Abstract
Introduction
Neural crest (NC)-like stem/progenitor cells provide an attractive cell source for regenerative medicine because of their multipotent property and ease of isolation from adult tissue. Although human umbilical cord blood (HUCB) is known to be a rich source of stem cells, the presence of the NC-like stem/progenitor cells in HUCB remains to be elucidated. In this study, we have isolated NC-like progenitor cells using an antibody to p75 neurotrophin receptor (p75NTR) and examined their phenotype and stem cell function in vitro.
Methods
To confirm whether p75NTR+ NC-derived cells are present in cord blood, flow cytometric analysis of cord blood derived from P0-Cre/Floxed-EGFP reporter mouse embryos was performed. Freshly isolated HUCB mononuclear cells was subjected to flow cytometry to detect p75NTR+ cells and determined their immunophenotype. HUCB p75NTR+ cells were then collected by immunomagnetic separation and their immunophenotype, clonogenic potential, gene expression profile, and multilineage differentiation potential were examined.
Results
NC-derived EGFP+ cells co-expressing p75NTR was detected in cord blood of P0-Cre/Floxed-EGFP reporter mice. We found that freshly isolated HUCB mononuclear cells contained 0.23% of p75NTR+ cells. Isolated p75NTR+ cells from HUCB efficiently formed neurospheres and could differentiate into neuronal and glial cell lineages. The p75NTR+ cells expressed a set of NC-associated genes and undifferentiated neural cell marker genes before and after the culture.
Conclusions
These findings revealed that HUCB contained the p75NTR+ NC-like progenitor cell population which have the self-renewal capacity and the potential to differentiate into both neuronal and glial cell lineages.
Keywords: Neural crest stem cells, Umbilical cord blood, p75 neurotrophin receptor, Cell therapy
1. Introduction
The neural crest (NC) is a transient embryonic cell population with stem cell-like properties that emerges from the dorsal neural plate border and migrates extensively and differentiates into diverse derivatives. NC contains neural crest stem cells (NCSCs) that have ability of self-renewal and differentiate into a many array of cell types [1]. Although NCSCs rapidly lose their multipotency during the course of development, many studies demonstrated that NC-like stem/progenitor cells can be found and isolated in the adult tissue including bone marrow [2,3], dorsal root ganglia [2,4], gut [5], skin [6,7], heart [8], cornea [9], carotid body [10], and dental pulp [11]. These adult tissue-derived NC-like stem/progenitor cells provide an attractive cell source for regenerative medicine because of their multipotent property, the ease of accessibility and less ethical issue for collection compared to embryo tissue-derived stem cells.
Biomarkers for NCSCs have been extensively studied and several critical markers were identified. Among them, p75 neurotrophin receptor (p75NTR), also known as low affinity nerve growth factor (LNGFR) and CD271, has been recognized as a robust marker of NCSCs [12]. p75NTR is a member of the tumor necrosis factor (TNF) superfamily of transmembrane glycoprotein receptors with important roles in regulating axon growth and neuron/oligodendrocyte survival. p75NTR has been used as a marker for cell sorting to isolate NCSCs from fetal and adult tissue such as dental pulp [13], esophageal keratinocytes [14], gut [5], and oral mucosa [15]. Furthermore, Immunoselection for p75NTR has been successfully employed to generate multipotent NCSCs from human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) [[16], [17], [18], [19]]. These findings indicate that p75NTR is a promising biomarker for human NCSCs.
Human umbilical cord blood (HUCB) is widely used as a cell source of hematopoietic stem cell transplantation to treat malignant and nonmalignant disorders. As a cell souse for cell-based therapies, cord blood has advantages such as easily accessible, lack of ethical issue for collection, and containing cells that are primitive and little exposed to immunological challenges. HUCB also contains other types of stem/progenitor cells that have multilineage differentiation potential including mesenchymal- and neuronal cell-lineages [20,21]. Furthermore, the presence of more primitive cell populations with an embryonic stem cell-like phenotype in HUCB has been reported [[22], [23], [24]].
In a genetic lineage tracing study using Protein-0 (P0)- and Wnt1-cre reporter mouse lines, Nagoshi et al. [2] reported that NCSCs enter fetal circulation from aorta-gonad-mesonephros region and migrate to bone marrow through fetal liver in the embryonic stage. However, the presence of neural crest-derived cells including NCSCs in HUCB remains to be elucidated. In the present study, we attempted to identify NC-like stem/progenitor cells in HUCB using an antibody to p75NTR and examined their phenotype and stem cell function in vitro. Herein, we identified a rare population of p75NTR+ cells in HUCB. We found that the isolated p75NTR+ cells had self-renewal capacity and neuronal and glial cell differentiation potential. These data provide the first evidence that HUCB harbor the NC-like progenitor cells that can be detected and isolated as p75NTR+ cells.
2. Materials and methods
2.1. Cells isolation
Experiments of HUCB were approved by the Committee of Medical Ethics of Nihon University School of Medicine and the Ethics Committee of the Japan Cord Blood Bank Network. Fresh HUCB harvests were obtained from preterm and term deliveries from Nihon University Itabashi Hospital, Tokyo, Japan after the informed consent of the mothers. According to the guidelines of the Japan Cord Blood Bank, HUCB was collected immediately after the delivery of the placenta and drained by gravity in the adjacent room, and allowed to flow into the sterile collection bag containing the anticoagulant citrate-phosphate dextrose (CBC-20, Nipro, Osaka, Japan) until the flow ceased. Mononuclear cells (MNCs) were isolated from HUCB by density gradient separation at 350 g for 30 min, using Lymphoprep (density: 1.077 g/cm3, Axis-Shield PoC AS, Oslo, Norway). The separated MNCs were washed three times with phosphate-buffered saline (PBS). The p75NTR+ cell fraction was isolated from a portion of MNCs by a magnetic cell separator MACS (Miltenyi Biotec, Bergisch Gladbach, Germany) according to a previously reported by Quirici et al. [25]. Briefly, the MNCs were incubated with FcR blocking reagent and p75NTR (CD271)-allophycocyanin (APC) antibody (Miltenyi Biotec) for 10 min at 4 °C, washed with 10 ml MACS buffer containing PBS, 0.5% bovine serum albumin (BSA) and 2 mM EDTA, then incubated with FcR blocking reagent and anti-APC microbeads (Miltenyi Biotec) for 15 min at 4 °C. The magnetic-labeled cells were passed through a large separation LS column (Miltenyi Biotec) in a strong magnetic field, then washed three times with the MACS buffer. The column was then removed from the magnetic field and eluted with 5 ml of the MACS buffer. Both eluted p75NTR+ and unabsorbed p75NTR− cells were counted and assessed the viability using hemocytometer. The purity of p75NTR+ cells was determined by flow cytometry using phycoerythrin (PE)-conjugated anti-human p75NTR (CD271) antibody (Miltenyi Biotec). Human bone marrow MNCs and human adipose-derived stem cells (ASCs) were purchased from Lonza (Walkersville, MD).
2.2. Cell culture
Neurosphere culture was performed as described previously [26]. Briefly, isolated HUCB p75NTR+ or p75NTR− cells were plated on low cell binding 96-well plates (Thermo Fisher Scientific, Waltham, MA) at a density of 3 × 105 per well in serum-free Dulbecco's modified Eagle's medium: Nutrient Mixture F-12 (DMEM/F12, 1:1, Invitrogen, Carlsbad, CA) supplemented with B27 supplement (Invitrogen), 20 ng/ml epidermal growth factor (EGF, R&D systems, Minneapolis, MN), 10 ng/ml fibroblast growth factor-2 (FGF-2, R&D systems), 10 ng/ml recombinant mouse leukemia inhibitory factor (LIF, Chemicon, Temecula, CA). For plastic adherence culture to detect mesenchymal stem cells (MSCs), cells were plated on plastic culture dishes in DMEM supplemented with 20% fetal bovine serum (FBS, JRH Bioscience, Lenexa, KS). The cells were incubated at 37 °C in a fully humidified atmosphere containing 5% CO2.
2.3. Flow cytometric analysis
Antibodies against the human antigens CD13-PE, CD31-PE, CD34-PE, CD44-fluorescein isothiocyanate (FITC), CD49d-PE, CD57-FITC, CD73-PE, CD90 (Thy-1)-pure, and CD105 (Endoglin)-pure were purchased from BD Biosciences (San Jose, CA), CD45-FITC was from Immunotech (Marseille, France), and CD133/1-PE, and CD271(p75NTR)-FITC, -PE, -APC were obtained from Miltenyi Biotec. For each antibody expression assessment, a total of 1 × 105 MNCs from HUCB and human bone marrow were resuspended in 100 μl PBS containing 0.5% BSA and 200 mM EDTA, incubated with rabbit serum for blocking nonspecific binding. The cells were then incubated with primary antibodies for 30 min on ice. Binding of unconjugated anti-CD90 and CD105 was detected by secondary staining with PE-conjugated anti-mouse IgG antibody (BD Biosciences). FITC- and PE-conjugated mouse IgGs were used as the control isotype at the same concentration as specific primary antibodies. The fluorescence intensity of the cells was evaluated by flow cytometry (FACSCalibur, BD Biosciences), and data was analyzed with CellQuest software (BD Biosciences).
2.4. Immunocytochemistry
For immunocytochemical analysis, the following primary antibodies were used: rabbit polyclonal anti-human Nestin (1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA), and mouse monoclonal anti-human Musashi-1 (clone 282613, 500 μg/ml, R&D Systems) (early neuronal progenitor markers), mouse monoclonal anti-human CD31 (clone JC70A, 1:40; DakoCytomation, Glostrup, Denmark), mouse monoclonal anti-human NGFR (p75NTR) (clone C40-1457, 1:200; BD Biosciences) (neural crest progenitor marker), rabbit polyclonal anti-βIII-tubulin (1:800; Abcam, Cambridge, UK), mouse monoclonal anti-neurofilament (NF) 200 (clone NE14, 1:100; Sigma–Aldrich, St. Louis, MO), and mouse monoclonal anti-microtubule associated protein (MAP2) (clone HM-2, 1:200; Sigma–Aldrich) (neuronal markers), rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) (1:800; DakoCytomation) (astrocyte marker), mouse monoclonal anti-O4 (clone O4, 1 mg/ml; R&D Systems) (oligodendrocyte marker), and mouse monoclonal anti-α-smooth muscle actin (ASMA) (clone 1A4, 1:500; DakoCytomation) (myofibroblast marker). For immunostaining of colonies, day 1 colonies were picked up from neurosphere culture then cytospun on slides by using cytospine 3 (Thermo Fisher Scientific) at 500 rpm for 5 min. Cells were fixed with 4% paraformaldehyde (PFA) for 2 h at 4 °C then treated with PBS-Triton X (0.1%) incubated for 5 min. Before incubation with primary antibodies, nonspecific binding was blocked with 1% normal goat serum in PBS for 1 h at room temperature. The cells were then incubated with primary antibodies overnight at 4 °C. After washing with PBS, cells were exposed to goat anti-mouse or anti-rabbit secondary antibodies conjugated to Alexa Fluor 488 or 594 (1:1000, Invitrogen) for 60 min at room temperature in the darkness. Cell nuclei were counterstained with 5 μg/ml Hoechst 33342 in dH2O for 30 min. After final washes the coverslip were mounted in Fluoromount-G (Southern Biotech, Birmingham, AL). For the bromodeoxyuridine (BrdU) incorporation assay, 20 μM of BrdU (Sigma–Aldrich) was added into the culture 4 h before fixation, fix with 70% ethanol for 30 min, then washed with 0.07 N NaOH followed with great amount of PBS. The cells were incubated with FITC-labeled anti-BrdU monoclonal antibody (BD Biosciences) for 1 h at room temperature. All cultures were examined using Nikon eclipse (TE 2000-U, Nikon, Tokyo, Japan) fluorescence microscope and a confocal laser scanning microscope Fluoview FV10i (Olympus, Tokyo, Japan). For quantitative analysis, the percentage of BrdU+ cells, Nestin+ cells, Musashi-1+ cells, p75NTR+ cells, and CD31+ cells were determined by counting the number of cells (nuclei) in randomly selected 5 spheres.
2.5. Reverse transcription-polymerase chain reaction
Total RNA was extracted from cells by using the RNeasy Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Semiquantitative reverse transcription (RT)-polymerase chain reaction (PCR) was performed for each gene (Table 1). Briefly, 1 μg RNA was reverse-transcribed into cDNA with random 9-mers with a Takara RNA PCR Kit (AMV) Ver. 3.0 (Takara Bio, Ohtsu, Japan) in a total reaction volume of 10 μl. Reverse-transcription product was amplified by PCR in a 50 μl reaction volume containing 0.2 μM of each forward and reverse primer sets, 5 units/ml of Takara Ex Taq (Takara Bio), 10× Ex Taq buffer, 25 mM MgCl2, and 2.5 mM dNTP mixture. The protocol for the thermal cycler (Applied Biosystems AB, Foster City, CA) was as follows: denaturation at 94 °C for 2 min followed by 28–38 cycles of 94 °C for 30 s, optimal annealing temperature at 60 °C for 30 s, and 72 °C for 1.5 min, with the reaction terminated by final 7 min incubation at 72 °C. β-actin served as the internal control. 10 μl of each PCR product was electrophoresed on an e-PAGEL (10%) (ATTO, Tokyo, Japan) and SYBER green (Cambrex, Walkersville, MD) stained cDNA were visualized under blue incident light at 460 nm using a LAS-3000 luminescent image analyzer (Fuji film, Tokyo, Japan).
Table 1.
Details of primers used for RT-PCR analysis.
| Gene | Sequence (5′→3′) | Size (bp) | |
|---|---|---|---|
| NGFR (p75NTR) | Forward | GCCAGGACAAGCAGAACAC | 367 |
| Reverse | GCCAGGATGGAGCAATAGAC | ||
| Nestin | Forward | ACACCTGTGCCAGCCTTTCTT | 376 |
| Reverse | TGAACACTCTAGACCCACCGGA | ||
| Musashi-1 | Forward | GACTCAGTTGGCAGACTACGCA | 129 |
| Reverse | TGAAAGTGACGAAGCCGAAAC | ||
| c-kit | Forward | GATCACGGAAAAGGCAGAAG | 123 |
| Reverse | GAGCGGTCAACAAGGAAAAG | ||
| TWIST1 | Forward | GTCCGCAGTCTTACGAGGAG | 159 |
| Reverse | CCAGCTTGAGGGTCTGAATC | ||
| SNAI2 (Slug) | Forward | TCGGACCCACACATTACCTT | 145 |
| Reverse | TGACCTGTCTGCAAATGCTC | ||
| SNAI1 | Forward | CTCTAGGCCCTGGCTGCTA | 153 |
| Reverse | GCCTGGCACTGGTACTTCTT | ||
| WNT1 | Forward | TGGAAGGAATCGTGCTTTGG | 275 |
| Reverse | GCATCTCGGAGAATACGGTC | ||
| FOXD3 | Forward | TCTGCGAGTTCATCAGCAAC | 103 |
| Reverse | TTGACGAAGCAGTCGTTGAG | ||
| TFAP2A (AP2α) | Forward | GTTACCCTGCTCACATCAC | 147 |
| Reverse | TCTTGTCACTTGCTCATTGG | ||
| SOX1 | Forward | ATAGCCAATGCCAGGTGCTC | 282 |
| Reverse | CCGTGAATACGATGAGTGTTACC | ||
| SOX9 | Forward | CGAACGCACATCAAGACG | 161 |
| Reverse | TTCTGGTGGTCGGTGTAGTC | ||
| SOX10 | Forward | TTGATGTGGCTGAGTTGGAC | 201 |
| Reverse | CTGTCTTCACCTGGGCTTTG | ||
| CD31 | Forward | GCAAAATGGGAAGAACCTGA | 250 |
| Reverse | CACTCCTTCCACCAACACCT | ||
| CD34 | Forward | AAAACGTGTTGCCTTGAACC | 209 |
| Reverse | TGGCCCAGAGAGACTAGAA | ||
| AC133 | Forward | TTGTGGCAAATCACCAGGTA | 162 |
| Reverse | TCAGATCTGTGAACGCCTTG | ||
| CDH2(Ncad) | Forward | ATCGTGTCTCAGGCTCCAAG | 377 |
| Reverse | ATGGCGAACCGTCCAGTAG | ||
| ENO2 (NSE) | Forward | TGGATGTTGCTGCCTCAG | 201 |
| Reverse | CATTGGCTGTGAACTTGGAC | ||
| SNCA | Forward | GTTGGAGGAGCAGTGGTGAC | 225 |
| Reverse | GGCTTCAGGTTCGTAGTCTTG | ||
| ACTB (βactin) | Forward | GAGAAAATCTGGCACCACA | 339 |
| Reverse | CTCGGTGAGGATCTTCATG | ||
2.6. Neurogenic differentiation
One day after seeding, p75NTR+ and p75NTR− HUCB cells were incubated for 24 h with alpha-modified minimum essential medium (α-MEM, Invitrogen) containing 1 mM beta-mercaptoethanol (βME), the media were removed, washed with PBS (pH 7.4) and replaced with new media consisting of α-MEM, 10% FBS (Sigma–Aldrich) and 35 ng/ml all-trans-retinoic acid (RA, Sigma–Aldrich) for 3 days. Cells were washed with PBS and transferred to α-MEM containing 10% FBS, 5 μM forskolin (FSK, Sigma–Aldrich), 20 ng/ml EGF, 10 ng/ml FGF-2 and 10 ng/ml brain-derived neurotrophic factor (BDNF, Sigma–Aldrich); then they were cultured in 48 well human fibronectin-coated plate (BD Biosciences) for 7 days. All cultures were visualized immunocytochemically for several neuronal markers according to description in “Immunocytochemistry”.
2.7. Glial cell differentiation
One day after seeding, p75NTR+ and p75NTR− HUCB cells were cultured in α-MEM containing 1 mM βME for 24 h then replaced with 35 ng/ml RA for 3 days. The medium used for promoting glial cells differentiation was: α-MEM containing 10% FBS, 5 μM FSK, 10 ng/ml FGF-2, 5 ng/ml recombinant human platelet derived growth factor-BB (PDGF-BB, R&D Systems) and 200 ng/ml recombinant human Neuregulin1-β1/Heregulin1-β1 EGF domain (R&D Systems); they were incubated in 48 well human fibronectin-coated plate for 7 days. All cultures were visualized immunocytochemically for several glial cell markers according to description in “Immunocytochemistry”.
2.8. Myofibroblast differentiation
p75NTR+ HUCB cells were plated on plastic culture dishes and cultured in DMEM supplemented with 5%FBS and 5 ng/ml recombinant human transforming growth factor-β1 (TGF-β1, R&D Systems) for 7 days. Human ASCs were used for this analysis as positive control cells. All cultures were visualized immunocytochemically for ASMA to detect myofibroblastic differentiation according to description in “Immunocytochemistry”.
2.9. Flow cytometric analysis of cord blood MNCs in P0-cre/floxed-EGFP mice embryos
Transgenic mice expressing Cre recombinase under control of the Protein-0 (P0) promoter (P0-Cre) [27] were mated with EGFP reporter mice (CAG-CATloxP/loxP-EGFP) [28] to obtain P0-Cre/Floxed-EGFP double-transgenic mice. Adult wild-type ICR mice were purchased for mating from CLEA Japan, Tokyo, Japan. All animal experiments were approved by the Animal Research and Care committee at the Nihon University and the experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of Nihon University. Pregnant P0-Cre/Floxed-EGFP mice were deeply anesthetized and sacrificed be cervical dislocation. Blood cells from E15.5 embryos were obtained by cutting the umbilical cord and allowing the blood to flow freely into PBS with 2 mM EDTA. Red blood cells were hemolyzed by Pharm Lyse (BD Biosciences) and remained MNCs were collected and washed twice, suspended in PBS with 0.5% BSA and 2 mM EDTA. Cells were stained as described previously [29]. Briefly, after blocking, MNCs were incubated with rabbit polyclonal anti mouse p75NTR antibodies (Millipore, Temecula, CA) for 30 min at 4 °C. Cells were washed three times, incubated with secondary antibody of anti-rabbit IgG Alexa Fluor 594 (Molecular probes, Karlsruhe, Germany) for 30 min at 4 °C. The stained cells (1 × 105) were analyzed by FACSCalibur flow cytometer (BD Biosciences). For negative isotype controls, rabbit IgG1 (BD Biosciences) was used. Dead cells were excluded from the plots on the basis of 7AAD staining. EGFP+ cells were identified by EGFP fluorescence.
3. Results
3.1. Umbilical cord blood contains p75NTR+ NC-derived cells in mice
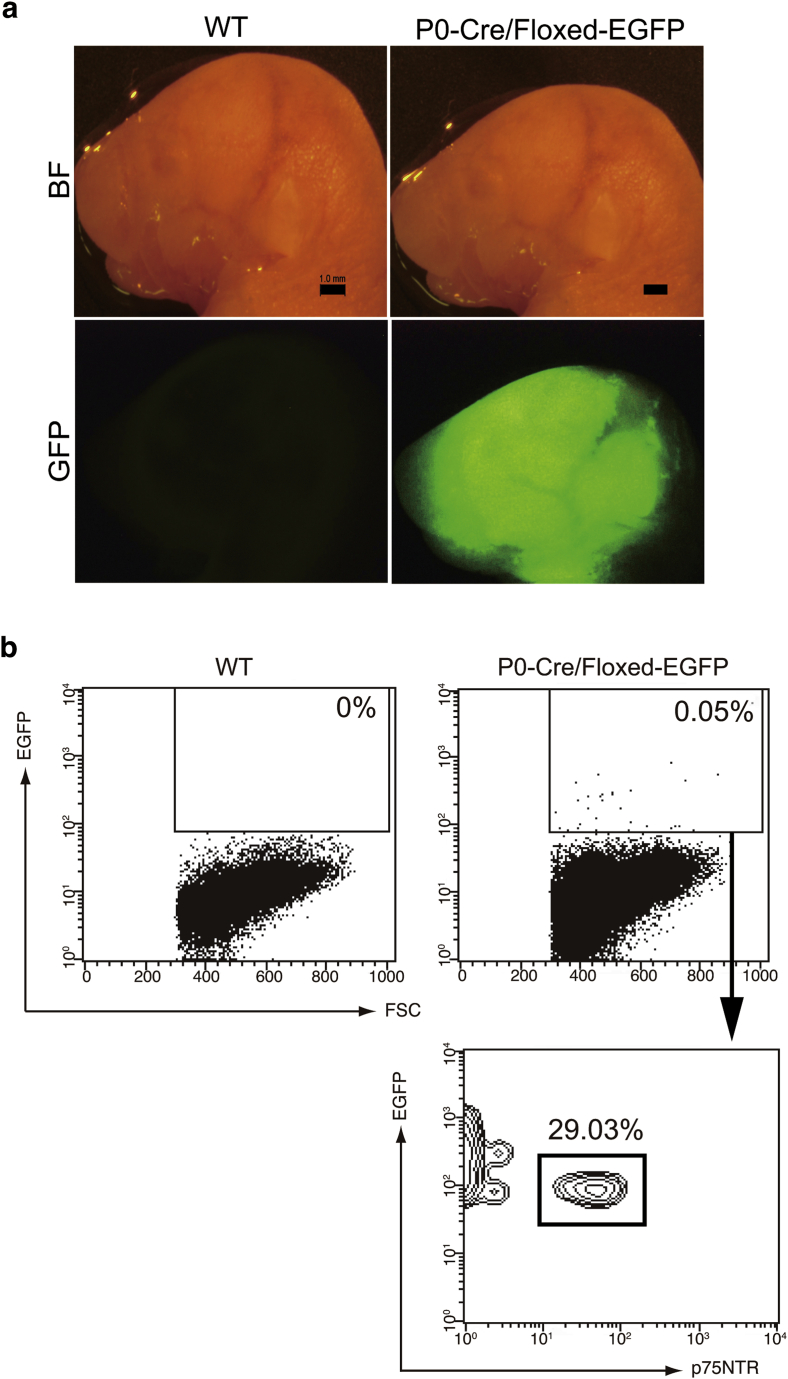
A previous lineage tracing study demonstrated that NC-derived cells migrate through the bloodstream to the bone marrow in fetal period [2]. To confirm whether NC-derived cells are present in umbilical cord blood, we examined flow cytometric analysis of umbilical cord blood in the P0-Cre/Floxed-EGFP mouse embryos, in which NC-derived cells express EGFP. At E15.5, positive EGFP fluorescence at frontal region of the head was observed in the P0-Cre/Floxed-EGFP mice (Fig. 1a). Flow cytometric analysis revealed that 0.05% of cord blood MNCs were positive for EGFP in the P0-Cre/Floxed-EGFP mice while no EGFP-positive cells were detected in the wild type mice (Fig. 1b). A certain number of the EGFP-positive cells co-expressed p75NTR (29.03%). Similar results were obtained from three independent experiments. These data confirm that NC-derived cells that express p75NTR are present in cord blood.
Fig. 1.
Detection of neural crest (NC)-derived cells in umbilical cord blood (UCB) from P0-Cre/Floxed-EGFP mice embryos. UCB mononuclear cells (MNCs) were collected from P0-Cre/Floxed-EGFP or wild type mice embryos at E15.5 and were subjected to flow cytometric analysis. (a) Macroscopic and fluorescent images of heads in P0-Cre/Floxed-EGFP and wild type (WT) mice embryo. Scale bars represent 1.0 mm. (b) Flow cytometric analysis for EGFP-expressing cells. Data are representative of at least three experiments.
3.2. HUCB MNCs contain a rare population of p75NTR+ cells that show a different immunophenotype from bone marrow p75NTR+ cells
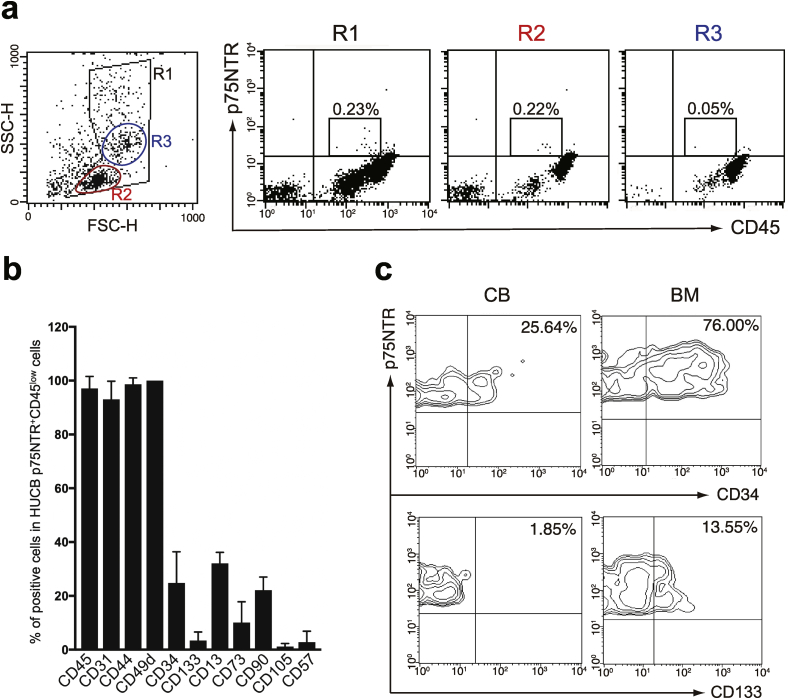
To date, p75NTR is known to be a robust marker for characterization and purification of NCSCs [12,30]. We examined whether p75NTR+ cells are present in HUCB. Flow cytometric analysis revealed that 0.23% of the freshly isolated HUCB MNCs showed immunoreactivity for p75NTR as shown in gated area R1 (Fig. 2a). The most of the p75NTR+ cells expressed low levels of CD45 and were distributed in the lymphocyte fraction (R2; 0.22%). Cell surface antigen profile of the p75NTR+ CD45low cells showed relatively high level of a vascular endothelial cell marker CD31 (93.1 ± 6.7%), a stem cell-associated marker CD44 (98.7 ± 2.3%), and a neural crest cell marker CD49d (100.0 ± 0.0%), whereas relatively low levels of hematopoietic stem/progenitor cell markers CD34 (24.8 ± 11.6%) and CD133 (3.4 ± 3.2%), and mesenchymal stem cell markers CD13 (32.1 ± 4.1%), CD73 (10.1 ± 7.7%), CD90 (22.1 ± 4.9%), and CD105 (1.2 ± 1.1%) (Fig. 2b). Interestingly, only 2.8 ± 1.0% cells expressed migratory neural crest cell marker CD57 (HNK-1).
Fig. 2.
Flow cytometric analysis of p75NTR+ cells in Human umbilical cord blood (HUCB) MNCs. (a) Freshly isolated MNCs from HUCB were subjected to flow cytometric analysis. (b) Cell surface antigens profile of p75NTR+ CD45low cells in HUCB MNCs. Data are shown as mean ± SD for three independent experiments. (c) Comparison of immunophenotype of p75NTR+ cells in HUCB MNCs and in human bone marrow (BM) MNCs. Data are representative of three independent experiments.
We next compared cell surface antigen profiles between HUCB p75NTR+ cells and bone marrow p75NTR+ cells. We found that p75NTR+ cells in bone marrow MNCs expressed higher levels of CD34 (76.00%) and CD133 (13.55%) compared to those in HUCB MNCs (Fig. 2c). In short, these data suggest that p75NTR+ cells were present in HUCB as a rare population, and the HUCB p75NTR+ cells showed different immunophenotype from the bone marrow p75NTR+ cells.
3.3. Isolation and enrichment of HUCB p75NTR+ cells by immunomagnetic separation
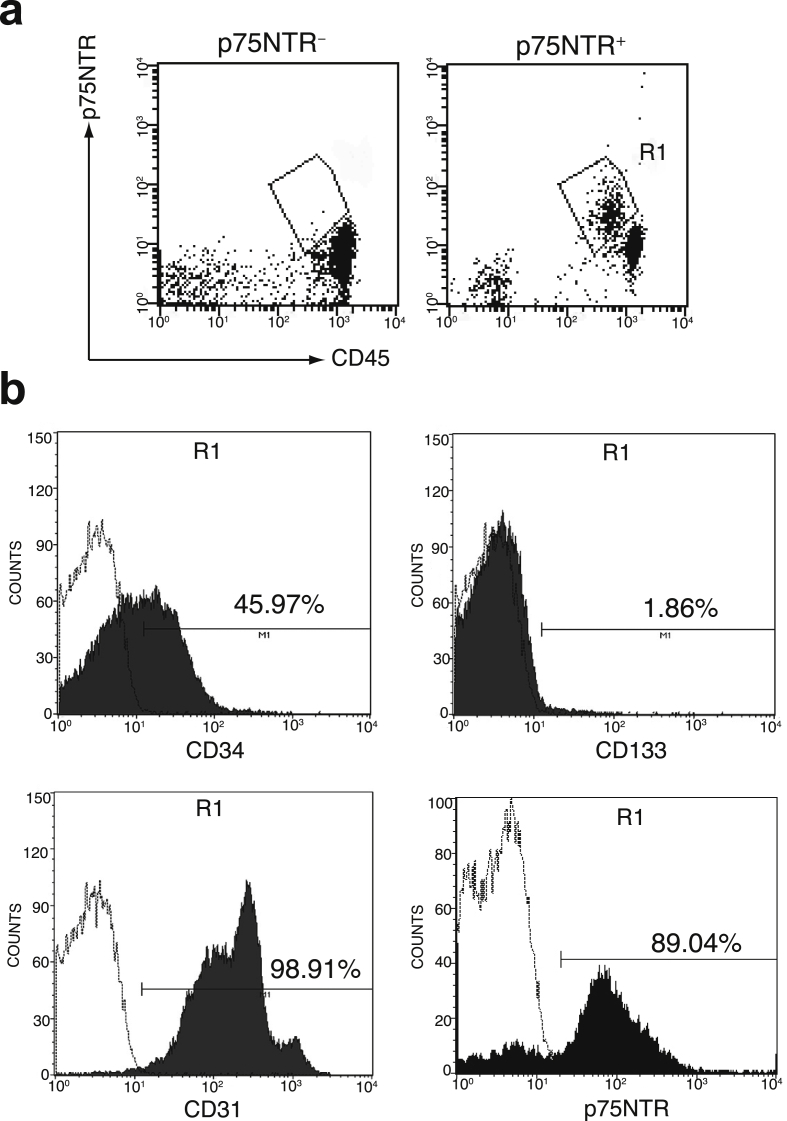
We next isolated HUCB p75NTR+ cells using a magnetic cell sorting system and examined their cell surface antigen profile. A total of 2–3 ×105 p75NTR+ cells were reproducibly collected from the HUCB MNCs. Flow cytometric analysis revealed that p75NTR+CD45low region as shown in gated area R1 was highly enriched in p75NTR+ cell fraction compared to p75NTR− cell fraction (Fig. 3a). We confirmed that the purity of p75NTR+ cells in the p75NTR+ cell fraction was 89.01 ± 0.77%. Because the p75NTR+ cell fraction was very small population in the HUCB MNCs, we again performed immunophenotypic analysis of the enriched p75NTR+ cells to determine more precisely their immunophenotype. As the result, the frequency of CD34+, CD133+, CD31+, and p75NTR+ cells in the gated p75NTR+CD45low cell fraction were 45.97%, 1.86%, 98.91%, and 89.04%, respectively (Fig. 3b). The immunophenotype was almost identical to that of the p75NTR+CD45low cells before isolation.
Fig. 3.
Flow cytometric analysis of enriched HUCB p75NTR+ cells. Freshly isolated p75NTR+ and p75NTR− cell fractions from HUCB MNCs by using magnetic cell separator technology were subjected to flow cytometric analysis. (a) Comparison of p75NTR+CD45low cell fraction (R1) in the p75NTR+ cells and the p75NTR− cells. (b) Representative histograms of cells surface antigens in the p75NTR+CD45low cells. Histograms show isotype control IgG staining profile (white) versus specific antibodies staining profile (black). Data are representative of at least three experiments.
3.4. Isolated HUCB p75NTR+ cells have neurosphere-forming capacity
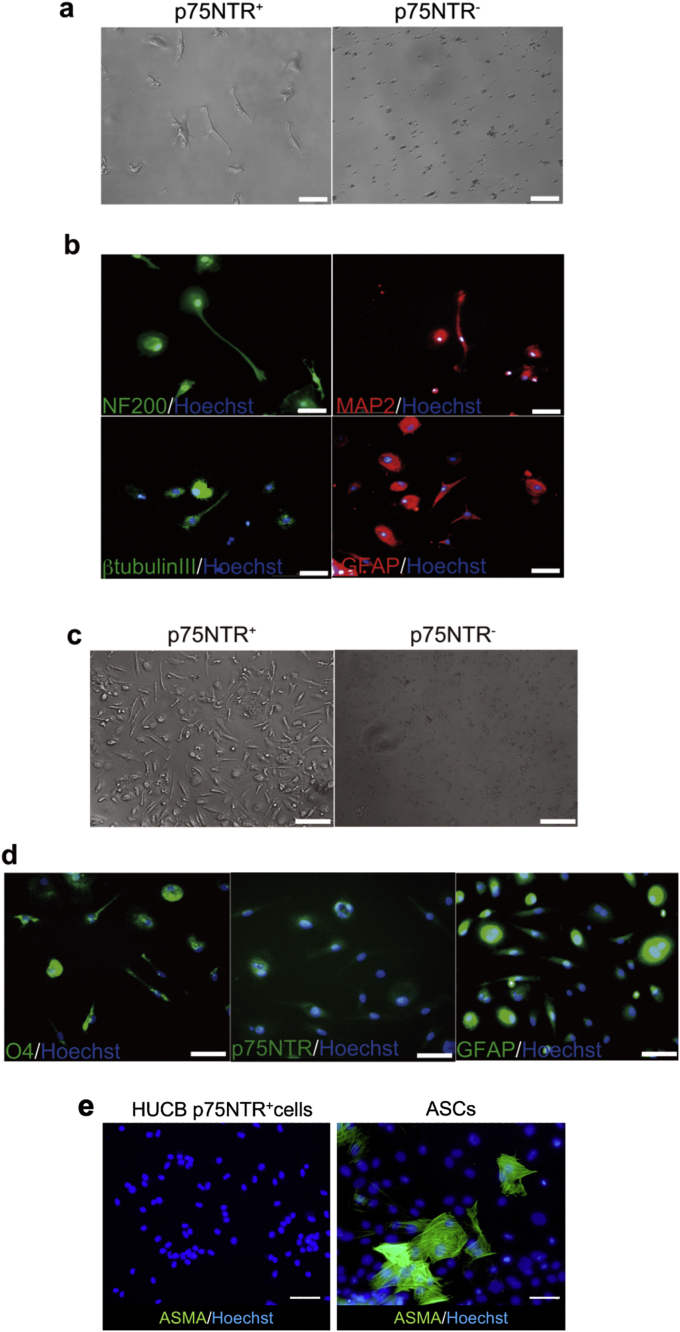
We next examined the clonogenic potential of the p75NTR+ cells isolated from HUCB MNCs. Two different culture conditions (neurosphere culture condition and MSC culture condition) were employed in the experiments. In the neurosphere culture condition, floating colonies that were morphologically similar to neurospheres were observed in the p75NTR+ cells by 24 h after plating. The spheres became larger and more varied in size by 7 days after plating (Fig. 4a). On the other hands, no or only very small colonies were detected in the p75NTR− cells. In the MSC culture condition, a few p75NTR+ cells adhered to plastic and formed elongated fibroblast-like morphology although the cells showed no proliferative and colony forming ability (Fig. 4a). The p75NTR− cells showed less adherence ability than the p75NTR+ cells.
Fig. 4.
Neurosphere culture of HUCB p75NTR+ cells. Freshly isolated p75NTR+ and p75NTR− cell fractions were cultured in a neurosphere culture condition or in a mesenchymal stem cell (MCS) culture condition. (a) Representative images of each cell fraction. (b) Bromodeoxyuridine (BrdU) incorporation in p75NTR+ cell-derived spheres. (c) Immunocytochemical analysis of p75NTR+ cell-derived spheres. Arrows represent p75NTR+ small spheres. (d) Representative images of secondary and tertiary spheres. Scale bars represent 50 μm.
We next performed immunocytochemical analysis of the spheres to determine their proliferation ability and immunophenotype. The BrdU incorporation assay revealed that BrdU-positive cells were detected in nuclei of the spheres (Fig. 4b), indicating the presence of proliferative cells. Quantitative analysis indicated that 38.0 ± 3.4% of total cells showed positive for BrdU. Immunostaining for stem/progenitor cell markers revealed that most of spheres strongly expressed the neuronal progenitor cell markers Nestin and Musashi-1 as well as p75NTR (Fig. 4c), suggesting the spheres contain neuroepithelial stem/progenitor cells. Notably, expression of p75NTR was observed almost all spheres even the sphere size is small (Fig. 4c arrows). The spheres also showed expression of CD31, as corresponding to the data of flow cytometric analysis. Quantitative analysis revealed that 69.0 ± 10.5% of total cells expressed Nestin, 49.6 ± 30.1% expressed Musashi-1, 95.9 ± 1.4% expressed p75NTR, and 75.4 ± 15.2% expressed CD31.
To assess the self-renewal capacity, we next tested whether sphere-forming cells could form new spheres. Primary spheres derived from p75NTR+ cells were mechanically dissociated into single-cell suspensions and reseeded at clone density in the neurosphere culture medium. The result showed that secondary and up to tertiary spheres could be obtained, although the sphere size became smaller (Fig. 4d). These data suggest that the p75NTR+ cells could form neurospheres with self-renewal capacity.
3.5. Gene expression profile of p75NTR+ cells and spheres
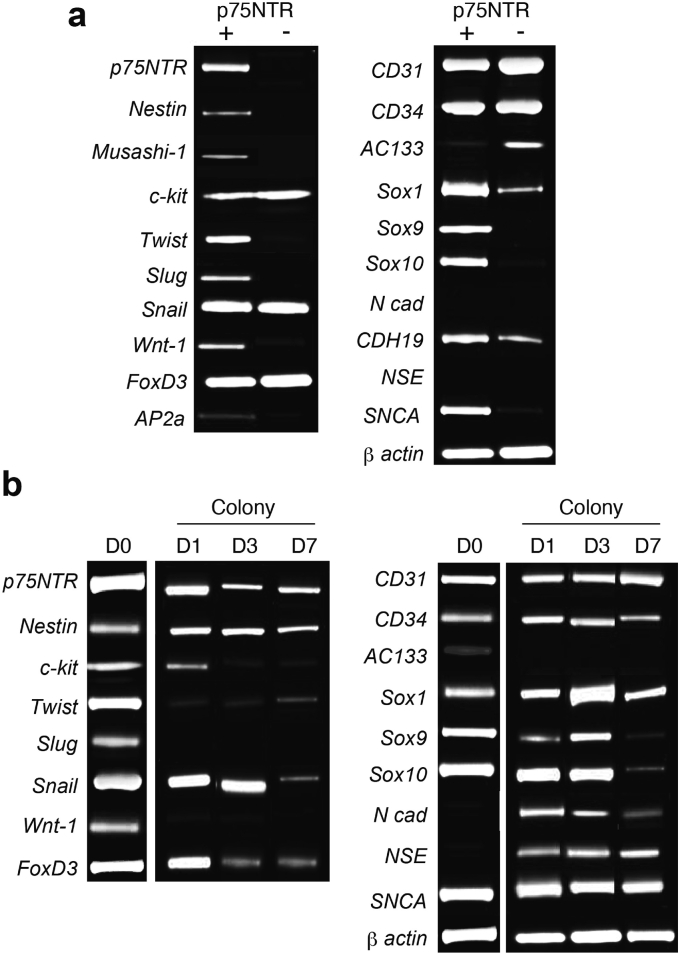
We next examined gene expression profile of the HUCB p75NTR+ cells and their derived spheres by RT-PCR analysis. We found that expression of Nestin and Musashi-1 was observed in the p75NTR+ cells but not in the p75NTR− cells (Fig. 5a). Hematopoietic stem cell markers c-Kit, and AC133 were expressed in the p75NTR− cells, whereas their expressions were less or absent in the p75NTR+ cells. Neural crest-associated genes Twist, Slug, Wnt-1, Sox9, and Sox10 were dominantly expressed in the p75NTR+ cells (Fig. 5a), whereas the other neural crest-associated genes Snail and FoxD3 were expressed both in the p75NTR+ cells and the p75NTR− cells at similar level. In addition, an early neural cell marker α-synuclein SNCA was specifically expressed in the p75NTR+ cells. In the p75NTR+ cell-derived spheres, expression of p75NTR, Nestin, Snail, FoxD3, CD31, CD34, Sox1, Sox9, Sox10 and SNCA was observed along culture with different levels (Fig. 5b). In contrast, expression of c-kit, Twist, Slug and Wnt-1 were rapidly reduced or lost in the spheres compared to those in the cells before culture. Expression of N-cadherin, a mesenchymal marker of epithelial mesenchymal transition, and neuron specific enolase (NSE), an early neural cell marker, were observed in the spheres even their expression was not detected before culture (Fig. 5b). These findings suggest that the HUCB p75NTR+ cells show a phenotypic feature of NC-derived cells.
Fig. 5.
RT-PCR analysis of HUCB p75NTR+ cells. Total RNA was extracted from freshly isolated p75NTR+ and p75NTR− cell fractions from HUCB MNCs. RT-PCR was then conducted to evaluate the mRNA expression of various genes including NC-associated markers. β-actin was loaded as an internal control. (a) Comparison of gene expression profile in the p75NTR+ and the p75NTR− cells. (b) Time course of gene expression changes in p75NTR+ cell-derived spheres during the neurosphere culture.
3.6. The HUCB p75NTR+ cells can differentiate into neuronal and glial cell types in vitro
We next investigated the differentiation potential of the HUCB p75NTR+ cells in vitro. When the p75NTR+ cells were cultured in the neurogenic differentiation medium containing EGF, FGF-2, and BDNF for 7 days, these cells showed morphological features of neural cells with long bipolar or multipolar extensions sand branching terminals (Fig. 6a). On the other hands, p75NTR− cells did not show any morphological changes under the same culture condition. Immunocytochemical analysis revealed that nearly all p75NTR+ cells expressed neuronal markers NF200, MAP2 and βIII-tubulin, and a astrocytes marker GFAP at 7 days after neurogenic induction (Fig. 6b). Expression of these marker proteins was absent in p75NTR− cells (data not shown). When the p75NTR+ cells were cultured in the glial cell differentiation medium containing FGF-2, FSK, PDGF-BB, and Neuregulin1-β1/Heregulin1-β1 EGF domain for 7 days, the cells showed in bipolar, spindle-shaped morphology (Fig. 6c). Immunocytochemical analysis revealed the p75NTR+ cells expressed O4 and GFAP, markers for oligodendrocytes and astrocytes, respectively (Fig. 6d). Expression of p75NTR was also retained in the p75NTR+ cells. On the other hands, p75NTR− cells did not show any morphological changes and immunoreactivity under the same culture condition. These data indicate that the HUCB p75NTR+ cells have ability to differentiate into neuronal and glial cell types.
Fig. 6.
Neurogenic differentiation ability of HUCB p75NTR+ cells. Freshly isolated p75NTR+ and p75NTR− cell fractions were cultured in neuronal cell or glial cell differentiation media for 7 days and assessed by immunocytochemistry. (a) Representative images of each fraction cultured in neuronal cell differentiation media. (b) Immunocytochemical analysis of p75NTR+ cells cultured in neuronal cell differentiation media. (c) Representative images of each fraction cultured in glial cell differentiation media. (d) Immunocytochemical analysis of p75NTR+ cells cultured in glial cell differentiation media. (e) Immunocytochemical analysis of p75NTR+ cells and human adipose-derived stem cells (ASCs) cultured in myofibroblastic differentiation media. Scale bars represent 50 μm.
It is known that NCSCs have the capacity to differentiate into myofibroblasts as well as neuronal and glial cells. Therefore, we examined the differentiation potential of the HUCB p75NTR+ cells for a smooth muscle cell lineage by culturing with 5% FBS/DMEM containing 5 ng/ml TGF-β. However, repeated immunocytochemical analysis revealed that the p75NTR+ cells did not exhibit myofibroblast phenotype in the culture, while myofibroblastic differentiation was observed in ASCs as positive control cells (Fig. 6e).
4. Discussion
In the present study, we demonstrated that HUCB contains neural crest-derived cell marker p75NTR expressing cells even at low frequencies (0.23% of the total HUCB MNCs). We also found that p75NTR+ cells isolated from HUCB efficiently form neurospheres and have ability to differentiate into neuronal and glial cell types. The enriched p75NTR+ cell population expressed a set of neural crest-associated genes and neuronal progenitor cell markers before and after the culture. Previous studies have characterized NCSCs by their capacity for self-renewal and their multilineage differentiation potential toward neuronal cells, glial cells, and myofibroblasts [31,32]. Our results showed that the differentiation potential of the HUCB p75NTR+ cells was restricted to neuronal and glial cell lineages, suggesting the p75NTR+ cells exhibit functional features of NC progenitor cells. To our knowledge, this is the first demonstration that HUCB contains NC-like progenitor cells.
In the mouse embryonic stage, NCSCs were reported to appear in fetal bloodstream at E12.5 and reach peak numbers at E15.5 and disappear by E18.5 after reaching bone marrow [2]. The timing coincides with that of hematopoietic stem cells. Although very small number of p75NTR+ cells was detected in term HUCB in this study, it is possible that preterm HUCB contains more NC-like stem/progenitor cells as similar as hematopoietic stem cells [33]. Further investigation is required to explore gestation period-dependent changes in the HUCB p75NTR+ cells.
Our flow cytometric analysis revealed the most p75NTR+ cells expressed a hematopoietic lineage marker CD45 at low level. Rogers et al. [34] reported that CD45+/lineage− cells in HUCB displayed multilineage differentiation potential including neural cell lineage. Jones et al. [35] reported that multipotent bone marrow MSCs are enriched within the CD45lowp75NTR+ cell population after anti-fibroblast microbeads selection. These findings suggest that the CD45low fraction are implicated in the multipotency of adult stem cells.
We found that the HUCB p75NTR+ cell population was phenotypically different from the bone marrow p75NTR+ cells. Quirici et al. [25] reported that positive selection using p75NTR antibody is a useful for enrichment of MSCs in bone marrow. However, our results suggest that the HUCB p75NTR+ cells contain little or no MSCs, because the HUCB p75NTR+ cells did not adhere and proliferate on a plastic dish in the MSC culture condition (Fig. 4a). To support our results, previous studies reported that p75NTR was not a useful marker for MSC isolation from HUCB [36,37].
In the present study, the isolated p75NTR+ cell fraction after immunomagnetic separation efficiently formed neurospheres, whereas the p75NTR− cell fraction did not form them. In addition, gene expression analysis revealed that expression of neural crest-associated genes such as p75NTR, Twist, Slug, Wnt-1, Sox9 and Sox10 was observed in the isolated p75NTR+ cell fraction but was absent in the p75NTR− cell fraction (Fig. 5a). These findings suggest efficient isolation and enrichment of NC-like progenitor cells in the p75NTR+ cell fraction. It should be considered that the p75NTR+ cells are heterogenous cell population containing non-NCSC populations. In fact, Betters et al. [38] reported that p75NTR could broadly label neural crest cells and other cell types in human embryos. Ouchi et al. [39] reported that human ESCs/iPSCs-derived NCSCs were efficiently enriched by positive selection using anti-p75NTR and anti-THY-1(CD90) antibodies. Because our flow cytometry data indicates that 22.1% of p75NTR+ cells were positive for CD90 (Fig. 1b), additional positive selection for CD90 may be useful to collect NC-like stem/progenitor cell population with higher purity.
Our RT-PCR data showed that the isolated p75NTR+ cells express various NC-associated marker genes, including the NCSC markers p75NTR and Sox10, the neural crest markers Snail, Slug, Twist, Sox9, and the early neuronal progenitor markers Nestin and Musashi1. The sustained expression of p75NTR, Nestin, and Sox10 in spheres generated from the p75NTR+ cells suggests that the property of NCSCs retains during the sphere culture. Among of the NCSC markers, Sox10 expression rapidly decreased at day 7 of the culture, whereas the early neural cell marker NSE expression increased at the time point. These findings suggest that multipotent property was lost and spontaneous neural lineage differentiation occurred at day 7 of the culture, because Sox10 has been shown to play an important role in the maintenance of NCSC multipotency [40,41].
Trilineage differentiation potential (neurons, glia, and myofibroblasts) has been accepted as a major functional property of NCSCs. Our in vitro differentiation assay data revealed that the HUCB p75NTR+ cells have ability to differentiate into neuronal and glial cell types but not myofibroblasts under appropriate culture conditions. It has been documented that trilineage differentiation potential of whisker pad and bone marrow-derived spheres were significantly restricted compared to dorsal root ganglia-derived spheres in the P0 and Wnt1-cre reporter mouse lines [2]. Thus, the differentiation potentials of NCSCs isolated from different tissue were intrinsically different. Recent studies demonstrated that Twist1 regulates neural crest fate toward a mesenchymal lineage [42]. Our data that Twist expression in the HUCB p75NTR+ cells quickly disappeared in the neurosphere culture (Fig. 4b) could explain the inability of myofibroblast differentiation.
As a practical source of NCSCs, HUCB have several advantages over other adult tissue such as bone marrow and skin. HUCB can be collected without painful procedure and has not been exposed to immunologic challenge. Furthermore, HUCB transplantation has been well established with great potential and no serious ethical issue by establishment of public umbilical cord blood banks in many countries. Therefore p75NTR+ NC-like progenitor cells in HUCB may provide an attractive cell source for allogeneic stem cell transplantation in patients with neurological disorders such as demyelinating disorders and spinal cord injury.
We acknowledge that there are limitations to this study. We failed long-term culture to expand the isolated p75NTR+ cells with maintaining stem cell properties. Recently, an optimized culture medium to maintain neural crest stemness has been developed [43]. Such technologies will help to improve the long-term culture condition for the HUCB p75NTR+ cells. In addition, we have not evaluated the functional properties of the HUCB p75NTR+ cells in vivo. Further studies are needed to clarify whether the HUCB p75NTR+ cells have the migration and the multilineage differentiation potential using appropriate animal models.
5. Conclusion
To our knowledge, this is first demonstration that HUCB contained p75NTR+ NC-like progenitor cells which have the self-renewal capacity and the potential to differentiate into both neuronal and glial cell lineages. Although the self-renewal and multilineage differentiation potential was restricted, the cell population is expected to provide attractive cell source for the treatment of neurological disorders.
Declaration of Competing Interest
The authors declare that there is no conflict of interests in this article.
Acknowledgements
This research was supported by JSPS KAKENHI Grant Number 20H03581, 17H04152, by AMED-supported Program for the Research Project for Practical Applications of Regenerative Medicine (19bk0104005h002), by MEXT-supported Program for the Strategic Research Foundation at Private Universities (S1411018) and by Nihon University President Grant Initiative (2018–2020). We thank Dr. Maiko Kato and Dr. Minako Kazama for providing excellent technical assistance in the flow cytometry, Dr. Yuji Iribe for providing technical assistance in the differentiation assay.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Liu J.A., Cheung M. Neural crest stem cells and their potential therapeutic applications. Dev Biol. 2016;419(2):199–216. doi: 10.1016/j.ydbio.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Nagoshi N., Shibata S., Kubota Y., Nakamura M., Nagai Y., Satoh E. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2(4):392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Shi H., Gong Y., Qiang L., Li X., Zhang S., Gao J. Derivation of Schwann cell precursors from neural crest cells resident in bone marrow for cell therapy to improve peripheral nerve regeneration. Biomaterials. 2016;89:25–37. doi: 10.1016/j.biomaterials.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Li H.Y., Say E.H., Zhou X.F. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cell. 2007;25(8):2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- 5.Kruger G.M., Mosher J.T., Bixby S., Joseph N., Iwashita T., Morrison S.J. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toma J.G., Akhavan M., Fernandes K.J., Barnabe-Heider F., Sadikot A., Kaplan D.R. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 7.Wong C.E., Paratore C., Dours-Zimmermann M.T., Rochat A., Pietri T., Suter U. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175(6):1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomita Y., Matsumura K., Wakamatsu Y., Matsuzaki Y., Shibuya I., Kawaguchi H. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170(7):1135–1146. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandl C., Florian C., Driemel O., Weber B.H., Morsczeck C. Identification of neural crest-derived stem cell-like cells from the corneal limbus of juvenile mice. Exp Eye Res. 2009;89(2):209–217. doi: 10.1016/j.exer.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Pardal R., Ortega-Saenz P., Duran R., Lopez-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131(2):364–377. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Janebodin K., Horst O.V., Ieronimakis N., Balasundaram G., Reesukumal K., Pratumvinit B. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PloS One. 2011;6(11) doi: 10.1371/journal.pone.0027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomellini E., Lagadec C., Polakowska R., Le Bourhis X. Role of p75 neurotrophin receptor in stem cell biology: more than just a marker. Cell Mol Life Sci. 2014;71(13):2467–2481. doi: 10.1007/s00018-014-1564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikami Y., Matsumoto T., Kano K., Toriumi T., Somei M., Honda M.J. Current status of drug therapies for osteoporosis and the search for stem cells adapted for bone regenerative medicine. Anat Sci Int. 2014;89(1):1–10. doi: 10.1007/s12565-013-0208-8. [DOI] [PubMed] [Google Scholar]
- 14.Totsuka T., Kanai T., Iiyama R., Uraushihara K., Yamazaki M., Okamoto R. Ameliorating effect of anti-inducible costimulator monoclonal antibody in a murine model of chronic colitis. Gastroenterology. 2003;124(2):410–421. doi: 10.1053/gast.2003.50050. [DOI] [PubMed] [Google Scholar]
- 15.Marynka-Kalmani K., Treves S., Yafee M., Rachima H., Gafni Y., Cohen M.A. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cell. 2010;28(5):984–995. doi: 10.1002/stem.425. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X., Gwye Y., McKeown S.J., Bronner-Fraser M., Lutzko C., Lawlor E.R. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18(7):1059–1070. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G., Chambers S.M., Tomishima M.J., Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5(4):688–701. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q., Spusta S.C., Mi R., Lassiter R.N., Stark M.R., Hoke A. Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl Med. 2012;1(4):266–278. doi: 10.5966/sctm.2011-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin H.S., Bicknese A.R., Chien S.N., Bogucki B.D., Quinn C.O., Wall D.A. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7(11):581–588. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T., Mugishima H. Non-hematopoietic stem cells in umbilical cord blood. Int J Stem Cells. 2009;2(2):83–89. doi: 10.15283/ijsc.2009.2.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuba-Surma E.K., Klich I., Greco N., Laughlin M.J., Ratajczak J., Ratajczak M.Z. Optimization of isolation and further characterization of umbilical-cord-blood-derived very small embryonic/epiblast-like stem cells (VSELs) Eur J Haematol. 2010;84(1):34–46. doi: 10.1111/j.1600-0609.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 23.McGuckin C.P., Forraz N. Potential for access to embryonic-like cells from human umbilical cord blood. Cell Prolif. 2008;41(Suppl 1):31–40. doi: 10.1111/j.1365-2184.2008.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J., Sun Y., Block T.J., Marinkovic M., Zhang Z.L., Chen R. Umbilical cord blood-derived non-hematopoietic stem cells retrieved and expanded on bone marrow-derived extracellular matrix display pluripotent characteristics. Stem Cell Res Ther. 2016;7(1):176. doi: 10.1186/s13287-016-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quirici N., Soligo D., Bossolasco P., Servida F., Lumini C., Deliliers G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30(7):783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 26.Toma J.G., McKenzie I.A., Bagli D., Miller F.D. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cell. 2005;23(6):727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi Y., Abe K., Mantani A., Hitoshi Y., Suzuki M., Osuzu F. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212(1):191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]
- 28.Kawamoto S., Niwa H., Tashiro F., Sano S., Kondoh G., Takeda J. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 2000;470(3):263–268. doi: 10.1016/s0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto T., Kano K., Kondo D., Fukuda N., Iribe Y., Tanaka N. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215(1):210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 30.Stemple D.L., Anderson D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71(6):973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 31.Morrison S.J., White P.M., Zock C., Anderson D.J. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96(5):737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 32.Shah N.M., Groves A.K., Anderson D.J. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85(3):331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 33.Shields L.E., Andrews R.G. Gestational age changes in circulating CD34+ hematopoietic stem/progenitor cells in fetal cord blood. Am J Obstet Gynecol. 1998;178(5):931–937. doi: 10.1016/s0002-9378(98)70526-5. [DOI] [PubMed] [Google Scholar]
- 34.Rogers I., Yamanaka N., Bielecki R., Wong C.J., Chua S., Yuen S. Identification and analysis of in vitro cultured CD45-positive cells capable of multi-lineage differentiation. Exp Cell Res. 2007;313(9):1839–1852. doi: 10.1016/j.yexcr.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Jones E.A., English A., Kinsey S.E., Straszynski L., Emery P., Ponchel F. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70(6):391–399. doi: 10.1002/cyto.b.20118. [DOI] [PubMed] [Google Scholar]
- 36.Attar A., Ghalyanchi Langeroudi A., Vassaghi A., Ahrari I., Maharlooei M.K., Monabati A. Role of CD271 enrichment in the isolation of mesenchymal stromal cells from umbilical cord blood. Cell Biol Int. 2013;37(9):1010–1015. doi: 10.1002/cbin.10117. [DOI] [PubMed] [Google Scholar]
- 37.Watson J.T., Foo T., Wu J., Moed B.R., Thorpe M., Schon L. CD271 as a marker for mesenchymal stem cells in bone marrow versus umbilical cord blood. Cells Tissues Organs. 2013;197(6):496–504. doi: 10.1159/000348794. [DOI] [PubMed] [Google Scholar]
- 38.Betters E., Liu Y., Kjaeldgaard A., Sundstrom E., Garcia-Castro M.I. Analysis of early human neural crest development. Dev Biol. 2010;344(2):578–592. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouchi T., Morikawa S., Shibata S., Fukuda K., Okuno H., Fujimura T. LNGFR(+)THY-1(+) human pluripotent stem cell-derived neural crest-like cells have the potential to develop into mesenchymal stem cells. Differentiation. 2016;92(5):270–280. doi: 10.1016/j.diff.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Britsch S., Goerich D.E., Riethmacher D., Peirano R.I., Rossner M., Nave K.A. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15(1):66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J., Lo L., Dormand E., Anderson D.J. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38(1):17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 42.Soldatov R., Kaucka M., Kastriti M.E., Petersen J., Chontorotzea T., Englmaier L. Spatiotemporal structure of cell fate decisions in murine neural crest. Science. 2019;364(6444) doi: 10.1126/science.aas9536. [DOI] [PubMed] [Google Scholar]
- 43.Mohlin S., Kerosuo L. In vitro maintenance of multipotent neural crest stem cells as crestospheres. Methods Mol Biol. 2019;2002:1–11. doi: 10.1007/7651_2018_180. [DOI] [PMC free article] [PubMed] [Google Scholar]