Abstract
OBJECTIVE
Patients with diabetes and chronic kidney disease (CKD) have increased susceptibility to acute kidney injury (AKI), but mechanisms are unclear. We investigated the association of glycemic control with risk of AKI.
RESEARCH DESIGN AND METHODS
In two observational cohorts of U.S. (Geisinger Health System, Danville, PA) and Swedish (Stockholm CREAtinine Measurements [SCREAM] project, Stockholm, Sweden) adults with type 2 diabetes and confirmed CKD stages G3–G5 undergoing routine care, we evaluated associations between baseline and time-varying hemoglobin A1c (HbA1c) with the incident AKI (defined as increase in creatinine ≥0.3 mg/dL over 48 h or 1.5 times creatinine over 7 days).
RESULTS
In the U.S. cohort, there were 22,877 patients (55% women) with a median age of 72 years and estimated glomerular filtration rate (eGFR) 52 mL/min/1.73 m2. In the Swedish cohort, there were 12,157 patients (50% women) with a median age of 77 years and eGFR 51 mL/min/1.73 m2. During 3.1 and 2.3 years of follow-up, 7,060 and 2,619 AKI events were recorded in the U.S. and Swedish cohorts, respectively. The adjusted association between baseline HbA1c and AKI was similar in both cohorts. Compared with baseline HbA1c 6–6.9% (42–52 mmol/mol), the hazard ratio for AKI in patients with HbA1c >9% (75 mmol/mol) was 1.29 (95% CI 1.18–1.41) in Geisinger and 1.33 (95% CI 1.13–1.57) in the Swedish cohort. Results were consistent in stratified analysis, when using death as competing risk, and when using time-varying HbA1c.
CONCLUSIONS
Higher HbA1c was associated with AKI in adults with type 2 diabetes and CKD, suggesting that improving glycemic control may reduce the risk of AKI.
Introduction
Chronic kidney disease (CKD) is present in up to 40% of patients with type 2 diabetes, contributing to elevated health care costs, poor quality of life, increased risk adverse drug events, and risk of death (1,2). The presence of CKD considerably increases the complexity of diabetes management (3,4). Patients with CKD and type 2 diabetes have increased risk of hypoglycemia (5,6). Due to the risks of hypoglycemia and challenges in patients in CKD, diabetes guidelines recommend less intensive glycemic control (a higher hemoglobin A1c [HbA1c] target) in patients with CKD compared with patients without (7–9).
Acute kidney injury (AKI), a sudden (over hours or days) deterioration in kidney function, occurs in 8–17% of hospital admissions (10) and is associated with poor in-hospital outcomes (11), increased length of hospital stay, and high health care costs (11,12). For patients with AKI who survive to hospital discharge, there continues to be an elevated risk of adverse outcomes, including subsequent admission with heart failure (11,13), progression to end-stage kidney disease (14), and death (15).
Both diabetes and CKD are independent predictors of AKI (16), with some (17,18) but not all (19) studies suggesting that AKI risk is highest in persons with both comorbidities. The reasons for the elevated AKI risk in diabetes and CKD have not been well explored, but chronic hyperglycemia may be a contributing factor. There is evidence from animal models that hyperglycemia may provoke mitochondrial dysfunction, apoptosis, and renal injury (20,21). There are also consistent clinical data associating hyperglycemia perioperatively or at intensive care unit admission with the risk of in-hospital or postoperative AKI (22–24). We performed an observational study in two parallel health care cohorts of patients from the United States and Sweden to test the hypothesis that high HbA1c values were associated with the risk of AKI.
Research Design and Methods
Data Sources and Study Population
The study populations consisted of parallel cohorts of patients with type 2 diabetes and CKD undergoing routine care in two health systems. The U.S. cohort was derived from half a million adults receiving primary care at Geisinger, a large, predominantly rural, integrated health care system serving 44 counties in central and northeast Pennsylvania. Deidentified patient data from inpatient and outpatient encounters from 1997 to 2019 were used in this analysis. The Swedish cohort is derived from the Stockholm CREAtinine Measurements (SCREAM) project, a health care extraction of all patients undergoing creatinine testing in Stockholm health care. Stockholm health care is the sole provider of universal care to >2 million residents (25). Deidentified data from inpatient and outpatient encounters from 2006 up to 2011 were used in this analysis and cross linked to several national data sources, with complete information on demographics, dispensed drugs, and vital status, with no loss to follow-up. Use of data for this study was approved by the Geisinger Medical Center Institutional Review Board, the Johns Hopkins Institutional Review Board, and the Stockholm Ethics Review Board.
In both health care systems, we included all adult (>18 years old) residents with type 2 diabetes and confirmed CKD stages G3–G5, in accordance with current Kidney Disease: Improving Global Outcomes (KDIGO) criteria (8). Type 2 diabetes was defined by the presence of a diagnosis (ICD-10 codes E11–E14 and ICD-9 code 250) and receiving antidiabetic pharmacotherapy but without initiation of insulin (Anatomical Therapeutic Chemical Classification of Medications code A10, except A10A). CKD stages G3–G5 were defined by the presence of at least two estimated glomerular filtration rates (eGFR) <60 mL/min/1.73 m2 from outpatient creatinine measurements (>3 months and <1 year apart). The day of the first available measurement for HbA1c after the previous two conditions were met was considered the index date of the study. Exclusion criteria included missing information on age and sex or undergoing kidney replacement therapy (chronic dialysis or kidney transplantation) at index date. A flowchart of the patient selection is illustrated in Supplementary Fig. 1.
Exposure and Covariates
HbA1c data were obtained from routine outpatient care records. For each patient, we extracted information on all HbA1c measurements available during observation and until the end of follow-up. HbA1c was updated during the follow-up period. When more than one HbA1c measurement was available within a 1-month interval, the median value of those measurements was computed. HbA1c values were categorized into five levels of glycemic control intensity (<6% [42 mmol/mol], 6–6.9% [42–52 mmol/mol], 7–7.9% [53–63 mmol/mol], 8–8.9% [64–74 mmol/mol], and ≥9% [75 mmol/mol]). We used HbA1c 6–6.9% (42–52 mmol/mol) as the reference category.
Study covariates included age, sex, laboratory values, comorbidities, and concomitant medications. Information on race/ethnicity was available in the U.S. cohort as reported in the electronic health care records, but this information was not available in the Swedish cohort, as collecting this information is not allowed by law in Sweden. For analyses evaluating baseline HbA1c levels, covariates were fixed. For analyses evaluating time-dependent HbA1c levels, age, sex, and ethnicity (Geisinger only) were fixed at the index date, and the remaining covariates were time-updated. The occurrence of diabetes complications and presence of comorbidities were identified through diagnoses or therapeutic procedure codes (see definitions in Supplementary Table 1). Information on medication use at the index date was ascertained via pharmacy dispensations (in the Swedish cohort) or prescriptions (in the U.S. cohort) (Supplementary Table 1), and thereafter every new dispensation/prescription was treated as a time-varying covariate. Given that hyperglycemia may cause diuresis and changes in volume status could be a cause of AKI, we extracted and included in our analyses baseline and time-varying information on diuretics and renin-angiotensin-aldosterone system inhibitor use, as well as hospitalizations due to heart failure. We also extracted information of outpatient measurements of serum (in the U.S. cohort) or plasma (in the Swedish cohort) creatinine, dipstick albuminuria, urinary albumin-to-creatinine ratio (ACR), and total as well as LDL-cholesterol (LDL-C) tests as performed during routine care. Laboratory tests were recorded as the nearest measurement before the index date (and up to 1 year prior) and updated during the follow-up in an identical manner to HbA1c. Creatinine values <0.28 and >16.96 mg/dL were considered implausible and discarded. Serum (plasma in the Swedish cohort) creatinine was used to calculate eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation (26) and classified into six categories: G1 (≥90 mL/min/1.73 m2), G2 (60–89 mL/min/1.73 m2), G3a (45–59 mL/min/1.73 m2), G3b (30–44 mL/min/1.73 m2), G4 (15–29 mL/min/1.73 m2), and G5 (<15 mL/min/1.73 m2) as per KDIGO criteria (8). Measurements of albuminuria were missing in many patients. In order to maximize the sample size, both ACR measurements and dipstick albuminuria measurements were extracted, and the closest prior to index date was used. ACR values were approximated (27) to albuminuria dipstick categories as follows: normal to mild (<3 mg/mmol), moderate (3–30 mg/mmol), and severe (≥30 mg/mmol) albuminuria. The process was repeated at each HbA1c measurement during follow-up. Finally, the frequency of health care utilization was evaluated as an additional measure of disease severity and defined at baseline as the number of visits to primary, secondary, and inpatient care during the preceding 6 months. Thereafter, it was updated at each HbA1c measurement.
Outcome
The primary outcome was the occurrence of AKI, defined by transient creatinine elevations (either outpatient or inpatient) as per KDIGO criteria (28), in creatinine ≥0.3 mg/dL over 48 h or >1.5 times over 7 days. The secondary outcome was all-cause mortality. The start of renal replacement therapy (kidney transplantation or initiation of chronic dialysis) was considered a censoring event. Death was treated as a competing risk for the analysis of AKI. Patients were followed until event, censoring, or end of follow-up (25 January 2019 in the U.S. cohort and 31 December 2012 in the Swedish cohort), whichever occurred first.
Statistical Analyses
Baseline characteristics overall and across HbA1c categories are presented as mean ± SD or median (interquartile interval [IQI]) for continuous variables and as count (and proportion) for categorical variables.
First, we evaluated the association between baseline HbA1c levels and study outcomes using Cox proportional hazards regression models, or cause-specific Cox regression. To graphically model the association between the baseline HbA1c (as a continuous variable) and study outcomes, we modeled HbA1c using natural cubic splines. Second, we evaluated the association between time-dependent HbA1c levels and study outcome, taking into account all subsequent HbA1c values measured during follow-up.
There were missing values for albuminuria and total cholesterol. In the U.S. cohort, the missingness for albuminuria measures was 22% at baseline and 9% during follow-up; the missingness for total cholesterol was 6% at baseline and 2% when including any measure during follow-up; and the missingness for LDL-C was 8% at baseline and 3% during follow-up. In the Swedish cohort, the missingness for albuminuria was 48% at baseline and 33% using follow-up measures; the missing rate for total cholesterol was 19% at baseline and 11% including follow-up; and the missingness for LDL-C was 32% at baseline and 32% during the whole follow-up. We used chained equations by classification and regression trees to impute five complete data sets for each outcome separately. The predictors included observed patient characteristics, the event indicator for the outcome, and the Nelson–Aalen estimator of the baseline and every updated HbA1c cumulative hazard. Adjusted analyses were performed on the five imputed data sets, and then the results were combined.
Subgroup analyses were performed by categories of age, sex, and eGFR. We also conducted sensitivity analyses to test the robustness of results. First, we used the Fine and Gray method to deal with competing risk of death. Second, we repeated the AKI outcome analysis after excluding patients with a clinical history of AKI. Third, we repeated the analysis with a more strict definition of AKI of severity stage 2 to 3 (i.e., 2.0–2.9 times higher creatinine within 7 days, 3.0 times higher creatinine within 7 days, increase in creatinine to ≥4.0 mg/dL [≥353.6 mmol/L] within 48 h, or initiation of kidney replacement therapy). Last, we repeated our analyses using the mean HbA1c value every 3 months during follow-up to assess if the functional form of time-varying HbA1c values influenced our results. Analyses were done using Stata SE 15 (in the U.S. cohort) and R 3.4.3 software (in the Swedish cohort).
Results
Baseline Characteristics
After applying inclusion and exclusion criteria, we included 22,877 (in the U.S. cohort) and 12,157 (in the Swedish cohort) adults with type 2 diabetes and CKD stages G3–G5 (Supplementary Fig. 1). Baseline characteristics for individuals in the U.S. and Swedish cohorts, overall and stratified by baseline HbA1c categories, are shown in Table 1 and Supplementary Table 2. The U.S. cohort was, on average, younger than the Swedish cohort (mean age 72 ± 10 vs. 77 ± 9 years) and had slightly more women (55% vs. 50%). In both cohorts, hypertension, cardiovascular diseases, and chronic obstructive pulmonary disease were the most common comorbidities. The majority of participants had CKD stage G3a, and the use of ACE inhibitors/angiotensin receptor blockers (ACEi/ARBs) was high. Fewer patients in the U.S. cohort were diagnosed with diabetic retinopathy compared with Sweden. Around 60% of patients used oral antihyperglycemics in both cohorts, and insulin use was lower in the United States.
Table 1.
Baseline characteristics of patients with type 2 diabetes and CKD at baseline, overall and by HbA1c categories in the U.S. (Geisinger Health System) cohort
| Covariates | Overall, N = 22,877 | <6% (42 mmol/mol), N = 3,038 (12.6%) | 6–6.9% (42–52 mmol/mol), N = 8,924 (37.3%) | 7–7.9% (53–63 mmol/mol), N = 6,256 (27.5%) | 8–8.9% (64–74 mmol/mol), N = 2,664 (12.5%) | ≥9% (75 mmol/mol), N = 1,995 (10.0%) |
|---|---|---|---|---|---|---|
| Age (mean [SD]), years | 72 (10) | 71 (11) | 73 (10) | 73 (10) | 71 (10) | 68 (11) |
| Women | 12,500 (54.6) | 1,688 (55.6) | 4,995 (56.0) | 3,408 (54.5) | 1,390 (52.2) | 1,019 (51.1) |
| Black | 349 (1.5) | 41 (1.3) | 114 (1.3) | 93 (1.5) | 40 (1.5) | 61 (3.1) |
| Diabetic retinopathy | 2,245 (9.8) | 194 (6.4) | 658 (7.4) | 629 (10.1) | 400 (15.0) | 364 (18.2) |
| Diabetic neuropathy | 4,315 (18.9) | 495 (16.3) | 1,393 (15.6) | 1,226 (19.6) | 648 (24.3) | 553 (27.7) |
| Recent history of hypoglycemia (1 year prior) | 282 (1.2) | 59 (1.9) | 105 (1.2) | 56 (0.9) | 40 (1.5) | 22 (1.1) |
| Hypertension | 20,632 (90.2) | 2,756 (90.7) | 8,089 (90.6) | 5,614 (89.7) | 2,395 (89.9) | 1,778 (89.1) |
| Myocardial infarction | 2,778 (12.1) | 329 (10.8) | 952 (10.7) | 814 (13.0) | 402 (15.1) | 281 (14.1) |
| Heart failure | 5,303 (23.2) | 718 (23.6) | 1,799 (20.2) | 1,443 (23.1) | 761 (28.6) | 582 (29.2) |
| Cerebrovascular disease | 3,628 (15.9) | 500 (16.5) | 1,436 (16.1) | 968 (15.5) | 409 (15.4) | 315 (15.8) |
| Peripheral vascular disease | 2,960 (12.9) | 339 (11.2) | 1,111 (12.4) | 837 (13.4) | 377 (14.2) | 296 (14.8) |
| Cancer | 4,671 (20.4) | 742 (24.4) | 1,909 (21.4) | 1,230 (19.7) | 485 (18.2) | 305 (15.3) |
| Chronic obstructive pulmonary disease | 6,528 (28.5) | 960 (31.6) | 2,566 (28.8) | 1,659 (26.5) | 760 (28.5) | 583 (29.2) |
| Liver disease | 1,755 (7.7) | 389 (12.8) | 640 (7.2) | 378 (6.0) | 181 (6.8) | 167 (8.4) |
| Hepatitis C | 180 (0.8) | 48 (1.6) | 59 (0.7) | 28 (0.4) | 20 (0.8) | 25 (1.3) |
| AKI history | 2,791 (12.2) | 524 (17.2) | 940 (10.5) | 636 (10.2) | 346 (13.0) | 345 (17.3) |
| Insulin | 4,484 (19.6) | 350 (11.5) | 1,049 (11.8) | 1,382 (22.1) | 873 (32.8) | 830 (41.6) |
| Oral antidiabetic drugs | 12,963 (56.7) | 1,709 (56.3) | 5,307 (59.5) | 3,561 (56.9) | 1,420 (53.3) | 966 (48.4) |
| Metformin | 8,357 (36.5) | 1,072 (35.3) | 3,535 (39.6) | 2,343 (37.5) | 877 (32.9) | 530 (26.6) |
| Metformin monotherapy | 3,733 (16.3) | 683 (22.5) | 1,975 (22.1) | 778 (12.4) | 189 (7.1) | 108 (5.4) |
| Sulfonylurea | 6,609 (28.9) | 731 (24.1) | 2,439 (27.3) | 1,988 (31.8) | 893 (33.5) | 558 (28.0) |
| Other (composite of below) | 3,010 (13.2) | 329 (10.8) | 1,057 (11.8) | 957 (15.3) | 379 (14.2) | 288 (14.4) |
| Thiazolidinediones | 1,170 (5.1) | 122 (4.0) | 453 (5.1) | 382 (6.1) | 132 (5.0) | 81 (4.1) |
| α-Glucosidase inhibitors | 75 (0.3) | 8 (0.3) | 27 (0.3) | 25 (0.4) | 7 (0.3) | 8 (0.4) |
| Glinides | 566 (2.5) | 61 (2.0) | 186 (2.1) | 178 (2.8) | 84 (3.2) | 57 (2.9) |
| GLP-1ra | 263 (1.1) | 28 (0.9) | 73 (0.8) | 82 (1.3) | 42 (1.6) | 38 (1.9) |
| DPP-4i | 1,573 (6.9) | 174 (5.7) | 546 (6.1) | 509 (8.1) | 191 (7.2) | 153 (7.7) |
| SGLT2i | 180 (0.8) | 12 (0.4) | 51 (0.6) | 47 (0.8) | 40 (1.5) | 30 (1.5) |
| ACEi/ARBs | 12,051 (52.7) | 1,503 (49.5) | 4,879 (54.7) | 3,347 (53.5) | 1,339 (50.3) | 983 (49.3) |
| Non–potassium-sparing diuretics | 9,759 (42.7) | 1,217 (40.1) | 3,849 (43.1) | 2,648 (42.3) | 1,145 (43.0) | 900 (45.1) |
| Potassium-sparing diuretics | 1,678 (7.3) | 215 (7.1) | 677 (7.6) | 413 (6.6) | 207 (7.8) | 166 (8.3) |
| Other antihypertensives | 15,446 (67.5) | 1,996 (65.7) | 6,151 (68.9) | 4,215 (67.4) | 1,748 (65.6) | 1,336 (67.0) |
| eGFR categories | ||||||
| G1 | 85 (0.4) | 13 (0.4) | 28 (0.3) | 25 (0.4) | 7 (0.3) | 12 (0.6) |
| G2 | 2,394 (10.5) | 392 (12.9) | 936 (10.5) | 604 (9.7) | 253 (9.5) | 209 (10.5) |
| G3a | 14,059 (61.5) | 1,747 (57.5) | 5,691 (63.8) | 3,946 (63.1) | 1,548 (58.1) | 1,127 (56.5) |
| G3b | 4,773 (20.9) | 612 (20.1) | 1,732 (19.4) | 1,304 (20.8) | 658 (24.7) | 467 (23.4) |
| G4 | 1,396 (6.1) | 228 (7.5) | 483 (5.4) | 342 (5.5) | 180 (6.8) | 163 (8.2) |
| G5 | 170 (0.7) | 46 (1.5) | 54 (0.6) | 35 (0.6) | 18 (0.7) | 17 (0.9) |
| Albuminuria categories | ||||||
| Normal to mild | 10,512 (59.3) | 1,393 (62.3) | 4,533 (64.9) | 2,868 (58.7) | 1,071 (51.2) | 647 (42.5) |
| Moderate | 5,085 (28.7) | 616 (27.6) | 1,838 (26.3) | 1,456 (29.8) | 655 (31.3) | 520 (34.1) |
| Severe | 2,115 (11.9) | 226 (10.1) | 610 (8.7) | 558 (11.4) | 365 (17.5) | 356 (23.4) |
| Total cholesterol (mean [SD]), mmol/L | 4.4 (1.1) | 4.3 (1.1) | 4.4 (1.1) | 4.4 (1.1) | 4.5 (1.2) | 4.8 (1.4) |
| LDL-C (mean [SD]), mmol/L | 2.8 (1.0) | 2.7 (1.0) | 2.8 (0.9) | 2.8 (1.0) | 2.9 (1.0) | 3.1 (1.2) |
| Primary care visits in the 6 months prior (median [IQI]) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–3) | 2 (1–4) | 2 (1–4) |
| Outpatient visits in the 6 months prior (median [IQI]) | 4 (2–7) | 4 (2–8) | 4 (2–7) | 4 (2–7) | 4 (2–7) | 4 (2–7) |
| Inpatient visits in the 6 months prior (median [IQI]) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
Data are n (%) unless otherwise indicated. DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1ra, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
At baseline, more patients in Sweden had HbA1c <6% (42 mmol/mol) than in the United States. There were more patients with HbA1c >8% (64 mmol/mol) in the United States compared with Sweden. In both cohorts, across categories of higher HbA1c, patients were progressively younger. The prevalence of comorbidities and diabetes complications was generally higher at higher HbA1c. In higher HbA1c categories, the use of oral antihyperglycemic drugs was less common and the use of insulin was more common (Table 1 and Supplementary Table 2).
HbA1c and the Risk of AKI
During a median follow-up of 3.1 (IQI 1.7–6.2) years in the U.S. Geisinger cohort and 2.3 (IQI 1.2–3.8) years in the Swedish cohort, 7,060 and 2,619 AKI events were recorded, respectively.
Compared with baseline HbA1c 6–6.9% (42–52 mmol/mol), higher HbA1c categories were associated with higher AKI risk (Table 2). For instance, baseline HbA1c ≥9% (75 mmol/mol) was associated with a 29% higher risk of AKI (hazard ratio [HR] 1.29 [95% CI 1.18–1.41]) in the U.S. cohort and a 33% higher risk (HR 1.33 [95% CI 1.13–1.57]) in the Swedish cohort. Using restricted cubic splines (Fig. 1A and B), the association between baseline HbA1c (continuous variable) and the risk of AKI was J-shaped, with both low and high baseline HbA1c levels being associated with higher AKI risk. In the Swedish cohort, the association between low HbA1c values and the risk of subsequent AKI appeared more flattened.
Table 2.
HRs (95% CIs) for the risk of AKI and all-cause death across categories of HbA1c
| HbA1c <6% (42 mmol/mol) | HbA1c 6–6.9% (42–52 mmol/mol) | HbA1c 7–7.9% (53–63 mmol/mol) | HbA1c 8–8.9% (64–74 mmol/mol) | HbA1c ≥ 9% (75 mmol/mol) | |
|---|---|---|---|---|---|
| AKI | |||||
| Geisinger U.S. cohort | |||||
| Number of events/N | 902/3,038 | 2,588/8,924 | 1,958/6,256 | 890/2,664 | 722/1,995 |
| Baseline HbA1c unadjusted | 1.21 (1.12–1.30) | 1.00 (reference) | 1.10 (1.04–1.17) | 1.36 (1.26–1.47) | 1.65 (1.52–1.79) |
| Baseline HbA1c adjusted | 1.02 (0.95–1.10) | 1.00 (reference) | 1.06 (1.00–1.12) | 1.16 (1.08–1.26) | 1.29 (1.18–1.41) |
| Time-varying HbA1c adjusted | 1.15 (1.07–1.23) | 1.00 (reference) | 0.99 (0.94–1.06) | 1.15 (1.06–1.24) | 1.25 (1.15–1.37) |
| Swedish SCREAM cohort | |||||
| Number of events | 1,098/5,391 | 661/3,283 | 445/1,901 | 214/871 | 201/711 |
| Baseline HbA1c unadjusted | 1.05 (0.95–1.16) | 1.00 (reference) | 1.19 (1.05–1.34) | 1.29 (1.11–1.51) | 1.60 (1.37–1.87) |
| Baseline HbA1c adjusted | 1.02 (0.93–1.13) | 1.00 (reference) | 1.12 (0.99–1.26) | 1.15 (0.99–1.35) | 1.33 (1.13–1.57) |
| Time-varying HbA1c adjusted | 1.06 (0.96–1.17) | 1.00 (reference) | 1.00 (0.88–1.14) | 1.15 (0.99–1.35) | 1.21 (1.03–1.43) |
| All-cause death | |||||
| Geisinger U.S. cohort | |||||
| Number of events/N | 1,200/3,038 | 3,466/8,924 | 2,751/6,256 | 1,239/2,664 | 907/1,995 |
| Baseline HbA1c unadjusted | 1.20 (1.12–1.28) | 1.00 (reference) | 1.13 (1.08–1.19) | 1.31 (1.23–1.40) | 1.40 (1.30–1.51) |
| Baseline HbA1c adjusted | 1.11 (1.04–1.19) | 1.00 (reference) | 1.09 (1.04–1.15) | 1.18 (1.11–1.27) | 1.30 (1.21–1.41) |
| Time-varying HbA1c adjusted | 1.07 (1.01–1.13) | 1.00 (reference) | 1.01 (0.95–1.06) | 1.08 (1.01–1.16) | 1.26 (1.16–1.36) |
| Swedish SCREAM cohort | |||||
| Number of events/N | 1,423/5,391 | 824/3,283 | 544/1,901 | 253/871 | 250/711 |
| Baseline HbA1c unadjusted | 1.11 (1.02–1.21) | 1.00 (reference) | 1.16 (1.04–1.29) | 1.20 (1.05–1.39) | 1.53 (1.33–1.76) |
| Baseline HbA1c adjusted | 1.11 (1.02–1.21) | 1.00 (reference) | 1.10 (0.99–1.23) | 1.17 (1.02–1.35) | 1.46 (1.26–1.68) |
| Time-varying HbA1c adjusted | 1.13 (1.03–1.23) | 1.00 (reference) | 1.02 (0.91–1.14) | 1.09 (0.95–1.26) | 1.21 (1.04–1.40) |
Adjustment for age, sex, ethnicity (for U.S. cohort only), diabetic retinopathy, diabetic neuropathy, history of hypoglycemia (1 year prior), hypertension, myocardial infarction, congestive heart failure, cerebrovascular disease, peripheral vascular disease, cancer, chronic obstructive pulmonary disease, liver disease, hepatitis C, history of AKI (for AKI only), metformin, sulfonylurea, other oral antidiabetic drugs, insulins, ACEi/ARBs, diuretics, other hypertensives, eGFR, albuminuria, total cholesterol, LDL-C, and number of primary care visits, outpatient visits, and inpatient visits in the 6 months prior.
Figure 1.
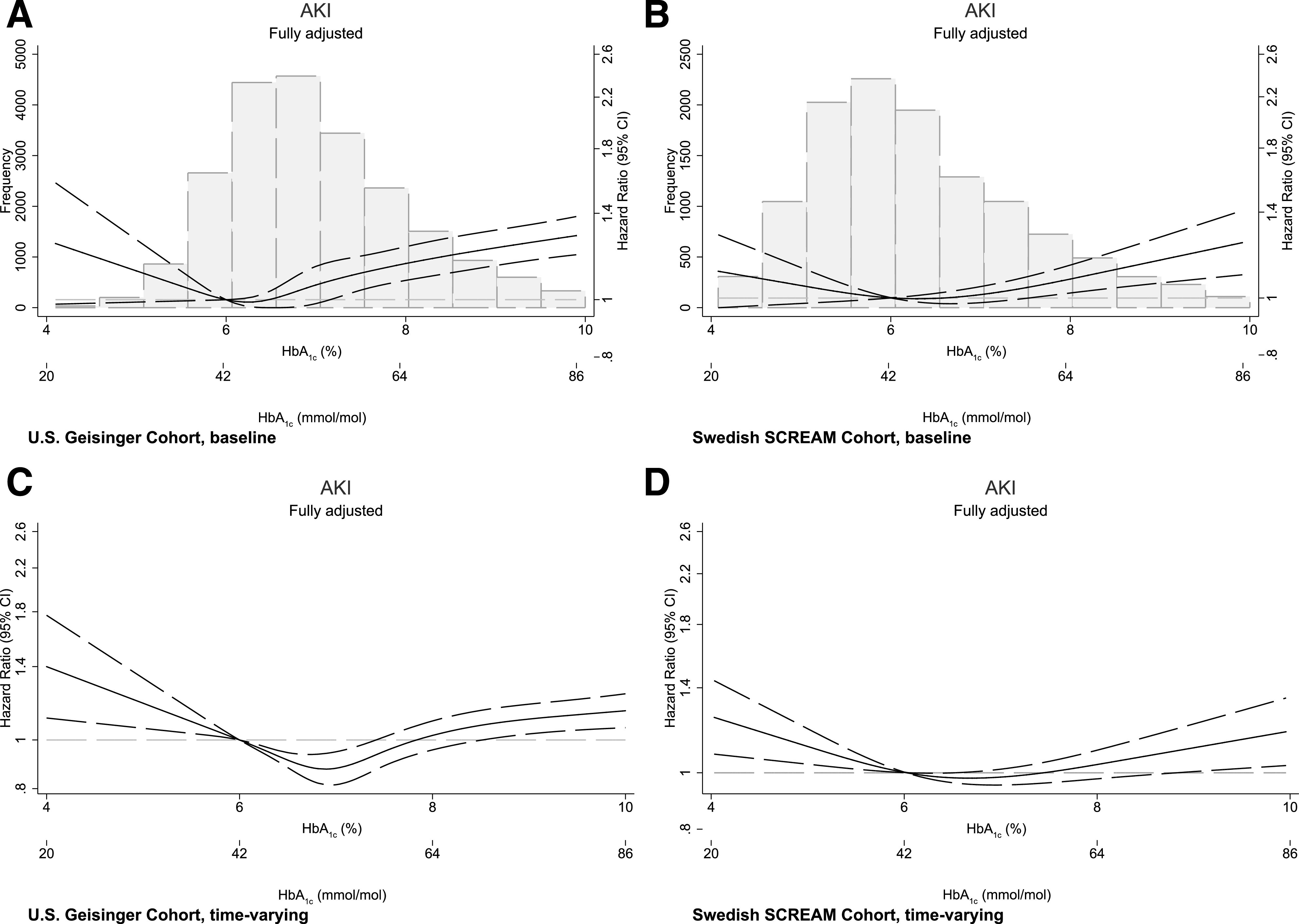
Associations between baseline (A and B) and time-varying (C and D) HbA1c values with the risk of AKI. Multivariable adjustment is detailed in the legend of Table 2.
In categorical analyses using time-varying HbA1c and compared with HbA1c 6–6.9% (42–52 mmol/mol), we also observed increased AKI risk for higher HbA1c categories (Table 2). We observed significantly increased AKI risk for the category of HbA1c ≥9% (75 mmol/mol) in both cohorts. The magnitude of AKI risk was similar between the two cohorts for the categories HbA1c 8–8.9% (64–74 mmol/mol) and HbA1c <6% (42 mmol/mol), but the risk was statistically significant only in the U.S. cohort. Using restricted cubic splines (Fig. 1C and D), we observed similar J-shaped associations, with AKI risks being lowest at HbA1c values between 6 (42 mmol/mol) and 7% (53 mmol/mol).
HbA1c and the Risk of Death
During a median follow-up of 4.0 (IQI 1.9–7.5) years in U.S. cohort and 2.8 (IQI 1.4–4.2) years in the Swedish cohort, 9,563 and 3,294 deaths were recorded, respectively.
Compared with baseline HbA1c level (6–6.9% [42–52 mmol/mol]), higher and lower HbA1c categories were associated with the risk of death (Table 2). For instance, baseline HbA1c level ≥9% (75 mmol/mol) was associated with increased risk of death in both the U.S. (HR 1.30 [95% CI 1.21–1.41]) and Swedish (HR 1.46 [95% CI 1.26–1.68]) cohorts; persons with baseline HbA1c <6% (42 mmol/mol) were also at higher risk of death in both cohorts (HR 1.11 [95% CI 1.04–1.19] in the U.S. and HR 1.11 [95% CI 1.02–1.21] in the Swedish cohort). Time-varying HbA1c analyses showed associations consistent with our baseline HbA1c analysis, as well as its graphical representation through cubic splines (Fig. 2).
Figure 2.
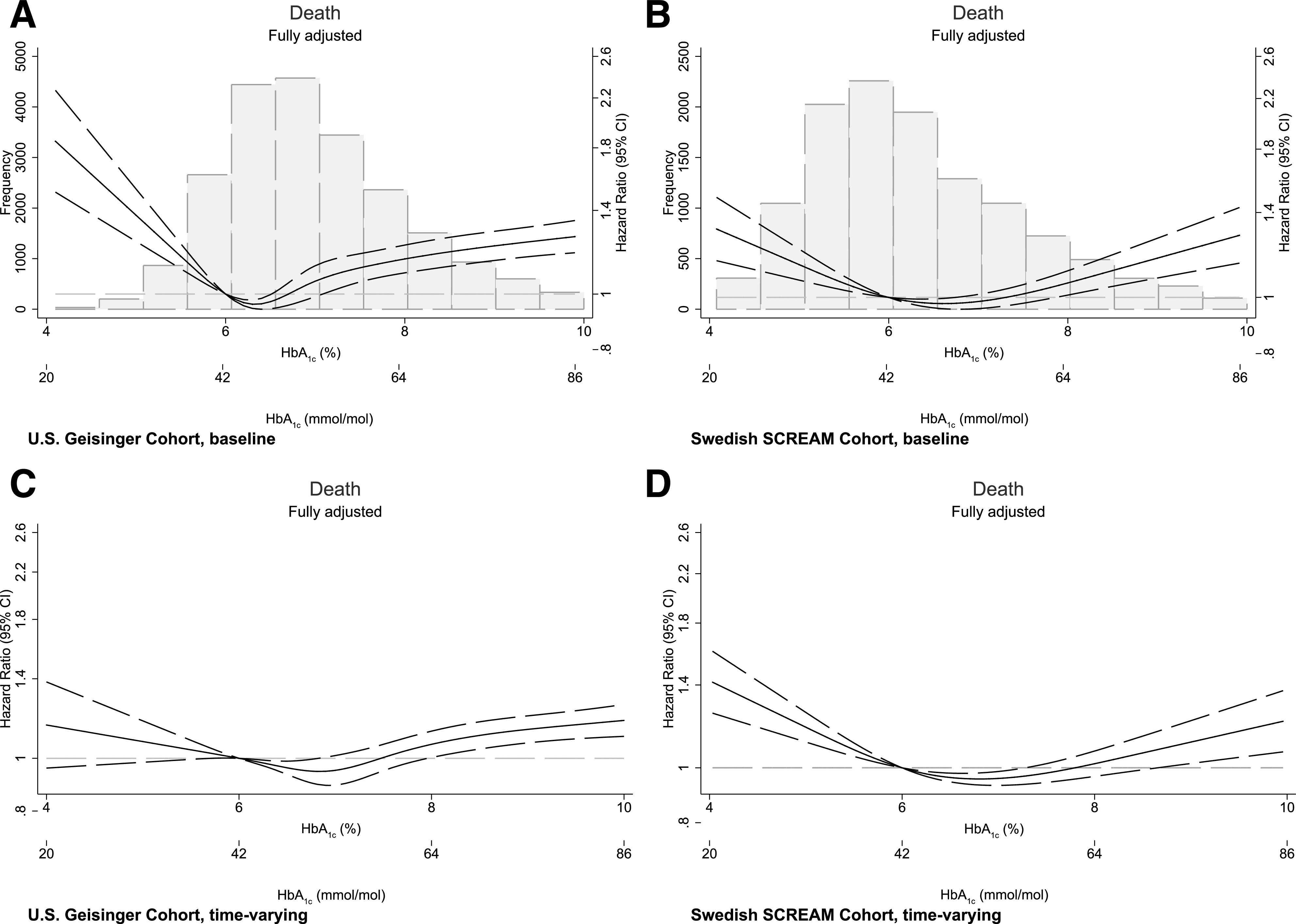
Associations between baseline (A and B) and time-varying (C and D) HbA1c values with the risk of all-cause death. Multivariable adjustment is detailed in the legend of Table 2.
Subgroup and Sensitivity Analyses
No effect modification was observed by eGFR, sex, and age strata, neither in baseline nor in time-varying designs (Supplementary Tables 3–6). Similar results were obtained by using the Fine and Gray method, excluding patients with clinical history of AKI at baseline, evaluating more severe AKI, and after averaging HbA1c in 3-month periods (Supplementary Tables 7–11).
Conclusions
In this study of two large, geographically diverse health systems, we found associations between poor glycemic control and the risk of AKI in persons with type 2 diabetes and CKD. This observation was consistent between the two health systems and also regardless of whether HbA1c was modeled as a time-fixed or time-varying exposure. Because AKI is common and a strong risk factor for progression of CKD and mortality, our results should be followed by investigations as to whether tighter control of hyperglycemia might reduce risk of AKI.
This study adds to the existing literature of glycemic control and AKI in the hospital setting by demonstrating an association between glycemic control in outpatient care and the occurrence of AKI (22–24,29). A previous study of patients undergoing coronary artery bypass grafting surgery showed that perioperative HbA1c ≥6.0% (42 mmol/mol) was more strongly associated with the occurrence of postoperative AKI than the presence of diabetes (24). In a case-matched, retrospective analysis of patients with diabetes undergoing partial nephrectomy, preoperative HbA1c >7% (53 mmol/mol) predicted the presence of postoperative AKI (29). In addition, some clinical trials have shown that maintaining normoglycemia with insulin in the intensive care unit reduces the incidence of AKI (30–32). Our results expand this previous evidence to the outpatient setting and were robust in analyses that explored a single HbA1c at baseline or all health care–assessed HbA1c measurements during observation. The latter may add some strength to our study, as it reduces exposure misclassification bias when exploring short-term outcomes such as AKI.
These findings are in line with mechanistic evidence suggesting a role for hyperglycemia in increasing AKI susceptibility in type 2 diabetes by inducing profibrotic and proinflammatory responses in the kidney (33–35). Recent studies have unveiled further mechanisms: using a rabbit model (20) of burn-induced prolonged critical illness in which blood glucose and insulin were independently regulated at normal or elevated levels, hyperglycemia caused cellular glucose overload in the kidneys, and this was accompanied by mitochondrial dysfunction and structural kidney injury. In another study (21), renal ischemia-reperfusion induced more severe AKI in diabetic mice than in nondiabetic mice, and the severity of AKI correlated with their blood glucose levels. Analysis of kidney tissues from these animals as well as of renal proximal tubular cells exposed to high-glucose medium showed that, mechanistically, the injury induced by hyperglycemia involved the activation of the intrinsic pathway of apoptosis on the mitochondria. Because administration of oral insulin to reduce blood glucose attenuated AKI sensitivity and the observed histopathological damages in the kidney tissue, the authors concluded that hyperglycemia is a triggering factor of AKI in type 2 diabetes.
Low HbA1c (<6% [42 mmol/mol]) was also associated with increased risk of AKI in some of our analyses, but this association was less consistent across analyses (baseline vs. time-dependent HbA1c) and cohorts (Sweden vs. United States). It is possible that this category may reflect confounding by underlying illness (36), although our comparison of comorbidities did not find striking differences in the HbA1c <6% (42 mmol/mol) group. We did not have data on weight loss, so we cannot rule out the possibility that patients were losing weight due to illness, which may have predisposed them to increased risk of AKI.
We report a J-shaped association between HbA1c and the risk of death, which is in line with previous observational studies. In a health system database analysis from Canada, HbA1c levels <6.5% (48 mmol/mol) and >9% (75 mmol/mol) were associated with higher risks of death (37). In Taiwan, HbA1c >9% (75 mmol/mol) was associated with the risk of cardiovascular events and death (38). In another analysis (39) from a health care provider in Ohio (United States), both HbA1c levels <6% (42 mmol/mol) and ≥9% (75 mmol/mol) were associated with higher risk of death.
The HbA1c associations reported in our study represent the patient’s underlying illness and the strategies used to reach HbA1c targets rather than the targets per se (e.g., confounding by indication). Results of observational studies are weaker than clinical trial evidence but can lend support to current guidelines. The 2020 KDIGO guidelines (9) recommend an individualized HbA1c target ranging from <6.5% (48 mmol/mol) to <8% (64 mmol/mol) in patients with type 2 diabetes and nondialysis-dependent CKD. The 2020 American Diabetes Association guidelines recommend less stringent HbA1c goals (such as <8% [64 mmol/mol]) for patients with extensive comorbid conditions or older adults with coexisting chronic illnesses (7), conditions that would define most of the patients in our analysis. Both documents emphasize the need to individualize HbA1c targets in an interactive process that includes individual assessment of risk, life expectancy, disease/therapy burden, and patient preferences. Our study adds to this evidence the observation that both high and low HbA1c levels may increase AKI risk. Because AKI is a strong risk factor for progressive CKD and mortality (15,16), these results should be followed by investigations as to whether tighter control of hyperglycemia might reduce risk of AKI.
Strengths of our study include the large sample sizes, replication of findings in two health systems of different coverage and health care reimbursement, and the use of both baseline and time-dependent exposure designs. Our analysis is not exempt from limitations: we lacked information for diet, BMI (in Sweden), duration of type 2 diabetes, volume status, or changes in drug dosages; the population was predominantly Caucasian, and racial/ethnic diversity is underrepresented. The periods of data collection differed between cohorts, and most included patients who were not taking the newer classes of antidiabetic medications such as sodium–glucose cotransporter 2 inhibitors, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide 1 receptor agonists.
To conclude, higher HbA1c (HbA1c >9% [75 mmol/mol]) was robustly associated with the risk of AKI in adults with type 2 diabetes and CKD, suggesting that improving glycemic control may also reduce the risk of AKI. Our findings provide evidence to consider HbA1c control for patients with CKD at high risk of AKI.
Article Information
Funding. Research reported in this publication was supported by Vetenskapsrådet 2019-01059 (principal investigator [PI]: J.J.C.) and National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK115534 and R01 DK100446 (PI: M.E.G.) and K01 DK121825 (PI: J.-I.S.).
The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data, and preparation or final approval of the manuscript before publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.E.G. and J.J.C. contributed to study conception. Y.X. and A.S. developed the analysis plan and performed data extraction and analysis. Y.X. drafted the first manuscript, which was reviewed by J.A., M.E., J.-I.S., E.S., A.C., M.E.G., and J.J.C. and brought to its final form. Y.X. and J.J.C. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This work was presented at the 57th European Renal Association–European Dialysis and Transplant Association Congress, 6–9 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12982916.
References
- 1.Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 2016;316:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkovic V, Agarwal R, Fioretto P, et al.; Conference Participants . Management of patients with diabetes and CKD: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2016;90:1175–1183 [DOI] [PubMed] [Google Scholar]
- 5.Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 2009;4:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weir MA, Gomes T, Mamdani M, et al. Impaired renal function modifies the risk of severe hypoglycaemia among users of insulin but not glyburide: a population-based nested case-control study. Nephrol Dial Transplant 2011;26:1888–1894 [DOI] [PubMed] [Google Scholar]
- 7.Introduction: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S1–S2 [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation KDOQI clinical practice guideline for diabetes and CKD: 2012 update [published correction appears in Am J Kidney Dis 2013;61:1049]. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease Improving Global Outcomes KDIGO Clinical Practice Guideline on the Management of Diabetes in CKD, 2019. Accessed September 24, 2020. Available from https://kdigo.org/wp-content/uploads/2018/03/KDIGO-Diabetes-Management-in-CKD_Public-Review.pdf [Google Scholar]
- 10.Sawhney S, Marks A, Fluck N, Levin A, Prescott G, Black C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017;69:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–3370 [DOI] [PubMed] [Google Scholar]
- 12.Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med 2017;12:70–76 [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Hsu C-Y, Yang J, et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol 2018;13:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girman CJ, Kou TD, Brodovicz K, et al. Risk of acute renal failure in patients with type 2 diabetes mellitus. Diabet Med 2012;29:614–621 [DOI] [PubMed] [Google Scholar]
- 17.Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015;5:e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patschan D, Müller GA. Acute kidney injury in diabetes mellitus. Int J Nephrol 2016;2016:6232909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James MT, Grams ME, Woodward M, et al.; CKD Prognosis Consortium . A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 2015;66:602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanhorebeek I, Gunst J, Ellger B, et al. Hyperglycemic kidney damage in an animal model of prolonged critical illness. Kidney Int 2009;76:512–520 [DOI] [PubMed] [Google Scholar]
- 21.Peng J, Li X, Zhang D, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int 2015;87:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isobe S, Yamada T, Yuba M, Hayashi M, Ishii H, Murohara T. Relationship between pre-procedural microalbuminuria and renal functional changes after coronary computed tomography in diabetic patients. J Cardiol 2017;69:666–670 [DOI] [PubMed] [Google Scholar]
- 23.Marenzi G, Cosentino N, Milazzo V, et al. Acute kidney injury in diabetic patients with acute myocardial infarction: role of acute and chronic glycemia. J Am Heart Assoc 2018;7:e008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oezkur M, Wagner M, Weismann D, et al. Chronic hyperglycemia is associated with acute kidney injury in patients undergoing CABG surgery--a cohort study. BMC Cardiovasc Disord 2015;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Runesson B, Gasparini A, Qureshi AR, et al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J 2016;9:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konta T, Hao Z, Takasaki S, et al. Clinical utility of trace proteinuria for microalbuminuria screening in the general population. Clin Exp Nephrol 2007;11:51–55 [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Lameire N, Aspelin P, et al.; Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138 [Google Scholar]
- 29.Kim NY, Hong JH, Koh DH, Lee J, Nam HJ, Kim SY. Effect of diabetes mellitus on acute kidney injury after minimally invasive partial nephrectomy: a case-matched retrospective analysis. J Clin Med 2019;8:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 31.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 32.Schetz M, Vanhorebeek I, Wouters PJ, Wilmer A, Van den Berghe G. Tight blood glucose control is renoprotective in critically ill patients. J Am Soc Nephrol 2008;19:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 34.Shi H, Patschan D, Epstein T, Goligorsky MS, Winaver J. Delayed recovery of renal regional blood flow in diabetic mice subjected to acute ischemic kidney injury. Am J Physiol Renal Physiol 2007;293:F1512–F1517 [DOI] [PubMed] [Google Scholar]
- 35.Mariappan MM, DeSilva K, Sorice GP, et al. Combined acute hyperglycemic and hyperinsulinemic clamp induced profibrotic and proinflammatory responses in the kidney. Am J Physiol Cell Physiol 2014;306:C202–C211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggarwal V, Schneider ALC, Selvin E. Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care 2012;35:2055–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shurraw S, Hemmelgarn B, Lin M, et al.; Alberta Kidney Disease Network . Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med 2011;171:1920–1927 [DOI] [PubMed] [Google Scholar]
- 38.Liao L-N, Li C-I, Liu C-S, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan Diabetes Study. PLoS One 2015;10:e0130828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navaneethan SD, Schold JD, Jolly SE, Arrigain S, Winkelmayer WC, Nally JV Jr. Diabetes control and the risks of ESRD and mortality in patients with CKD. Am J Kidney Dis 2017;70:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]


