Abstract
Introduction
Mesenchymal stem cells (MSCs) are promising therapeutic tools in regenerative medicine. In particularly adipose tissue derived MSC (AMSC) has powerful potential for the therapeutics of rheumatoid arthritis (RA) because these cells can control immune balance. RA systemically occurs autoimmune disease. Interestingly, IL-1 receptor antagonist deficient (IL-1ra−/−) mice induce inflammation in joints like RA. In RA therapy, although AMSC improves the inflammation activity, it is little known to play roles of extracellular microvesicles (EV) for improvement of RA. To clarify the MSC-derived EVs are involved amelioration mechanisms for RA by themselves, we examined the functional effects of development for RA by AMSC-EVs.
Methods
We isolated AMSCs derived mice adipose tissue and purified EVs from the culture supernatant of AMSCs. To examine whether EVs can improve RA, we administrated EVs or AMSCs to IL-1ra knockout mice as RA model mice. We analyzed EVs-included factor by western blot methods and RA improvement effect by ELISA.
Results
In this study, we showed that the swellings of joints on mice in wild type AMSC and that in AMSC-EVs decreased than that in IL-1ra−/− mice-AMSC-EVs and in none-treated. We detected IL-1ra expression in AMSC-EVs in wild type mice but not that in IL-1ra−/− mice. Proinflammatory cytokine expression changes in mice showed in AMSCs and AMSC-EVs, but no apparent differences cytokine expressions were detected in IL-1ra−/− mice.
Conclusions
In this study, we concluded that MSCs might improve RA by the transferring of factors such as IL-1ra, which are included their MSC derived- EVs.
Keywords: Adipose-derived mesenchymal stem cells, Extracellular vesicle, IL-1 receptor antagonist, Arthritis
Abbreviations: AMSC, Adipose-derived mesenchymal stem cell; EV, Extracellular vesicle; IL-1ra, IL-1 receptor antagonist; TNFα, Tumor Necrosis Factor α; IFNγ, Interferon γ; HE, Hematoxylin Eosin
Highlights
-
•
Adipose tissue derived MSC (AMSC)s improves arthritis.
-
•
AMSCs derived Extracellular vesicles (EVs) alone improve arthritis.
-
•
AMSCs derived EVs contain IL-1 receptor antagonist.
1. Introduction
Mesenchymal stem cells (MSCs) are promising therapy tools for a variety of human diseases. MSCs are isolated from several tissues, including bone marrow, synovium, umbilical cord, and adipose tissue [[1], [2], [3], [4], [5]]. These cells possess immunomodulatory and tissue repair properties and their use for the management of RA [6]. It has been reported that bone marrow-derived-MSCs improves Graft versus host disease (GVHD) by bone marrow transplantation and heart failure [7,8]. Furthermore, it is known that in autoimmune disease, MSCs lead to improve their symptoms [9,10]. Although the mechanism for autoimmune amelioration by MSCs is well studied [[11], [12], [13]], however, it has remained unclear about the therapeutic effects of EVs secreted from MSCs in RA.
EVs, which include exosomes, are extracellular vesicles with a size of about 50–1000 nm in diameter derived from a cell and play roles to intercellular communication vehicles for modulating or mediating processes [[14], [15], [16], [17], [18]]. EVs consist of the membrane of the original cell, and they include proteins, mRNAs, and microRNAs. These factors, which enclosed in EVs, and EV membrane components such as membrane surface molecules and lectins, are expected to apply for molecular target therapy for cancer or autoimmune diseases [19,20].
Furthermore, EV included exosomes are expected to use of drug delivery systems for anticancer reagents. We previously reported that the exosomes, which bearing of the breast cancer affinity molecules, and contained anticancer microRNA, induced to tumor suppression [21]. In this way, EVs are expected for biomaterials of therapeutics.
Interleukin (IL)-1 receptor antagonist (ra) is an inhibitor of IL-1, which is a proinflammatory cytokine that plays important roles in inflammation [[22], [23], [24], [25], [26]]. IL-1ra blocks to bind with IL-1 and its receptor and inhibits the secretion of proinflammatory cytokines such as IL-β, IL-6, and TNFα expression. Horai et al. reported that IL-1ra gene-deficient mice induce autoimmunity and joint-specific inflammation, such as RA [27].
Regenerative therapy for RA by MSCs improves inflammation on joints in RA animal models. In this study, we demonstrated that EVs, which derived AMSCs, involved in the RA improvement with AMSCs. EVs derived from AMSCs existed in their vesicles, and this factor suppressed the expression of inflammatory cytokines. From our results, we concluded that AMSCs might improve RA by secreting EVs from the cells, and these cells and EVs co-operatively replace immunosuppressed factors, and AMSCs and only EVs (cell-free) might be useful for RA regenerative therapy.
2. Materials and methods
2.1. Adipose tissue-derived mesenchymal stem cells (AMSCs) and EVs
AMSCs were purified from adipose tissue on abdomens in BALB/c mice (WT) or IL-1ra−/−one. And adipose tissues from mouse bodies were minced and digested with 5 μg/ml of collagenase type P (Sigma–Aldrich, MI, USA) for 1 h at 37 °C. After the cells were collected by centrifugation and culture in 20%FCS-Dulbecco MEM contained 100 U/ml of penicillin and 100 μg/ml of streptomycin, GlutaMax (Gibco-Thermo Fisher Scientific, MA, USA), Non-Essential Amino Acid (Gibco) for three days. The culture medium was removed, and the adherent cells were MSCs. The EVs were purified from the culture media of AMSCs from IL-1ra−/− mice or WT mice with ExoEasy Maxi kit (Qiagen, Hilden, Germany).
2.2. Electron microscope of microvesicles
The isolated EVs were collected by ultracenrifugation and EVs samples were absorbed onto glow discharged carbon-coated grids. The solution was blotted of and negatively stained with 4% ammonium molybdate. Micrographs were recorded with JEM 1200 EX II (JEOL Ltd., Tokyo, Japan) electron microscope.
2.3. Tracking analysis for microvesicles by Nanosight
Contents and size for the EVs were analyzed with NanoSight. The diluted samples from AMSCs were analyzed with NanoSight LM-10 HSBF (Malvern Panalytical, Malvern, UK).
2.4. Mice and arthritis animal models
For the RA model mouse, we used IL-1ra−/− BALB/c mice, which generated backcross to BALB/cA strain mice for eight generations. Then, heterozygous mice were intercrossed with each other to obtain homozygous mutant mice [27]. IL-1ra−/−/BALB/c mice and BALB/c mice of males from 8 weeks to 12 weeks old were purchased Nihon SLC Co. (Shizuoka, Japan).
Animal experiments in this study were by the Animal Experimentation Ethics Committee of Tokyo Medical University. 12-week old mice were the administration of 5 μg EVs per body or 1 × 106 AMSCs per body by tail vein weekly for four weeks. Analysis of swelling of hiding paws in mice measured the thickness of paws.
2.5. Histology
The hind paws of mice were removed from their bodies and fixed with 10% buffered formalin and incubated with decalcification solution, and then specimens were embedded paraffin wax. The sections were stained with hematoxylin and eosin for cartridges.
2.6. Cytokines assay
The levels of IL-1β, TNFα, INFγ and IL-1ra in murine sera were measured with Quantikine ELISA assay systems (R & D Systems, Inc.). The sera from mice were collected from blood in mice, which were received four weeks after the treatments with AMSCs or EVs. The assay was performed according to the manufacturer's instructions. For signal detection, we used with microplate reader iMark (Bio-Rad Co. Hercules, CA, USA).
2.7. Western blot analysis
The lysates of AMSCs and AMSC-derived EVs were extracted with CelLytic (Sigma Aldrich). The protein samples were suspended in sodium dodecyl sulfate (SDS) loading buffer. After boiling, the 10 μg of protein was run on 15% SDS–polyacrylamide gel electrophoresis gels and then transferred to Immobilon membranes (Millipore, Bed-ford, MA, USA) by semidry blotting. The membranes were probed with anti-IL-1 receptor antagonist clone #694204 (R & D Systems, Minneapolis, MN, USA) or anti-CD63 (Abiocode, Agoura Hills, CA, USA) antibodies using standard techniques. The signals were visualized using the ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ, USA) and detected on LAS-3000 mini (Fujifilm Co., Tokyo, Japan).
2.8. Evaluation of arthritis
In the assessment of improvement for RA, the swelling of hind paws in mice was measured by a scale at the time after the 4th treatment.
2.9. Statistics
Statistics were evaluated with student t-test analysis, and all experiments were done three times, independently.
3. Results
3.1. AMSCs and EVs
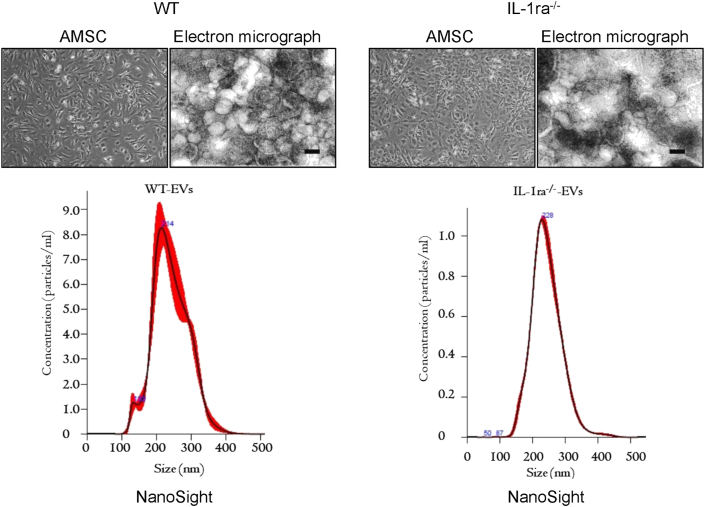
AMSCs were isolated from adipose tissue on the abdomen in BALB/c wild type mice or IL-1ra−/−BALB/c mice. At three days after the initiating culture, adherent cells on plates were maintained as MSCs. The morphology of the adherent cells was spindle shape like fibroblasts (Fig. 1). EVs were isolated from the culture media containing 20% FCS-excluded-vesicles both AMSCs in WT mice and IL-1ra−/− mice. The purified EVs from AMSC derived IL-1ra−/− mice or WT mice were no differences in size and numbers in both. Nano tracking analysis data showed that the size of EVs derived from WT and IL-1ra−/− cells are 218 nm and 230 nm in diameter, respectively. Moreover, electron microscopic data revealed the size was about 200 nm in diameter of the vesicles derived both cells (Fig. 1). In this study, we obtained 30 μg of EVs protein amount from adipose tissue one mouse.
Fig. 1.
AMSCs and EVs derived from mouse adipose tissues. The left in the upper panels are the morphology of Adipose-derived mesenchymal stem cells (AMSC)s derived from the Wild type of mice (BALB/c; WT) and IL-1ra deficient (IL-1ra−/−) mice. AMSCs are cells attached to a culture dish. The morphologies are at six days after the beginning of their culture. The light in the upper panels are the electron micrograph of the extracellular vesicle (EV)s by negative staining. White particles in the pictures are EVs. The size bar in the pictures is 200 nm. The lower panels are the data for the size and distribution of EVs by nanoparticle tracking analysis with Nano Sight.
3.2. RA model mouse and therapeutics by AMSC-derived EVs
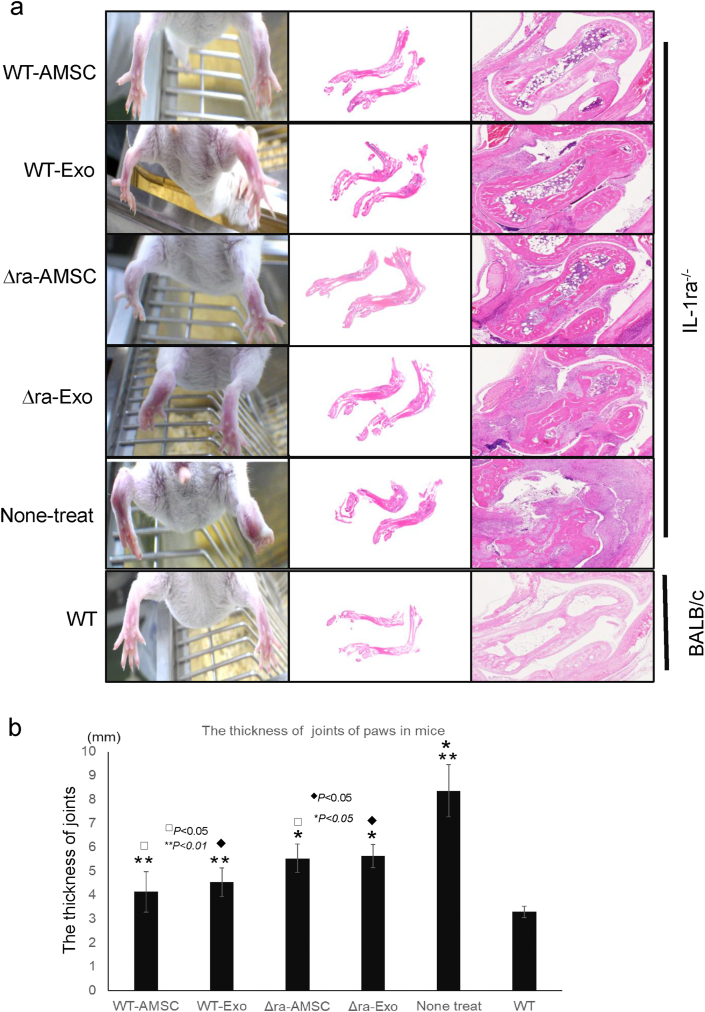
IL-1ra−/− mouse naturally appears swelling, such as RA in hind paws at 8–10 weeks old. In first, whether AMSC-derived EVs were involved in the RA improvement, we examined the evaluation for RA ameliorates using IL-1ra−/− mice by AMSC-EVs. AMSCs possess the potential for cartilage repair in mice, but the EVs unclear remain about the functions and mechanisms to therapy of RA. To characterize the effect of the improvement of therapy for RA by the EVs derived from AMSCs, we tried to effect inhibition for swelling of hind paws by the administration of the EVs into tail vein in mice. The thickness of joins in AMSC-derived EVs and IL-1ra−/− mice-derived EVs was thinner than non-treated mice (Fig. 2). And histological analysis of tissues from hind paws in mice revealed prominent synovial hyperplasia, with some pannus formation in the ankle in None-treat mice (Fig. 2a). The histological findings of cartilage tissues on paws in mice showed the cartilages of their ankles in WT-AMSC, and WT-EVs increased than in IL-1ra−/− --AMSCs, IL-1ra−/− EVs or none-treat (Fig. 2a). And the thickness of joins in WT-AMSC and -EVs decreased than in IL-1ra−/− -AMSC or -EVs (Fig. 2b).
Fig. 2.
AMSCs and EVs improve arthritis of joints in mice. The hind paws of IL-1ra−/− mice, which were administrated AMSCc or EVs, are shown in pictures. The sections of hind paws at a week after the last administration were stained with hematoxylin and eosin. The right panel shows histological appearance of synovial membrane and cartridge surface in mice (a). The thickness of joints of paws in mice was measured a week after the last administration (b). Data were means ± SEM (n = 10 to 15). P-value was determined by unpaired two-tailed student's t-test.
3.3. Inflammatory cytokines expression changes
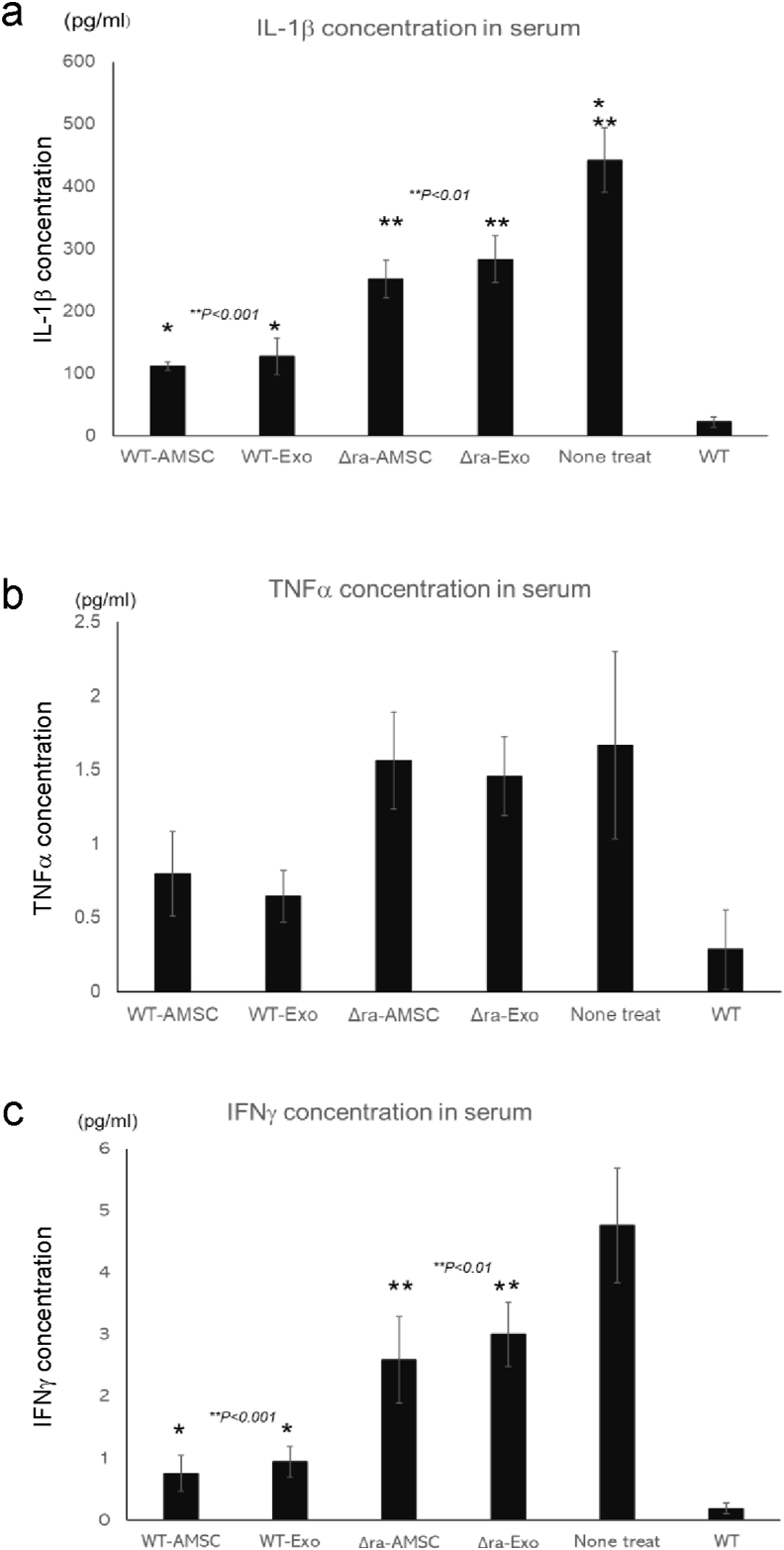
Because inflammatory cytokine concentrations in sera of IL-1ra−/− mice, which possess the swelling on paws, are at high levels, whether AMSC and EVs can affect the cytokine expression. So, we performed inflammatory cytokine assay in the sera from IL-1ra−/− mice, which were the treatment of AMSC or their EVs. IL-1β and IFNγ, which is one of the proinflammatory cytokines, expression in WT-AMSCs and WT-EVs groups than in IL-1ra−/− -AMSC or -EVs (Fig.3a, c). The data of TNFα concentration in sera showed the low expressions in WT-AMSC and -EVs than in IL-1ra−/− -AMSC or -EVs (Fig. 3b).
Fig. 3.
AMSCs and EVs suppress the secretion of inflammatory cytokines in arthritis mice. Inflammatory cytokines concentrations in sera of AMSCs or EVs administrated mice. Data were means ± SEM (n = 5) (a: IL-1β, b: TNFα, c: IFNγ).
3.4. EVs contain RA recovery factor
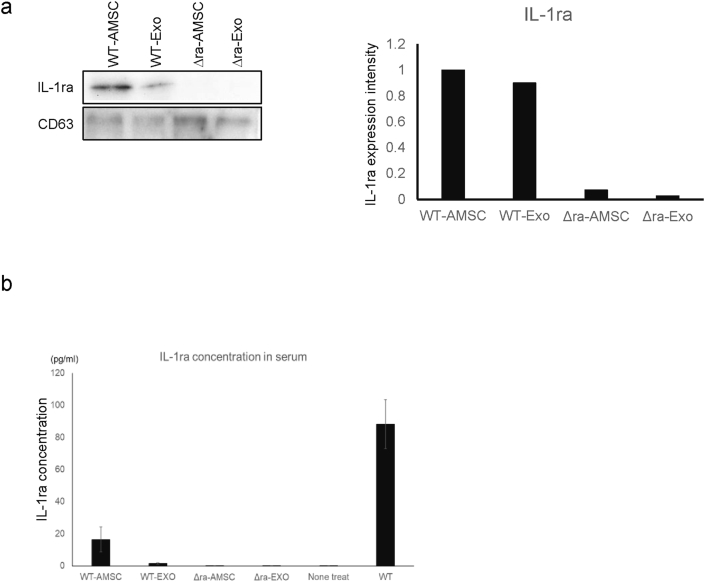
The improvements for inflammatory in mice paws have seemed in AMSC, and EVs derived wild type mice. Because the difference between wild type mouse and IL-1ra−/− mice is the expression of IL-1ra, we examined whether IL-1ra−/− -EVs contain IL-1ra in their vesicles by western blot with IL-1ra specific antibody. The data showed the band was detected in WT-AMSC and -EVs, but not recognized it in IL-1ra−/− -AMSC and -EVs (Fig. 4a). In the next, we tried to assay IL-1ra concentration in the sera of the administrated mice with ELISA. The data revealed that we could not detect IL-1ra in IL-1ra−/− -AMSC and -EVs, on the other hand, we recognized it in WT-AMSC and -EVs (Fig. 4b).
Fig. 4.
Adipose tissue-derived AMSCs and EVs include IL-1ra. a, Western blot for IL-1ra of AMSCs and EVs are shown. CD63 was used as the loading control of the samples. IL-1ra expression in each sample normalized IL-1ra expression intensity/CD63 expression intensity and then the expression intensity of IL-1ra revealed the intensity of WT-AMSC as 1. b, IL-1ra concentration in sera of AMSCs or EVs administrated mice.
From these results, we concluded that the inflammatory improvement for RA model mouse by AMSC was concerned with EVs- derived from AMSCs.
4. Discussions
MSCs are promising of therapeutics for autoimmune disease, cancer, and wound because these cells possess the functions of differentiation for osteocytes, adipocytes, or chondrocytes and mediation to a broad spectrum of immunoregulatory activity and dampen innate and adaptive immune responses [[28], [29], [30], [31]]. And for RA, clinical trials of regenerative medicine applied MSCs by animal model experiments showed effective inflammatory suppression [[32], [33], [34]]. MSCs are established derived from bone marrow, adipose tissue, and other tissues. Adipose-derived MSCs are contained lots of stem cells in the tissue than in bone marrow [35]. In this time, we obtained the preparations of AMSCs derived from adipose tissue in mice. We established AMSCs derived from two types of origin mice, which were IL-1ra gene deficiency or not. No apparent differences in the two kinds of AMSCs were in the morphology of cell (Fig. 1) and cell proliferation. These AMSCs secreted the same size of nano microvesicles in their culture media (Fig. 1). From the data of nano tracking analysis and electron microscopic, we thought these vesicles as EVs. EVs are nano-size in the diameter of microvesicles and with double lipid membrane that consists of some factors, such as mRNAs, miRNAs, and proteins like enzymes in their vesicles. These contents of EVs are thought to play critical roles in the improvement of disease and wound with regenerative therapy.
In this study, we examined whether EVs concerned the suppression of the inflammatory reaction of RA, not only MSCs but also EVs. MSCs can suppress inflammatory responses in murine RA models, so these cells are promising of rheumatoid therapy. We examined the efficiency of RA with AMSCs derived murine adipose tissue. The swellings of joint on mice reduced in AMSCs treated mice (Fig. 1). Our AMSCs possess the potentials for the suppression of inflammatory reaction the same as other MSCs. Next, whether our AMSCs derived EVs can influence the inflammatory suppression of arthritis, we tried to treat the EVs into the RA model. Our data showed that only EVs treatment, not with AMSCs, could decrease the swelling of paws on RA model mice (Fig. 2). Because AMSCs express IL-1ra in cells, the administration of these cells into the IL-1ra deficient mouse reduces the swelling of hind paws in this mouse. We considered that if the EVs derived from WT-AMSCs have IL-1ra in their vesicles, it will get the same results as the treatment of WT-AMSC to the IL-1ra−/− RA model mice. These results showed the reduced swelling of hind paws with WT-EVs same well as WT-AMSCs. Therefore, we thought that WT-EVs possess the factors which can improve RA. The western bolt analysis data revealed that the IL-1ra band was detected in not only WT-AMSCs but also in WT-EVs, on the other hand, not identified in IL-1ra −/− WT-AMSCs and lL-1 ra −/− EVs. From these results, we considered that the inflammatory improvement of RA by AMSC was involved with the factor- derived from AMSC not only but from their EVs also. Indeed, in IL-1ra −/− AMSCs and their EVs did not show the data which were demonstrated, such as wild type cells and their EVs.
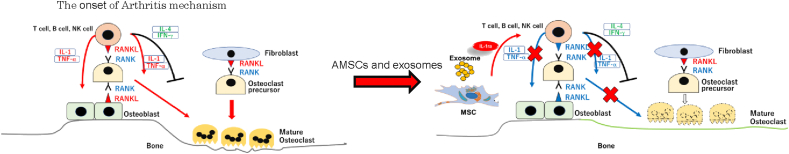
IL-1ra, which is one of the inflammatory cytokine members, blocks the binding of IL-1α or -β to their ligand, and represses the activation of IL-1 receptor by the interleukins [23]. So, we examined to clarify whether the IL-1ra, which were derived from AMSCs and their EVs, involve inhibiting the inflammatory signals in IL-1ra deficient mice. IL-1ra at a low level or the inadequate induces activation of IL-1 receptor signaling by binding of IL-1α or -β and then, the low levels of these cytokines in serum on mice. Our ELISA data for cytokines in sera on mice, which were the treatment of WT-AMSCs and their EVs, showed IL-1-β concentration was lower compared with lL-1 ra−/−-AMSCs and their EVs. Our data demonstrated that WT-EVs possess the effect of the suppression of inflammatory activation the same as WT-AMSCs. After the low level of IL-1ra concentration induces the activation of IL-1 signaling, and then, activated T cells, and B cells are caused. And the activated lymphocytes secrete IFNγ and inflammatory cytokines, and these cytokines make change RANKL (receptor activator of nuclear factor-kappa B ligand) to the form [[36], [37], [38], [39], [40], [41]]. Activated RANK binds to RANKL on osteoclasts and leading to maturation of osteoclasts. In this study, we confirmed that IFNγ and IL-1β were secreted at a low level by IL-1ra, which derived AMSCs and EVs, so we concluded that AMSCs and EVs induced immunosuppressive for inflammatory reaction in RA model mice (Fig. 5).
Fig. 5.
AMSCs- and EVs- included IL-1ra improve arthritis. AMSCs- and EVs- included IL-1ra suppresses IL-1 and TNFα secretions. Inflammatory cytokines depletion induces the inactivation of osteoclasts.
Moreover, we evaluated whether AMSC-derived EVs play a role in the improvement of RA with AMSCs. Our data revealed that EVs possessed the immunosuppressive factors in their vesicles the same as AMSCs. WT- EVs alone treatment to RA mice induced the suppression of IL-1β and going down for swelling of paws on mice. In regenerative therapy, it has to resolve the problems to the implementation for clinical treatment because iPS cells and ES cells develop to tumor formation such as teratoma in vivo. On the other hand, because it is known that MSCs are not a formation of tumor in vivo, MSCs are superior to iPS cells and ES cells in safety for tumorigenesis [42]. Although it is thought that MSCs are no problems for regenerative medicine, the modified MSCs with miRNAs or gene transformed maybe change their characters. Therefore, EVs are useful for cell-free therapy. EV-derived MSCs are no difficulties that need to be resolved for tumorigenesis of cells because EVs are not growth or proliferation themselves. Recently it was reported that EVs are possible to use as cargo for nuclear drugs such as mRNA or miRNA for the anticancer reagent to delivery systems in cancer therapy. Previously, we reported that the exosomes bearing the peptide, which affinity to breast cancer cell membrane-molecules, were delivered anticancer miRNA to breast cancer cells. And the modified exosomes, which encoded anticancer miRNA, suppressed breast cancer cells [21].
Because in this study, we demonstrated that only EVs without AMSCs played roles useful to immunosuppression for RA, it may be a possibility of cell-free treatment with EVs (Fig. 2).
According to the western blot data, IL-1ra existed on EVs derived from AMSCs (Fig. 4a). Therefore, IL-1ra replacement by AMSCs or EVs in RA model mice serves as an improvement of their severe status in RA.
It is known that the diseases which cause the low level or dysfunction of IL-1ra are RA, psoriasis, autoimmune, and cardiovascular disease [5,43,44]. In the past two decades, the recombinant IL-1ra, which called anakinra, is applied for trial to therapy of these diseases. RA has approved anakinra to treatment [4]. In this study, we obtained the same data by the treatment of AMSCs and EVs. Because anakinra, a recombinant IL-1 receptor antagonist, has anti-inflammatory actions and used in the therapy of RA and inflammatory arthritis, anakinra is associated with a low rate of enzyme elevations. But because anakinra is a recombinant drug, it is unknown whether it's stable of this reagent in vivo. Moreover, it needs daily treatment for therapy by anakinra, but in our study, we treated with AMSCs and EVs by a weekly in RA model mice. From this fact, we consider that IL-1ra in vivo, which is included AMSCs and EVs, are stable compared with anakinra.
In this study, we concluded that EVs derived from AMSCs contain IL-1ra in their vesicles.
Authors' contributions
M.T and S.K designed this study.
K.T, M.T, K.S, A.I, S.M, K.K, and S.U examined and analyzed data.
K.T, M.T, and M.K wrote the manuscript.
Declaration of competing interest
The authors declare no conflicts of interest in association with the present study.
Acknowledgments
This research was partially supported by the Japan Society for the Promotion of Sciences; Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan, Grant number: 19K08382 and 17H04067.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Campagnoli C., Roberts I.A., Kumar S., Bennett P.R., Bellantuono I., Fisk N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 2.Lee O.K., Kuo T.K., Chen W.M., Lee K.D., Hsieh S.L., Chen T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 3.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens M., Singh J.A. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36(6):1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 5.Arend W.P. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4–5):323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez M.A., Gonzalez-Rey E., Rico L., Buscher D., Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60(4):1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 7.Baron F., Lechanteur C., Willems E., Bruck F., Baudoux E., Seidel L. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2010;16(6):838–847. doi: 10.1016/j.bbmt.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Introna M., Lucchini G., Dander E., Galimberti S., Rovelli A., Balduzzi A. Treatment of Graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. 2014;20(3):375–381. doi: 10.1016/j.bbmt.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Yanez R., Lamana M.L., Garcia-Castro J., Colmenero I., Ramirez M., Bueren J.A. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cell. 2006;24(11):2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 10.Sun L., Akiyama K., Zhang H., Yamaza T., Hou Y., Zhao S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cell. 2009;27(6):1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Wang L., Cong X., Liu G., Zhou J., Bai B. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cell Dev. 2013;22(24):3192–3202. doi: 10.1089/scd.2013.0023. [DOI] [PubMed] [Google Scholar]
- 12.Liu X.J., Zhang J.F., Sun B., Peng H.S., Kong Q.F., Bai S.S. Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-beta and interleukin-6. Clin Exp Immunol. 2009;158(1):37–44. doi: 10.1111/j.1365-2249.2009.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang J., Zhang H., Hua B., Wang H., Lu L., Shi S. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69(8):1423–1429. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 14.Simpson R.J., Lim J.W., Moritz R.L., Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6(3):267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 15.Kramer-Albers E.M., Hill A.F. Extracellular vesicles: interneural shuttles of complex messages. Curr Opin Neurobiol. 2016;39:101–107. doi: 10.1016/j.conb.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Olaizola P., Lee-Law P.Y., Arbelaiz A., Perugorria M.J., Bujanda L. MicroRNAs and extracellular vesicles in cholangiopathies. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1293–1307. doi: 10.1016/j.bbadis.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Saha B., Momen-Heravi F., Kodys K., Szabo G. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem. 2016;291(1):149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiera G., Di Liegro C.M., Di Liegro I. Extracellular membrane vesicles as vehicles for brain cell-to-cell interactions in physiological as well as pathological conditions. BioMed Res Int. 2015;2015:152926. doi: 10.1155/2015/152926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhome R., Goh R.W., Bullock M.D., Pillar N., Thirdborough S.M., Mellone M. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging (Albany NY) 2017;9(12):2666–2694. doi: 10.18632/aging.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo N., Akiyoshi K., Shiku H. Exosome-mediated regulation of tumor immunology. Canc Sci. 2018;109(10):2998–3004. doi: 10.1111/cas.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno S., Takanashi M M., Sudo K., Ueda S., Ishikawa A., Matsuyama N. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassoy E.Y., Towne J.E., Gabay C. Regulation and function of interleukin-36 cytokines. Immunol Rev. 2018;281(1):169–178. doi: 10.1111/imr.12610. [DOI] [PubMed] [Google Scholar]
- 23.Gabay C., Lamacchia C., Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6(4):232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 24.Palomo J., Dietrich D., Martin P., Palmer G., Gabay C. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76(1):25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Weber A., Wasiliew P., Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3(105):cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 26.Yazdi A.S., Ghoreschi K. The interleukin-1 family. Adv Exp Med Biol. 2016;941:21–29. doi: 10.1007/978-94-024-0921-5_2. [DOI] [PubMed] [Google Scholar]
- 27.Horai R., Asano M., Sudo K., Kanuka H., Suzuki M., Nishihara M. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187(9):1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassano J.M., Schnabel L.V., Goodale M.B., Fortier L.A. Inflammatory licensed equine MSCs are chondroprotective and exhibit enhanced immunomodulation in an inflammatory environment. Stem Cell Res Ther. 2018;9(1):82. doi: 10.1186/s13287-018-0840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miteva K., Pappritz K., Sosnowski M., El-Shafeey M., Muller I., Dong F. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of Coxsackievirus B3-induced inflammatory cardiomyopathy. Sci Rep. 2018;8(1):2820. doi: 10.1038/s41598-018-20686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X., Hao H., Han Q., Song X., Liu J., Dong L. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8(1):241. doi: 10.1186/s13287-017-0668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volarevic V., Gazdic M., Simovic Markovic, B., Jovicic N., Djonov V., Arsenijevic N. Mesenchymal stem cell-derived factors: immuno-modulatory effects and therapeutic potential. Biofactors. 2017;43(5):633–644. doi: 10.1002/biof.1374. [DOI] [PubMed] [Google Scholar]
- 32.Ichiseki T., Shimazaki M., Ueda Y., Ueda S., Tsuchiya M., Souma D. Intraarticularly-Injected mesenchymal stem cells stimulate anti-inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate-induced rat arthritis model. Int J Mol Sci. 2018;19(1) doi: 10.3390/ijms19010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kay A.G., Long G., Tyler G., Stefan A., Broadfoot S.J., Piccinini A.M. Mesenchymal stem cell-conditioned medium reduces disease severity and immune responses in inflammatory arthritis. Sci Rep. 2017;7(1):18019. doi: 10.1038/s41598-017-18144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R., Zhang Y., Zheng X., Peng S., Yuan K., Zhang X. Synergistic suppression of autoimmune arthritis through concurrent treatment with tolerogenic DC and MSC. Sci Rep. 2017;7:43188. doi: 10.1038/srep43188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blazquez-Prunera A., Diez J.M., Gajardo R., Grancha S. Human mesenchymal stem cells maintain their phenotype, multipotentiality, and genetic stability when cultured using a defined xeno-free human plasma fraction. Stem Cell Res Ther. 2017;8(1):103. doi: 10.1186/s13287-017-0552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotake S., Nanke Y., Mogi M., Kawamoto M., Furuya T., Yago T. IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35(11):3353–3363. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 37.Lee E.Y., Seo M., Juhnn Y.S., Kim J.Y., Hong Y.J., Lee Y.J. Potential role and mechanism of IFN-gamma inducible protein-10 on receptor activator of nuclear factor kappa-B ligand (RANKL) expression in rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R104. doi: 10.1186/ar3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ota Y., Niiro H., Ota S., Ueki N., Tsuzuki H., Nakayama T. Generation mechanism of RANKL(+) effector memory B cells: relevance to the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2016;18:67. doi: 10.1186/s13075-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romas E., Gillespie M.T., Martin T.J. Involvement of receptor activator of NFkB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30(2):340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 40.Takayanagi H., Sato K., Takaoka A., Tanioguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005;208:181–193. doi: 10.1111/j.0105-2896.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 41.Udagawa N., Kotake S., Kamatani N., Takahashi N., Suda T. The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res. 2002;4(5):281–289. doi: 10.1186/ar431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang G., Miao Y.L., Zhang Y., Liu S., Kou Z., Ding J. Linking incomplete reprogramming to the improved pluripotency of murine embryonal carcinoma cell-derived pluripotent stem cells. PloS One. 2010;5(4) doi: 10.1371/journal.pone.0010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertani I., Iori V., Trusel M., Maroso M., Foray C., Mantovani S. Inhibition of IL-1beta signaling normalizes NMDA-dependent neurotransmission and reduces seizure susceptibility in a mouse model of creutzfeldt-jakob disease. J Neurosci. 2017;37(43):10278–10289. doi: 10.1523/JNEUROSCI.1301-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckley L.F., Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39(22):2063–2069. doi: 10.1093/eurheartj/ehy128. [DOI] [PubMed] [Google Scholar]