Abstract
Objective
This study aimed to investigate effects of TGF-β1-containing exosomes derived from bone marrow mesenchymal stem cells (BMSC) on cell function of rotator cuff tenocytes and its implication to rotator cuff tear.
Methods
The primary BMSC and rotator cuff tenocytes were extracted and cultured. Identification of BMSC were performed by observing cell morphology and measurement of surface biomarkers by flow cytometry. BMSC-derived exosomes were extracted and identified by using electron microscopy, nanoparticle-tracking analysis (NTA) and western blotting. Cell proliferation and cell cycle were measured by CCK-8 assay and flow cytometry assay, respectively. Transwell assay was used for detection of tenocytes migration. The fibrotic activity of tenocytes was determined via qPCR and western blotting assays.
Results
BMSC and BMSC-derived exosomes were successfully extracted. Treatment of BMSC-derived exosomes or TGF-β1 promoted cell proliferation, migration and increased cell ratio of (S + G2/M) phases in tenocytes, as well as enhanced the expression levels of fibrotic activity associated proteins. However, inhibition of TGF-β1 by transfection of sh-TGF-β1 or treatment of TGFβR I/II inhibitor partially reversed the impact of BMSC-derived exosomes on tenocytes function.
Conclusion
Taken together, TGF-β1-containing exosomes derived from BMSC promoted proliferation, migration and fibrotic activity in rotator cuff tenocytes, providing a new direction for treatment of rotator cuff tendon healing.
Keywords: BMSC, Exosomes, TGF-β1, Rotator cuff tear, Proliferation, Migration, Fibrotic activity
Abbreviations: BMSC, Bone mesenchymal stem cells; TGF-β1, Transforming growth factors β1; TGF-βR I/II, Transforming growth factors β1 receptor type I/II; qPCR, Quantitative reverse-transcription polymerase chain reaction; FBS, Fetal bovine serum; DMEM, Dulbecco's modified Eagle's medium; SDS-PAGE, Sodium dodecyl sulfate polyacrylamide gel electrophoresis; Col I, Collagen I; Col III, Collagen III; α-SMA, α-smooth muscle actin; Scx, Scleraxis; Tnc, Tenascin C; PVDF, Polyvinylidene fluoride; CCK8, Cell counting kit-8; Smad7, Mothers against decapentaplegic homolog 7
1. Introduction
Rotator cuff tear is a commonly clinical problem in elderly patients, which can cause either acute or chronic tear condition in rotator cuff tear as well as other tissue injuries [1,2]. Since there are a lot of healing failure factors which increases repair failure risk, such as tendon degeneration, tear size and patients age [[3], [4], [5]], chronic rotator cuff tear injury is still difficult to heal and easy to recur. Thus, to find novel potential treatment strategies for rotator cuff tear is of great significance.
Tenocytes possessed typically quiescent, non-dividing feature and are reported to be involved in rotator cuff disease [6,7]. It was found tenocytes proliferation and migration took part in tendon repair [8]. In recent years, novel repair technologies are explored in order to reduce the repair failure rate of rotator cuff tear [9,10]. However, the post-surgery rotator cuff re-tear from poor tendon bone quality is not eradicated, so this challenge clinical problem is urgent to be solved.
Some reports demonstrated that engineered mesenchymal stem cells (MSC) can reduce rotator cuff re-tear. It was found after pioglitazone pretreatment, the healing effect of MSC on tendon repair was increased [11]. Another study also showed MSC with overexpression of VEGFA had a positive function on tendon-bone healing of rotator cuff tear [12]. Moreover, exosomes mediated intercellular communication and biomolecules transporter (protein, DNA and RNA families) function as crucial regulators in multiple biological processes. MSC-derived exosomes could target inflammatory cells by suppressing inflammatory mediators and NLRP3 inflammasome activation [13]. It was also reported that lipopolysaccharide (LPS)-induced acute lung injury was inhibited by MSC-exosomes with overexpression of miR-30b-3p [14]. Besides, some reports demonstrated that MSC exosomes have potential function in tissue regeneration [15]. However, there is little report on bone marrow mesenchymal stem cell (BMSC)-derived exosomes targeting rotator cuff tenocytes.
Transforming growth factor β1 (TGF-β1) is an important cytokine in cell activities and participates in the regulation of healing of tendon tear [16]. Furthermore, studies demonstrated TGF-β1 could promote tenocytes proliferation [17]. However, the role of TGF-β1 in tenocytes migration and fibrotic activity is not supported by sufficient roofs.
In the present study, we firstly demonstrated that BMSC derived exosomes with rich TGF-β1 might regulate tenocyte migration and fibrotic activity as well as tenocytes proliferation. The results might provide novel insights on rotator cuff tear healing therapy.
2. Materials and method
2.1. Animals and primary isolation and culture of rat tenocytes
Rotator cuff tendons were obtained from healthy Sprague Dawley (SD) rats purchased from Shanghai SLAKE Experiment Animal Limited Company (Shanghai, China). Briefly, all animals were kept in a light-controlled room under a 12 h/12 h light/dark cycle and controlled temperature (23–25 °C), and had free access to food and water according to the Guide for the Care and Use of Laboratory Animals. The experiments were approved by the Institutional Animal Care and Use Committee of Beijing Friendship Hospital, Capital Medical University.
After obtaining the rotator cuff tendons, tendons were cut into small pieces of 2–3 mm3, distributed into six-well culture plates, followed by addition of 2.5 ml Dulbecco's modified Eagle's medium (DMEM, Gibco, Gaithersburg, MD, USA) with 10% FBS (Hyclone, Logan, UT). The culture plate was placed in a humidified 5% CO2 incubator at 37 °C for 5 days. After tenocytes are trypsinized, the tenocytes were cultured in DMEM with 10% FBS at 5% CO2 and 37 °C, the cells from passage 3 were used in this study.
2.2. Isolation and identification of BMSC
BMSC were isolated from femurs by flushing the femurs and tibias of the rats with D-Hanks' solution. The cells were placed in low-glucose DMEM supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. 24 h after plating, non-adherent cells are removed by replacing the medium. When the BMSC conform 70%–80% confluence, BMSC were split (0.05% trypsin/EDTA) and further enriched by passage cultures. Cells from passage 3 were used in this study. For identification of BMSC, surface markers of CD44, CD90, CD45 and CD34 were characterized by flow cytometry analysis as described previously [11].
2.3. Cell transfection
To silence the expression of TGF-β1, short hairpin RNA against TGF-β1 (sh-TGF-β1) and the negative control shRNA (sh-NC) were purchased from RiboBio, Guangzhou, China. Cells were transfected with sh-TGF-β1 (5 nM) or sh-NC (5 nM) by using Lipofectamine 3000 (Invitrogen, Waltham, MA, USA). The transfection efficacy was evaluated by quantitative real-time PCR (qPCR) assay after 48 h of transfection.
2.4. Extraction and identification of BMSC-derived exosomes
For extraction of BMSC-derived exosomes, after 48 h of transfection, the supernatant was collected, centrifuged and resuspended in 200 μl PBS as described elsewhere [18]. After fixed with 2% paraformaldehyde and loaded on parafilm, the exosome samples were observed under a transmission electron microscopy (TEM; JEM-1400PLUS, Japan) at 100 KV and the nanoparticle-tracking analysis (NTA) software was used to evaluate the size of exosomes.
A BCA protein assay kit (Beyotime, China) was used to measure the protein concentration of the exosomes. The identification of exosomes was performed by Western blot assay for analysis of exosome markers CD9, CD63, CD81 and TSG101. Briefly, exosome proteins were separated by 10% SDS-PAGE, transferred onto PVDF membrane and then incubated with primary antibodies of anti-CD9 (ab92726, Abcam) and anti-CD63 (ab108950, Abcam), anti-CD81 (ab109201, Abcam) and anti-TSG101 (ab125011, Abcam) at 4 °C overnight, following with incubation of corresponding secondary antibodies at 37 °C for 45 min. Proteins were visualized on an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE). For treatment of tenocytes, the extracted exosomes (20 μg/ml) were added into the plates and the control cells were untreated.
2.5. CCK-8 assay
The proliferation of tenocytes was determined using CCK-8 assay. Briefly, tenocytes (1 × 104 cells/well) were cultured overnight in 96-well plates. After treatment, cells were treated with 10 μl of CCK-8 reagent (Dojindo, Japan) for 4 h at room temperature. Then absorbance was recorded on a microplate reader at a wavelength of 450 nm.
2.6. Cell cycle analysis
For cell cycle analysis, the tenocytes were digested by trypsin, washed with PBS, and fixed in 70% ethanol. Then, fixed cells were stained using PI (20 μg/ml; BD Biosciences, MA, USA) for 20 min according to manufacturer's instruction. The cell cycle was analyzed with a FACS Canto flow cytometer (BD Biosciences, USA).
2.7. Transwell assay
For analysis of cell migration, the cell transwell assay was conducted. Briefly, 1 × 104 cells in 0.2 ml free-serum DMEM medium were seeded into the insert upper-chamber with the non-coated membrane (24-well insert; pore size 8 μm, Corning, USA). Cells were cultured in serum-free media for 24 h, stained with 0.1% crystal violet, and then counted and photographed.
2.8. qPCR assay
The expression levels of Collagen I (Col I), Collagen III (Col III), α-smooth muscle actin (α-SMA), Scleraxis (Scx), Tenascin C (TnC) and TGF-β1 were determined by qPCR. Briefly, Cell sample RNAs were extracted using TRIzol reagent (Tiangen Biotech, Beijing, China). Then RNA was converted to cDNA using a Prime-Script™ One Step RT-qPCR kit (Takara Biotechnology Co., Ltd., Dalian, China). PCR reaction was performed using SYBR Green Master Mix (Solarbio Science & Technology Co., Ltd., Beijing, China) in an Applied Biosystems 7500 Real Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). GAPDH was used as the internal control. The relative expression level was calculated by the 2−ΔΔCt method.
2.9. Western blot assay
Western blot assay was conducted for determination of Col I, Col III, α-SMA, Scx, TnC and TGF-β1. Similar to the procedure of determine of exosome proteins, samples were separated with 10% SDS-PAGE, transferred onto a PVDF membrane, blocked by non-fat milk for 1 h at room temperature and incubated with the following primary antibodies at 4 °C overnight: anti-β-actin (ab8227, Abcam) anti-Col I (ab34710, Abcam), anti-Col III (ab7778, Abcam), anti-α-SMA (ab32575, Abcam), anti-Scx (ab58655, Abcam) and anti-TnC (ab108930, Abcam), TGF-β1 (ab92486, Abcam). The samples were then incubated with corresponding secondary antibody (Goat Anti-Rabbit IgG H&L, ab205718, Abcam) at 37 °C for 45 min. β-actin was used as the internal control. Protein brands were visualized by Super Signal West Pico Chemiluminescent Substrate kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and the ImageJ software (Rasband; NIH, USA) was used for quantification of the bands to assess relative proteins expression.
2.10. Statistical analysis
Experiments were performed in triplicate. Statistical differences were analyzed by Student's t-test for two groups and one-way analysis of variance (ANOVA) followed by Tukey's correction for multiple comparisons using GraphPad Prism software (GraphPad Software, California).
3. Results
3.1. Identification of phenotype in BMSC and BMSC-derived exosomes
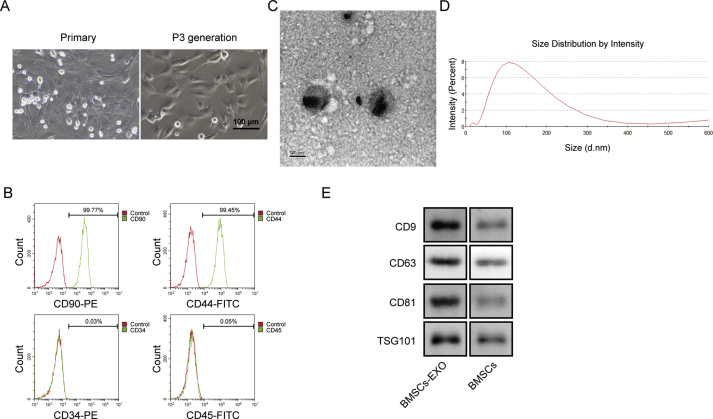
First, we identified the BMSC and BMSC-derived exosomes. As shown in Fig. 1A, the cell morphology was shuttle, spindle and polygon as typical morphology of BMSC. The results of Flow cytometry revealed that obtained cells were positive for CD90 and CD44, and negative for CD45 and CD34, indicating the characterization of BMSC (Fig. 1B). The TEM photograph showed the extracted exosomes of round and elliptical vesicles with a complete double-layer membrane and low electron density materials inside (Fig. 1C). The exosomes size analysis revealed the mean diameter of the exosomes within the range of 30–150 nm (Fig. 1D). Finally, the marker proteins of CD9, CD63, CD81 and TSG101 were all expressed in exosomes with significantly higher levels than the BMSC (Fig. 1E). All these results indicated the successful isolation of BMSC and BMSC-derived exosomes.
Fig. 1.
Identification of morphology and phenotype in BMSC and BMSC-derived exosomes. A) Cell morphology was photographed under optical microscope. B) Cell surface biomarkers of CD90, CD44, CD34 and CD45 were measured by flow cytometry. C) Morphology of BMSC-derived exosomes was observed using TEM. Scale bars, 100 nm. D) Dimeter was calculated using NTA software. E) Expression of exosomal biomarkers CD9, CD63, CD81 and TSG101 was determined by western blot.
3.2. BMSC-derived exosomes promoted proliferation, migration and fibrosis of tenocytes
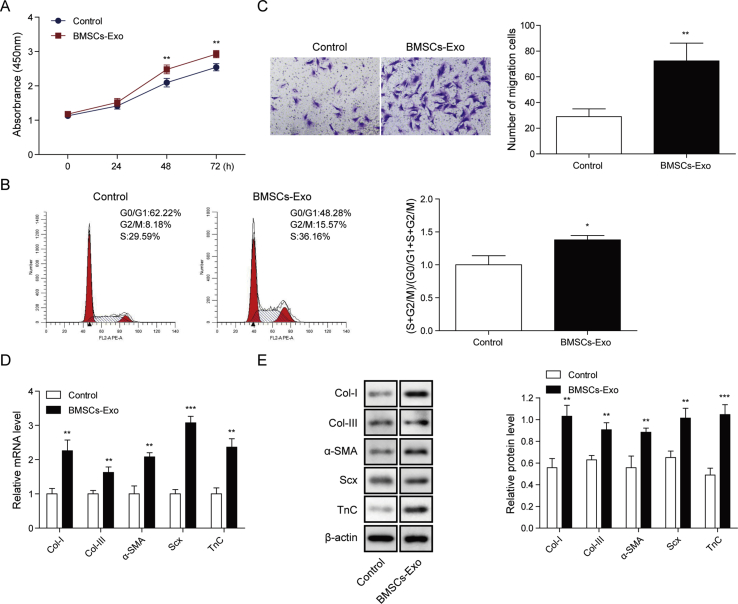
Then we evaluated the effects of BMSC-derived exosomes on cell function of tenocytes. Results showed that the cell proliferation of tenocytes was remarkably promoted by treatment of BMSC-derived exosomes (Fig. 2A). Meanwhile, exosomes treatment also markedly induced the ratio of (S + G2/M) phase cells (Fig. 2B). Transwell assay demonstrated that cells treated with BMSC-derived exosomes had significantly stronger migration ability than the control cells (Fig. 2C). Additionally, both the mRNA and protein levels of Col I, Col III, α-SMA, Scx and TnC were dramatically enhanced by treatment of BMSC-derived exosomes (Fig. 2D and E). Collectively, these findings suggested that the BMSC-derived exosomes could promote cell proliferation, migration and fibrosis of tenocytes.
Fig. 2.
BMSC-derived exosomes promoted proliferation, migration and fibrosis of tenocytes. A) Cell proliferation of tenocytes was determined by CCK-8 assay for both control cells and cells treated by BMSC-derived exosomes. B) Cell cycle of different groups of tenocytes was measured by flow cytometry. C) Cell migration was determined using transwell assay. D) and E) Expression levels of Col I, Col III, α-SMA, Scx and TnC were determined by qPCR and western blot. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.3. TGF-β1 was expressed in BMSC-derived exosomes and was enhanced in exosomes treated tenocytes
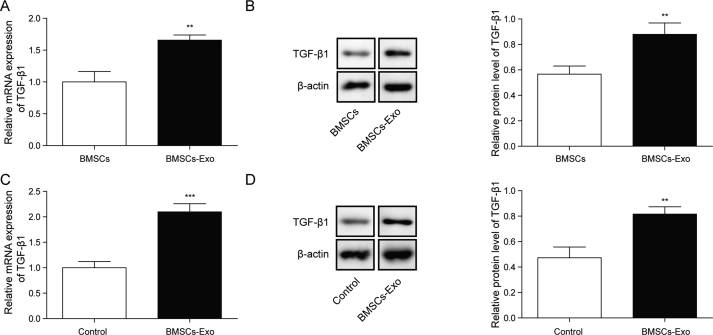
Then the expression of TGF-β1 in BMSC-derived exosomes was determined. It was observed that both mRNA and protein levels of TGF-β1 were significantly higher in BMSC-derived exosomes than that in BMSC (Fig. 3A and B). Similarly, in tenocytes treated by BMSC-derived exosomes, the expression of TGF-β1 was remarkably increased in comparison with the untreated cells at both mRNA and protein levels (Fig. 3C and D). Taken together, TGF-β1 was expressed in BMSC-derived exosomes and BMSC-derived exosomes might promote the expression of TGF-β1 in tenocytes.
Fig. 3.
TGF-β1 was expressed in BMSC-derived exosomes and was enhanced in exosomes treated tenocytes. A-B) mRNA and protein levels of TGF-β1 were determined by qPCR and Western blot in BMSC or BMSC-derived exosomes. C) and D) mRNA and protein levels of TGF-β1 were determined by qPCR and Western blot in control tenocytes or tenocytes treated with BMSC-derived exosomes. ∗∗P < 0.01, ∗∗∗P < 0.001.
3.4. Knockdown of TGF-β1 suppressed the effects of BMSC-derived exosomes on proliferation, migration and fibrosis of tenocytes
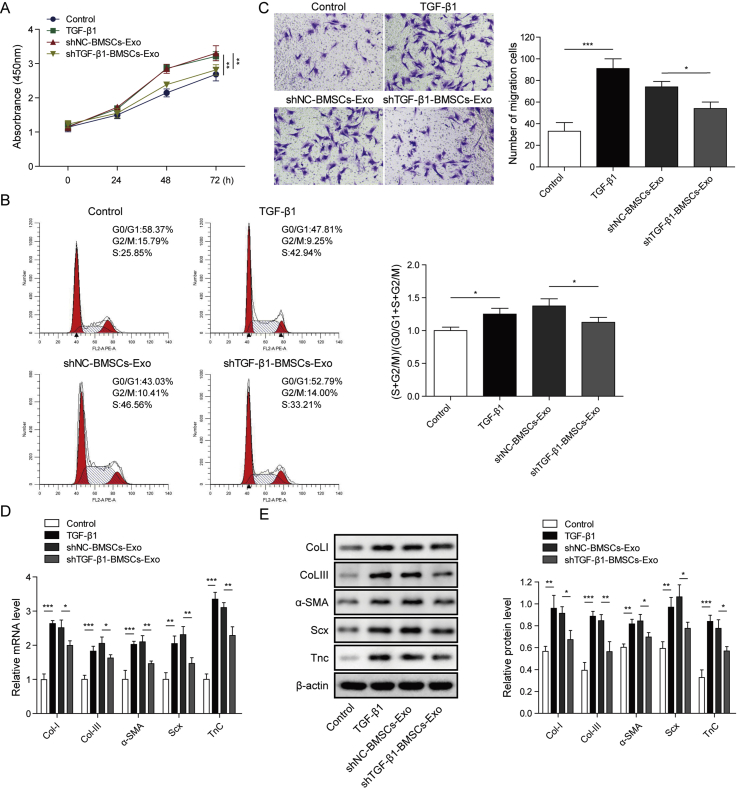
To further investigated the role of TGF-β1 in cell function of tenocytes, we used sh-TGF-β1 to knock down its expression (not shown). When cells were treated with BMSC-derived exosomes or TGF-β1 (1 ng/ml), the cell proliferation, cell migration ability and the ratio of (S + G2/M) phase cells were all remarkably enhanced compared with the untreated cells (Fig. 4A–C). However, the knockdown of TGF-β1 by transfection with sh-TGF-β1 dramatically reversed all the above effects. Besides, both treatment of BMSC-derived exosomes or TGF-β1 significantly increased the expression levels of Col I, Col III, α-SMA, Scx and TnC (Fig. 4D and E). And the transfection of sh-TGF-β1 remarkably decreased the above protein levels which was increased by BMSC-derived exosomes. All these results demonstrated that TGF-β1 participated in the process of cell proliferation, migration and fibrosis of tenocytes and the effects of BMSC-derived exosomes on cell function might be through regulation of TGF-β1.
Fig. 4.
Knockdown of TGF-β1 in suppressed the effects of BMSC-derived exosomes on proliferation, migration and fibrosis of tenocytes. A) Cell proliferation of tenocytes was determined by CCK-8 assay. B) Cell cycle of different groups of tenocytes was measured by flow cytometry. C) Cell migration was determined using transwell assay. D) and E) mRNA and protein levels of Col I, Col III, α-SMA, Scx and TnC were determined by qPCR and western blot. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
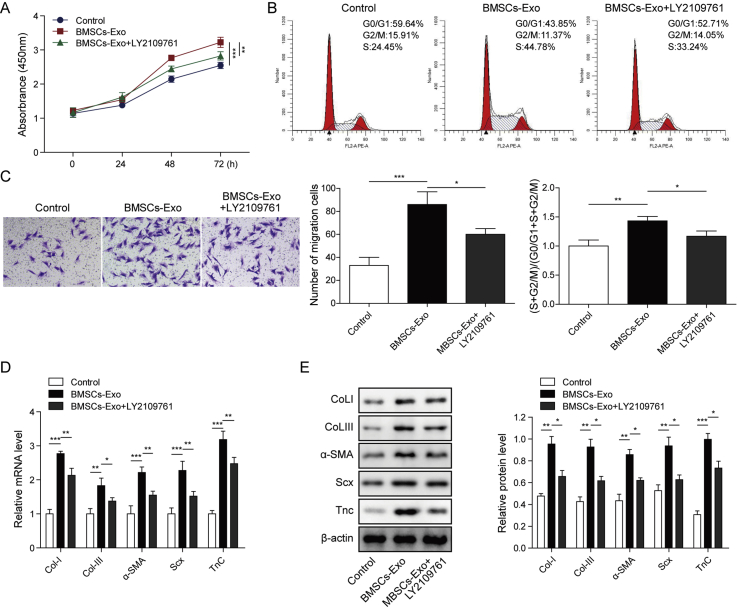
3.5. BMSC-derived exosomes regulated cell function of tenocytes through TGF-β1 signaling
Finally, we confirmed the role of TGF-β1 in exosomes regulated-cell function of tenocytes. The TGFβR I/II inhibitor LY2109761 (2 μM) was used to suppress TGFβ signaling. As shown in Fig. 5A, cell proliferation which was remarkably promoted by BMSC-derived exosomes was significantly inhibited by treatment of LY2109761. Besides, BMSC-derived exosomes enhanced ratio of (S + G2/M) phase cells, cell migration and the protein levels of Col I, Col III, α-SMA, Scx and TnC, while treatment of LY2109761 reversed all these effects (Fig. 5B–E). These data provided evidence that BMSC-derived exosomes regulated cell function tenocytes through activation of TGF-β1 signaling.
Fig. 5.
BMSC-derived exosomes regulated cell function of tenocytes through TGF-β1 signaling. A) Cell proliferation of tenocytes was determined by CCK-8 assay for cells treated with BMSC-derived exosomes and LY2108761 or alone. B) Cell cycle of different groups of tenocytes was measured by flow cytometry. C) Cell migration was determined using transwell assay. D) and E) mRNA and protein levels of Col I, Col III, α-SMA, Scx and TnC were determined by qPCR and western blot. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
4. Discussion
Despite numerous studies, the treatment and recovery of rotator cuff tear tendon injury are still challenging in clinic. Thus, the illumination of novel molecular mechanisms, as well as potential new treatment methods for rotator cuff tear tendon injury are urgent. In recent years, exosomes are reported in many studies as a delivery system to transport specific molecules to the target cells or organs. Zhang et al. showed BMSC-derived exosomal miRNAs could promote metastasis and epithelial stromal transformation of lung cancer cells [18]. It was also found MSC-derived exosomes suppressed cell apoptosis of chondrocytes by regulation of p38, ERK, and Akt pathways [19]. In the present study, we demonstrated for the first time that BMSC-derived exosomes contained TGF-β1 and could promote proliferation, migration and fibrotic activity in rotator cuff tenocytes.
BMSC-derived exosomes have been reported in many diseases and bioprocesses as potential treatment methods, including tendon function. Chen et al. reported that MSC-conditioned medium had a potential ability on tendon injury healing through promotion of proliferation and migration in tendon cells [20]. It was also found the BMSC-derived secretome combined with an engineering therapy could improve chronic massive rotator cuff tear [21]. Besides, exosomes could also mediate intercellular communication and biomolecules transporter (protein, DNA and RNA families) function as crucial regulator [22]. Studies also demonstrated that BMSC could improve recovery of traumatic brain injury [23], spinal cord injury [24] and regeneration of cochlear fibrocytes [25]. However, the functional roles of exosomes derived from BMSC in rotator cuff tear are not known. In our study, we demonstrated for the first time that BMSC-derived exosomes could promote cell proliferation, migration and fibrotic activity in tenocytes of rotator cuff, providing a potential treatment approach for rotator cuff tear.
TGF-β1 is a widely known factor which can facilitate the fibrosis. In a recent study, TGF-β signaling was found to be essential for tenocytes recruitment and functional neonatal tendon regeneration [26]. Another research demonstrated that substance P and acetylcholine could promote proliferation of tenocytes through activation of TGF-β1 [17]. Similar results were observed in effects of substance P on tenocytes through CCN2 dependent TGF-β signaling pathways [27]. Besides, in a recent research, Xu et al. found that TGF-β existed in tenocyte-derived exosomes, and tenocyte-derived exosomes could activate TGF-β signaling and mediated the tenogenic differentiation of mesenchymal stem cells [28]. Jiang et al. also demonstrated that human BMSC-derived exosomes stimulated cutaneous wound healing through activating TGF-β3 and mothers against decapentaplegic homolog 7 (Smad7) signaling pathway [29]. On the other hand, many researches have proven that exosomes express membrane-associated TGF-β1, which could interact with its receptor on the recipient cell [30,31]. In a recent research, Zhu et al. demonstrated that TGF-β1 mRNA in exosomes served a role between macrophages and mesangial cells by activating the TGF-β1/Smad3 pathway [32]. In an early study, analysis of tumor exosomes revealed membrane-associated TGF-β1 participated in the regulation of cell proliferation [33]. In this study, we also found TGF-β1 could promote cell proliferation, migration and fibrotic activity of tenocytes, furthermore, we observed TGF-β1 was expressed in BMSC-derived exosomes. Importantly, blocking exosomes associated TGF-β1 with its receptor inhibitor was sufficient to significantly reduce the role of BMSC-derived exosomes in tenocytes, further supporting these findings.
In conclusion, we conducted an in vitro study to investigate the effects of BMSC-derived exosomes on cell function of tenocytes in rotator cuff. Results showed TGF-β1-containing exosomes derived BMSC could promote proliferation, migration and fibrotic activity in rotator cuff tenocytes, indicating novel potential treatment methods for rotator cuff tear tendon injury. Nevertheless, some limitations to this study still exist. Animal studies using rotator cuff tear models and damaged cell experiments are needed to confirm the therapeutic effects of BMSC-derived exosomes in the future.
Ethics approval
The experiments were approved by the Institutional Animal Care and Use Committee of Beijing Friendship Hospital, Capital Medical University.
Authors' contributions
Guarantor of integrity of the entire study: Jia Li; Study concepts: Jia Li; Study design: Jia Li, Ai Guo; Definition of intellectual content: Jia Li; Experimental studies: Jia Li, Zheng-Peng Liu; Data acquisition: Zheng-Peng Liu; Data analysis: Zheng-Peng Liu; Statistical analysis: Cong Xu; Manuscript preparation: Jia Li; Manuscript editing: Ai Guo; Manuscript review: Ai Guo.
Declaration of Competing Interest
The authors have no conflicting financial interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Iwata E., Shigematsu H., Inoue K., Egawa T., Tanaka M., Okuda A. Biceps-related physical findings are useful to prevent misdiagnosis of cervical spondylotic amyotrophy as a rotator cuff tear. Asian Spine J. 2018;12(1):69–73. doi: 10.4184/asj.2018.12.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bureau N.J., Deslauriers M., Lepage-Saucier M., Rouleau D.M., Roy A., Tétreault P. Rotator cuff tear morphologic parameters at magnetic resonance imaging: relationship with muscle atrophy and fatty infiltration and patient-reported function and health-related quality of life. J Comput Assist Tomogr. 2018;42(5):784–791. doi: 10.1097/RCT.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 3.Le B.T.N., Wu X.L., Lam P.H., Murrell G.A.C. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134–1142. doi: 10.1177/0363546514525336. [DOI] [PubMed] [Google Scholar]
- 4.Barry J.J., Lansdown D.A., Cheung S., Feeley B.T., Ma C.B. The relationship between tear severity, fatty infiltration, and muscle atrophy in the supraspinatus. J Shoulder Elbow Surg. 2013;22(1):18–25. doi: 10.1016/j.jse.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Oh J.H., Kim S.H., Ji H.M., Jo K.H., Bin S.W., Gong H.S. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthrosc J Arthrosc Relat Surg. 2009;25(1):30–39. doi: 10.1016/j.arthro.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Schwab L.M., Blanch P., Young M. Autologous tenocyte implantation into shoulder tendon pathology in an elite swimmer. Phys Ther Sport. 2018;29:19–25. doi: 10.1016/j.ptsp.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Lundgreen K., Lian O.B., Engebretsen L., Scott A. Tenocyte apoptosis in the torn rotator cuff: a primary or secondary pathological event? Br J Sports Med. 2011;45(13):1035–1039. doi: 10.1136/bjsm.2010.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger D.R., Centeno C.J., Steinmetz N.J. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Jt Res. 2019;8(1):32–40. doi: 10.1302/2046-3758.81.BJR-2018-0164.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nassos J.T., ElAttrache N.S., Angel M.J., Tibone J.E., Limpisvasti O., Lee T.Q. A watertight construct in arthroscopic rotator cuff repair. Compar Study. 2012;21(5):589–596. doi: 10.1016/j.jse.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Brown M.J., Pula D.A., Kluczynski M.A., Mashtare T., Bisson L.J. Does suture technique affect Re-rupture in arthroscopic rotator cuff repair? A meta-analysis. Arthrosc J Arthrosc Relat Surg. 2015;31(8):1576–1582. doi: 10.1016/j.arthro.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Kim W., Lee S.K., Kwon Y.W., Chung S.G., Kim S. Pioglitazone-primed mesenchymal stem cells stimulate cell proliferation, collagen synthesis and matrix gene expression in tenocytes. Int J Mol Sci. 2019;20(3):472. doi: 10.3390/ijms20030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q., Sun W.X., Zhang Z.F. High expression of VEGFA in MSCs promotes tendon-bone healing of rotator cuff tear via microRNA-205-5p. Eur Rev Med Pharmacol Sci. 2019;23(10):4081–4088. doi: 10.26355/eurrev_201905_17909. [DOI] [PubMed] [Google Scholar]
- 13.Xia C., Zeng Z., Fang B., Tao M., Gu C., Zheng L. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1–15. doi: 10.1016/j.freeradbiomed.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Yi X., Wei X., Lv H., An Y., Li L., Lu P. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383(2):111454. doi: 10.1016/j.yexcr.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y., Shi H., Nomi A., Lei-Lei Z., Zhang B., Qian H. Mesenchymal stem cell–derived extracellular vesicles: a new impetus of promoting angiogenesis in tissue regeneration. Cytotherapy. 2019;21(5):497–508. doi: 10.1016/j.jcyt.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Pauly S., Klatte-Schulz F., Stahnke K., Scheibel M., Wildemann B. The effect of autologous platelet rich plasma on tenocytes of the human rotator cuff. BMC Muscoskel Disord. 2018;19(1):422. doi: 10.1186/s12891-018-2339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong G., Backman L.J., Alfredson H., Scott A., Danielson P. The effects of substance P and acetylcholine on human tenocyte proliferation converge mechanistically via TGF-β1. PloS One. 2017;12(3) doi: 10.1371/journal.pone.0174101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Zheng Y., Hou L., Zhou Z., Huang Y., Zhang Y. Exosomes derived from maxillary BMSCs enhanced the osteogenesis in iliac BMSCs. Oral Dis. 2020;26(1):131–144. doi: 10.1111/odi.13202. [DOI] [PubMed] [Google Scholar]
- 19.Qi H., Liu D.-P., Xiao D.-W., Tian D.C., Su Y.W., Jin S.F. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. Vitro Anim Cell Dev Biol. 2019;55(3):203–210. doi: 10.1007/s11626-019-00330-x. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q., Liang Q., Zhuang W., Zhou J., Zhang B., Xu P. Tenocyte proliferation and migration promoted by rat bone marrow mesenchymal stem cell-derived conditioned medium. Biotechnol Lett. 2018;40(1):215–224. doi: 10.1007/s10529-017-2446-7. [DOI] [PubMed] [Google Scholar]
- 21.Sevivas N., Teixeira F.G., Portugal R., Direito-Santos B., Espregueira-Mendes J., Oliveira F.J. Mesenchymal stem cell secretome improves tendon cell viability in vitro and tendon-bone healing in vivo when a tissue engineering strategy is used in a rat model of chronic massive rotator cuff tear. Am J Sports Med. 2018;46(2):449–459. doi: 10.1177/0363546517735850. [DOI] [PubMed] [Google Scholar]
- 22.Kim C., Lee K. Polydiacetylene (PDA) liposome-based immunosensor for the detection of exosomes. Biomacromolecules. 2019;20(9):3392–3398. doi: 10.1021/acs.biomac.9b00641. [DOI] [PubMed] [Google Scholar]
- 23.Yao M., Gao F., Xu R., Zhang J., Chen Y., Guan F. A dual-enzymatically cross-linked injectable gelatin hydrogel loaded with BMSC improves neurological function recovery of traumatic brain injury in rats. Biomater Sci. 2019;7(10):4088–4098. doi: 10.1039/c9bm00749k. [DOI] [PubMed] [Google Scholar]
- 24.Bai S., Zhou H., Wu L. Bone marrow stromal cells improved functional recovery in spinal cord injury rats partly via the Toll-like receptor-4/nuclear factor-κB signaling pathway. Exp Therap Med. 2019;17(1):444–448. doi: 10.3892/etm.2018.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kada S., Hamaguchi K., Ito J., Omori K., Nakagawa T. Bone marrow stromal cells accelerate hearing recovery via regeneration or maintenance of cochlear fibrocytes in mouse spiral ligaments. Anat Rec. 2020;303(3):478–486. doi: 10.1002/ar.24063. [DOI] [PubMed] [Google Scholar]
- 26.Kaji D.A., Howell K.L., Huang A.H. TGFβ signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. BioRxiv. 2019:767467. doi: 10.7554/eLife.51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frara N., Fisher P.W., Zhao Y., Tarr J.T., Amin M., Popoff S.N. Substance P increases CCN2 dependent on TGF-beta yet Collagen Type I via TGF-beta1 dependent and independent pathways in tenocytes. Connect Tissue Res. 2018;59(1):30–34. doi: 10.1080/03008207.2017.1297809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu T., Xu M., Bai J., Lin J., Yu B., Liu Y. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology. 2019;71(1):57–65. doi: 10.1007/s10616-018-0264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang T., Wang Z., Sun J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res Ther. 2020;11(1):1–10. doi: 10.1186/s13287-020-01723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu J., Hui Q., Li S., Zhang X., Zhu W., Huang L. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/smad pathway. PloS One. 2012;7(12) doi: 10.1371/journal.pone.0052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra L., Derynck R., Mishra B. Transforming growth factor-β signaling in stem cells and cancer. Science. 2005;310(5745):68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 32.Qi-jin Z., Mei Z., Xing-xin X., Xiao-Ming M., Yong-Gui W. Exosomes from high glucose–treated macrophages activate glomerular mesangial cells via TGF-β1/Smad3 pathway in vivo and in vitro. Faseb J. 2019;33(8):9279–9290. doi: 10.1096/fj.201802427RRR. [DOI] [PubMed] [Google Scholar]
- 33.Clayton A., Mitchell J.P., Court J., Mason M.D., Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Canc Res. 2007;67(15):7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]