Abstract
Background/purpose
Oral candidiasis is the most common fungal infection of the oral cavity and has become a focus of attention in recent years because of its association with highly topical immunosuppressive conditions. The aims of this study were to determine the value of microbiological, clinical and histological parameters of Candida albicans colonisation of the dorsal tongue surface as indicators of disease severity, and to evaluate therapeutic response to different formulations of nystatin.
Materials and methods
We used 84 males, 2-month-old Sprague–Dawley sialoadenectomized rats. Different formulations of nystatin were used to evaluate the therapeutic response. The animals were randomized to 2 groups with each of 42 animals and received the experimental treatments from day 17–22.
Results
100% of the rats showed evidence of infection. At 5 and 10 days of starting treatment with nystatin + chitosan, and at 10 days of starting nystatin + orabase, the number of animals with positive dorsal tongue culture decreased significantly (p < 0.05), acting the Nystatin + chitosan more rapidly against Candida. In the control group, the percentage of normal papillae on day 22 and 27 was 83.33% (SD = 1.50) and 79.08% (SD = 2.30), respectively. Significant differences were observed in the mean O'Grady score at 5 and 10 days (p < 0.0001).
Conclusion
The model has been shown to be effective in inducing infection, and that the combination of nystatin and chitosan yielded the best therapeutic outcomes at both 5 and 10 days after infection.
Keywords: Experimental models, Salivary glands, Nystatine, C-albicans, Oral candidiasis
Introduction
Oral candidiasis or oral thrush is an infectious disease caused by an increase in Candida colonisation and invasion of oral tissues that occurs when physical barriers and host defences have been weakened. It is the most common fungal infection of the oral cavity and the most prevalent strain is Candida albicans. Most clinicians consider oral candidiasis to be synonymous with C. albicans infection.1
Oral candidiasis has become a focus of attention in recent years because of its association with highly topical immunosuppressive conditions, such as those derived from human immunodeficiency virus (HIV) infection.2 At least 80% of AIDS patients and one third of patients with HIV infection develop concomitant Candida infection.3 Systemic diseases, such as diabetes,4 and the wide array of pharmacological treatments (antibiotics, immunosuppressants and psychotropic drugs) used by the general population have also helped increase the prevalence of this disease. In addition, the high prevalence (31%) of dry mouth (xerostomia) in the over-65s, due to comorbidities and use of pharmacological therapies, explains the presence of this disease in this segment of the population.5
The study of oral candidiasis is particularly challenging due to the number of predisposing factors, which tend to confound the results of published studies. One way to overcome this is to use experimental animal models that simulate human disease. This approach gives investigators access to a series of genetically uniform individuals that are subject to the same environmental conditions. It also avoids the bias that genetic variability, immunological status, differences in diet and lifestyle, dental prostheses, variations in salivary flow and composition, medical treatments, and lack of cooperation can cause in human studies.6
On this premise, we developed a rodent model of oral candidiasis that reproduces the clinical manifestations of the disease in humans. The aims of the study were to develop a homogeneous and reproducible model of oral candidiasis due to C. albicans in sialoadenectomized rats; to determine the value of microbiological, clinical and histological parameters of C. albicans colonisation of the dorsal tongue surface as indicators of disease severity; and to evaluate the therapeutic response to different formulations of nystatin.
Material and methods
C. albicans colonisation model
Animals
We used 84 male, 2-month-old Sprague–Dawley rats (Interfauna Ibérica, S.A., Barcelona, Spain), weighing between 180 and 200 g. Absence of C. albicans was confirmed after samples from the oral cavity of each rat had been cultured in yeast extract peptone dextrose (YEPD) medium, as it has already been described.15 Seven days before the start of the experiments, the animals were housed in individual filter-top cages in an aseptic chamber maintained at between 21 and 22 °C, with 12/12 h artificial light–dark cycle. They were given ad libitum food (Panlab Diet A.03) and sterile water.
Xerostomia induction
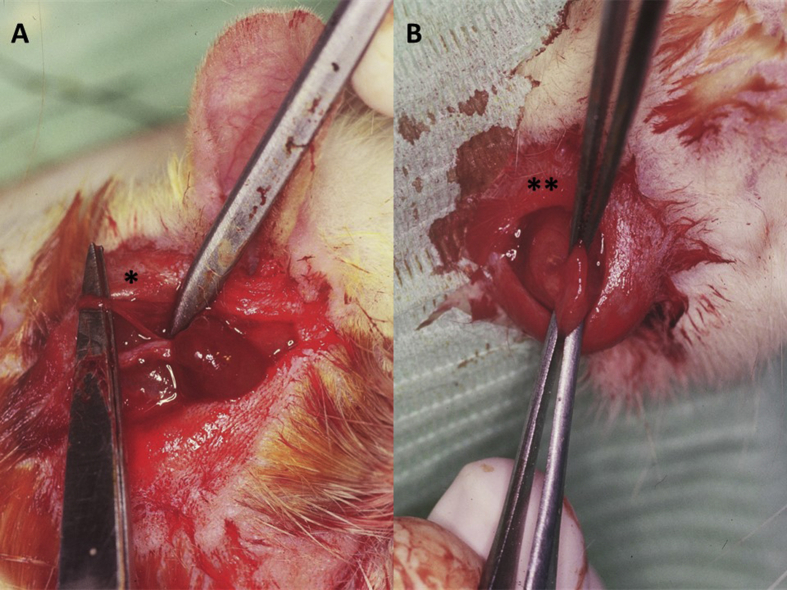
Sialoadenectomy was performed to induce xerostomia and facilitate the development of oral candidiasis. The rats were anesthetised with 40 mg/kg ketamine (Ketolar, Parke Davis, Barcelona, Spain) and 1 mg/kg diazepam (Valium, Roche, Madrid, Spain) before undergoing excision of the sublingual and submaxillary salivary glands and ligation of the Stensen or parotid ducts (Fig. 1A and B). A 1 cm incision was made below the external auditory canal and extended in a straight line to the external angle of the eye, following the course of the exorbital lacrimal gland, which overlaps the parotid gland and covers the start of the parotid duct, through the masseter muscle. After the gland was separated from the buccal and mandibular branches of the facial nerve, it was tied with Catgut® (Braun Surgycal, S.A., Barcelona, Spain). To remove the submaxillary and sublingual glands, the lower border of the hyoid bone and the manubrium sterni (caudally) were located by palpation, and an incision was made along an imaginary line between both points. Next, the submaxillary and sublingual glands were exposed and removed en bloc, given their close anatomical relationship. Before removal of the glands, the hilae were tied off with Catgut®. The incision was then closed in layers using 3.0 suture (Ethicon Ltd, Edinburgh, United Kingdom).
Figure 1.
Experimental rats undergoing (A) ligature of Stensen ducts (∗) and (B) removal of submaxillary glands (∗∗).
Two months after surgery, candidiasis was induced. This interval was considered sufficient to allow the surgical wound to heal and for clinical signs of xerostomia to be confirmed.7,8
Induction of oral candidiasis
C. albicans was obtained from erythematous oral lesions in a patient and identified by the Microbiology Department of the University Hospital, Santiago de Compostela. The Candida strain was plated on YEPD growth medium at room temperature. The microorganisms isolated were identified as C. albicans using the germ tube and chlamydospore formation test. To prepare C. albicans for inoculation, the colonies were suspended in sterile buffered saline, washed and centrifuged (×2), and resuspended in the same saline solution. The inoculum was adjusted to a concentration of 3 × 108 CFU/ml based on the optical density at 300 nm (OD300) measured using a densitometer Lumeton Colorimeter 401A (Photovolt Instruments Inc. St. Louis Park, MN, USA).
The rats were infected by topical application of the inoculum on the dorsal tongue on 2 consecutive days. Swabs Assure (ref. 81,005) (Sultan Chemists Inc., Englewood, NJ, USA) saturated with 0.1 ml of fresh suspension were used to impregnate the dorsal tongue with inoculum. C. albicans infection was confirmed 17 days after inoculation and at the end of the experiment. Induction of oral candidiasis protocol was developed following Samaranayake et al. considerations.9 The fungus was collected by introducing a collection swab in the oral cavity and rotating it on the dorsal tongue. Samples were seeded on sabouraud dextrose agar, and incubated at 37 °C (Estufa Microbiológica Selecta, Barcelona, Spain) for 48 h, after which time the colonies were identified.
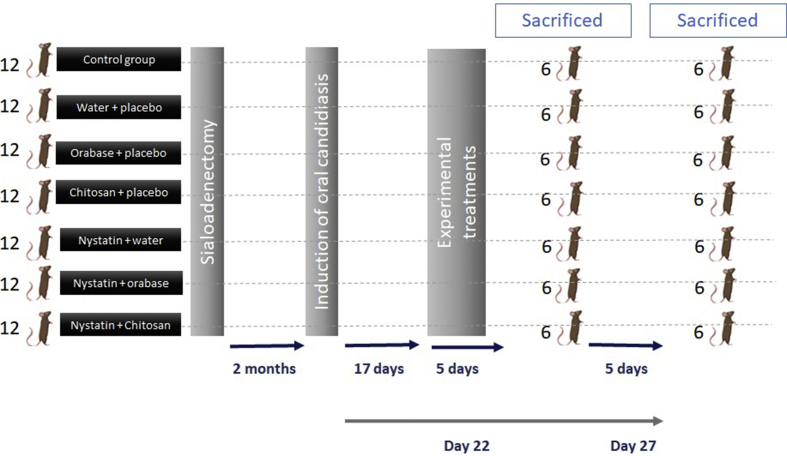
Experimental protocol
The dorsal tongue was examined macroscopically everyday from the time of inoculation until the end of the experiment. The latency period and the size and characteristics of the lesions were recorded over the course of the study. On day 17, the presence of C. albicans in the oral cavity of the rats was confirmed by culture of swab samples. Experimental treatments were applied from day 17–22. The animals were then randomised to 2 groups (sacrificed at 5 or 10 days) with each of 42 animals (See flow chart, Fig. 2). These groups were stablished to determine hyphae invasion according to evolutionary process. The animals were sacrificed using CO2/N2 and the dorsal tongue was photographed in situ (10x).9
Figure 2.
Flow chart of methodological design.
Evaluation of the disease model
Candida infection was evaluated on the basis of a positive culture from the surface of the tongue, the lingual surface affected by the lesion, the change in the number and morphology of the papillae, and hyphal invasion of the stratum corneum of the lingual epithelium.
Clinical manifestations
As described above, the dorsal tongue was inspected daily from the time of inoculation until the end of the experiment, which allowed us to study the size and characteristics of the lesion. The size of the lesion (μm) was quantified using digital image analysis (Técnicas Médicas Mab, Barcelona, Spain) of the photographs taken on the day of sacrifice, measuring the percentage of affected surface area (absence of papillae and/or smaller papillae) with respect to the total surface of the tongue.
Histological study
The tongues were excised immediately after the animal was sacrificed and were hemissected along the sagittal plane to allow each sample to be studied under an optical microscope. The hemissected tongues were fixed in 2.5% phosphate-buffered formaldehyde solution with 0.1 M Sorensen's buffer at 4 °C, dehydrated in incremental solutions of alcohol and embedded in paraffin. Sagittal 5 μm slices were made and stained with haematoxylin-eosin (HE) and periodic acid-Schiff (PAS). The following parameters from 3 successive HE- and PAS-stained slices were measured as indicators of C. albicans infection using digital image analysis.
Affected lingual surface
This was measured on HE-stained slices, quantifying tissue injury according to the presence of normal, atrophic, and hypertrophic papillae, taking into account the number and morphological characteristics of the papillae of the dorsal tongue per microscopic field (magnification ×40). The image analysis system was calibrated for the unit of measurement (μm) used, and the presence of atrophic or hypertrophic papillae was established by calculating the average papilla size (μm) captured by the lens and measuring their width at mid height per microscopic field.
Measurements were taken from the entire dorsal tongue surface, between the anterior tongue and the start of the pharynx, taking the lingual glands (serous glands or Von Ebner's gland) embedded in the intrinsic muscles of the tongue as an anatomical landmark.
Quantification of hyphae
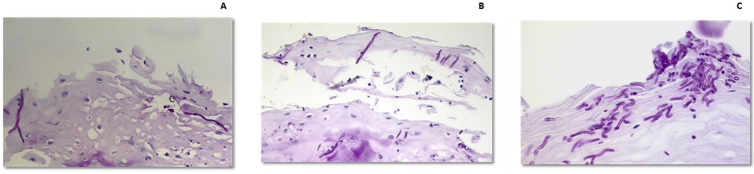
A semi-quantitative 5-point scale (absence of hyphae = 0; maximum invasion, more than 50 hyphae per microscopic field [magnification ×40] = 4) was used to assess the extent of epithelium hyphal invasion. Invasion was graded as 1 for the presence of 1–5 hyphae, 2 for 6 to 15 hyphae, and 3 for 16 to 50 hyphae (O'Grady and Reade scale) (Fig. 3).10 The process was double-blinded, and measurements were taken from 3 successive PAS-stained slices taken from each sample.
Figure 3.
O'Grady and Reade scale. A) Grade 1, presence of 1–5 hyphae; B) Grade 2, presence of 6–15 hyphae; C) Grade 3 presence of 16–50 hyphae.
Study treatments
The topical antifungal drug nystatin combined with different excipients was studied: a) No excipient: Nystatin in aqueous suspension at a concentration of 100,000 IU/ml (Mycostatin, Squibb, Madrid, Spain) was administered at a dose of 0.5 ml/rat/day. b) With excipient: i) Nystatin (Squibb) dissolved in orabase (Colgate-Hoyt Laboratories, Norwood, MA, USA) at a concentration of 100,000 IU/ml, administered at a dose of 0.5 ml per animal per day; ii) Nystatin (Squibb) (0.3% w/w) dissolved in an aqueous solution (1.20% w/w) of chitosan glutamate (Sea Cure G110) (Pronova Laboratories, Drammer, Norway) with a viscosity of less than 100 cP at 25 °C. Each animal received 1.55 ml per day; iii) Controls and placebos: the same dose of the different excipients tested were administered (water, orabase and chitosan). c) Controls received no treatment. This gave us 1 control group, 3 placebo groups and 3 treatment groups with 6 rats in each group at 5 days, and 6 rats in each group at 10 days of treatment.
Statistical analysis
The presence of C. albicans on the dorsal tongue and the percentage of clinical lesion area in respect of the dorsal tongue surface was quantified using the Student's t test. The degree of hyphal invasion of the epithelium was evaluated using the Wilcoxon test for paired data and the Kruskal–Wallis test for multiple comparisons. Significance was set at p < 0.05.
Ethics statement
C. albicans was obtained from erythematous oral lesions in a patient and identified by the Microbiology Department of the University Hospital, Santiago de Compostela, specifically for this study. The sample were anonymized before its use. The Ethics Committee of Clinical Hospital of Santiago de Compostela and the Ethics Committee of University of Santiago de Compostela have given their approved. Bioethics Committee of Experimental Animal Studies of the University of Santiago de Compostela approved this study (Ref. CEEA/132/16) and met the relevant regional and European Union requirements (protocol number: AELUOO1/14/INVMED/OUTROS [04]/FMG/07) for the care and use of research animals. Animal experiments complied with the ARRIVE guidelines, was carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Results
Presence of C. albicans in the oral cavity
Culture of the dorsal tongue samples at days 17, 22 and 27 showed the presence of C. albicans in all the inoculated animals. However, at 5 and 10 days of starting treatment with nystatin + chitosan, and at 10 days of starting nystatin + orabase, the number of animals with positive dorsal tongue culture decreased (p < 0.05). Nystatin + chitosan appears to act more rapidly against Candida.
Clinical evaluation of the lesion
The first clinically objective changes were observed on day 13 post-inoculation, and by day 17, 100% of the rats showed evidence of infection, and treatment was started. From day 17, we observed a slight decrease in the percentage of rats with clinical evidence of lesions: 83.3% and 66.6% of the rats showed clinical evidence of lesions on days 22 and 27, respectively.
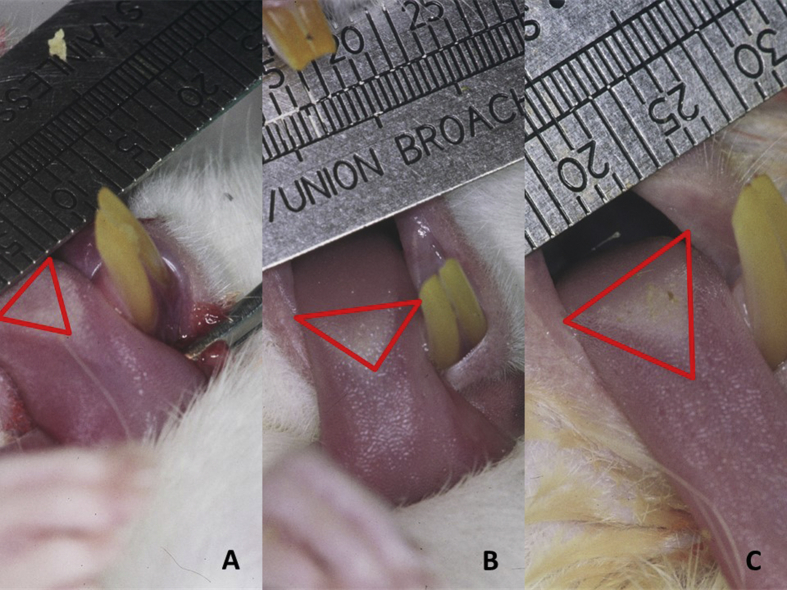
Digitalization and analysis of the surface of the tongue allowed us to measure the density of the entire lesion area (Fig. 4), discriminating between areas with no papillae and/or smaller papillae. In controls, 4.88% (SD = 0.41) and 3.90% (SD = 0.53) of the surface of the tongue was affected on days 22 and 27, respectively. A significant reduction in the lesion area was observed between both days in all groups (Table 1).
Figure 4.
Digitalization technique used to evaluate clinical lesions. (A) Nystatin with chitosan. (B) Nystatin alone. (C) Placebo.
Table 1.
Clinical evaluation of candidiasis lesion on the dorsal tongue as a % of affected surface area with respect to the total surface area of the tongue.
| Group | 5 Days % (SD) | 10 Days % (SD) |
|---|---|---|
| Control | 4.88 (0.41) | 3.90 (0.53) ♦ |
| Placebo water | 4.93 (0.09) | 3.95 (0.80) ♦ |
| Placebo orabase | 4.96 (0.79) | 3.90 (0.13) ♦ |
| Placebo chitosan | 4.72 (0.14) | 3.82 (0.98) ♦ |
| Nystatin + water | 3.87 (0.62) ▪ | 3.26 (0.23) ♦ ▪ |
| Nystatin + orabase | 1.20 (0.42) ▪ ♥ | 0.69 (0.42) ♦ ▪ ♥ |
| Nystatin + chitosan | 0.67 (0.25) ▪ ♥ | 0.14 (0.12) ♦ ▪ ♥ ♣ |
♦ Significant differences between groups treated for 5 or 10 days.
▪ Significant differences between groups treated with nystatin and control/placebo groups.
♥ Significant differences between groups treated with nystatin + orabase or nystatin + chitosan and groups treated with nystatin + water.
♣ Significant differences between groups treated with nystatin + chitosan and groups treated with nystatin + orabase.
In all (100%) cases, the lesion occurred only on the junction between the anterior 2 thirds and the posterior third of the tongue.
Histology
A digital image analysis system was used to estimate the number and average size (μm) of the papillae per microscopic field in each histological sample (3 from each side of the tongue) according to their topographical distribution, measured from the anterior to the posterior edge of the tongue. In the control group, the percentage of normal papillae on day 22 and 27 was 83.33% (SD = 1.50) and 79.08% (SD = 2.30), respectively. The number of normal papillae increased over time in each study group (Fig. 3), being higher in the nystatin + chitosan, nystatin + orabase, and nystatin + chitosan groups at 10 days (Table 2) (see Fig. 5).
Table 2.
Percentage of normal papillae at 5 and 10 days, according to the type of treatment.
| Group | 5 Days % (SD) | 10 Days % (SD) |
|---|---|---|
| Control | 83.33 (1.50) | 79.08 (2.30) |
| Placebo water | 82.83 (0.75) | 80.84 (0.98) |
| Placebo orabase | 82.33 (0.81) | 79.65 (1.25) |
| Placebo chitosan | 83.60 (1.51) | 78.32 (0.63) ♦ |
| Nystatin + water | 84.33 (1.21) | 93.16 (2.13) ♦ ▪ |
| Nystatin + orabase | 87.33 (0.81) ▪ ♥ | 96.66 (0.81) ♦ ▪ ♥ |
| Nystatin + chitosan | 92.00 (02.80) ▪ ♥ ♣ | 98.16 (0.40) ♦ ▪ ♥ ♣ |
♦ Significant differences between groups treated for 5 or 10 days.
▪ Significant differences between groups treated with nystatin and control/placebo groups.
♥ Significant differences between groups treated with nystatin + orabase or nystatin + chitosan and groups treated with nystatin + water.
♣ Significant differences between groups treated with nystatin + chitosan and groups treated with nystatin + orabase.
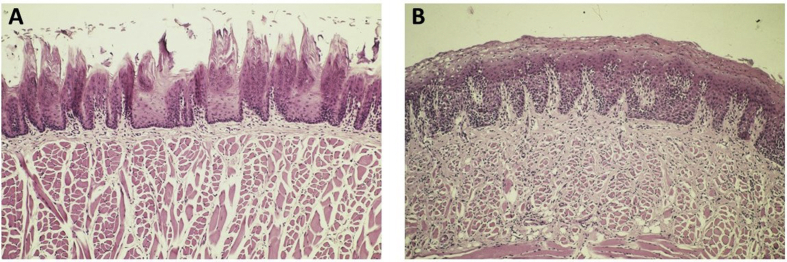
Figure 5.
Histopathological microphotographs of the tongue of rats. (A) Lingual papillae before inoculation (hematoxylin and eosin stain, 10 × ). (B) Depapillated area at 17 days (hematoxylin and eosin stain, 10 × ).
Hyphal invasion of the epithelium
Hyphal invasion of the stratum corneum was observed in the affected area, particularly in the region between the anterior 2 thirds and the posterior third of the tongue. A slight decrease in this parameter was observed after 5 days of evolution: on day 22, 50% of control group rats presented grade 3 invasion, and the other 50% grade 2; while on day 27, 50% of control rats presented grade 2 invasion and the other 50% grade 1. Table 3 shows the mean values determined in each group. Significant differences were observed in the mean score at 5 and 10 days (p < 0.0001) and, after applying the Bonferroni post-hoc test, between the nystatin + chitosan group and all other groups, between the nystatin + orabase group and the control groups, and between the orabase placebo group and the nystatin + water and the nystatin + chitosan groups.
Table 3.
Hyphal invasion of epithelium based on the O'Grady and Reade scale at 5 and 10 days, by type of treatment and multiple comparisons. The last column refers to the group or groups with significant differences. R: Rat.
| O'Grady and Reade classification | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 5 Days |
10 Days |
Mean (SD) | Significant Groups Comparisons (p ≤ 0.01) | ||||||||||
| R1 | R2 | R3 | R4 | R5 | R6 | R1 | R2 | R3 | R4 | R5 | R6 | |||
| Control | 3 | 2 | 3 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2.00 (0.74) | Nystatin + orabase |
| Water placebo | 2 | 3 | 2 | 2 | 3 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 1.92 (0.67) | Nystatin + chitosan |
| Orabase placebo | 2 | 2 | 3 | 3 | 3 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 2.00 (0.74) | Nystatin + orabase//Nystatin + chitosan |
| Chitosan placebo | 2 | 2 | 2 | 3 | 3 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 1.92 (0.67) | Nystatin + chitosan |
| Nystatin + water | 2 | 3 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1.42 (0.90) | Nystatin + chitosan |
| Nystatin + orabase | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1.00 (0.74) | Control//Orabase placebo |
| Nystatin + chitosan | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0.42 (0.52) | All |
| Total | 1.52 (0.90) | |||||||||||||
Discussion
In this study, we used Sprague–Dawley rats to develop a model of oral candidiasis that reproduces the clinical manifestations of the disease in humans. This was reached successfully as all animals (100%) developed candidiasis.
As described above, a number of predisposing factors are involved in the pathogenesis of oral candidiasis. These have already been described in other studies in which experimental models were developed to study clinical infection in humans.9,11 Since rats are not naturally colonized by C. albicans,12 animal models use various pharmacological treatments, such as antibiotics and immunosuppressants13,14 or psychological stress as auditory stressor,15 to promote fungal growth. Because of its broad spectrum, tetracycline has been the most widely used antibiotic in this setting.16 The effect of this drug on the development of candidiasis, when administered before inoculation of the germ and/or during evolution of the disease, has been amply documented.17 This effect has been attributed to the suppression of organisms competing for nutrients and adhesion sites, although tetracycline could also either reduce the host's ability to expel the fungi or enhance colonisation. Although tetracycline has been shown to prolong C. albicans colonisation of the oral cavity of rats and to contribute to establishing the infection, other authors concluded that tetracycline was not needed to induce infection if a sufficiently virulent strain of C. albicans was used.6 We think that although studies have reported a higher incidence and persistence of pseudomembranous lesions in mice treated with antibiotics and immunosuppressants,6 these strategies can introduce considerable variability.
One of the features most widely studied in the different experimental models of this disease is xerostomia. For this reason, we chose to reduce salivary flow as a predisposing factor for oral candidiasis.18 Saliva is part of the localised secretory or mucosal immune system that protects mucosal surfaces. It is one of the primary effectors of this system due to both its physical action (clearing and breaking up fungal and bacterial colonies) and its immunological (e.g., IgA secretion) and enzymatic (e.g., lysozyme, lactoperoxidase) mechanisms. It also has an important buffer effect that helps maintain pH levels at around 7.25. Hence, reducing and/or altering salivary flow is an important predisposing factor for oral candidiasis in both humans19,20 and animals.21
The presence of oral candidiasis was evaluated using clinical, histological and microbiological parameters. We compared the percentage of grossly visible lingual involvement, and the percentage of normal versus atrophic papillae in the histological specimens similar to Junquera et al.22 We did not perform a colony count, as other authors before,14,23,24 because isolation of Candida in the oral cavity of the rat is only indicative of carriage, not of clinical infection.
The higher incidence of lesions obtained in our study can, at least in part, be attributed to the Candida inoculation technique. In most studies, the inoculum is deposited in the entire oral cavity using a syringe or micropipette,25 due to rats cannot wash with mouth rinse, this suggests that much of the inoculum is spat out or swallowed by the rat. In contrast, we applied the inoculum to the dorsal surface of the tongue using a swab. The pressure exerted when rubbing the swab on the tongue favours penetration and retention of the inoculum between the lingual papillae. This prevents the rat from swallowing or spitting it out, and ensures that it is not lost during chewing. Moreover, although we used the same, or similar, volume of inoculum, it was distributed on a smaller surface (the dorsal tongue instead of the entire oral cavity), thus increasing the concentration of inoculum administered per surface area. We also avoided anaesthetic drug for colonization by Candida because according Takakura et al.14 the infection severity degree is correlated with the length of the sedated period.
We speculate that the high percentage of gross lesions obtained in our study from relatively few inoculations could be due to decreasing salivary flow by means of surgical sialoadenectomy, and using a strain isolated from an atrophic, clinically more virulent lesion.
The culture results of dorsal tongue samples obtained after 5 or 10 days of treatment differed according to the pharmacological treatment administered. For example, cultures from the groups treated with nystatin dissolved in water were 100% positive at both time points. Although the nystatin + orabase combination did not eliminate the pathogen at 5 days, it did succeed at 10 days in one of the rat models. Nystatin + chitosan showed greater efficacy compared to other excipients, obtaining negative cultures in 2 rats after 5 days of treatment, and in 4 rats after 10 days.
Gross examination of the dorsal tongue allowed us to determine the point at which the lesions appeared and their subsequent evolution over the study period. In all (100%) cases, the lesion was limited to the junction between the anterior 2 thirds and the posterior third of the tongue, which is characterised in normal conditions by the presence of dense, elongated papillae (giant conical papillae) that form a raised, whitish triangle.
The calculation of the affected tongue surface was expressed as a percentage of the total tongue surface area. We observed a significant reduction in the lesion area after 5 and 10 days of treatment.
As described above, the lesion is characterized histologically by destruction of papillae, hyper- or parakeratosis, acanthosis, chronic inflammatory infiltrate in underlying connective tissue, and pseudomycelia invasion of the superficial layers of the epithelium. These characteristics are similar to those found in the lingual candidiasis models developed by different research groups,7 and are accompanied by regenerative phenomena as the lesion evolves.
The aforementioned digital image analysis system was used to estimate the number and topographical distribution of the papillae per microscopic field in each sample (3 from each side of the tongue), measured from the anterior to the posterior edge of the tongue. Some decrease was observed, although differences were only significant in the case of chitosan. These differences could be attributed to our inability to accurately distinguish between papillae under destruction and in the process of regeneration using digital image analysis. Thus, an area that appears repaired under gross examination could in fact be populated with papillae in the regeneration phase, which are considered pathological because they have not reached normal length. This suspicion was confirmed to a large extent by microscopic examination of the histological samples. In short, the decrease in the number of normal papillae observed in the aforementioned groups could not be considered an indicator of greater disease severity.
The last parameter analysed was hyphal invasion of the stratum corneum. This was observed in all treatment groups, particularly in the junction between the anterior 2 thirds and the posterior third of the tongue, which is where the clinical lesions occur. The O'Grady and Reade10 penetration scale allowed us to quantify this invasion and observe a slight decrease in the depth of penetration at 5 and 10 days after the start of treatment. Treatment with nystatin in an aqueous solution significantly reduced the degree of hyphal invasion, with peak reduction occurring after 10 days. This decrease is greater when combined with orabase, and even more so with chitosan.
The best clinical and histological results were found in the group treated with nystatin + chitosan: 100% clinical cure at 5 and 10 days; 92% and 98.6% of papillae normalised at 5 and 10 days, respectively; grade 1 hyphal invasion in 50% of rats and grade 0 in the other 50% at 5 days, and grade 1 in 33.3% and grade 0 in 66.6% at 10 days. Culture was negative in 33.3% of rats after 5 days of treatment and in 66.6% after 10 days.
This improvement may be due to the intrinsic qualities of the biomaterial: low viscosity, low water absorption, and a positive charge that favours binding with the negatively charged surface of the mucosa.26
One limitation of our study is that hyphal penetration is associated with hyposalivation but also with many other factors, such as diet and, above all, the immune system. The data on hyphae penetration after 10 days of treatment on control group, highlight the host immune role in the regulation of candidiasis. Even so, we consider that the treatment can help to improve candidiasis in patients with any other physiological state.
Other limitation is the intrinsic characteristics of an experimental model in rats, although it has many advantages (as low maintenance cost, easy inoculation and sample collection), candidiasis has a multifactorial aetiology (hormonal origin, carbohydrates consumption, immunity state, etc.) and not only reduced saliva rates. Otherwise, this experimental model implies an easy tool to control and manipulate assays necessary to prove new treatments and improve Candidiasis knowledge.
In this experimental study, we have developed a homogeneous and reproducible model of oral candidiasis in Candida-free rats. The model has been shown to be effective in inducing infection, with clinical and histological evidence of lesions in found in all rats inoculated with C. albicans. All the parameters used for candidiasis evaluation, namely, culture of samples collected from the dorsal tongue, calculation of the affected tongue surface, and histological studies (percentage of normal papillae and hyphal invasion of the stratum corneum), show that the combination of nystatin and chitosan yielded the best therapeutic outcomes at both 5 and 10 days after infection.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Junqueira J.C., Vilela S.F.G., Rossoni R.D. Oral colonization by yeasts in HIV-positive patients in Brazil. Rev Inst Med Trop Sao Paulo. 2012;1:17–24. doi: 10.1590/s0036-46652012000100004. [DOI] [PubMed] [Google Scholar]
- 2.de Almeida V.L., Lima I.F.P., Ziegelmann P.K. Impact of highly active antiretroviral therapy on the prevalence of oral lesions in HIV-positive patients: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2017;11:1497–1504. doi: 10.1016/j.ijom.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Marukutira T., Huprikar S., Azie N. Clinical characteristics and outcomes in 303 HIV-infected patients with invasive fungal infections: data from the Prospective Antifungal Therapy Alliance registry, a multicenter, observational study. HIV AIDS (Auckl) 2014;6:39–47. doi: 10.2147/HIV.S53910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampath A., Weerasekera M., Dilhari A. Type 2 diabetes mellitus and oral Candida colonization: analysis of risk factors in a Sri Lankan cohort. Acta Odontol Scand. 2019;30:1–9. doi: 10.1080/00016357.2019.1607547. [DOI] [PubMed] [Google Scholar]
- 5.Nadig S.D., Ashwathappa D.T., Manjunath M. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J Oral Maxillofac Pathol. 2017;2:316. doi: 10.4103/jomfp.JOMFP_231_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa A., Pereira C.A., Junqueira J.C. Recent mouse and rat methods for the study of experimental oral candidiasis. Virulence. 2013;5:391–399. doi: 10.4161/viru.25199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Totti M.A.G., dos Santos E.B., de Almeida O.P. Oral candidosis by Candida albicans in normal and xerostomic mice. Braz Oral Res. 2004;3:202–207. doi: 10.1590/s1806-83242004000300005. [DOI] [PubMed] [Google Scholar]
- 8.Jorge A.O., Totti M.A., de Almeida O.P. Effect of sialoadenectomy on the carriage of Candida albicans in the mouths of rats. J Oral Pathol Med. 1993;3:138–140. doi: 10.1111/j.1600-0714.1993.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 9.Samaranayake Y.H., Samaranayake L.P. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001;2:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Grady J.F., Reade P.C. Role of thermal trauma in experimental oral mucosal Candida infections in rats. J Oral Pathol Med. 1993;3:132–137. doi: 10.1111/j.1600-0714.1993.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 11.Junqueira J.C. Models hosts for the study of oral candidiasis. Adv Exp Med Biol. 2012;710:95–105. doi: 10.1007/978-1-4419-5638-5_10. [DOI] [PubMed] [Google Scholar]
- 12.Solis N.V., Filler S.G. Mouse model of oropharyngeal candidiasis. Nat Protoc. 2012;4:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chami N., Chami F., Bennis S. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz J Infect Dis. 2004;3:217–226. doi: 10.1590/s1413-86702004000300005. [DOI] [PubMed] [Google Scholar]
- 14.Takakura N., Sato Y., Ishibashi H. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol. 2003;5:321–326. doi: 10.1111/j.1348-0421.2003.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 15.Núñez M.J., Novío S., Suárez J.A. Effects of psychological stress and fluoxetine on development of oral candidiasis in rats. Clin Vaccine Immunol. 2010;4:668–673. doi: 10.1128/CVI.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junqueira J.C., Martins J.S., Faria R.L. Photodynamic therapy for the treatment of buccal candidiasis in rats. Laser Med Sci. 2009;6:877–884. doi: 10.1007/s10103-009-0673-4. [DOI] [PubMed] [Google Scholar]
- 17.Farah C.S., Lynch N., McCullough M.J. Oral fungal infections: an update for the general practitioner. Aust Dent J. 2010;1:48–54. doi: 10.1111/j.1834-7819.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 18.Totti M.A.G., Santos E.B., Almedia O.P. Implantation and permanency of Candida albicans in the oral cavity of normal sialodenectomized mice after a single inoculation of yeast. Braz J Oral Sci. 2002;1:133–136. [Google Scholar]
- 19.Psianou K., Panagoulias I., Papanastasiou A.D. Clinical and immunological parameters of Sjögren's syndrome. Autoimmun Rev. 2018;10:1053–1064. doi: 10.1016/j.autrev.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Kroese F.G.M., Haacke E.A., Bombardieri M. The role of salivary gland histopathology in primary Sjögren's syndrome: promises and pitfalls. Clin Exp Rheumatol. 2018;3:222–233. [PubMed] [Google Scholar]
- 21.Ramírez I., Soley M. Submandibular salivary glands: influence on growth rate and life span in mice. J Physiol Biochem. 2011;2:225–233. doi: 10.1007/s13105-010-0067-x. [DOI] [PubMed] [Google Scholar]
- 22.Junqueira J.C., Colombo C.E.D., Martins J.S. Experimental candidosis and recovery of Candida albicans from the oral cavity of ovariectomized rats. Microbiol Immunol. 2005;3:199–207. doi: 10.1111/j.1348-0421.2005.tb03721.x. [DOI] [PubMed] [Google Scholar]
- 23.Martins J.S., Junqueira J.C., Faria R.L. Antimicrobial photodynamic therapy in rat experimental candidiasis: evaluation of pathogenicity factors of Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;1:71–77. doi: 10.1016/j.tripleo.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Borges Pereira A.C., Campos Rasteiro V.M., Hashimoto da Silva. Effect of erythrosine- and LED-mediated photodynamic therapy on buccal candidiasis infection of immunosuppressed mice and Candida albicans adherence to buccal epithelial cells. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;1:67–74. doi: 10.1016/j.oooo.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Conti H.R., Huppler A.R., Whibley N. Animal models for candidiasis. Curr Protoc Im. 2014;105:1–17. doi: 10.1002/0471142735.im1906s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collado-González M., González Espinosa Y., Goycoolea F.M. Interaction between chitosan and mucin: fundamentals and applications. Biomimetics. 2019;4:2. doi: 10.3390/biomimetics4020032. [DOI] [PMC free article] [PubMed] [Google Scholar]