Abstract
Hand–foot syndrome (HFS) is a skin toxicity that occurs in areas of compressed skin. HFS manifests mainly in insensitive palms and the soles of the feet or in erythematous areas on the extremities caused by chemotherapy, which may be related to the dosage. This paper reports a case of HFS caused by liposomal doxorubicin. A 64-year-old Asian woman presented with severe erythema, ulceration, pruritus, and edema-related pain in her back, hands, and feet after receiving four cycles of liposomal doxorubicin. Clinicians and a pharmacist analyzed and evaluated the patient’s adverse reactions. After symptomatic treatment and patient education, her HFS symptoms were significantly relieved. The purpose of this study was to raise clinical awareness regarding adverse events following liposomal doxorubicin injection, and to provide new ideas for the clinical treatment of these adverse events.
Keywords: Liposomal doxorubicin, hand–foot syndrome, prevention and treatment strategy, palmar–plantar erythrodysesthesia, adverse drug event, erythema
Introduction
Hand–foot syndrome (HFS), also known as palmar–plantar erythrodysesthesia (PPE), is most often an adverse skin reaction caused by antineoplastic drugs. The first symptoms are pruritus and hyperemia of the palms and the plantar skin, pain in the fingertips and toes, skin redness and swelling, and numbness. Some patients may have blisters, desquamation, and exudation, and in other patients, the drug administration interval must be extended for 1 to 2 weeks, and/or the chemotherapy dose must be reduced or treatment must be interrupted. Seriously-affected patients may even lose their ability to perform self-care. The liposomal doxorubicin that our patient received is encapsulated in liposomes, with methoxypolyethylene glycol on the surface. The incidence of HFS caused by liposomal doxorubicin is relatively low, but the symptoms are obvious and easily cause patients physiological and psychological discomfort.1,2 The purpose of this paper was to raise clinical awareness of the adverse events related to liposomal doxorubicin injection, and to provide new ideas for the clinical treatment of these adverse events.
Case Report
A 64-year-old Asian woman had ovarian cancer for more than 2 years and a history of cephalosporin allergy and rheumatoid arthritis for 10 years. Owing to disease progression, the patient began a regimen of liposomal doxorubicin (40 mg, qd, intravenous (IV) drip infusion) + carboplatin (370 mg, qd, IV drip infusion) on 9 January 2020. The second, third, and fourth chemotherapy treatments were given on 3 February, 24 February, and 16 March 2020, respectively. On 30 March 2020, large areas of erythema, ulceration, and exudation appeared on her back, hands, and feet (Figure 1 and Figure 2). On 13 April and 4 May 2020, the patient received single chemotherapy with liposomal doxorubicin (40 mg, qd, IV drip infusion), but the erythema did not improve. The clinicians then consulted a dermatologist who considered that the lesions represented Tinea corporis infection. Calamine lotion and terbinafine cream were prescribed as treatments, but the patient reported no improvement after 1 week of therapy. At this point, the clinicians and a pharmacist considered an association between the chemotherapy drugs and the adverse events, caused mainly by the liposomal doxorubicin. According to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0,3 the patient’s adverse reaction was diagnosed as grade 3; HFS caused by liposomal doxorubicin.
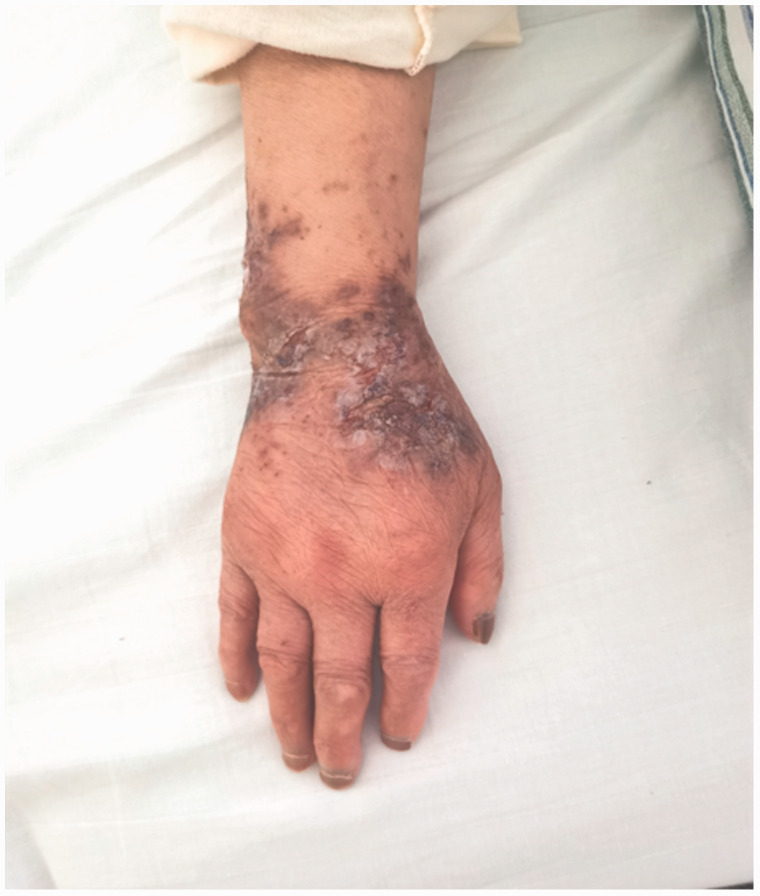
Figure 1.
Photograph showing the acute stage of erythema on the patient’s right hand.
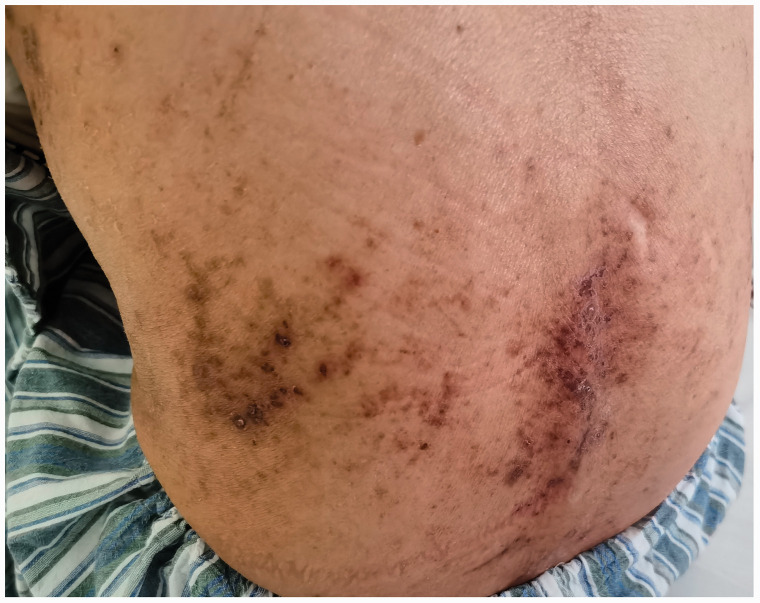
Figure 2.
Photograph showing the acute stage of erythema on the patient's back.
Currently, there are no standard guidelines for the prevention and treatment of HFS caused by liposomal doxorubicin. Corticosteroids and nonsteroidal antiinflammatory drugs are generally accepted drugs that can decrease the inflammatory response. According to the manufacturer’s drug insert for liposomal doxorubicin, some patients suffering liposomal doxorubicin-related PPE require oral dexamethasone or topical corticosteroid. Urea cream, hydrocortisone butyrate cream (us. ext., tid) and celecoxib capsules (p.o., 100 mg, bid) were prescribed for the patient. After 14 days of treatment, the erythema on her hands and feet had obviously disappeared, and all blisters had dried and crusted. Pruritus and edema-associated pain were also relieved (Figure 3 and Figure 4). After her symptoms improved, chemotherapy was restarted without liposomal doxorubicin. The chemotherapy regimen was changed to docetaxel and bevacizumab injection, and no significant adverse events occurred with this therapy. The patient was discharged from the hospital and took oral vitamin B6 as an outpatient. She was instructed to avoid local and systemic irritation and to perform the following: 1. face washing with a soft cloth; avoiding chemicals, rough fabrics, and chemical detergents (e.g., laundry detergent); hand washing without hot water; keeping the skin of her hands and feet moisturized; and using alcohol-free emollients (e.g., urea ointment); 2. avoiding friction and pressure on her hands and feet and wearing appropriately fitted shoes; 3. avoiding hot and spicy foods; and 4. avoiding sun exposure to the skin. If possible, she was instructed to apply fresh aloe, if available, to her hands and feet 3 to 4 times per day. One month after discharge, we re-evaluated the patient, and the erythema on her back, hands, and feet had resolved well.
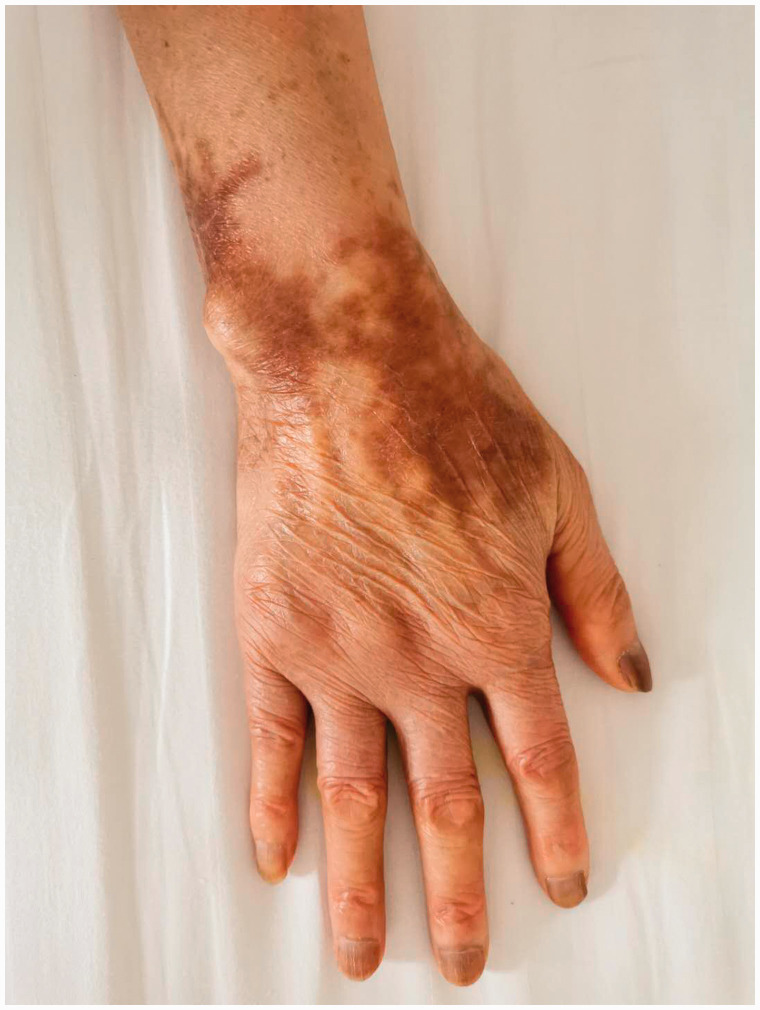
Figure 3.
Photograph showing the resolving erythema on the patient's right hand.
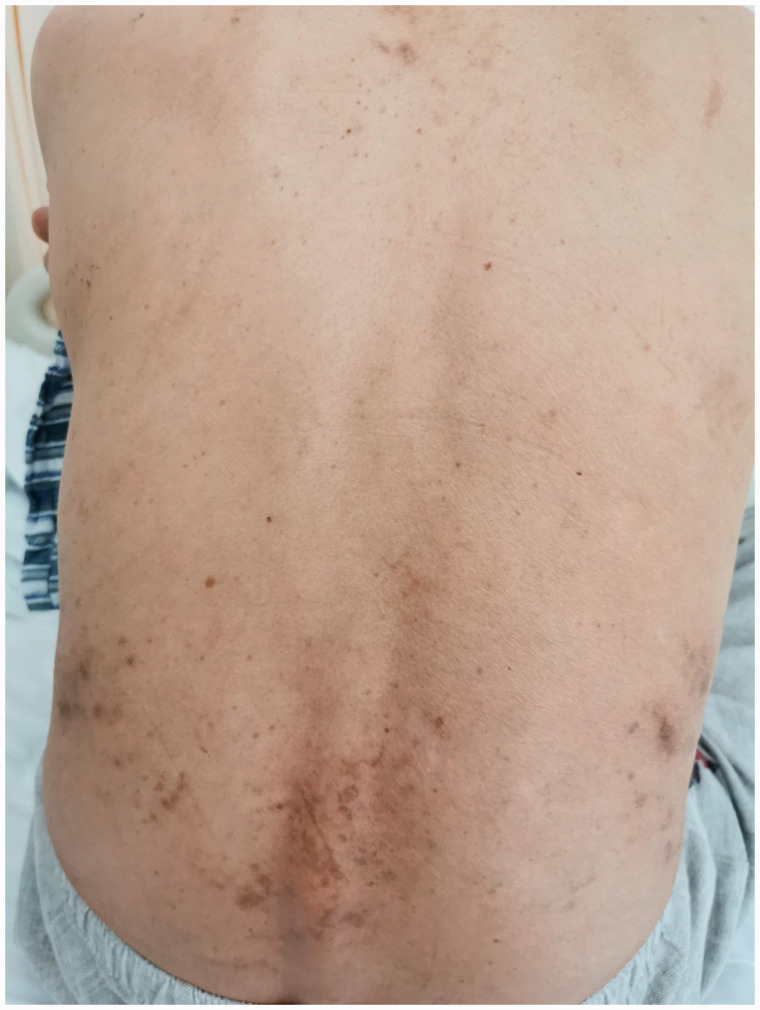
Figure 4.
Photograph showing the resolving erythema on the patient’s back.
Discussion
PPE is a type of HFS. PPE is a less common adverse reaction (incidence <5%) to liposomal doxorubicin, and presents as painful erythema. The common accompanying symptoms of PPE are oral candidiasis, nausea and vomiting, weight loss, skin rash, oral ulcers, dyspnea, abdominal pain, vasodilation, dizziness, anorexia, constipation, retinitis, and consciousness disturbance. Generally, this reactions appears after ≥6 weeks of drug therapy. In most patients, the symptoms resolve from 1 to 2 weeks after drug withdrawal.
The main factors associated with HFS are gender (higher incidence rate in women), drug dosage, some malignant tumors, and genetic polymorphism. With grade 1 HFS, medication dose reduction or stopping the medication is often not required. With ≥grade 2 HFS, treatment must stop, symptoms must be actively treated, and physicians must consider whether to reduce the medication dose.4 The patient in this article was an Asian woman who suffered grade 3 HFS caused by liposomal doxorubicin. After drug withdrawal and symptomatic treatment, her symptoms improved significantly. Her chemotherapy regimen was then changed, and she experienced no repeat HFS episodes.
Compared with doxorubicin, liposomal doxorubicin has higher and longer accumulation concentrations in vivo; therefore, the related incidence of HFS is higher than with non-liposomal doxorubicin. Charrois et al.5 found that the half-life of doxorubicin in patients’ palms and soles of the feet was significantly prolonged with liposomal doxorubicin, and that the hydrophilic liposomal coating increased drug excretion through sweat, resulting in the accumulation of drugs in the ducts of eccrine glands. The corneum layer then acted as a drug reservoir, resulting in increased drug concentrations, which easily let to free-radical generation. High levels of free radicals can reduce the skin’s antioxidant capacity, which leads to HFS.6 Yokomichi et al.7 studied HFS caused by liposomal doxorubicin in an animal model. The results showed that doxorubicin can interact with copper ions in the skin to produce reactive oxygen species (ROS). ROS can attack keratinocytes, and release inflammatory cytokines, such as interleukin (IL)-8, IL-1β, IL-1α, and IL-6, which induce keratinocyte apoptosis. Blocking the production of ROS to prevent and treat HFS is an area of future research.8 Liposomal doxorubicin and its metabolites reach the skin surface through sweat. The palms and the soles of the feet have rich capillary networks, and because the skin in these regions is easily and repeatedly rubbed or traumatized, blood flow is increased. In addition, these area have high-density and widely-distributed secretory glands; therefore, these areas may also have high concentrations of chemotherapy drugs.9 This means that HFS can be prevented and treated by reducing friction and trauma to the hands and feet, avoiding sweat secretion, and inhibiting inflammatory reactions.
Currently, there are no standard guidelines to prevent and treat HFS caused by liposomal doxorubicin. However, the following aspects have significance for the clinical treatment of such adverse reactions:
Pre-medication patient education: Liposomal doxorubicin is a relatively new dosage form, and patients may have concerns about its efficacy and safety. Therefore, patients should be informed of the advantages, costs, methods of administration, precautions, possible adverse events and preventive measures prior to receiving the drug, which can reduce negative psychological effects. After effective patient education and providing appropriate psychological support, patients are more likely to agree to the treatment.
Local cooling: Mangili et al.10 reported that in patients treated with liposomal doxorubicin, the incidence and severity of HFS could be significantly reduced by applying ice packs to the wrist and ankle joints during drug administration. The incidence of HFS was 7.1% in the ice-pack-application group and 36% in the control group. The decreased incidence of HFS may be related to vasoconstriction induced by the ice and decreased drug accumulation in the limbs.
Vitamins: Vail et al.11 observed an effect of vitamin B6 in HFS in dogs in a randomized, double-blind and placebo-controlled trial. During chemotherapy with liposomal doxorubicin with concurrent oral vitamin B6 administration, the severity score of the HFS clinical signs decreased significantly. In the placebo group, the severity of HFS and the incidence of chemotherapy cessation were 4.2 times higher than the incidence in the vitamin B6 group. However, there was no significant decrease in the histopathological grade in the treatment group. The authors suggested that vitamin B6 does not completely alleviate HFS, but that the vitamin can slow the progression of toxicity and prevent treatment delay or interruption. One study reported12 that after 1 week of oral administration of vitamin E (300 mg/D), grade 2 and 3 HFS improved significantly.
Corticosteroids: Drake et al.13 evaluated the efficacy of dexamethasone in the treatment of HFS caused by liposomal doxorubicin in a prospective and placebo-controlled trial. The results showed that in patients with gynecological tumors treated with dexamethasone, five cycles of chemotherapy could be completed according to the original dose and administration cycle. In the control group without dexamethasone, the chemotherapy drug doses were reduced or treatment was delayed to varying degrees. The mechanism underlying how dexamethasone relieves HFS is unclear, and may be related to reducing the inflammatory response.
Dimethyl sulfoxide (DMSO): Lopez et al.14 reported that 99% DMSO (topical, four times/day, 14 consecutive days) successfully resolved grade 3 HFS caused by liposomal doxorubicin. DMSO can transport free doxorubicin into the circulatory system and acts as an antioxidant, preventing doxorubicin toxicity in soft tissues.
Traditional Chinese medicine: Oral Chinese medicines for the treatment of HFS mainly include angelica, Astragalus, and white peony. External lotions are the main Chinese medicine form for promoting blood circulation and removing blood stasis, among which safflower, Ligusticum, and Caulis spatholobi are frequently used.15,16 However, high-quality randomized controlled studies of the efficacy and safety of traditional Chinese medicine in the treatment of HFS are lacking, and further study is needed.
In this paper, we summarized a case of severe HFS caused by liposomal doxorubicin, which was treated correctly and timely using effective communication with the patient and appropriate evaluation by the clinicians and the clinical pharmacist. The patient achieved a good outcome, and the case provides a reference for the clinical treatment of patients with similar adverse events.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethics statement: This study was approved by the Ethics Committee of Shanghai Tenth People’s Hospital, and the patient provided written informed consent to publish her case (including the publication of her images).
Funding: This study was funded by the Shanghai Key Clinical Specialist Construction Programs (to FMS) and “Cultivation program in the National Academy of Natural Sciences” supported by Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai [No. 04.03.17.046].
ORCID iD: Chenxu Ni https://orcid.org/0000-0003-3551-1663
References
- 1.Guerriero KA, Wilson SR, Boutagy NE, et al. Cutaneous toxicity in a laboratory beagle (Canis lupus familiaris) after chronic administration of doxorubicin hydrochloride. Comp Med 2018; 68: 56–62. [PMC free article] [PubMed] [Google Scholar]
- 2.Jung S, Sehouli J, Patzelt A, et al. Influence of mechanical stress on palmoplantar erythrodysesthesia – a case report. Oncol Res Treat 2015; 38: 42–44. [DOI] [PubMed] [Google Scholar]
- 3.Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27, 2017. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
- 4.Nikolaou V, Syrigos K, Saif MW. Incidence and implications of chemotherapy related hand–foot syndrome. Expert Opin Drug Saf 2016; 15: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 5.Charrois GJR, Allen TM. Multiple injections of pegylated liposomal doxorubicin: pharmacokinetics and therapeutic activity. J Pharmacol Exp Ther 2003; 306: 1058–1067. [DOI] [PubMed] [Google Scholar]
- 6.Bun S, Yunokawa M, Tamaki Y, et al. Symptom management: the utility of regional cooling for hand–foot syndrome induced by pegylated liposomal doxorubicin in ovarian cancer. Support Care Cancer 2018; 26: 2161–2166. [DOI] [PubMed] [Google Scholar]
- 7.Yokomichi N, Nagasawa T, Coler-Reilly A, et al. Pathogenesis of hand–foot syndrome induced by PEG-modified liposomal doxorubicin. Hum Cell 2013; 26: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milano G, Etienne-Grimaldi MC, Mari M, et al. Candidate mechanisms for capecitabine-related hand–foot syndrome. Br J Clin Pharmacol 2008; 66: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin JI, Wang G, Smith WJ, et al. Revealing dynamics of accumulation of systemically injected liposomes in the skin by intravital microscopy. ACS Nano 2017; 11: 11584–11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangili G, Petrone M, Gentile C, et al. Prevention strategies in palmar–plantar erythrodysesthesia onset: the role of regional cooling. Gynecol Oncol 2008; 108: 332–335. [DOI] [PubMed] [Google Scholar]
- 11.Vail DM, Chun R, Tham DH, et al. Efficacy of pyridoxine to ameliorate the cutaneous toxicity associated with doxorubicin containing pegylated (Stealth) liposomes: a randomized double-blind clinical trial using a canine model. Clin Cancer Res 1998; 4: 1567–1571. [PubMed] [Google Scholar]
- 12.Kara IO, Sahin B, Erkisi M. Palmar–plantar erythrodysesthesia due to docetaxel-capecitabine therapy is treated with vitamin E without dose reduction. Breast 2006; 15: 414–424. [DOI] [PubMed] [Google Scholar]
- 13.Drake RD, Lin WM, King M, et al. Oral dexamethasone attenuates Doxil-induced palmar–plantar erythrodysesthesias in patients with recurrent gynecologic malignancies. Gynecol Oncol 2004; 94: 320–324. [DOI] [PubMed] [Google Scholar]
- 14.Lopez AM, Wallace L, Dorr RT, et al. Topical DMSO treatment for pegylated liposomal doxorubicin-induced palmar–plantar erythrodysesthesia. Cancer Chemother Pharmacol 1999; 44: 303–306. [DOI] [PubMed] [Google Scholar]
- 15.McLellan B, Ciardiello F, Lacouture ME, et al. Regorafenib-associated hand–foot skin reaction: practical advice on diagnosis, prevention, and management. Ann Oncol 2015; 26: 2017–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao C, Chen J, Yu B, et al. Effect of modified taohongsiwu decoction on patients with chemotherapy-induced hand-foot syndrome. J Tradit Chin Med 2014; 34: 10–14. [DOI] [PubMed] [Google Scholar]