Abstract
OBJECTIVE
We aimed to compare cardiovascular (CV) events, all-cause mortality, and CV mortality rates among adults with and without diabetes in countries with differing levels of income.
RESEARCH DESIGN AND METHODS
The Prospective Urban Rural Epidemiology (PURE) study enrolled 143,567 adults aged 35–70 years from 4 high-income countries (HIC), 12 middle-income countries (MIC), and 5 low-income countries (LIC). The mean follow-up was 9.0 ± 3.0 years.
RESULTS
Among those with diabetes, CVD rates (LIC 10.3, MIC 9.2, HIC 8.3 per 1,000 person-years, P < 0.001), all-cause mortality (LIC 13.8, MIC 7.2, HIC 4.2 per 1,000 person-years, P < 0.001), and CV mortality (LIC 5.7, MIC 2.2, HIC 1.0 per 1,000 person-years, P < 0.001) were considerably higher in LIC compared with MIC and HIC. Within LIC, mortality was higher in those in the lowest tertile of wealth index (low 14.7%, middle 10.8%, and high 6.5%). In contrast to HIC and MIC, the increased CV mortality in those with diabetes in LIC remained unchanged even after adjustment for behavioral risk factors and treatments (hazard ratio [95% CI] 1.89 [1.58–2.27] to 1.78 [1.36–2.34]).
CONCLUSIONS
CVD rates, all-cause mortality, and CV mortality were markedly higher among those with diabetes in LIC compared with MIC and HIC with mortality risk remaining unchanged even after adjustment for risk factors and treatments. There is an urgent need to improve access to care to those with diabetes in LIC to reduce the excess mortality rates, particularly among those in the poorer strata of society.
Introduction
Diabetes affects ∼460 million individuals globally (1) and was the cause of more than 5 million deaths in 2017 (2) with cardiovascular disease (CVD) accounting for most of this mortality. Diabetes has been well known to lead to increased CV and all-cause mortality (3,4). Recent reports from high-income countries (HIC) suggest that the incidence of CVD and, particularly, CV mortality among those with diabetes might be decreasing, possibly due to the use of statins and antiplatelet agents, a decrease in smoking rates, and better glucose and blood pressure (BP) control apart from access to high-quality health care (5–8). However, there are few data from middle-income countries (MIC) and particularly from low-income countries (LIC), which collectively experience the largest burden of diabetes and CVD. No study has compared risk factors, treatments, and CV outcomes including CV mortality in individuals with and without diabetes from a large number of countries at different levels of income or from different regions of the world using standardized methods. Additionally, there are few data on the impacts of behavioral risk factors and treatments in reducing the excess all-cause and CVD mortality among people with diabetes and whether the latter vary across HIC, MIC, and LIC. We aimed to compare CV events and all-cause and CV mortality rates among adults with and without diabetes in countries with differing levels of income.
Research Design and Methods
Study Design and Sample Selection
The detailed methodology of the Prospective Urban Rural Epidemiology (PURE) study has been published (9–12) and is described in the Supplementary Appendix 1. Briefly, PURE recruited 151,365 people from 631 communities in 21 countries. Countries were classified as HIC, MIC, and LIC based on World Bank classification for 2006. The HICs studied included Canada, Saudi Arabia, Sweden, and United Arab Emirates; MIC included Argentina, Brazil, Chile, China, Colombia, Iran, Malaysia, Palestine, Philippines, Poland, South Africa, and Turkey; and LIC included Bangladesh, India, Pakistan, Tanzania, and Zimbabwe. Within each country, rural and urban communities were selected based on prespecified criteria. For selection of both urban and rural communities, the national definition of the country was used. Rural communities were selected such that they were isolated (distance of >50 km or lack easy access to commuter transportation) from urban centers. However, the ability to process blood samples was also considered, e.g., villages in rural developing countries needed to be within a 45-min drive of an appropriate facility. The feasibility for long-term follow-up was also considered. For urban communities, the sites with a stable population, such as residential colonies related to specific work sites in developing countries, were chosen. Households were selected using an unbiased approach to sampling with the aim of providing a broadly representative sample of the community. The study was coordinated by the Population Health Research Institute (Hamilton Health Sciences, Hamilton, Ontario, Canada) and was approved by relevant institutional research ethics boards at all sites.
Selection of Households and Individuals
Within each community, sampling was designed to achieve a broadly representative sample of that community of adults aged between 35 and 70 years. The choice of sampling frame within each center was based on both representativeness and feasibility of long-term follow-up, following broad study guidelines. Once a community was identified, where possible, common and standardized approaches were applied to the enumeration of households, identification of individuals, recruitment procedures, and data collection. The method of approaching households differed between regions. For example, in rural areas of India and China, a community announcement was made to the village with the help of a community leader, followed by in-person door-to-door visits of all households. In contrast, in Canada, initial contact was by mail followed by telephone calls inviting members of the households to a central clinic. For each approach, at least three attempts at contact were made. Households were eligible if at least one member of the household was between the ages of 35 and 70 years and if the household members intended to continue living in their current home for a further 4 years. All individuals within these households between 35 and 70 years providing written informed consent were enrolled. When a household refused to participate, demographics and simple self-report risk factor data were recorded in a nonresponder form.
Procedures
All participants provided written informed consent. Standardized questionnaires were used to collect information about demographic factors, socioeconomic status, health behaviors, health history, and medication use. Smoking status was categorized as never, former, or current smoker. Weight, height, waist and hip circumferences, and BP were recorded. Socioeconomic status was assessed using household wealth index. Household wealth, calculated at the household level and with household data, was defined by an index on the basis of ownership of assets and housing characteristics (13) that has been validated in several countries and documented to be a robust measure of wealth, consistent with measures of income and expenditure. Detailed follow-up occurred at 3, 6, and 9 years at which times clinical events and deaths were recorded. Standardized case report forms were used to record data on major CV events and mortality during follow-up, which were adjudicated in each country by trained physicians using standard definitions. Data were electronically transferred to the Population Health Research Institute, Canada where further quality control checks were undertaken.
Participants were considered to have diabetes if they had been previously diagnosed by a physician and/or if they had a fasting plasma glucose level of 126 mg/dL (7.0 mmol/L) or greater or were being treated with a glucose-lowering drug (14). Before the venous puncture, the health professional verified that the participant fasted for at least 8 h (no food or beverages, excluding water). Blood was centrifuged within 2 h of collection at the local site. Samples were kept on ice until centrifugation. Plasma was either immediately analyzed for glucose locally or stored at –20°C to –70°C and then subsequently shipped in temperature-controlled containers for central measurements. Plasma glucose was measured by standardized enzymatic methods using hexokinase or glucose oxidase. Hypertension was defined as those with either a history of hypertension, current use of antihypertensive medication, and/or a BP >140/90 mmHg.
The mean follow-up period was 9.0 ± 3.0 years (total of 1.176 million years of follow-up) and varied based on the date when recruitment began at each site or country. During the follow-up period, contact was made at least every 3 years (and in some countries annually) with every participant by telephone or face-to-face interviews with the local research team. Information on events of interest (including available documentation) were obtained from participants, family, and from hospital or clinic records whenever possible. Standardized case report forms, death certificates, medical records, and verbal autopsies were used to capture details about major CV events and death during follow-up. These were then adjudicated by trained physicians by review of all information using standardized definitions. Verbal autopsy reports were obtained when the cause of death could not be ascertained from available medical records (15).
Outcome Ascertainment
The main clinical outcomes included in the analyses in this article are major CVD (myocardial infarction, stroke, or heart failure), deaths categorized as all-cause mortality (including CV death and non-CV death categorized further by cause, such as infections, cancers, respiratory disease, pregnancy/delivery/puerperium, injury, or other causes), or CV mortality (including sudden unexpected CV death, fatal myocardial infarction, fatal stroke, fatal congestive heart failure, and death due to other CVDs) The definitions of events are detailed in Supplementary Appendix 2.
Statistical Analyses
Of the recruited 151,365 individuals in PURE, those with CVD at baseline were removed, and the analysis was performed on n = 143,567 individuals. Continuous variables were summarized as means and SDs, and categorical variables were given as numbers and percentages. Age-adjusted prevalence of diabetes was calculated using logistic regression. Person-years of follow-up were calculated from the baseline examination until the event developed or death occurred or until the last examination, whichever came first. Event rates were calculated as a rate per 1,000 person-years of follow-up in individuals with diabetes (self-reported and newly diagnosed) and individuals without diabetes. Hazard ratios (HR) and 95% CIs were calculated using a multivariable Cox frailty analysis with random intercepts to account for the correlation of observations within centers (which therefore also accounted for clustering at region and country levels). The assumption for the models were tested using Supremum test and Kaplan-Meier survival estimates of the survival function for continuous and categorical variables, respectively (16). All models were adjusted for age, sex, ethnicity, and center as a random effect (model 1). Additionally, multivariable models were first adjusted for behavior-related and clinical parameters, such as low physical activity, smoking, BMI, hypertension, and baseline CVD (model 2). Finally, in model 3, we adjusted for medications (antidiabetes drugs, BP- and lipid-lowering drugs, and aspirin). Stratification analyses were performed by 1) geographic regions (North America/Europe, South America, Middle East, Africa, South Asia, South East Asia, and China); 2) self-reported diabetes (those taking and not taking medication) and newly diagnosed diabetes; and 3) tertiles of individual wealth index. P value < 0.05 was considered significant. Data were analyzed with SAS version 9.4.
Results
Participant Baseline Characteristics
Of the 143,567 participants included in the study, 16,286 were from HIC; 94,385 were from MIC; and 32,896 were from LIC. The mean age of the overall population was 50.3 ± 9.9 years, and HIC participants were older than those from MIC or LIC (P for trend, P < 0.001).
The baseline characteristics of study participants stratified by country income categories and diabetes status are presented in Table 1. The age-adjusted prevalence of diabetes in the overall population was 12.8% (95% CI 12.8–12.9%) with the highest rates in LIC followed by HIC and MIC. Individuals with diabetes were significantly older within country categories. Among those with diabetes, there were significantly more women in MIC (women 61.5% vs. men 38.5%) and LIC (women 54.1% vs. men 45.9%) but not in HIC (women 49.3% vs. men 50.7%). The median durations of follow-up (interquartile range [IQR]) in HIC, MIC, and LIC were 9.0 (6.5–9.3), 8.8 (6.3–9.7), and 9.4 (6.7–11.9), respectively. Mean BP was significantly higher in those with diabetes compared with those without across country income categories (P < 0.001). BMI, waist circumference, hip circumference, and waist-to-hip ratio were also significantly higher in those with diabetes compared with those without in all three country income categories. Individuals with diabetes in HIC had a more favorable lipid profile (lower total and LDL cholesterol) compared with those without diabetes, while this was not seen in MIC and LIC. Among those with diabetes, current use of tobacco was highest in MIC (16.4%) followed by LIC (15.6%) and HIC (11.8%). Alcohol consumption was significantly lower in those with diabetes in HIC and MIC but not in LIC.
Table 1.
Baseline characteristics of participants by country income categories and diabetes status
| HIC (n = 16,286) | MIC (n = 94,385) | LIC (n = 32,896) | ||||
|---|---|---|---|---|---|---|
| No DM (n = 14,330) | Prevalent DM (n = 1,955) | No DM (n = 85,206) | Prevalent DM (n = 9,161) | No DM (n = 28,413) | Prevalent DM (n = 4,476) | |
| Age-adjusted prevalence of diabetes, % (95% CI) | 0 (0) | 13.3 (13.1–13.4) | 0 (0) | 11.5 (11.4–11.6) | 0 (0) | 15.4 (15.2–15.5) |
| Age (years) | 51.3 ± 9.5 | 54.8 ± 9.0** | 50.3 ± 9.8 | 55.0 ± 8.9** | 47.7 ± 10.3 | 52.1 ± 9.8** |
| Sex, male | 6,426 (44.8) | 992 (50.7)** | 34,395 (40.4) | 3,526 (38.5)** | 11,953 (42.1) | 2,055 (45.9)** |
| Duration of follow-up (years) | 9.0 (6.6–9.3) | 8.8 (4.1–9.3)** | 8.8 (6.3–9.7) | 8.7 (6.2–9.4)** | 8.8 (6.3–9.7) | 8.7 (6.2–9.4)** |
| Systolic BP (mmHg) | 128.3 ± 19.6 | 136.1 ± 19.3** | 132.1 ± 23.1 | 139.6 ± 23.5** | 125 ± 21.4 | 134.9 ± 22.3** |
| Diastolic BP (mmHg) | 81.3 ± 12.2 | 83.6 ± 12.1* | 82.1 ± 16.8 | 84 ± 14.2** | 79.8 ± 12.9 | 83.1 ± 13.2** |
| BMI (kg/m2) | 27.3 ± 5.2 | 31.6 ± 5.9** | 25.9 ± 4.9 | 28.9 ± 5.6** | 23.0 ± 4.9 | 25.3 ± 4.4** |
| Waist (cm) | 89 ± 13.8 | 101.8 ± 14.1** | 84.7 ± 12.5 | 93.7 ± 13.3** | 77.3 ± 13.2 | 85.5 ± 11.6** |
| Waist-to-hip ratio | 0.87 ± 0.09 | 0.94 ± 0.09** | 0.87 ± 0.08 | 0.92 ± 0.08** | 0.86 ± 0.09 | 0.91 ± 0.09** |
| Cholesterol (mmol/L) (n = 113,898) | 5.3 ± 1.0 | 4.8 ± 1.1** | 4.9 ± 1.0 | 5.1 ± 1.1** | 4.5 ± 1.0 | 4.8 ± 1.0** |
| Triglycerides (mmol/L) (n = 113,826) | 1.3 ± 0.8 | 1.8 ± 1.1** | 1.6 ± 1.1 | 2.1 ± 1.4** | 1.4 ± 0.9 | 1.6 ± 1.0** |
| HDL cholesterol (mmol/L) (n = 113,513) | 1.4 ± 0.4 | 1.2 ± 0.3** | 1.2 ± 0.3 | 1.1 ± 0.3** | 1.2 ± 0.4 | 1.2 ± 0.3 |
| LDL cholesterol (mmol/L) (n = 112,908) | 3.3 ± 0.9 | 2.9 ± 1** | 3 ± 0.9 | 3.2 ± 0.9** | 3.1 ± 1.1 | 3.3 ± 1.2** |
| Total cholesterol to HDL ratio (n = 112,884) | 4.0 ± 1.2 | 4.4 ± 1.3** | 4.3 ± 1.2 | 4.8 ± 1.4** | 4.1 ± 1.3 | 4.4 ± 1.4** |
| Current use of tobacco | 1,959 (13.7) | 231 (11.8)** | 18,624 (22.0) | 1,491 (16.4)** | 6,597 (23.3) | 699 (15.6)** |
| Current use of alcohol | 9,953 (69.5) | 673 (34.4)** | 21,735 (26.0) | 1,664 (18.8)** | 2,783 (9.8) | 468 (10.5)** |
| On glucose-lowering agentsa | 0 (0) | 1,032 (71.9) | 0 (0) | 3,484 (55.4) | 0 (0) | 821 (28.7) |
| Oral hypoglycemic agentsa | 0 (0) | 947 (65.9) | 0 (0) | 3,272 (52.0) | 0 (0) | 763 (26.6) |
| Insulina | 0 (0) | 178 (12.4) | 0 (0) | 371 (5.9) | 0 (0) | 79 (2.8) |
| On BP-lowering medication | 1,934 (13.5) | 819 (41.9)** | 10,995 (12.9) | 3,051 (33.3)** | 905 (3.2) | 453 (10.1)** |
| On cholesterol-lowering medication | 1,198 (8.4) | 830 (42.5)** | 2,224 (2.6) | 1,266 (13.8)** | 132 (0.5) | 93 (2.1)** |
| On aspirin | 903 (6.3) | 477 (24.4)** | 1,960 (2.3) | 715 (7.8)** | 92 (0.3) | 58 (1.3)** |
Data are mean ± SD, n (%), or median (IQR) unless otherwise indicated. DM, self-reported diabetes taking antidiabetes medications or fasting glucose ≥7.0 mmol/L; non-DM, not on diabetes medication and fasting glucose <7 mmol/L. *P < 0.05 and **P < 0.001 compared with individuals without diabetes; P < 0.001 for all comparisons between the income country groups in both those with diabetes and no diabetes (except for diastolic BP, P = 0.004).
Percentage calculated for the self-reported diabetes individuals, HIC (n = 1,436), MIC (n = 6,289) and LIC (n = 2,864).
Overall, 50.4% of those with diabetes were on glucose-lowering medication (5.9% on insulin); 27.7% were on BP-lowering medication; 14.0% were on cholesterol-lowering medication; and 8.0% were on aspirin. Use of all medications was highest in HIC (30.6%), followed by MIC (21.2%) and LIC (7.0%) (P for trend < 0.001). The overall use of glucose-lowering agents among those with diabetes was 71.9% in HIC, 55.4% in MIC, and 28.7% in LIC with lower use of both insulin and oral hypoglycemic agents in LIC. Among those with hypertension, 78.0% of HIC, 65.1% of MIC, and 24.9% of LIC were on BP-lowering medication.
Supplementary Table 1 shows the baseline characteristics of participants with and without diabetes by geographic region. Individuals with diabetes were significantly older in all regions compared with those without. Mean BP, BMI, waist circumference, hip circumference, and waist-to-hip ratio were significantly higher in those with diabetes compared with those without, across the regions. Individuals with diabetes in Africa had a more favorable lipid profile (lower total and LDL cholesterol) compared with individuals with diabetes in other regions, while higher levels of lipids were seen among South East Asia region. Current use of tobacco and alcohol consumption were significantly lower in those with diabetes in all regions, except for alcohol use in patients from South Asia, which was higher. The proportion of individuals with diabetes on glucose-lowering medication was highest in the Middle East (71.5%) and lowest in South Asia (29.1%). Use of BP-lowering, cholesterol-lowering, and aspirin medications were higher among those with diabetes in all regions, and among those with diabetes, the use of these medications was highest in North America/Europe and lowest in South Asia.
Age- and Sex-Adjusted Event Rates: Major CVD, All-Cause Mortality, and CV Mortality
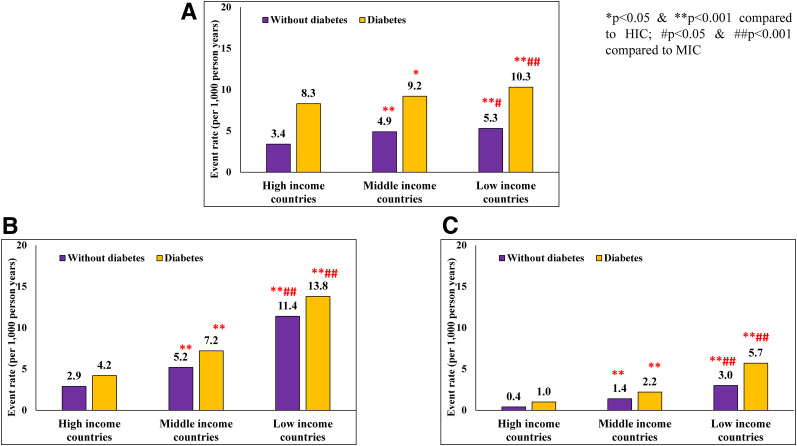
The age-adjusted incidence of major CVD (per 1,000 person-years) is shown in Fig. 1A. In those without diabetes, CVD rates were highest in LIC (5.3) followed by MIC (4.9) and HIC (3.4) (P < 0.001). A similar pattern was seen among those with diabetes: 10.3, 9.2, and 8.3 in LIC, MIC, and HIC, respectively (P < 0.001). In all regions, the CVD rates were significantly higher in those with diabetes compared with rates in those without diabetes (P < 0.001).
Figure 1.
Age- and sex-adjusted event rates (per 1,000 person-years) for major CVD (A), all-cause mortality (B), and CV mortality (C) in individuals with and without diabetes by country income categories.
Figure 1B shows that in all countries irrespective of income categories, all-cause mortality was higher among those with diabetes compared with those without diabetes (P < 0.001). Among those without diabetes, all-cause mortality significantly increased from HIC (2.9%) to MIC (5.2%) and LIC (11.4%) (P for trend, P < 0.001). Similar patterns in all-cause mortality were seen in those with diabetes: HIC 4.2%, MIC 7.2%, and LIC 13.8% (P for trend < 0.001). It is of interest that all-cause mortality among those without diabetes in LIC (11.4%) was higher than those with diabetes in HIC (4.2%) (P < 0.001). Figure 1C shows that similar patterns were seen with CV mortality with much higher rates in LIC and MIC compared with HIC. Again, the CV mortality among those without diabetes in LIC (3.0%) exceeded those with diabetes in HIC (1.0%).
Table 2 shows the age- and sex-adjusted event rates (per 1,000 person-years) among those with self-reported and newly diagnosed diabetes by country income categories. The event rates of major CVD were significantly higher in self-reported diabetes compared with newly diagnosed diabetes across all country income categories. Similar results were seen for all-cause and CV mortality with rates in self-reported diabetes being more than double of those among newly diagnosed diabetes. All event rates were highest in LIC, followed by MIC; and they were the lowest in HIC.
Table 2.
Age- and sex-adjusted event rates (per 1,000 person-years) among diabetes (known and newly diagnosed) and by country income categories
| Risk factor | HIC | MIC | LIC | |||
|---|---|---|---|---|---|---|
| Self-reported diabetes | Newly diagnosed diabetes | Self-reported diabetes | Newly diagnosed diabetes | Self-reported diabetes | Newly diagnosed diabetes | |
| Major CVD | 9.7 (7.8–11.7) | 5.5 (3.4–7.7)** | 10.0 (9.0–11.0) | 8.0 (6.9–9.1)** | 12.6 (11.1–14.2) | 6.4 (5.2–7.7)** |
| All-cause mortality | 5.0 (3.8–6.3) | 2.1 (0.8–3.3)** | 9.8 (8.7–10.8) | 2.9 (2.2–3.6)** | 18.1 (16.3–20) | 7.9 (6.1–9.6)** |
| CV mortality | 1.3 (0.7–2.0) | 0 (0–0) | 3.0 (2.5–3.6) | 0.7 (0.4–1)** | 7.6 (6.5–8.8) | 2.3 (1.6–3.1)** |
Values are presented as age- and sex-adjusted event rates per 1,000 person-years (95% CI).
P < 0.001 compared with self-reported diabetes.
Similar results are presented in Supplementary Table 2, in which the age- and sex-adjusted event rates among those with self-reported diabetes who were on medication are compared with those who were not, across country income categories. It can be seen that with respect to major CVD, the rate was highest in the participants who had physician-diagnosed diabetes and were on medication compared with those who had physician-diagnosed diabetes but were not on medication and those with newly diagnosed diabetes in all three regions, namely, HIC, MIC, and LIC. Similar trends were seen with respect to all-cause mortality and CV mortality, suggesting that the patients with newly diagnosed diabetes had milder diabetes and CVD risk. However, the CV mortality in the LIC group, even among those who were physician diagnosed and were on medication, was 6.2 times higher in LIC than in HIC (event rates per 1,000 person-years [95% CI] LIC 8.7 [5.5–11.8] vs. HIC 1.4 [0.6–2.1]), suggesting that, despite treatment, LIC participants with diabetes had much higher CV mortality. Similar results were seen for all-cause mortality (LIC 21.0 [16.3–25.7] vs. HIC 5.0 [3.6–6.4]).
HRs for All-Cause and CV Mortality
Table 3 shows the HRs for all cause and CV mortality for those with diabetes compared with those without. HRs were adjusted for age, sex, ethnicity, and center (model 1). The next model adjusted for behavioral risk factors like physical inactivity, smoking, BMI, and hypertension (model 2). Finally, model 3 adjusted for various treatments (BP- and lipid-lowering drugs, antidiabetes drugs, and aspirin).
Table 3.
HRs for major CVD, all-cause mortality, and CV mortality in HIC, MIC, and LIC
| n | HIC | MIC | LIC | |
|---|---|---|---|---|
| Major CVD, n (%) | 498 (3.1) | 3,743 (4.1) | 1,719 (5.4) | |
| Model 1 | 124,067 | 2.13 (1.72–2.64) | 1.92 (1.77–2.09) | 1.94 (1.69–2.22) |
| Model 2 | 106,924 | 1.84 (1.46–2.33) | 1.74 (1.59–1.91) | 1.86 (1.57–2.21) |
| Model 3 | 106,924 | 1.59 (1.17–2.15) | 1.70 (1.52–1.89) | 1.82 (1.50–2.20) |
| All-cause mortality, n (%) | 395 (2.4) | 3,818 (4.2) | 3,378 (10.5) | |
| Model 1 | 124,067 | 1.36 (1.05–1.78) | 1.44 (1.32–1.58) | 1.33 (1.18–1.49) |
| Model 2 | 106,924 | 1.20 (0.90–1.61) | 1.37 (1.24–1.52) | 1.51 (1.31–1.75) |
| Model 3 | 106,924 | 0.99 (0.68–1.45) | 1.18 (1.03–1.34) | 1.40 (1.18–1.66) |
| CV mortality, n (%) | 59 (0.4) | 1,068 (1.2) | 999 (3.1) | |
| Model 1 | 124,067 | 1.84 (1.01–3.37) | 1.64 (1.39–1.94) | 1.89 (1.58–2.27) |
| Model 2 | 106,924 | 1.44 (0.76–2.75) | 1.48 (1.24–1.78) | 1.89 (1.48–2.40) |
| Model 3 | 106,924 | 1.63 (0.76–3.48) | 1.14 (0.90–1.45) | 1.78 (1.36–2.34) |
Data are HR (95% CI) unless otherwise noted. Reference group: individuals without diabetes. Model 1: adjusted for age, sex, ethnicity, and center (as random effect). Model 2: model 1 + behavioral factors (physical activity level, smoking), BMI, and hypertension. Model 3: model 2 + treatments (BP-, cholesterol-, and glucose-lowering agents and aspirin).
In HIC, there was a substantial attenuation of risk for all-cause mortality among those with diabetes, and the HRs decreased from 1.36 (95% CI 1.05–1.78) in model 1 (i.e., adjustment for only sex and age) to 1.20 (0.90–1.61) in model 2 (adjustment for risk factors) to 0.99 (0.68–1.45) in model 3 (additional adjustment for treatments). Among MIC, the attenuation was less marked from 1.44 in model 1 to 1.37 in model 2 and to 1.18 in model 3. Among LIC, there was no attenuation with 1.33, 1.51, and 1.40 in models 1, 2, and 3, respectively.
Similar findings were seen with respect to CV mortality. In HIC, the HR for CV mortality decreased from 1.84 in model 1 to 1.63 in model 3. Among MIC also, there was attenuation from 1.64 to 1.14. In the case of LIC, there was virtually no attenuation 1.89 (models 1 and 2) to 1.78 (model 3) after adjustment across the models.
Similar results were seen when the HRs for both all-cause mortality and CV mortality were stratified based on geographic regions representing HIC, MIC, and LIC (North America/Europe, South America, Middle East, Africa, South Asia, South East Asia, and China) (Supplementary Table 3).
Supplementary Table 4 shows the sensitivity analysis of HRs for all-cause and CV mortality for those with diabetes compared with those without, stratified by physician diagnosis (for those on and not on medication) and newly diagnosed diabetes across country income categories. The hazards of all-cause and CV mortality were significantly higher in those with self-reported (for both those taking and not taking medication) compared with newly diagnosed diabetes. However, the attenuation patterns remained the same after adjustment for various factors including drug treatment for BP, lipids, and aspirin (maximum in HIC and least in LIC) with maximum attenuation seen in HIC, less seen in MIC, and virtually no attenuation in LIC. This suggests that the excess CV mortality in LIC is probably independent of these factors. It may also suggest suboptimal control of these risk factors in LIC.
Supplementary Table 5 shows the HRs for all-cause and CV mortality by tertiles of individual wealth index and country income categories. In LIC, those in the lowest tertile of the wealth index (lowest personal wealth) had the highest hazards of all-cause and CV mortality compared with the middle and high tertiles even after adjustment of all confounders (model 3). However, no clear patterns were seen in HIC and MIC, when stratified based on tertiles of wealth.
Conclusions
Our study has some novel findings. CVD rates, all-cause mortality, and CV mortality were markedly higher among those with diabetes in LIC compared with MIC and HIC, with the risk being higher for those with self-reported diabetes as compared to those with newly diagnosed diabetes. While there was a marked attenuation of hazards for all-cause and CV mortality after adjustment for baseline risk factors and treatments in HIC and some attenuation in MIC, there was virtually no attenuation in LIC. Hazards for all-cause and CV mortality were highest among individuals in the lowest tertile of personal wealth index in LIC, but not in HIC or MIC.
Recent studies from affluent countries such as the U.S., U.K., Canada, and Sweden suggest that there has been a marked decline in CV mortality rates among individuals with diabetes (17–20). The National Health and Nutrition Examination Survey (NHANES) (21) showed a declining trend in mortality from vascular disease among individuals with diabetes in the U.S. over the past 30 years, and this has been corroborated by a more recent study of 963,648 U.S. veterans with diabetes (3). Another study in Sweden also indicated that excess mortality due to diabetes was much lower in 2005–2011 compared with earlier periods (before 2005) (4). It is likely that more aggressive treatment of CV risk factors, such as hypertension and dyslipidemia, as well as general improvements in the management of acute CV events and heart failure could have contributed to the decline in mortality rates in these countries.
The higher mortality among individuals in LIC, in general, has already been reported in an earlier publication from PURE (11). However, in that article, differences between individuals with and without diabetes were not considered, which is the main focus of the present work. At least part of the difference between LIC, MIC, and HIC could be related to differences in access to health care systems. We report here on the abysmally low use of antidiabetes, antihypertensive, and lipid-lowering drugs by patients in LIC compared with those in MIC and HIC even among those with CVD and diabetes. It has been shown that long-term survivors with diabetes have better glycemic, BP, and lipid control compared with nonsurvivors (22). Our results, therefore, suggest that there is an urgent need to ensure access, and adherence to, CVD risk–lowering medications if the mortality rates in LIC and MIC are to improve to the levels currently seen in HIC.
Earlier analyses of the PURE data have shown that availability, affordability, and usage of medications for CVD prevention, including antihypertensive agents and essential diabetes drugs were low in LIC (23–26). While ensuring wider availability and more widespread use of these agents are essential first steps in reducing CV mortality in these countries, our results also suggest that these interventions by themselves may not reduce the risk of adverse outcomes to levels comparable to HIC, as the association between diabetes and CV mortality appears to be independent of these factors in LIC. It is also possible that individuals in LIC are not prescribed these agents at optimal doses or at the optimal time, which is an example of therapeutic inertia. This is likely for financial reasons (as most patients pay out of pocket for medicines) or patient/provider beliefs regarding the safety and efficacy of these agents. In this context, our finding that individuals with self-reported diabetes have higher hazards of all-cause and CV mortality compared with those with newly diagnosed diabetes points to the deleterious effects of prolonged and (possibly uncontrolled) hyperglycemia and related metabolic derangements on CV outcomes, and it makes a case for more widespread screening of the population for diabetes so that these risk factors can be identified early and treated appropriately.
Moreover, within the LIC, key procedures for acute coronary events (such as percutaneous coronary angioplasty or coronary artery bypass grafting) are performed less often in individuals of lower socioeconomic classes (27,28), which offers a possible explanation for the high mortality rates observed among individuals in the lowest tertile of personal wealth in these countries, explaining the highest hazards of all-cause and CV mortality in this subgroup. The probable reason why among the HIC and MIC there were no clear mortality rates with respect to wealth index could be that there is a more equitable distribution of health in those countries due to availability of social security systems, insurance and other facilities for people living in these countries. This is at least in part a reflection of the very low funding of government health care programs and the absence of universal health insurance systems or social support mechanisms in LIC. This issue needs to be addressed urgently by increased provision of quality care through a universal health care package and improved access to quality health care systems if mortality rates due to CVD are to be decreased in LIC, particularly among those with diabetes.
Our study has several strengths. Earlier studies comparing mortality rates between individuals with and without diabetes have been conducted in single countries and were largely confined to wealthier parts of the world. Our study is the first to have prospectively addressed this question in a large number of countries from five continents, across a broad range of income categories, thereby making our results more globally applicable. Additional strengths of the study include the use of standardized methods for selection of subjects, definitions and follow-up rates.
Our study also has a few limitations. We did not use strict proportional sampling in each country, so our data cannot be considered fully representative of the individual countries. However, in a previous article, we demonstrated similarities in the mean ages and mortality rates in the PURE population compared with national data from various countries (10). Thus, there are unlikely to be major biases in the selection of participants. Next, the study enrolled ∼11% of people from HIC, but this did not include populations from the U.S. and several countries in Europe (partly because these countries already have substantial epidemiologic data and partly because of the higher costs of conducting the study in these regions). However, a few European countries (e.g., Germany) have now joined the study through collaborative partnerships, and so in the future, there could be further data from the richer countries. Nevertheless, given that substantial data already exist from Europe and North America and that the data from the included populations from Canada, Sweden, and Poland are unlikely to be substantially dissimilar to that of the U.S. or other countries in Europe, our results add to the literature and are broadly applicable. Thirdly, there could have been some underreporting of CV events in LIC on account of lack of access to diagnostic and treatment facilities in these countries, although differences in ascertaining events would not have affected our data on mortality rates and might imply that CV event rates in LIC are, in fact, higher than what we have observed. However, we did systematically contact each participant (up to six times during each follow-up cycle) and inquire about events, hospitalizations, visits to physicians, and use of medications to minimize variations in the reporting of events to the extent possible. Fourthly, there may have been additional confounders of the associations between diabetes and CVD and all-cause and CV mortality that were not accounted for in the various models used in the analysis. Another limitation is that no glucose tolerance tests were done, as this is impractical in large population studies especially involving multiple LIC with a significant proportion of participants enrolled from rural and remote areas. However, 77% of the population across countries had blood tests done, and so, our results are unlikely to be significantly biased in this respect.
In conclusion, our results indicate that CVD rates remain higher in those with diabetes across all regions. However, among those with diabetes, all-cause mortality, and CV mortality are disproportionately higher in LIC and MIC compared with HIC. The risk of all-cause and CV mortality is highest among those with self-reported diabetes across country categories. However, when stratified according to tertiles of wealth, individuals in the lowest tertile of personal wealth index in LIC had the highest hazards for all-cause and CV mortality compared with the upper two tertiles of wealth, whereas such a clear pattern was not observed in MIC and HIC. The increased CV mortality in those with diabetes in LIC remains unchanged even after adjustment for behavioral risk factors and treatments, whereas in MIC and HIC, there is some attenuation of the risk. These facts underscore the urgent need to improve access to quality diagnostic and therapeutic health care in those with diabetes in LIC especially in the poorer strata of these countries so that the excess mortality rates could be reduced.
Article Information
Acknowledgments. The authors acknowledge the contribution of the PURE Project Office Staff, National Coordinators, Investigators, and Key Staff. A complete list of the contributors and the institutions in each country is found in the Supplementary Material online. Argentina: Fundacion Estudios Clínicos Latino America; Bangladesh: Independent University, Bangladesh and Mitra and Associates; Brazil: Unilever Health Institute, Brazil; Canada: Public Health Agency of Canada and Champlain Cardiovascular Disease Prevention Network; Chile: Universidad de la Frontera; China: National Center for Cardiovascular Diseases and ThinkTank Research Center for Health Development; Colombia: Colciencias (grant 6566-04-18062 and grant 6517-777-58228); India: Indian Council of Medical Research; Malaysia: Ministry of Science, Technology and Innovation of Malaysia (grant 100-IRDC/BIOTEK 16/6/21 [13/2007] and 07-05-IFN-BPH 010), Ministry of Higher Education of Malaysia (grant 600-RMI/LRGS/5/3 [2/2011]), Universiti Teknologi MARA, Universiti Kebangsaan Malaysia (UKM-Hejim-Komuniti-15-2010); Occupied Palestinian Territory: the United Nations Relief and Works Agency for Palestine Refugees in the Near East, International Development Research Centre, Canada; Philippines: Philippine Council for Health Research and Development; Poland: Polish Ministry of Science and Higher Education (grant 290/W-PURE/2008/0), Wroclaw Medical University; Saudi Arabia: Saudi Heart Association, Dr. Mohammad Alfagih Hospital, The Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia (research group RG-1436-013); South Africa: The North-West University, South African-Netherlands Programme for Alternatives in Development, National Research Foundation, Medical Research Council of South Africa, The South Africa Sugar Association, Faculty of Community and Health Sciences; Sweden: grants from the Swedish state under the Agreement concerning research and education of doctors, the Swedish Heart and Lung Foundation, the Swedish Research Council, the Swedish Council for Health, Working Life and Welfare, King Gustaf V’s and Queen Victoria Freemasons’ Foundation, AFA Insurance; Turkey: Metabolic Syndrome Society, AstraZeneca, Sanofi Aventis; United Arab Emirates: Sheikh Hamdan Bin Rashid Al Maktoum Award For Medical Sciences and Dubai Health Authority, Dubai.
Funding and Duality of Interest. S.Y. is supported by the Marion W. Burke endowed chair of the Heart and Stroke Foundation of Ontario. This work was supported by the Population Health Research Institute, the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, support from Canadian Institutes of Health Research’s Strategy for Patient-Oriented Research, through the Ontario Strategy for Patient-Oriented Research Support Unit, as well as the Ontario Ministry of Health and Long-Term Care and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca (Canada), Sanofi (France and Canada), Boehringer Ingelheim (Germany and Canada), Servier, and Glaxo‐SmithKline, and additional contributions from Novartis and King Pharma and from various national or local organizations in participating countries. No other potential conflicts of interest relevant to this article were reported.
The funders and sponsors had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, in the preparation, review, or approval of the manuscript, or in the decision to submit the manuscript for publication.
Author Contributions. R.M.A. and V.M. were involved in the analysis and interpretation of the data and wrote the first and subsequent drafts of the manuscript. S.R. coordinated the worldwide PURE study and reviewed and commented on the drafts of the manuscript. U.V. was involved in the statistical analyses. S.Y. designed the study, conceived and initiated PURE, supervised its conduct and data analysis, and provided comments on all drafts. All authors coordinated the study, collected data in their respective countries, and/or provided comments on drafts of the manuscript. S.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Footnotes
R.M.A. and V.M. are joint first authors.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12985481.
References
- 1.International Diabetes Federation IDF Diabetes Atlas, 9th edition, 2019. Accessed 6 December 2019. Available from https://www.diabetesatlas.org/en//
- 2.World Health Organization Diabetes. Accessed October. 20172018 Available from https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 3.Raghavan S, Vassy JL, Ho YL, et al. . Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc 2019;8:e011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tancredi M, Rosengren A, Svensson AM, et al. . Excess mortality among persons with type 2 diabetes. N Engl J Med 2015;373:1720–1732 [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Coady S, Sorlie PD, et al. . Trends in cardiovascular complications of diabetes. JAMA 2004;292:2495–2499 [DOI] [PubMed] [Google Scholar]
- 6.Carson AP, Tanner RM, Yun H, et al. . Declines in coronary heart disease incidence and mortality among middle-aged adults with and without diabetes. Ann Epidemiol 2014;24:581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng M, Freeman MK, Fleming TD, et al. . Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA 2014;311:183–192 [DOI] [PubMed] [Google Scholar]
- 8.Rawshani A, Rawshani A, Franzén S, et al. . Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018;379:633–644 [DOI] [PubMed] [Google Scholar]
- 9.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S; PURE Investigators-Writing Group . The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J 2009;158:1–7.e1 [DOI] [PubMed] [Google Scholar]
- 10.Corsi DJ, Subramanian SV, Chow CK, et al. . Prospective Urban Rural Epidemiology (PURE) study: baseline characteristics of the household sample and comparative analyses with national data in 17 countries. Am Heart J 2013;166:636–646.e4 [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Rangarajan S, Teo K, et al.; PURE Investigators . Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818–827 [DOI] [PubMed] [Google Scholar]
- 12.Miller V, Mente A, Dehghan M, et al.; Prospective Urban Rural Epidemiology (PURE) study investigators . Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet 2017;390:2037–2049 [DOI] [PubMed] [Google Scholar]
- 13.Gupta R, Islam S, Mony P, et al. . Socioeconomic factors and use of secondary preventive therapies for cardiovascular diseases in South Asia: the PURE study. Eur J Prev Cardiol 2015;22:1261–1271 [DOI] [PubMed] [Google Scholar]
- 14.WHO Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva, Switzerland, World Health Organization, 2006 [Google Scholar]
- 15.Dehghan M, Mente A, Rangarajan S, et al.; Prospective Urban Rural Epidemiology (PURE) study investigators . Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2018;392:2288–2297 [DOI] [PubMed] [Google Scholar]
- 16.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526 [Google Scholar]
- 17.Gregg EW, Cheng YJ, Saydah S, et al. . Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care 2012;35:1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 2007;147:149–155 [DOI] [PubMed] [Google Scholar]
- 19.Lind M, Garcia-Rodriguez LA, Booth GL, et al. . Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia 2013;56:2601–2608 [DOI] [PubMed] [Google Scholar]
- 20.Jansson SP, Andersson DK, Svärdsudd K. Mortality trends in subjects with and without diabetes during 33 years of follow-up. Diabetes Care 2010;33:551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujimoto T, Kajio H, Sugiyama T. Favourable changes in mortality in people with diabetes: US NHANES 1999-2010. Diabetes Obes Metab 2018;20:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan V, Shanthi Rani CS, Amutha A, et al. . Clinical profile of long-term survivors and nonsurvivors with type 2 diabetes. Diabetes Care 2013;36:2190–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatib R, McKee M, Shannon H, et al.; PURE study investigators . Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet 2016;387:61–69 [DOI] [PubMed] [Google Scholar]
- 24.Attaei MW, Khatib R, McKee M, et al.; PURE study investigators . Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet Public Health 2017;2:e411–e419 [DOI] [PubMed] [Google Scholar]
- 25.Chow CK, Ramasundarahettige C, Hu W, et al.; PURE investigators . Availability and affordability of essential medicines for diabetes across high-income, middle-income, and low-income countries: a prospective epidemiological study. Lancet Diabetes Endocrinol 2018;6:798–808 [DOI] [PubMed] [Google Scholar]
- 26.Murphy A, Palafox B, O’Donnell O, et al. . Inequalities in the use of secondary prevention of cardiovascular disease by socioeconomic status: evidence from the PURE observational study. Lancet Glob Health 2018;6:e292–e301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xavier D, Pais P, Devereaux PJ, et al.; CREATE registry investigators . Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet 2008;371:1435–1442 [DOI] [PubMed] [Google Scholar]
- 28.Langhorne P, O’Donnell MJ, Chin SL, et al.; INTERSTROKE collaborators . Practice patterns and outcomes after stroke across countries at different economic levels (INTERSTROKE): an international observational study. Lancet 2018;391:2019–2027 [DOI] [PubMed] [Google Scholar]