Abstract
Objective
This study evaluated the characteristics of opioid prescriptions, including prescriber specialty, given to opioid-naïve patients and their association with chronic use.
Design
Cross-sectional analysis of the Ohio prescription drug monitoring program from January 2010 to November 2017.
Setting
Ohio, USA.
Subjects
Patients who had no opioid prescriptions from 2010 to 2012 and a first-time prescription from January 2013 to November 2016.
Methods
Chronic use was defined as at least six opioid prescriptions in one year and either one or more years between the first and last prescription or an average of ≤30 days not covered by an opioid during that year.
Results
A total of 4,252,809 opioid-naïve patients received their first opioid prescription between 2013 and 2016; 364,947 (8.6%) met the definition for chronic use. Those who developed chronic use were older (51.7 vs 45.6 years) and more likely to be female (53.6% vs 52.8%), and their first prescription had higher pill quantities (44.9 vs 30.2), higher morphine milligram equivalents (MME; 355.3 vs 200.0), and was more likely to be an extended-release formulation (2.9% vs 0.7%, all P < 0.001). When compared with internal medicine, the adjusted odds of chronic use were highest with anesthesiology (odds ratio [OR] = 1.46) and neurology (OR = 1.43) and lowest with ophthalmology (OR = 0.33) and gynecology (OR = 0.37).
Conclusions
Eight point six percent of opioid-naïve individuals who received an opioid prescription developed chronic use. This rate varied depending on the specialty of the provider who wrote the prescription. The risk of chronic use increased with higher MME content of the initial prescription and use of extended-release opioids.
Keywords: Opioids, Chronic Pain, American Board of Medical Specialties
Introduction
Even in the era of the modern opioid overdose and death epidemic, opioids remain a mainstay of treatment for acutely painful conditions, such as after procedures or in the setting of severe trauma. In current medical practice, guidelines like those from the Centers for Disease Control and Prevention (CDC) state, “When opioids are needed for acute pain, clinicians should prescribe opioids at the lowest effective dose and for no longer than the expected duration of pain severe enough to require opioids to minimize unintentional initiation of long-term opioid use” [1]. For prescribers treating these patients with acute pain, the conversion of a previously opioid-naïve patient to a long-term user because of an initial prescription should be of the utmost concern, as chronic opioid use for noncancer pain is associated with increased risk of overdose and death and has been shown to provide only minimal benefit but more side effects for patients with chronic noncancer pain [2–4].
Patients also need to know, as part of informed consent and shared decision-making, what their risk of long-term opioid use is after an initial prescription. A study by Shah et al. found that one-year continued opioid use for patients prescribed at least one day of opioids overall was 6.0%, and that number rose significantly for initial prescriptions with longer days’ supply [5]. A study of older adults undergoing short-stay surgery found that patients who received an opioid prescription around the time of their surgery were 44% more likely to become long-term opioid users at one year compared with those who did not receive an opioid [6]. The effect appears to be dose dependent. Deyo et al. found that 5.0% of opioid-naïve patients prescribed opioids became chronic users, and those who received between 400 and 799 morphine milligram equivalents (MME)—equivalent to about 53 to 107 pills of oxycodone 5 mg—had a threefold higher odds of developing chronic use compared with those who received prescriptions for <120 MME (about 16 pills of oxycodone 5 mg) [7]. Furthermore, patients in this study who were prescribed long-acting opioids had a higher risk of converting to long-term use.
To mitigate the possibility of chronic use, guidelines recommend alternatives to opioids whenever possible [8]. When using an opioid for acute noncancer pain, prescribers should write for the smallest amount possible to still maintain adequate analgesia but avoid conversion to chronic opioid use. Recognizing the dose-dependent relationship, the CDC guidelines recommended prescribing three or fewer days’ supply for pain not related to surgery or trauma and state that more than a seven-day supply will rarely be needed [1]. After surgery, initiatives like the Michigan Opioid Prescriber Engagement Network recommend standard opioid pill quantities based on analysis of actual patient opioid use after surgery [9]. The group recommends 10 pills or fewer for common procedures like laparoscopic appendectomy, laparoscopic cholecystectomy, and hernia repair.
In our past work, we ascertained that there are different opioid prescribing behaviors when comparing the various medical specialties [10]. Learning the difference in prescribing practices between specialties is helpful to be able to target educational interventions and guidelines. For example, if a significant number of previously opioid-naïve patients converted to chronic use after prescriptions by emergency physicians (e.g., at the time of an acute injury) or orthopedic surgeons (e.g., after an acute surgery), it would raise the possibility for creation of guidelines and further analysis specific to those specialties. Likewise, clinical settings and patient conditions vary markedly depending on the specialty of the prescriber, making generalized guidelines difficult to apply.
The goal of our study was to determine the prescription and prescriber specialty characteristics of an initial prescription that is then associated with long-term opioid use in opioid-naïve patients. Different than previous studies, we stratify prescriptions by specialty to learn the prescribing patterns and chronic opioid use conversion rates of those individual specialties when available. We use an entire state’s prescription drug monitoring program (PDMP) data in order to capture nearly every opioid prescription dispensed in the state, including those paid out of pocket without insurance, which is different than prior studies, which used select payer databases or individual hospital or health system data. These findings provide a deeper understanding about which specialties are writing prescriptions to opioid-naïve patients and what the characteristics of those prescriptions are that are associated with subsequent chronic opioid use.
Methods
Study Data Source
This was a cross-sectional study of the Ohio Prescription Drug Monitoring Program (PDMP) database, also known as the Ohio Automated Rx Reporting System (OARRS). Data from January 1, 2010, to November 30, 2017, were analyzed. This data set includes all controlled substance (schedule II–V) prescriptions filled in Ohio, regardless of pharmacy, location, or payer (including self-pay). Prescriptions dispensed in out-of-state retail pharmacies are not included. However, if the prescription is mailed into the state, it is required to be reported to OARRS. The study was deemed “not human research” by the Partners Human Research Committee.
Dataset Construction
The following fields were extracted from the PDMP data set: unique identifier for each patient, patient age and gender; payment type; name of the drug, its strength, and its formulation (including if it was extended-release/long-acting); pill quantities and days’ supply; date on which the prescription was filled; and specialty of the prescriber. Days’ supply is defined as the number of days the prescription should last if taken per prescriber’s orders without considering “as needed” instructions. Our methodology for categorizing prescriber specialties has been previously described [10]. Briefly, specialties were collapsed into broad categories. A specialty of “other” was assigned to specialties like radiology, pathology, and psychiatry, which prescribed very infrequently. If the field was not present in the data, specialty was defined as “missing.” “Advanced practice providers” indicates nonphysician individuals with the ability to write a prescription, namely nurse practitioners and physician assistants. During the dates of this study, the Ohio PDMP included specialty information for about two-thirds of its providers. When present, this was determined by what the prescriber self-reported at the time of PDMP registration or was reported by their professional licensure board, and this information is absent at random across specialties. Only the primary specialty was considered for this classification.
We analyzed filled prescriptions for patients aged five to 99 years. We included the pill form of the nine most commonly prescribed opioids in Ohio during the study period: hydrocodone, oxycodone, tramadol, codeine, hydromorphone, meperidine, methadone, morphine, and oxymorphone. Liquid formulations (e.g., cough syrup–containing opioids) or nonoral formulations such as patches were not included.
Each prescription was converted to a total MME dose using previously published methodology [11]. To account for outliers in the data, we excluded the following: 1) pill quantities of fewer than four pills and ≥99% percentile for each specialty; 2) pill quantities that were not integers; 3) prescriptions for patients younger than age five or older than age 99 years; and 4) prescriptions with days’ supply of <0 or >90.
Chronic Use Definition
There are several definitions of long-term opioid use in the literature, including those used in the studies by Shah and Deyo [5, 7]. Shah et al. defined chronic use as a patient having >180 days of opioid use and no gap of >30 days. Deyo et al. defined long-term use as the presence of six or more opioid fills in the year following the initial 30-day period in which at least one opioid was prescribed. Yet another definition is opioid prescribing lasting >90 days and ≥120 total days’ supply or 10 or more prescriptions in the year after the initial prescription was filled [11, 12]. There is no consensus about which definition is the best, but a validation study determined that the Deyo methodology yielded 6.2% for chronic use and the Shah methodology yielded 16.8% for chronic use, so there are differences [13].
After extensively considering these and other definitions, our group decided on the following methodology to determine chronic use: a) the patient had to have a first opioid prescription between January 1, 2013, and November 30, 2016, but not have had a prescription in the database between 2010 and 2012; b) the patient had to have six opioid prescriptions for the studied opioids in one year (including the index prescription) and one or both of the following: ≥365 days between the first and last prescription or an average of ≤30 days between refills without a prescription. Our justification is that, as the data were available, we wanted to ensure that the patient was opioid-naïve from the perspective of our data set for a minimum of three years, and we wanted to include reasonable factors from Shah (minimal gaps in opioid prescriptions over the year) and Deyo (at least six prescriptions in one year). We included patients until November 2016 to allow a 365-day follow-up in the data (December 2016 to November 2017). We also concurred with Deyo et al. that patients on chronic opioids should have at least six prescriptions per year. Finally, because we did not want to include someone who had six prescriptions in a brief time period, we required either one or more years between the first and last prescription or an average of ≤30 days in which the patient was not covered by an opioid during that year. Our data also permitted a longer “washout” period than prior studies. Overall, we expected our rates of “chronic use” to be lower than both prior definitions given our stricter criteria.
Data Analysis
After defining these criteria, a data set was created that contained only the first (index) prescription for each patient; the primary outcome was the proportion of patients who met the definition for chronic use. The first step in the analysis was descriptive, comparing patient demographics and prescription characteristics between prescriptions associated with chronic use or not. The Student t test was used to compare continuous variables, and the chi-square statistic was used for categorical variables. A multivariable logistic regression model adjusting for age, sex, payment type, extended-release formulation, month and year filled, and MME of the prescription was created to determine the adjusted odds ratio for chronic use. Huber/White/sandwich estimator was used to calculate robust standard errors using all the variables in the model. Finally, association of chronic use stratified by MME of the prescription was plotted for first prescriptions overall, a reference specialty (internal medicine), and then select specialties that typically prescribe opioids for acute pain (dentistry, emergency medicine, gynecology, orthopedics, and specialty surgery). Data were analyzed with SAS and JMP (SAS Institute, Inc., Cary, NC, USA) and StataMP (version 13; StataCorp, College Station, TX, USA). The graph was plotted with Microsoft Excel (Microsoft Corp, Redmond, WA, USA).
Results
There were 4,252,809 opioid-naïve patients who were dispensed an opioid prescription from 2013 to 2016. Of these, 364,947 (8.6%) met our definition for chronic use. Table 1 summarizes patient demographic and index prescription characteristics, comparing those who developed chronic use with those who did not. Overall, those who developed chronic use had higher pill quantities (44.9 vs 30.2), higher days’ supply (12.3 vs 6.8), were older (51.7 vs 45.6 years), were more likely to be female (53.6% vs 52.8%), and were more likely to be prescribed an extended-release formulation (2.9% vs 0.7%, all P < 0.001). There were also differences based on form of payment. Table 2 shows the active ingredient of the index prescription stratified by those that preceded chronic use or not, compared with a reference level (codeine).
Table 1.
Comparison of demographic characteristics and index opioid prescription characteristics associated with no chronic use and with chronic use
| No Chronic Use, N = 3,887,862 (91.4%) | Chronic Use, N = 364,947 (8.6%) | P Value | ||
|---|---|---|---|---|
| Age, y | 45.6 ± 20.1 | 51.7 ± 18.4 | <0.001 | |
| Sex | Male | 1,827,574 (47.0) | 168,943 (46.3) | <0.001 |
| Female | 2,053,847 (52.8) | 195,495 (53.6) | ||
| Missing | 6,441 (0.2) | 509 (0.1) | ||
| Payment | Cash | 545,064 (14.0) | 49,171 (13.5) | <0.001 |
| Medicaid | 236,660 (6.1) | 26,637 (7.3) | ||
| Medicare | 400,544 (10.3) | 53,344 (14.6) | ||
| Commercial | 2,671,945 (68.7) | 224,503 (61.5) | ||
| Other | 33,649 (8.7) | 11,292 (3.1) | ||
| Pill quantity | 30.2 ± 30.7 | 44.9 ± 45.2 | <0.001 | |
| Days’ supply | 6.8 ± 10.6 | 12.3 ± 16.6 | <0.001 | |
| Morphine milligram equivalents | 200.0 ± 408.7 | 355.3 ± 810.5 | <0.001 | |
| Extended-release | Yes | 27,254 (0.7) | 10,543 (2.9) | <0.001 |
| Year filled | 2013 | 1,069,420 (27.5) | 136,522 (37.4) | <0.001 |
| 2014 | 990,103 (25.5) | 108,892 (29.8) | ||
| 2015 | 988,544 (25.4) | 83,837 (23.0) | ||
| 2016 | 839,795 (21.6) | 35,696 (9.8) | ||
Data are presented as mean ± SD or No. (%).
Table 2.
Comparison of active ingredient in first-time opioid prescriptions that was associated with development of chronic use or not; reference level is codeine
| Active Ingredient | No Chronic Use, No. (%) | Chronic Use, No. (%) | Odds Ratio (95% CI) |
|---|---|---|---|
| Codeine | 305,919 (94.4) | 18,157 (5.6) | Reference |
| Hydrocodone | 2,001,030 (92.7) | 157,488 (7.3) | 1.33 (1.31–1.35) |
| Meperidine | 1,954 (91.9) | 173 (8.1) | 1.49 (1.28–1.74) |
| Oxycodone | 974,653 (91.3) | 94,837 (8.7) | 1.64 (1.61–1.67) |
| Hydromorphone | 8,745 (86.2) | 1,402 (13.8) | 2.04 (1.92–2.16) |
| Tramadol | 579,931 (87.0) | 86,840 (13.0) | 2.52 (2.48–2.57) |
| Oxymorphone | 1,003 (73.3) | 366 (26.7) | 6.15 (5.45–6.93) |
| Morphine | 11,505 (72.7) | 4,310 (27.3) | 6.31 (6.08–6.56) |
| Methadone | 3,122 (69.4) | 1,374 (30.6) | 7.41 (6.95–7.91) |
Specialty information of the prescriber was available for 2,906,808 (68.4%) prescriptions and is provided in Tables 3 and 4. Overall chronic use was 8.6%, and for those with a missing specialty it was 9.3%. Pill quantities, MME, and days’ supply varied markedly depending on specialty, with emergency physicians and dentists prescribing fewer pills and physical medicine/rehabilitation, hematologist/oncologists, and neurologists providing higher pill quantities. Rates of chronic use after the first prescription varied between 2.4% (first prescription from dentistry) and 20.8% (first prescription from neurology).
Table 3.
Characteristics of opioid prescriptions to opioid-naïve patients stratified by primary specialty of the prescriber, ordered by percent meeting the definition for chronic use
| Specialty | No. | Pill Quantity, Mean ± SD | Days’ Supply, Mean ± SD | Extended Release, No. (%) | Morphine Milligram Equivalents, Mean ± SD | Chronic Opioid Use, No. (%) |
|---|---|---|---|---|---|---|
| Total | 4,252,809 | 31.5 ± 32.4 | 7.2 ± 11.3 | 37,797 (0.9) | 213.3 ± 459.3 | 364,947 (8.6) |
| Neurology | 5,195 (0.1) | 53.4 ± 40.9 | 16.3 ± 12.5 | 181 (3.5) | 502.4 ± 1,078.2 | 1,081 (20.8) |
| Anesthesiology | 27,561 (0.6) | 49.5 ± 38.8 | 15.0 ± 12.8 | 1,251 (4.5) | 457.2 ± 784.9 | 5,623 (20.4) |
| Physical medicine | 18,863 (0.4) | 55.9 ± 33.7 | 14.1 ± 10.4 | 679 (3.6) | 429.0 ± 614.2 | 3,420 (18.1) |
| Orthopedics | 109,885 (2.6) | 43.7 ± 21.0 | 6.8 ± 5.0 | 1,536 (1.4) | 286.1 ± 242.0 | 14,657 (13.3) |
| Hematology/oncology | 11,029 (0.3) | 54.2 ± 36.3 | 11.1 ± 9.0 | 818 (7.4) | 467.8 ± 717.0 | 1,433 (13.0) |
| Internal medicine | 824,657 (19.4) | 34.1 ± 30.7 | 9.0 ± 10.0 | 8,204 (1.0) | 231.8 ± 356.5 | 97,062 (11.8) |
| Advanced practice providers | 143,025 (3.4) | 30.4 ± 33.5 | 7.0 ± 11.2 | 1,787 (1.2) | 202.8 ± 382.9 | 12,721 (8.9) |
| Other | 337,314 (7.9) | 26.8 ± 19.6 | 4.8 ± 4.6 | 2,212 (0.7) | 178.6 ± 199.5 | 28,366 (8.4) |
| Emergency medicine | 449,864 (10.6) | 16.7 ± 8.3 | 3.4 ± 2.5 | 525 (0.1) | 95.6 ± 81.4 | 35,132 (7.8) |
| Addiction medicine | 625 (0.0) | 36.9 ± 28.0 | 10.0 ± 9.6 | 19 (3.0) | 265.3 ± 519.4 | 44 (7.0) |
| Pediatrics | 24,422 (0.6) | 24.1 ± 20.7 | 5.7 ± 6.6 | 167 (0.7) | 143.1 ± 210.8 | 1,705 (7.0) |
| Psychiatry | 10,690 (0.3) | 21.2 ± 18.3 | 4.7 ± 6.0 | 49 (0.5) | 132.0 ± 329.0 | 647 (6.1) |
| Radiology | 10,507 (0.2) | 26.9 ± 20.8 | 5.5 ± 6.4 | 34 (0.3) | 167.1 ± 167.0 | 579 (5.5) |
| Dermatology | 9,144 (0.2) | 17.9 ± 12.4 | 3.7 ± 4.1 | 7 (0.1) | 97.3 ± 83.5 | 444 (4.9) |
| Specialty surgery | 468,039 (11.0) | 33.0 ± 18.6 | 5.3 ± 4.2 | 1,659 (0.4) | 214.4 ± 175.2 | 22,943 (4.9) |
| Gynecology | 152,088 (3.6) | 27.3 ± 13.0 | 4.4 ± 3.4 | 163 (0.1) | 181.2 ± 144.0 | 6,559 (4.3) |
| Ophthalmology | 14,621 (0.3) | 18.2 ± 10.8 | 3.5 ± 3.6 | 11 (0.1) | 104.6 ± 74.7 | 513 (3.5) |
| Dentistry | 289,279 (6.8) | 16.8 ± 6.1 | 3.2 ± 1.6 | 23 (0.0) | 94.5 ± 50.2 | 6,966 (2.4) |
| Missing | 1,346,001 (31.6) | 37.7 ± 45.1 | 9.8 ± 16.8 | 18,666 (1.4) | 278.3 ± 705.8 | 125,488 (9.3) |
Table 4.
Days’ supply (number and percentage) of first opioid prescriptions written by each specialty
| Specialty | 0 to 1 Days, No. (%) | 2 to 3 Days, No. (%) | 4 to 7 Days, No. (%) | 8 to 10 Days, No. (%) | 11 to 14 Days, No. (%) | 15 to 30 Days, No. (%) | 31 to 90 Days, No. (%) | Total, No. |
|---|---|---|---|---|---|---|---|---|
| Total | 117,799 (4.1) | 1,177,786 (40.5) | 1,094,239 (37.6) | 227,205 (7.8) | 42,260 (1.5) | 237,776 (8.2) | 9,743 (0.3) | 2,906,808 |
| Addiction medicine | 7 (1.1) | 79 (12.6) | 332 (53.1) | 55 (8.8) | 12 (1.9) | 133 (21.3) | 7 (1.1) | 625 |
| Anesthesiology | 346 (1.3) | 5,856 (21.2) | 7,332 (26.6) | 1,118 (4.1) | 822 (3.0) | 11,869 (43.1) | 218 (0.8) | 27,561 |
| Dentistry | 14,824 (5.1) | 172,208 (59.5) | 99,917 (34.5) | 1,831 (0.6) | 118 (0.0) | 366 (0.1) | 15 (0.0) | 289,279 |
| Dermatology | 720 (7.9) | 5,336 (58.4) | 2,613 (28.6) | 255 (2.8) | 33 (0.4) | 167 (1.8) | 20 (0.2) | 9,144 |
| Emergency medicine | 36,856 (8.2) | 260,696 (57.9) | 140,109 (31.1) | 7,875 (1.8) | 780 (0.2) | 3,382 (0.8) | 166 (0.0) | 449,864 |
| Gynecology | 6,398 (4.2) | 63,591 (41.8) | 68,792 (45.2) | 10,066 (6.6) | 911 (0.6) | 2,249 (1.5) | 81 (0.1) | 152,088 |
| Hematology/oncology | 202 (1.8) | 1,362 (12.3) | 3,392 (30.8) | 2,171 (19.7) | 705 (6.4) | 3,121 (28.3) | 76 (0.7) | 11,029 |
| Internal medicine | 26,126 (3.2) | 242,750 (29.4) | 274,435 (33.3) | 101,290 (12.3) | 1,9474 (2.4) | 154,634 (18.8) | 5,948 (0.7) | 824,657 |
| Advanced practice provider | 6,350 (4.4) | 63,668 (44.5) | 45,805 (32.0) | 9,992 (7.0) | 2,433 (1.7) | 12,743 (8.9) | 2,034 (1.4) | 143,025 |
| Neurology | 52 (1.0) | 586 (11.3) | 1,101 (21.2) | 738 (14.2) | 224 (4.3) | 2,418 (46.5) | 76 (1.5) | 5,195 |
| Ophthalmology | 1,383 (9.5) | 8,557 (58.5) | 4,109 (28.1) | 275 (1.9) | 39 (0.3) | 243 (1.7) | 15 (0.1) | 14,621 |
| Orthopedics | 818 (0.7) | 20,046 (18.2) | 59,296 (54.0) | 18,036 (16.4) | 3,552 (3.2) | 7,983 (7.3) | 154 (0.1) | 109,885 |
| Other | 11,447 (3.4) | 152,622 (45.2) | 135,740 (40.2) | 21,790 (6.5) | 3,845 (1.1) | 11,504 (3.4) | 366 (0.1) | 337,314 |
| Pediatrics | 946 (3.9) | 11,023 (45.1) | 8,856 (36.3) | 1,449 (5.9) | 247 (1.0) | 1,848 (7.6) | 53 (0.2) | 24,422 |
| Physical medicine | 109 (0.6) | 1,368 (7.3) | 5,270 (27.9) | 3,635 (19.3) | 971 (5.1) | 7,417 (39.3) | 93 (0.5) | 18,863 |
| Psychiatry | 501 (4.7) | 5,920 (55.4) | 3,256 (30.5) | 349 (3.3) | 100 (0.9) | 528 (4.9) | 36 (0.3) | 10,690 |
| Radiology | 511 (4.9) | 4,484 (42.7) | 4,075 (38.8) | 516 (4.9) | 143 (1.4) | 738 (7.0) | 40 (0.4) | 10,507 |
| Specialty surgery | 10,203 (2.2) | 157,634 (33.7) | 229,809 (49.1) | 45,764 (9.8) | 7,851 (1.7) | 16,433 (3.5) | 345 (0.1) | 468,039 |
Table 5 is the result of the multivariable model predicting chronic opioid use. There are several notable findings: 1) compared with those aged 18–30 years, the age range of 51–65 years has the highest rate of developing chronic use (odds ratio [OR] = 1.78); 2) females had a higher rate of chronic use than males (OR = 1.06); 3) major medical coverage (OR = 3.33) and workers’ compensation (OR = 1.95) were more likely to develop chronic use compared with commercially insured; 4) an extended-release formulation as a first prescription increased the odds of chronic use by 80% compared with non-extended-release (OR = 1.79). There were also differences depending on the month and year of the index prescription, with those written in 2016 being much less likely to have chronic use (OR = 0.31) compared with those written in 2013. The odds of developing chronic use increased substantially with increasing MME of the prescription. Finally, when compared with a reference of internal medicine, development of chronic use was most likely when the index prescription came from an anesthesiologist (OR = 1.46), neurology (OR = 1.43), or physical medicine (OR = 1.29) and least likely when the index prescription came from ophthalmology (OR = 0.33), gynecology (OR = 0.37), or dentistry (OR = 0.39).
Table 5.
Multivariable model of developing chronic opioid use
| Odds Ratio | P > z | 95% Confidence Interval | ||
|---|---|---|---|---|
| Age categories, y | ||||
| 18–30 | Reference | |||
| 5–17 | 0.56 | <0.01 | 0.54 | 0.57 |
| 31–50 | 1.57 | <0.01 | 1.55 | 1.58 |
| 51–65 | 1.78 | <0.01 | 1.76 | 1.80 |
| 66–99 | 1.53 | <0.01 | 1.51 | 1.55 |
| Sex | ||||
| Male | Reference | |||
| Female | 1.06 | <0.01 | 1.05 | 1.07 |
| Missing | 1.28 | <0.01 | 1.17 | 1.41 |
| Payment type | ||||
| Commercial | Reference | |||
| Private pay | 1.03 | <0.01 | 1.02 | 1.05 |
| Medicare | 1.12 | <0.01 | 1.11 | 1.14 |
| Medicaid | 1.72 | <0.01 | 1.70 | 1.74 |
| Workers’ compensation | 1.95 | <0.01 | 1.84 | 2.05 |
| Major medical | 3.33 | <0.01 | 3.25 | 3.42 |
| Extended-release | ||||
| No | Reference | |||
| Yes | 1.79 | <0.01 | 1.74 | 1.84 |
| Month filled | ||||
| January | Reference | |||
| February | 0.98 | 0.03 | 0.97 | 1.00 |
| March | 0.94 | <0.01 | 0.93 | 0.96 |
| April | 1.04 | <0.01 | 1.02 | 1.06 |
| May | 0.89 | <0.01 | 0.88 | 0.91 |
| June | 0.86 | <0.01 | 0.85 | 0.88 |
| July | 0.84 | <0.01 | 0.83 | 0.86 |
| August | 0.82 | <0.01 | 0.81 | 0.84 |
| September | 0.79 | <0.01 | 0.78 | 0.81 |
| October | 0.75 | <0.01 | 0.74 | 0.76 |
| November | 0.65 | <0.01 | 0.64 | 0.66 |
| December | 0.70 | <0.01 | 0.69 | 0.71 |
| Year filled | ||||
| 2013 | Reference | |||
| 2014 | 0.88 | <0.01 | 0.87 | 0.89 |
| 2015 | 0.60 | <0.01 | 0.60 | 0.61 |
| 2016 | 0.31 | <0.01 | 0.31 | 0.32 |
| Prescription morphine milligram equivalents | ||||
| 9–50 | Reference | |||
| 51–100 | 0.91 | <0.01 | 0.90 | 0.92 |
| 101–150 | 1.17 | <0.01 | 1.15 | 1.18 |
| 151–200 | 1.26 | <0.01 | 1.23 | 1.28 |
| 201–250 | 1.30 | <0.01 | 1.28 | 1.33 |
| 251–300 | 1.81 | <0.01 | 1.78 | 1.84 |
| 301–350 | 1.55 | <0.01 | 1.48 | 1.62 |
| 351–400 | 1.53 | <0.01 | 1.48 | 1.58 |
| 401–450 | 2.20 | <0.01 | 2.15 | 2.24 |
| 451–500 | 2.05 | <0.01 | 1.96 | 2.14 |
| >500 | 2.90 | <0.01 | 2.85 | 2.95 |
| Specialty of prescriber | ||||
| Internal medicine | Reference | |||
| Ophthalmology | 0.33 | <0.01 | 0.3 | 0.36 |
| Gynecology | 0.37 | <0.01 | 0.36 | 0.38 |
| Dentistry | 0.39 | <0.01 | 0.38 | 0.4 |
| Specialty surgery | 0.44 | <0.01 | 0.43 | 0.45 |
| Dermatology | 0.45 | <0.01 | 0.41 | 0.5 |
| Radiology | 0.49 | <0.01 | 0.45 | 0.53 |
| Psychiatry | 0.54 | <0.01 | 0.5 | 0.58 |
| Other | 0.60 | <0.01 | 0.59 | 0.61 |
| Missing | 0.67 | <0.01 | 0.66 | 0.67 |
| Orthopedics | 0.74 | <0.01 | 0.72 | 0.75 |
| Pediatrics | 0.74 | <0.01 | 0.7 | 0.78 |
| Hematology/oncology | 0.78 | <0.01 | 0.73 | 0.82 |
| Midlevel | 0.84 | <0.01 | 0.82 | 0.86 |
| Emergency medicine | 0.85 | <0.01 | 0.84 | 0.86 |
| Addiction medicine | 1.02 | 0.91 | 0.76 | 1.37 |
| Physical medicine | 1.29 | <0.01 | 1.24 | 1.34 |
| Neurology | 1.43 | <0.01 | 1.33 | 1.54 |
| Anesthesiology | 1.46 | <0.01 | 1.42 | 1.51 |
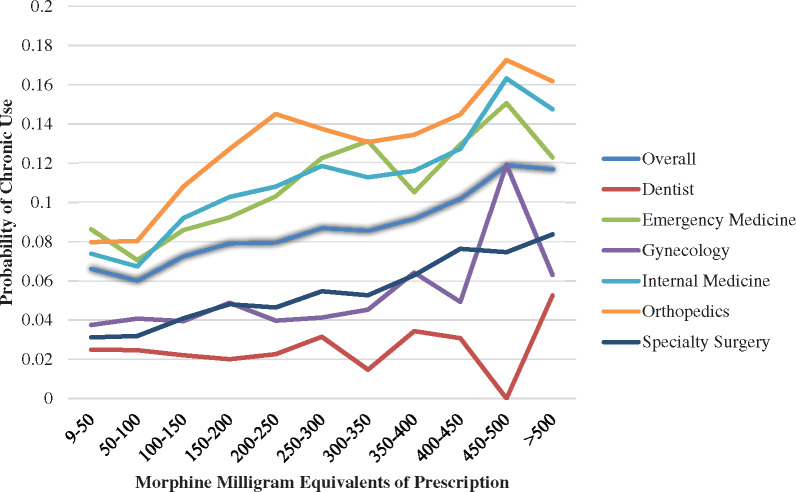
Figure 1 shows the relationship between MME content of the first prescription and probability of developing chronic use. Prescriptions are binned in 50 MME categories, starting with the smallest amount in our data set (MME [9]), displayed overall (dark blue line), for a reference specialty (internal medicine), and for several specialties that typically prescribe opioids for acute pain (dentistry, emergency medicine, gynecology, orthopedics, and specialty surgery). Only prescriptions with days’ supply ≤14 were included to capture prescriptions most likely to be for acute issues. Even with higher MME prescriptions, those written by dentists were the least associated with chronic use, while those written by orthopedic surgeons were the most associated with chronic use, particularly when the prescription was for >200 MME.
Figure 1.
Probability of chronic opioid use based on morphine milligram equivalent content of first-time opioid prescriptions overall and for select specialties. Only prescriptions for ≤14 days were considered.
Discussion
This study evaluated the characteristics of first opioid prescription preceding chronic use and includes detailed information about the prescriptions themselves and specialty information on a state-wide scale. The use of the Ohio PDMP allowed evaluation of nearly all the state’s population filling controlled prescriptions regardless of payer or pharmacy used. Several findings emerged that were novel and confirmed prior work.
For patient demographics, on the adjusted analysis, females had slightly higher odds of chronic use compared with males, and mid-aged and older patients (≥31 years) were more likely to develop chronic use than younger patients (≤30 years). Those age >65 years had 53% increased odds of developing chronic use when compared with those aged 18–30 years. Similar findings were also recently described in a study of an insurance database that found that fill rates of opioid prescriptions were disproportionately higher among men and women aged ≥65 years and women of all ages [14]. Higher presence of chronic use in older patients may reflect the presence of more comorbidities, but it is concerning given that opioids should be a last-line pain treatment modality for older patients [15]. Patients who self-paid for their first prescription did not have a notably different rate of developing chronic use (OR = 1.03 when compared with commercial insurance). This is an unexpected finding, as self-payment is typically thought of as a risk factor for opioid “shopping” behavior [16]. However, those who filled prescriptions using Medicaid were more likely to develop chronic use compared with those with commercial insurance, concordant with a Maine study that found a greater utilization of public insurance by patients with chronic pain [17]. Although major medical insurance and workers’ compensation insurance were used infrequently, both were associated with a much higher odds of chronic risk, a finding that warrants further investigation.
A key finding, which confirms prior research, is the simple principle that the greater the total morphine equivalents contained in the initial prescription, the greater the associated chance of developing chronic use [5, 7, 12, 13, 18]. Pill quantities, days’ supply, and MME per prescription are directly related and were all higher for patients who developed chronic use in this study. The direction of causality is difficult to establish because it may be that patients with higher MME prescriptions had more severe pain (e.g., major trauma or complicated surgery) that did not resolve after the initial prescription, but it is also possible that it was the characteristics of the first prescription that led to chronic use. Also, that there were differences in chronic use for the same MME categories indicates that there may be differences in the underlying patient populations treated by the different specialties. At a minimum, these findings call for standardization of prescribing after common similar surgical procedures [9,19].
When evaluating the active ingredient of the medications prescribed, it is interesting that oxycodone and hydrocodone had similar associations with developing chronic use (8.7% vs 7.3% of the prescriptions). Though when compared with codeine, hydrocodone had an odds ratio for association of long-term use of 1.33, while the odds ratio for oxycodone was 1.64. The fact that oxycodone was more likely to be associated with chronic use compared with hydrocodone may be related to its “likability” and higher abuse liability than hydrocodone [20, 21]. Tramadol, conversely, is sometimes thought of as a safer opioid and is recommended for older patients who fail nonopioid therapy for pain control, so its use may have increased over time, yet its association with chronic use when given as a first-time prescription was 13.0% and it had an odds ratio of 2.52 when compared with codeine. Tramadol has abuse potential and significant adverse effects. In older patients, particularly, use of tramadol (compared with no opioid use) is associated with increased risk of multiple emergency department visits, falls and fractures, cardiovascular disease hospitalizations, safety event hospitalizations, and mortality [15, 22, 23]. That tramadol was more common with chronic use in this study may be another warning against its use. In fact, one study looking at chronic use of tramadol after acute pain concluded, “Providers should use as much caution when prescribing tramadol in the setting of acute pain as for other short acting opioids” [24]. Other medications (oxymorphone, morphine, and methadone) were much more likely to be associated with long-term use, but these prescriptions were very rare (0.5% of all index prescriptions) and it is hypothesized that these prescriptions were unlikely to be first opioid prescriptions but rather misclassifications, as described in the limitations below. Overall, future research and guidelines could be more specific not just about how many pills to prescribe but also could compare effectiveness and abuse potential of different opioids for common acutely painful conditions.
The primary outcome of the study was to compare opioid prescribing stratified by specialty. As specialty information was available for about two-thirds of the prescriptions, it is not feasible to directly compare numbers of prescriptions written by each specialty, but it is possible to compare the characteristics of prescriptions written when the specialty was identified. The specialties whose prescriptions were least likely to be associated with chronic use were ophthalmology, gynecology, and dentistry. Dentists have been a source of recent attention, as they prescribe about one in 10 opioid prescriptions in the United States and historically have overprescribed opioids [25, 26]. Dental patients who take opioids after dental procedures report both increased pain and no difference in satisfaction compared with those who do not take opioids [27]. The fact that the baseline chronic use even for higher-MME prescriptions was relatively low is reassuring, given that opioids prescribed for dental issues should typically be for time-limited conditions.
Another specialty of interest is emergency medicine, which has been the focus of many targeted guidelines [29–31]. The emergency department (ED) is a place commonly thought of as being the source of acute prescriptions, and one study found that about half of patients with opioid use disorder had their first exposure from a legitimate prescription, and about a third of those came from the ED [32]. Our data demonstrate that about 7.8% of first-time opioid prescriptions from the ED were associated with chronic use. This is a notable statistic: About one out of every 13 opioid prescriptions to opioid-naïve patients written by emergency physicians will lead to chronic use. Still, this is far lower than some other specialties, similar to what has been described previously [12]. As the MME curves in Figure 1 demonstrate, there is a dose-dependent component in which smaller prescription MMEs are less likely to be associated with chronic use, which validates many guideline recommendations, including those from the CDC [1].
We also focused on prescriptions written by orthopedic surgeons, which are more likely to be written postsurgically. After surgery, in general, patients tend to use substantially fewer opioids than are prescribed, which may lead to nonmedical use or diversion of remaining pills [19]. This is also specifically the case after total hip and knee arthroplasty [33]. Therefore, it is essential to “right-size” the prescription after surgery to balance appropriate analgesia with mitigating development of opioid use disorder or diversion. Prior research has shown that 4% of opioid-naïve adult orthopedic surgery patients at one center developed chronic use, which was associated with having a prescription MME >675 mg [34]. Our study shows that prescriptions to opioid-naïve patients by orthopedic surgeons were associated with a 13.3% rate of chronic use, and the rate was high even with smaller-MME prescriptions.
Finally, the prescriptions that were most likely to be associated with chronic use were by anesthesiology, neurology, and physical medicine/rehabilitation. These providers tended to have longer days’ supply for their first prescription, with about half for ≥15 days. We hypothesize that patients receiving opioids from these specialties are already chronic pain sufferers, and the opioid use may have been initiated by these specialists after other options did not work. A limitation of PDMP data is that they do not contain the indication for the opioid prescription, which limits interpretation of appropriateness for these prescriptions. Overall, we are hopeful that individual specialty societies and groups will use information like this to create guidance that is most applicable to their unique practices and educate on the risk of acute opioid prescribing leading to chronic use.
There are several other limitations of this study to consider. First, this was an analysis of one state’s data. Prescriptions written in Ohio but filled in a retail pharmacy in another state will not appear. Likewise, there would be a classification error if a patient who had no prior opioid prescription in our data set had prescriptions written in another state. The PDMP is an administrative data set that relies on pharmacists inputting data at the time of prescription dispensation. There may have been errors in data entry. We removed outliers that appeared to be nonsensical, but some errors may have remained. The specialty information may not reflect actual practice. For example, a neurologist may work in a pain clinic or a pediatrician may work in an ED. We were likewise unable to determine the practice location of advanced practice providers. We did not consider opioid prescriptions for liquid formulations or patches, which may have altered the results if included. We were only able to characterize prescribed opioids but could not capture illicit or diverted opioid use. Finally, the age of the data should be taken into consideration, as many prescribing guidelines and prescribing practices may have changed after 2016.
Conclusions
In general, about one in 12 opioid-naïve individuals who receive an opioid prescription will develop chronic use. This rate varies depending on the specialty of the provider who wrote the index prescription, which may reflect the patient populations that these specialties treat. The risk of chronic use increased with higher MME content of the initial prescription and use of extended-release opioids. This information can be used by prescribers who should avoid opioid prescribing for acute pain whenever possible, right-size prescriptions when opioids are indicated for acute pain, and inform patients when prescribing opioids following the principles of shared decision-making.
Funding sources: This work was funded by National Institutes of Health grant 1-R01-DA044167. Additional support for statistical analysis was provided by an unrestricted grant from the ADK Charities. The funding sources were not involved in the study design, collection, analysis, or interpretation of the data, writing of the report, or the decision to submit the report for publication. The conclusions in this article are those of the authors and do not necessarily represent the official position of the funders.
Conflicts of interest: The authors have no financial conflicts of interest to declare.
Prior presentations: Preliminary results of this study were presented at the 2019 American College of Emergency Physicians Scientific Assembly, Denver, Colorado, USA.
References
- 1. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 2010;152(2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305(13):1315–21. [DOI] [PubMed] [Google Scholar]
- 4. Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: A systematic review and meta-analysis. JAMA 2018;320(23):2448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah A, Hayes CJ, Martin BC.. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66(10):265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM.. Long-term analgesic use after low-risk surgery: A retrospective cohort study. Arch Intern Med 2012;172(5):425–30. [DOI] [PubMed] [Google Scholar]
- 7. Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naïve patients: A statewide retrospective cohort study. J Gen Intern Med 2017;32(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colorado Hospital Association. Colorado ALTO Project. Available at: https://cha.com/opioid-safety/colorado-alto-project (accessed July 12, 2020).
- 9.Michigan Opioid Prescribing Engagement Network. Prescribing recommendations. Available at: https://michigan-open.org/prescribing-recommendations/ (accessed July 2020).
- 10. Weiner SG, Baker O, Rodgers AF, et al. Opioid prescriptions by specialty in Ohio, 2010-2014. Pain Med 2018;19(5):978–89. [DOI] [PubMed] [Google Scholar]
- 11. Korff MV, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24(6):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeffery MM, Hooten WM, Hess EP, et al. Opioid prescribing for opioid-naive patients in emergency departments and other settings: Characteristics of prescriptions and association with long-term use. Ann Emerg Med 2018;71(3):326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hadlandsmyth K, Lund BC, Mosher HJ.. Associations between initial opioid exposure and the likelihood for long-term use. J Am Pharm Assoc (2003) 2019;59(1):17–22. [DOI] [PubMed] [Google Scholar]
- 14. Schieber LZ, Guy GP Jr, Seth P, Losby JL.. Variation in adult outpatient opioid prescription dispensing by age and sex - United States, 2008-2018. MMWR Morb Mortal Wkly Rep 2020;69(11):298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Makris UE, Abrams RC, Gurland B, Reid MC.. Management of persistent pain in the older patient: A clinical review. JAMA 2014;312(8):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC.. Opioid shopping behavior: How often, how soon, which drugs, and what payment method. J Clin Pharmacol 2013;53(1):112–7. [DOI] [PubMed] [Google Scholar]
- 17. Malon J, Shah P, Koh WY, Cattabriga G, Li E, Cao L.. Characterizing the demographics of chronic pain patients in the state of Maine using the Maine all payer claims database. BMC Public Health 2018;18(1):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Webster BS, Verma SK, Gatchel RJ.. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976) 2007;32(19):2127–32. [DOI] [PubMed] [Google Scholar]
- 19. Feinberg AE, Chesney TR, Srikandarajah S, Acuna SA, McLeod RS; Best Practice in Surgery Group. Opioid use after discharge in postoperative patients: A systematic review. Ann Surg 2018;267(6):1056–62. [DOI] [PubMed] [Google Scholar]
- 20. Wightman R, Perrone J, Portelli I, Nelson L.. Likeability and abuse liability of commonly prescribed opioids. J Med Toxicol 2012;8(4):335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Remillard D, Kaye AD, McAnally H.. Oxycodone’s unparalleled addictive potential: Is it time for a moratorium? Curr Pain Headache Rep 2019;23(2):15. [DOI] [PubMed] [Google Scholar]
- 22. Bush DM. Emergency Department Visits for Drug Misuse or Abuse Involving the Pain Medication Tramadol. The CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [PubMed]
- 23. Musich S, Wang SS, Schaeffer JA, Slindee L, Kraemer S, Yeh CS.. Safety events associated with tramadol use among older adults with osteoarthritis. Popul Health Manag. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thiels CA, Habermann EB, Hooten WM, Jeffery MM.. Chronic use of tramadol after acute pain episode: Cohort study. BMJ 2019;365:l1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suda KJ, Zhou J, Rowan SA, et al. Overprescribing of opioids to adults by dentists in the U.S., 2011-2015. Am J Prev Med 2020;58(4):473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suda KJ, Durkin MJ, Calip GS, et al. Comparison of opioid prescribing by dentists in the United States and England. JAMA Netw Open 2019;2(5):e194303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nalliah RP, Sloss KR, Kenney BC, et al. Association of opioid use with pain and satisfaction after dental extraction. JAMA Netw Open 2020;3(3):e200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weiner SG, Baker O, Poon SJ, et al. The effect of opioid prescribing guidelines on prescriptions by emergency physicians in Ohio. Ann Emerg Med 2017;70(6):799–808.e1. [DOI] [PubMed] [Google Scholar]
- 30. Weiner SG, Yannopoulos PF, Lu C.. Chronic pain patients’ impressions of an emergency department opioid prescribing guideline poster. Pain Med 2015;16(9):1759–63. [DOI] [PubMed] [Google Scholar]
- 31. Weiner SG, Perrone J, Nelson LS.. Centering the pendulum: The evolution of emergency medicine opioid prescribing guidelines. Ann Emerg Med 2013;62(3):241–3. [DOI] [PubMed] [Google Scholar]
- 32. Butler MM, Ancona RM, Beauchamp GA, et al. Emergency department prescription opioids as an initial exposure preceding addiction. Ann Emerg Med 2016;68(2):202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roberts KC, Moser SE, Collins AC, et al. Prescribing and consumption of opioids after primary, unilateral total hip and knee arthroplasty in opioid-naive patients. J Arthroplasty 2020;35(4):960–5.e1. [DOI] [PubMed] [Google Scholar]
- 34. Lanzillotta-Rangeley J, Clark A, Christianson A, Kalarchian MA.. Association of prescription opioid exposure and patient factors with prolonged postoperative opioid use in opioid-naïve patients. AANA J 2020;88(1):18–26. [PubMed] [Google Scholar]