Abstract
OBJECTIVE
To explore the effects of empagliflozin on the incidence of obstructive sleep apnea (OSA) and its effects on metabolic, cardiovascular (CV), and renal outcomes among participants with or without OSA in the EMPA-REG OUTCOME trial.
RESEARCH DESIGN AND METHODS
Participants with diabetes and CV disease were randomized to empagliflozin (10 and 25 mg) or placebo daily in addition to standard of care. OSA was assessed by investigator report using Medical Dictionary for Regulatory Activities version 18.0, and CV outcomes were independently adjudicated. Analyses were performed using multivariable-adjusted Cox regression models.
RESULTS
OSA was reported in 391 of 7,020 (5.6%) participants at baseline. Those with OSA were more likely to be male (83% vs. 71%) and to have moderate to severe obesity (BMI ≥35 kg/m2; 55% vs. 18%). Over a median of 3.1 years, empagliflozin had similar placebo-adjusted reductions in HbA1c, waist circumference, and systolic blood pressure, regardless of OSA status, but a larger effect on weight (adjusted mean ± SE difference at week 52: OSA vs. no OSA −2.9 ± 0.5 vs. −1.9 ± 0.1 kg). Incidence of 3-point major adverse CV events, CV death, heart failure hospitalization, and incident or worsening nephropathy in the placebo group was 1.2- to 2.0-fold higher for those with baseline OSA compared with those without. Empagliflozin significantly reduced the risk for outcomes regardless of OSA status (P-interaction all >0.05). Fifty patients reported a new diagnosis of OSA through 7 days after medication discontinuation, and this occurred less often with empagliflozin treatment (hazard ratio 0.48 [95% CI 0.27, 0.83]).
CONCLUSIONS
In EMPA-REG OUTCOME, participants with OSA had greater comorbidity and higher frequency of CV and renal events. Empagliflozin had favorable effects on risk factors and CV and renal outcomes regardless of preexisting OSA and may also reduce the risk for new-onset OSA.
Introduction
Obstructive sleep apnea (OSA) is a common clinical syndrome characterized by intermittent sleep state–dependent collapse of the upper airway, resulting in periodic cessations or reductions in airflow and subsequent hypoxia, hypercapnia, or frequent arousals from sleep (1). Its prevalence in adults is conservatively estimated to be ∼3% in women and 10% in men, with higher prevalence with advancing age (e.g., at 50–70 years, 9% in women and 17% in men) (2). OSA is a multifaceted syndrome caused by conditions that reduce the size of the resting pharynx or increase airway collapsibility. Since increased adipose tissue within the soft tissues of the head and neck compromises upper-airway dimensions and makes the airway more prone to collapse during sleep, obesity is the most prevalent risk factor, with OSA reported in >40% of adults with BMI ≥30 kg/m2; other risk factors include male sex, metabolic syndrome, and type 2 diabetes (1). Certain medications, such as opioids, benzodiazepines, and testosterone, may also worsen or induce OSA (3).
OSA and type 2 diabetes frequently occur together, and both are associated with adverse metabolic consequences that increase the risk for cardiovascular disease (CVD) (4), including stroke, myocardial infarction, hospitalization for heart failure (HHF), cardiovascular (CV) death, and all-cause mortality (5,6), as well as the risk for chronic kidney disease (7). Furthermore, OSA may worsen glucose tolerance, impair diabetes management, and lead to poor glycemic control (8). There are no pharmacological therapies currently approved for the treatment of OSA (9), and therapies for OSA have historically been designed to reduce the frequency of sleep-disordered breathing events (e.g., with continuous positive airway pressure [CPAP] devices or surgical procedures) (1). An effective treatment measure, however, involves treatment of the underlying metabolic cause (e.g., obesity), and with guideline-recommended weight loss, OSA has been reported to resolve in >50% of patients (10). Unfortunately, effective and sustained weight loss in people with or at risk for type 2 diabetes using lifestyle approaches alone remains challenging (11).
Empagliflozin is a potent and selective sodium–glucose cotransporter 2 (SGLT2) inhibitor used for the treatment of type 2 diabetes that can significantly improve multiple metabolic factors associated with OSA, such as glycosylated hemoglobin (HbA1c) (12,13), systolic blood pressure (SBP) (14), waist circumference, and body weight (15). In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME), a randomized placebo-controlled CV outcomes trial of individuals with type 2 diabetes and established CVD, empagliflozin reduced the risk of 3-point major adverse CV events (3P-MACE) by 14%, CV death by 38%, HHF by 35% (16), and incident or worsening nephropathy by 39% (17). There are currently no randomized controlled trial data of SGLT2 inhibitors reporting outcomes related to OSA. Therefore, we performed a post hoc analysis of the EMPA-REG OUTCOME trial, investigating the prevalence of OSA in the trial cohort; the effects of empagliflozin on metabolic, CV, and renal outcomes according to baseline OSA status; and the effect of empagliflozin on new-onset OSA during the course of the trial among participants without OSA at baseline.
Research Design and Methods
Trial Population and Design
The design and conduct of the EMPA-REG OUTCOME study have been published previously (16). Briefly, participants with type 2 diabetes (HbA1c 7.0–9.0% [53–75 mmol/mol] for those who are medication naive and 7.0–10.0% [53–86 mmol/mol] for those on stable glucose-lowering therapy), established CVD, and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 were randomized to receive empagliflozin 10 mg, empagliflozin 25 mg, or placebo once daily in addition to standard of care and followed for a median of 3.1 years. Background glucose-lowering therapy remained unchanged for the first 12 weeks and was subsequently adjusted at the investigator’s discretion to achieve glycemic control. Independent ethics committees or institutional review boards approved the clinical protocol, and participants provided written informed consent before study initiation.
OSA
Data on the presence of OSA were collected at baseline and during follow-up on the basis of study investigator reports conforming to the Medical Dictionary for Regulatory Activities (MedDRA) version 18.0, a standardized and clinically validated international medical terminology code. Reporting used the single preferred term sleep apnea syndrome (https://bioportal.bioontology.org/ontologies/MEDDRA?p=classes&conceptid=10040979), which included the following synonyms: hypopnea syndrome, obstructive sleep apnea syndrome, central sleep apnea syndrome, sleep apnea, obstructive sleep apnea hypopnea syndrome, hypopnea syndrome, apnea syndrome, sleep apnea, sleep apnea syndrome, obstructive sleep apnea syndrome, apnea syndrome, sleep apnea syndromes, central sleep apnea syndrome, and obstructive sleep apnea hypopnea syndrome, as previously applied by others (18).
Primary Outcome and Further CVD and Renal End Points
All CVD outcome events, HHFs, and deaths were prospectively adjudicated by independent, blinded clinical events committees. The primary outcome of the main trial was the composite outcome 3P-MACE (death as a result of CV causes, nonfatal myocardial infarction, or nonfatal stroke), with secondary outcomes including HHF, all-cause death, and incident or worsening nephropathy. The latter was defined as progression to macroalbuminuria (urinary albumin-to-creatinine ratio [UACR] >300 mg albumin/g creatinine); a doubling of the serum creatinine level accompanied by an eGFR of ≤45 mL/min/1.73 m2, as calculated by the MDRD equation; the initiation of continuous renal replacement therapy; or death as a result of renal disease. Additional renal outcomes included progression to macroalbuminuria (in patients who did not have macroalbuminuria at baseline) and changes in UACR over time. Participants who prematurely discontinued study medication were followed for ascertainment of CV outcomes and vital status.
Statistical Analysis
All analyses were performed in participants treated with one or more doses of study medication (modified intention-to-treat [mITT] approach), and all empagliflozin treatment analyses were performed using the pooled empagliflozin treatment groups (10 and 25 mg). Baseline characteristics of those with and without OSA at baseline are presented as mean ± SD, and categorical variables are presented as numbers and proportions. Effects of empagliflozin versus placebo on cardiometabolic risk factors (HbA1c, weight, waist circumference, SBP, and log[UACR]) by OSA status at baseline were performed using a mixed-effects repeated-measures model that included terms for the baseline parameter in question and baseline HbA1c as linear covariates and geographical region, BMI categories, number of weeks reachable with a postrandomization measurement of the parameter of interest, baseline eGFR categories, treatment, visit, OSA at baseline, treatment-by-visit interaction, visit-by-OSA at baseline interaction, treatment-by-OSA at baseline interaction, treatment-by-visit-by OSA at baseline interaction, baseline HbA1c-by-visit interaction, and baseline parameter in question-by-visit interaction as fixed effects. Treatment effects on risks for CVD, HHF, mortality, and renal outcomes between treatment groups by OSA status at baseline were assessed using a Cox proportional hazards model that included terms for age, sex, baseline BMI category, baseline HbA1c category, baseline eGFR category, geographical region, treatment, OSA, and treatment-by-OSA interaction.
Kaplan-Meier estimates for time to first OSA event on treatment for participants without OSA at baseline were plotted on the basis of investigator-reported events until treatment end plus an additional 7 days, and a sensitivity analysis using all reported OSA events until study end was done (mITT analysis) to determine consistency of results. Hazard ratios (HRs) comparing time to first OSA were based on a Cox regression model with terms for age, sex, baseline BMI categories, baseline HbA1c categories, baseline eGFR categories, geographical region, and treatment.
Baseline characteristics in those who developed OSA versus those who did not were also evaluated, and an exploratory mediation analysis was performed to evaluate whether a treatment-related change in conventional OSA risk factors over time could explain the observed difference in new onset of OSA between empagliflozin and placebo, as previously conducted to analyze mediation effects of empagliflozin on CV deaths (19) (Supplementary Appendix, Section A). Comparisons were not adjusted for multiple testing, and P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Of 7,020 patients with type 2 diabetes and CVD, OSA was reported at baseline in 391 (5.6% overall, placebo 5.4%, pooled empagliflozin doses 5.7%). Compared with participants without OSA at baseline, those with OSA were more likely to be male (82.9% vs. 70.8%), have moderate to severe obesity (BMI ≥35 kg/m2) (55.2% vs. 18.2%), and have greater prevalence of coronary artery disease (88.0% vs. 74.9%). Furthermore, those with OSA had longer diabetes duration, higher rates of insulin use (64.2% vs. 47.3%), and more prevalent use of antihypertensive therapies (98.5% vs. 94.8%) (Table 1).
Table 1.
Baseline characteristics of participants with and without reported prevalent OSA
| Participants without OSA | Participants with OSA | |||
|---|---|---|---|---|
| Empagliflozin (n = 4,421) | Placebo (n = 2,208) | Empagliflozin (n = 266) | Placebo (n = 125) | |
| Male sex | 3,120 (70.6) | 1,572 (71.2) | 216 (81.2) | 108 (86.4) |
| Age (years) | 63.1 ± 8.6 | 63.2 ± 8.9 | 63.7 ± 7.7 | 63.7 ± 7.3 |
| ≥75 | 405 (9.2) | 220 (10.0) | 19 (7.1) | 8 (6.4) |
| Region | ||||
| Europe | 1,841 (41.6) | 927 (42.0) | 85 (32.0) | 32 (25.6) |
| North America | 766 (17.3) | 381 (17.3) | 166 (62.4) | 81 (64.8) |
| Latin America | 720 (16.3) | 354 (16.0) | 1 (0.4) | 6 (4.8) |
| Africa | 206 (4.7) | 100 (4.5) | 5 (1.9) | 2 (1.6) |
| Asia | 888 (20.1) | 446 (20.2) | 9 (3.4) | 4 (3.2) |
| Diabetes duration (years) | ||||
| ≤1 | 125 (2.8) | 50 (2.3) | 3 (1.1) | 2 (1.6) |
| >1–5 | 682 (15.4) | 360 (16.3) | 30 (11.3) | 11 (8.8) |
| >5–10 | 1,101 (24.9) | 543 (24.6) | 74 (27.8) | 28 (22.4) |
| >10 | 2,513 (56.8) | 1,255 (56.8) | 159 (59.8) | 84 (67.2) |
| HbA1c (%) | 8.1 ± 0.9 | 8.1 ± 0.9 | 8.1 ± 0.9 | 8.1 ± 0.8 |
| ≥9.0 (≥75 mmol/mol) | 760 (17.2) | 358 (16.2) | 52 (19.5) | 24 (19.2) |
| Diabetes medications | ||||
| Metformin | 3,271 (74.0) | 1,653 (74.9) | 188 (70.7) | 81 (64.8) |
| Sulfonylurea | 1,924 (43.5) | 952 (43.1) | 90 (33.8) | 40 (32.0) |
| Insulin | 2,084 (47.1) | 1,052 (47.6) | 168 (63.2) | 83 (66.4) |
| TZD | 186 (4.2) | 97 (4.4) | 12 (4.5) | 4 (3.2) |
| DPP-4 inhibitors | 493 (11.2) | 252 (11.4) | 36 (13.5) | 15 (12.0) |
| GLP-1 receptor analogs | 100 (2.3) | 56 (2.5) | 26 (9.8) | 14 (11.2) |
| eGFR (MDRD) (mL/min/1.73 m2) | 74.5 ± 21.6 | 74.1 ± 21.1 | 68.0 ± 20.2 | 68.4 ± 20.0 |
| <60 | 1,115 (25.2) | 557 (25.2) | 97 (36.5) | 50 (40.0) |
| UACR (mg/g), median (IQR) | 17.7 (7.1, 70.7) | 17.7 (7.1, 74.3) | 16.8 (6.2, 80.4) | 18.6 (6.2, 74.3) |
| UACR (mg/g) | ||||
| <30 | 2,638 (59.7) | 1,310 (59.3) | 151 (56.8) | 72 (57.6) |
| 30–300 | 1,263 (28.6) | 635 (28.8) | 75 (28.2) | 40 (32.0) |
| >300 | 475 (10.7) | 247 (11.2) | 34 (12.8) | 13 (10.4) |
| Missing | 45 (1.0) | 16 (0.7) | 6 (2.3) | 0 (0.0) |
| BMI (kg/m2) | 30.3 ± 5.1 | 30.4 ± 5.1 | 35.5 ± 5.0 | 36.0 ± 4.9 |
| ≥35 | 808 (18.3) | 401 (18.2) | 143 (53.8) | 73 (58.4) |
| Weight (kg) | 85.1 ± 18.4 | 85.4 ± 18.4 | 104.6 ± 18.1 | 107.6 ± 18.0 |
| Waist circumference (cm) | 104.0 ± 13.4 | 104.2 ± 13.6 | 116.5 ± 12.6 | 119.3 ± 13.0 |
| Coronary artery disease | 3,315 (75.0) | 1,649 (74.7) | 230 (86.5) | 114 (91.2) |
| Myocardial infarction | 2,078 (47.0) | 1,025 (46.4) | 112 (42.1) | 58 (46.4) |
| Stroke | 1,032 (23.3) | 535 (24.2) | 52 (19.5) | 18 (14.4) |
| Peripheral arterial disease | 927 (21.0) | 458 (20.7) | 55 (20.7) | 21 (16.8) |
| Heart failure | 424 (9.6) | 224 (10.1) | 38 (14.3) | 20 (16.0) |
| Current smoking | 602 (13.6) | 289 (13.1) | 25 (9.4) | 13 (10.4) |
| SBP (mmHg) | 135.3 ± 16.9 | 135.9 ± 17.3 | 133.8 ± 17.9 | 133.1 ± 15.0 |
| DBP (mmHg) | 76.7 ± 9.7 | 76.9 ± 10.1 | 75.0 ± 9.5 | 75.2 ± 10.6 |
| Any antihypertensives | 4,184 (94.6) | 2,098 (95.0) | 262 (98.5) | 123 (98.4) |
| ACEi/ARBs | 3,571 (80.8) | 1,759 (79.7) | 227 (85.3) | 109 (87.2) |
| β-Blockers | 2,855 (64.6) | 1,403 (63.5) | 201 (75.6) | 95 (76.0) |
| Diuretics | 1,877 (42.5) | 914 (41.4) | 170 (63.9) | 74 (59.2) |
| Antihypertensives (n) | ||||
| 0 | 237 (5.4) | 110 (5.0) | 4 (1.5) | 2 (1.6) |
| 1 | 832 (18.8) | 423 (19.2) | 29 (10.9) | 11 (8.8) |
| 2 | 1,379 (31.2) | 737 (33.4) | 72 (27.1) | 36 (28.8) |
| 3 | 1,160 (26.2) | 548 (24.8) | 82 (30.8) | 51 (40.8) |
| >3 | 813 (18.4) | 390 (17.7) | 79 (29.7) | 25 (20.0) |
| Total cholesterol (mg/dL) | 164.3 ± 44.4 | 162.6 ± 43.5 | 150.5 ± 38.3 | 149.6 ± 31.8 |
| LDL cholesterol (mg/dL) | 86.6 ± 36.1 | 85.4 ± 35.6 | 74.2 ± 30.7 | 74.5 ± 27.9 |
| HDL cholesterol (mg/dL) | 44.8 ± 11.9 | 44.3 ± 11.4 | 40.4 ± 11.3 | 40.0 ± 8.9 |
| Triglycerides (mg/dL), median (IQR) | 141.7 (102.7, 195.7) | 139.9 (104.5, 200.2) | 158.5 (116.0, 217.0) | 160.3 (110.7, 217.0) |
| Any lipid-lowering medications | 3,580 (81.0) | 1,755 (79.5) | 240 (90.2) | 109 (87.2) |
| Statins | 3,404 (77.0) | 1,672 (75.7) | 226 (85.0) | 101 (80.8) |
| Fibrates | 386 (8.7) | 178 (8.1) | 45 (16.9) | 21 (16.8) |
| Ezetimibe | 171 (3.9) | 73 (3.3) | 18 (6.8) | 8 (6.4) |
| Aspirin | 3,646 (82.5) | 1,818 (82.3) | 230 (86.5) | 109 (87.2) |
| Vitamin K antagonists | 242 (5.5) | 146 (6.6) | 24 (9.0) | 10 (8.0) |
Data are n (%) or mean ± SD unless otherwise indicated. ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; IQR, interquartile range; TZD, thiazolidinedione.
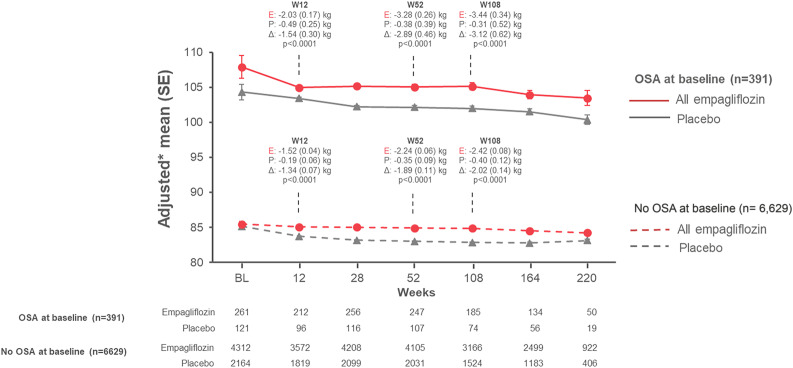
Over a median 3.1 years, empagliflozin led to similar placebo-adjusted reductions in HbA1c, waist circumference, SBP, and UACR (Supplementary Appendix, Section B, and Supplementary Figs. B1–B4), regardless of OSA status at baseline. However, there was a trend for a larger effect of empagliflozin on weight reduction (adjusted for baseline weight) in patients with OSA compared with those without OSA (adjusted mean ± SE difference vs. placebo from baseline at week 52, −2.9 ± 0.5 vs. −1.9 ± 0.1 kg, and at week 108, −3.1 ± 0.6 vs. −2.0 ± 0.1 kg, respectively) (Fig. 1).
Figure 1.
Effects of empagliflozin treatment on body weight by OSA status at baseline. Model reflects a mixed-model repeated-measures analysis that included terms for baseline weight and baseline HbA1c as linear covariates and baseline eGFR category, geographical region, baseline BMI category, number of weeks reachable with a postrandomization weight measurement, treatment, visit, sleep apnea syndrome at baseline, treatment-by-visit interaction, visit-by-sleep apnea syndrome at baseline interaction, treatment-by-sleep apnea syndrome at baseline interaction, treatment-by-visit by sleep apnea syndrome at baseline interaction, baseline HbA1c-by-visit interaction, and baseline weight-by-visit interaction as fixed effects. P values indicated are for differences in change between empagliflozin and placebo at respective time points. *Adjusted mean (SE) changes from baseline by OSA status at baseline. BL, baseline; E, empagliflozin; P, placebo; W, week.
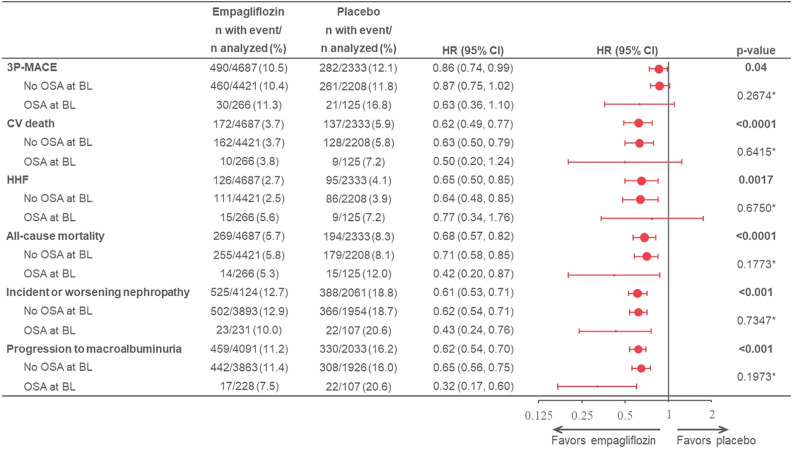
During the course of the trial, individuals with baseline OSA in the placebo group experienced 1.2- to 2.0-fold higher incidence rates for 3P-MACE (OSA vs. no OSA 6.49 vs. 4.27/100 patient-years), CV death (2.57 vs. 1.99/100 patient-years), HHF (2.71 vs. 1.38/100 patient-years), all-cause mortality (4.29 vs. 2.78/100 patient-years), incident or worsening nephropathy (9.16 vs. 7.52/100 patient-years), and progression to macroalbuminuria (9.08 vs. 6.36/100 patient-years) (Supplementary Appendix, Section C, and Supplementary Fig. C1). Empagliflozin reduced the risk for CV, HHF, mortality, and renal outcomes regardless of presence of OSA at baseline, with no evidence for heterogeneity of effect (P-interaction for all >0.05) (Fig. 2), except for the risk of progression to macroalbuminuria. Here, there was some indication for a larger magnitude of benefit among those with OSA at baseline (HR 0.32 [95% CI 0.17, 0.60]) versus no OSA at baseline (0.65 [0.56, 0.75]; P-interaction = 0.0312).
Figure 2.
Effects of empagliflozin treatment on CV, HHF, mortality, and renal outcomes by OSA status at baseline (BL). Events and HRs (95% CIs) for empagliflozin treatment on 3P-MACE, CV death, HHF, all-cause mortality, incident and worsening nephropathy, and progression to macroalbuminuria both overall (indicated in bold) and by OSA status at BL. Cox model includes age, sex, region, BL eGFR, BL BMI, BL HbA1c, treatment, OSA at BL, and treatment-by-OSA at BL interaction. *P-interaction.
Adverse events during trial follow-up among those with and without OSA at baseline are reported in Supplementary Appendix, Section D, and Supplementary Table D1 and were consistent with observations reported previously (16), regardless of OSA status at baseline. Of note, the reported frequency of nocturia among those with OSA at baseline between those treated with empagliflozin and placebo appeared to be similar (1.9% with empagliflozin vs. 3.2% with placebo).
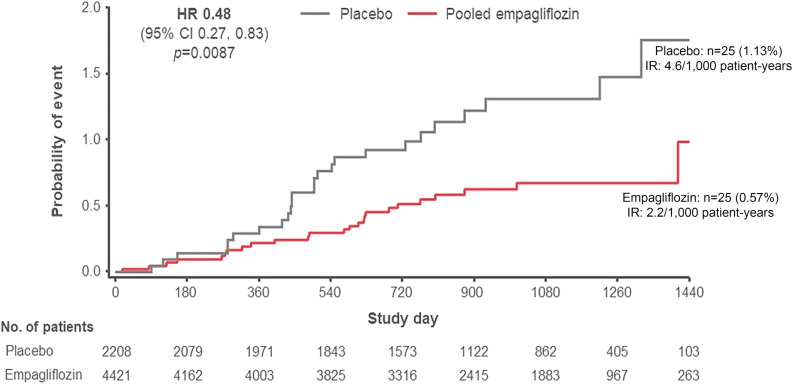
Fifty participants (representing 25 new cases among 4,421 [0.57%] without OSA at baseline in the empagliflozin group and 25 new cases among 2,208 [1.13%] without OSA at baseline in the placebo group) reported to develop new-onset OSA during the trial until 7 days after study drug discontinuation. Those treated with empagliflozin were 52% less likely to report new OSA (Fig. 3) compared with those treated with placebo (HR 0.48 [95% CI 0.27, 0.83]), with consistent effects also seen in the sensitivity mITT analysis (0.52 [0.30, 0.89]). Baseline characteristics of those who developed OSA (Supplementary Appendix, Section E, and Supplementary Table E1) suggested that these participants had a greater cardiometabolic burden than those who did not develop OSA. In an exploratory analysis for evaluating possible mediators of the treatment difference on new-onset OSA, the effects of empagliflozin on conventional OSA risk factors only partially explained the lower frequency for reporting of new OSA (Supplementary Appendix, Section F, and Supplementary Table F1). Weight reduction alone mediated 16.5%; HbA1c reduction alone, 12.2%; hematocrit increase alone, 9.4%; and SBP reduction alone, 8.9%. In a multivariable analysis, only 22.4% of the decreased-onset OSA in the empagliflozin arm was mediated by the treatment’s effect on all four conventional risk factors combined.
Figure 3.
Kaplan-Meier estimates for time to first OSA event on treatment for patients without OSA at baseline. OSA events are based on investigator-reported events (MedDRA version 18.0) until treatment end plus an additional 7 days. HRs are based on a Cox regression model with terms for age, sex, baseline BMI categories, HbA1c categories, eGFR categories, geographical region, and treatment. Empagliflozin estimates correspond to pooled treatment groups. IR, incidence rate.
Conclusions
In the EMPA-REG OUTCOME randomized placebo-controlled CV outcomes trial involving participants with type 2 diabetes and established CVD, we found that ∼6% of participants had OSA at baseline and that those with OSA experienced higher overall CV and renal event rates during the trial. We observed that empagliflozin improved multiple cardiometabolic risk markers, with a larger effect to reduce body weight among those with prevalent OSA. Furthermore, empagliflozin significantly reduced the risk for CV, HHF, mortality outcomes, and incident or worsening nephropathy, regardless of OSA status, consistent with the results in the overall study population. There was some evidence of a larger magnitude of effect on reducing the progression to macroalbuminuria. Furthermore, we found that empagliflozin may reduce the incidence of new-onset OSA among those without OSA at baseline and that this effect might be only partially attributable to its effects on conventional OSA risk factors. To our knowledge, this is the first report of outcomes related to OSA in a randomized controlled trial of SGLT2 inhibition. Given the increasing global burden of type 2 diabetes and obesity (20) and the lack of effective pharmacological treatments for OSA, these observations should prompt further study of SGLT2 inhibition as a therapeutic strategy for patients with type 2 diabetes and OSA.
The 6% prevalence of OSA in EMPA-REG OUTCOME is lower than expected on the basis of a study population with a mean age of 63 years and mean BMI of 30.6 kg/m2 (21). This may be because OSA is systematically underdiagnosed since it is notoriously difficult to diagnose and classify (22) and often not prioritized during busy clinic visits (23–25), leading to low reporting in trials (26). Nevertheless, as reported by others (27), we found in individuals with type 2 diabetes a consistently higher cardiometabolic burden associated with baseline OSA (e.g., higher BMI, higher utilization of insulin and antihypertensive medication, more prevalent CVD, and higher event rates for CV and heart failure outcomes).
From a mechanistic perspective, OSA in type 2 diabetes is believed to contribute to both intermittent hypoxia during sleep and frequent fragmentation of sleep patterns, which in turn promote deleterious effects on metabolic health. Multiple mechanistic pathways have been implicated, including increased sympathetic activation, oxidative stress, systemic inflammation, hypothalamic pituitary axis activation, and adipokine dysregulation (28), with each of these factors more highly prevalent in obesogenic states (29). Indeed, a small, open-label, single-arm study in Japanese patients with moderate to severe OSA, obesity, and diabetes showed improved metabolic parameters and less oxygen desaturation events with low-dose dapagliflozin administered over 24 weeks (30). In the current trial, empagliflozin treatment improved several metabolic end points in participants with OSA, with a trend for a larger effect on weight loss among those with baseline OSA, and may also reduce new-onset OSA. Empagliflozin is known to improve body weight and several indices of visceral adiposity (15), with greater effects on weight loss among those with higher baseline weight, which may in part explain its effects on metabolic parameters and reduced risk for new-onset OSA. However, we adjusted for baseline BMI in our analysis and continued to observe a lower frequency of new-onset OSA, suggesting that the observed weight loss may have included changes in total body water in addition to adipose tissue. Indeed, empagliflozin has modest diuretic and natriuretic effects and may improve OSA symptoms by reducing intrathoracic fluid retention (31) and rostral fluid shifts from the legs (32) and influence sodium handling by the kidneys (33). An alternative consideration is that a modest increase in urinary frequency with empagliflozin treatment, manifested nocturnally as nocturia, might have led to interrupted sleep cycles and decreasing the duration of rapid eye movement (REM) sleep. REM sleep is a higher risk period for OSA events (34), and a decrease in REM sleep would lead to fewer observed apneic events, leading to a potential ascertainment bias. However, since the frequency of nocturia in EMPA-REG OUTCOME was relatively balanced between treatment groups, we do not believe that this is a likely explanation for the results.
There are no pharmacological therapies currently approved for the treatment of OSA (9), and most investigations have focused on medications that can influence mechanisms like increasing the tone in the upper-airway dilator muscles, increasing ventilatory drive, reducing the proportion of REM sleep, increasing cholinergic tone during sleep, increasing arousal threshold, reducing airway resistance, or reducing surface tension in the upper airway. Several medication classes that are known to have beneficial effects on body weight and/or glycemic control have been studied in randomized controlled trials with varying results (9). Extended-release phentermine/topiramate (approved for long-term weight reduction) was investigated in a single-center study of 45 patients with moderate to severe OSA not receiving CPAP therapy. After 28 weeks of therapy, extended-release phentermine/topiramate reduced weight by ∼10% (placebo corrected ∼6%) and improved change in the apnea-hypopnea index (AHI) by ∼15 events (35). Liraglutide 3.0 mg (also approved for long-term weight reduction) was studied in 359 patients with moderate to severe OSA not receiving CPAP therapy; after 32 weeks of therapy, liraglutide reduced weight by ∼4% (vs. placebo) and significantly reduced the AHI by approximately six events (36). Although post hoc and not directly comparable to these studies, in the EMPA-REG OUTCOME trial, empagliflozin reduced weight by ∼2.5–3.0 kg in patients with baseline OSA (∼2.5% from baseline), yet empagliflozin treatment was associated with a 52% lower reported frequency of new-onset OSA.
With regard to mechanisms, when we performed a mediation analysis to further investigate what factors may have contributed to the lower hazard for OSA, we found that only ∼22% of the lower risk was attributable to the combined change in conventional risk factors, with the majority being due to weight loss. Thus, the majority of the empagliflozin effect on lower incidence of OSA may be mediated directly or indirectly by empagliflozin through factors not assessed in the trial.
There are several strengths of this study over prior work that add to our understanding of OSA, type 2 diabetes, and CVD. To our knowledge, this is the first report of outcomes related to OSA in a randomized controlled trial of SGLT2 inhibition, albeit in a post hoc, exploratory analysis. Second, all CV outcomes were prospectively adjudicated by independent, blinded clinical events committees, ensuring accurate and complete event reporting. Third, use of a mediation analysis allowed us to explore potential explanatory factors contributing to our prospective findings of lower new-onset OSA frequency with empagliflozin. Several limitations also merit comment. First, since the diagnosis of OSA was based on patient/investigator report rather than on systematic prospective polysomnography, the reported diagnosis is likely to be accurate/specific (i.e., low likelihood of false positives) but underidentified in patients without a prior sleep study. Thus, the prevalence of OSA in our population was lower than that expected in the general population, although underreporting in patients without a prior formal sleep study should be independent of randomized treatment. As such, it should not introduce a bias in the measured relative risk reduction between the two treatment arms. Second, since AHI was not prospectively measured in EMPA-REG OUTCOME, we are unable to comment on OSA severity with empagliflozin treatment. Third, we are unable to report on how participants were treated (CPAP vs. other treatments) for OSA during the course of the trial and how adherent they were to OSA therapies. Consequently, we are unable to determine to what extent a lack of sleep apnea treatment may have hindered weight loss and associated cardiometabolic benefits, thereby influencing our results. Fourth, since there are no AHI data to verify sleep apnea type or severity, and the MedDRA identifier code does not differentiate between OSA and central sleep apnea (CSA), we are unable to report the differential effects of empagliflozin on OSA versus CSA. However, since the prevalence of OSA is substantially higher than CSA in the general population, and both entities have adverse consequences, the overall findings remain pertinent. In summary, our findings are exploratory and should be understood to be hypothesis generating only; further prospective assessment of SGLT2 inhibition in OSA should be performed to confirm our results.
In conclusion, patients with type 2 diabetes, established CVD, and baseline OSA had greater cardiometabolic comorbidity and higher frequency of 3P-MACE, HHF, mortality, and renal outcomes in the EMPA-REG OUTCOME trial. Empagliflozin had favorable effects on cardiometabolic risk factors and consistently reduced risk for 3P-MACE, HHF, mortality, and renal outcomes in those with and without OSA at baseline. Furthermore, we found that empagliflozin could reduce the risk for new-onset OSA and that this additional potential benefit of empagliflozin could be partially independent of its effects on traditional OSA risk factors. Prospectively powered randomized controlled trials, including objective assessments of OSA using polysomnography (e.g., AHI, apnea severity, sleep efficiency, periodic limb movement, differentiation of OSA vs. CSA) are needed to further inform the effectiveness and limitations of novel therapies such as SGLT2 inhibition for OSA to treat residual CV risk related to OSA in this high-risk patient population.
Article Information
Acknowledgments. The authors thank the investigators, coordinators, and patients who participated in this trial. The authors acknowledge Matt Smith and Giles Brooke from Envision Scientific Solutions for graphical support (Figs. 1–3 and Supplementary Figs. B1–B4), supported financially by Boehringer Ingelheim.
Funding and Duality of Interest. This study was sponsored by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance. I.J.N. is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23-DK-106520 and the Dedman Family Scholarship in Clinical Care from the University of Texas Southwestern. I.J.N. reports speakers’ fees and consultancy honoraria from the Boehringer Ingelheim/Lilly Alliance and research support from Novo Nordisk. B.E. reports personal fees (expert panels, lectures) from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme (MSD), Mundipharma, Navamedic, Novo Nordisk, and RLS Global and grants and personal fees from Sanofi, all outside the submitted work. T.K. has received speakers’ fees from Boehringer Ingelheim. N.M. has given lectures for Amgen, Boehringer Ingelheim, Sanofi, MSD, Bristol-Myers Squibb, AstraZeneca, Eli Lilly, and Novo Nordisk; has received unrestricted research grants from Boehringer Ingelheim; and has served as an advisor for Amgen, Bayer, Boehringer Ingelheim, Sanofi, MSD, Bristol-Myers Squibb, AstraZeneca, and Novo Nordisk. In addition, he has served in trial leadership for Boehringer Ingelheim and Novo Nordisk. B.Z. has received consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi. S.E.I. has consulted for or served on clinical trial steering, executive, or publications committees for Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Sanofi/Lexicon Pharmaceuticals, Merck, vTv Therapeutics, and Abbott/Alere. C.W. has received grant support, fees for advisory services, and lecture fees from Boehringer Ingelheim; advisory services fees from Bayer, MSD, and Mundipharma; and lecture fees from Eli Lilly and AstraZeneca. I.Z. is an employee of Boehringer Ingelheim. O.E.J. was employed by Boehringer Ingelheim at the time of this writing. No other potential conflicts of interest relevant to this article were reported.
Boehringer Ingelheim was involved in the design and conduct of the study; collection, analysis, and interpretation of data; and preparation of the manuscript.
Author Contributions. I.J.N. and O.E.J. prepared the first draft and subsequent versions of the manuscript, which was reviewed and revised by all the authors, who approve the final version of the manuscript. I.J.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Thoracic Society 2020 Virtual Conference, 15–20 May 2020, and the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
Clinical trial reg. no. NCT01131676, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.12970550.
H.K.Y. and O.E.J. contributed equally to this work.
References
- 1.Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med 2019;380:1442–1449 [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jullian-Desayes I, Revol B, Chareyre E, et al. Impact of concomitant medications on obstructive sleep apnoea. Br J Clin Pharmacol 2017;83:688–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strausz S, Havulinna AS, Tuomi T, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ Open 2018;8:e022752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041 [DOI] [PubMed] [Google Scholar]
- 6.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 2010;14:131–136 [DOI] [PubMed] [Google Scholar]
- 7.Al Mawed S, Unruh M. Diabetic kidney disease and obstructive sleep apnea: a new frontier? Curr Opin Pulm Med 2016;22:80–88 [DOI] [PubMed] [Google Scholar]
- 8.Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest 2017;152:1070–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2013;3:CD003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudgel DW, Patel SR, Ahasic AM, et al.; American Thoracic Society Assembly on Sleep and Respiratory Neurobiology . The role of weight management in the treatment of adult obstructive sleep apnea. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e70–e87 [DOI] [PubMed] [Google Scholar]
- 11.Delahanty LM, Trief PM, Cibula DA, Weinstock RS. Barriers to weight loss and physical activity, and coach approaches to addressing barriers, in a real-world adaptation of the DPP lifestyle intervention: a process analysis. Diabetes Educ 2019;45:596–606 [DOI] [PubMed] [Google Scholar]
- 12.Häring HU, Merker L, Seewaldt-Becker E, et al.; EMPA-REG METSU Trial Investigators . Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013;36:3396–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs CS, Seshiah V, Swallow R, et al.; EMPA-REG PIO™ Trial Investigators . Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014;16:147–158 [DOI] [PubMed] [Google Scholar]
- 14.Tikkanen I, Narko K, Zeller C, et al.; EMPA-REG BP Investigators . Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015;38:420–428 [DOI] [PubMed] [Google Scholar]
- 15.Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 2016;13:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 17.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 18.Linselle M, Sommet A, Bondon-Guitton E, et al. Can drugs induce or aggravate sleep apneas? A case-noncase study in VigiBase®, the WHO pharmacovigilance database. Fundam Clin Pharmacol 2017;31:359–366 [DOI] [PubMed] [Google Scholar]
- 19.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018;41:356–363 [DOI] [PubMed] [Google Scholar]
- 20.Afshin A, Forouzanfar MH, Reitsma MB, et al.; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escourrou P, Grote L, Penzel T, et al.; ESADA Study Group . The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res 2015;24:730–738 [DOI] [PubMed] [Google Scholar]
- 23.Costa LE, Uchôa CH, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart 2015;101:1288–1292 [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro JP, Araújo A, Vieira C, et al. Undiagnosed risk of obstructive sleep apnea in obese individuals in a primary health care context. Acta Med Port 2020;33:161–165 [DOI] [PubMed] [Google Scholar]
- 25.Storgaard H, Mortensen B, Almdal T, Laub M, Tarnow L. At least one in three people with type 2 diabetes mellitus referred to a diabetes centre has symptomatic obstructive sleep apnoea. Diabet Med 2014;31:1460–1467 [DOI] [PubMed] [Google Scholar]
- 26.Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017;317:415–433 [DOI] [PubMed] [Google Scholar]
- 27.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol 2013;62:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jehan S, Myers AK, Zizi F, Pandi-Perumal SR, Jean-Louis G, McFarlane SI. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: epidemiology and pathophysiologic insights. Sleep Med Disord 2018;2:52–58 [PMC free article] [PubMed] [Google Scholar]
- 29.Valaiyapathi B, Calhoun DA. Role of mineralocorticoid receptors in obstructive sleep apnea and metabolic syndrome. Curr Hypertens Rep 2018;20:23. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa S, Miyake T, Senba H, et al. The effectiveness of dapagliflozin for sleep-disordered breathing among Japanese patients with obesity and type 2 diabetes mellitus. Endocr J 2018;65:953–961 [DOI] [PubMed] [Google Scholar]
- 31.Pearse SG, Cowie MR, Sharma R, Vazir A. Sleep-disordered breathing in heart failure. Eur Cardiol 2015;10:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasai T. Fluid retention and rostral fluid shift in sleep-disordered breathing. Curr Hypertens Rev 2016;12:32–42 [DOI] [PubMed] [Google Scholar]
- 33.de Albuquerque Rocha N, Neeland IJ, McCullough PA, Toto RD, McGuire DK. Effects of sodium glucose co-transporter 2 inhibitors on the kidney. Diab Vasc Dis Res 2018;15:375–386 [DOI] [PubMed] [Google Scholar]
- 34.Alzoubaidi M, Mokhlesi B. Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr Opin Pulm Med 2016;22:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep (Basel) 2012;35:1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes 2016;40:1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]