Abstract
Background/purpose
Glass ionomers undergo degradation when exposed to fluoride, which changes the physico-chemical characteristics of the materials. The purpose of this study was to evaluate the surface changes of resin-modified glass ionomer (RMGI) when immersed in a sodium fluoride (NaF) solution according to pH and time.
Materials and methods
120 RMGI specimens were prepared, and 30 specimens were placed in four types of storage solutions for four weeks; pH 7 artificial saliva with or without 0.2% NaF (As7 and NaF7), pH 5 artificial saliva with or without 0.2% NaF (As5 and NaF5). Interferometry and microscopy were performed to evaluate the surface roughness and topography, while spectroscopy was used to analyze the chemical composition changes.
Results
Rougher topography and increased roughness was exhibited in NaF groups, owing to the disintegration of the polysalt matrix. Reduced Sr and F was exhibited in all groups, whereas NaF group showed a decrease in Al and inorganic components.
Conclusion
This study suggest that excessive use of fluoride therapy could lead to severe degradation of RMGI.
Keywords: Chemical composition, Fluoride, RMGI, Surface degradation, Surface roughness
Capsule-type RMGI (Fuji II LC capsule, GC Co., Tokyo, Japan) was used in the present study to minimize error in manual mixing. Four types of storage solutions were used in the study: pH 7 artificial saliva (As7); pH 7 artificial saliva containing 0.2% NaF (NaF7); pH 5 artificial saliva (As5); and pH 5 artificial saliva containing 0.2% NaF (NaF5). Artificial saliva was generated according to Macknight-Hane and Whitford (1992) formula,24 and the amount of cellulose was reduced to 2.00 g/L to adjust the viscosity.
Introduction
First introduced in 1960s, glass ionomers (GIs) have been widely used in dentistry as restorative materials, liners, bases, fissure sealants, and bonding agents for orthodontic brackets.1 GI shows several clinical advantages over other restorative materials, such as adhesion to tooth structure, biocompatibility, fluoride release, anti-cariogenic properties, and low coefficients of thermal expansion.2, 3, 4 However, GI exhibits poor physical-mechanical properties compared with resin composites, including insufficient strength, inadequate toughness, and low wear resistance.3,5 Therefore, in order to improve the mechanical properties of conventional GI, resin-modified glass ionomers (RMGIs) were developed.2
RMGI contains the same essential components as conventional glass ionomers but also includes a monomer component, 2-hydroxyethyl methacrylate (HEMA), and an associated initiator, camphorquinone.1 Therefore, the setting of RMGI is processed by an acid–base reaction and an additional polymerization reaction.6 RMGI exhibits better immediate setting and greater strength and aesthetic enhancement compared with conventional GI, while retaining one of the major advantages of GI, the release and uptake of fluoride and other ions.7,8 It has been suggested that RMGI (GI) releases Al, Ca, P, and F ions in an aqueous environment, exhibiting anti-cariogenic properties and remineralization;9 meanwhile, the topical application of fluoride to GI has been shown to enhance fluoride release through the uptake of fluoride.10 However, whether fluoride released from GI can improve the prevention or inhibition of secondary caries compared with non-fluoridated materials remain controversial,11,12 and previous studies have reported that fluoride-releasing restorative materials seem to show surface degradation when exposed to fluoride.13, 14, 15, 16, 17 The topical application of acidulated phosphate fluoride to restorative materials seems to cause surface degradation, with increased surface roughness and a change in its morphology.13,15 Accordingly, the application of neutral fluoride, instead of acidic fluoride agents, has been suggested;18,19 however, even a neutral 2% NaF application14,17 and immersion in neutral 0.2% NaF solution have resulted in surface degradation of GI.20
The surface degradation of RMGI (GI) when exposed to fluoride is a result of chemical erosion due to the disintegration of the polysalt matrix between glass particles. This was reported to occur on glass ionomer with fluoride-containing glass, whereas those containing no fluoride showed little or no surface disintegration.17,20 The degradation and subsequent increased roughness of the RMGI (GI) surface would cause additional plaque accumulation, wear, discoloration of restoration, and eventually, the formation of secondary caries, affecting its longevity.21,22 However, studies on the physico-chemical characteristics of dental materials are limited;23 furthermore, the changes in the chemical composition of the surface undergoing structural modification require consideration.20
Therefore, the purpose of this study is to evaluate the effects of time and pH on the surface degradation of RMGI, induced by immersion in 0.2% NaF solution, by analyzing its surface topography, roughness, and chemical composition changes. The null hypothesis of this study was that pH and time would not affect surface degradation of RMGI induced by NaF solution.
Materials and methods
Experimental design
Capsule-type RMGI (Fuji II LC capsule, GC Co., Tokyo, Japan) was used in the present study to minimize error in manual mixing. Four types of storage solutions were used in the study: pH 7 artificial saliva (As7); pH 7 artificial saliva containing 0.2% NaF (NaF7); pH 5 artificial saliva (As5); and pH 5 artificial saliva containing 0.2% NaF (NaF5). Artificial saliva was generated according to Macknight-Hane and Whitford (1992) formula,24 and the amount of cellulose was reduced to 2.00 g/L to adjust the viscosity.
To make 0.2% NaF concentration of artificial saliva, 10,000 ppm of NaF solution was diluted and mixed with the artificial saliva. The pH of the four solutions was adjusted to 7 and 5 with KOH and lactic acid, respectively. Then, 40 ml of each solution was inserted in a 50 ml conical tube. The composition of the artificial saliva and RMGI used in this study is shown in Table 1.
Table 1.
Composition of the materials used in this study.
| Materials | Fluoride | pH | Composition (g/L) | Manufacturer | |
|---|---|---|---|---|---|
| Artificial saliva | As7 | – | 7 | Methyl-p-hydroxybenzoate 2.00 Sodium Carboxymethyl Cellulose 2.00 KCl 0.625 MgCl2·6H2O 0.059 CaCl2·2H2O 0.166 K2HPO4 0.804 KH2PO4 0.326 |
– |
| NaF7 | 0.2% NaF | 7 | |||
| As5 | – | 5 | |||
| NaF5 | 0.2% NaF | 5 | |||
| Resin modified -glass ionomer | Fuji II LC capsule | – | – | Powder: fluoroaluminosilicate glass Liquid: HEMA, UDMA, polyacrylic acid, water, camphorquinone |
GC CO., Tokyo, Japan |
As5: pH 5 artificial saliva, As7: pH 7 artificial saliva, NaF5: pH 5 artificial saliva containing 0.2% NaF, NaF7: pH 7 artificial saliva containing 0.2% NaF.
RMGI specimen preparation
First, 120 specimens of RMGI were prepared according to the manufacturer's instructions. A Fuji II LC capsule was mixed for 10 s with amalgamator (D-650, TPC Advanced Technology Inc., City of Industry, CA, USA) and inserted into a Teflon mold (5 mm diameter and 2 mm depth). After the material was filled, a mylar strip was pressed onto the surface and light-cured with an LED light-curing unit (B&LiteS, B&L Biotech, Ansan, Korea) with irradiance of 800 mW/cm2 for 20 s on each side. The specimens were polished with sandpaper using a polisher (J-POS2, JISICO, Seoul, Korea) with 1000 and 2000 grit sandpaper under continuous water irrigation. To standardize the polishing procedure, it was performed by the same operator. The polished specimens were stored in 100% humidity for 24 h for initial polymerization.
Immersion in NaF solution
The RMGI specimens were randomly divided into four groups (As7, NaF7, As5, NaF5) and placed in a 50 mL conical tube, containing 40 mL of the corresponding storage medium. To imitate the dynamic conditions of mouthwash, the specimens of each group were placed in a conical tube, which was placed in a shaker (Trayster basic, IKA®, Staufen, Germany) at 20 rpm, to allow slight mechanical contact between the specimens. The storage medium was changed every 2 day, and this procedure was repeated for 28 days. During the immersion period, the shaker containing the specimen was stored at 37 ± 1 °C.
3D surface topography and surface roughness
At the end of every week during the immersion period, three specimens from each group were randomly selected and washed with distilled water for analysis of the surface roughness and chemical composition and observation of the surface topography.
Three-dimensional surface morphology features and the surface roughness of the specimens were observed with non-contact optical profilometry, white light interferometry (WLI) (NV-2400, Nanosystem, Daejeon, Korea). Three random fields were selected for each specimen. A 20× interferometric objective lens with 1.0× field-of-view lenses were used, and the scan area was measured at 320×240 μm.2 The surface roughness parameter Ra, the average roughness, and Rt, the maximum distance between peaks and valleys were measured.
2D surface topography and surface components
Two-dimensional surface morphology features and the constituent elements of the RMGI surface were observed with scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS). The surfaces of the specimen were sputter-coated with Au in an ion Coater (SPT-20, COXEM, Daejeon, Korea) and examined using SEM (EM-30AX, COXEM, Daejeon, Korea) equipped with EDS at an accelerating voltage of 20 kV. On three randomly selected spots, SEM images were taken at 2000× magnification, and EDS was performed at 500× magnification.
Statistical analysis
Differences in the surface roughness values were analyzed by the Kruskal–Wallis test and Mann–Whitney test using SPSS version 25 (IBM CO., Armonk, NY, USA), and a Bonferroni correction for multiple comparisons was performed (P < 0.008).
Results
Surface roughness
Table 2 and Table 3 shows Ra and Rt values of RMGI specimens immersed in different storage solutions for 4 weeks. Surface degradation by 0.2% NaF significantly increased the surface roughness of RMGI. The Ra and Rt values of RMGI immersed in NaF group were higher than those for specimens immersed in As group, regardless of pH. The Ra and Rt values of As5 were significantly lower than those of NaF7 and NaF5, and that value of As7 was significantly lower than that of NaF7 for all time periods. However, the multiple-comparison Bonferroni test showed no significant difference between As7 and NaF5 after two weeks. After four weeks, NaF group showed a significant increase in surface roughness compared with the baseline (0 d), whereas As group did not show a significant difference in either Ra or Rt. The Ra and Rt values were observed to be highest in NaF7, followed by NaF5, As7, and As5.
Table 2.
Statistical comparison of mean (SD) surface roughness Ra values (μm) of RMGI specimens.
| Solution | baseline | 1 week | 2 weeks | 3 weeks | 4 weeks |
|---|---|---|---|---|---|
| As7 | 0.70 (0.28)a | 0.83 (0.05)A,a | 0.91 (0.07)AC,a | 0.92 (0.17)AC,a | 0.96 (0.16)AC,a |
| NaF7 | 0.70 (0.28)a | 1.60 (0.10)B,b | 1.78 (0.14)B,b | 1.81 (0.19)B,b | 1.77 (0.35)B,b |
| As5 | 0.70 (0.28)a | 0.78 (0.22)A,a | 0.74 (0.17)A,a | 0.56 (0.08)A,a | 0.56 (0.12)A,a |
| NaF5 | 0.70 (0.28)a | 1.49 (0.13)B,b | 1.50 (0.14)BC,b | 1.65 (0.07)BC,b | 1.59 (0.17)BC,b |
Superscripts with different letters indicates statistically significant difference (P < 0.008).
A, B, C Superscripts with uppercase letters are used for comparison between solution in the same column.
a, b Superscripts with lowercase letters are used for comparison between time within the same row.
As5: pH 5 artificial saliva, As7: pH 7 artificial saliva, NaF5: pH 5 artificial saliva containing 0.2% NaF, NaF7: pH 7 artificial saliva containing 0.2% NaF.
Table 3.
Statistical comparison of mean (SD) surface roughness Rt values (μm) of RMGI specimens.
| Solution | baseline | 1 week | 2 weeks | 3 weeks | 4 weeks |
|---|---|---|---|---|---|
| As7 | 5.53 (0.28)a | 7.65 (2.55)A,a | 8.13 (2.47)AC,a | 7.96 (1.93)AC,a | 8.23 (4.19)AC,a |
| NaF7 | 5.53 (0.28)a | 11.60 (3.65)B,b | 11.75 (3.21)B,b | 11.16 (2.49)B,b | 10.41 (4.32)BC,b |
| As5 | 5.53 (0.28)a | 6.88 (2.18)A,a | 6.95 (2.55)A,a | 6.04 (1.92)A,a | 6.64 (2.23)A,a |
| NaF5 | 5.53 (0.28)a | 10.21 (2.85)B,b | 11.09 (4.32)BC,b | 10.13 (1.98)BC,b | 10.14 (2.12)BC,b |
Superscripts with different letters indicates statistically significant difference (P < 0.008).
A, B, C Superscripts with uppercase letters are used for comparison between solution in the same column.
a, b Superscripts with lowercase letters are used for comparison between time within the same row.
As5: pH 5 artificial saliva, As7: pH 7 artificial saliva, NaF5: pH 5 artificial saliva containing 0.2% NaF, NaF7: pH 7 artificial saliva containing 0.2% NaF.
Surface superficial morphology by 2D and 3D image
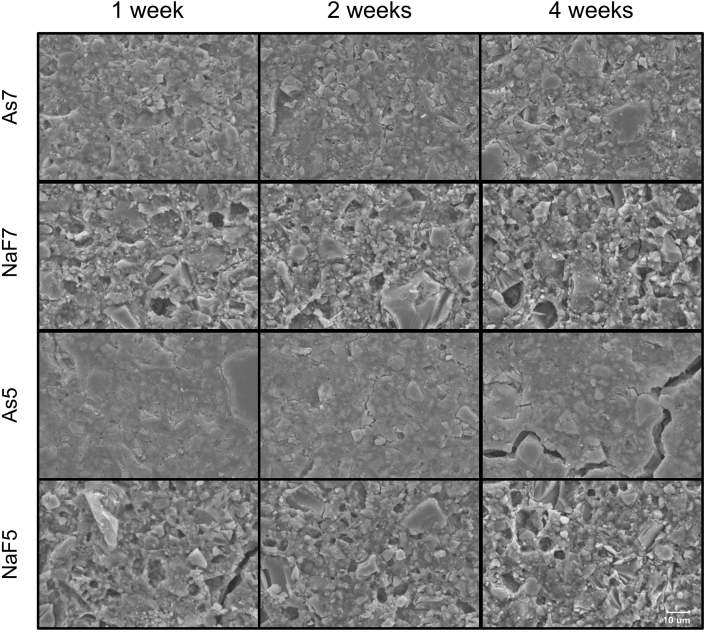
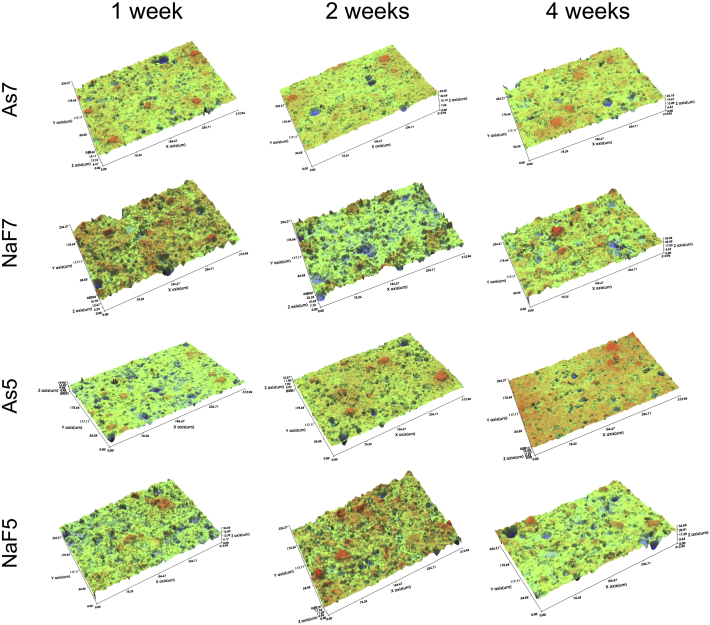
The surface superficial morphology was observed by SEM (Fig. 2) and WLI (Fig. 3), yielding 2D and 3D images, respectively. The baseline images are shown in Fig. 1, and Figure 2, Figure 3 shows surface changes after 1 week, 2 weeks, and 4 weeks of immersion. Severe destruction of the material surface was seen in the specimen stored in NaF solution. SEM analysis showed large cracked glass particles and eroded smaller glass particles in NaF group. The filler particles were more exposed and partially covered by the matrix. In As group, a relatively flat surface with fewer pores was observed in both SEM and WLI images.
Figure 2.
SEM observation of RMGI specimen after immersion in solution at 1 week, 2 weeks, and 4 weeks (2000× magnification). Exposure to NaF produced severe destruction on the RMGI surface, more glass particles exposed and irregular pores seen in NaF group. As5: pH 5 artificial saliva, As7: pH 7 artificial saliva, NaF5: pH 5 artificial saliva containing 0.2% NaF, NaF7: pH 7 artificial saliva containing 0.2% NaF, SEM: scanning electron microscopy.
Figure 3.
WLI observation of RMGI specimen after immersion in solution at 1 week, 2 weeks, and 4 weeks. 3D images show surface topography at the microscale level. Roughened surface can be seen in NaF group. As5: pH 5 artificial saliva, As7: pH 7 artificial saliva, NaF5: pH 5 artificial saliva containing 0.2% NaF, NaF7: pH 7 artificial saliva containing 0.2% NaF, WLI: white light interferometry.
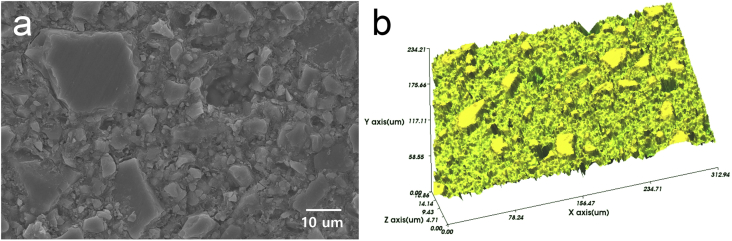
Figure 1.
Baseline 2D and 3D surface images of the RMGI specimen before immersion. (a) SEM observation (2000× magnification) and (b) WLI observation. SEM: scanning electron microscopy, WLI: white light interferometry.
Surface chemical composition
EDS analysis of RMGI immersed in various solutions at baseline, 1 week, and 4 weeks are presented in Table 4. The main inorganic elements constituting RMGI were F, Al, Si, and Sr. Compared to the control, F and Sr was decreased in all groups, Al was decreased in NaF group, and Si was similar in all groups. Some elements, besides those of the control RMGI composition, were detected depending on the solution. Na was seen in only NaF groups; meanwhile, Ca was seen in only As groups, and K was found in all groups. The ratio of organic to inorganic elements was lower in NaF group than As group.
Table 4.
Surface chemical composition (weight%) of RMGI immersed in different solutions for baseline, 1 week, and 4 weeks.
| Composition | C (Organic fraction) | O | F | Na | Al | Si | K | Ca | Sr | Inorganic fraction | Or: Non-Or | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 22.21 | 26.68 | 9.64 | – | 9.79 | 11.29 | – | – | 20.39 | 51.11 | 1 : 2.30 | |
| 1 week | As7 | 19.17 | 29.67 | 9.12 | – | 9.85 | 10.30 | 0.81 | 1.34 | 19.75 | 51.16 | 1 : 2.67 |
| NaF7 | 24.37 | 28.68 | 8.13 | 0.58 | 8.10 | 10.18 | 0.83 | – | 19.13 | 46.95 | 1 : 1.93 | |
| As5 | 17.72 | 33.87 | 9.13 | – | 9.45 | 11.45 | 1.96 | 1.16 | 15.26 | 48.41 | 1 : 2.73 | |
| NaF5 | 24.43 | 28.34 | 8.34 | 0.72 | 8.08 | 10.18 | 0.81 | – | 19.10 | 47.23 | 1 : 1.93 | |
| 4 weeks | As7 | 17.24 | 33.29 | 8.76 | – | 9.28 | 9.88 | 1.17 | 2.95 | 17.42 | 49.47 | 1 : 2.87 |
| NaF7 | 24.74 | 30.27 | 8.05 | 0.55 | 7.62 | 10.04 | 1.18 | – | 17.57 | 44.99 | 1 : 1.82 | |
| As5 | 18.81 | 36.68 | 7.85 | – | 9.96 | 11.73 | 1.68 | 0.60 | 12.70 | 44.51 | 1 : 2.37 | |
| NaF5 | 24.93 | 31.40 | 8.19 | 0.66 | 7.39 | 9.73 | 1.09 | – | 16.61 | 43.67 | 1 : 1.75 | |
As5: pH 5 artificial saliva, As7: pH 7 artificial saliva, NaF5: pH 5 artificial saliva containing 0.2% NaF, NaF7: pH 7 artificial saliva containing 0.2% NaF.
Discussion
In the present study, changes in the surface characteristics of topography, roughness, and chemical composition in the RMGI specimen undergoing prolonged surface degradation by 0.2% NaF solution was analyzed. An RMGI specimen was used, because as a dynamic rotating storage condition was devised to imitate mouthwash, the resulting mechanical wear to the surface during immersion would affect the chemical degradation of conventional GI, owing to its lower wear resistance.5 As the surface degradation by fluoride has already been reported to increase the surface roughness of RMGI (GI),13,14,17 our study attempted to visualize and observe the 2D and 3D images of the overall topography of the roughened surface through WLI and SEM methods. WLI is a computerized optical interference microscopy, which is fast, non-destructive, and accurate in analyzing three-dimensional surface structure at the microscale level;25 meanwhile, SEM was performed to obtain qualitative information regarding the variations on the surface with 2D imaging at the nanoscale level.8 In both WLI and SEM images, severe degradation of the surface was observed in NaF group. Due to gradual disintegration of the polysalt matrix in NaF group, more filler particles were exposed and polyacid matrix was destroyed, resulting rougher topography than As group (Figure 2, Figure 3). In As group, a swollen matrix appearance was seen and it was more profound in As5. This is in agreement with Nicholson et al. who reported that light-cured glass ionomers prepared with HEMA copolymers behave like hydrogels and absorb water,26 and RMGI showing a smooth and intact surface with an undisturbed matrix when absorbing water.19
Surface roughness parameters Ra and Rt significantly increased after surface degradation, reflecting the topographic images of the roughened surface. The Ra and Rt values of NaF group were significantly higher than those of the baseline and also higher than that of As group during the immersion periods. However, there was limitation to the increase in the roughness values, as the Ra and Rt values did not show a significant increase from 1 week to 4 weeks. This may be due to the dynamic storage condition, which was implemented to imitate oral mouthwash, affecting the surface roughness of RMGI to a maximum degree within a week. Surface degradation of RMGI (GI) by fluoride has been known to increase surface roughness;13 however, according to the results of this study, after reaching its maximum surface roughness by prolonged degradation, disintegration occurred without a further increase in the roughness value. As shown in the Ra and Rt values, it may be assumed that the maximum increase in the surface roughness by surface degradation is associated with the size of the particle. The average RMGI particle size used in this study is approximately 5.9 μm,4 and the median Rt values of 0.2% NaF group were approximately twice the size of the particle.
In the previous study, the surface degradation of restorative materials by fluoride was suggested to be greater under acidic conditions than neutral conditions.19 However, in this study, a pH difference between 5 and 7 did not affect the surface roughness of the RMGI specimen. It is possible that the degradation of the surface by fluoride is correlated with the acid resistance properties of the material. A pH of 5 was not acidic enough to increase the surface roughness of GI based materials,27,28 and this acid resistance of the specimen likewise did not make a difference in NaF group in this study.
Surface degradation is caused by a selective attack by alkali metal fluoride to the polysalt matrix between the glass particles.14,17,29 In the setting reaction of RMGI (GI), the acid–base reaction destroys the glass particles and releases cations such as Al and Ca. These released cations form chelates with the carboxylate groups of the polymer, producing crosslinks in the polymer network and forming a polysalt matrix.2 The Al-based crosslink is crucial to the hydrolytic stability of these cements,29 and when coming into contact with fluoride, the concentration of fluoride in the cement gradually increases, while the fluoride ion competes with carboxylate groups to form complexes with the Al3+ ions. The formation of higher-order fluoride complexes [Al(H2O)6-nFn]3−n with n ≥ 2 decreases the number of ionic crosslinks and site-bounded aluminum, causing a gradual disintegration of the polysalt matrix.17 It is suggested that this chemical erosion occurs on fluoride-containing glass20,29 and is affected by the composition of the cement, concentration of the fluoride solution, time, and frequency of immersion.10,17
The chemical composition changes in the RMGI surface due to prolonged surface degradation was evaluated by EDS. EDS is a reproducible, reliable, and precise method for identifying and quantifying the major components or compounds present in a material.30 In this study, results of the EDS analysis showed detectable changes in the surface composition in all groups. The inorganic composition of RMGI used in this study was primarily F, Al, Si, and Sr. After immersion, Na, K, and Ca were detected, depending on the immersed solution. K in artificial saliva was absorbed by RMGI in both NaF and As groups, but Ca was only absorbed in As group. GI cements are able to take up Ca ions in natural saliva and develop a harder surface.31 The ion exchange between Sr in cement and Ca from saliva is reported to occur to maintain electrolytic balance, Ca diffuse into the hydrogel matrix and form an intermediate Ca-rich layer that hardens the surface of GI, although the exchange mechanism has not yet been identified.7 As Ca was detected on only As group, it is suggested that when chemical erosion by fluoride occurs on the surface of RMGI, Ca could not be exchanged with Sr or absorbed to the surface, assuming due to the disintegration of the matrix. In the oral environment, Ca promotes the remineralization of the tooth in acidic conditions, diffused from plaque to saliva.32 According to the results of this study, in acidic conditions, uptake of diffused Ca to the RMGI would decrease, as surface absorption of Ca was higher in As7 than in As5. For Sr, which is known to enhance the re-mineralizing effects of fluoride, the level of Sr release was reported to far exceed that of F or Al ions in GI cements,7 and tend to be released further under acidic conditions.33 Likewise, Sr concentration in this study decreased in all groups, decreasing further over time. It was observed to be affected by pH and degradation, as it was the lowest in As5, and the decrease in NaF5 was reduced. The concentration of Al, which forms networks in the glass and responsible for the stability of the cement,34 was decreased under sustained degradation, with a greater decrease after 4 weeks. Petrovic and Markovic have reported an increase in surface F concentration after immersion in NaF solution,33,35 but in this study, the F concentration in NaF group did not increase compared with the control or As group. The F concentration in the surface was decreased in all groups at the 4 week period. The lowest F concentration was seen in As5, as RMGI (GI) released more fluoride in an acidic environment,35 and the effect of pH was not detectable in NaF group. In an environment where sustained surface degradation occurs, F uptake in the surface may not be detected. However, as F release is related to GI degradation,36 a considerable amount of F release can be expected in NaF group, owing to the severe surface degradation detected by SEM and WLI. Overall, after degradation, inorganic components of the surface was decreased and this could suggest the weakening of the surface mechanical properties of the RMGI (GI).5
The present study assessed the roughness, 2D and 3D topographic images, and chemical composition of RMGI surface after the prolonged degradation by fluoride. The null hypothesis that pH and time would not affect surface degradation of RMGI induced by NaF solution was rejected. While the roughness value maintained a certain level during the four-week degradation period, the chemical components of the surface continued to change over time and pH in all storage conditions. Changes in composition by NaF degradation showed a decrease in inorganic elements that enhance physical properties at the surface, and fluoride concentration did not seem to increase. The use of fluoride is recommended for caries management and prevention as it shows remineralization effects and antimicrobial activity against bacteria and biofilms.37 However, based on the results of the study, when RMGI or GI restoration is present in the oral cavity, the indiscriminating use of fluoride to force fluoride recharge of the restoration may increase the risk of increased surface roughness and affect the surface elemental composition, eventually deteriorating the restoration. Even neutral NaF solution can cause surface degradation of the RMGI, which degraded surface would alter restoration more readily eroded under intraoral chemical and physical conditions, and the roughened surface would lead to increased bacterial accumulations.19 Therefore, a comprehensive understanding of the interactions between restorative material, oral environment, and fluoride treatment should be considered in dental practice. Although the prolonged degradation of RMGI in this study may not be relevant to a clinical situation, this present study provides more information on the chemical and topographic characteristics of the surface degradation phenomenon on RMGI. Further studies on surface degradation are needed at shorter time periods with different types of fluoride-releasing materials and in various solutions under both in vivo and in vitro conditions.
Within the limitations of in vitro study, the results of this study suggest that the surface morphological structure and chemical composition of RMGI can be affected by fluoride solution. Following surface degradation, the disintegration of the polyacid matrix on the surface was observed with WLI and SEM, with significantly increased surface roughness. It was suggested that the surface roughness value is related to the particle size; meanwhile, the effects of time and pH were insignificant. The chemical composition of the surface was also changed through sustained degradation. The changes in the elements varied individually, affected by both pH and time. The physicochemical characteristics of the restorative material should be considered to increase the longevity of the material and establish a treatment plan suitable to the patient.
Declaration of competing interest
The authors have no conflicts of interest related to this article.
Acknowledgements
The authors would like to acknowledge Sung-Hoon Lee (Department of Microbiology and Immunology, College of dentistry, Dankook University, Republic of Korea) for discussions and support.
References
- 1.Sidhu S.K., Nicholson J.W. A review of glass-ionomer cements for clinical dentistry. J Funct Biomater. 2016;7:16–30. doi: 10.3390/jfb7030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoroushi M., Keshani F. A review of glass-ionomers: from conventional glass-ionomer to bioactive glass-ionomer. Dent Res J. 2013;10:411–420. [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho F.G., Sampaio C.S., Fucio S.B., Carlo H.L., Correr-Sobrinho L., Puppin-Rontani R.M. Effect of chemical and mechanical degradation on surface roughness of three glass ionomers and a nanofilled resin composite. Operat Dent. 2012;37:509–517. doi: 10.2341/10-406-L. [DOI] [PubMed] [Google Scholar]
- 4.Bala O., Arisu H.D., Yikilgan I., Arslan S., Gullu A. Evaluation of surface roughness and hardness of different glass ionomer cements. Eur J Dermatol. 2012;6:79–86. [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues D.S., Buciumeanu M., Martinelli A.E. Mechanical strength and wear of dental glass-ionomer and resin composites affected by porosity and chemical composition. J Bio- Tribo-Corrosion. 2015;1:24–32. [Google Scholar]
- 6.Berzins D.W., Abey S., Costache M.C., Wilkie C.A., Roberts H.W. Resin-modified glass-ionomer setting reaction competition. J Dent Res. 2010;89:82–86. doi: 10.1177/0022034509355919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngo H. Glass-ionomer cements as restorative and preventive materials. Dent Clin North Am. 2010;54:551–563. doi: 10.1016/j.cden.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Osorio E., Osorio R., Zanotto E.D., Peitl O., Toledano-Osorio M., Toledano M. SEM and AFM characterization of surface of two RMGICs for degradation before and after modification with bioactive glass ceramic. J Adhes Sci Technol. 2015;30:621–632. [Google Scholar]
- 9.Yli-Urpo H., Vallittu P.K., Närhi T.O., Forsback A.P., Väkiparta M. Release of silica, calcium, phosphorus, and fluoride from glass ionomer cement containing bioactive glass. J Biomater Appl. 2004;19:5–20. doi: 10.1177/0085328204044538. [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir-Ozenen D., Sungurtekin E., Issever H., Sandalli N. Surface roughness of fluoride-releasing restorative materials after topical fluoride application. Eur J Paediatr Dent. 2013;14:68–72. [PubMed] [Google Scholar]
- 11.Wiegand A., Buchalla W., Attin T. Review on fluoride-releasing restorative materials-Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–362. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Manhart J., Chen H.Y., Hamm G., Hickel R. Review of the clinical survival of direct and indirect restorations in posterior teeth of the permanent dentition. Operat Dent. 2004;29:481–508. [PubMed] [Google Scholar]
- 13.Benderli Y., Gökçe K., Kazak M. Effect of APF gel on micromorphology of resin modified glass–ionomer cements and flowable compomers. J Oral Rehabil. 2005;32:669–675. doi: 10.1111/j.1365-2842.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- 14.Alkesen J.P., Afseth J., Rolla G. In vitro damage to glass-ionomer cements by fluoride ion. Caries Res. 1987;66:844. [Google Scholar]
- 15.Yip H.K., To W.M., Smales R.J. Effects of artificial saliva and APF gel on the surface roughness of newer glass ionomer cements. Operat Dent. 2004;29:661–668. [PubMed] [Google Scholar]
- 16.Khosla E., Kuriakose S., Suderasen C. Surface micromorphological changes of glass ionomer following application of 1.23% acidulated phosphate fluoride: a scanning electron microscope study. Indian J Dent Res. 2014;25:493–498. doi: 10.4103/0970-9290.142545. [DOI] [PubMed] [Google Scholar]
- 17.De Witte A.M., De Maeyer E.A., Verbeeck R.M. Surface roughening of glass ionomer cements by neutral NaF solutions. Biomaterials. 2003;24:1995–2000. doi: 10.1016/s0142-9612(02)00617-8. [DOI] [PubMed] [Google Scholar]
- 18.Triana R.T., Millan C.P., Barrio J.G., Garcia-Godoy F. Effect of APF gel on light-cured glass ionomer cements: an SEM study. J Clin Pediatr Dent. 1994;18:109–113. [PubMed] [Google Scholar]
- 19.Dionysopoulos P., Gerasimou P., Tolidis K. The effect of home-use fluoride gels on glass–ionomer, compomer and composite resin restorations. J Oral Rehabil. 2003;30:683–689. doi: 10.1046/j.1365-2842.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- 20.Hadley P.C., Billington R.W., Pearson G.J., Williams J.A. Effect of monovalent ions in glass ionomer cements on their interaction with sodium fluoride solution. Biomaterials. 2000;21:97–102. doi: 10.1016/s0142-9612(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 21.Yeh S.T., Wang H.T., Liao H.Y. The roughness, microhardness, and surface analysis of nanocomposites after application of topical fluoride gels. Dent Mater. 2011;27:187–196. doi: 10.1016/j.dental.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Bohner L.O., de Godoi A.P., Ahmed A.S., Neto P.T., Catirse A.B. Surface roughness of restorative materials after immersion in mouthwashes. Eur J Gen Dent. 2016;5:111–114. [Google Scholar]
- 23.Carlen A., Nikdel K., Wennerberg A., Holmberg K., Olsson J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials. 2001;22:481–487. doi: 10.1016/s0142-9612(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 24.McKnight-Hanes C., Whitford G.M. Fluoride release from three glass ionomer materials and the effects of varnishing with or without finishing. Caries Res. 1992;26:345–350. doi: 10.1159/000261466. [DOI] [PubMed] [Google Scholar]
- 25.Etxeberria M., Escuin T., Vinas M., Ascaso C. Useful surface parameters for biomaterial discrimination. Scanning. 2015;37:429–437. doi: 10.1002/sca.21232. [DOI] [PubMed] [Google Scholar]
- 26.Beriat N.C., Nalbant D. Water absorption and HEMA release of resin-modified glass-ionomers. Eur J Dermatol. 2009;3:267–272. [PMC free article] [PubMed] [Google Scholar]
- 27.Beresescu G., Brezeanu L.C. Effect of artificial saliva on the surface roughness of glass-ionomer cements. Sci Bull Petru Maior Univ Targu Mures. 2011;8:134–136. [Google Scholar]
- 28.Mohamed-Tahir M.A., Yap A.U. Effects of pH on the surface texture of glass ionomer based/containing restorative materials. Operat Dent. 2004;29:586–591. [PubMed] [Google Scholar]
- 29.Billington R.W., Hadley P.C., Towler M.R., Pearson G.J., Williams J.A. Effects of adding sodium and fluoride ions to glass ionomer on its interactions with sodium fluoride solution. Biomaterials. 2000;21:377–383. doi: 10.1016/s0142-9612(99)00199-4. [DOI] [PubMed] [Google Scholar]
- 30.Saxena S., Tiwari S. Energy dispersive X-ray microanalysis, fluoride release, and antimicrobial properties of glass ionomer cements indicated for atraumatic restorative treatment. J Int Soc Prev Community Dent. 2016;6:366–372. doi: 10.4103/2231-0762.186790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada K., Tosaki S., Hirota K., Hume W.R. Surface hardness change of restorative filling materials stored in saliva. Dent Mater. 2001;17:34–39. doi: 10.1016/s0109-5641(00)00053-1. [DOI] [PubMed] [Google Scholar]
- 32.Baumann T., Bereiter R., Lussi A., Carvalho T.S. The effect of different salivary calcium concentrations on the erosion protection conferred by the salivary pellicle. Sci Rep. 2017;11:1–9. doi: 10.1038/s41598-017-13367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovic B., Markovic D., Kojic S. Characterization of glass ionomer cements stored in various solutions. Materiali in Tehnologije. 2019;53:285–293. [Google Scholar]
- 34.Billington R.W., Williams J.A., Pearson G.J. Ion processes in glass ionomer cements. J Dent. 2006;34:544–555. doi: 10.1016/j.jdent.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Markovic D., Petrovic B.B., Peric T.O. Fluoride content and recharge ability of five glassionomer dental materials. BMC Oral Health. 2008;8:21–28. doi: 10.1186/1472-6831-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandolfi M.G., Chersoni S., Acquaviva G.L., Piana G., Prati C., Mongiorgi R. Fluoride release and absorption at different pH from glass-ionomer cements. Dent Mater. 2006;22:441–449. doi: 10.1016/j.dental.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Marquis R.E. Antimicrobial actions of fluoride for oral bacteria. Can J Microbiol. 1995;41:955–964. doi: 10.1139/m95-133. [DOI] [PubMed] [Google Scholar]