Abstract
Introduction
Basic fibroblast growth factor (bFGF) is a promising cytokine in regenerative therapy for spinal cord injury. In this study, recombinant canine bFGF (rc-bFGF) was synthesized for clinical use in dogs, and the ability of rc-bFGF to differentiate canine bone marrow mesenchymal stem cells (BMSCs) into functional neurons was investigated.
Methods
The rc-bFGF was synthesized using a wheat germ cell-free protein synthesis system. The expression of rc-bFGF mRNA in the purification process was confirmed using a reverse transcription-polymerase chain reaction (RT-PCR). Western blotting was performed to confirm the antigenic property of the purified protein. To verify function of the purified protein, phosphorylation of extracellular signal-regulated kinase (ERK) was examined by in vitro assay using HEK293 cells. To compare the neuronal differentiation capacity of canine BMSCs in response to treatment with rc-bFGF, the cells were divided into the following four groups: control, undifferentiated, rh-bFGF, and rc-bFGF groups. After neuronal induction, the percentage of cells that had changed to a neuron-like morphology and the mRNA expression of neuronal markers were evaluated. Furthermore, to assess the function of the canine BMSCs after neuronal induction, changes in the intracellular Ca2+ concentrations after stimulation with KCl and l-glutamate were examined.
Results
The protein synthesized in this study was rc-bFGF and functioned as bFGF, from the results of RT-PCR, western blotting, and the expression of pERK in HEK293 cells. Canine BMSCs acquired a neuron-like morphology and expressed mRNAs of neuronal markers after neuronal induction in the rh-bFGF and the rc-bFGF groups. These results were more marked in the rc-bFGF group than in the other groups. Furthermore, an increase in intracellular Ca2+ concentrations was observed after the stimulation of KCl and l-glutamate in the rc-bFGF group, same as in the rh-bFGF group.
Conclusions
A functional rc-bFGF was successfully synthesized, and rc-bFGF induced the differentiation of canine BMSCs into voltage- and glutamate-responsive neuron-like cells. Our purified rc-bFGF may contribute, on its own, or in combination with canine BMSCs, to regenerative therapy for spinal cord injury in dogs.
Keywords: Basic fibroblast growth factor, Bone marrow, Dog, Differentiation, Neuron, Mesenchymal stem cell
Abbreviations: BMSCs, bone marrow mesenchymal stem cells; bFGF, basic fibroblast growth factor; FGFR, basic fibroblast growth factor receptor; PI3K, phosphatidylinositol 3-kinase; mRNA, messenger ribonucleic acid; cDNA, complementary DNA; RT-PCR, reverse transcription-polymerase chain reaction; PCR, polymerase chain reaction; HRP, horseradish peroxidase; ERK, extracellular signal-regulated kinase; pERK, phosphorylated extracellular signal-regulated kinase; HEK293, human embryonic kidney cells 293; αMEM, alpha modified eagle minimum essential medium; FBS, fatal bovine serum; EDTA, ethylenediaminetetraacetic acid; GUSB, β-glucuronidase; PBS, phosphate buffered saline
Highlights
-
•
Functional rc-bFGF was successfully synthesized.
-
•
rc-bFGF induced the differentiation of canine BMSCs into neuron-like cells.
-
•
rc-bFGF may aid in regenerative therapy of spinal cord injury in dogs.
1. Introduction
Bone marrow mesenchymal stem cells (BMSCs) are plastic-adherent cells, in cultures of bone marrow aspirates, and are a non-hematopoietic cell population with multiple differentiation potential [[1], [2], [3]]. BMSCs can differentiate into bone, cartilage and fat [2,3], as well as into neuronal cells lineage [[4], [5], [6]]. From this background, BMSCs have been investigated as one possible stem cell source for regenerative therapy of the central nervous system [[7], [8], [9], [10], [11]]. In veterinary medicine, there have been many basic researches on cell therapy using BMSCs, focusing on spinal cord injury [6,[12], [13], [14], [15], [16]]. To date, intramedullary, intradural or intravenous administration of mesenchymal stem cells has been performed in dogs with spinal cord injury, and a certain of therapeutic effects have been reported [[17], [18], [19], [20], [21]].
Although the mechanism of repair of the injured spinal cord by mesenchymal stem cells is not fully understood, three mechanisms have been proposed: tissue repair via humoral factors (the paracrine effect), cell replacement by stem cells, and axon elongation and recanalization from engrafted stem cells [9,10,22]. Currently, the prevailing mechanism is the repair of the injured spinal cord by the paracrine effect [10,23].
Traumatic spinal cord injury is often accompanied by necrosis of the center of the spinal cord [24]. Cell therapy using only undifferentiated mesenchymal stem cells is limited to complete repair of the injured spinal cord. If mesenchymal stem cells can be induced to differentiate into functional neurons, they can become a cell source to replace damaged neurons, and further improve therapeutic outcomes. Therefore, we think that one of the key issues is to investigate the neuronal differentiation potential of mesenchymal stem cells.
In our laboratory, the neuronal differentiation potential of canine BMSCs has already been thoroughly investigated using previously reported neural induction media. When canine BMSCs were cultured using Neurobasal-A medium supplemented with recombinant human basic fibroblast growth factor (rh-bFGF), they changed to a neuron-like morphology which they maintained for a long time [14]. The neuron-like cells expressed messenger ribonucleic acid (mRNAs) and proteins associated with neurons, and also were positive against neuronal markers by immunocytochemistry [14]. In addition, when the neuron-like cells were stimulated by KCl or l-glutamate, an increase in intracellular Ca2+ concentration was observed by Ca2+ imaging, suggesting that these cells have a physiological function [14]. Furthermore, it was revealed that the FGFR-2/PI3K/Akt signaling pathway was involved in the bFGF-induced neuronal differentiation of canine BMSCs [15]. These results suggested that bFGF may have potential in differentiation canine BMSCs into functional neuron-like cells, and also in the differentiation of endogenous neural stem cells in the spinal cord into functional neurons. The results give us reason to believe that cytokine therapy using bFGF may become an effective method in regenerative therapy for spinal cord injury.
In canine medicine, the clinical application of bFGF for neuronal regenerative therapy ideally involves the use of recombinant canine bFGF (rc-bFGF) rather than rh-bFGF, because the base sequence of rc-bFGF is not the same as that of rh-bFGF. To the best of our knowledge, there have been no reports of the synthesis of functional rc-bFGF and its effect on neuronal differentiation of canine BMSCs. Therefore, in the present study, rc-bFGF for clinical use was synthesized using a cell-free protein synthesis technique, which did not use E. coli during synthesis and was less affected by endotoxin. Furthermore, the ability of rc-bFGF to differentiate canine BMSCs into functional neurons was also investigated.
2. Materials and methods
2.1. Plasmid and sequence for rc-bFGF
The open reading frame of canine bFGF was amplified by PCR with the primers 5′-EcoRV-canine-bFGF (5′-GGAAGATATCATGGCAGCCGGGAGCATCAC-3′) and 3′-Spe Ⅰ-canine-bFGF (5′-CCTTACTAGTTCAGCTCTTAGCAGACATTG-3′) using a full-length complementary DNA (cDNA) clone from the canine epithelial cell line (XM_003432481.2), as a template. The amplified PCR product was digested with EcoRV and Spe Ⅰ, and then subcloned into EcoRV and Spe Ⅰ digested pEU-E01-His-TEV-MCS vector (Cellfree Sciences, Matsuyama, Ehime, Japan) including His-tag at the N-terminus. The insertion of the canine bFGF gene into the protein expression vector was confirmed by sequence analysis.
2.2. Synthesis and purification of rc-bFGF
In this study, the His-tag fusion recombinant canine bFGF (rc-bFGF) was synthesized in a wheat germ cell-free protein expression system using the WEPRO7240H Expression Kit (CellFree Sciences). The synthesized rc-bFGF was purified using Ni Sepharose™ High Performance (GE Healthcare, Bio-Sciences, Tokyo, Japan) and concentrated with a centrifugal filter (Amicon Ultra-0.5, Merck KgaA, Darmstadt, Germany). All processes for protein synthesis and affinity purification were carried out by an automated protein synthesizer (Protemist DT Ⅱ, CellFree Sciences) according to the manufacturer's instructions.
2.3. Confirmation of purified protein as bFGF
The expression of rc-bFGF mRNA in the purification process was confirmed using a reverse transcription-polymerase chain reaction (RT-PCR). Briefly: total RNA was extracted from the reaction solution after protein synthesis. The first-strand cDNA synthesis was performed with 100 ng of total RNA using the AccessQuick™ RT-PCR system (Promega, Madison, WI, U.S.A.). Then, PCR was performed with PrimeSTAR HS DNA polymerase (TaKaRa Bio Inc., Ohtsu, Shiga, Japan) and primers (forward primer: 5′-ATAGAATTCatggcagccgggagcatcaccac-3′; reverse primer: 5′-ATACTCGAGtcagctcttagcagacattggaaga-3′). The PCR was conducted using a Biometra T3000 Thermal cycler (Biometra GmbH, Göttingen, Germany). The PCR conditions were as follows: 1 cycle of denaturing at 98 °C for 10 s, annealing at 70 °C for 10 s, extension at 72 °C for 60 s, and indicated cycles of denaturing at 98 °C for 10 s, annealing and extension at 72 °C for 60 s.
Western blotting was then performed to confirm that the purified protein was bFGF, as described previously [25,26]. Briefly: our purified protein that synthesized using a wheat germ cell-free protein synthesis system, rc-bFGF that was previously synthesized using E. coli, and commercially available rh-bFGF (FUJIFILM Wako Pure Chemical Co., Tokyo, Japan) were subjected to SDS-PAGE. These separated proteins in the gels were transferred to Immobilon-P transfer membranes (Merck KGaA) using the transfer system (Trans-Blot Turbo, Bio-Rad Laboratories, Inc. Hercules, CA, U.S.A.). The membrane was blocked with 5% skim milk (FUJIFILM Wako Pure Chemical Co.). The membranes were incubated with the anti-rabbit monoclonal bFGF antibody (Cell Signaling Technology Inc., Danvers, MA, U.S.A.). This monoclonal antibody has been confirmed to cross rc-bFGF synthesized using E. coli in our preliminary study. After washing, the membranes were incubated with HRP-linked secondary antibody and the proteins were detected using a Chemi-Lumi One assay kit (Nacalai Tesque Inc., Kyoto, Japan).
To verify that the purified protein functioned as bFGF, the protein was added to HEK293 cells and phosphorylation of extracellular signal-regulated kinase (ERK) was detected by western blotting assay [26]. Briefly: HEK293 cells were cultured in a 6-well cell culture plate with DMEM (Wako Pure Chemical, Osaka, Japan) supplemented with 10% FBS (Biowest, Nuaillé, France) and 1% penicillin and streptomycin solution (Nacarai Tesque Inc.) at 37 °C under 5% CO2 and 95% air for 48 h. In this study, HEK293 cells were stimulated by 5 ng/mL and 15 ng/mL of our purified protein, and 5 ng/mL of commercially available rh-bFGF (FUJIFILM Wako Pure Chemical Co.) for 15 min. A sample that was not stimulated by rh-bFGF was used as control. After the stimulation, HEK293 cells were detached from the culture plate and the proteins were extracted after treatment with phosphatase inhibitor cocktail (PhosSTOP; Roche, Indianapolis, IN, U.S.A.). Western blotting was carried out using anti-mouse monoclonal ERK antibody (Santa Cruz Biotechnology Inc., Dallas, TX, U.S.A.), and anti-mouse monoclonal pERK antibody (Santa Cruz Biotechnology Inc.), as mentioned above.
2.4. Culture of canine BMSCs
Canine BMSCs were isolated as described previously [6,13,14]. Briefly: canine bone marrow was aspirated from the humerus under general anesthesia. This study protocols was approved by Nihon University Animal Care and Use Committee (AP12B015). Mononuclear cells were then separated by density gradient centrifugation using Histopaque-1077 (Sigma–Aldrich Inc., St. Louis, MO, U.S.A.). Following collection, the mononuclear cells were transferred to a 25-cm2 plastic culture flask (Corning Incorporated Life Sciences, Lowell, MA, U.S.A.), and static-cultured in an incubator at 5% CO2 and 37 °C using α-MEM (Life Technologies Co., Carlsbad, CA, U.S.A.) with 10% FBS (Life Technologies Co.). On the fourth day of culture, nonadherent cells were removed when the culture medium was replaced, thus isolating canine BMSCs. Canine BMSCs were harvested using 0.25% trypsin–EDTA (Life Technologies Co.), once they reached approximately 90% confluence. The second-passage canine BMSCs were used for all the following experiments.
2.5. Neuronal induction of canine BMSCs
The second-passage canine BMSCs were placed in a 25-cm2 plastic culture flask (Corning Inc. Life Sciences) at a density of 4000 cells/cm2 and were cultured with αMEM containing 10% FBS for 24 h. Neuronal induction of the canine BMSCs was conducted according to our previously reported method [14,15]. Briefly: the medium was changed to Neurobasal-A medium (Life Technologies Co.) containing 2% B-27 supplement (Life Technologies Co.) and 100 ng/mL rh-bFGF (rh-bFGF group; n = 6) or 100 ng/mL rc-bFGF (rc-bFGF group; n = 6). Canine BMSCs were also cultured with αMEM containing 10% FBS (control group; n = 6), and with Neurobasal-A medium containing 2% B27 supplement only (undifferentiated group; n = 6). These culture media were changed every 3 days. The morphology of these cells was monitored under an inverted microscope at 1, 3, 5, 7, 9, and 10 days of neuronal induction. At 10 days of neuronal induction, the percentage of canine BMSCs that had changed to a neuron-like morphology was calculated for each group.
2.6. Real-time RT-PCR
Total RNA was extracted from the canine BMSCs before and at 10 days of neuronal induction by using TRIzol® reagent (Life Technologies Co.) in each group. The first-strand cDNA synthesis was carried out with 500 ng of total RNA using PrimeScript® RT Master Mix (TaKaRa Bio Inc.). Real-time RT-PCRs were performed with 2 μl of the first-strand cDNA using canine-specific primer sets for neuronal markers (Table 1) and SYBR® Premix Ex Taq™ II (TaKaRa Bio Inc.). The PCR was conducted using Thermal Cycler Dice® Real Time System II (TaKaRa Bio Inc.). The PCR reactions consisted of 1 cycle of denaturing at 95 °C for 30 s, 40 cycles of denaturing at 95 °C for 5 s and annealing and extension at 60 °C for 30 s. The results were analyzed by the second derivative method and the comparative cycle threshold (ΔΔCt) method using TP900 DiceRealTime v4.02B (TaKaRa Bio Inc.). Amplification of GUSB from the same amount of cDNA was used as an endogenous control, and the amplification of the cDNA from non-treated canine BMSCs was used as a calibration standard.
Table 1.
Canine specific primer sets used to investigate the mRNA expression of neuronal makers.
| Gene name | Primer sequences |
|---|---|
| NEFL | F:5′-TGAATATCATGGGCAGAAGTGGAA-3′ |
| R:5′-GGTCAGGATTGCAGGCAACA-3′ | |
| NEFH | F:5′-GGAGGTTCCTGCCAAGGTGA-3′ |
| R:5′-CTCTGCTGCTTTGCTGGGTTC-3′ | |
| MAP2 | F:5′-AAGCATCAACCTGCTCGAATCC-3′ |
| R:5′-GCTTAGCGAGTGCAGCAGTGAC-3′ | |
| TUBB3 | F:5′-TACAACGCCACGCTGTCCA-3′ |
| R:5′-CTTGAGAGTGCGGAAGCAGATG-3′ | |
| GUSB | F:5′-ACATCGACGACATCACCGTCA-3′ |
| R:5′-GGAAGTGTTCACTGCCCTGGA-3′ |
2.7. Ca2+ imaging
Ca2+ imaging of canine BMSCs after neuronal induction was performed according to our previously reported method [14]. Briefly: canine BMSCs from each group were seeded on 35-mm glass base dishes at a density of 4000 cells/cm2. At 10 days of the neuronal induction, the cells were incubated with Neurobasal-A medium containing 2% B-27 supplement and 4.0 μM Fluo 3-AM (Dojindo Lab., Kumamoto, Japan) for 30 min at 37 °C in the dark. Following incubation, the cells were washed twice in PBS. After washing, the culture medium was changed to a Ca2+ imaging buffer (containing 120 mM NaCl, 5 mM KCl, 0.96 mM NaH2PO4, 1 mM MgCl2, 11.1 mM glucose, 1 mM CaCl2, 1 mg/mL BSA and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; pH 7.4). The glass base dishes with the fluorescent dye-loaded cells were placed at room temperature on the stage of a confocal laser scanning microscope (LSM510; Carl Zeiss, Oberkochen, Germany). Images were captured every 2 s, in a time lapse sequence. After baseline images were acquired, the cells were stimulated with 50 mM KCl (FUJIFILM Wako Pure Chemical Co.) or 100 μM l-glutamate (FUJIFILM Wako Pure Chemical Co.). The relative change in intracellular Ca2+ concentration over time was expressed as the change in fluorescence relative to baseline.
2.8. Statistical analysis
The data for these experiments were calculated as mean ± standard error. Statistical analyses were performed using GraphPad Prism version 6.0 for Macintosh (GraphPad Software Inc., San Diego, CA, U.S.A.). The data from the groups were analyzed using one-way analysis of variance, and Tukey's test was used as post hoc analysis. Data before and after neuronal induction were analyzed using an un-paired student t-test. Values of p less than 0.05 were considered significant.
3. Results
3.1. Synthesis and confirmation of rc-bFGF
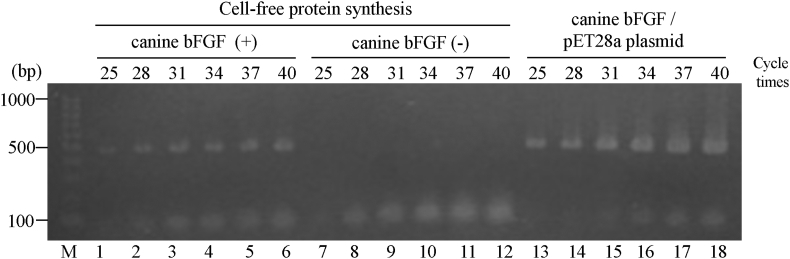
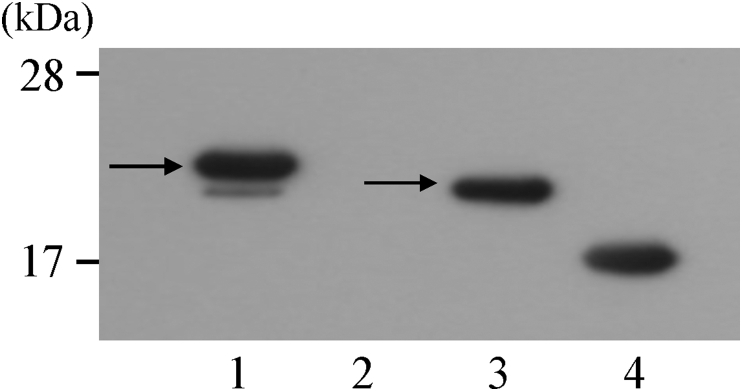
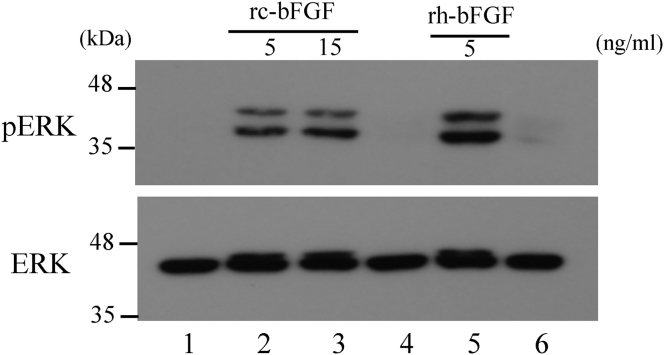
Sequence analysis confirmed that the vector for the wheat germ cell-free protein synthesis system inserted the canine bFGF gene at the target site. When RT-PCR was performed using mRNA in the purification process of rc-bFGF by the cell-free protein synthesis system, bands formed in the same position as a positive control using the pET28a plasmid for synthesis by E. coli (Fig. 1). Thus, it was confirmed that canine bFGF mRNA had been synthesized in the protein synthesis process using this system. SDS-PAGE revealed that the purified protein had expected molecular weight. Western blotting of purified protein showed the formation of band stained by anti-bFGF monoclonal antibody at positions similar to that of rc-bFGF synthesised using E. coli (Fig. 2). When the purified protein was added to HEK293 cells, the expression of pERK was confirmed in the same way as was observed following the stimulation by commercially available rh-bFGF (Fig. 3). These results suggested that the purified protein was rc-bFGF and functioned as bFGF.
Fig. 1.
The expression of rc-bFGF mRNA in the purification process of rc-bFGF by the cell-free protein synthesis system. Lane 1–6: the results of RT-PCR in reaction solution after synthesis of canine bFGF. Lane 7-12: the results of RT-PCR in reaction solution without synthesis of canine bFGF (negative control). Lane 13–18: the results of PCR in the pET28a plasmid for synthesis by E. coli (positive control).
Fig. 2.
Western blotting of purified protein. Band formation was observed at almost same position as rc-bFGF synthesized using E. coli (arrows). Lane 1: reaction solution after synthesis of canine bFGF. Lane 2: reaction solution without synthesis of canine bFGF (negative control). Lane 3: rc-bFGF synthesized using E. coli, Lane 4: commercially available rh-bFGF (positive control).
Fig. 3.
Phosphorylation of ERK in HEK293 cells after stimulation by purified protein. pERK expression was observed after stimulation of HEK293 cells by our purified protein, as it was after stimulation by commercially available rh-bFGF. Lane 1: extract from HEK293 cells after no stimulation of rc-bFGF or rh-bFGF (negative control). Lane 2: extract from HEK293 cells after stimulation by 5 ng/mL of rc-bFGF. Lane 3: extract from HEK293 cells after stimulation by 15 ng/mL of rc-bFGF. Lane 4: rc-bFGF synthesized solution only. Lane 5: extract from HEK293 cells after stimulation by 5 ng/mL of commercially available rh-bFGF (positive control). Lane 6: blotting buffer.
3.2. Neuronal induction of canine BMSCs
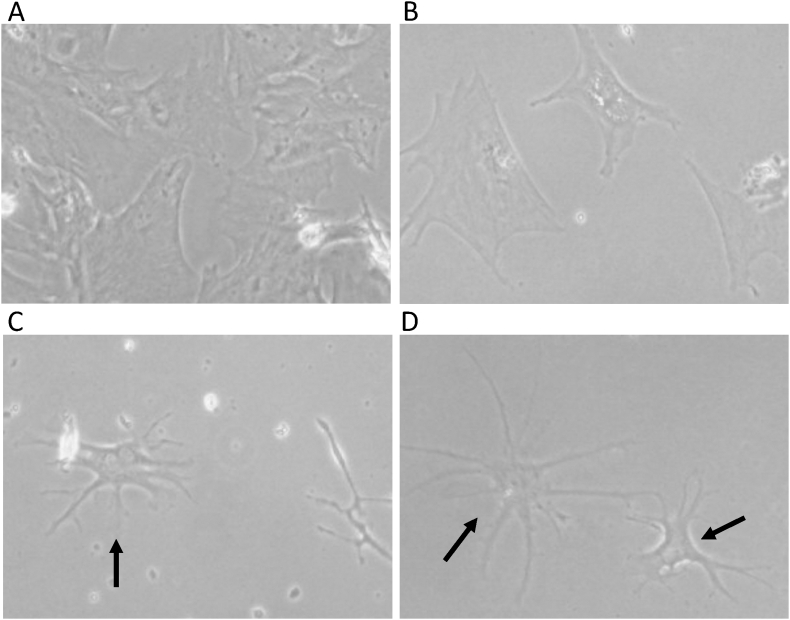
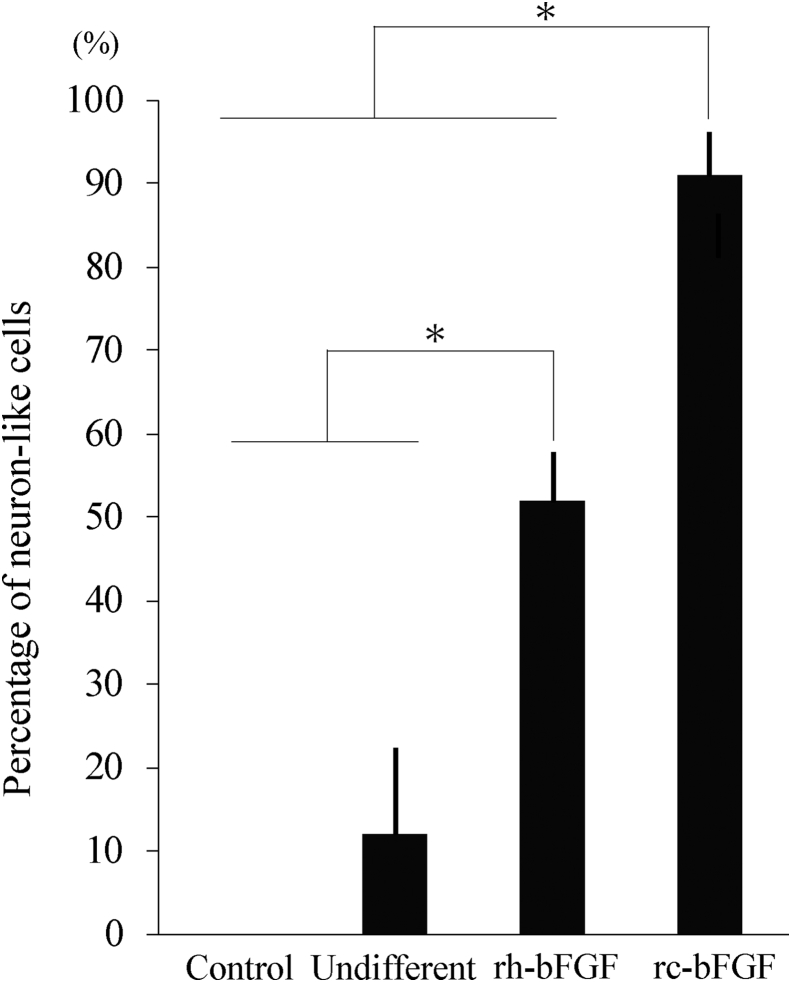
Canine BMSCs in the rh-bFGF and rc-bFGF groups were markedly changed to a neuron-like morphology after neuronal induction (Fig. 4). However, there was no change in morphology in the control group, and little in the undifferentiated group. Among the groups, neuron-like cells were found the earliest in the rc-bFGF group and could be clearly observed at 3 days of neuronal induction. The percentage of canine BMSCs that changed to a neuron-like morphology at 10 days of neuronal induction in the control, undifferentiated, rh-bFGF and rc-bFGF groups was 0 ± 0%, 12.2 ± 1.9%, 52.1 ± 1.7% and 91.2 ± 1.9%, respectively (Fig. 5). In the rc-bFGF group, the percentage was significantly higher than that in the other groups (Fig. 5).
Fig. 4.
The morphologies of canine BMSCs at 10 days of neuronal induction. Canine BMSCs were changed to a neuron-like morphology after neuronal induction in the rh-bFGF and the rc-bFGF groups (arrows). A: control group, B: undifferentiated group, C: rh-bFGF group, and D: rc-bFGF group.
Fig. 5.
The percentage of canine BMSCs that had changed to a neuron-like morphology at 10 days of neuronal induction. Error bars show mean ± standard error (n = 6 in each group). Asterisks indicate statistical difference among the groups (P < 0.05). Control; control group, Undifferent; undifferentiated group, rh-bFGF; rh-bFGF group, rc-bFGF; rc-bFGF group.
3.3. The mRNA expressions of neuronal markers
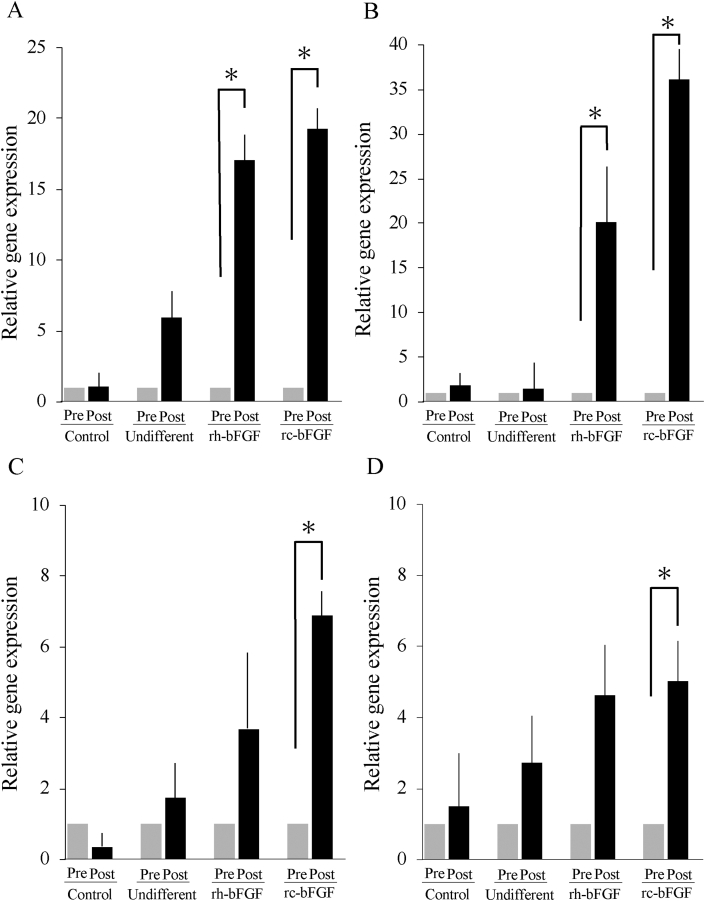
In the rc-bFGF and rh-bFGF groups, mRNA expression of NEFL and NEFH was significantly increased after neuronal induction (Fig. 6A and B). A significant increase in mRNA expression of MAP2 and TUBB3 was observed after neuronal induction only in the rc-bFGF group (Fig. 6C and D). Conversely, there was almost no increase in mRNA expression for neuronal markers after neuronal induction in the control and undifferentiated groups (Fig. 6).
Fig. 6.
The mRNA expression of neuronal markers pre- and post-neuronal induction. A: NEFL, B: NEFH, C: MAP2, and D: TUBB3. Error bars show mean ± standard error (n = 6 in each group). Asterisks indicate statistical difference between pre- and post-neuronal induction (P < 0.05). Control; control group, Undifferent; undifferentiated group, rh-bFGF; rh-bFGF group, rc-bFGF; rc-bFGF group.
3.4. Ca2+ imaging of neuron-like cells differentiated from canine BMSCs
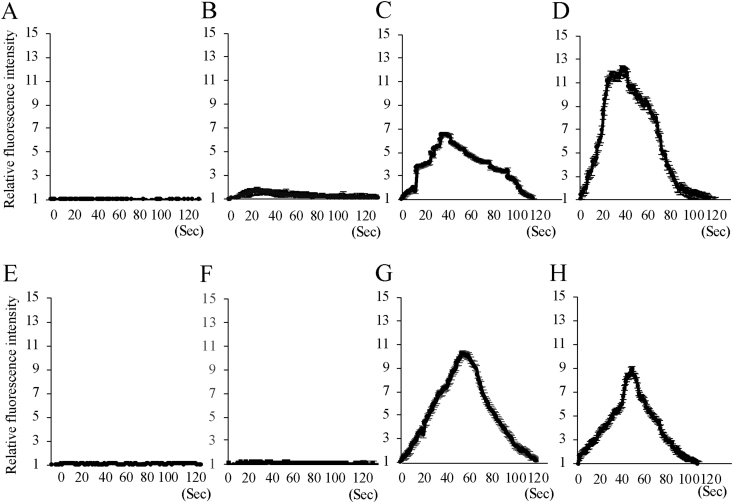
In the rh-bFGF and rc-bFGF groups, depolarization stimulation of canine BMSCs using 50 mM KCl at 10 days of neuronal induction resulted in an increase in intracellular Ca2+ concentration (Fig. 7). In addition, 100 μM of l-glutamate also evoked a rise in intracellular Ca2+ concentrations in the rh-bFGF and rc-bFGF groups (Fig. 7). Conversely, KCl and l-glutamate had almost no effect on the intracellular Ca2+ concentration in the control and the undifferentiated groups (Fig. 7).
Fig. 7.
Relative fluorescence intensity of intracellular Ca2+ concentration after the stimulation by 50 mM KCl (A, B, C, D) or 100 μM l-glutamate (E, F, G, H). A and E: Control group, B and F: Undifferentiated group, C and G: rh-bFGF group, D and H: rc-bFGF group.
4. Discussion
The synthesis of rc-FGF has been tried in some veterinary medicine institutions, but, to the best of our knowledge, there have been no reports on the synthesis of rc-bFGF and detailed investigations of its function. In our experience, the purification of rc-bFGF synthesized by E. coli has been relatively inefficient, and the elimination of endotoxin has presented difficulties. Therefore, to synthesize rc-bFGF for clinical applications, it is desirable to avoid using pathogens such as E coli. and that contains only a minimal amount of endotoxin in the final purified solution. For these reasons, in the present study, a wheat germ cell-free protein synthesis system was employed to synthesize rc-bFGF.
In the present study, the recombinant protein of full-length rc-bFGF was synthesized with reference to canine genetic information (XM_003432481.2). The amino acid sequence of bFGF used in this study was 98% homologous to that of human bFGF. In 1986, the DNA of the human bFGF gene was sequenced and bFGF was identified as a protein with a molecular weight of 18 kDa [27]. Then, the molecular weight of most commercially available rh-FGF is 16–18 kDa. Subsequently, additional forms of bFGF of 22, 22.5, and 24 kDa in humans, and 20.5 and 21 kDa in rodents, were discovered and are referred to as high molecular weight bFGF [28]. In this study, the molecular weight of our purified protein was consistent with previous bFGF results. In addition, when western blotting was performed using a monoclonal antibody that has been proven to confirm rc-bFGF synthesized by E. coli, the band of our purified protein was observed in the almost same position as that of rc-bFGF. These results suggested that our purified protein was rc-bFGF and that molecular weight was slightly larger than that of commercially available rh-bFGF.
It has been reported that bFGF strongly stimulates ERK (MAPK) signaling and phosphorylates ERK [29]. Therefore, to confirm that our purified protein functions as bFGF, the expression of pERK after stimulation by our purified protein was examined. The results of this study indicated that our purified protein functioned as bFGF, and functional rc-bFGF was successfully synthesized. It is known that bFGF promotes angiogenesis, vascular remodeling, wound healing, bone development and remodeling, and neuronal differentiation [30,31]. In addition, rh-bFGF is already approved in Japan for wound healing and for periodontitis in humans [32,33]. Therefore, we suggest that our purified rc-bFGF also has the potential to contribute to the treatment of related conditions in dogs.
Several studies have reported the usefulness of bFGF in the differentiation of BMSCs into neurons [[34], [35], [36], [37]]. In mouse BMSCs treated with bFGF, neuron-specific proteins and voltage-dependent channels were expressed, and neuron-like K+ outward currents were detected [34]. This led us to investigate the differentiation of functional neurons from canine BMSCs for its potential as a possible treatment for spinal cord injury. When canine BMSCs were induced to differentiate into neurons using a neural induction medium containing rh-bFGF, they changed to a neuron-like morphology and expressed mRNA and proteins related to neuronal markers [14]. In addition, Ca2+ imaging demonstrated that canine BMSCs differentiated into voltage- and glutamate-responsive neuron-like cells [14]. Thus, our previous study was the first successfully to differentiate canine BMSCs into functional neuron-like cells. Furthermore, the results of this study suggested that rc-bFGF was also effective in differentiating canine BMSCs into functional neuron-like cells, and that it was more effective than rh-bFGF.
The therapeutic effect of BMSCs on spinal cord injury is thought to be predominantly due to anti-inflammatory effects and to tissue repair by trophic effects [10,23]. However, there is doubt whether transplanted undifferentiated BMSCs can differentiate into functional neurons or can survive for long at the site of injury [9]. Studies on the transplantation of neurally induced mesenchymal stem cells into spinal cord injury animal models have been performed and have been shown to produce a better outcome than with undifferentiated cells [38,39]. Therefore, neuron-like cells differentiated using the methods in this study may help to improve treatment outcomes in spinal cord injury in dogs.
It has been reported that some neuron-like cells after grafting into injury site appeared to lose the neural phenotypes and instead transdifferentiated into myelin-forming cells [40]. Therefore, further investigation will be required, into the timing of transplantation, the behavior of the transplanted neuron-like cells, and whether they are used in combination with undifferentiated BMSCs, to refine the clinical applications of these cells in the treatment of spinal cord injury.
Our purified rc-bFGF may be promising as a treatment for spinal cord injury in canine medicine. Many studies have demonstrated that bFGF has biological functions such as angiogenesis, tissue healing and regeneration of injured nerves [30,31,41]. In addition, bFGF has been shown to inhibit apoptosis of neurons and to exhibit a neuroprotective effect [41,42]. The administration of bFGF increased both the survival of neurons and neurogenesis in spinal cord injury models [43,44]. In the present study, rc-bFGF was able to differentiate mesenchymal stem cells into functional neuron-like cells, suggesting that it may also have the ability to differentiate endogenous neural stem cells in spinal cord into functional neurons. Furthermore, it is reported that bFGF treatment improved locomotor function in spinal cord injury model, via axonal regeneration [45]. Our purified rc-bFGF may be effective in repairing injured spinal cord in dogs, by a similar mechanism.
Recently, the efficacy of transplantation with a combination of bFGF and BMSCs has been demonstrated in spinal cord injury models [46]. Therefore, a combination of rc-bFGF and canine BMSCs would be a candidate as a treatment strategy for spinal cord injury in dogs. Clinical applications of rc-bFGF in dogs will require consideration of the route of administration, the need for carriers, and the possible combination of rc-bFGF and canine BMSCs.
5. Conclusions
In our study, a functional rc-bFGF was successfully synthesized using a wheat germ cell-free protein synthesis system. The rc-bFGF induced the differentiation of canine BMSCs into voltage- and glutamate-responsive neuron-like cells. Administration of rc-bFGF alone or in combination with canine BMSCs may contribute to regenerative therapy of spinal cord injury in dogs.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach, Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cryotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Woodbury D., Schwarz E.J., Prockop D.J., Black I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 6.Edamura K., Kuriyama K., Kato K., Nakano R., Teshima K., Asano K. Proliferation capacity, neuronal differentiation potency and microstructures after the differentiation of canine bone marrow stromal cells into neurons. J Vet Med Sci. 2012;74:923–927. doi: 10.1292/jvms.11-0388. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y., Chopp M., Chen J., Wang L., Gautam S.C., Xu X.X. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cerebr Blood Flow Metabol. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Ohta M., Suzuki Y., Noda T., Ejiri Y., Dezawa M., Kataoka K. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Wright K.T., El Masri W., Osman A., Chowdhury J., Johnson W.E. Concise review: bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cell. 2011;29:169–178. doi: 10.1002/stem.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forosyak S., Jendelova P., Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochem. 2013;95:2257–2270. doi: 10.1016/j.biochi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Anbari F., Khalili M.A., Bahrami A.R., Khoradmehr A., Sadeghian F., Fesahat F. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen Res. 2014;9:919–923. doi: 10.4103/1673-5374.133133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung D.I., Ha J., Kang B.T., Kim J.W., Quan F.S., Lee J.H. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009;285:67–77. doi: 10.1016/j.jns.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Nakano R., Edamura K., Sugiya H., Narita T., Okabayashi K., Moritomo T. Evaluation of mRNA expression levels and electrophysiological function of neuron-like cells derived from canine bone marrow stromal cells. Am J Vet Res. 2013;74:1311–1320. doi: 10.2460/ajvr.74.10.1311. [DOI] [PubMed] [Google Scholar]
- 14.Nakano R., Edamura K., Nakayama T., Teshima K., Asano K., Narita T. Differentiation of canine bone marrow stromal cells into voltage- and glutamate-responsive neuron-like cells by basic fibroblast growth factor. J Vet Med Sci. 2015;77:27–35. doi: 10.1292/jvms.14-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano R., Edamura K., Nakayama T., Narita T., Okabayashi K., Sugiya H. Fibroblast growth factor receptor-2 contributes to the basic fibroblast growth factor-induced neuronal differentiation in canine bone marrow stromal cells via phosphoinositide 3-kinase/Akt signaling pathway. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G.H., Shi H.J., Che M.T., Huang M.Y., Wei Q.S., Feng B. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials. 2018;181:15–34. doi: 10.1016/j.biomaterials.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K. Safety of autologous bone marrow stromal cell transplantation in dogs with acute spinal cord injury. Vet Surg. 2012;41:437–442. doi: 10.1111/j.1532-950X.2011.00959.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y., Jo S.H., Kim W.H., Kweon O.K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res Ther. 2015;6:229. doi: 10.1186/s13287-015-0236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machill B.G., Borjesson D.L., Sieber-Blum M., Nolta J.A., Sturges B.K. Stem cells in canine spinal cord injury -promise for regenerative therapy in a large animal model of human disease. Stem cell Rev Rep. 2015;11:180–193. doi: 10.1007/s12015-014-9553-9. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman A.M., Dow S.W. Stem cell trials using companion animal disease models. Stem Cell. 2016;34:1709–1729. doi: 10.1002/stem.2377. [DOI] [PubMed] [Google Scholar]
- 21.Besalti O., Aktas Z., Can P., Akpinar E., Elcin A.E., Elcin Y.M. The use of autologous neurogenically-induced bone marrow derived mesenchymal stem cells for the treatment of paraplegic dogs without nociception due to spinal trauma. J Vet Med Sci. 2016;78:1465–1473. doi: 10.1292/jvms.15-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L., Lin H., Bai S., Zehng L., Zhan X. Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration. Neurochem Int. 2018;115:80–84. doi: 10.1016/j.neuint.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Mukhamedshina Y.O., Gracheve O.A., Mukhutdinova D.M., Chelyshev Y.A., Rizvanov A.A. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14:227–237. doi: 10.4103/1673-5374.244778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mothe A.J., Tator C.H. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31:701–713. doi: 10.1016/j.ijdevneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Masuhiro Y., Kayama K., Fukushima A., Baba K., Kamiya Y., Gotoh M. SOCS-3 inhibits E2F/DP-1 transcriptional activity and cell cycle progression via interaction with DP-1. J Biol Chem. 2008;283:31575–31583. doi: 10.1074/jbc.M800328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oho M., Nakano R., Nakayama R., Sakurai W., Miyamoto A., Masuhiro Y. TIPE2 (tumor necrosis factor α-induced protein 8-like 2) is a novel negative regulator of TAK1 signal. J Biol Chem. 2016;291:22650–22660. doi: 10.1074/jbc.M116.733451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham J.A., Whang J.L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986;5:2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu P.J., Ferrari G., Galloway A.C., Mignatti P., Pintucci G. Basic fibroblast growth factor (FGF-2): the high molecular weight form come of age. J Cell Biochem. 2007;100:1100–1108. doi: 10.1002/jcb.21116. [DOI] [PubMed] [Google Scholar]
- 29.Yim D., Ghosh S., Guy G.R., Virshup D.M. Casein kinase 1 regulates sprout 2 in FGF-ERK signaling. Oncogene. 2015;34:474–484. doi: 10.1038/onc.2013.564. [DOI] [PubMed] [Google Scholar]
- 30.Ornitz D.M., Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. revies3005.1-3005.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun Y.R., Won J.E., Jeon E., Lee S., Kang W., Jo H. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010 doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui Q., Zi J., Li X., Liu C., Wang X. FGF Family: From drug development to clinical application. Int J Mol Sci. 2018;19:1875. doi: 10.3390/ijms19071875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito A., Bizenjima T., Takeuchi T., Suzuki E., Sato M., Yoshikawa K. Treatment of intrabony periodontal defects using rhFGF-2 in combination with deproteinized bovine bone material or rhFGF-2 alone: a6-month randomized controlled trial. J Clin Periodontol. 2019;46:332–341. doi: 10.1111/jcpe.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H., Xia Y., Lu S.Q., Soong T.W., Feng Z.W. Basic fibroblast growth factor-induced neural differentiation of mouse bone marrow stromal cells requires FGFR-1, MAPK/ERK, and transcription factor AP-1. J Biol Chem. 2008;283:5287–5295. doi: 10.1074/jbc.M706917200. [DOI] [PubMed] [Google Scholar]
- 35.Guan M., Xu Y., Wang W., Lin S. Differentiation into neurons of rat bone marrow-derived mesenchymal stem cells. Eur Cytokine Netw. 2014;25:58–63. doi: 10.1684/ecn.2014.0357. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y., Zhang Y., Tian K., Xun C., Wang S., Lu D. Effects of nerve growth factor and basic fibroblast growth factor dual gene modification on rat bone marrow mesenchymal stem cell differentiation into neuron-like cells in vitro. Mol Med Rep. 2016;13:49–58. doi: 10.3892/mmr.2015.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M., Zhao Y., Gao Y., Hao P., Shang J., Duan H. Differentiation of bone marrow mesenchymal stem cells into neural linage cells induced by bFGF-chitosan controlled release system. BioMed Res Int. 2019 doi: 10.1155/2019/5086297. ID 5086297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S.S., Lee Y.J., Lee S.H., Lee D., Choi K., Kim W.H. Functional recovery after spinal cord injury in dogs treated with a combination of matrigel and neural-induced adipose-derived mesenchymal stem cells. Cryotherapy. 2012;14:584–597. doi: 10.3109/14653249.2012.658913. [DOI] [PubMed] [Google Scholar]
- 39.Yazdani S.O., Pedram M., Hafizi M., Kabiri M., Soleimani M., Dehghan M.M. A comparison between neurally induced bone marrow derived mesenchymal stem cells and olfactory ensheathing glial cells to repair spinal cord injuries in rat. Tissue Cell. 2012;44:205–213. doi: 10.1016/j.tice.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Qui X.C., Jin H., Zhang R.Y., Ding Y., Zheng X., Lai B.Q. Donor mesenchymal stem cell-derived neural-like cells transdifferentiate into myelin-forming cells and promote axon regeneration in rat spinal cord transection. Stem Cell Res Ther. 2015;6:105. doi: 10.1186/s13287-015-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan L., Tian F.R., Zhu Ge D.L., Zhu Ge Q.C., Shen B.X., Jin B.H. Implantable porous gelatin microspheres sustained release of bFGF and improved its neuroprotective effect on rats after spinal cord injury. PloS One. 2017;12 doi: 10.1371/journal.pone.0173814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee T.T., Green B.A., Dietrich W.D., Yezierski R.P. Neuroprotective effects of basic fibroblast growth factor following spinal cord contusion injury in the rat. J Neurotrauma. 1999;16:347–356. doi: 10.1089/neu.1999.16.347. [DOI] [PubMed] [Google Scholar]
- 43.Rabchevsky A.G., Fugaccia I., Turner A.F., Blades D.A., Mattson M.P., Scheff S.W. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000;164:280–291. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H.Y., Wang Z.G., Wu F.Z., Kong X.X., Yang J., Lin B.B. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neuroboiol. 2013;48:452–464. doi: 10.1007/s12035-013-8432-8. [DOI] [PubMed] [Google Scholar]
- 45.Furukawa S., Furukawa Y. FGF-2-treatment improves locomotor function via axonal regeneration in the transected rat spinal cord. Brain Nerve. 2007;59:1333–1339. [PubMed] [Google Scholar]
- 46.Huang X.R., Xu H., Zhang Y., Jiang Y.B., Xia C.L., Fang S.C. Repair effect of bFGF combined with bone marrow mesenchymal stem cells on spinal cord injury in rats. ZhongGuo Gu Shang. 2019;32:653–657. doi: 10.3969/j.issn.1003-0034.2019.07.013. [DOI] [PubMed] [Google Scholar]