Abstract
Abstract Background/purpose
Sprouty (SPRY) has four isoforms, SPRY1–4, and its deficiency produces haphazard ‘sprouting’ of tracheal tubules. This study investigated SPRY1 protein expression in human oral potentially malignant disorders (OPMDs) and oral squamous cell carcinomas (OSCCs).
Materials and methods
90 OSCCs, 10 OPMDs with malignant transformation (MT), 17 OPMDs without MT, and six normal oral mucosa (NOM) tissue samples were subjected to immunohistochemical staining. Three human oral cancer cell lines (OCCLs), an oral precancer cell line (DOK), and a primary culture of normal oral keratinocytes (HOK) were used for western blotting.
Results
Significantly increased expression of SPRY1 protein from NOM and OPMD without MT to OSCC was observed. The protein expressions of SPRY1 in OCCLs were significantly enhanced as compared with DOK and HOK. Increased phosphor/total-ERK expression was observed in OCCLs as compared with HOK. A significantly increased SPRY1 protein level was noted in OPMDs with MT as compared with those without MT, in addition to a significant increase in DOK in comparison with HOK.
Conclusion
Our results indicated that overexpression of SPRY1 protein is potentially associated with human oral squamous cell carcinogenesis.
Keywords: SPRY1, Oral squamous cell carcinoma, Oral potentially malignant disorders
Introduction
Oral squamous cell carcinoma (OSCC) is the most common human oral epithelial malignancy, comprising approximately 3% of all newly-confirmed cancer cases.1,2 In Taiwan, OSCC represents the fourth most common cancer, and is also the fourth leading cause of death due to cancer in males.3,4 Perhaps due to diagnosis at a late stage, the five-year survival rate of OSCC patients is low,5 highlighting the necessity to improve our understanding of the pathogenesis of human oral squamous cell carcinogenesis.6 Moreover, a proportion of OSCC cases undergo malignant transformation (MT) from a spectrum of oral potentially malignant disorders (OPMDs).7,8 Hence, avoidance of MT in patients with OPMDs is imperative.9
Sprouty (SPRY) is an intracellular protein that has four isoforms (SPRY1–4) and is related to the modulation of branching in tracheal development, and absence of SPRY could induce haphazard ‘sprouting’ of tracheal tubules.10 SPRY has been found to be an inhibitor protein that participates in changing of the components of the Ras/ERK (receptor tyrosine kinase, RTK) pathway.11 Binding of SPRY to the plasma membrane leads to inhibition of mitogen-activated protein kinase (MAPK) signaling.12, 13, 14, 15, 16, 17 Additionally, the targets for SPRY repression could vary, as it regulates upstream of RAS or downstream at the level of RAF.18, 19, 20, 21 On the other hand, SPRY has been confirmed as being not only capable of suppressing MAPK activation, but also of upregulating the MAPK pathway.22, 23, 24, 25
To the best of our knowledge, SPRY1 expression has not been documented in human oral squamous cell carcinogenesis. So, the present study aimed to investigate the SPRY1 expression in human OPMDs and OSCCs.
Material and methods
SPRY1 expression in human OSCCs and OPMDs
Immunohistochemistry
Tissue specimens from 90 primary OSCC patients with the habits of drinking alcohol, chewing betel quid, and smoking cigarettes under the approval of the Ethics Committee for Scientific Research on Human Beings of the institution (KMUHIRB-E(II)-20150269) were fixed in 10% neutral buffered formalin solution, dehydrated in graded alcohols, cleaned in xylene, and embedded in paraffin for subsequent immunohistochemical staining. Six normal oral mucosal tissues acquired from healthy persons without the aforementioned oral habits that are risk factors for oral malignancy were employed as controls. Tissue samples were also obtained from 27 human OPMD patients (including patients with hyperkeratosis/epithelial hyperplasia with or without oral epithelial dysplasia, oral submucous fibrosis, and verrucous hyperplasia) for whom 10 patients exhibited MT and 17 who did not.
Deparaffinization of paraffin-embedded 4-μm-thick tissue sections was performed using xylene solutions, and rehydration was achieved using graded alcohols. Then, the tissue sections were treated three times with microwave radiation in a 10 mM citrate buffer (pH 6.0) for 5 min each time. The sections were subsequently immersed in methanol with 0.3% H2O2 for 45 min to block the endogenous peroxidase activity, and incubated in normal goat serum to reduce non-specific binding. Sections were finally incubated for 60 min at room temperature with primary anti-SPRY1 antibody (ABGENT, San Diego, CA, USA; Cat. No. ALS14077; 1:100). The sections were then processed using the standard avidin–biotin peroxidase complex method in accordance with the manufacturer's protocol (Vector Laboratories).26 Diaminobenzidine (Roche; Cat. No. 1718096) was used as a chromogen, and hematoxylin was used for counterstaining. Each set of experiments included a human colon squamous cell carcinoma specimen known to express SPRY1 protein, which served as a positive control and ensured the reproducibility of the staining process. Negative controls were employed using the same procedure, with omission of the primary antibody.
The scores of the percentage of positive staining (P) were categorized as follows: 0 (<1%); 1 (1–24%); 2 (25–49%); 3 (50–74%); and 4 (75–100%), whereas the scores for the intensity of staining (I) were designated as 0, no staining; 1, light yellow color (weak staining); 2, brown color (moderately strong staining); and 3, dark brown color (strong staining). The total score (S) was then calculated as P × I for each section.27 All stained slides were observed and quantified using the semi-automated image analysis software Image J, Version 1.51e, to determine the patterns of positive staining microscopically.
Cell cultures
Three human oral cancer cell lines (OECM1, SAS, and Ca922) were used in the current study. OECM128 and SAS29 were respectively derived from primary gingiva squamous cell carcinoma with OECM1 being obtained from a Taiwanese betel-quid chewer, and SAS29 shown extensive epidermal growth receptor expression. Ca92230 was obtained from a Japanese patient with high-grade primary squamous cell carcinoma. Ca922, OECM1, and SAS cells were cultured in high-glucose DMEM (HyClone, Logan, UT, USA) together with 10% fetal bovine serum (HyClone) and 1% penicillin–streptomycin (HyClone) at 37 °C in a 5% CO2 incubator. In addition, for primary culture, normal human oral keratinocytes (HOK) (ScienCell, USA) were cultured in Oral Keratinocyte Medium (OKM, ScienCell). The culture medium was replaced every three days.
A human oral precancer cell line DOK31 derived from mild to moderate dysplastic oral keratinocytes from the tongue of a Caucasian patient with a keratin profile similar to the original dysplasia, which has been shown to be non-tumorigenic in athymic nude mice, was cultured in high-glucose DMEM (HyClone) with the addition of 10% fetal bovine serum (HyClone), 2 mM glutamine (HyClone), 5 μg/ml hydrocortisone (HyClone), and 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C in a 5% CO2 incubator.
Western blot analysis of human oral cancer cell lines and oral precancer cell line
After rinsing Ca922, OECM1, SAS, DOK, and HOK cells with PBS (Sigma-Aldrich, St Louis, MO, USA) followed by treatment with Radio-Immunoprecipitation Assay (RIPA) lysis buffer, the lysates were centrifuged at 4 °C, 14,000 rpm, for 15 min. The protein concentration was then quantified using a Thermo Pierce Protein Assay Kit. Equal amounts of protein were denatured with sodium dodecyl sulfate (SDS) running buffer (Sigma-Aldrich) and β-mercaptoethanol (Sigma-Aldrich). The samples were then analyzed using 10% SDS-plyacrylamide gel electrophoresis (PAGE) (Sigma-Aldrich) gels, and the proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Sigma-Aldrich) using Bio-Rad's transblot with SPRY1 primary antibody (ABGENT; 1:1000), with an observed molecular weight of 35 kDa; and GAPDH (Sigma-Aldrich; 1:1000), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Sigma-Aldrich; 1:5000). The protein level was measured using a Fuji LAS-4000 lumino image analyzer (Fuji Photo Film Co., Tokyo, Japan). The ratio was normalized to the GAPDH signal.
Further, samples were analyzed using 10% SDS-PAGE (Sigma-Aldrich) gels, and the proteins were transmitted onto a PVDF membrane (Sigma-Aldrich) using Bio-Rad's transblot with primary antibodies against phosphor-ERK (Boster Biological Technology, CA, USA; Cat. No. P00104; 1:1000) and total-ERK (Boster Biological Technology; Cat. No. P00104; 1:1000), with species specificity for human tissues and an observed molecular weight of 42–44 kDa; and β-actin (Sigma-Aldrich; 1:1000), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Sigma-Aldrich; 1:5000). The amount of protein was measured using a Fuji LAS-4000 lumino image analyzer (Fuji Photo Film Co.). The ratio was normalized to the β-actin signal.
Statistical analyses
All statistical analyses were performed using the SAS Statistical Package (Version 9.1.3, SAS Institute Inc.). Nonparametric Kruskal–Wallis tests were employed to compare the immunohistochemical expressions of SPRY1 protein and to analyze the results of western blots. Statistical significance was considered when the P value was less than 0.05.
Results
SPRY1 expression in human OSCCs
Immunohistochemistry
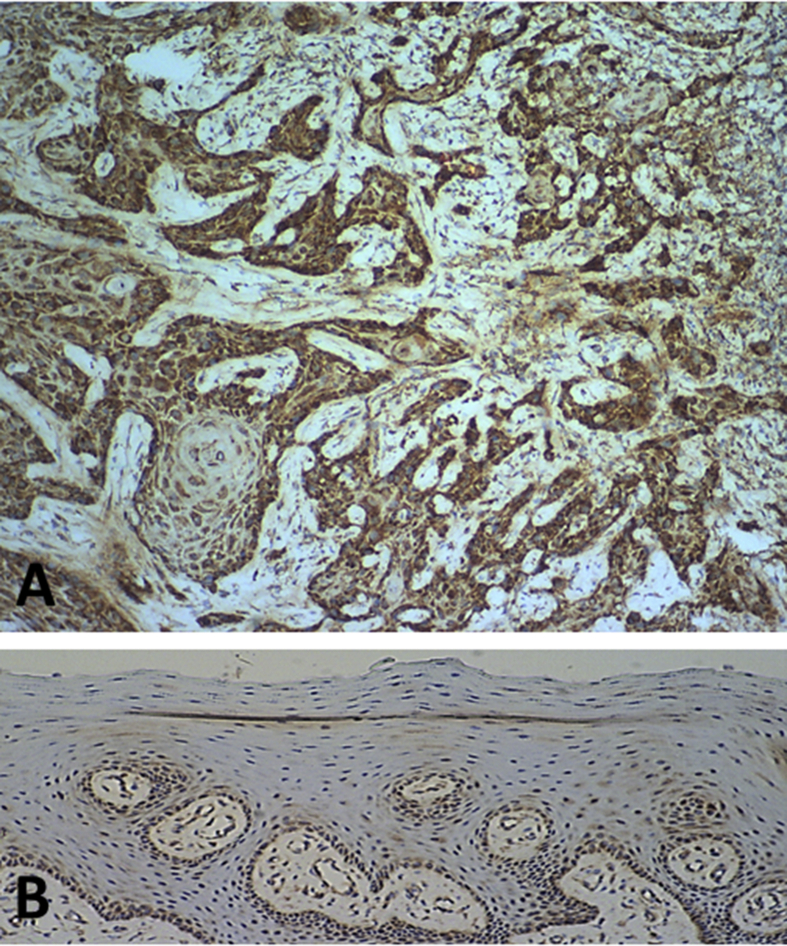
Positive staining of SPRY1 protein was present in 90 cases of OSCC (Fig. 1A) and in six normal oral mucosa samples (Fig. 1B). A significant increase was noted in the mean IS of the OSCC cases (mean IS = 4.18) in comparison with that of the normal oral mucosa samples (mean IS = 1.50) (P = 0.001).
Figure 1.
Immunohistochemical staining of SPRY1 protein in human oral squamous cell carcinoma and normal oral mucosa. Representative strong immunohistochemical staining of SPRY1 protein in human oral squamous cell carcinoma (A, ×100) and weak staining of SPRY1 protein in normal oral mucosa (B, ×100).
The statistical comparison of the mean IS of SPRY1 protein in OSCC patients with histopathological features and oral risk factors is summarized in the Supplementary Table. A significant increase in the mean IS of SPRY1 protein in the male OSCC patients was observed as compared with the female patients (P = 0.044). The mean IS of SPRY1 protein in the T2, T3, and T4 patients was increased in comparison with the T1 patients, and an increased mean IS was also observed in stage II, III, and IV patients as compared with stage I patients; however, neither of these increases were of statistical significance. The mean IS of SPRY1 protein for patients with lymph node metastasis was nearly the same as for patients without lymph node metastasis. An increase in the mean IS of SPRY1 protein was noted in OSCC patients with the habits of drinking alcohol, chewing betel quid, and smoking cigarettes as compared with patients without these oral habits, but statistical significance was not identified.
Western blot
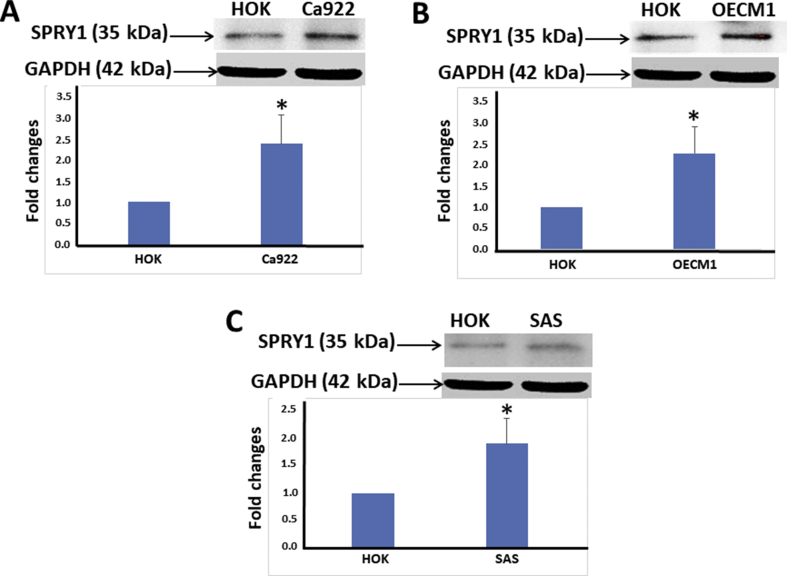
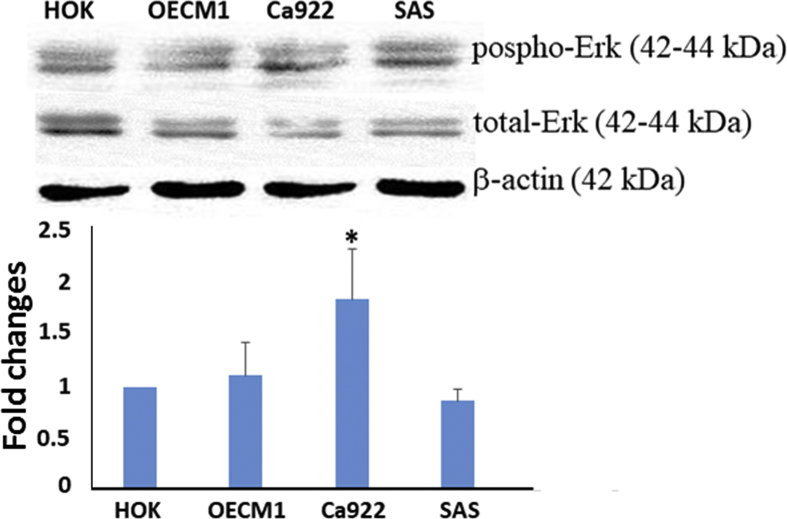
Western blot analysis demonstrated statistically significant overexpression of SPRY1 protein in the three human oral cancer cell lines (Ca922, OECM1, and SAS) as compared with primary culture of HOK (P < 0.05) (Fig. 2A–C). On the other hand, overexpression of phospho-ERK (normalized to total-ERK) was noted in OECM1 and Ca922 cells as compared with primary culture of HOK, with only Ca922 showing statistical significance (P < 0.05); however, there was a slight decrease in expression in SAS in comparison with primary culture of HOK (P > 0.05) (Fig. 3).
Figure 2.
Western blot analyses: SPRY1 protein expression in human oral cancer cell lines as compared with a primary culture of normal oral mucosa (HOK). Upregulation of SPRY1 protein expression in human oral cancer cell lines (A, Ca922; B, OCEM1; C, SAS) as compared with a HOK. Results were quantified using densitometric analysis, normalized to the level of GAPDH, and expressed as a fold change relative to the normal oral mucosa. Bars represent means ± standard deviation of the mean (∗P < 0.05). A representative result of three independent experiments is shown.
Figure 3.
Western blot analyses: phosphor-ERK and total-ERK in human oral cancer cell lines as compared with a primary culture of normal oral mucosa (HOK). Upregulation of phosphor-ERK (normalized to total-ERK) in OECM1 and Ca922 cell lines as compared with HOK, and a slightly decreased expression in SAS as compared with HOK. Results were quantified using densitometric analysis, normalized to the level of β-actin, and expressed as a fold change relative to the normal oral mucosa. Bars represent means ± standard deviation of the mean (∗P < 0.05). A representative result of three independent experiments is shown.
SPRY1 expression in human OPMDs
Immunohistochemistry
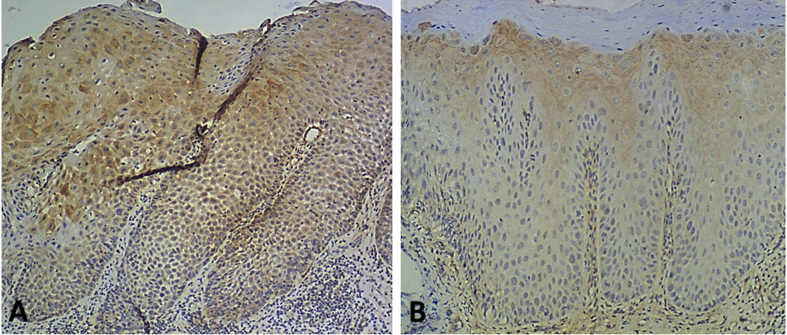
Positive staining of SPRY1 protein was observed in all cases of human OPMD with and without MT (Fig. 4A & B). The mean IS of SPRY1 protein in the human OPMDs with and without MT was 3.30 and 1.53, respectively, a significant increase being noted when comparing the two mean IS values (P < 0.001). On the other hand, SPRY1 protein expression in human OSCC tissue specimens was significantly increased in comparison with human OPMD tissue specimens without MT (P < 0.001). Moreover, SPRY1 protein expression in human OPMD tissue specimens with MT was significantly increased as compared with normal mucosa tissue specimens (P < 0.05).
Figure 4.
Immunohistochemical staining of SPRY1 protein in human oral potentially malignant disorders (OPMDs) with and without malignant transformation (MT). Representative stronger immunohistochemical staining of SPRY1 protein in a human OPMD with MT (A, ×100) and weaker staining of SPRY1 protein in a human OPMD without MT (B, ×100).
Western blot
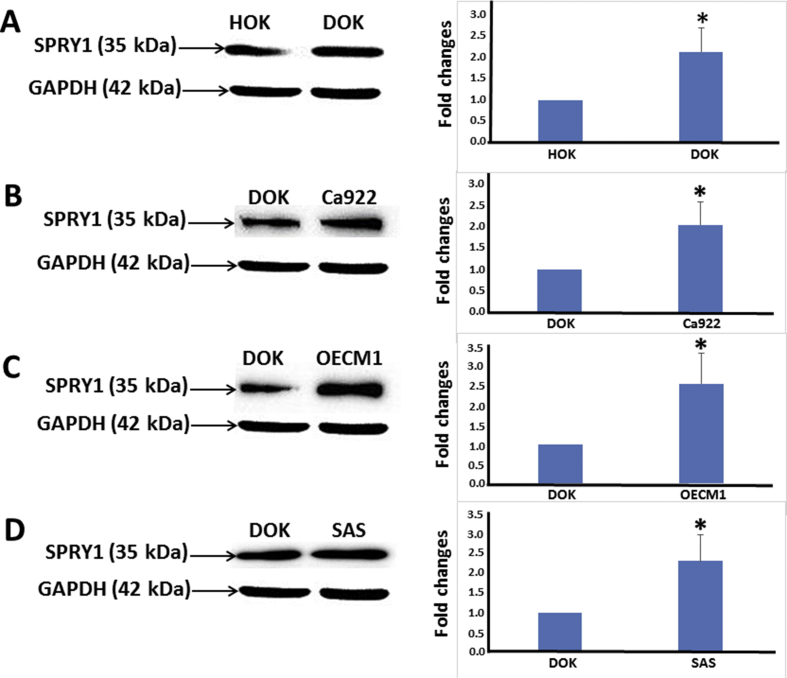
Western blot analysis of human oral premalignant cell line DOK indicated overexpression of SPRY1 protein (Fig. 5A) as compared with the primary culture of HOK, this difference being of statistical significance (P < 0.05). On the other hand, statistically significant upregulation of SPRY1 protein (Fig. 5B–D) expression was also noted in human oral cancer cell lines Ca922, OECM1, and SAS as compared with DOK (P < 0.05).
Figure 5.
Western blot analyses: SPRY1 protein expression in human oral premalignant cell line DOK as compared with a primary culture of human normal oral mucosa (HOK) and with human oral cancer cell lines. Upregulation of SPRY1 protein expression was observed for DOK as compared with HOK (A). Upregulation of SPRY1 protein expression in human oral cancer cell lines Ca922 (B), OECM1 (C), and SAS (D) as compared with DOK. Results were quantified using densitometric analysis, normalized to the level of GAPDH, and expressed as a fold change relative to the normal oral mucosa (A) and to human oral cancer cell lines (B–D). Bars represent means ± standard deviation of the mean (∗P < 0.05). A representative result of three independent experiments is shown.
Discussion
Significant upregulation of SPRY1 protein expression in human OSCC tissue specimens in comparison with human normal oral mucosa was confirmed by immunohistochemical staining in the current study. Additionally, the in vivo data demonstrating overexpression of SPRY1 protein in human OSCC as compared with normal oral mucosa were consistent with the in vitro findings in human oral cancer cell lines when compared with a human normal oral mucosa primary culture (HOK). Thus, to our knowledge, this was the first study to demonstrate SPRY1 overexpression in human oral squamous cell carcinoma.
Upregulation of SPRY1 protein expression was noted in human oral cancer cell lines as compared with human oral premalignant cell line (DOK). Significant overexpression of SPRY1 protein was also observed in DOK as compared with HOK in the present study. Furthermore, SPRY1 protein expression in human OSCC tissue specimens was significantly increased in comparison with human OPMD tissue specimens without MT in the current study. Moreover, SPRY1 protein expression in human OPMD tissue specimens with MT was significantly increased as compared with normal mucosa tissue specimens. So, collectively, to the best of our knowledge, we have reported for the first time the upregulation of SPRY1 protein in human oral squamous cell carcinogenesis.
A statistically higher SPRY1 protein expression was noted in human OPMDs with MT in comparison with cases without MT in our investigation. Thus, the aforementioned experimental evidence derived in the current study indicated that SPRY1 is possibly involved in human OSCC formation, and could have a potential association with MT of human OPMD.
In contrast to the findings of the present study, SPRY1 protein has been demonstrated to be downregulated in breast cancer32,33 and prostate cancer34,35 as compared with normal tissues; however, compatible with the results of this study, a significant proportion of cancerous tissues exhibited overexpression of SPRY1 as compared with the corresponding normal epithelium in the same study on prostate cancer.35 Additionally, consistent with the results of the current study, enhanced SPRY1 protein expression has also been reported in ovarian cancer cell lines36 and also with our previous study on SPRY2 on human oral squamous cell carcinogenesis.37
An elevated SPRY1 protein expression in parallel with an enhanced phospho-ERK expression was noted in only two oral cancer cell lines in the current investigation, indicating that other potential mechanisms could also be present. Further studies exploring other possible mechanisms involved in the overexpression of SPRY1 protein are warranted.
In conclusion, our results indicated that SPRY1 overexpression is positively associated with OSCC formation. Nevertheless, the potential genetic and epigenetic alterations of the SPRY1 gene associated with human oral squamous cell carcinogenesis remain uncertain.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgment
The work was supported by a grant from the Ministry of Science and Technology, Taiwan (MOST-107-2314-B-037-045), and grant from the Ministry of Health and Welfare (MOHW108-TDU-B-212-124016, Health and welfare surcharge of tobacco products) of Taiwan.
Contributor Information
Shyng-Shiou Yuan, Email: yuanssf@ms33.hinet.net.
Yuk-Kwan Chen, Email: k0285@ms22.hinet.net.
Appendix.
Supplementary Table.
Statistical comparison of immunohistochemical expression of SPRY1 protein in human oral squamous cell carcinomas, including gender, oral risk factors and histopathological features.
| Number | Mean | Standard deviation | P value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 84 | 4.32143 | 2.49897 | 0.0435 |
| Female | 6 | 2.16667 | 2.31667 | |
| Alcohol drinking | ||||
| No | 21 | 3.33333 | 2.05757 | 0.1137 |
| Yes | 67 | 4.31343 | 2.55965 | |
| Betel-quid chewing | ||||
| No | 14 | 3.28571 | 2.16364 | 0.1921 |
| Yes | 74 | 4.22973 | 2.51324 | |
| Cigarette smoking | ||||
| No | 11 | 2.81818 | 1.77866 | 0.0704 |
| Yes | 77 | 4.25974 | 2.51523 | |
| T (tumor size) | ||||
| T1 | 37 | 3.75676 | 2.47662 | 0.1893 |
| T2+T3+T4 | 53 | 4.4717 | 2.55406 | |
| N (lymph node metastasis) | ||||
| Yes | 23 | 4.17391 | 1.99208 | 0.9933 |
| No | 67 | 4.1791 | 2.7076 | |
| TNM stage | ||||
| I | 32 | 3.875 | 2.62433 | 0.5905 |
| II | 17 | 4.76471 | 2.63461 | |
| III | 10 | 4.7 | 1.94651 | |
| IV | 31 | 4 | 2.58199 | |
References
- 1.Tang H., Wu Z., Zhang J., Su B. Salivary lncRNA as a potential marker for oral squamous cell carcinoma diagnosis. Mol Med Rep. 2013;7:761–766. doi: 10.3892/mmr.2012.1254. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y.S., Rees T., Wright J. A review of research on salivary biomarkers for oral cancer detection. Clin Transl Med. 2014;3:3. doi: 10.1186/2001-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tovosia S., Chen P.H., Ko A.M., Tu H.P., Tsai P.C., Ko Y.C. Prevalence and associated factors of betel quid use in the Solomon Islands: a hyperendemic area for oral and pharyngeal cancer. Am J Trop Med Hyg. 2007;77:586–590. [PubMed] [Google Scholar]
- 4.Health Promotion Administration, Ministry of Health and Welfare . 2016. Cancer Registry Annual Report, Taiwan. [Google Scholar]
- 5.Scott S.E., Grunfeld E.A., McGurk M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol. 2005;41:396–403. doi: 10.1016/j.oraloncology.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.K., Huang H.C., Lin L.M., Lin C.C. Primary oral squamous cell carcinomas: an analysis of 703 cases in southern Taiwan. Oral Oncol. 1999;35:173–179. doi: 10.1016/s1368-8375(98)00101-8. [DOI] [PubMed] [Google Scholar]
- 7.Hsue S.S., Wang W.C., Chen C.H., Lin C.C., Chen Y.K., Lin L.M. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: a follow-up study based in a Taiwanese hospital. J Oral Pathol Med. 2007;36:25–29. doi: 10.1111/j.1600-0714.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y.Y., Tail Y.H., Wang W.C. Malignant transformation in 5071 southern Taiwanese patients with potentially malignant oral mucosal disorders. BMC Oral Health. 2014;14:99. doi: 10.1186/1472-6831-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45:317–323. doi: 10.1016/j.oraloncology.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Hacohen N., Kramer S., Sutherland D., Hiromi Y., Krasnow M.A. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 11.Lo T.L., Fong C.W., Yusoff P. Sprouty and cancer: the first terms report. Cancer Lett. 2006;242:141–150. doi: 10.1016/j.canlet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Tefft J.D., Lee M., Smith S. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–222. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- 13.de Maximy A.A., Nakatake Y., Moncada S., Itoh N., Thiery J.P., Bellusci S. Cloning and expression pattern of a mouse homologue of Drosophila sprouty in the mouse embryo. Mech Dev. 1999;81:213–216. doi: 10.1016/s0925-4773(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 14.Minowada G., Jarvis L.A., Chi C.L. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 15.Cabrita M.A., Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 16.Guy G.R., Wong E.S., Yusoff P. Sprouty: how does the branch manager work? J Cell Sci. 2003;116:3061–3068. doi: 10.1242/jcs.00652. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.J., Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–450. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- 18.Gross I., Bassit B., Benezra M., Licht J.D. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J Biol Chem. 2001;276:46460–46468. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- 19.Hanafusa H., Torii S., Yasunaga T., Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.C., Putnam A.J., Miranti C.K. Overexpression of sprouty 2 inhibits HGF/SF-mediated cell growth, invasion, migration, and cytokinesis. Oncogene. 2004;23:5193–5202. doi: 10.1038/sj.onc.1207646. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki A., Taketomi T., Kato R. Mammalian Sprouty4 suppresses ras-independent ERK activation by binding to Raf1. Nat Cell Biol. 2003;5:427–432. doi: 10.1038/ncb978. [DOI] [PubMed] [Google Scholar]
- 22.Lim J., Yusoff P., Wong E.S. The cysteine-rich sprouty translocation domain targets mitogen-activated protein kinase inhibitory proteins to phosphatidylinositol 4,5-bisphosphate in plasma membranes. Mol Cell Biol. 2002;22:7953–7966. doi: 10.1128/MCB.22.22.7953-7966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin C., Litvak V., Medvedovsky H., Zwang Y., Lev S., Yarden Y. Sprouty fine-tunes EGF signaling through interlinked positive and negative feedback loops. Curr Biol. 2003;13:297–307. doi: 10.1016/s0960-9822(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 24.Wong E.S., Lim J., Low B.C., Chen Q., Guy G.R. Evidence for direct interaction between sprouty and Cbl. J Biol Chem. 2001;276:5866–5875. doi: 10.1074/jbc.M006945200. [DOI] [PubMed] [Google Scholar]
- 25.Grose R., Dickson C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev. 2005;16:179–186. doi: 10.1016/j.cytogfr.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Hsu S.M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 27.Sarbia M., Loberg C., Wolter M. Expression of bcl-2 and amplification of c-myc are frequent in basaloid squamous cell carcinomas of the esophagus. Am J Pathol. 1999;155:1027–1032. doi: 10.1016/S0002-9440(10)65203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C.Y., Meng C.L. Regulation of PG synthase by EGF and PDGF in human oral, breast, stomach, and fibrosarcoma cancer cell lines. J Dent Res. 1994;73:1407–1415. doi: 10.1177/00220345940730080301. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K., Kanazawa H., Akiyama Y. Establishment and characterization of a cell line (SAS) from poorly differentiated human squamous cell carcinoma of the tongue. J Jpn Stomatol Soc. 1989;38:20–28. [Google Scholar]
- 30.Kimura Y. Studies on lactate dehydrogenase isoenzymes in a cell line (Ca 9-22) derived from carcinoma of the gingiva. Kokubyo Gakkai Zasshi. 1978;45:20–35. doi: 10.5357/koubyou.45.20. [DOI] [PubMed] [Google Scholar]
- 31.Chang S.E., Foster S., Betts D., Marnock W.E. DOK, a cell line established from human dysplastic oral mucosa, shows a partially transformed non-malignant phenotype. Int J Cancer. 1992;52:896–902. doi: 10.1002/ijc.2910520612. [DOI] [PubMed] [Google Scholar]
- 32.Lo T.L., Yusoff P., Fong C.W. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Res. 2004;64:6127–6136. doi: 10.1158/0008-5472.CAN-04-1207. [DOI] [PubMed] [Google Scholar]
- 33.Mekkawy A.H., Pourgholami M.H., Morris D.L. Human Sprouty1 suppresses growth, migration, and invasion in human breast cancer cells. Tumour Biol. 2014;35:5037–5048. doi: 10.1007/s13277-014-1665-y. [DOI] [PubMed] [Google Scholar]
- 34.Fritzsche S., Kenzelmann M., Hoffmann M.J. Concomitant down-regulation of SPRY1 and SPRY2 in prostate carcinoma. Endocr Relat Cancer. 2006;13:839–849. doi: 10.1677/erc.1.01190. [DOI] [PubMed] [Google Scholar]
- 35.Kwabi-Addo B., Wang J., Erdem H. The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res. 2004;64:4728–4735. doi: 10.1158/0008-5472.CAN-03-3759. [DOI] [PubMed] [Google Scholar]
- 36.Moghaddam S.M., Amini A., Wei A.Q., Pourgholami M.H., Morris D.L. Initial report on differential expression of sprouty proteins 1 and 2 in human epithelial ovarian cancer cell lines. J Oncol. 2012;2012:373826. doi: 10.1155/2012/373826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao P.H., Wang Y.Y., Wang W.C. Overexpression of sprouty2 in human oral squamous cell carcinogenesis. Arch Oral Biol. 2018;87:131–134. doi: 10.1016/j.archoralbio.2017.12.021. [DOI] [PubMed] [Google Scholar]