Abstract
Background/purpose
Macrophage migration inhibitory factor (MIF) is a multifunctional cytokine that contributes to the progression of several cancers. MIF overexpression has been reported in head and neck squamous cell carcinoma (HNSCC) patients. However, the exact role of MIF in HNSCC is not fully understood. Our aim was to evaluate the amount of secreted MIF and the role of MIF in the proliferation, cell cycle, and apoptosis in HNSCC cell lines.
Materials and methods
Genetically matched HNSCC cell lines derived from primary (HN18 and HN30) and metastatic sites (HN17 and HN31) from the same patient were used in this study. The MIF levels in conditioned media from the HNSCC cell lines were evaluated using ELISA. The HNSCC cell lines were treated with recombinant MIF at concentrations 25, 50 and 100 ng/ml, and cell proliferation was evaluated by MTT assay. A proliferative dose of MIF was used to treat the cells then, cell cycle, and apoptotic status were determined by flow cytometry.
Results
The HNSCC-secreted MIF concentration ranged from 49.33 to 973 pg/ml. Exogenous MIF (25 ng/ml) significantly increased HN18, HN30, and HN31 cell proliferation. Moreover, MIF induced cell cycle progression and inhibited apoptosis in these cells. However, MIF did not affect growth or apoptosis in HN17 cell.
Conclusion
MIF secreted from the HNSCC cell lines were evaluated. Exogenous MIF promotes various effects on proliferation, cell cycle, and apoptosis in HNSCC cells.
Keywords: Apoptosis, Cell cycle, HNSCC cell Lines, Macrophage migration inhibitory factor, Proliferation
Introduction
Head and neck squamous cell carcinoma (HNSCC) initiates from the squamous cells lining the mucosal epithelium of the upper aerodigestive tract, including the oral cavity, pharynx, larynx, and sinonasal tract.1 HNSCC is the sixth most common malignancy with an incidence of over 500,000 new cases per year worldwide. Although the understanding of HNSCC mechanisms has improved in the past few decades, current therapies are not always successful.2 Therefore, research interest has focused on discovering new prognostic markers and targeted therapy adapted to the biomolecular profile of the patients with tumors.3
Macrophage migration inhibitory factor (MIF) was originally identified as a molecule secreted from activated T lymphocytes that inhibited the random migration of macrophages.4 MIF is constitutively expressed in a variety of cells, such as eosinophils, neutrophils, monocytes, macrophages, B and T lymphocytes, endothelial, epithelial, and neuronal cells.5 MIF regulates both physiological and pathological conditions. MIF is a multifunctional molecule that promotes cell proliferation, inhibits apoptosis, regulates cell differentiation, and induces inflammation. However, some of MIF's functions can result in an oncogenic microenvironment that promotes cancer development.6 MIF is overexpressed in several types of human cancer tissues, including breast, prostate, liver, lung, colon, bladder, ovarian, pancreas, and lymphocytic leukemia B cells.7 The production of MIF is secreted by cancer cells and activated in autocrine mode, resulting in increased cytokine, chemokine, and angiogenic factor production that leads to tumor growth and metastasis.8, 9, 10 MIF induces most of the hallmark cancer activities. In melanoma, bladder cancer, and esophageal squamous cell carcinoma, MIF has a proliferative effect by stimulating cell cycle progression.11, 12, 13 Moreover, MIF has been shown to enhance cancer invasion and metastasis in nasopharyngeal carcinoma, breast cancer, and osteosarcoma.9,14,15
Similar to other cancers, MIF was overexpressed mainly in the tumor cells and significantly increased in the serum and saliva from HNSCC patients.16, 17, 18 MIF overexpression was significantly correlated with a poor prognosis. In addition, MIF-induced neutrophils promoted oral cancer cell migration.19 Compared with other cancers, investigations into the effect of MIF in the HNSCC microenvironment is limited and its role remains unclear. Based on the evidence concerning the effect of MIF in various cancers, we hypothesized that MIF could promote the proliferation of HNSCC cells by inducing cell cycle progression and inhibiting apoptosis. The aim of this study was to assess if MIF is secreted by primary and metastatic HNSCC cell lines. Moreover, the effect of MIF on proliferation, cell cycle status, and apoptosis were evaluated in these HNSCC cell lines.
Materials and methods
Cell culture
Genetically matched HNSCC cell lines derived from primary and metastatic lesions at different clinical stages from the same patient were used in this study.20 HN18 and HN17 cells were obtained from primary tongue lesions and neck dissections (T2N2M0), respectively. HN30 and HN31 cells were obtained from primary pharynx lesions and lymph node metastases (T3N0M0), respectively. The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen, Grand Island, NY, USA) supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen), and an antimycotic. The cells were cultured in a 37ᵒC and 5% CO2 incubator. The cells were passaged using 0.25% trypsin-EDTA when 90–100% confluent. Only cultures with at least 95% cell viability were used in the experiments.
Determination of secreted MIF amount by ELISA
Conditioned media from the HNSCC cells were collected and stored at −80 °C until analyzed. The MIF concentration was measured using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
MTT assay
HNSCC cell lines were seeded (5000 cells/well) in 96-well plates and incubated in a 37ᵒC and 5% CO2 incubator. The cells were serum starved in serum-free DMEM for 24 h and treated with 25, 50, or 100 ng/ml human recombinant MIF (PeproTech, Hamburg, Germany). Cells in serum-free DMEM served as control. After 24 h incubation, the amount of viable cells in each treatment group was determined using the thiazolyl blue tetrazolium bromide (MTT, Sigma, St. Louis, MA, USA) assay. The medium was removed, 150 μL fresh medium was added, followed by adding 2 mg/ml MTT solution (50 μL/well). The plates were incubated for 4 h in a 37 °C and 5% CO2 incubator. The precipitated formazan crystals were solubilized in DMSO (200 μL/well). The absorbance (Abs) of the resulting solution was measured at 570 nm by a microplate reader (Tecan Trading, Salzburg, Austria) and converted to percent cell proliferation compared with that of the control. Cell proliferation (%) was determined as follows: cell proliferation (%) = (mean Abs570treated cells - mean Abs570blank)/(mean Abs570control cells - mean Abs570blank) × 100. Three independent experiments were performed.
Cell cycle analysis by flow cytometry
The cells (5 × 105/well in 6-well plates) were synchronized at G1 phase of the cell cycle by culturing them in serum-free DMEM for 24 h. The G1 phase-arrested cells were treated with 25 ng/ml recombinant MIF for 24 h. The cells were harvested, centrifuged at 200×g for 5 min, and washed twice with cold phosphate-buffered saline (PBS). The cell pellets were fixed in 70% ethanol and incubated for 15 min at −20 °C. The cells were then washed twice with cold PBS, centrifuged at 200×g for 5 min and RNA was eliminated by incubating with 10 μg/μl DNase-free RNaseA (USB Corporation, Cleveland, OH, USA) at 37 °C for 30 min. After washing twice with PBS and centrifuging at 200×g for 5 min, the cells were incubated for 15 min at 4 °C in the dark with 1 mg/ml propidium iodide (Sigma). The distribution of the cells in each stage of the cell cycle was quantified in a flow cytometer and CyteExpert software (Cytoflex®, Beckman Coulter, Indianapolis, IN, USA). The percentage of the HNSCC cell lines in the S phase of the cell cycle was measured and compared between conditions with and without recombinant MIF treatment. Three independent experiments were performed.
Apoptosis assessment by flow cytometry
The HNSCC cells were treated with 25 ng/ml recombinant MIF for 24 h. The cells were harvested, centrifuged at 200×g for 5 min, and washed twice with cold PBS. The apoptotic cells were assessed using Annexin A5-FITC/7-AAD kits (Beckman Coulter) according to the manufacturer's instructions. The number of apoptotic HNSCC cells was quantified in a flow cytometer using CyteExpert software (Cytoflex®, Beckman Coulter). The percentage of apoptotic cells were measured and compared between conditions with and without recombinant MIF treatment. Three independent experiments were performed.
Statistical analysis
The results are presented as means and standard error of the mean (SEM). The difference between the means of more than two groups was analyzed using one-way ANOVA followed by the Dunnett's multiple comparisons test. The unpaired t-test was used to compare means between two groups. The statistical analysis was performed with Prism GraphPad 7.0 (GraphPad Software, La Jolla, CA, USA). The significance level was set at 0.05.
Results
Endogenous MIF secreted from the HNSCC cell lines
The HNSCC cell lines used in this study all secreted MIF, ranging from 49.33 to 973 pg/ml (Table 1). The HN18 and HN30 cells secreted MIF at 49.33 and 860 pg/ml, respectively. Moreover, the HN17 and HN31 lines secreted MIF up to 973 and 950.2 pg/ml, respectively.
Table 1.
HNSCC cell-secreted MIF.
| HNSCC cell lines | Secreted MIF (pg/ml) |
|---|---|
| HN18 | 49.33 ± 27.9 |
| HN17 | 973.0 ± 26 |
| HN30 | 860.0 ± 204.4 |
| HN31 | 950.2 ± 70.4 |
The concentration of macrophage migration inhibitory factor (MIF) is shown as mean ± SEM.
Proliferative effect of exogenous MIF on the HNSCC cell lines
Recombinant MIF was used to evaluate the proliferative effect of exogenous MIF in the tumor microenvironment on the HNSCC cells. The HNSCC cell lines were treated with 25, 50, or 100 ng/ml recombinant MIF. The results indicated that 25 and 50 ng/ml MIF significantly dose-dependently increased HN18, HN30, and HN31 cell proliferation, compared with control (P < 0.05) (Fig. 1A, C, and D). When the MIF concentration was increased to 100 ng/ml, the proliferation of these cell lines declined to baseline. However, when treated with exogenous MIF at all tested concentrations, there was no significant change in proliferation in the HN17 cell line (Fig. 1B). Based on these results, 25 ng/ml MIF was used in the subsequent experiments.
Figure 1.
Proliferative effect of MIF on the HNSCC cell lines. MTT assay was performed to evaluate the proliferative effect of recombinant MIF on (A) HN18, (B) HN17, (C) HN30, and (D) HN31 cells. Bars represent mean ± SEM percentage cell proliferation (n = 3). ∗P < 0.05 compared with the control.
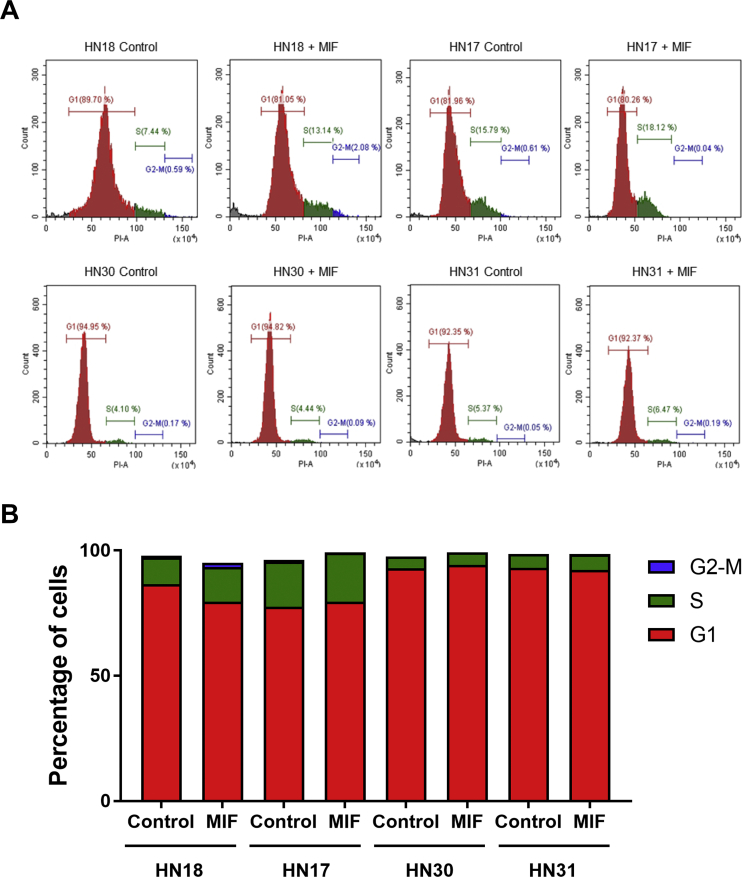
Effect of exogenous MIF on cell cycle status in the HNSCC cell lines
Recombinant MIF (25 ng/ml) was used to determine the cell cycle status in the HNSCC cell lines. The histograms demonstrated the DNA distribution in the cell cycle of the HNSCC cell lines compared between control and MIF treatment (Fig. 2A). When treated with MIF, the DNA content in the S phase of the cell cycle in HN18 cells increased (Fig. 2B). In contrast, the DNA content in the S phase of the HN17, HN30 and HN31 cells were not changed after MIF exposure.
Figure 2.
Effect of MIF on cell cycle in the HNSCC cell lines. (A) The cell cycle distribution was determined after MIF treatment compared with control. (B) The percentage of cells in each phase of the cell cycle was calculated in the MIF-treated condition compared with control.
Effect of exogenous MIF on apoptosis in the HNSCC cell lines
The cell death status of the HNSCC cells treated with 25 ng/ml recombinant MIF and control were analyzed (Fig. 3A). The apoptotic fold-change of the MIF-treated HN18, HN30, and HN31 cells were significantly decreased compared with the control (P < 0.05) (Fig. 3B). However, MIF demonstrated no effect on the apoptotic status in the HN17 cells.
Figure 3.
Effect of MIF on apoptosis in the HNSCC cell lines. (A) Apoptotic status of the MIF-treated cells compared with control. (B) The apoptotic fold-change in the MIF-treated cells compared with control. Bars represent mean ± SEM apoptotic fold change (n = 3). ∗P < 0.05 compared with the control.
Discussion
Cancer has a unique microenvironment composed of cancer cells, tumor-associated cells, growth factors, matrix metalloproteinases, and inflammatory cytokines.21 In the HNSCC microenvironment, modulating the inflammatory response is an important factor that contributes to tumor progression.22 MIF is a potent inflammatory cytokine that is associated with poor prognosis in head and neck cancer patients.23 Previous studies reported that HNSCC cells are the major source of MIF secreted in the tumor microenvironment.24 In the present study, we evaluated the amount of endogenous MIF secreted by both primary and metastatic HNSCC cell lines. The HNSCC-secreted MIF ranged from 49.33 to 973.0 pg/ml. The higher concentrations were found in the metastatic cell lines. The MIF level in the serum of HNSCC patients ranged from 433 pg/ml‒21.3 ng/ml.17 However, another study reported that the serum MIF in oral squamous cell carcinoma patients was approximately 30–50 ng/ml.18 Our findings are in line with the MIF concentrations found in clinical studies. Although several types of cancer cells have been proposed as the major source of secreted MIF, in vitro, effects were found when using exogenous MIF at a higher concentration than that of the autocrine MIF. The effective doses of MIF that induced proliferation of bladder and gastric cancers in vitro were 25–100 ng/ml.11,25 The present study found that 25 and 50 ng/ml recombinant MIF significantly induced HN18, HN30 and HN31 cell proliferation. Feedback inhibition effects were observed in these cell lines when the MIF concentration was increased to 100 ng/ml. Moreover, 25 ng/ml recombinant MIF promoted cell cycle progression and inhibited apoptosis in the cell lines. The MIF concentration may influence tumorigenic responses in different tumors.
The mechanism of MIF has been proposed to occur by stimulating the CXCR4 (CD74) receptor to sustain cell survival and promote cell cycle progression and angiogenesis in several cancers.24 Overexpression of CXCR4 on HNSCC cells has been found in tissues and cell lines, including HN30 and HN31 cells.26,27 The tumorigenic activities of MIF found in the present study may be associated with the CXCR4 signaling pathway. Although the HN17 cell endogenously secreted a high concentration of MIF, exogenous MIF showed no effect on proliferation, cell cycle, or apoptosis in this cell line. It should be noted that there is a variety of HNSCC cells that respond to MIF stimulation. Our findings initiate the understanding of how MIF functions in the HNSCC hallmark activities, such as proliferation, cell cycle progression, and apoptosis inhibition. However, further studies are needed to determine the exact mechanism of MIF in HNSCC progression.
MIF is a pro-tumorigenic cytokine that is mainly secreted by cancer cells. Our study confirmed that the evaluated HNSCC cells all secreted MIF as high as in the reported cancers. MIF promoted proliferation and the cell cycle, and inhibited apoptosis in the HN18, HN30, and HN31 cells. However, MIF was unable to stimulate HN17 cell proliferation as found in the other cell lines used in this study. In addition to the overexpression of MIF in clinical studies, the present study highlights the variety of MIF effects on growth and apoptosis among HNSCC cell lines. To confirm the MIF mechanism, further studies are required to dissect the MIF downstream signaling pathways in HNSCC cells.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This research was funded by Faculty of Dentistry, Thammasat University (Grant no. 2560). The authors thank Professor Silvio Gutkind (Department of Pharmacology, University of California San Diego, USA) for providing the HNSCC cell lines. We thank Dr. Kanyarat Krajangmontree and Dr. Akarapong Boontankun for technical assistance.
References
- 1.Argiris A., Karamouzis M.V., Raben D., Ferris R.L. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsahafi E., Begg K., Amelio I. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto V.N., Thylur D.S., Bauschard M., Schmale I., Sinha U.K. Overcoming radioresistance in head and neck squamous cell carcinoma. Oral Oncol. 2016;63:44–51. doi: 10.1016/j.oraloncology.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bloom B.R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 5.Lolis E., Bucala R. Macrophage migration inhibitory factor. Expert Opin Ther Targets. 2003;7:153–164. doi: 10.1517/14728222.7.2.153. [DOI] [PubMed] [Google Scholar]
- 6.Gordon-Weeks A.N., Lim S.Y., Yuzhalin A.E., Jones K., Muschel R. Macrophage migration inhibitory factor: a key cytokine and therapeutic target in colon cancer. Cytokine Growth Factor Rev. 2015;26:451–461. doi: 10.1016/j.cytogfr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Nobre C.C., de Araujo J.M., Fernandes T.A. Macrophage migration inhibitory factor (MIF): biological activities and relation with cancer. Pathol Oncol Res. 2017;23:235–244. doi: 10.1007/s12253-016-0138-6. [DOI] [PubMed] [Google Scholar]
- 8.Hagemann T., Robinson S.C., Thompson R.G., Charles K., Kulbe H., Balkwill F.R. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Canc Therapeut. 2007;6:1993–2002. doi: 10.1158/1535-7163.MCT-07-0118. [DOI] [PubMed] [Google Scholar]
- 9.Pei X.J., Wu T.T., Li B., Tian X.Y., Li Z., Yang Q.X. Increased expression of macrophage migration inhibitory factor and DJ-1 contribute to cell invasion and metastasis of nasopharyngeal carcinoma. Int J Med Sci. 2014;11:106–115. doi: 10.7150/ijms.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Wang R., Huang A. Upregulation of macrophage migration inhibitory factor promotes tumor metastasis and correlates with poor prognosis of pancreatic ductal adenocarcinoma. Oncol Rep. 2018;40:2628–2636. doi: 10.3892/or.2018.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhary S., Hegde P., Pruitt J.R. Macrophage migratory inhibitory factor promotes bladder cancer progression via increasing proliferation and angiogenesis. Carcinogenesis. 2013;34:2891–2899. doi: 10.1093/carcin/bgt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu R.M., Sun D.N., Jiao Y.L. Macrophage migration inhibitory factor promotes tumor aggressiveness of esophageal squamous cell carcinoma via activation of Akt and inactivation of GSK3beta. Canc Lett. 2018;412:289–296. doi: 10.1016/j.canlet.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira C.S., de Bock C.E., Molloy T.J. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC Canc. 2014;14:630. doi: 10.1186/1471-2407-14-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv W., Chen N., Lin Y. Macrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/NF kappa B axis. Canc Lett. 2016;375:245–255. doi: 10.1016/j.canlet.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang C., Zhou X., Li W. Macrophage migration inhibitory factor promotes osteosarcoma growth and lung metastasis through activating the RAS/MAPK pathway. Canc Lett. 2017;403:271–279. doi: 10.1016/j.canlet.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Franca C.M., Batista A.C., Borra R.C. Macrophage migration inhibitory factor and oral cancer. J Oral Pathol Med. 2013;42:368–373. doi: 10.1111/jop.12011. [DOI] [PubMed] [Google Scholar]
- 17.Kindt N., Preillon J., Kaltner H. Macrophage migration inhibitory factor in head and neck squamous cell carcinoma: clinical and experimental studies. J Canc Res Clin Oncol. 2013;139:727–737. doi: 10.1007/s00432-013-1375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MB D.E.S., Curioni O.A., Kanda J.L., MB D.E.C. Serum and salivary macrophage migration inhibitory factor in patients with oral squamous cell carcinoma. Oncol Lett. 2014;8:2267–2275. doi: 10.3892/ol.2014.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumitru C.A., Gholaman H., Trellakis S. Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. Int J Canc. 2011;129:859–869. doi: 10.1002/ijc.25991. [DOI] [PubMed] [Google Scholar]
- 20.Cardinali M., Pietraszkiewicz H., Ensley J.F., Robbins K.C. Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int J Canc. 1995;61:98–103. doi: 10.1002/ijc.2910610117. [DOI] [PubMed] [Google Scholar]
- 21.Utispan K., Koontongkaew S. Fibroblasts and macrophages: key players in the head and neck cancer microenvironment. J Oral Biosci. 2017;59:23–30. [Google Scholar]
- 22.Feller L., Altini M., Lemmer J. Inflammation in the context of oral cancer. Oral Oncol. 2013;49:887–892. doi: 10.1016/j.oraloncology.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Wang S.S., Cen X., Liang X.H., Tang Y.L. Macrophage migration inhibitory factor: a potential driver and biomarker for head and neck squamous cell carcinoma. Oncotarget. 2017;8:10650–10661. doi: 10.18632/oncotarget.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechien J.R., Nassri A., Kindt N., Brown D.N., Journe F., Saussez S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: a systematic review. Head Neck. 2017;39:2573–2584. doi: 10.1002/hed.24939. [DOI] [PubMed] [Google Scholar]
- 25.Li G.Q., Xie J., Lei X.Y., Zhang L. Macrophage migration inhibitory factor regulates proliferation of gastric cancer cells via the PI3K/Akt pathway. World J Gastroenterol. 2009;15:5541–5548. doi: 10.3748/wjg.15.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kindt N., Lechien J.R., Nonclercq D., Laurent G., Saussez S. Involvement of CD74 in head and neck squamous cell carcinomas. J Canc Res Clin Oncol. 2014;140:937–947. doi: 10.1007/s00432-014-1648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koontongkaew S., Amornphimoltham P., Monthanpisut P., Saensuk T., Leelakriangsak M. Fibroblasts and extracellular matrix differently modulate MMP activation by primary and metastatic head and neck cancer cells. Med Oncol. 2012;29:690–703. doi: 10.1007/s12032-011-9871-6. [DOI] [PubMed] [Google Scholar]