Abstract
Experimental evidence indicates that cannabidiol (CBD) induces anxiolytic and antiepileptic effects through the activation of 5-HT1A receptors. These receptors are coupled to Gi/o proteins and induce inhibitory effects. At present, the interaction of CBD with 5-HT1A receptors in the human brain is unknown. The aim of this study focused on evaluating the interaction between CBD and 5-HT1A receptors in cell membranes obtained from the hippocampus and temporal neocortex of autopsies and patients with drug-resistant mesial temporal lobe epilepsy (DR-MTLE). Cell membranes were isolated from the hippocampus and temporal neocortex of a group of patients with DR-MTLE who were submitted to epilepsy surgery (n = 11) and from a group of autopsies (n = 11). The [3H]-8-OH-DPAT binding assay was used to determine the pharmacological interaction of CBD with 5-HT1A receptors. The [35S]-GTPγS assay was used to investigate the CBD-induced activation of Gi/o proteins through its action on 5-HT1A receptors.The CBD affinity (pKi) for 5-HT1A receptors was similar for autopsies and patients with DR-MTLE (hippocampus: 4.29 and 4.47, respectively; temporal neocortex: 4.67 and 4.74, respectively). Concerning the [35S]-GTPγS assay, no statistically significant changes were observed for both hippocampal and neocortical tissue (p > 0.05) at low CBD concentrations (1 pM to 10 μM). In contrast, at high concentrations (100 μM), CBD reduced the constitutive activity of Gi/o proteins of autopsies and DR-MTLE patients (hippocampus: 39.2% and 39.6%, respectively; temporal neocortex: 35.2% and 24.4%, respectively). These changes were partially reversed in the presence of WAY-100635, an antagonist of 5-HT1A receptors, in the autopsy group (hippocampus, 59.8%, p < 0.0001; temporal neocortex, 71.5%, p < 0.0001) and the group of patients with DR-MTLE (hippocampus, 53.7%, p < 0.0001; temporal neocortex, 68.5%, p < 0.001). Our results show that CBD interacts with human 5-HT1A receptors of the hippocampus and temporal neocortex. At low concentrations, the effect of CBD upon Gi/o protein activation is limited. However, at high concentrations, CBD acts as an inverse agonist of 5-HT1A receptors. This effect could modify neuronal excitation and epileptic seizures in patients with DR-MTLE.
Keywords: serotonin, 5-HT1A receptor, hippocampus, mesial temporal lobe epilepsy, drug-resistant epilepsy, cannabidiol, temporal neocortex
Introduction
Cannabidiol (CBD), the main non-psychoactive component of Cannabis plants (Russo, 2017), has a terpenophenolic structure hydroxylated at carbons 1 and 3 (Jones et al., 1977). This structure gives CBD lipophilic properties, which allow its passage across the blood–brain barrier (Calapai et al., 2020).
CBD induces antiepileptic effects in humans and experimental models (Silvestro et al., 2019). In patients with Dravet- or Lennox-Gastaut syndromes, the administration of CBD reduces the frequency and severity of the seizures (Maa and Figi, 2014; Thiele et al., 2018; Lazaridis et al., 2019). In patients with drug-resistant temporal lobe epilepsy (DR-TLE), the coadministration of CBD with antiseizure drugs reduces the number and intensity of epileptic seizures (Cunha et al., 1980). CBD administration also decreases seizure activity and neuronal hyperexcitability in experimental models of temporal lobe epilepsy (Khan et al., 2018; Patra et al., 2019). Moreover, CBD produces anxiolytic effects in humans and experimental models (Shannon et al., 2019). CBD induces antidepressant effects that are more evident when tissue serotonin levels are high (Sales et al., 2018). These effects are partially explained because CBD interacts with 5-hydroxytryptamine1A (5-HT1A) receptors (Magen et al., 2010). In cultured Chinese hamster ovary (CHO) cells expressing 5-HT1A receptors, CBD showed micromolar affinity in displacing [3H]-8-OH-DPAT from 5-HT1A receptors, increased [35S]-GTPγS binding in this Gi/o protein-coupled receptor, and reduced cAMP concentration. According to these results, the authors concluded that CBD behaves as an agonist of 5-HT1A receptors (Russo et al., 2005). These effects were not reproduced when cell membranes obtained from rat brainstem were exposed to CBD. However, this phytocannabinoid enhanced the ability of 8-OH-DPAT, an agonist of 5-HT1A receptors, to stimulate [35S]-GTPγS binding. These results suggest that CBD shows an allosteric interaction with 5-HT1A receptors (Rock et al., 2012).
At present, the interaction of CBD with 5-HT1A receptors in the human brain is not known. Indeed, changes of this interaction induced by drug-resistant epilepsy may represent a condition that augments or reduces the efficacy of CBD to control the seizure activity. The aim of this study focused on evaluating the interaction of CBD with 5-HT1A receptors in cell membranes obtained from the hippocampus and temporal neocortex of patients with drug-resistant mesial TLE (DR-MTLE). The results were compared with brain tissue with no neurological disorders obtained from autopsies.
Materials and Methods
Patients with DR-MTLE
Patients with DR-MTLE underwent an extensive presurgical evaluation that included video electroencephalogram (EEG) and magnetic resonance imaging (MRI) in the Epilepsy Clinic of the General Hospital from Mexico Dr. Eduardo Liceaga. Four serial EEGs were conducted to determine the presence and location of epileptiform activity. T1–T2-weighted MRI served to identify mesial sclerosis. Patients with cortical dysplasia or neocortical TLE were excluded from the study. After the presurgical evaluation, 11 patients with DR-MTLE (five females and six males) were included in the present study. The scientific and ethics committees from all the institutions involved approved this experimental protocol (authorization number DI/15/403/03/32). Written informed consent was obtained from all participants.
A standard anterior temporal lobectomy ipsilateral to the epileptogenic zone was performed in every patient 48 h after the last seizure occurrence. During the surgical procedure, samples from the hippocampus and temporal neocortex were collected immediately after resection and stored at −70°C (Table 1).
Table 1.
Clinical data of patients with drug-resistant mesial temporal lobe epilepsy (DR-MTLE).
| ID | Gender | Age (years) | Age of seizure onset (years) | Duration of epilepsy (years) | Frequency of seizures (per month) | Side of focus | Precipitating factors | ASD before surgery |
|---|---|---|---|---|---|---|---|---|
| HUM-138 | F | 37 | 4 | 33 | 75 | Left | Febrile seizures in childhood | PHT/CBZ/VA/ OXC/CLN |
| HUM-154 | F | 9 | 3 | 6 | 7 | Right | Febrile seizures in childhood | VA/TOP/CBZ/ PHB/LEV/LAM |
| HUM-155 | M | 31 | 12 | 19 | 20 | Left | Febrile seizures in childhood | PHT/VA/CBZ/LAM |
| HUM- 161 | M | 19 | 0.16 | 19 | 2 | Left | Febrile seizures in childhood | PHT/VA/CBZ/TOP |
| HUM-162 | M | 56 | 18 | 38 | 30 | Right | Temporal-occipital AVM | CBZ/DZP/PHT/ PHB/LEV/VA |
| HUM-168 | M | 28 | 15 | 13 | 15 | Bilateral | TBI and mother with epilepsy | CBZ/CLN/LEV |
| HUM-169 | M | 46 | 12 | 34 | 15 | Right | TBI | PHT/CBZ |
| HUM-173 | F | 46 | 8 | 38 | 2 | Right | Unknown | PHT/OXC |
| HUM-184 | F | 28 | 13 | 15 | 16 | Bilateral | Unknown | LAM/VA/CBZ/LEV |
| HUM-192 | M | 41 | 32 | 9 | 10 | Left | Father with epilepsy | LAM/VA/CBZ/LEV |
| HUM-193 | F | 22 | 11 | 11 | 4 | Right | Febrile seizures in childhood | LAM/VA/LEV/TOP |
ASD, antiseizure drugs; AVM, arteriovenous malformation; CBZ, carbamazepine; CLN, clonazepam; F, female; LAM, lamotrigine; LEV, levetiracetam; M, male; OXC, oxcarbazepine; PHB, phenobarbital; PHT, phenytoin; TBI, traumatic brain injury; TOP, topiramate; VA, valproic acid.
Autopsies
Samples from the hippocampus and temporal neocortex were obtained from 11 autopsies (three females and eight males, 37.1 ± 17.8 years of age). Death was not associated with neurological disorders, for which brain tissues were analyzed and considered as controls. Autopsy samples were collected with a postmortem interval (PMI) of 15.91 ± 3.11 h. The samples were frozen immediately after resection and stored at −70°C. Autopsies were performed at the Institute of Forensic Sciences in Mexico City (Table 2).
Table 2.
Clinical data of autopsies.
| ID | Gender | Age (years) | Cause of death | PMI (h) |
|---|---|---|---|---|
| A2 | M | 29 | Polycontusion | 18 |
| A3 | M | 30 | Ballistic trauma | 14 |
| A7 | M | 45 | Suffocation | 18 |
| A8 | M | 73 | Complications associated with diabetes | 15 |
| A10 | M | 36 | Ballistic trauma | 12 |
| A11 | F | 12 | Suffocation | 14 |
| A12 | F | 40 | Ballistic trauma | 20 |
| A14 | F | 45 | Unknown | 10 |
| A16 | M | 57 | Myocardial infarction | 18 |
| A17 | M | 25 | Penetrating wound in thorax | 18 |
| A21 | M | 16 | Suffocation | 18 |
F, female; h, hours; M, male; PMI, postmortem interval.
Radioligand Displacement Assay
To assess the interaction of CBD with 5-HT1A receptors, we conducted radioligand displacement assays and evaluated the ability of CBD to displace a radioactively labeled ligand bound to these receptors. Cell membranes were obtained as previously described, with some modifications (Benyhe et al., 1997). Briefly, brain tissue (~500 mg) was homogenized in ice-cold 50 mM Tris–HCl buffer (pH 7.4). Subsequently, it was centrifuged at 15,000 rpm for 25 min at 4°C. The resulting pellet was resuspended in 50 mM Tris–HCl buffer (pH 7.4) and incubated for 30 min at 35°C. At the end of the incubation, the preparation was centrifuged under the conditions previously indicated. The resultant pellet was resuspended in buffer (50 mM Tris–HCl, 1 mM EGTA, and 5 mM MgCl2•6H2O, pH 7.4), and protein concentration was determined by the Bradford method (Bradford, 1976).
The radioligand displacement assay was performed in a final reaction volume of 500 μl that contained 250 μg of protein of the membrane suspension and increasing concentrations of CBD (10 nM to 10 mM). The assay was carried out in the presence of [3H]-8-OH-DPAT at 0.7 nM. This ligand has a high affinity for 5-HT1A (Ki = 0.56) and low affinity for other receptors (Ki ranging from 41.9 to >10,000; Middlemiss and Fozard, 1983; Schlegel and Peroutka, 1986; Brown et al., 1990; Pauwels et al., 1996; Kleven et al., 1997). According to the Ki of [3H]-8-OH-DPAT for the different receptors, we expected that the results obtained represent the interaction of this ligand on 5-HT1A receptors.
The mixture was incubated for 45 min at 35°C. Non-specific binding was determined in the presence of the 5-HT1A receptor antagonist WAY-100635 (10 μM). The reaction was terminated by rapid filtration on a Brandel M-48 multifilter through a Whatman GF/C glass fiber filter followed by three washes with ice-cold buffer (50 mM Tris–HCl, pH 7.4). Radioligand binding was determined as disintegrations per minute (DPM) values (Beckman LS6000SC scintillation counter), which were normalized with respect to the maximum binding. Data were fitted to a non-linear regression to determine the inhibitory concentration 50 (IC50) with the model Y = Bottom + (Top − Bottom)/(1 + 10(LogIC50-X) * HillSlope) using Prism software (GraphPad Software, Inc.). The same equation was used to determine the Hill coefficient, which gives information on the number of interacting sites and possible allosteric interactions (Prinz, 2010). The Cheng–Prusoff equation was used to determine the inhibition constant (Ki) considering the dissociation constant (Kd) of [3H]-8-OH-DPAT equal to 0.46 nM (Cheng and Prusoff, 1973; Cusack et al., 1994). Data are expressed as the mean ± standard error of the mean (SEM).
[35S]-GTPγS Binding Assay
5-HT1A receptors are highly expressed Gi/o protein-coupled receptors that activate inhibitory pathways. Considering that CBD acts on several Gi/o protein-coupled receptors, such as CB1, CB2, opioid, and 5-HT1A, among others (Alves et al., 2020), we initially evaluated if CBD was able to activate the Gi/o protein and if a specific antagonist to 5-HT1A receptors was able to prevent such effect. The [35S]-GTPγS binding assay was used for this purpose.
Brain tissue was homogenized in buffer (50 mM Tris–HCl, 1 mM EGTA, and 3 mM MgCl2•6H2O, pH 7.4). The homogenate was centrifuged at 20,000 rpm for 45 min at 4°C. The resulting pellet was resuspended in buffer (50 mM Tris–HCl, 0.2 mM EGTA, 9 mM MgCl2•6H2O, and 150 mM NaCl, pH 7.4) and centrifuged under the same conditions. The resulting pellet was resuspended in buffer once more, and protein concentration was determined as described above (see “Radioligand Displacement Assay” section).
The [35S]-GTPγS binding assay was carried out as previously described (Spetea et al., 1998; Cuellar-Herrera et al., 2014), with minor variations. Briefly, cell membranes (10 μg of protein) were incubated at 30°C for 60 min in a reaction tube that contained Tris–EGTA buffer [Tris–50 mM HCl, 1 mM EGTA, 3 mM MgCl2•6H2O, 100 mM NaCl, and 0.1% (w/v) albumin; pH 7.4], GDP (100 μM), [35S]-GTPγS (100 μM), and increasing concentrations of CBD (1 pM to 100 μM). Total binding was measured in the absence of CBD. Non-specific binding was estimated in the presence of unlabeled GTPγS (100 μM). Data were analyzed as specific binding that resulted from subtracting non-specific binding from total binding. If any effect was obtained, WAY-100635 (100 μM) was included in the assay to determine the participation of 5-HT1A receptors. The reaction was terminated by rapid filtration on a Brandel M-48 multifilter through a Whatman GF/B glass fiber filters followed by three washes with ice-cold buffer (50 mM Tris–HCl and 5 mM MgCl2•6H2O, pH 7.4). A concentration–effect curve was built with the percentage of activation calculated considering the basal binding (in the absence of stimulation) as zero. Data were analyzed by a two-way ANOVA, taking group and CBD concentration as factors (concentration–effect curve), or a one-way ANOVA (effect of 100 μM in presence or absence of WAY-100635). p values < 0.05 were considered statistically significant.
Results
CBD Acts on 5-HT1A Receptors in Human Brain Tissue
In the hippocampal tissue obtained from autopsies, CBD displaced [3H]-8-OH-DPAT from its binding sites in a concentration-dependent manner (IC50 = 129.40 ± 9.40 μM; pKi = 4.29 ± 0.03). In this tissue, the Hill coefficient was 3.37 ± 0.27. The radioligand displacement assay on the hippocampal tissue of patients with DR-MTLE revealed similar values (IC50 = 93.61 ± 18.25 μM, p = 0.1194; pKi = 4.47 ± 0.09, p = 0.0748) as those observed in the autopsies. However, in the group of DR-MTLE patients, the Hill coefficient was higher (4.84 ± 0.35, p < 0.05) than in the group of autopsies.
In the temporal neocortex, we observed similar values in both DR-MTLE and autopsy groups (Figure 1). In the autopsy group, CBD displacement values (IC50 = 54.34 ± 3.66 μM; pKi = 4.67 ± 0.03; Hill coefficient = 4.67 ± 0.93) were found within the same range of concentration as in the group of patients with DR-MTLE (IC50 = 46.84 ± 6.30 μM, p = 0.334; pKi = 4.74 ± 0.05, p = 0.2643; Hill coefficient = 3.18 ± 0.57, p = 0.2091; Table 3).
Figure 1.
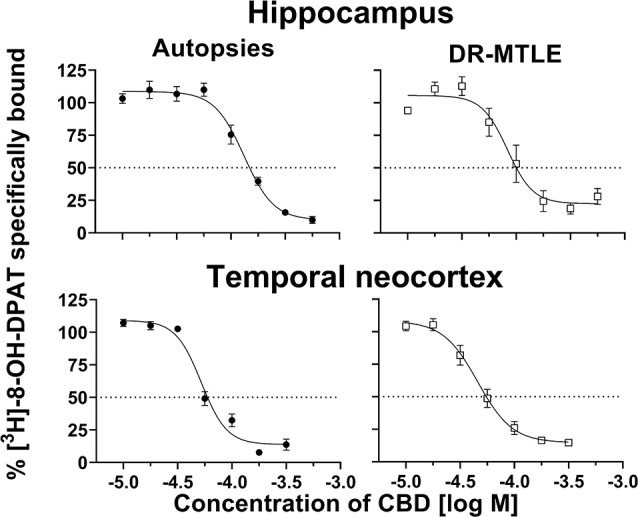
Effects of cannabidiol (CBD) on specific binding of [3H]-8-OH-DPAT to cell membranes obtained from the hippocampus (top panels) and temporal neocortex (bottom panels) of autopsies and patients with drug-resistant mesial temporal lobe epilepsy (MTLE; left and right panels, respectively). Symbols represent the mean ± Standard Error of the Mean (SEM) of five experiments. The dotted lines indicate 50% inhibition of specific binding. Curves were fitted to a model of four parameters (see “Materials and Methods” section).
Table 3.
Data obtained from the radioligand displacement assay in the hippocampus and temporal neocortex of autopsies and patients with drug resistance mesial lobe epilepsy.
| Brain structure | Group | IC50 (μM) | Ki (μM) | pKi | Hill coefficient |
|---|---|---|---|---|---|
| Hippocampus | Autopsies | 129.40 ± 9.40 | 51.31 ± 3.73 | 4.29 ± 0.03 | 3.37 ± 0.27 |
| Patients | 93.61 ± 18.25 | 37.12 ± 7.24 | 4.47 ± 0.09 | 4.84 ± 0.35* | |
| Temporal neocortex | Autopsies | 54.34 ± 3.66 | 21.55 ± 1.45 | 4.67 ± 0.03 | 4.67 ± 0.93 |
| Patients | 46.84 ± 6.30 | 18.57 ± 2.50 | 4.74 ± 0.05 | 3.18 ± 0.57 |
IC50, inhibitory concentration 50; Ki, inhibition constant; pKi, inverse logarithm of Ki. Ki was determined from IC50 according to the Cheng–Prusoff equation (1973): Ki = IC50/(1 + [radioligand]/KD). Values are expressed as the mean ± SEM of five experiments. *p < 0.05 vs. autopsies.
CBD Modifies the Activity of Gi/o Protein-Coupled Receptors
In the autopsies, CBD did not produce significant changes in the binding of [35S]-GTPγS at low concentrations (1 pM to 10 μM, p > 0.05), neither in the hippocampus nor in the temporal neocortex. However, at high concentrations (100 μM), CBD reduced [35S]-GTPγS binding, which was 39.2% and 35.2% lower in the hippocampus and temporal neocortex, respectively, than the basal levels (p < 0.05 and p < 0.01, respectively; Figure 2).
Figure 2.
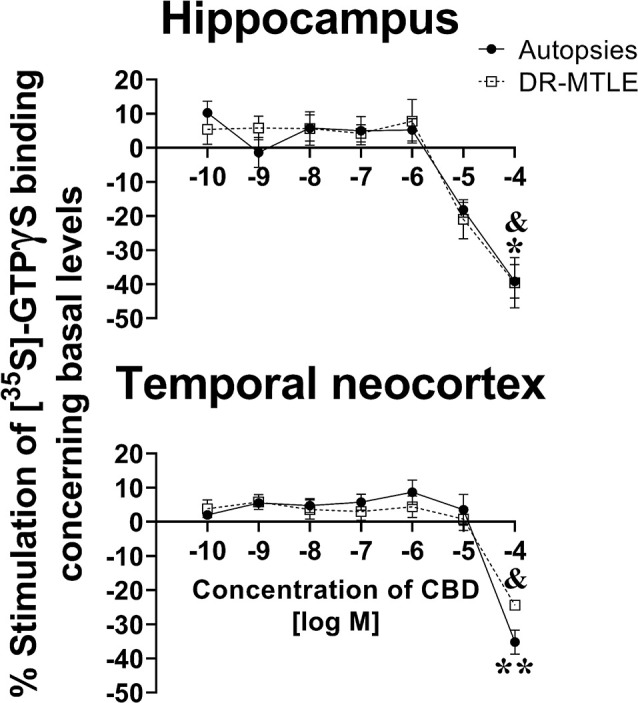
Effect of increasing concentrations of CBD on [35S]-GTPγS binding in cell membranes obtained from the hippocampus (top panels) and the temporal neocortex (bottom panels) of autopsies and patients with drug-resistant MTLE. Values are expressed as the mean ± SEM. *p < 0.05, **p < 0.01 (autopsies vs. baseline values); &p < 0.05 MTLE vs. baseline values.
When evaluating the brain tissue of patients with DR-MTLE, the [35S]-GTPγS assay revealed similar results as those observed in the group of autopsies. No changes were detected at low concentrations (1 pM to 10 μM, p > 0.05), whereas 100 μM CBD induced a significant reduction of [35S]-GTPγS binding in the hippocampus and temporal neocortex (39.6% and 24.4% lower, respectively, when compared to basal binding levels, p < 0.05; Figure 2). In comparison with the autopsy group, a less evident [35S]-GTPγS binding decrease induced by CBD was observed in the temporal neocortex of the DR-MTLE group (p < 0.05).
The decrease in the constitutive activity of Gi/o proteins induced by a high concentration of CBD was partially reversed in the presence of WAY-100635 at 100 μM in both the autopsy group (hippocampus, 59.8%; p < 0.0001; temporal neocortex, 71.5%; p < 0.0001) and the DR-MTLE group (hippocampus, 53.7%; p < 0.0001; temporal neocortex, 68.5%; p < 0.001; Figure 3).
Figure 3.
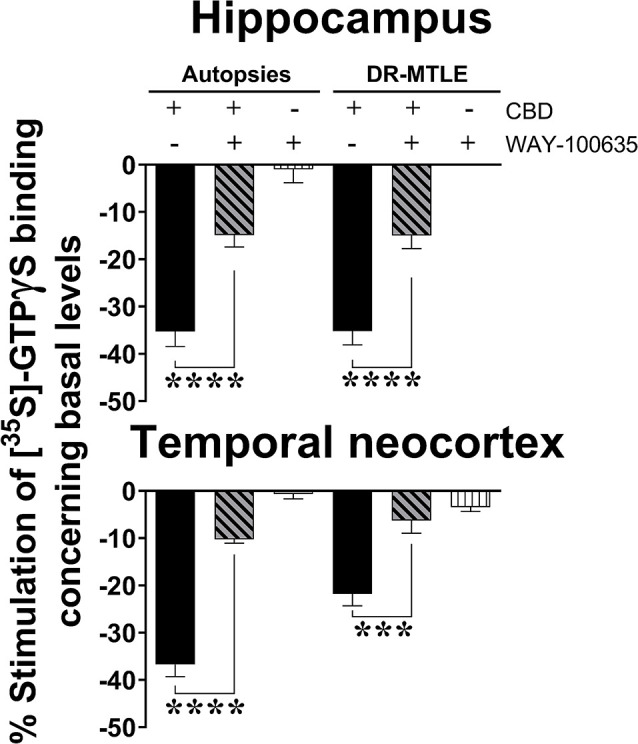
Effect of CBD (100 μM) on the constitutive activity of Gi/o proteins alone and combined with WAY100635 on [35S]-GTPγS binding in cell membranes obtained from the hippocampus (top panel) and temporal neocortex (bottom panel) of autopsies and patients with drug-resistant MTLE. Values are expressed as the mean ± SEM. ***p < 0.001; ****p < 0.0001.
Discussion
The radioligand displacement assay results support CBD affinity for 5-HT1A receptors in the brain tissue obtained from autopsies and patients with DR-MTLE. Furthermore, the experiments using [35S]-GTPγS revealed that CBD decreased the constitutive activity of Gi/o protein-coupled receptors at high concentrations and an antagonist of 5-HT1A receptors significantly reversed this effect.
Our experiments showed that CBD displaces [3H]-8-OH-DPAT from its binding site on 5-HT1A receptors in a concentration-dependent manner in human hippocampal and neocortical samples. These findings support the affinity of CBD for 5-HT1A receptors in the human brain. Indeed, they are in agreement with previous studies indicating that the exposure to CBD at micromolar concentrations displaced [3H]-8-OH-DPAT from its binding site in CHO cells expressing the human 5-HT1A receptor (Russo et al., 2005).
The present results indicate that CBD interacts with 5-HT1A receptors at high concentrations regardless of its lower affinity (pKi ≈ 4.5) in comparison with other ligands such as serotonin (pKi = 9.2), 8-OH-DPAT (pKi = 8.0), or WAY-100635 (pKi = 8.7; U.S. National Library of Medicine). However, the Hill coefficients obtained suggest that CBD is an allosteric modulator of 5-HT1A receptors (Hill, 1910; Goutelle et al., 2008). This condition may facilitate the binding of endogenous ligands and agonists to 5-HT1A receptors (May et al., 2007; Saleh et al., 2018). Indeed, the allosteric condition of CBD may represent a novel therapeutic strategy to influence the effects of 5-HT1A receptors (Azam et al., 2020).
Radioligand binding assay revealed differences in IC50 of CBD between hippocampus and temporal neocortex. It also showed the inversion of the Hill coefficient profile between autopsies and patients when temporal neocortex and hippocampus were compared. The results obtained suggest that activation of 5-HT1A receptors by CBD induces different functional consequences within these brain regions and depending on the pathological condition. It is known that variations of Hill coefficients are associated with conformational changes of the receptor and the number of binding sites (Colquhoun, 1998; Prinz, 2010). We found that the group of DR-MTLE patients showed a higher Hill coefficient in the hippocampus, the brain structure that mainly develops aberrant changes due to epilepsy (Bartolomei et al., 2008). This result indicates that epilepsy might be producing changes in the binding sites for CBD on the 5-HT1A receptors in the hippocampus (Lolkema and Slotboom, 2015). This finding is consistent with a previous study in which the Hill coefficient increase was associated with neuronal hyperexcitability and seizure activity due to a conformational change in the γ2 subunit of the GABAA receptor (Migita et al., 2013). Further binding kinetics studies are necessary to determine changes mediating the enhanced Hill coefficient in 5-HT1A receptors in the hippocampus of patients with DR-MTLE.
Regarding the [35S]-GTPγS assay, low concentrations of CBD did not modify the activity of Gi/o proteins in cell membranes obtained from the human brain, neither in autopsies nor in patients with DR-MTLE. These results are similar to those previously reported by Rock et al. (2012), who described that Gi/o protein-coupled receptors in rat brainstem membranes were not activated at low concentrations of CBD. This finding could be explained because the receptor–transducer selectivity was not evaluated. This is an important limitation of the experimental procedure used in the present study since the [35S]-GTPγS assay evaluates all the interactions of Gi/o protein-coupled receptors. Another possibility is that, at low concentrations of CBD, an equilibrium state between the activation and inhibition of Gi/o proteins is achieved, which is a common condition for the Gi/o protein-coupled receptors (Seyedabadi et al., 2019).
The constitutive activity of 5-HT1A receptors is susceptible to the effect of inverse agonists (Newman-Tancredi et al., 1997; Milligan, 2003). We found that high concentrations of CBD (100 μM) decreased the binding of [35S]-GTPγS to Gi/o proteins below the baseline values. Therefore, CBD reduced the constitutive activity of receptors coupled to Gi/o proteins. These changes were partially reversed when cell membranes were exposed to CBD in the presence of WAY100635, an antagonist of 5-HT1A receptors. The results obtained indicated that at high concentrations, CBD modifies the constitutive activity of 5-HT1A receptors acting as an inverse agonist.
Inverse agonists could indirectly increase the signalling of targeted receptors through the increase in the proportion of receptors on the cellular surface (Abbas et al., 2007; Kumar et al., 2019). Therefore, the continuous administration of inverse agonists of 5-HT1A receptors induces antiallodynic effects due to inverse tolerance (Deseure et al., 2003). According to this information, the continuous administration of CBD as an inverse agonist of 5-HT1A receptors may represent a therapeutic strategy to augment the signaling of these and other Gi/o protein-coupled receptors involved in neuroprotection. Additional experiments are essential to support this notion.
It is known that CBD is an inverse agonist of other Gi/o protein-coupled receptors, such as CB2 receptors (Thomas et al., 2007) and GPR3 and GPR6 orphan receptors (Laun and Song, 2017). In the present study, WAY-100635 partially blocked the CBD-induced decreased binding of [35S]-GTPγS to Gi/o proteins. WAY-100635 is considered the quintessential antagonist of the 5-HT1A receptors (Ki = 2.2 nM). However, although with lower affinity values, it is also an antagonist of other receptors such as 5-HT2A (Ki = 6260 nM), 5-HT2B (Ki = 24 nM), and D2-like (Ki 16.4–940 nM; Chemel et al., 2006). Therefore, WAY-100635 could be blocking the constitutive activity of 5-HT1A, 5-HT2A, 5-HT2B, and D2-like receptors as well. On the other hand, [3H]-8-OH-DPAT has low affinity for 5-HT7 (Thomas et al., 1998) and α1 receptors (Yoshio et al., 2001). Then, some of the results obtained can involve the action of CBD on these receptors. Future experiments should be conducted to investigate the effects of CBD on binding and constitutive activity of different type of receptors in the brain of patients with epilepsy. These experiments should include kinetic binding studies and displacement experiments (pseudo-competition experiments) in the presence and absence of CBD.
5-HT1A receptors play an important role in cerebral functions, and they are considered targets to develop novel therapeutic strategies. They show heterogeneous distribution, including pre- and postsynaptic localization, as well as cross-talk with different types of 5-HT and other neurotransmitter receptors. Dysfunction of 5-HT1A receptors is associated with psychiatric disorders such as anxiety and depression (Popova and Naumenko, 2013). Experimental evidence indicates decline of 5-HT1A receptor binding in the brain of patients with epilepsy (Toczek et al., 2003; Theodore et al., 2012). It is suggested that 5-HT1A receptors on astrocytes represent a potential therapeutic target for the treatment of neurodegenerative disorders (Miyazaki and Asanuma, 2016). An important limitation of the present study is that the current methodology does not allow one to identify in which type of cells the 5-HT1A receptors were evaluated. More experiments are needed to investigate the effects of CBD on specific brain cells and its therapeutic relevance.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of the Hospital General de Mexico and Centro de Investigacion y de Estudios Avanzados. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LR conceived and designed the study and wrote the manuscript. CM-A carried out the experiments and analyzed the results. FC-C carried out the experiments. FV performed the neurosurgery of patients. AV and GA-C identified and evaluated the patients with epilepsy. MC-H collected samples, participated in the analysis of the results, and wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to HempMeds, LLC, USA, who donated the cannabidiol.
Footnotes
Funding. This study was supported by the National Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología, CONACyT; grant A3-S-26782 and scholarship 489763 to CM-A).
References
- Abbas S. Y., Nogueira M. I., Azmitia E. C. (2007). Antagonist-induced increase in 5-HT1A-receptor expression in adult rat hippocampus and cortex. Synapse 61, 531–539. 10.1002/syn.20399 [DOI] [PubMed] [Google Scholar]
- Alves P., Amaral C., Teixeira N., Correia-da-Silva G. (2020). Cannabis sativa: much more beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 157:104822. 10.1016/j.phrs.2020.104822 [DOI] [PubMed] [Google Scholar]
- Azam S., Haque M., Jakaria M., Jo S., Kim I., Choi D. (2020). G-Protein-coupled receptors in CNS: a potential therapeutic target for intervention in neurodegenerative disorders and associated cognitive deficits. Cells 9:506. 10.3390/cells9020506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F., Chauvel P., Wendling F. (2008). Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain 131, 1818–1830. 10.1093/brain/awn111 [DOI] [PubMed] [Google Scholar]
- Benyhe S., Farkas J., Tó th G., Wollemann M. (1997). Met5-enkephalin-Arg6-Phe7, an endogenous neuropeptide, binds to multiple opioid and nonopioid sites in rat brain. J. Neurosci. Res. 48, 249–258. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. 10.1006/abio.1976.9999 [DOI] [PubMed] [Google Scholar]
- Brown C., MacKinnon A., McGrath J., Spedding M., Kilpatrick A. (1990). Heterogeneity of α2-adrenoceptors in rat cortex but not human platelets can be defined by 8-OH-DPAT, RU 24969 and methysergide. Br. J. Pharmacol. 99, 481–486. 10.1111/j.1476-5381.1990.tb12954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai F., Cardia L., Sorbara E. E., Navarra M., Gangemi S., Calapai G., et al. (2020). Cannabinoids, blood-brain barrier and brain disposition. Pharmaceutics 12:265. 10.3390/pharmaceutics12030265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemel B. R., Roth B. L., Armbruster B., Watts V. J., Nichols D. E. (2006). WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology 188, 244–251. 10.1007/s00213-006-0490-4 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. (1973). Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108. 10.1016/0006-2952(73)90196-2 [DOI] [PubMed] [Google Scholar]
- Colquhoun D. (1998). Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 125, 924–947. 10.1038/sj.bjp.0702164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Herrera M., Velasco A. L., Velasco F., Trejo D., Alonso- Vanegas M., Nuche- Bricaire A., et al. (2014). Alterations of 5-HT1A receptor-induced G-protein functional activation and relationship to memory deficits in patients with pharmacoresistant temporal lobe epilepsy. Epilepsy Res. 108, 1853–1863. 10.1016/j.eplepsyres.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Cunha J. M., Carlini E. A., Pereira A. E., Ramos O. L., Pimentel C., Gagliardi R., et al. (1980). Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21, 175–185. 10.1159/000137430 [DOI] [PubMed] [Google Scholar]
- Cusack B., Nelson A., Richelson E. (1994). Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology 114, 559–565. 10.1007/BF02244985 [DOI] [PubMed] [Google Scholar]
- Deseure K., Koek W., Adriaensen H., Colpaert F. C. (2003). Continuous administration of the 5-hydroxytryptamine1A agonist (3-Chloro-4-fluoro-phenyl)-[4-fluoro-4-[[(5-methyl-pyridin-2-ylmethyl)-amino]-methyl]piperidin-1-yl]-methadone (F 13640) attenuates allodynia-like behavior in a rat model of trigeminal neuropathic pain. J. Pharmacol. Exp. Ther. 306, 505–514. 10.1124/jpet.103.050286 [DOI] [PubMed] [Google Scholar]
- Goutelle S., Maurin M., Rougier F., Barbaut X., Bourguignon L., Ducher M., et al. (2008). The Hill equation: a review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 22, 633–648. 10.1111/j.1472-8206.2008.00633.x [DOI] [PubMed] [Google Scholar]
- Hill A. V. (1910). The possible effects of the aggregation of the molecules of hemoglobin on its dissociation curves. J. Physiol. 40, 4–7. Available online at: http://jp.physoc.org/content/40/supplement/i.shortJ. Accessed November 17. [Google Scholar]
- Jones P. G., Falvello L., Kennard O., Sheldrick G. M., Mechoulam R. (1977). Cannabidiol. Acta Cryst. B33, 3211–3214. 10.1107/S0567740877010577 [DOI] [Google Scholar]
- Khan A. A., Shekh- Ahmad T., Khalil A., Walker M. C., Ali A. B. (2018). Cannabidiol exerts antiepileptic effects by restoring hippocampal interneuron functions in a temporal lobe epilepsy model. Br. J. Pharmacol. 175, 2097–2115. 10.1111/bph.14202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven M., Assié M., Koek W. (1997). Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J. Pharmacol. Exp. Ther. 282, 747–759. [PubMed] [Google Scholar]
- Kumar G. A., Sarkar P., Jafurulla M., Singh S. P., Srinivas G., Pande G., et al. (2019). Exploring endocytosis and intracellular trafficking of the human serotonin1A receptor. Biochemistry 58, 2628–2641. 10.1021/acs.biochem.9b00033 [DOI] [PubMed] [Google Scholar]
- Laun A. S., Song Z. H. (2017). GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem. Biophys. Res. Commun. 490, 17–21. 10.1016/j.bbrc.2017.05.165 [DOI] [PubMed] [Google Scholar]
- Lazaridis D., Eraikhuemen N., Williams K., Lovince J. (2019). Treatment of seizures associated with Lennox-Gastaut and Dravet syndromes: a focus on cannabidiol oral solution. P T 44, 255–266. [PMC free article] [PubMed] [Google Scholar]
- Lolkema J. S., Slotboom D. J. (2015). The Hill analysis and co-ion-driven transporter kinetics. J. Gen. Physiol. 145, 565–574. 10.1085/jgp.201411332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa E., Figi P. (2014). The case for medical marijuana in epilepsy. Epilepsia 55, 783–786. 10.1111/epi.12610 [DOI] [PubMed] [Google Scholar]
- Magen I., Avraham Y., Ackerman Z., Vorobiev L., Mechoulam R., Berry E. M. (2010). Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 159, 950–957. 10.1111/j.1476-5381.2009.00589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T., Leach K., Sexton P. M., Christopoulos A. (2007). Christopoulos, A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 47, 1–51. 10.1146/annurev.pharmtox.47.120505.105159 [DOI] [PubMed] [Google Scholar]
- Middlemiss D., Fozard J. (1983). 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1A recognition site. Eur. J. Pharmacol. 90, 151–153. 10.1146/annurev.pharmtox.47.120505.105159 [DOI] [PubMed] [Google Scholar]
- Migita K., Yamada J., Nikaido Y., Shi X., Kaneko S., Hirose S., et al. (2013). Properties of a novel GABAA receptor γ2 subunit mutation associated with seizures. J. Pharmacol. Sci. 121, 84–87. 10.1254/jphs.12222sc [DOI] [PubMed] [Google Scholar]
- Milligan G. (2003). Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol. Pharmacol. 64, 1271–1276. 10.1124/mol.64.6.1271 [DOI] [PubMed] [Google Scholar]
- Miyazaki I., Asanuma M. (2016). Serotonin 1A receptors on astrocytes as a potential target for the treatment of Parkinson’s disease. Curr. Med. Chem. 23, 686–700. 10.2174/0929867323666160122115057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A., Conte C., Chaput C., Spedding M., Millan M. J. (1997). Inhibition of the constitutive activity of human 5-HT1A receptors by the inverse agonist, spiperone but not the neutral antagonist, WAY 100,635. Br. J. Pharmacol. 120, 737–739. 10.1038/sj.bjp.0701025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra P. H., Barker- Haliski M., White H. S., Whalley B. J., Glyn S., Sandhu H., et al. (2019). Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia 60, 303–314. 10.1111/epi.14629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels P., Palmier C., Wurch T., Colpaert F. (1996). Pharmacology of cloned human 5-HT1D receptor-mediated functional responses in stably transfected rat C6-glial cell lines: further evidence differentiating human 5-HT1D and 5-HT1B receptors. Naunyn Schmiedebergs Arch. Pharmacol. 353, 144–156. 10.1007/BF00168751 [DOI] [PubMed] [Google Scholar]
- Popova N., Naumenko V. (2013). 5-HT1A receptor as a key player in the brain 5-HT system. Rev. Neurosci. 24, 1–14. 10.1515/revneuro-2012-0082 [DOI] [PubMed] [Google Scholar]
- Prinz H. (2010). Hill coefficients, dose-response curves and allosteric mechanisms. J. Chem. Biol. 3, 37–44. 10.1007/s12154-009-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock E. M., Bolognini D., Limebeer C. L., Cascio M. G., Anavi-Goffer S., Fletcher P. J., et al. (2012). Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165, 2620–2634. 10.1111/j.1476-5381.2011.01621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E. B. (2017). Cannabidiol claims and misconceptions. Trends Pharmacol. Sci. 38, 198–201. 10.1016/j.tips.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Russo E. B., Burnett A., Hall B., Parker K. K. (2005). Agonistic properties of cannabidiol at 5-HT1A receptors. Neurochem. Res. 30, 1037–1043. 10.1007/s11064-005-6978-1 [DOI] [PubMed] [Google Scholar]
- Saleh N., Hucke O., Kramer G., Schmidt E., Montel F., Lipinski R., et al. (2018). Multiple binding sites contribute to the mechanism of mixed agonistic and positive allosteric modulators of the cannabinoid CB1 receptor. Angew. Chem. Int. Ed. Engl. 57, 2580–2585. 10.1002/anie.201708764 [DOI] [PubMed] [Google Scholar]
- Sales A. J., Crestani C. C., Guimarã es F. S., Joca S. R. L. (2018). Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Prog. Neuropsychopharmacol. Biol. Psychiatry 86, 255–261. 10.1016/j.pnpbp.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Seyedabadi M., Ghahremani M. H., Albert P. R. (2019). Biased signaling of G protein coupled receptors (GPCRs): molecular determinants of GPCR/transducer selectivity and therapeutic potential. Pharmacol. Ther. 200, 148–178. 10.1016/j.pharmthera.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Shannon S., Lewis N., Lee H., Hughes S. (2019). Cannabidiol in anxiety and sleep: a large case series. Perm. J. 23, 18–041. 10.7812/TPP/18-041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel J., Peroutka S. (1986). Nucleotide interactions with 5-HT1A binding sites directly labeled by [3H]-8-hydroxy-2-(di)-n-propylamino) tetralin ([3H]-8-OH-DPAT). Biochem. Pharmacol. 35, 1943–1949. 10.1016/0006-2952(86)90725-2 [DOI] [PubMed] [Google Scholar]
- Silvestro S., Mammana S., Cavalli E., Bramanti P., Mazzon E. (2019). Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules 24:1459. 10.3390/molecules24081459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea M., Monory K., Tömbö ly C., Tó th G., Tzavara E., Benyhe S., et al. (1998). In vitro binding and signaling profile of the novel mu-opioid receptor agonist endomorphin 2 in rat brain membranes. Biochem. Biophys. Res. Commun. 250, 720–725. 10.1006/bbrc.1998.9395 [DOI] [PubMed] [Google Scholar]
- Theodore W., Wiggs E., Martinez A., Dustin I., Khan O., Appel S., et al. (2012). Serotonin 1A receptors, depression and memory in temporal lobe epilepsy. Epilepsia 53, 129–133. 10.1111/j.1528-1167.2011.03309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele E. A., Marsh E. D., French J. A., Mazurkiewicz-Beldzinska M., Benbadis S. R., Joshi C., et al. (2018). GWPCARE4 Study Group. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 1085–1096. 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- Thomas A., Baillie G. L., Phillips A. M., Razdan R. K., Ross R. A., Pertwee R. G. (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623. 10.1038/sj.bjp.0707133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. R., Gittins S. A., Collin L. L., Middlemiss D. N., Riley G., Hagan J., et al. (1998). Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br. J. Pharmacol. 124, 1300–1306. 10.1038/sj.bjp.0701946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczek M., Carson R., Lang L., Ma Y., Spanaki M., Der M., et al. (2003). PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology 60, 749–756. 10.1212/01.wnl.0000049930.93113.20 [DOI] [PubMed] [Google Scholar]
- Yoshio R., Taniguchi T., Itoh H., Muramatsu I. (2001). Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn. J. Pharmacol. 86, 189–195. 10.1254/jjp.86.189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


