Abstract
Background/purpose
Various questionnaires have been validated as methods for screening of neuropathic pain, but none have been established for the orofacial region. Although chronic pain and depression are likely to comorbid, few studies have examined the relationship between orofacial chronic pain and depression. Therefore, we evaluated the potential of the Japanese Version of PainDETECT as an assessment tool for neuropathic pain associated with burning mouth syndrome (BMS) and persistent idiopathic facial pain (PIFP). We also evaluated the depression scale such as Beck's Depression Inventory (BDI: a subjective index) and Hamilton Depression Rating Scale (HDRS: an objective index) with BMS or PIFP.
Materials and methods
As a target, we administered the Japanese version of the PainDETECT questionnaire to the BMS (29 patients) and PIFP (17 patients). As a control, patients with post-extraction pain (typical nociceptive pain, (EXT) 16 patients) were also participated. We performed BDI and HDRS with BMS or PIFP.
Results
Although PainDETECT final score was significantly higher in BMS [median: 10] compared with PIFP [6] and EXT [5] (p < 0.05), PainDETECT final scores for all groups were lower than the cutoff value for the possibility of neuropathic pain. HDRS was significantly higher in the BMS than the PIFP. There were no significant differences between the BMS and PIFP in BDI.
Conclusion
Under the limitations of current research design, the Japanese version of the PainDETECT questionnaire does not show sufficient potential as pain assessment tool for patients with BMS and PIFP. BMS is comorbid with depression objectively when compared with PIFP.
Keywords: PainDETECT, Burning mouth syndrome, Persistent idiopathic facial pain, Beck's depression inventory, Hamilton depression rating scale
Introduction
Various types of chronic orofacial pain of unknown origin exist. The pathologies associated with these conditions, which include burning mouth syndrome (BMS) and persistent idiopathic facial pain (PIFP), remain unclear. Recently, it was hypothesized that these conditions may actually represent neuropathic pain. Although they may be caused by peripheral sensitization, central sensitization, or impaired upper central nervous control function,1 pathophysiological diversity was observed, as we previously reported.2
Various questionnaires have been introduced and validated as methods for screening for diagnosis of neuropathic pain.3, 4, 5 These questionnaires offer excellent usability, do not require special examination, and are low-cost. Furthermore, they are considered as accurate and cost-effective means of diagnosing neuropathic pain.6 PainDETECT, one such questionnaire, was originally developed in Germany as a screening tool for neuropathic pain in patients with chronic lower back pain, and its reliability and validity have been examined.7 Furthermore, the reliability and validity of the Japanese version of the PainDETECT has also been proved.8 In recent years, some practitioners have used the PainDETECT to diagnose neuropathic pain in patients with BMS.9,10 However, we were unable to locate any reports of the Japanese version of the PainDETECT used with patients with PIFP. Furthermore, there were no reports of the Japanese version of the PainDETECT used with patients with post-extraction pain (EXT) (typical nociceptive pain) as control group to assess the validity for assessment tool for using PainDETECT as an orofacial region.
Chronic pain represented by neuropathic pain can be comorbid with mental illness such as depression and can also affect various aspects of patients' daily lifestyles, such as housework and employment, which immensely lowers patients’ quality of life.11
Although chronic pain and depression are likely to comorbid, few studies have examined the relationship between chronic pain in the orofacial region and depression.
In this study, although the number of cases is limited, we evaluated the potential of the Japanese version of the PainDETECT questionnaire as a pain assessment tool for patients with BMS and PIFP. We also performed depression rating scale to the patients with BMS and PIFP and also evaluated the correlation between the PainDETECT final score and depression rating scale.
Materials and methods
Ethical guidelines
This study was approved by the Ethical Committee of the School of Dentistry, Aichi Gakuin University and the Ethics Review Committee of Nagoya University Graduate School of Medicine (Approval No. 372: Aichi Gakuin University, Approval No. 2004-0234: Nagoya University). This study was performed with sufficient consideration of the protection of personal information. We have followed the Helsinki Declaration and have followed the guidelines in this investigation.
Study design and patients
As a target group, the subjects comprised 46 patients diagnosed with either BMS (n = 29) or PIFP (n = 17) who were treated at the Liaison Clinic, Department of Oral and Maxillofacial Surgery, Aichi Gakuin University Dental Hospital (coordinated with the Department of Psychiatry, Nagoya University Graduate School of Medicine) and who consented to participate in the study. The diagnostic criteria for BMS and PIFP were based on the International Classification of Headache Disorders 3rd edition (Table 1).12 As a control group, to assess the validity of PainDETECT as an orofacial region, the patients with EXT (the day after tooth extraction, n = 16) were also participated in this study. We administered the Japanese version of the PainDETECT questionnaire to these subjects. A trained psychiatrist performed psychiatric evaluations on all subjects using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).13 In order to make a psychiatric diagnosis based on DSM-5, we used the structured clinical interview. The psychiatrist and dentist are providing treatment in the same clinic, and the psychiatric and dental diagnosis were performed in the same place and on the same time.
Table 1.
Diagnostic criteria of BMS and PIFP based on the beta version of the International Classification of Headache Disorder.
| BMS | PIFP |
|---|---|
| A. Oral pain fulfilling criteria B and C | A. Facial and/or oral pain fulfilling criteria B and C |
| B. Recurring daily for 2 h per day for > 3 months | B. Recurring daily for 2 h per day for > 3 months |
C. Pain has both of following characteristics:
|
C. Pain has both of following characteristics:
|
| D. Oral mucosa is of normal appearance and clinical examination including sensory testing is normal | D. Clinical neurological examination is normal |
| E. Not better accounted for by another ICHD-3 diagnosis | E. A dental cause has been excluded by appropriate investigations |
| F. Not better accounted for by another ICHD-3 diagnosis |
BMS: Burning Mouth Syndrome, PIFP: Persistent Idiopathic Facial Pain.
Outcome measures
We additionally collected information related to age, sex, and duration period. The Japanese version of the PainDETECT questionnaire was used to individually evaluate burning, tingling, allodynia, electric shock-like pain, pain on cold/hot stimulation, numbness, and pain by pressure. These were evaluated on a six-point Likert scale (max = 5 points, min = 0 points) and the total scores (max = 35 points, min = 0 points) of these seven items were calculated. Total score was adjusted based on persistent pain with fluctuations (±0 if present), persistent pain with pain attacks (−1 if present), pain attacks without interceding pain (+1 if present), pain attacks with interceding pain (+1 if present), and radiating pain (+2 if present) to calculate the final score (max = 38 points, min = 0 points). This was considered as the PainDETECT final score. The distribution of PainDETECT final score means as follows. 0–12: Possibility of neuropathic pain is less than 15%. 13–19: Including element of neuropathic pain. 20–38: Elements of neuropathic pain exceed 90%. Additionally, we evaluated pain intensity using an 11-step numerical rating scale (NRS), according to present, average, and maximum values. To evaluate depression in the 46 patients with BMS and PIFP, we used Beck's Depression Inventory (BDI: cutoff value 10 or less) as a subjective index and the Hamilton Depression (HDRS: cutoff value 7 or less), which uses semi-structured interviews by a trained psychiatrist, as a highly-precise, objective index.14,15
Statistical analysis
Data are expressed as median [Interquartile range: IQR] or number. Since the sample size is small and the data didn't follow normal distribution, we adopted the median value instead of the average value. All statistical analyses of recorded data were performed using the Excel statistical software package (Ekuseru-Toukei 2010; Social Survey Research Information Co., Ltd., Tokyo, Japan). The Kruskal–Wallis test and multiple comparisons using the Steel-Dwass were used for age, pain intensity, and PainDETECT final score. Mann–Whitney's U-test was used for duration, BDI total score, and HDRS total score. The chi-square test of independence was used for psychiatric diagnosis, pain course pattern, radiating pain, and the number of patients in each score range of PainDETECT final score. Spearman's rank-order correlation coefficient was used to investigate the correlation between PainDETECT final score and BDI total score or HDRS total score. The level of statistical significance was set at p < 0.05.
Results
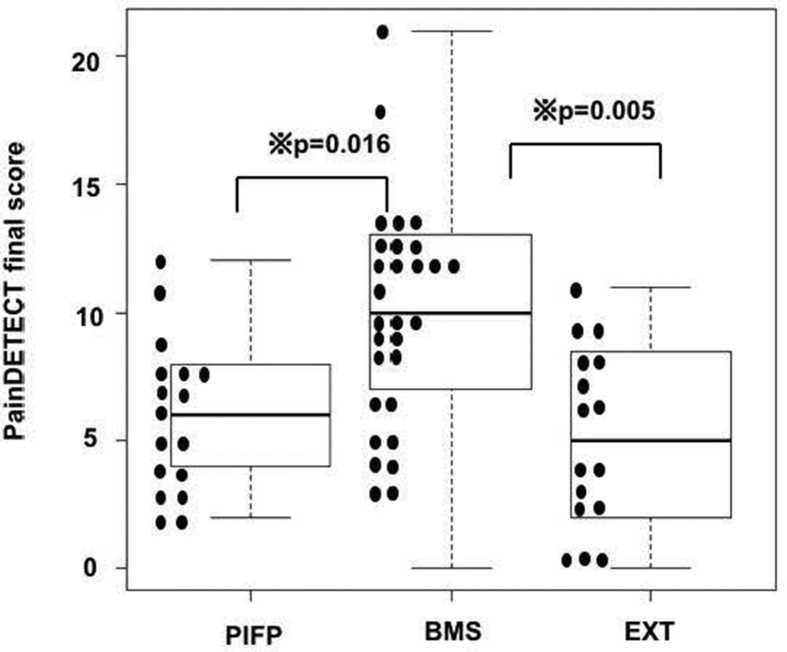
Demographic data are summarized in Table 2. Age was significantly higher in the BMS ([median (IQR)]) [65 (61–65)] than in the PIFP [52 (43.5–61)] (p = 0.003) and EXT [40 (30–43.5)] group (p < 0.001). HDRS total score was significantly higher in the BMS [7 (3–12.75)] than in the PIFP [3.5 (2.75–7)] groups (p = 0.04). There were no significant differences between each group in sex, examination period, BDI total score, and psychiatric diagnosis. Summaries of patient responses are presented in Table 3. Pain course patterns and radiating pain are summarized in Table 4; there were no significant differences between each group. Scores of pain intensity by NRS and the PainDETECT final score are summarized in Table 5. Using the Kruskal-Wallis test, PainDETECT final score was significantly different among the 3 groups. In multiple comparisons using the Steel-Dwass, PainDETECT final score was significantly higher in the BMS [10 (7–13)] than in the PIFP [6 (4–8)] (p = 0.016) and EXT [5 (2–8)] (p = 0.005) (Fig. 1). There were significant differences in the number of patients in each range of the PainDETECT final score (Table 6). (p≦0.033). Spearman's correlations between the PainDETECT final score and BDI total score, or HDRS total score are summarized in Table 7. There was little significant correlation between PainDETECT final score and BDI or HDRS scores in either group.
Table 2.
Characteristics of patients in this study.
| BMS | PIFP | EXT | P Value | |
|---|---|---|---|---|
| Patient's demographics | ||||
| Age (year) | 65 [61–65] | 52 [43.5–61] | 40 [36–43.5] | P = 0.003 (BMS vs PIFP) P < 0.001(BMS vs EXT) P = 0.015(PIFP vs EXT) |
| Male/Female | 3/26 | 2/15 | 2/14 | NS P = 0.75 |
| Duration (months) | 11 [9–11] | 11 [10–11] | NS P = 0.94 | |
| BDI | 14 [8–23] | 9 [5–15] | NS P = 0.11 | |
| HDRS | 7 [3–12.75] | 3.5 [2.75–7] | P = 0.04 | |
| Psychiatric Diagnosis | ||||
| Major depressive Disorder | 1 | 2 | NS P = 0.27 | |
| Somatic Symptom Disorder with Predominant Pain | 23 | 15 | NS P = 0.44 | |
| Somatic Symptom Disorder (other than those above) | 1 | 0 | NS P = 0.43 | |
| Major depressive Disorder + Somatic Symptom Disorder with Predominant Pain | 4 | 0 | NS P = 0.10 | |
Data are expressed as median {IQR} or number. NS: Not significant; BMS: Burning Mouth Syndrome, PIFP: Persistent Idiopathic Facial Pain; EXT: Post-extraction pain.
Table 3.
PainDETECT Questionnaire-Japaneses version (PDQ-J): summary of patient responses.
| BMS | PIFP | EXT | P Value | |
|---|---|---|---|---|
| Burning | 3 [2–3] | 0 [0–1] | 0 [0–0.25] | P < 0.001(BMS vs PIFP and EXT) |
| Tingling | 1 [0–3] | 1 [0–2] | 0 [0–1] | P = 0.002 (BMS vs EXT) |
| Allodynia | 0 [0–1] | 1 [0–2] | 1 [0–2] | NS P = 0.21 |
| Electric shock-like pain | 0 [0–0.25] | 0 [0-0] | 0 [0-0] | NS P = 0.81 |
| Pain on cold/hot stimulation | 0 [0–2] | 0 [0–1] | 0 [0–1] | NS P = 0.86 |
| Numbness | 2 [1–2] | 1 [0–1] | 0 [0–1] | P = 0.032(BMS vs PIFP) P = 0.01(BMS vs EXT) |
| Pain by pressure | 1 [0–1] | 2 [1–2] | 2 [2–3] | P = 0.015 (BMS vs EXT) |
Data are expressed as median [IQR]. NS: Not significant; BMS: Burning Mouth Syndrome, PIFP: Persistent Idiopathic Facial Pain; EXT: Post-extraction pain.
Table 4.
PainDETECT Questionnaire-Japaneses version (PDQ-J): the number of pain course pattern and radiating pain.
| BMS | PIFP | EXT | P Value | |
|---|---|---|---|---|
| Pain course pattern | ||||
| Persistent pain with slight fluctuations | 18 | 11 | 14 | NS P = 0.18 |
| Persistent pain with pain attacks | 5 | 1 | 1 | NS P = 0.38 |
| Pain attacks without pain between pain | 3 | 2 | 1 | NS P = 0.85 |
| Pain attacks with between them | 3 | 3 | 0 | NS P = 0.22 |
| Radiating pain | ||||
| Yes | 5 | 2 | 0 | NS P = 0.21 |
| No | 24 | 15 | 16 | |
NS: Not significant; BMS: Burning Mouth Syndrome, PIFP: Persistent Idiopathic Facial Pain; EXT: Post-extraction pain.
Table 5.
Scores of pain intensity and PainDETECT.
| BMS | PIFP | EXT | P Value | |
|---|---|---|---|---|
| Pain intensity-NRS (present) | 4 [3–7] | 5 [3–8] | 3 [2–4] | NS P = 0.055 |
| Pain intensity-NRS (average) | 6 [4–7] | 5 [3–6] | 1.5 [0–3] | P < 0.001 (BMS and PIFP vs EXT) |
| Pain intensity-NRS (maximum) | 8 [6–9] | 7 [5–9] | 3 [0–6] | P = 0.003 (PIFP vs EXT) P = 0.026 (BMS vs EXT) |
| PainDETECT | 10 [7–13] | 6 [4–8] | 5 [2–8] | P = 0.005 (BMS vs EXT) P = 0.016 (BMS vs PIFP) |
Data are expressed as median [IQR]; NS: Not significant. NRS: Numerical Rating Scale; BMS: Burning Mouth Syndrome, PIFP: Persistent Idiopathic Facial Pain; EXT: Post-extraction pain.
Figure 1.
The patient distribution of PainDETECT final score in each group. PainDETECT final score was significantly higher in the BMS [10 (7–13)] than in the PIFP [6 (4–8)] (p = 0.016) and EXT [5 (2–8)] (p = 0.005).
Table 6.
The number of patients in each score range of PainDETECT.
| BMS | PIFP | EXT | P Value | |
|---|---|---|---|---|
| 0–12 (Nociceptive) | 21 | 17 | 16 | P = 0.027 (BMS vs PIFP) P = 0.032 (BMS vs EXT) |
| 13–19 (Mixed) | 7 | 0 | 0 | P = 0.027 (BMS vs PIFP) P = 0.033 (BMS vs EXT) |
| 20–38 (Neuropathic) | 1 | 0 | 0 | NS P = 0.56 |
0–12: Possibility of neuropathic pain is less than 15%. 13–19: Including element of neuropathic pain. 20–38: Elements of neuropathic pain exceed 90%. NS: Not significant. BMS: Burning Mouth Syndrome, PIFP: Persistent Idiopathic Facial Pain. EXT: Post-extraction pain.
Table 7.
Spearman's correlation between PainDETECT and BDI or HAMD.
| BMS | PIFP | |
|---|---|---|
| BDI | 0.18 | −0.08 |
| HDRS | 0.09 | 0.12 |
0∼±0.2 There is little correlation, ±0.2∼±0.4 There is somewhat correlation, ±0.4∼±0.7 There is a correlation, ±0.7∼±0.9 There is a strong correlation, ±0.9∼±1.0 There is a very strong correlation; BMS: Burning Mouth Syndrome, PIFP: Persistent Idiopathic Facial Pain;BDI, Beck's Depression Inventory; HDRS, Hamilton Depression Rating Scale.
Discussion
Although this study is a preliminary, ours is the first study to use the Japanese version of PainDETECT for BMS and PIFP, and to examine the association between depression and PainDETECT score. Our results indicated that 21 patients (72%) in the BMS group and 17 (100%) patients in the PIFP group had less than a 15% possibility of neuropathic pain elements. This result is similar to a prior report that found the PainDETECT questionnaire to be unsuitable as an assessment tool for BMS.9 Relying solely on the PainDETECT final score may erroneously lead one to the conclusion that BMS and PIFP pain are not neuropathic but rather nociceptive in nature. This result may have occurred because the PainDETECT was not originally developed for assessing neuropathic pain in the orofacial region. If PainDETECT is to be used to assess the orofacial region, its current cutoff value is not ideal for pain screening.
Chronic pain can be classified from a variety of different angles. When classifying by pain factors, there are nociceptive pain, neuropathic pain, psychosocial pain and others. When the pain becomes chronic, the cause is seldom due to one of these three and in many cases, it is a complex mixed pain condition involving several causing factors.16 In the present study, although both the BMS and PIFP lacked macroscopic organic changes, our results indicated a probability of nociceptive pain elements.
In terms of chronic pain and depression, although both BMS and PIFP have low scores, our results showed that values of the HDRS, which is an objective index of depression, were significantly higher in the BMS group than in the PIFP group. Meanwhile, no significant differences were seen between the two groups for BDI, which is a subjective index of depression. This suggested that BMS was comorbid with depression objectively, while PIPF was associated with milder depression than BMS, despite resulting from similar kinds of chronic pain. The present study revealed that BDI or HDRS was little correlated with PainDETECT. This demonstrates that depression based on BDI and HDRS score is not always severe just because the PainDETECT score is high. Our results may also demonstrate that the intensity of depression is not related to the diagnostic type of pain. In this study, the results for age, sex ratio and duration of BMS are almost the same as those of previous studies.9,10 However, comparison of the BMS and PIFP groups showed that ages were significantly higher among BMS patients. This strongly suggested that PIFP develops in younger age than other forms of chronic pain in the orofacial region.
There are several limitations to the present study. First, the sample size is relatively small. Second, the number of examples and age are not matched in each group. Although we will continue clinical research in a larger sample size and examining a patient's medication history may provide a more useful assessment, we hope that the results of this study will help clarify the mechanism of pain in BMS and PIFP.
We applied the Japanese version of the PainDETECT questionnaire to patients with BMS and PIFP. Under the limitations of current research design, the Japanese version of the PainDETECT questionnaire does not show sufficient potential as pain assessment tool for patients with BMS and PIFP. BMS is comorbid with depression objectively when compared with PIFP.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the School of Dentistry, Aichi Gakuin University and the Ethics Review Committee of Nagoya University Graduate School of Medicine (Approval No. 372: Aichi Gakuin University, Approval No. 2004-0234: Nagoya University). Written informed consent was obtained from all the patients.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
Aiji Sato, Hiroyuki Kimura, Tatsuya Tokura, Mikiko Ito and Tomoya Miyauchi wrote this manuscript under supervision of Yumi Nakano, Norio Ozaki, Eri Umemura, Takashi Tonoike and Masahiro Okuda. Aiji Sato, Hiroyuki Kimura, Tatsuya Tokura, Tomoya Miyauchi, Eri Umemura, Mikiko Ito and Norio Ozaki designed the study. Aiji Sato, Shinichi Kishi and Norio Ozaki performed the investigation and analyzed the data. Eri Umemura, Mikiko Ito, Shinichi Kishi, Yumi Nakano, Masahiro Okuda, Tonoike Takashi, Norio Ozaki, Hiroyuki Kimura, Takashi Tonoike, Tomoya Miyauchi and Norio Ozaki made substantial contribution to the interruption of the data. Norio Ozaki and Masahiro Okuda was responsible for the study design, writing of the manuscript, analysis and interpretation of the data. All authors have read and approved the final manuscript.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
The authors would like to thank the Department of Oral and Maxillofacial Surgery at Aichi Gakuin University Dental Hospital for their help in recruiting patients for this study. This study was fully supported by a governmental Grant-in-Aid grant for Scientific Research (C, No. 24591703) and a governmental Grant-in-Aid for Young Scientists (B, No. 19K17108) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and the Japanese Society for the Promotion of Science.
References
- 1.Charleston L., 4th Burning mouth syndrome: a review of recent literature. Curr Pain Headache Rep. 2013;17:336. doi: 10.1007/s11916-013-0336-9. [DOI] [PubMed] [Google Scholar]
- 2.Ito M., Tokura T., Yoshida K. Five patients with burning mouth syndrome in whom an antidepressant (serotonin-noradrenaline reuptake inhibitor) was not effective, but pregabalin markedly relieved pain. Clin Neuropharmacol. 2015;38:158–161. doi: 10.1097/WNF.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 3.Bennett M. The lanss pain scale: the leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 4.Bennett M.I., Smith B.H., Torrance N., Potter J. The s-lanss score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6:149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Krause S.J., Backonja M.M. Development of a neuropathic questionnaire. Clin J Pain. 2003;19:306–314. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M.I., Attal N., Backonja M.M. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203. doi: 10.1016/j.pain.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Freyhagen R., Baron R., Gockel U., Tolle T.R. Paindetect: a new screening questionnaire to detect neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 8.Matsubayashi Y., Takeshita K., Sumitani M. Validity and reliability of the Japanese version of the painDETECT questionnaire: a multicenter observational study. PloS One. 2013;30 doi: 10.1371/journal.pone.0068013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heo J.Y., Ok S.M., Ahn Y.W., Ko M.Y., Jeong S.H. The application of neuropathic pain questionnaires in burning mouth syndrome patients. J Oral Facial Pain Headache. 2015;29:177–182. doi: 10.11607/ofph.1326. [DOI] [PubMed] [Google Scholar]
- 10.Lopez J.P., Molino P.D., Parra P.P., Valenzuela S. Neuropathic pain in patients with burning mouth syndrome evaluated using paindetect. Pain Med. 2017;18:1528–1533. doi: 10.1093/pm/pnw304. [DOI] [PubMed] [Google Scholar]
- 11.TurK D.C., Wilson H.D., Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377:2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. 3rd ed. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association . Amer Psychiatric; Washington DC: 2013. Diagnostic and statistical manual of mental disorders DSM-5. [Google Scholar]
- 14.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatr. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The committee for clinical practice guideline for chronic pain: clinical practice guideline for chronic pain. Shinko Trading Co.Ltd; Tokyo: 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.