Abstract
OBJECTIVE
Diabetes and obesity are highly prevalent among hospitalized patients with coronavirus disease 2019 (COVID-19), but little is known about their contributions to early COVID-19 outcomes. We tested the hypothesis that diabetes is a risk factor for poor early outcomes, after adjustment for obesity, among a cohort of patients hospitalized with COVID-19.
RESEARCH DESIGN AND METHODS
We used data from the Massachusetts General Hospital (MGH) COVID-19 Data Registry of patients hospitalized with COVID-19 between 11 March 2020 and 30 April 2020. Primary outcomes were admission to the intensive care unit (ICU), need for mechanical ventilation, and death within 14 days of presentation to care. Logistic regression models were adjusted for demographic characteristics, obesity, and relevant comorbidities.
RESULTS
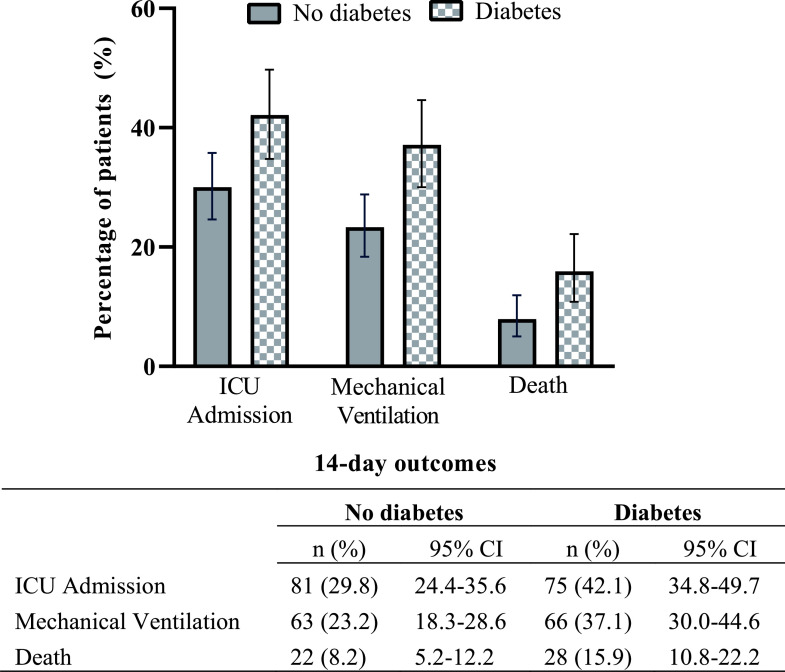
Among 450 patients, 178 (39.6%) had diabetes—mostly type 2 diabetes. Among patients with diabetes versus patients without diabetes, a higher proportion was admitted to the ICU (42.1% vs. 29.8%, respectively, P = 0.007), required mechanical ventilation (37.1% vs. 23.2%, P = 0.001), and died (15.9% vs. 7.9%, P = 0.009). In multivariable logistic regression models, diabetes was associated with greater odds of ICU admission (odds ratio 1.59 [95% CI 1.01–2.52]), mechanical ventilation (1.97 [1.21–3.20]), and death (2.02 [1.01–4.03]) at 14 days. Obesity was associated with greater odds of ICU admission (2.16 [1.20–3.88]) and mechanical ventilation (2.13 [1.14–4.00]) but not with death.
CONCLUSIONS
Among hospitalized patients with COVID-19, diabetes was associated with poor early outcomes, after adjustment for obesity. These findings can help inform patient-centered care decision making for people with diabetes at risk for COVID-19.
Introduction
Diabetes is one of the most common chronic conditions in the U.S., currently estimated to affect 34.2 million people (10.5% of the U.S. population) (1). In addition to the well-documented adverse health outcomes associated with this disease, diabetes has also emerged as a commonly reported risk factor among people hospitalized with coronavirus disease 2019 (COVID-19), caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2–7). Following initial reports of COVID-19 in Washington state (January 2020) (8), COVID-19 has spread rapidly and resulted in >3.0 million cases and nearly 135,000 deaths in the U.S. as of July 2020 (9). The convergence of these two epidemics has raised critical questions about the relationship between diabetes and COVID-19 severity (10,11), particularly in light of the increasing recognition that obesity, which is also highly prevalent in the U.S., is also a strong risk factor for severe COVID-19 (12–14).
Consistent with early reports emerging from China (15,16), several U.S. studies have confirmed a high prevalence of diabetes and obesity among hospitalized patients with COVID-19 (4,13,17). However, few studies have evaluated whether patients with diabetes who are hospitalized with COVID-19 are more likely to experience poor early outcomes (4,11) and whether this association remains after adjustment for obesity status (4,13). Given that ∼61% of people with diabetes in the U.S. have obesity (1), understanding the contribution of these comorbidities to early outcomes among people hospitalized with COVID-19 is critically important to inform patient-centered care decision making for people with these conditions. In this study, we tested the hypothesis that diabetes is associated with poor early outcomes of hospitalization with COVID-19, even after obesity and other relevant clinical characteristics are accounted for.
Research Design and Methods
Data Source and Sample
This study used data from the Massachusetts General Hospital (MGH) COVID-19 Data Registry. Patients were eligible for inclusion in the registry if they presented to care (defined as the first contact with the health care system with COVID-19 symptoms) and were subsequently hospitalized at MGH with PCR-confirmed SARS-CoV-2 infection between 11 March 2020 and 30 April 2020. Over the study period, MGH had the highest total number of hospitalized patients with COVID-19 in Massachusetts, which in turn has had one of the largest COVID-19 case numbers in the U.S. as of June 2020 (9,18). All patients included in our study were followed for 14 days from the date of initial presentation to care with COVID-19. General inpatient diabetes at MGH is managed mostly by the primary medical team with basal-bolus supplemental insulin or supplemental insulin alone for mild hyperglycemia, whereas insulin drips are favored in the intensive care unit (ICU) setting. Specialty consultation is widely available for challenging cases.
Additional registry data were collected by performance of manual chart review for key aspects of the past medical history and details of the hospitalization. Chart review was performed by physicians and research nurses and their assistants. All members of the chart review team underwent detailed training on the standard operating procedure for data extraction, which was also subjected to quality checks to ensure data accuracy and minimize missing variables. In addition, height, weight, and BMI as well as hemoglobin A1c (HbA1c) obtained during the hospitalization and blood glucose at admission during the index hospitalization were obtained electronically through the Enterprise Data Warehouse (EDW), a repository that was derived from the Epic electronic medical records system. For patients with diabetes who did not have an HbA1c measured during the index hospitalization, we obtained HbA1c values directly from the historical electronic medical record if available within the year preceding the index hospitalization for inclusion in a subgroup analysis of the relationship between baseline glycemic control and 14-day outcomes. The registry and this research were approved by the Partners HealthCare Institutional Review Board (protocol number 2020P000829).
Definitions of Diabetes, Obesity, and Clinical Covariates
Diabetes was defined based on the following criteria: 1) past medical history of diabetes as documented in the medical record and manually retrieved on chart review, 2) HbA1c ≥6.5% during the index hospitalization, or 3) random blood glucose ≥200 mg/dL at admission to the hospital with supportive history by chart review. Only four individuals had a presenting random blood glucose of ≥200 mg/dL in the absence of a recent elevated HbA1c or chart review diagnosis of diabetes. Two endocrinologists adjudicated these cases and determined that two of the four individuals had diabetes. The distribution of diabetes diagnoses based on these criteria is provided in the Supplementary Materials (p. 2). BMI, defined as weight in kilograms divided by the square of height in meters, was available from the EDW and was defined using standard BMI thresholds of <18.5 kg/m2 for underweight, 18.5–24.9 kg/m2 for normal weight, 25.0–29.9 kg/m2 for overweight, and ≥30.0 kg/m2 for obese. Additional analyses with subcategorization of the BMI ≥30.0 kg/m2 group into ≥30.0–34.9 kg/m2 and ≥35.0 kg/m2 are provided in the Supplementary Materials (p. 7). Models were adjusted for race/ethnicity (available from the EDW based on self-report) and defined as non-Hispanic White, non-Hispanic African American, Hispanic, other, and unknown. Finally, we manually extracted comorbidities that have commonly been reported among hospitalized patients with COVID-19 (17): coronary artery disease (CAD) or myocardial infarction (MI), congestive heart failure (CHF) (including both heart failure with preserved ejection fraction and heart failure with reduced ejection fraction), hypertension, renal disease, liver disease, chronic obstructive pulmonary disease (COPD) or asthma, and active malignancy, using standard clinical diagnostic criteria.
Statistical Analysis
The primary outcomes in this study were 1) admission to the ICU, 2) need for mechanical ventilation, and 3) death among hospitalized patients with COVID-19 within 14 days of presentation to care. These states were not mutually exclusive, so an individual could experience more than one of these outcomes within the 14-day period. The exposure of interest was diabetes as defined above and, as a secondary exposure of interest, BMI category. We first described differences in the demographic and health characteristics by diabetes status. We used t tests to compare means and χ2 tests to compare proportions. We then stratified the cohort by diabetes status and BMI category and compared the proportions of people with and without diabetes within each BMI category for all three outcomes using χ2 tests. To test the hypothesis that poor baseline glycemic control would be associated with severe COVID-19, we conducted a subanalysis of the group with diabetes and compared the proportion of people by outcome according to HbA1c values obtained either during the hospitalization if available or using the most recent value available in the year preceding the index hospitalization. HbA1c was categorized as poor glycemic control (HbA1c >9.0% according to HEDIS definition [19]) versus better glycemic control: ≤7.0% and >7.0–9.0%. Lastly, we conducted univariate and multivariable logistic regression to evaluate the association of diabetes, BMI category, and each of the primary outcomes. Multivariable models were additionally adjusted for age, sex, race/ethnicity, and comorbidities. Given that there were individuals who died prior to being mechanically ventilated, we conducted sensitivity analyses that excluded these individuals as well as a composite outcome “mechanical ventilation or death,” which we provide in the Supplementary Materials (p.6). In all analyses, results were considered statistically significant if the corresponding P value was <0.05. Analyses were conducted in Stata v. 15.1.
Results
This article reports on 450 people who were eligible for inclusion in the MGH COVID-19 Data Registry and were hospitalized at MGH with SARS-CoV-2 infection between 11 March 2020 and 30 April 2020. The baseline characteristics of the study population are summarized in Table 1. Of the 450 patients, 178 (39.6%) had diabetes, the vast majority of which was type 2 diabetes (mean HbA1c 8.1%). Patients with diabetes were older than those without diabetes (mean age 66.7 vs. 61.6 years), and 42.1% of patients with diabetes were age ≥70 years (Table 1). There was a higher proportion of patients with diabetes who had obesity (51.4%, χ2df = 2 = 9.6, P = 0.008) compared with the proportion of those without diabetes (36.8%). A higher proportion of patients with diabetes had a past medical history of CHF, hypertension, and renal disease compared with the proportion of patients without diabetes (Table 1).
Table 1.
Characteristics of 450 hospitalized people with COVID-19 and with or without diabetes
| No diabetes | Diabetes | P | |
|---|---|---|---|
| (N = 272) | (N = 178) | ||
| Age (years), mean ± SD | 61.1 ± 18.8 | 66.7 ± 14.2 | 0.007 |
| Age (years), n (%) | |||
| <50 | 84 (31) | 20 (11.2) | <0.001 |
| 50–59 | 41 (15.1) | 41 (23.0) | |
| 60–69 | 40 (14.8) | 42 (23.6) | |
| ≥70 | 106 (39.1) | 75 (42.1) | |
| Sex, n (%) | |||
| Male | 149 (54.8) | 110 (61.8) | 0.14 |
| Female | 123 (45.2) | 68 (38.2) | |
| HbA1c (%), mean ± SD | — | 8.1 ± 2.0 | |
| Race/ethnicity, n (%) | |||
| White | 136 (50.8) | 89 (50.3) | 0.26 |
| Hispanic | 83 (30.5) | 41 (23.0) | |
| African American | 18 (6.6) | 19 (10.7) | |
| Other | 8 (2.9) | 7 (3.9) | |
| Unknown/missing | 27 (9.9) | 22 (12.4) | |
| BMI category, n (%) | |||
| Underweight/normal | 67 (24.6) | 36 (20.3) | 0.008 |
| Overweight | 105 (38.3) | 50 (28.3) | |
| Obese | 100 (36.8) | 91 (51.4) | |
| Comorbidities, n (%) | |||
| CAD or MI | 47 (17.3) | 44 (24.7) | 0.06 |
| CHF | 23 (8.4) | 29 (16.3) | 0.01 |
| Hypertension | 44 (24.7) | 134 (75.3) | <0.001 |
| COPD/asthma | 66 (24.3) | 46 (25.8) | 0.71 |
| Cancer (active) | 17 (6.3) | 4 (2.3) | 0.05 |
| Liver disease | 25 (9.4) | 21 (11.9) | 0.41 |
| Renal disease | 40 (14.9) | 46 (26.0) | 0.004 |
t tests were used to compare means and χ2 tests were used to compare proportions. BMI categorization: <18.5 kg/m2 for underweight, >18.5–24.9 kg/m2 for normal weight, >25.0–29.9 kg/m2 for overweight, and >30.0 kg/m2 for obese. The majority of diabetes cases were type 2 diabetes; only two patients in the cohort had type 1 diabetes.
Outcomes by Diabetes, BMI Category, and Baseline Glycemic Control
There were 156 ICU admissions, 129 people who required mechanical ventilation, and 49 deaths during the first 14 days after presentation to care. Among patients with diabetes versus patients without diabetes, a higher proportion was admitted to the ICU (42.1% vs. 29.8%, respectively, χ2df = 1 = 7.3, P = 0.007), had mechanical ventilation (37.1% vs. 23.2%, χ2df = 1 = 10.2, P = 0.001), or died (15.9% vs 7.9%, χ2df = 1 = 6.8, P = 0.009) over this time horizon (Fig. 1). The numerical counts for patients requiring ICU admission and mechanical ventilation were higher for people with diabetes than for people without diabetes within each BMI category, but pairwise comparisons of the proportions did not achieve statistical significance (Fig. 2). In a subanalysis of people with diabetes and HbA1c (n = 168), the proportion of people who required ICU admission and mechanical ventilation was greater among people with HbA1c >9.0 (52.5% and 47.5%, respectively) than among people with HbA1c >7.0–9.0% (40.3% and 36.1%, respectively) and ≤7.0% (41.4% and 33.9%, respectively), but the differences did not achieve statistical significance at the 0.05 level (Supplementary Materials, p. 3). Multivariable logistic regression models that include baseline glycemic control as a dichotomous variable are provided in the Supplementary Materials (p. 4).
Figure 1.
Fourteen-day outcomes among 450 hospitalized patients with COVID-19, according to diabetes status. Three outcomes are shown on the x-axis, and the percentage of patients in each outcome group is shown on the y-axis. Within each outcome, all pairwise χ2 tests were P < 0.05.
Figure 2.
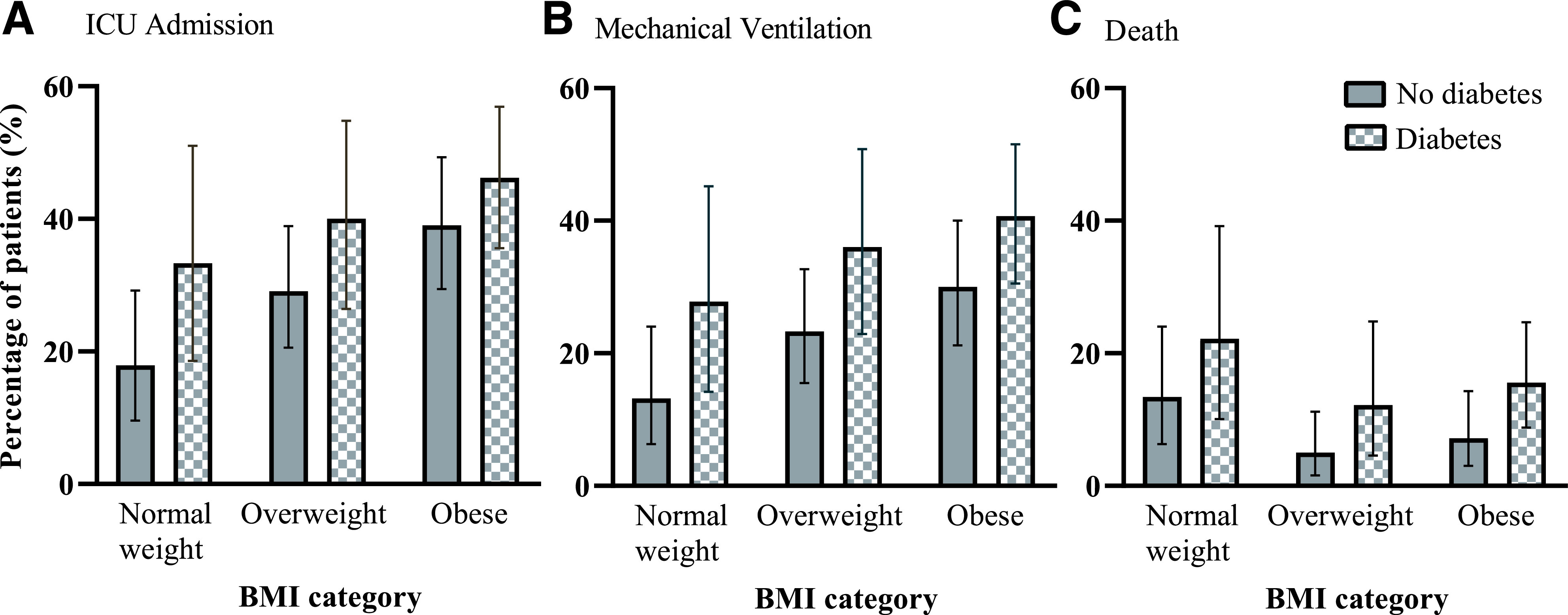
Fourteen-day outcomes among 450 hospitalized patients with COVID-19, according to diabetes status and BMI category. Each panel represents an outcome: ICU admission (A), mechanical ventilation (B), and death (C). Three BMI categories are shown on the x-axis, and the percentage of patients in each BMI category is shown on the y-axis. Within each BMI category, all pairwise χ2 testing was not statistically significant.
Logistic Regression Models of ICU Admission, Mechanical Ventilation, and Death
Univariate models are provided in the Supplementary Materials (p. 5). In multivariable logistic regression models, diabetes was associated with greater odds of ICU admission (odds ratio [OR] 1.59 [95% CI 1.01–2.52]), mechanical ventilation (1.97 [1.21–3.20]), and death (2.02 [1.01–4.03]) after adjustment for age-group, sex, race/ethnicity, BMI category, and medical comorbidities (Table 2). Obesity was associated with greater odds of ICU admission (2.16 [1.20–3.88]) and mechanical ventilation (2.13 [1.14–4.00]) but not with greater odds of death (1.10 [0.50–2.45]) (Table 2). Age ≥70 years was associated with increased odds of death (12.66 [1.50–106.56]), after adjustment for all other characteristics. Male sex (1.60 [1.00–2.57]) and Hispanic ethnicity (1.94 [1.08–3.44]) were associated with need for mechanical ventilation as compared with female sex and White/Non-Hispanic race/ethnicity, respectively. Interaction terms for diabetes and obesity in the models of both ICU admission and need for mechanical ventilation were tested and were not statistically significant. In a sensitivity analysis that excluded people who died without having mechanical ventilation, the association between diabetes and mechanical ventilation (OR 2.01 [1.24–3.25]) and obesity and mechanical ventilation (2.01 [1.05–3.82]) remained of a magnitude and significance similar to those in the primary analysis (Supplementary Materials, p. 6). Multivariable logistic regression analysis with additional subcategorization of the BMI group ≥30.0 kg/m2 showed that BMI ≥30.0–34.9 kg/m2 remained associated with greater odds of ICU admission (2.20 [1.14–4.27]) and mechanical ventilation (2.32 [1.15–4.68]), but the ≥35.0 kg/m2 category was only associated with greater odds of ICU admission (2.11 [1.09–4.09]) (Supplementary Materials, p. 7).
Table 2.
Multivariable logistic regression analysis of 14-day outcomes among 450 hospitalized people with COVID-19
| 14-day outcomes, OR (95% CI) | |||
|---|---|---|---|
| ICU admission | Mechanical ventilation | Death | |
| (N = 436) | (N = 436) | (N = 417) | |
| Diabetes | 1.59 (1.01–2.52) | 1.97 (1.21–3.20) | 2.02 (1.01–4.03) |
| BMI category | |||
| Overweight | 1.42 (0.78–2.58) | 1.42 (0.75–2.71) | 0.60 (0.25–1.44) |
| Obese | 2.16 (1.20–3.88) | 2.13 (1.14–4.00) | 1.10 (0.50–2.45) |
| Age (years) | |||
| 50–59 | 1.13 (0.57–2.24) | 1.02 (0.50–2.08) | 3.74 (0.40–34.54) |
| 60–69 | 1.22 (0.60–2.49) | 1.20 (0.57–2.54) | 4.57 (0.50–41.9) |
| ≥70 | 1.41 (0.69–2.85) | 1.72 (0.82–3.61) | 12.66 (1.50–106.56) |
| Male | 1.36 (0.88–2.12) | 1.60 (1.00–2.57) | 1.35 (0.66–2.75) |
| Race/ethnicity | |||
| Hispanic | 1.60 (0.93–2.76) | 1.94 (1.08–3.44) | 0.44 (0.15–1.25) |
| African American | 1.36 (0.62–2.97) | 1.47 (0.64–3.40) | 0.35 (0.07–1.62) |
| Other | 2.30 (0.76–6.98) | 1.72 (0.53–5.60) | — |
| Unknown/missing | 2.75 (1.38–5.47) | 2.34 (1.15–4.76) | 0.68 (0.21–2.18) |
| CAD or MI | 0.63 (0.33–1.19) | 0.55 (0.27–1.11) | 0.71 (0.31–1.61) |
| CHF | 1.65 (0.77–3.51) | 0.98 (0.42–2.26) | 1.94 (0.78–4.85) |
| Hypertension | 0.96 (0.57–1.60) | 0.78 (0.45–1.35) | 1.33 (0.57–3.10) |
| COPD/asthma | 0.66 (0.39–1.11) | 0.55 (0.31–0.98) | 0.75 (0.35–1.60) |
| Cancer (active) | 0.60 (0.18–1.94) | 0.55 (0.15–2.05) | 1.57 (0.46–5.36) |
| Liver disease | 1.13 (0.57–2.23) | 1.10 (0.54–2.25) | 0.91 (0.27–3.03) |
| Renal disease | 0.89 (0.49–1.64) | 1.09 (0.58–2.09) | 1.22 (0.55–2.73) |
Reference groups: “no disease” for diabetes and all comorbidities, normal weight for BMI category, age <50 years, female sex, and non-Hispanic White. Events per outcome: 156 people were admitted to the ICU, 129 people were mechanically ventilated, and 49 people died within 14 days of presentation to care.
Conclusions
In this observational study of 450 patients hospitalized with COVID-19, we found that compared with proportions of patients without diabetes, greater proportions of patients with diabetes were admitted to the ICU, required mechanical ventilation, and died within 14 days of presentation to care. We also found that diabetes was associated with 1.6-fold increased odds of ICU admission, 2.0-fold increased odds of mechanical ventilation, and 2.0-fold increased odds of death within 14 days from presentation to care, after adjustment for obesity. These findings add to the growing body of literature about the relationship of diabetes, obesity, and COVID-19 severity among people hospitalized with COVID-19 in the U.S. and expand our current understanding of the contributions of these two conditions to early outcomes among people hospitalized with COVID-19. Given the high prevalence of diabetes and obesity in the U.S. (1) and the rapidly rising case numbers of COVID-19 (9), these findings have important implications for patient-centered care decision making for people with metabolic disease and COVID-19.
Several other important findings emerged from our analysis. First, we found that the proportion of people with and without diabetes who were admitted to the ICU and who required mechanical ventilation was higher in magnitude with increasing BMI category. We also found that obesity was associated with 2.2-fold increased odds of ICU admission and 2.1-fold increased odds of mechanical ventilation compared with normal weight, after adjustment for diabetes, age, sex, race/ethnicity, and major comorbidities. These findings are consistent with numerous studies reporting that obesity is a strong risk factor for severe COVID-19 (12–14) and add to the current literature by showing that this association persists at lower BMI thresholds than previously reported in a U.S. population (4,13).
Second, we did not find a significant difference in the association between poor baseline glycemic control, as measured by HbA1c >9.0 at hospitalization or within the year prior to admission, and early outcomes in this cohort; however, the proportion of patients with severe COVID-19 was higher in magnitude among patients with poor glycemic control compared with that among patients with better glycemic control. Although there has been little study of prehospitalization HbA1c and COVID-19 outcomes, mounting evidence suggests that poor glycemic control among hospitalized patients with COVID-19 is associated with adverse outcomes, including higher mortality (20–23). Since the lack of an association between baseline glycemic control and early outcomes may have been limited by sample size in our study, the question of whether baseline glycemic control is associated with poor early COVID-19 outcomes remains a critically important one in consideration of the implications for risk stratification for this patient population.
Third, we found that Hispanic ethnicity was associated with greater odds of mechanical ventilation. This finding is consistent with recent data showing that ethnic minorities in the U.S. have a greater risk of poor COVID-19 outcomes (24–27). However, understanding the relationship between race/ethnicity and COVID-19 severity requires a more complex framework than afforded by the scope of this analysis. Given the limited data on racial/ethnic disparities and COVID-19 severity, future studies are needed to better understand these findings. Finally, the lack of an association between other major comorbidities and COVID-19 severity is also consistent with most U.S.-based adjusted analyses to date (4,13) but may also be related to limited power, since these comorbidities were less common than diabetes and obesity in our sample.
Our findings are consistent with numerous studies that have reported a high prevalence of diabetes among people with severe COVID-19 (2,4,5,7,13,15,28,29) and add to the growing literature regarding the intersection of these epidemics by showing that the relationship between diabetes and increased odds of three key measures of COVID-19 severity at 14 days remains after adjustment for obesity. While several international studies have analyzed the association between diabetes and severe COVID-19 (30,31), only a few studies have presented this association according to stratified outcomes among patients hospitalized with COVID-19 (32,33). In their cross-sectional analysis of 51,633 Mexican subjects with COVID-19, Bello-Chavolla et al. (30) showed that diabetes (self-reported in 18.3% of subjects) and obesity (self-reported in 20.7% of subjects) were associated with greater odds of hospitalization, ICU admission, and mechanical ventilation. With use of a mechanistic COVID-19 lethality score, early-onset diabetes (defined as diabetes among individuals younger than 40 years of age) also emerged as an important predictor of mortality (hazard ratio 2.86 [95% CI 2.19–3.76]). In a similar study of 50,411 Mexican individuals with COVID-19, Gutierrez and Bertozzi reported that diabetes was associated with a 1.21 odds of mechanical ventilation and 1.95 odds of death (32). Although the effect size of these associations was larger in our study, it is possible that the risk was underestimated in the study by Gutierrez and Bertozzi due to use of self-reported diabetes diagnosis only and the possible misclassification of individuals without diabetes who in fact had diabetes.
Few studies evaluating the association between diabetes and COVID-19 severity have been published to date in a U.S. population. In a longitudinal cohort study of 2,729 patients hospitalized with COVID-19 in New York, Petrilli et al. (4) showed that diabetes and obesity (≥40 kg/m2) were associated with 1.2 and 1.5 increased odds of critical illness, respectively. However, Petrilli et al. defined critical illness as a composite of care in the ICU, use of mechanical ventilation, discharge to hospice, or death, thus capturing a composite outcome. While composite outcomes are based in an assumption that components have the same directional relationship with the treatment or exposure, our analysis suggests that components of this outcome may have different relationships with diabetes and obesity. Palaiodimos et al. (13) analyzed data on the first 200 patients admitted with COVID-19 to a tertiary hospital in the Bronx, New York. Patients were followed for 3 weeks after admission to the hospital. In contrast to the findings of our study, only BMI ≥35 kg/m2 was associated with poor COVID-19 outcomes (13). Although diabetes did not emerge as a risk factor for severe COVID-19 in their study, the lack of an association may have been due to limitations of a small sample size of people with diabetes (n = 79).
Aside from previously studied mechanisms that predispose people with diabetes to higher risk of infections, such as impaired immunity (33,34), several mechanisms have been proposed suggesting a specific association between diabetes and SARS-CoV-2 illness severity (2). Rao, Lau, and So (35) showed that diabetes and related traits are associated with increased expression of ACE2, the receptor for SARS-CoV-2, which could influence susceptibility and severity of infection. In addition, cytokines may be another mechanistic link between diabetes and SARS-CoV-2 illness severity. For instance, interleukin-6 (IL-6), a pleiotropic cytokine that is increased in diabetes and metabolic disorders (10), has also emerged as a predictor of poor prognosis among people with COVID-19 (36). However, because most studies are preliminary, additional work is needed to understand mechanisms underlying the association between diabetes and increased COVID-19 severity (7).
One of the strengths of this study is that manual chart review allowed for detailed patient information to be incorporated into the analysis, including the adjudication of diabetes status by endocrinologists. However, this study had several limitations. First, the study was conducted at a single institution in Massachusetts, which had a different COVID-19 prevalence, rate of COVID-19 case increase, and ICU bed capacity (18) than the sites of other studies referenced here (4,13). Second, the sample size for this study was limited, which may have affected our ability to detect significant differences, especially in the association between prehospitalization glycemic control and the outcomes of interest. However, despite the small sample size, the completeness of data allowed us to rigorously evaluate the primary exposures, outcomes, and covariates, including medical comorbidities. Another important limitation is that people who died without escalation of care may have opted against admission to the ICU, for instance, due to do-not-resuscitate/do-not-intubate code status, hence reflecting their outcome in the context of their goals of care rather than as part of their course throughout the hospitalization with COVID-19. However, in sensitivity analyses that excluded these individuals and in one that included a composite outcome of mechanical ventilation or death, the magnitude and significance of the main associations reported remained. Lastly, the observational data and methodological approach used in this study identified potentially important associations but do not establish causal relationships.
The convergence of the diabetes and COVID-19 epidemics has raised critical concerns regarding the heightened illness severity associated with these conditions (10,11). This is a cause for particular concern in the U.S., where the prevalence of metabolic disease is high and may place many people at greater risk of developing severe COVID-19 should they become infected with SARS-CoV-2. In this study, we showed that diabetes was associated with poor early outcomes among hospitalized patients with COVID-19, after adjustment for obesity, which was also a strong risk factor for poor early outcomes. Our findings can help guide risk mitigation efforts and patient-centered care decision making for people with diabetes and obesity, particularly in areas of the U.S. with a high prevalence of diabetes and obesity that are in early phases of the SARS-CoV-2 outbreak. Future research is urgently needed to better understand the mechanisms underlying the relationship between diabetes, obesity, and COVID-19 severity and approaches to prevent and treat people with metabolic disease and COVID-19.
Article Information
Acknowledgments. The authors thank the COVID-19 Data Registry Team at MGH for their leadership in creating this valuable resource. In particular, the authors thank the numerous manual chart reviewers and experienced data managers for their methodological expertise, time, and effort.
Funding. Support for the MGH COVID-19 Data Registry was provided by the MGH Division of Clinical Research. J.S. and S.J.C. are supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant T32DK007028. J.P. is supported by NIDDK grant 5T32DK007191-45. V.A.T. is supported by National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), grant R01HL132786 and National Institute of Allergy and Infectious Diseases, NIH, grant R01AG062393. J.M.-G. is supported by National Institute of Allergy and Infectious Diseases grant T32AI007433. I.V.B. is supported by National Institute of Allergy and Infectious Diseases grant K24AI141036. A.S.F. is supported by National Institute of General Medical Sciences, NIH, grant R01GM127862. J.H. is supported by National Institute on Aging grant R01AG062282 and NIDDK grant R01DK085070.
The contents of this research are solely the responsibility of the authors.
Duality of Interest. D.J.W. reports serving on a data monitoring committee for Novo Nordisk. A close family member of S.J.C. is employed by a Johnson & Johnson company. J.B.M. is an Academic Associate for Quest Diagnostics. J.H. has consulted for several health care systems. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.-G., J.S., J.P., D.J.W., and J.B.M. co-conceived the study. J.M.-G., J.S., J.P., D.J.W., and J.B.M. led the analysis, which was carried out by J.M.-G. A.S.F. provided additional input on the analysis. J.M.-G., J.S., and J.P. wrote the first draft of the manuscript, and all authors provided critical input on multiple iterations. All authors approved the final version. J.M.-G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
J.S. and J.P. made equal contributions.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12766001.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2020. Accessed 16 July 2020. Available from https://www.cdc.gov/diabetes/data/statistics-report/index.html [Google Scholar]
- 2.Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr 2020;14:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020;81:e16–e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wang S, Sun L, Qin G. Prevalence of diabetes mellitus in 2019 novel coronavirus: a meta-analysis. Diabetes Res Clin Pract 2020;164:108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shabto JM, Loerinc L, O’Keefe GA, O’Keefe J. Characteristics and outcomes of COVID-19 positive patients with diabetes managed as outpatients. Diabetes Res Clin Pract 2020;164:108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holshue ML, DeBolt C, Lindquist S, et al.; Washington State 2019-nCoV Case Investigation Team . First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC Coronavirus Disease 2019 (COVID-19) in the U.S.: Cases, Data & Surveillance: Cases in the U.S. Atlanta, GA, Centers for Disease Control and Prevention, 2020. Accessed 4 May 2020. Available from https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [Google Scholar]
- 10.Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes/Metabolism Research and Reviews. 31 March 2020 [Epub ahead of print] DOI: 10.1002/dmrr.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riddle MC, Buse JB, Franks PW, et al. COVID-19 in people with diabetes: urgently needed lessons from early reports. Diabetes Care 2020;43:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020;43:1392–1398 [DOI] [PubMed] [Google Scholar]
- 13.Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020;108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Zheng KI, Wang X-B, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 2020;43:e72–e74 [DOI] [PubMed] [Google Scholar]
- 15.Guan WJ, Ni ZY, Hu Y, et al.; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S, Hirsch JS, Narasimhan M, et al.; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID-19 Response Reporting Commonwealth of Massachusetts. Accessed 15 June 2020. Available from https://www.mass.gov/info-details/covid-19-response-reporting
- 19.HEDIS Measures and Technical Resources Washington, DC, National Committee for Quality Assurance. Accessed 4 June 2020. Available from https://www.ncqa.org/hedis/measures/
- 20.Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract 2020;164:108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol 2020;14:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, She Z-G, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068–1077.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Huang J, Zhu G, et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: a retrospective cohort study. BMJ Open Diabetes Res Care 2020;8:e001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood) 2020;39:1253–1262 [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Educ Behav 2020;47:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVID-19: Data: Summary City of New York. Accessed 29 May 2020. Available from https://www1.nyc.gov/site/doh/covid/covid-19-data.page
- 27.Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020;323:2466–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect 2020;26:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab 2020;105:dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denova-Gutiérrez E, Lopez-Gatell H, Alomia-Zegarra JL, et al. The association between obesity, type 2 diabetes, and hypertension with severe COVID-19 on admission among Mexicans. Obesity (Silver Spring). 1 July 2020. [Epub ahead of print]. DOI: 10.1002/oby.22946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez JP, Bertozzi SM. Non-communicable diseases and inequalities increase risk of death among COVID-19 patients in Mexico. medRxiv. 29 May 2020. [preprint]. DOI: 10.1101/2020.05.27.20115204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011;6:e23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci 2016;351:201–211 [DOI] [PubMed] [Google Scholar]
- 35.Rao S, Lau A, So H-C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care 2020;43:1416–1426 [DOI] [PubMed] [Google Scholar]
- 36.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]