidt RA, Mohan A, Pariser D, Stoner MC, Shah P, Chen L, Zhang H, Field DJ, Modjeski KL, Toth S, Morrell CN. Hypoxia and ischemia promote a maladaptive platelet phenotype. Arterioscler Thromb Vasc Biol. 2018;38:1594–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron SJ, Morrell CN, Bao C, Swaim AF, Rodriguez A, Lowenstein CJ. A novel anti-inflammatory effect for high density lipoprotein. PLoS One. 2015;10:e0144372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu L, Chang L, Zhang Y, Zhai L, Zhang S, Qi Z, Yan H, Yan Y, Luo X, Zhang S, Wang Y, Kunapuli SP, Ye H, Ding Z. Platelets express activated p2y12 receptor in patients with diabetes mellitus. Circulation. 2017;136:817–833 [DOI] [PubMed] [Google Scholar]
- 4.Wisman PP, Teraa M, de Borst GJ, Verhaar MC, Roest M, Moll FL. Baseline platelet activation and reactivity in patients with critical limb ischemia. PLoS One. 2015;10:e0131356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeymer U, Riedel K, Hahn M. Medical therapy and recurrent ischemic events in high risk patients surviving their myocardial infarction for at least 12 months: Comparison of patients with st elevation versus non-st elevation myocardial infarction. Cardiol Ther. 2017;6:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA Jr., Granger CB, Flather MD, Budaj A, Quill A, Gore JM, Investigators G. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA. 2007;297:1892–1900 [DOI] [PubMed] [Google Scholar]
- 7.Chan MY, Sun JL, Newby LK, Shaw LK, Lin M, Peterson ED, Califf RM, Kong DF, Roe MT. Long-term mortality of patients undergoing cardiac catheterization for st-elevation and non-st-elevation myocardial infarction. Circulation. 2009;119:3110–3117 [DOI] [PubMed] [Google Scholar]
- 8.Gupta T, Kolte D, Khera S. et al. Contemporary sex-based differences by age in presenting characteristics, use of an early invasive strategy, and inhospital mortality in patients with non-st-segment-elevation myocardial infarction in the united states. Circ Cardiovasc Interv. 2018;11:e005735. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JFF, Roncaglioni MC, Morimoto T, Mehta Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet. 2018;392:387–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker DM, Segal J, Vaidya D, Yanek LR, Herrera-Galeano JE, Bray PF, Moy TF, Becker LC, Faraday N. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. 2006;295:1420–1427 [DOI] [PubMed] [Google Scholar]
- 11.Faraday N, Goldschmidt-Clermont PJ, Bray PF. Gender differences in platelet gpiib-iiia activation. Thromb Haemost. 1997;77:748–754 [PubMed] [Google Scholar]
- 12.Schmidt RA, Morrell CN, Ling FS, Simlote P, Fernandez G, Rich DQ, Adler D, Gervase J, Cameron SJ. The platelet phenotype in patients with st-segment elevation myocardial infarction is different from non-st-segment elevation myocardial infarction. Transl Res. 2018;195:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camacho P, Fan H, Liu Z, He JQ. Small mammalian animal models of heart disease. Am J Cardiovasc Dis. 2016;6:70–80 [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron SJ, Ture SK, Mickelsen D, Chakrabarti E, Modjeski KL, McNitt S, Seaberry M, Field DJ, Le NT, Abe J, Morrell CN. Platelet extracellular regulated protein kinase 5 is a redox switch and triggers maladaptive platelet responses and myocardial infarct expansion. Circulation. 2015;132:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jantzen HM, Milstone DS, Gousset L, Conley PB, Mortensen RM. Impaired activation of murine platelets lacking g alpha(i2). J Clin Invest. 2001;108:477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy KL, Traynelis SF, Hepler JR. Par1 and par2 couple to overlapping and distinct sets of g proteins and linked signaling pathways to differentially regulate cell physiology. Mol Pharmacol. 2010;77:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Adams T, Zhi H, Yu M, Wen R, Newman PJ, Wang D, Newman DK. Restoration of responsiveness of phospholipase cgamma2-deficient platelets by enforced expression of phospholipase cgamma1. PLoS One. 2015;10:e0119739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter G, Ji Q. Phospholipase c-gamma as a signal-transducing element. Exp Cell Res. 1999;253:15–24 [DOI] [PubMed] [Google Scholar]
- 20.DeFilippis ECB, Singh A, Biery DW. et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: The mass general brigham young-mi registry. . Eur Heart J. 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokes KY, Granger DN. Platelets: A critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590:1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Covic L, Gresser AL, Kuliopulos A. Biphasic kinetics of activation and signaling for par1 and par4 thrombin receptors in platelets. Biochemistry. 2000;39:5458–5467 [DOI] [PubMed] [Google Scholar]
- 23.Han X, Nieman MT. Protease activated receptor 4: A backup receptor or a dark horse as a target in antiplatelet therapy? Ann Transl Med. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwertz DW, Beck JM, Kowalski JM, Ross JD. Sex differences in the response of rat heart ventricle to calcium. Biol Res Nurs. 2004;5:286–298 [DOI] [PubMed] [Google Scholar]
- 25.Grandy SA, Howlett SE. Cardiac excitation-contraction coupling is altered in myocytes from aged male mice but not in cells from aged female mice. Am J Physiol Heart Circ Physiol. 2006;291:H2362–2370 [DOI] [PubMed] [Google Scholar]
- 26.Howlett SE. Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am J Physiol Heart Circ Physiol. 2010;298:H659–670 [DOI] [PubMed] [Google Scholar]
- 27.Ward JA, Esa N, Pidikiti R, Freedman JE, Keaney JF, Tanriverdi K, Vitseva O, Ambros V, Lee R, McManus DD. Circulating cell and plasma microrna profiles differ between non-st-segment and st-segment-elevation myocardial infarction. Fam Med Med Sci Res. 2013;2:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eicher JD, Wakabayashi Y, Vitseva O, Esa N, Yang Y, Zhu J, Freedman JE, McManus DD, Johnson AD. Characterization of the platelet transcriptome by rna sequencing in patients with acute myocardial infarction. Platelets. 2016;27:230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuliopulos A, Gurbel PA, Rade JJ, Kimmelstiel CD, Turner SE, Bliden KP, Fletcher EK, Cox DH, Covic L, Investigators T-P. Par1 (protease-activated receptor 1) pepducin therapy targeting myocardial necrosis in coronary artery disease and acute coronary syndrome patients undergoing cardiac catheterization: A randomized, placebo-controlled, phase 2 study. Arterioscler Thromb Vasc Biol. 2020:ATVBAHA120315168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, Ray KK. Effect of aspirin on vascular and nonvascular outcomes: Meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–216 [DOI] [PubMed] [Google Scholar]
- 31.Ikeda Y, Shimada K, Teramoto T, Uchiyama S, Yamazaki T, Oikawa S, Sugawara M, Ando K, Murata M, Yokoyama K, Ishizuka N. Low-dose aspirin for primary prevention of cardiovascular events in japanese patients 60 years or older with atherosclerotic risk factors: A randomized clinical trial. JAMA. 2014;312:2510–2520 [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304 [DOI] [PubMed] [Google Scholar]
- 33.Ni R, Vaezzadeh N, Zhou J, Weitz JI, Cattaneo M, Gross PL. Effect of different doses of acetylsalicylic acid on the antithrombotic activity of clopidogrel in a mouse arterial thrombosis model. Arterioscler Thromb Vasc Biol. 2018;38:2338–2344 [DOI] [PubMed] [Google Scholar]
- 34.McEvoy JW, Ibrahim K, Kickler TS. et al. Effect of intravenous fentanyl on ticagrelor absorption and platelet inhibition among patients undergoing percutaneous coronary intervention: The pacify randomized clinical trial (platelet aggregation with ticagrelor inhibition and fentanyl). Circulation. 2018;137:307–309 [DOI] [PubMed] [Google Scholar]
- 35.Viviani Anselmi C, Briguori C, Roncarati R, Papa L, Visconti G, Focaccio A, De Micco F, Latronico MV, Pagnotta P, Condorelli G. Routine assessment of on-clopidogrel platelet reactivity and gene polymorphisms in predicting clinical outcome following drug-eluting stent implantation in patients with stable coronary artery disease. JACC Cardiovasc Interv. 2013;6:1166–1175 [DOI] [PubMed] [Google Scholar]
- 36.Bhatt DL, Grosser T, Dong JF, Logan D, Jeske W, Angiolillo DJ, Frelinger AL 3rd, Lei L, Liang J, Moore JE, Cryer B, Marathi U. Enteric coating and aspirin nonresponsiveness in patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2017;69:603–612 [DOI] [PubMed] [Google Scholar]
- 37.Tricoci P, Huang Z, Held C. et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33 [DOI] [PubMed] [Google Scholar]
- 38.Bonaca MP, Gutierrez JA, Creager MA, Scirica BM, Olin J, Murphy SA, Braunwald E, Morrow DA. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: Results from the trial to assess the effects of vorapaxar in preventing heart attack and stroke in patients with atherosclerosis-thrombolysis in myocardial infarction 50 (tra2 degrees p-timi 50). Circulation. 2016;133:997–1005 [DOI] [PubMed] [Google Scholar]
- 39.Devereaux PJ, Duceppe E, Guyatt G. et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (manage): An international, randomised, placebo-controlled trial. Lancet. 2018;391:2325–2334 [DOI] [PubMed] [Google Scholar]
- 40.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM, Investigators AAT. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19 [DOI] [PubMed] [Google Scholar]
- 41.Anand SS, Bosch J, Eikelboom JW. et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229 [DOI] [PubMed] [Google Scholar]
- 42.Eikelboom JW, Connolly SJ, Bosch J. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330 [DOI] [PubMed] [Google Scholar]
- 43.Samama MM. The mechanism of action of rivaroxaban--an oral, direct factor xa inhibitor--compared with other anticoagulants. Thromb Res. 2011;127:497–504 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.