Abstract
Background/purpose
Dental pulp stem cells can be isolated from human teeth with deep caries (cDPSCs), but their biological characteristics are still unclear. The aim of this study was to investigate the angiogenic potential of cDPSCs and compare them to dental pulp stem cells from human normal teeth (nDPSCs).
Materials and methods
Cells were isolated from human pulp tissue of normal and infected teeth with deep caries. Basic mesenchymal stem cell (MSC) characterization was conducted. Colony forming units and proliferation ability were evaluated in nDPSCs and cDPSCs. Expression of VEGF in both tissues and cells was examined by immunohistochemical staining. After stimulating nDPSCs and cDPSCs with an angiogenic medium, angiogenic markers were evaluated by qRT-PCR and western blotting. Finally, tube formation assays were used to evaluate the in vitro angiogenesis potential of both cell populations.
Results
Both nDPSCs and cDPSCs possessed typical MSC characteristics. cDPSCs had enhanced colony formation and proliferation capacities than nDPSCs did. The expression of VEGF was higher in pulp tissue from teeth with deep caries and cDPSCs than in normal tissue and nDPSCs. When both cell types were grown in vitro under angiogenic conditions, cDPSCs expressed a higher level of angiogenic markers and showed a stronger angiogenesis potential than nDPSCs did.
Conclusion
cDPSCs maintained MSC traits and presented a higher angiogenesis potential than nDPSCs.
Keywords: Angiogenesis, Deep caries, Pulp regeneration, Dental pulp stem cells
Introduction
Dental pulp is highly vascularized, vulnerable to external insults, and loose connective tissue surrounded by rigid dentin. When the pulp is in a state of irreversible inflammation, it is removed and replaced by gutta-percha.1 However, root fracture and postoperative pain may occur after the treatment, leading to a higher rate of tooth extraction.2,3 Hence, pulp regeneration is a better treatment compared to the root canal treatment.
Since the pulp is situated between the rigid dentin walls, its blood supply is provided through the small apical foramen, a surface even smaller than 1 mm. Thus, during cell-based pulp regeneration, angiogenesis is a challenging and crucial process because of the restricted access to the apical foramen.4 Dental pulp stem cells isolated from normal human teeth (nDPSCs) may contribute to angiogenesis during pulp regeneration by a paracrine effect, and even by differentiating themselves into an endothelial lineage. nDPSCs can express many angiogenic factors, including vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and stromal cell-derived factor 1 (SDF-1), promoting endothelial cell migration, and facilitating in vitro and in vivo endothelial tubulogenesis.5,6 When cultured under angiogenic conditions, nDPSCs can differentiate into endothelial-like cells, forming vascular networks.7, 8, 9
nDPSCs are promising for pulp regeneration. To date, a couple of clinical studies showed nDPSCs successfully regenerated dental pulp after transplantation in the presence of pulpitis, and injured teeth.10,11 However, their wide-scale use is limited by their availability. Additionally, stem cells can be isolated from pulp tissue under unfavorable conditions, including from teeth with deep caries, which are usually considered a medical waste, and thus, discarded.12 In the pulp of teeth with deep caries, noxious stimuli may activate different cells, triggering a series of inflammatory responses, and releasing cytokines such as interleukin (IL)-4, IL-6, and IL-10.13, 14, 15 The local inflammatory microenvironment may alter the characteristics of nDPSCs.16 Limited research has been performed with dental pulp stem cells isolated from human teeth with deep caries (cDPSCs). Therefore, whether cDPSCs can be used in tissue engineering remains unknown. Our previous study showed that cDPSCs possessed enhanced proliferation and mineralization capacities, compared to nDPSCs.12 However, the biological characteristics of cDPSCs still need further exploration. The angiogenesis properties of cDPSCs in an inflammatory microenvironment remain unknown. This study aimed to examine the angiogenesis capacity of cDPSCs and to better and further understand their implication in pulp regeneration.
Materials and methods
Cell culture
Freshly extracted normal and deep carious human teeth were collected from patients with 20–25 years old. All patient-related procedures in this study were approved by the Ethics Committee of Nanfang Hospital, and the informed written consent of the patients was obtained. Their demographic data was shown in Table 1. Normal dental pulp (n = 5) was separated from healthy human premolars or third molars that were previously extracted during orthodontic procedures or impaction treatments. Deep caries (n = 5) were diagnosed based on clinical examinations, clinical symptoms, and X-ray imaging confirming that lesions were at least 2 mm from the pulp tissue. Patients were included if there was no history of spontaneous and intense pain. nDPSCs and cDPSCs were isolated and cultured following the methods described by Li et al.17 nDPSCs and cDPSCs were passaged until they were 80% confluent. Cell phenotypes were analyzed by flow cytometry using CD90/PE, CD105/FITC, CD29/PE, CD44/FITC, and CD45/PE antibodies (Pharmingen, BD Biosciences, San Diego, CA, USA). Both cells were induced in the direction of lipogenesis, osteogenesis and chondrogenesis according to the manufacturer's protocol (Cyagen Biosciences, Chicago, IL,USA). Cells at passage three were used for all subsequent experiments.
Table 1.
The demographic data of normal and carious groups.
| Identity | Age (years) | Gender | Cold test | History of spontaneous and intense pain | X-ray (distance between the lesion and the pulp tissue) |
|---|---|---|---|---|---|
| N01 | 23 | Female | Normal | No | No lesion |
| N02 | 22 | Male | Normal | No | No lesion |
| N03 | 25 | Female | Normal | No | No lesion |
| N04 | 21 | Female | Normal | No | No lesion |
| N05 | 23 | Male | Normal | No | No lesion |
| C01 | 25 | Male | Normal | No | 2.4 mm |
| C02 | 24 | Male | Normal | No | 2.3 mm |
| C03 | 22 | Female | Normal | No | 2.3 mm |
| C04 | 24 | Female | Normal | No | 2.1 mm |
| C05 | 20 | Male | Normal | No | 2.2 mm |
N, normal group; C, carious group.
Colony-forming and proliferation assays
nDPSCs and cDPSCs from the third passage were maintained in culture dishes at 1500 cells/dish. After 14 days, cells were stained using Giemsa (Sigma–Aldrich, St. Louis, MO, USA). Colonies were identified as clusters of >50 cells and the total number of colonies was recorded. For proliferation assays, nDPSCs and cDPSCs were plated on 96-well plates at 1000 cells/well and evaluated by CCK8 assays on days 1, 3, 5, 7, and 9.
Immunohistochemical staining
Pulp tissues from freshly extracted healthy teeth and teeth with deep caries were fixed in 4% paraformaldehyde for immunohistochemical staining. For immunocytochemical staining, nDPSCs and cDPSCs were seeded on 6-well plates at 2.0 × 105 cells/well in basal medium. When cells reached 80% confluence, they were washed three times with PBS and fixed in 4% paraformaldehyde. Pulp tissues and cells were stained with a VEGF-specific antibody (Abcam, Burlingame, CA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
nDPSCs and cDPSCs were cultured on 6-well plates to 80% confluence. The medium was then replaced with angiogenic medium (EGM-2; Lonza, Basel, Switzerland). Total RNA was extracted on days 3, 7, and 14 using TRIzol Reagent (Invitrogen; Life Technologies, Carlsbad, CA, USA) and used as a template to generate cDNA using a reverse transcription kit (Invitrogen). Gene expression levels were then determined for VEGF, PDGF, SDF-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using primer sequences shown in Table 2. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using a SYBR Select Master Mix (Life Technologies) with the ABI 7500 Real-time Polymerase Chain Reaction System (ABI, Foster City, CA, USA) following the manufacturer's instructions. Relative expression was calculated using the cycle threshold (Ct) difference between GAPDH and each transcript with the ΔΔCt method.
Table 2.
Primer sets used for qRT-PCR.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| VEGF | CTACCTCCACCATGCCAAGT | CACACAGGATGGCTTGAAGA |
| PDGF | ACACGAGCAGTGTCAAGTGC | GGCTCATCCTCACCTCACAT |
| SDF-1 | GGCTCATCCTCACCTCACAT | ACACACACACCTGGTCCTCA |
| GAPDH | TCACCAGGGCTGCTTTTAAC | GACAAGCTTCCCGTTCTCAG |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PDGF, platelet derived growth factor; SDF-1, stromal cell-derived factor 1; VEGF, vascular endothelial growth factor.
Western blot analysis
Total protein was extracted from cultured cells after the induction of angiogenesis on days 3, 7, and 14, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). The membrane was incubated with a rabbit monoclonal anti-VEGF antibody (1:1000; Abcam, Burlingame, CA) overnight at 4 °C. Proteins were visualized using an IRDye 800CW goat anti-rabbit immunoglobulin G (1:15000; LI-COR, Lincoln, NE, USA) secondary antibody. The membrane was scanned using an Odyssey V3.0 scanner (LI-COR).
In vitro angiogenesis tube formation assay
Each well in 96–well plate was coated with 50 μL Matrigel solution (BD Biosciences, Franklin Lakes, NJ) and then placed at 37 °C for 1 h nDPSCs and cDPSCs were cultured in an angiogenic medium for 7 days, and then trypsinized and seeded onto Matrigel coated wells at 5 × 104 cells/well. A volume of 150 μL EGM-2 medium was added to each well and incubated at 37 °C. The formation of vascular-like structures was observed at 4 h by Nikon inverted microscope. Representative images were documented and the analysis of the vascular network was performed by ImageJ software.
Statistical analysis
Results were evaluated by analysis of variance and independent t tests by using SPSS 19.0 software (IBM, Armonk, NY, USA). The significance threshold was P < 0.05.
Results
Characterization of nDPSCs and cDPSCs
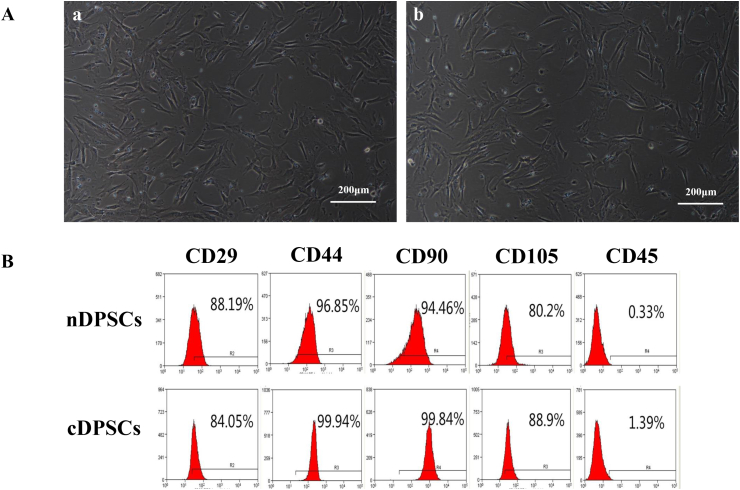
We successfully isolated and cultured stem cells from normal and deep carious pulps, and then we examined their basic characteristics. There was no difference in the morphology of nDPSCs and cDPSCs; both were spindle-shaped and fibroblast-like (Fig. 1A). Based on flow cytometry, both nDPSCs and cDPSCs showed high expression of mesenchymal stem cell markers and low expression of hematopoietic cell markers. The proportions of nDPSCs positive for CD29, CD44, CD90, CD105, and CD45 were 88.19%, 96.85%, 94.46%, 80.2%, and 0.33%, respectively. For cDPSCs, the proportions of these surface markers were 84.05%, 99.94%, 99.84%, 88.9%, and 1.39%, respectively (Fig. 1B). Both cell types displayed multi-directional differentiation potential (data not shown).These results suggested that both nDPSCs and cDPSCs had mesenchymal stem cell characteristics.
Figure 1.
The isolation and characterization of nDPSCs and cDPSCs. (A–a) nDPSCs at passage 3. (A-b) cDPSCs at passage 3.cDPSCs showed a similar morphology to nDPSCs. (B)A flow cytometry-based immunophenotype analysis of nDPSCs and cDPSCs from passage 3. Cells were incubated with specific monoclonal antibodies against cell surface marker antigens CD29, CD44, CD90, CD105, and CD45 prior to analysis.
Proliferation capacity of nDPSCs and cDPSCs
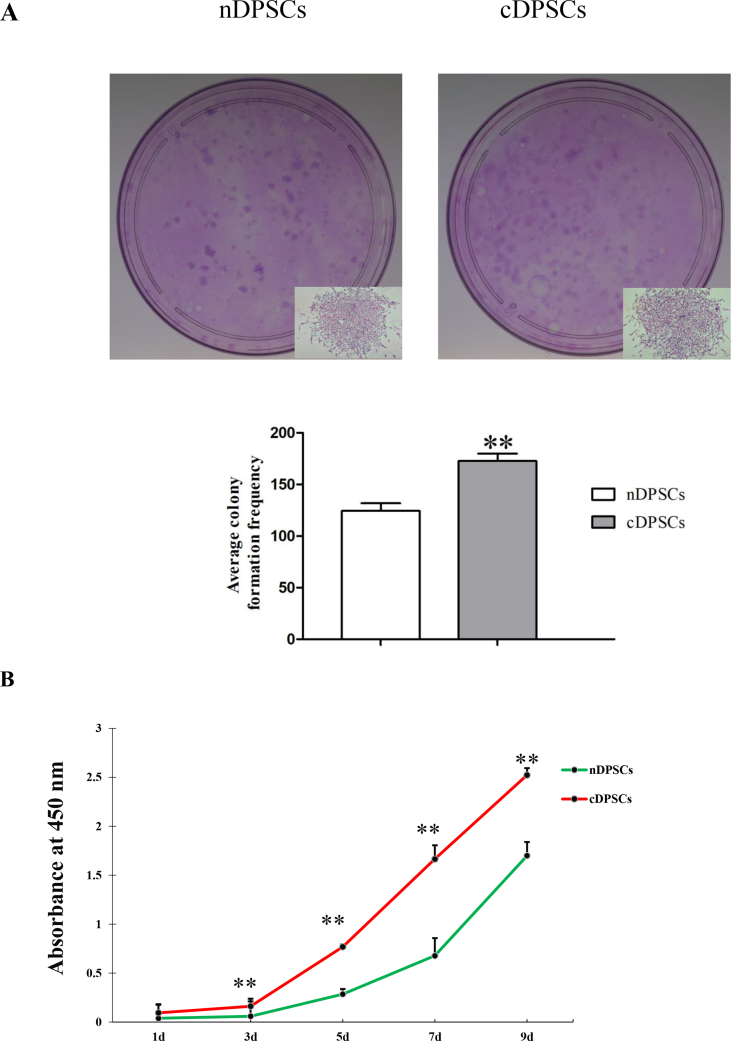
Cell proliferation ability is the foundation of tissue repair and regeneration, so we further explored the proliferation ability of these cells by colony-formation and CCK8 assays. Significantly more colony-forming units were obtained with cDPSCs than with nDPSCs; thus, cDPSCs had an enhanced colony formation ability (P < 0.01; Fig. 2A). CCK8 assays showed that both nDPSCs and cDPSCs entered a logarithmic growth phase by day 3, with no apparent stationary phase. On day 3, the proliferation rates of both nDPSCs and cDPSCs diverged, with cDPSCs showing significantly greater proliferation, and this difference was maintained on days 5 and 9 (P < 0.01; Fig. 2B). These results indicated that cDPSCs clearly had a greater proliferation capacity than nDPSCs did.
Figure 2.
Proliferation of nDPSCs and cDPSCs. (A) Colony-forming units of nDPSCs and cDPSCs. cDPSCs had a higher average colony formation frequency than nDPSCs. (B) CCK8 assay at 1, 3, 5, 7 and 9 days after cell seeding. After day 3, proliferation rates in cDPSCs were greater than nDPSCs (∗∗P < 0.01).
Marker expressions on nDPSCs and cDPSCs during angiogenesis
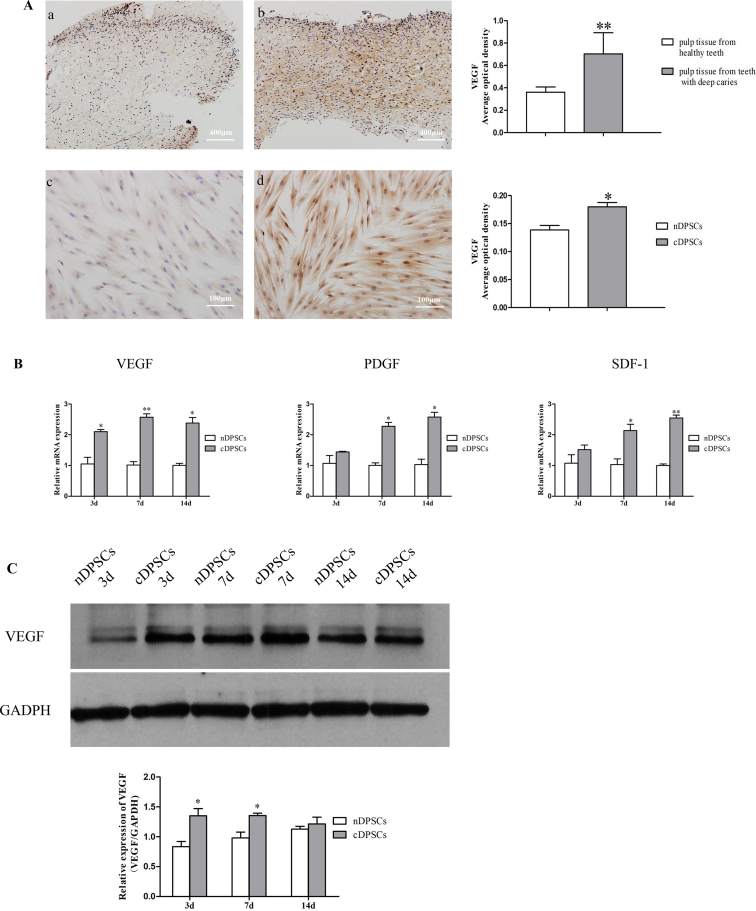
Angiogenic-related factors play an important role in the process of angiogenesis, especially VEGF. We examined the expression of VEGF in normal and deep carious pulps, as well as in nDPSCs and cDPSCs under basal medium by immunohistochemical staining. Results showed that the expression of VEGF was higher in deep carious pulp and cDPSCs than in normal samples (P < 0.05; Fig. 3A). Then, nDPSCs and cDPSCs were cultured in conditions that induced angiogenesis. We next assessed angiogenic-related gene expression by qRT-PCR on days 3, 7, and 14. PDGF and SDF-1 transcript levels were significantly different on days 7 and 14 (P < 0.05; Fig. 3B). VEGF expression significantly differed between cell types at all time points. Then we studied VEGF expression at the protein level by western blotting. Similar results were obtained using total protein preparations and western blotting (P < 0.05; Fig. 3C). The vascular development-related factors were more highly expressed in cDPSCs than in nDPSCs, suggesting that cDPSCs possessed increased angiogenesis potential.
Figure 3.
Comparison of the expression of angiogenic markers in nDPSCs and cDPSCs. (A–a) Healthy human dental pulp tissues were stained using a VEGF-specific antibody. (A-b) Dental pulp tissue derived from teeth with deep caries were also stained to show VEGF. (A-c) nDPSCs and (A-d) cDPSCs stained using a VEGF-specific antibody. The expression of VEGF was higher in pulp tissue from teeth with deep carious and cDPSCs. (B) The expression levels of angiogenesis markers (VEGF, PDGF, and SDF-1) were also found to be higher in cDPSCs compared to nDPSCs. (C) Expression levels of VEGF revealed by western blotting (∗P < 0.05,∗∗P < 0.01).
Analysis of vascular-like network formation
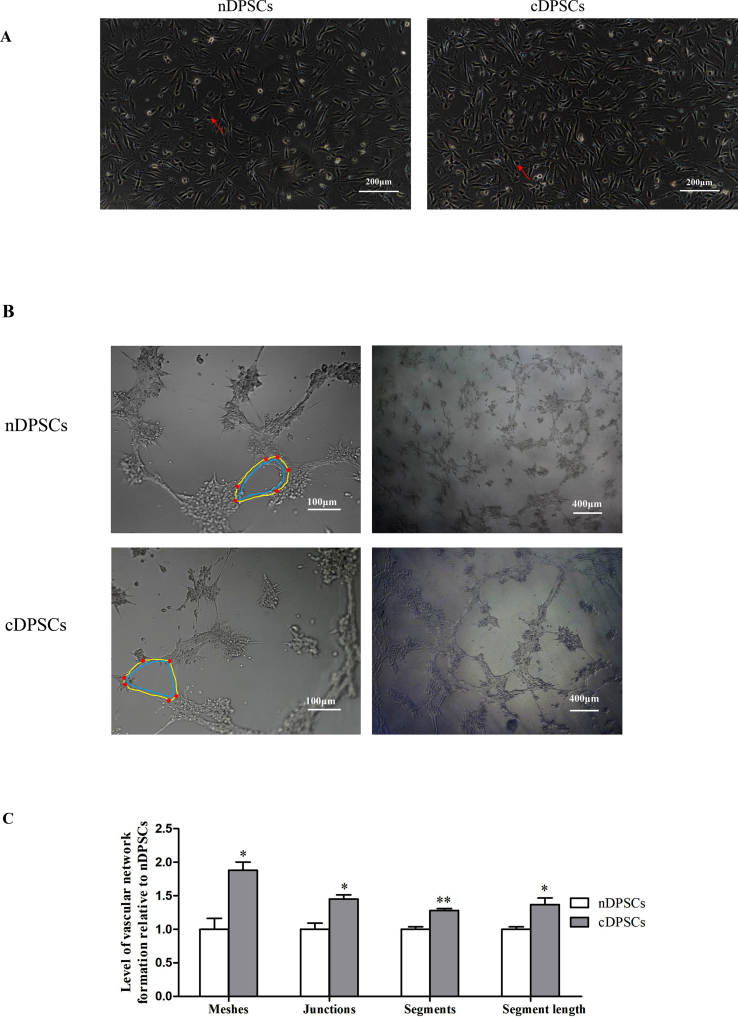
After the induction of angiogenesis, nDPSCs and cDPSCs showed slight morphological changes (Fig. 4A). Furthermore, nDPSCs and cDPSCs under angiogenic medium were able to form vascular-like networks on Matrigel. Both induced cell populations formed vascular-like networks at similar conditions (Fig. 4B). However, considering the number of meshes, junctions and segments, as well as the segment length between both cell populations, cDPSCs showed an enhanced in vitro angiogenesis potential compared to that of nDPSCs (P < 0.05; Fig. 4C).
Figure 4.
Angiogenesis capacities of nDPSCs and cDPSCs. (A) The morphologies of nDPSCs and cDPSCs under angiogenic medium. Some of them were more round and ovoid (arrow heads). (B) nDPSCs and cDPSCs under angiogenic medium were able to form vascular-like networks on Matrigel. Segments (yellow) were elements delimited by two junctions (red). Meshes (blue) were areas enclosed by segments. (C) Quantitative analysis of tubular structures at 4 h cDPSCs presented an enhanced angiogenesis capacity (∗P < 0.05,∗∗P < 0.01).
Discussion
As nDPSCs possess favorable proliferation and differentiation properties, they are regarded as ideal cells for pulp regeneration. With improved growing conditions and further research, investigators have isolated and cultivated stem cells from pulp tissue under an inflammatory microenvironment. These cells exhibited mesenchymal stem cell characteristics, but with different proliferation and differentiation properties when compared to nDPSCs.18, 19, 20, 21, 22 However, whether these stem cells can be used in tissue engineering remains unknown. In our study, we isolated and cultured nDPSCs and cDPSCs, then verified the stem cell characteristics of these cells by flow cytometry and multi-directional differentiation experiments. Results indicated that cDPSCs were mesenchymal stem cells as nDPSCs. Meanwhile, cDPSCs still showed an enhanced proliferation capacity, compared to that of nDPSCs, which was consistent with previous observations.12,19 Therefore, cDPSCs may have the potential to be used for pulp regeneration. Angiogenesis is the foundation of pulp regeneration, but this process is restricted by apical foramen. Thus, in this study, we further aimed to compare the angiogenesis ability of cDPSCs with that of nDPSCs.
Angiogenesis is a process that new blood vessels form from pre-existing vessels, and is regulated by multiple angiogenic growth factors.23,24 VEGF, the most potent and specific angiogenic factor, promotes vasodilatation, increases vascular permeability, and induces capillary formation.25,26 In this study, the expression of VEGF was higher in pulp tissue from teeth with deep caries than in normal tissue, as evidenced by immunohistochemical staining. This may indicate that pulp tissue of teeth with deep caries presented more active angiogenesis activity than healthy tissue did. Consistently, previous studies have reported that blood vessel density and the expression of VEGF were increased in the dental pulp of teeth with caries than in healthy tissue.27 In our study, the expression of VEGF was also higher in cDPSCs than in nDPSCs when they were cultured in vitro. Then we further investigated the angiogenesis characteristics of cDPSCs. Our qRT-PCR data showed significantly greater expression of angiogenesis-related factors, including VEGF, SDF-1, and PDGF, in cDPSCs than in nDPSCs. Among these factors, VEGF expression was the most significantly different. Accordingly, we assessed its expression at the protein level, which correlated with the trend of gene expression.
The expression of angiogenic factors was higher in cDPSCs. These angiogenic factors synergized to form a local microenvironment, and promote cell–cell chemoattraction and interaction, angiogenesis and maturation.28 When cariogenic bacteria invade the dentin and pulp, host cells recognize bacterial components via pathogen-associated molecular patterns, recruiting and activating immune cells, and thus, leading to increased expression of pro-inflammatory cytokines and chemokines.29,30 Exposure to an inflammatory environment leads to chronic hypoxia in the pulp.31 Hypoxia is the driving force of angiogenesis.32 Under hypoxic conditions, the paracrine angiogenic activity of stem cells was stimulated, resulting in higher expression of angiogenesis factors, such as VEGF, SDF-1, and PDGF.33, 34, 35 This may explain the stronger angiogenesis property of cDPSCs. Whether the this property will disappear with prolongation of culture time in vitro remains unknown. To better understand the environment of cDPSCs cultured in vitro, more inflammatory factors needed to de detected. The cross talk between inflammation and angiogenesis warrants further investigation.
Stem cells can not only promote angiogenesis through a paracrine fashion, but can also differentiate themselves into endothelial-like cells.5, 6, 7, 8, 9 When nDPSCs and cDPSCs were cultured under angiogenic medium, their morphologies slightly changed. And they behaved like endothelial cells which form vascular networks on Matrigel. Quantitative analysis showed that cDPSCs had an enhanced vascular-like network formation capability than nDPSCs. Whether nDPSCs and cDPSCs can differentiate into endothelial cells needed more experimental validation, including phenotypic detection.
In conclusion, the present study showed that cDPSCs and nDPSCs possessed a phenotype similar to mesenchymal stem cells. However, cDPSCs owned enhanced proliferation and angiogenesis capacities. Thus, cDPSCs, which are often discarded, have potential implications in pulp regeneration.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (grant no. 81870755, grant no. 81670986 and grant no. 81700956).
Contributor Information
Wenan Xu, Email: venus_200@163.com.
Buling Wu, Email: wubuling623@163.com.
References
- 1.Hargreaves K.M., Diogenes A., Teixeira F.B. Treatment options: biological basis of regenerative endodontic procedures. J Endod. 2013;39:S30–S43. doi: 10.1016/j.joen.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moule A.J., Kahler B. Diagnosis and management of teeth with vertical root fractures. Aust Dent J. 1999;44:75–87. doi: 10.1111/j.1834-7819.1999.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 3.Nixdorf D.R., Moana-Filho E.J., Law A.S., McGuire L.A., Hodges J.S., John M.T. Frequency of nonodontogenic pain after endodontic therapy: a systematic review and meta-analysis. J Endod. 2010;36:1494–1498. doi: 10.1016/j.joen.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dissanayaka W.L., Zhang C. The role of vasculature engineering in dental pulp regeneration. J Endod. 2017;43:S102–S106. doi: 10.1016/j.joen.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Hilkens P., Fanton Y., Martens W. Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res. 2014;12:778–790. doi: 10.1016/j.scr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Merckx G., Hosseinkhani B., Kuypers S. Angiogenic effects of human dental pulp and bone marrow-derived mesenchymal stromal cells and their extracellular vesicles. Cells. 2020;9:1–22. doi: 10.3390/cells9020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L., Dissanayaka W.L., Zhang C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin Oral Invest. 2019;23:2497–2509. doi: 10.1007/s00784-018-2699-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J., Sun C. SENP1/HIF-1α axis works in angiogenesis of human dental pulp stem cells. J Biochem Mol Toxicol. 2020 doi: 10.1002/jbt.22436. [DOI] [PubMed] [Google Scholar]
- 9.Aksel H., Huang G.T. Human and swine dental pulp stem cells form a vascularlike network after angiogenic differentiation in comparison with endothelial cells: a quantitative analysis. J Endod. 2017;43:588–595. doi: 10.1016/j.joen.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakashima M., Iohara K., Murakami M. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 2017;8:1–13. doi: 10.1186/s13287-017-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xuan K., Li B. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. 2018;10:1–13. doi: 10.1126/scitranslmed.aaf3227. [DOI] [PubMed] [Google Scholar]
- 12.Ma D., Gao J., Yue J., Yan W., Fang F., Wu B. Changes in proliferation and osteogenic differentiation of stem cells from deep caries in vitro. J Endod. 2012;38:796–802. doi: 10.1016/j.joen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Farges J.C., Keller J.F., Carrouel F. Odontoblasts in the dental pulp immune response. J Exp Zool B Mol Dev Evol. 2009;312b:425–436. doi: 10.1002/jez.b.21259. [DOI] [PubMed] [Google Scholar]
- 14.Cooper P.R., Chicca I.J., Holder M.J., Milward M.R. Inflammation and regeneration in the dentin-pulp complex: net gain or net loss? J Endod. 2017;43:S87–S94. doi: 10.1016/j.joen.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 15.McLachlan J.L., Sloan A.J., Smith A.J., Landini G., Cooper P.R. S100 and cytokine expression in caries. Infect Immun. 2004;72:4102–4108. doi: 10.1128/IAI.72.7.4102-4108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper P.R., Holder M.J., Smith A.J. Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. J Endod. 2014;40:S46–S51. doi: 10.1016/j.joen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Li X., Hou J., Wu B., Chen T., Luo A. Effects of platelet-rich plasma and cell coculture on angiogenesis in human dental pulp stem cells and endothelial progenitor cells. J Endod. 2014;40:1810–1814. doi: 10.1016/j.joen.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Sun H.H., Chen B., Zhu Q.L. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials. 2014;35:9459–9472. doi: 10.1016/j.biomaterials.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Alkharobi H., Beattie J., Meade J., Devine D., El-Gendy R. Dental pulp cells isolated from teeth with superficial caries retain an inflammatory phenotype and display an enhanced matrix mineralization potential. Front Physiol. 2017;8:1–11. doi: 10.3389/fphys.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomasello L., Mauceri R., Coppola A. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: a potential application for bone formation. Stem Cell Res Ther. 2017;8:1–15. doi: 10.1186/s13287-017-0633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S., Zhang Q.Z., Karabucak B., Le A.D. DPSCs from inflamed pulp modulate macrophage function via the TNF-alpha/Ido Axis. J Dent Res. 2016;95:1274–1281. doi: 10.1177/0022034516657817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alongi D.J., Yamaza T., Song Y. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010;5:617–631. doi: 10.2217/rme.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semenza G.L. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 24.Rombouts C., Giraud T., Jeanneau C., About I. Pulp vascularization during tooth development, regeneration, and therapy. J Dent Res. 2017;96:137–144. doi: 10.1177/0022034516671688. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 27.Soden R.I., Botero T.M., Hanks C.T., Nor J.E. Angiogenic signaling triggered by cariogenic bacteria in pulp cells. J Dent Res. 2009;88:835–840. doi: 10.1177/0022034509341946. [DOI] [PubMed] [Google Scholar]
- 28.Gharaei M.A., Xue Y., Mustafa K., Lie S.A., Fristad I. Human dental pulp stromal cell conditioned medium alters endothelial cell behavior. 2018;9:1–12. doi: 10.1186/s13287-018-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang J.H., Shin H.W., Lee J.M., Lee H.W., Kim E.C., Park S.H. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediat Inflamm. 2015;2015:794143. doi: 10.1155/2015/794143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bindal P., Ramasamy T.S., Kasim N.H.A., Gnanasegaran N., Chai W.L. Immune responses of human dental pulp stem cells in lipopolysaccharide-induced microenvironment. Cell Biol Int. 2018;42:832–840. doi: 10.1002/cbin.10938. [DOI] [PubMed] [Google Scholar]
- 31.Janjic K., Samiei M., Moritz A., Agis H. The influence of pro-inflammatory factors on sclerostin and dickkopf-1 production in human dental pulp cells under hypoxic conditions. Front Bioeng Biotechnol. 2019;7:1–7. doi: 10.3389/fbioe.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bronckaers A., Hilkens P., Fanton Y. Angiogenic properties of human dental pulp stem cells. PloS One. 2013;8 doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez K.H., Garcia N.A., Ontoria O.I., Ciria M., Montero J.A., Sepulveda P. Hypoxia inducible factor-1alpha potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. 2017;35:1747–1759. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 34.Bakopoulou A., Kritis A., Andreadis D. Angiogenic potential and secretome of human apical papilla mesenchymal stem cells in various stress microenvironments. Stem Cell Dev. 2015;24:2496–2512. doi: 10.1089/scd.2015.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan C., Wang P., Zhu L. Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Eng. 2015;21:1163–1172. doi: 10.1089/ten.tea.2014.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]