Abstract
Background/purpose
Overdenture with single symphyseal implant is one of the useful clinical approach for elderly edentulous patients. We aimed to evaluate edentulous patients with regards to the relationship between dimensions, bone characteristics, cancellous densities, and cortical thickness of the mandibular symphyseal region using cone beam computed tomography (CBCT).
Material and methods
We analyzed 103 CBCT from pre-implant investigations. All included patients were healthy without any disorders affecting bone metabolism. We performed 13 measurements for each patient: 9 of height, width, and thickness (mm) and 4 of density (Hounsfield Units; HU). Fisher's exact test was applied to examine the association between two categorical variables, the Pearson correlation coefficient to measure the strength of linear relationship between two quantitative variables. We used the two-sample Student's t-test to compare mean symphysis height between men and women, the paired Student's t-test to compare mean lingual and buccal cortical thickness. For all tests, the threshold of significance was fixed at 5%.
Results
Men and women significantly differed with regards to mean total symphysis height (p = 0.004) and the distribution of Cawood and Howell classifications (p = 0.033). Symphysis height was negatively correlated with mean density of cancellous bone (r = −0.453, p < 0.001). Mean lingual cortical thickness significantly differed from mean vestibular thickness (p < 0.001, paired Student's t-test).
Conclusion
Present findings supported that symphyseal measurements are parameters that reflect the symphysis bone characteristics, and can guide the choice of a suitable implant design.
Keywords: Bone densities, CBCT, Edentulous patient, Mandibular symphysis
Introduction
For over 100 years, fully edentulous patients have been treated using removable complete dentures, often with satisfaction, but sometimes with disappointing results. In 2002, the McGill consensus established that the minimum treatment for edentulous patients should include the use of two inter-foraminal implants and an overdenture. This clinical approach has improved patients’ levels of satisfaction and masticatory efficacity; however, the implant overdenture is often too expensive, and the surgery is difficult for aged patients. To address these issues, in 1997, Cordioli et al. proposed the use of a single implant in the middle of the symphysis.1 Since then, denture sets supported by a single implant have been successfully developed.2, 3, 4
The loss of anterior mandibular teeth induces many changes of the symphysis, particularly regarding its shape and volume. These changes have been well described in the literature.5,6 They mainly occur during the first 6 months after tooth extractions,7,8 and often involve the loss of up to 50% of the initial volume.9, 10, 11, 12
In the present study, we aimed to analyze the anatomical and morphological characteristics of the median symphyseal region in fully edentulous patients. Those two characteristics are not dissociable and yet to date no study has been studied simultaneously these two aspects of the symphyseal region. Analysis of these bone characteristics by cone beam computed tomography (CBCT) allow a practitioner to select the best implant design to achieve good primary stability and quick osteointegration. The null hypothesis was that the degree of median symphyseal modifications related to edentulism is not related the genre and with the bone characteristics.
Materials and methods
This study was conducted at La Timone University Hospital, Marseille, France, between 2016 and 2019, with approval from the Ethical Committee of this institution. The study enrolled 103 patients from the implant department, including 46 women (mean age, 68.2 ± 9.2 years) and 57 men (mean age, 71.2 ± 9 years). Inclusion criteria were an edentulous mandible for at least 3 months, and no health problems in terms of any disease that could affect bone metabolism. Patients were excluded if they had a history of injury, mandibular surgery, surgical sequelae, dysmorphic disorders, or diseases affecting muscle and skeletal function or development. All CBCT were performed by the same operator using the same imaging device (PlanMeca ProMax 3D, Helsinki, Finland/Classic voltage 120 kV; intensity, 160 mA; slice thickness, 0.625 mm; interval, 0.4 mm; DLP 215 mGy-cm). These scans were obtained as part of routine pre-implant investigations, and thus the enrolled patients did not receive any additional radiation due to participation in the study.
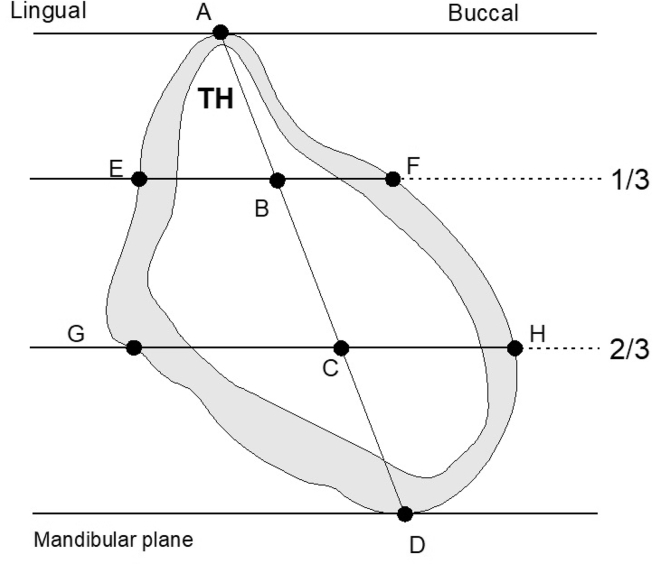
From the CBCT images for each patient, we selected the median section of the mandibular symphysis, i.e., the section lying an equal distance from each mental foramen. For each patient, we obtained 13 measurements of the selected median section: 9 measurements of height, width, and thickness in mm; and 4 measurements of density in Hounsfield units (HU). Fig. 1 shows the location of linear measurements on a cross-section of the mandible as rendered in a coronal CBCT image corrected for orientation.
Figure 1.
Median cross-section of the symphysis and the studied measurements. Bone densities were recorded at points A, B, C, and D. A, peak of the ridge; D, mandibular base; TH, Total Height; E, upper lingual; F, upper buccal; G, lower lingual; and H, lower buccal.
We analyzed three characteristics of the symphysis: dimensions (height and width), density, and thickness. Lingual and buccal cortical thickness measurements were completed from border to border of the cortical bone and using six defined sites: A, peak of the ridge; D, mandibular base; E, upper lingual; F, upper buccal; G, lower lingual; and H, lower buccal. Section height was determined by a segment passing through the maximum height of the symphysis. We also recorded the total height (TH), upper-third width (EF), and lower-third width (GH) of the mandible (Table 1). The lower point (D) was used to measure basal cortical thickness. Two lines were drawn parallel to the mandibular plane and through the section at the upper-third (EF) and lower-third (GH) of the total height (Table 2, Table 3). All sections, except those through the symphysis, were inclined lingually from the mandibular base to the alveolar ridge. Bone densities in HU were also recorded at 4 points (A, B, C, D). The study of bone density and cortical thickness is an important parameter in choosing the type of implant and its diameter. All measurements were performed using DTX Studio Design software (Nobel Biocare™, Envista Holdings Corporation, Brea, Cal, USA) and inserted in an Excel table.
Table 1.
Summary statistics of symphysis dimensions.
| Measurement | n | Symphysis dimensions (mm) |
||
|---|---|---|---|---|
| [min, max] | mean (SD) | median [Q1, Q3] | ||
| Total height (TH) | 103 | [10.30, 34.20] | 23.12 (4.66) | 23.30 [20.10, 26.50] |
| Width (EF) | 103 | [3.60, 17] | 10.63 (2.46) | 10.20 [9.10, 12.40] |
| Width (GH) | 103 | [7, 17.60] | 13.30 (2.19) | 13.30 [12, 14.70] |
Table 2.
Summary statistics of bone density at points A, B, C, and D.
| Point | n | Bone density (HU) |
||
|---|---|---|---|---|
| [min–max] | mean ± SD | median [Q1–Q3] | ||
| A | 103 | [131–1698] | 962.63 ± 357.52 | 936 [698–1281] |
| B | 103 | [87–1802] | 752.40 ± 352.20 | 752 [510–1030] |
| C | 103 | [102–1644] | 1082.36 ± 340.57 | 1156 [842–1331] |
| D | 103 | [838–1958] | 1525.11 ± 219.20 | 1546 [1354–1659] |
Table 3.
Summary statistics of cortical bone thickness at points A, E, F, G, H, and D.
| Point | n | Cortical bone thickness (mm) |
||
|---|---|---|---|---|
| [min–max] | mean ± SD | median [Q1–Q3] | ||
| A | 83 | [0–5.20] | 0.93 ± 0.84 | 0.80 [0.30–1.40] |
| E | 80 | [0.90–5] | 2.75 ± 0.87 | 2.65 [2.10–3.20] |
| F | 80 | [0.40–5.10] | 1.72 ± 0.91 | 1.40 [1.10–2.10] |
| G | 38 | [2.20–9.70] | 4.28 ± 1.50 | 4.35 [3.20–5.30] |
| H | 38 | [0.90–4.80] | 2.32 ± 0.88 | 2.15 [1.70–2.90] |
| D | 83 | [1.80–19.40] | 7.23 ± 3.60 | 7.10 [4.30–9.50] |
Statistical analysis
We performed a detailed descriptive analysis using SAS 9.4 software (SAS Institute Inc.) and R Software version 3.5.2 (R Foundation for Statistical Computing). Qualitative variables were presented as number and percentage (n, %). Quantitative variables were expressed as number, range, mean, SD, and medians sorted at the 25th and 75th percentiles (interquartile intervals) (Table 1, Table 2, Table 3).
The Fisher's exact test was used to compare the distribution of Cawood and Howell classifications between men and women. The Pearson correlation coefficient was used to measure the strength of the linear relationship between two quantitative variables. We used the two-sample Student's t-test to compare the mean symphysis height between men and women, and the paired Student's t-test to compare the mean lingual and buccal cortical thickness and mean cortical thickness at the minimum and maximum symphysis heights. All statistical tests were two-sided and the significance level was set at 0.05.
Results
This study included a total of 103 patients, with an age range of 44–90 years. The overall mean age of the sample was 69.8 ± 9.2 years. The sample included 46 women (mean age, 68.2 ± 9.2 years) and 57 men (mean age, 71.2 ± 9 years).
Symphysis dimensions (height and width)
In the total population, the mean total symphysis height was 23.12 ± 4.66 mm, with a minimum of 10.30 mm and a maximum of 34.20 mm. Mean symphysis height was 24.30 ± 4.10 mm in men, and 21.65 ± 4.90 mm in women, which was a significant difference (p = 0.004) (Table 1). The maximum width observed was 17.60 mm (measured at GH), and the minimum width was 3.60 mm (measured at EF). Mean symphysis widths were 10.63 ± 2.46 mm at EF, and 13.30 ± 2.19 mm at GH.
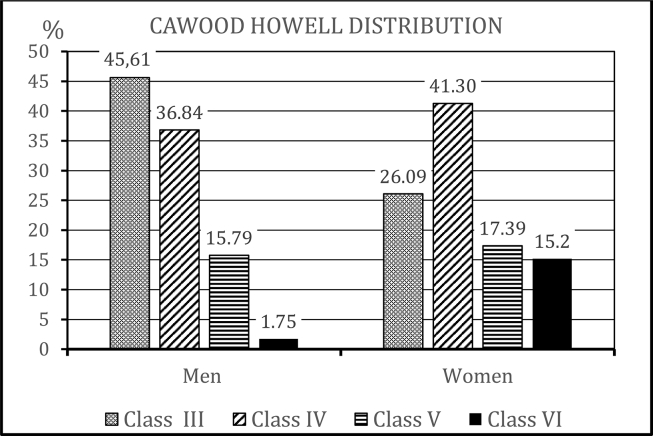
According to the symphysis classification of Cawood and Howell,10 our sample was distributed as follows: 36.9% class III (n = 38), 38.8% class IV (n = 40), 16.5% class V (n = 17), and 7.8% class VI (n = 8). Based on the inclusion criteria of our study, our study cohort did not include any patients in classes I or II. We also analyzed the distribution of Cawood and Howell classifications according to sex, and found that the distributions significantly differed between men and women (p = 0.033, Fisher's exact test) (Fig. 2).
Figure 2.
Distribution of Cawood and Howell classifications, according to sex (57 men and 46 women). No included patients were class I or II, due to the inclusion criteria.
Bone density
Mean densities varied according to the bone structures studied and the points measured. For cortical bone, the mean density was 962.63 ± 357.52 HU at point A, compared with 1525.11 ± 219.20 HU at point D. For cancellous bone, the mean density was 752.40 ± 352.20 HU at point B, and 1082.36 ± 340.57 HU at point C (Table 2).
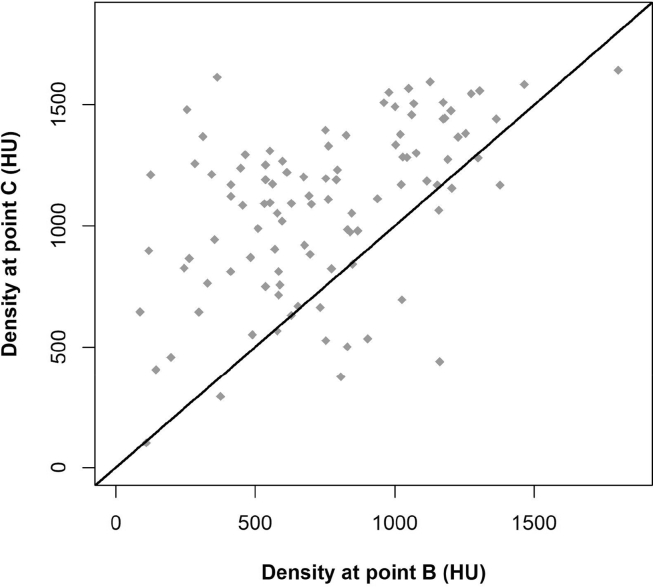
We found that 77.70% of patients (n = 80) had cancellous bone and cortical bone between points E and F, compared to 36.90% (n = 38) between G and H. The remaining patients had only cortical bone along the entire width. Comparison of densities at points B and C revealed that the density was greater at point C in 84.5% of patients (Fig. 3).
Figure 3.
Comparison of densities in Hounsfield units (HU) at points B and C.Scatterplot shows density at point C (y-axis) against density at point B (x-axis) and line y = x (red line).
Cortical thickness
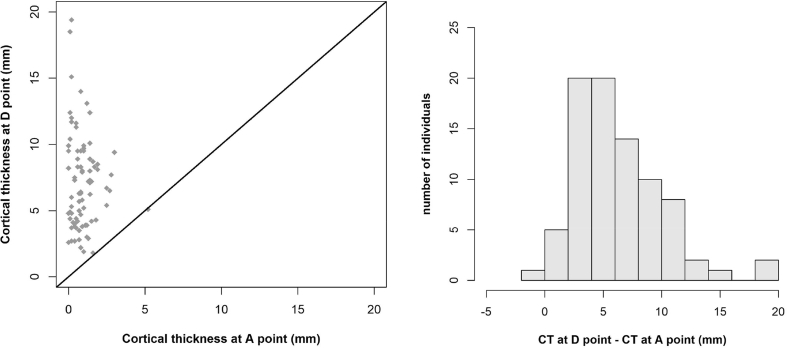
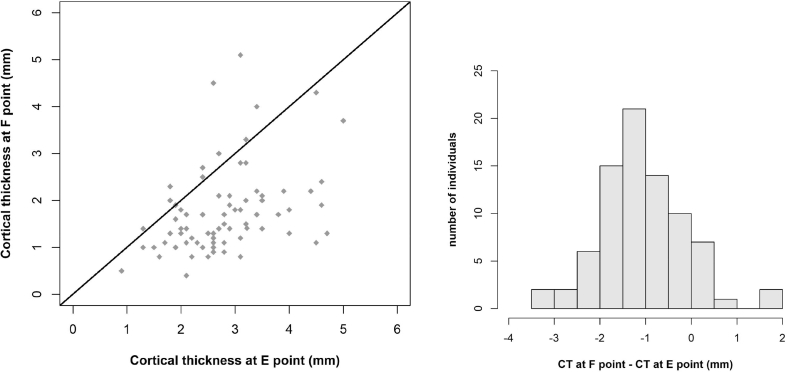
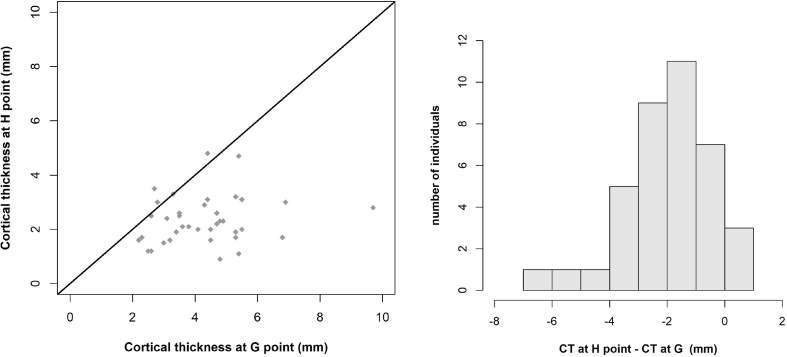
Measurements of cortical thickness revealed many differences between the studied points, from point A (0.93 ± 0.84 mm) to point D (7.23 ± 3.60 mm) (Table 3). Using the paired Student's t-test revealed that the mean cortical thickness at E was significantly different from the mean cortical thickness at F (2.75 vs 1.72 mm, P < 0.001). This comparison was conducted in a sample of 80 patients rather than 103, as we excluded patients in whom E = F, i.e., patients who had only cortical bone at EF. We also found that mean cortical thickness differed significantly at point G compared with point H (4.28 vs 2.32 mm, P < 0.001). This analysis was conducted in 38 patients, excluding those in whom G = H, i.e., who had only cortical bone at GH. Figure 4, Figure 5, Figure 6 show the statistical results and the paired comparisons of cortical thickness.
Figure 4.
Comparison of cortical thickness (CT) at points A and D (n = 83). Scatterplot shows CT at point D (y-axis) against CT at point A (x-axis) and line y = x (red line) and a histogram of the difference (CT at point D − CT at point A).
Figure 5.
Comparison of cortical thickness (CT) at points E and F (n = 80). Scatterplot shows CT at point F (y-axis) against CT at point E (x-axis) and line y = x (orange line) and histogram of difference (CT at point F – CT at point E).
Figure 6.
Comparison of cortical thickness at points G and H (n = 38). Scatterplot shows CT at point H (y-axis) against CT at point G (x-axis) and line y = x (orange line) and histogram of difference (CT at point H – CT at point G).
Of the 103 patients, 80.6% (n = 83) had cancellous bone between points A and D, 77.7% (n = 80) between E and F, and 36.9% (n = 38) between G and H. Among the 83 patients with cancellous bone between points A and D, 98.8% had cortical thickness at D that was greater than or equal to A. Of the 80 patients with cancellous bone between points E and F, 86.2% (n = 69) had a greater or equal cortical thickness at E compared to F. Among the 38 patients with cancellous bone between points G and H, 89.5% (n = 34) had a cortical thickness at G that was greater than or equal to H. Paired Student's t-tests were used to analyze differences in mean cortical thickness between points A and D (n = 83), E and F (n = 80), and G and H (n = 38), and all differences were significant (p < 0.001).
Correlation
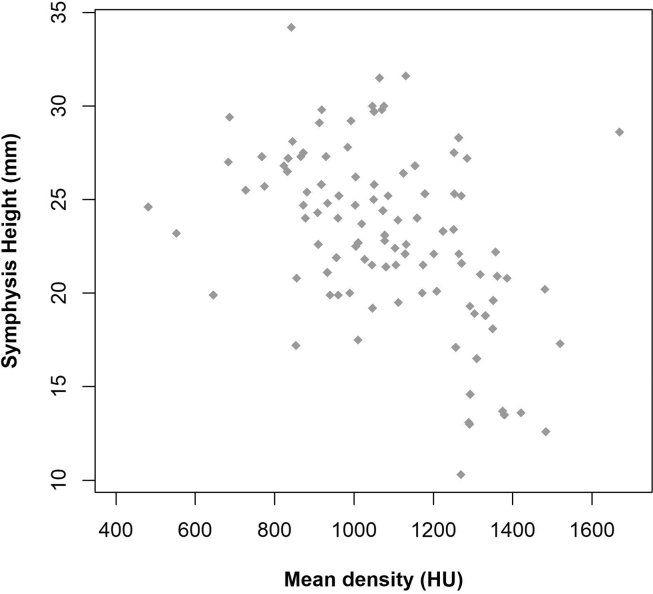
Analysis of the correlation between symphysis height and bone density at points A, B, C, and D revealed a negative correlation at points A (r = −0.33; p < 0.001), B (r = −0.33; p < 0.001), and C (r = −0.43; p < 0.001), and no correlation at point D (r = −0.06; p = 0.558). At points A, B, C, and D, a decrease in mean symphysis height was accompanied by an increased mean bone density. Fig. 7 shows the relationship between symphysis height and mean density (DA, DB, DC, and DD). We identified a significant and negative correlation between these two measures (r = −0.45; p < 0.001).
Figure 7.
Relationship between symphysis height and mean density (DA, DB, DC, and DD).
Discussion
Patient bone characteristics were initially evaluated with the goal of choosing the best place for the implant relative to the cortical density and trabecular spaces. However, nowadays the paradigm has changed: The prosthetic conception induces the choice of implant position whose characteristics (form, surface, thread) are in accordance with the underlying bone characteristics. Our study included a large sample of 103 edentulous patients evaluated by CBCT. However, it is difficult to compare our results with those of prior studies, since previous samples have included dentate or edentate mandibles, and living or deceased patients. Some prior investigations have been conducted on anatomical structures—for example, Ulm et al. performed histological research on four parts of the mandible from 128 patients (68 females and 60 males; mean age, 77.58 years),13 Cawood Howell studied 300 dry skulls,10 and Schwartz-Dabney conducted a cadaver study of 10 edentulous mandibles.6 Katranji examined 28 dissected mandibles, from a population that was 68% male and 32% female, with an average age of 73.1 years.14 Merrot et al. studied mandibular changes in 67 maxillary and mandibular edentate elderly patients and 43 dentate elderly patients, but did not differentiate between the sites.12 Guevara-Perez studied a series of 12 cadaveric mandibles, and Blahout et al. examined 41 mandibular halves.15,16
Many other clinical studies have been performed among patients using X-ray devices—for example, Tallgren reported cephalometry findings, and Truitt reported findings from CT scanning.17,18 Swasty et al. studied 113 individuals with ages of 10–19 years; Foosiri examined 51 individuals, including 21 males with a mean age of 29.9 years and 30 females with a mean age of 25.44 years; and Derya et al. studied 196 patients of 20–45 years of age.19,20
Since 1998, CBCT has been used extensively in odontology due to its low cost, ease of use, and low irradiation level, meeting the ALARA (As Low As Reasonably Achievable) principle.21 Notably, Jacobs et al. have stated a preference for following the ALADAIP (As low as Diagnostically Acceptable being Indication-oriented and Patient-specific) principle.22 However, in a literature review, De Vos found that the available data do not confirm the perception of the low irradiation level with CBCT, since the doses are related to the utilized imaging devices, and the irradiation doses vary between 41 and 53 μSv.21,23 In our present study, each examination was conducted by the same operator and using the same calibrated CBCT imaging unit (Planmeca).
Diagnostic imaging is based on evaluation of the gray level of hard and soft tissues obtained with CT or CBCT devices. With CBCT, the degree of X-ray attenuation is shown by gray scale (voxel value, VV). Mah reported data indicating a strong linear relationship between the gray scales in CBCT and HU in CT.24 Razi et al. stated that gray scale in CBCT is the standard for measuring bone density before implant treatment, and that this method is recommended because of the lower radiation dose and cost compared to CT scanning.25 However, Cassetta reported that a conversion ratio must be applied to the VV to more accurately define the bone density with CBCT.26
First published in 1985, the Lekholm & Zarb classification is a subjective classification system that uses gray levels to assess the cortical bone/cancellous bone ratio. However, its subjective aspect has been clearly demonstrated in numerous studies,27 and this approach has evolved over time. Using CBCT data, Al-Ekrish et al. developed a modification of the classification of the Lekholm & Zarb classification, which included five classes.28 Since the works of Misch, computed tomography has been considered a valid and precise method of measuring cancellous and cortical bone density.29,30
The degree of mandibular bone loss, particularly symphysis bone loss, is affected by a number of factors, including the initial clinical conditions and the healing process of the sockets. In a systematic review, Van der Weijden et al. showed that among dentate patients, during the post-extraction healing period, the mean changes affecting alveolar bone resulted in a clinical width loss of 3.87 mm, which was greater than height loss detected through both clinical evaluation (1.67–2.03 mm) and radiographically (1.53 mm).8 Tan et al. reported similar findings, with greater horizontal bone loss than vertical bone loss.7 These changes all occurred within the first 6 months after extraction. Bone remodeling is also influenced by mechanical and functional stresses, as described by Atwood and Tallgren and by Cawood and Howell.11,10 These authors also reported greater losses in width than in height, but with the greatest impact in the basal part of the mandibular body.31,28 In our present study, Cawood-Howell classes I and II were not represented, and patients were most frequently class IV (n = 40), followed by class III (n = 38).
In another study of the interforaminal region, the most marked atrophy-induced changes were observed in the mandibular height. The examined mandibles showed a very significant decrease in height of 46–57%, and over 60% in extreme cases. The data obtained from this cross-sectional study revealed that about one-third of the original bone height was lost at a relatively early stage in the resorption process. During all later stages of resorption, the mandible lost only one-fifth of its original bone substance. Moreover, the degree of alveolar ridge resorption was not associated with the patient's age, but depended on the time elapsed after extractions.16
In our present study, we compared lingual and basal cortical thicknesses with vestibular and superior thicknesses, and our findings were in agreement with those of Lestrel et al.5 They reported that the cortical thickness of the lower border of the mandible tended to slightly increase with age, regardless of whether the patient was dentate or edentate. They also demonstrated increased cortical thickness at the lower aspect of the mandibular symphysis in edentate patients.5 Accordingly, Schwartz-Dabney and Dechow reported an increased cortical thickness on the lingual aspect in edentate mandibles compared with dentate mandibles.6 Among dentate patients, the symphysis cortical compartment appeared to remain stable, even if this stability was no longer present distally.
It has been proposed that mandibular stability may principally depend on cortical bone and not cancellous bone.13 Within the region of the incisors, the cortical compartment has been reported to undergo no significant change; however, different results were obtained for distal aspects of the mandible.16 Although cancellous bone remodeling after tooth loss is a well-known phenomenon, structural changes at sites distant from the alveolar process have been scarcely discussed in the literature.6 Ridge resorption has often been considered a localized phenomenon, restricted to cancellous bone. One study examined the remodeling changes on the lower border of the mandible and the mandibular symphysis related to use of prostheses and aging.6 The results were in agreement with other findings, concluding that three-dimensional structural changes may occur in cortical bone, whereas density showed little change.6 In our present study, we observed considerable variability in height and width between individuals, while the densities at the same points (A, B, C, and D) varied little between individuals. However, one prior study has shown variations in density among different regions of the mandible (incisor, canine, premolar, and molar).29 These variations were related to functional differences in the studied regions, particularly the muscular insertions.
Schwartz-Dabney and Dechow found that the thickness of cortical bone in the mandible, and its density and elastic properties, exhibited a unique regional variation, with distinct findings between the symphysis and in the mandibular body.6 Park et al. reported mandibular cortical density of between 810 and 1580 HU in alveolar bone, and of between 1320 and 1560 HU in basal bone.32 This was in agreement with our present findings.
There remains controversy surrounding the impact of tooth loss on mandibular density. One study extended its investigations to other regions of the mandible, where no significant differences were found between regions. This absence of difference suggests that cortical bone density at various points is maintained after tooth loss, despite changes in structure, rigidity, and anisotropy.6
Considering the limits of this study, the following conclusions may be drawn. The reduction of symphyseal size is sex-related, with women showing greater reduction. The decrease of symphyseal height is accompanied by an increased density of the central and lower portion of cancellous bone, and the thickness of the lingual and lower parts of cortical bone. Symphysis size is an important parameter for selecting the design (form, thread, surface) of the implant to ensure optimal primary stability and quick osteointegration.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Cordioli G., Majzoub Z., Castagna S. Mandibular overdentures anchored to single implants: a five-year prospective study. J Prosthet Dent. 1997;78:159–165. doi: 10.1016/s0022-3913(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 2.Cheng T., Ma L.I., Liu X.L. Use of a single implant to retain mandibular overdenture: a preliminary clinical trial of 13 cases. J Dent Sci. 2012;7:261–266. [Google Scholar]
- 3.Krennmair G., Ulm C. The symphyseal single-tooth implant for anchorage of a mandibular complete denture in geriatric patients: a clinical report. Int J Oral Maxillofac Implants. 2001;16:98–104. [PubMed] [Google Scholar]
- 4.Nogueira T.E., Dias D.R., Leles C.R. Mandibular complete denture versus single-implant overdenture: a systematic review of patient-reported outcomes. J Oral Rehabil. 2017;44:1004–1016. doi: 10.1111/joor.12550. [DOI] [PubMed] [Google Scholar]
- 5.Lestrel P.E., Kapur K.K., Chauncey H.H. A cephalometric study of mandibular cortical bone thickness in dentulous persons and denture wearers. J Prosthet Dent. 1980;43:89–94. doi: 10.1016/0022-3913(80)90360-1. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz-Dabney C.L., Dechow P.C. Edentulation alters material properties of cortical bone in the human mandible. J Dent Res. 2002;81:613–617. doi: 10.1177/154405910208100907. [DOI] [PubMed] [Google Scholar]
- 7.Tan W.L., Wong T.L.T., Wong M.C.M., Lang N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23:1–21. doi: 10.1111/j.1600-0501.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- 8.Van der Weijden F., Dell'Acqua F., Slot D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review. J Clin Periodontol. 2009;36:1048–1058. doi: 10.1111/j.1600-051X.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 9.Atwood D.A. Postextraction changes in the adult mandible as illustrated by microradiographs of midsagittal sections and serial cephalometric roentgenograms. J Prosthet Dent. 1963;13:810–824. [Google Scholar]
- 10.Cawood J.I., Howell R.A. A classification of the edentulous jaws. Int J Oral Maxillofac Surg. 1988;17:232–236. doi: 10.1016/s0901-5027(88)80047-x. [DOI] [PubMed] [Google Scholar]
- 11.Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: a mixed-longitudinal study covering 25 years. J Prosthet Dent. 2003;89:427–435. doi: 10.1016/s0022-3913(03)00158-6. [DOI] [PubMed] [Google Scholar]
- 12.Merrot O., Vacher C., Merrot S., Godlewski G., Frigard B., Goudot P. Changes in the edentate mandible in the elderly. Surg Radiol Anat. 2005;27:265–270. doi: 10.1007/s00276-005-0323-x. [DOI] [PubMed] [Google Scholar]
- 13.Ulm C., Tepper G., Blahout R., Rausch-Fan X., Hienz S., Matejka M. Characteristic features of trabecular bone in edentulous mandibles. Clin Oral Implants Res. 2009;20:594–600. doi: 10.1111/j.1600-0501.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 14.Katranji A., Misch K., Wang H.-L. Cortical bone thickness in dentate and edentulous human cadavers. J Periodontol. 2007;78:874–878. doi: 10.1902/jop.2007.060342. [DOI] [PubMed] [Google Scholar]
- 15.Guevara Perez S.V., de la Rosa Castolo G., Thollon L., Behr M. A 3D characterization method of geometric variation in edentulous mandibles. Morphologie. 2018;102:255–262. doi: 10.1016/j.morpho.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Blahout R.M.S., Hienz S., Solar P., Matejka M.H., Ulm C.W. Quantification of bone resorption in the interforaminal region of the atrophic mandible. Int J Oral Maxillofac Implants. 2007;22:609–615. [PubMed] [Google Scholar]
- 17.Tallgren A., Solow B. Age differences in adult dentoalveolar heights. Eur J Orthod. 1991;13:149–156. doi: 10.1093/ejo/13.2.149. [DOI] [PubMed] [Google Scholar]
- 18.Truitt H.P., Altman A., Boyne P.J. Use of computer tomography in subperiosteal implant therapy. J Prosthet Dent. 1988;59:474–477. doi: 10.1016/0022-3913(88)90045-5. [DOI] [PubMed] [Google Scholar]
- 19.Swasty D., Lee J.S., Huang J.C. Anthropometric analysis of the human mandibular cortical bone as assessed by cone-beam computed tomography. J Oral Maxillofac Surg. 2009;67:491–500. doi: 10.1016/j.joms.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 20.Foosiri P., Mahatumarat K., Panmekiate S. Relationship between mandibular symphysis dimensions and mandibular anterior alveolar bone thickness as assessed with cone-beam computed tomography. Dent Press J Orthod. 2018;23:54–62. doi: 10.1590/2177-6709.23.1.054-062.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vos W., Casselman J., Swennen G.R.J. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg. 2009;38:609–625. doi: 10.1016/j.ijom.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs R., Salmon B., Codari M., Hassan B., Bornstein M.M. Cone beam computed tomography in implant dentistry: recommendations for clinical use. BMC Oral Health. 2018;18:88–104. doi: 10.1186/s12903-018-0523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dessel J., Nicolielo L.F.P., Huang Y. Quantification of bone quality using different cone beam computed tomography devices: accuracy assessment for edentulous human mandibles. Eur J Oral Implant. 2016;9:411–424. [PubMed] [Google Scholar]
- 24.Mah P., Reeves T.E., McDavid W.D. Deriving Hounsfield units using grey levels in cone beam computed tomography. Dentomaxillofacial Radiol. 2010;39:323–335. doi: 10.1259/dmfr/19603304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razi T., Emamverdizadeh P., Nilavar N., Razi S. Comparison of the Hounsfield unit in CT scan with the gray level in cone-beam CT. J Dent Res Dent Clin Dent Prospects. 2019;13:177–182. doi: 10.15171/joddd.2019.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassetta M., Giansanti M., Di Mambro A., Stefanelli L. Accuracy of positioning of implants inserted using a mucosa-supported stereolithographic surgical guide in the edentulous maxilla and mandible. Int J Oral Maxillofac Implants. 2014;29:1071–1078. doi: 10.11607/jomi.3329. [DOI] [PubMed] [Google Scholar]
- 27.Mesquita Júnior E.J., Vieta A.I., Taba Júnior M., Faria P.E.P. Correlation of radiographic analysis during initial planning and tactile perception during the placement of implants. Br J Oral Maxillofac Surg. 2017;55:17–21. doi: 10.1016/j.bjoms.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Al-Ekrish A., Widmann G., Alfadda S. Revised, computed tomography–based Lekholm and Zarb jawbone quality classification. Int J Prosthodont (IJP) 2018;31:342–345. doi: 10.11607/ijp.5714. [DOI] [PubMed] [Google Scholar]
- 29.Attili S., Surapaneni H., Kasina S.P., Kumar V.H.C., Balusu S., Barla S.C. To evaluate the bone mineral density in mandible of edentulous patients using computed tomography: an in vivo study. J Int Oral Health. 2015;7:22–26. [PMC free article] [PubMed] [Google Scholar]
- 30.Park J., Shin S., Lee J. Bar versus ball attachments for maxillary four-implant retained overdentures: a randomized controlled trial. Clin Oral Implants Res. 2019;30:1076–1084. doi: 10.1111/clr.13521. [DOI] [PubMed] [Google Scholar]
- 31.Chrcanovic B.R., Albrektsson T., Wennerberg A. Survival and complications of zygomatic implants: an updated systematic review. J Oral Maxillofac Surg. 2016;74:1949–1964. doi: 10.1016/j.joms.2016.06.166. [DOI] [PubMed] [Google Scholar]
- 32.Park H.-S., Lee Y.-J., Jeong S.-H., Kwon T.-G. Density of the alveolar and basal bones of the maxilla and the mandible. Am J Orthod Dentofacial Orthop. 2008;133:30–37. doi: 10.1016/j.ajodo.2006.01.044. [DOI] [PubMed] [Google Scholar]