Abstract
As soon as saliva contacts the teeth surface, salivary proteins adhere to the tooth surface to form acquired salivary pellicle. The formation of this acquired salivary pellicle is a dynamic and selective process of macromolecular adsorption and desorption. Although acquired salivary pellicle contains proteins and peptides, it also contains lipids, and other macro-molecules, all of which contribute to its protective properties. Acquired salivary pellicle is related to the development of common oral diseases, such as erosion, dental caries, and periodontal disease. Acquired salivary pellicle acts as a natural barrier to prevent a tooth's surface from making direct contact with acids and to protect it from erosive demineralization. It contributes to the control of dental erosion by modulating calcium and phosphate concentrations on the tooth surface. It also influences the initial colonizer of oral biofilm and affects the transportation pathway of the acidic products of cariogenic bacteria, which affects the development of dental caries. In addition, it influences periodontal disease by acting on the colonization of periodontal pathogens. This paper's aim is to provide an overview of the acquired salivary pellicle, highlighting its composition, structure, function, role in common oral diseases, and modification for the prevention of oral diseases.
Keywords: Saliva, Pellicle, Prevention, Cariology, Caries, Erosion
Introduction
Alexander Nasmyth discovered acquired salivary pellicle, or acquired enamel pellicle, in 1839.1, 2, 3, 4 It is a pellicle layer that forms when saliva contacts tooth surfaces.1,2 This organic film is formed via the selective adsorption of salivary biopolymers on the surface of a tooth.2,3 It is constructed in a structure of acellular multiple layers.5,6 All of the tooth surfaces are covered with acquired salivary pellicle in the oral cavity.5,6 The pellicle acts as the connection between dental hard tissue and oral environments.5,6 Because acquired salivary pellicle plays a key role in the maintenance of oral health, it has attracted great attention in dental research. Therefore, this review's aim is to provide an overview of the acquired salivary pellicle on tooth surfaces.
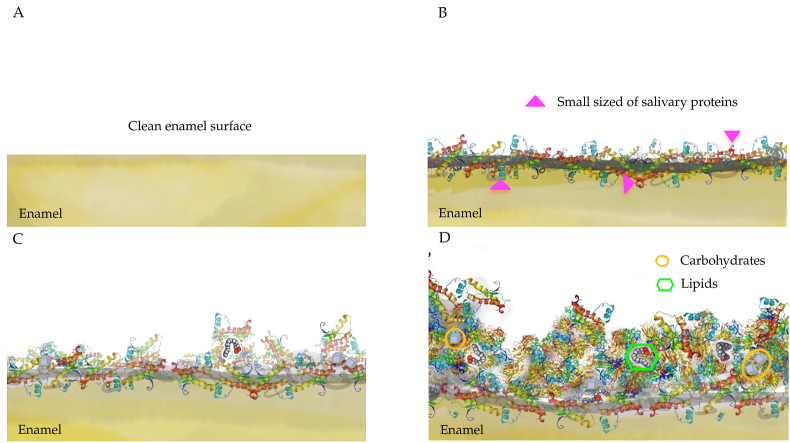
Acquired salivary pellicle formation is a dynamic and selective process that the adsorption and desorption processes influence.2,3 Fig. 1 shows the stages of acquired salivary pellicle formation. The precursors of acquired salivary pellicle proteins, such as proline-rich proteins, statherin, and histatins, attach to a tooth's surface in seconds to a couple of minutes in the initial stage.7,8 They have a calcium-binding domain that can be attached to the hydroxyapatite crystals at the tooth surface.8,9 The thickness of the pellicle reaches about 10–20 nm in just a few minutes after formation.1, 2, 3,10 In the developing stage, more salivary proteins aggregate with the precursor proteins of the salivary pellicle 30–45 min after the initial attachment. Protein–protein interactions occur on the tooth surface.3,11 The proteins aggregate in globular form, and the diameter of the protein globe gradually increases in this stage.11 In the maturation stage, high-molecular-weight mucins gradually adhere to the pellicle.12 The diameter of the constructed globular structure and the thickness of the salivary pellicle continue to increase until the maturation is reached.2,10,11 The pellicle reaches equilibrium between adsorption and desorption within 90–120 min, and the thickness of a mature salivary pellicle is approximately 100–1000 nm.1, 2, 3,10
Figure 1.
Formation of acquired salivary pellicle A: Clean enamel surface B: Initial stage: attachment of precursor proteins (seconds to a couple of minutes) C: Developing stage: protein–protein reaction (within 45 min) D: Maturation stage: the equilibrium between adsorption and desorption (within 120 min).
Several factors affect the formation of the salivary pellicle. The type of protein molecule determines the protein–protein interaction in the developing stage.3 Moreover, studies have revealed that a salivary pellicle with a prolonged formation time has an enhanced protective property.3 The improved protective property is a result of the increased thickness of the pellicle, an improved dynamic condition of the environment, and the active re-modelling reaction of the pellicle.1,3
Composition and structure of mature salivary pellicle
Components of acquired salivary pellicle originate mainly from salivary gland secretion, gingival crevicular fluid, products from the oral mucosa, and products from oral microorganisms.3,10 Acquired salivary pellicle mainly consist of proteins, lipids, and other macromolecules, such as carbohydrates.2,10 Major components of acquired salivary pellicle are salivary proteins and glycoproteins.3,5 Some amino acids are also present in acquired salivary pellicle.13 The composition of proteins and peptides are summarized in Table 1. The proteins provide acquired salivary pellicle with the function of the immune response, antimicrobial effect, and remineralization process.13 The functions of quite a number of proteins in acquired salivary pellicle remain uninvestigated.14
Table 1.
Components of acquired salivary pellicle and their functions.
| Components | Functions (Reference) |
|---|---|
| Proteins and peptides | |
| Carbonic anhydrase I, II, VI |
|
| Albumin |
|
| Fibrinogen |
|
| Fibronectin |
|
| Lactoferrin |
|
| Lysozyme |
|
| Myeloperoxidase/Peroxidase |
|
| Alpha-amylase |
|
| Mucin MG1, MG2 |
|
| Immunoglobulins: sIgA, IgGa | |
| Acidic proline-rich proteins | |
| Statherin | |
| Histatins | |
| Mucin 5CB, C7 | |
| Cystatin | |
| Lipids | |
| Cholesterol, Cholesterol esters, Glycerides, Phosphatidylcholine, Sphingomyelin, Phosphatidylethanolamine | |
| Carbohydrates | |
| Fucose, Galactose, Galactosamine, Lactose, Glucosamine, Glucose, Mannose, Manose, Rhamnose |
|
sIgA = secretory Immunoglobulin A; IgG = Immunoglobulin G.
Other components of acquired salivary pellicle include lipids, which include glycolipids and phospholipids.15 They normally originate from the major salivary glands.15 Lipids make up approximately 25% of the pellicle.16 (Table 1) The lipids collaborate with other components to contribute to the permeability of the acquired salivary pellicle.17,18 Permeability is the basis of the pellicle's resistance to the acid in the oral cavity.17,18 Thus, acquired salivary pellicle retards the diffusion of lactic acid to the enamel pellicle.17,18 Furthermore, lipids influence the ultrastructure of a pellicle and modify the outer layer of the pellicle via lipid micelles.17 Lipids also affect the initial stage of bacteria adhesion to the tooth surface.17 The hydrophobic property of lipids and lipophilic substances in acquired salivary pellicle impedes the attachment of microorganisms such as Streptococcus mutans.15, 16, 17
Acquired salivary pellicle also contains carbohydrates, which are macromolecules derived mainly from the submandibular and parotid glands.2,19 Most of the carbohydrates in acquired salivary pellicle exist in the form of complex compounds, such as glycoproteins and glycolipids (Table 1).2 The carbohydrates in acquired salivary pellicle, particularly glucose, may come from salivary glycoprotein and the glucans of bacteria.2,10 The function of carbohydrates in acquired salivary pellicle has not been well investigated.2,10 According to limited studies, the carbohydrates in acquired salivary pellicle may act as the nutrient supply for biofilm. They may also contribute to the protective barrier of the acquired salivary pellicle.2,10
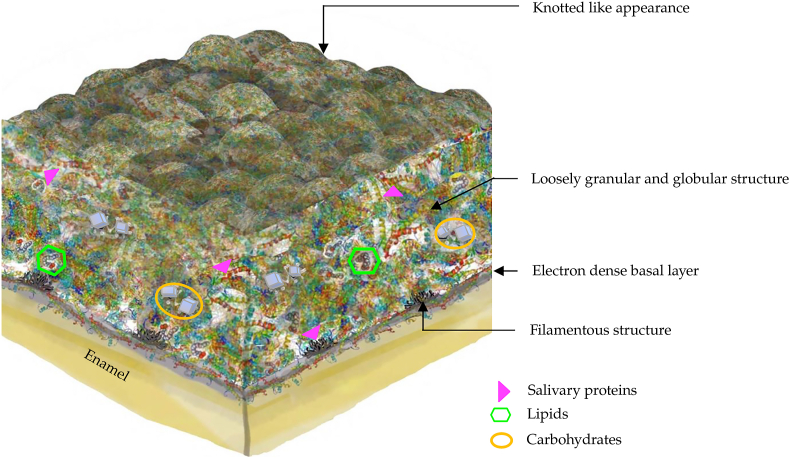
Acquired salivary pellicle displays a heterogeneous ultrastructure appearance with a globular and pore-like structure on the pellicle surface (Fig. 2).1,2 The base of the pellicle is an electron-dense basal layer, which can be observed via electron microscopy.2,10 The basal layer of the pellicle adheres to the enamel surface with filamentous structures.2,10 In addition, the middle layer of the acquired salivary pellicle shows a loosely granular and globular appearance.1,2,20 The surface layer of the pellicle is constructed of densely aggregated proteins,1 illustrating a knotted globular surface.10
Figure 2.
The schematic structure of acquired salivary pellicle.
Several factors affect the composition and structure of a mature acquired salivary, including the circadian rhythm, the compositions of the whole saliva, the location in the mouth, the proteolytic ability of the oral fluid, the oral pathological conditions, and the oral microorganic composition.3,11 The circadian rhythm changes the protein concentration in saliva, the composition of the saliva, and the flow rate of the saliva.3 These changes in the saliva have an influence on the pellicle composition.13,21 In fact, previous studies showed that morning pellicle compositions vary due to circadian effects.13,21
Saliva from various salivary glands has different physical and chemical properties.20 The parotid salivary gland secretes a high quantity of amylase and proline-rich proteins, whereas the sublingual salivary gland secretes a high quantity of mucins and lysozyme.20 The property of saliva differs if the proportion of saliva from various salivary glands changes.1, 2, 3 It therefore affects the composition, thickness, and ultrastructure of the acquired salivary pellicle.1, 2, 3
Mature salivary pellicles have varying levels of thickness in different locations in the oral cavity.2 In the self-cleansing area, the thickness of an acquired salivary pellicle is approximately 30–100 nm.2 The thickness of the pellicle is related to whether the teeth are located in the area with saliva accumulation.22 The thickest pellicle is located at the lingual surface of the lower teeth, whereas the thinnest pellicle is located at the palatal surface of the upper teeth.1,9,11 The location in the oral cavity also affects the composition and ultrastructure of the salivary pellicles by affecting the saliva supply, salivary flow rate, and shear forces of the local environment.1,9,11 Studies have revealed that pellicle forms in varying regions of the dental arches were composed of different proteins.21
The proteolytic ability of oral fluid may also alter the properties of salivary proteins and influence the maturation of salivary pellicles.3 Oral pathological conditions, especially gingivitis, result in an increasing level of crevicular fluid flow and plasma proteins.3,23 They affect the formation of acquired salivary pellicle and modify the primary colonization of oral microorganisms.3,23
The composition of oral microorganisms, such as S. mutans, Streptococcus gordonii, and Streptococcus oralis, may affect the level of carbohydrates in the salivary pellicle, particularly glucose.10
The function of salivary pellicle
Acquired salivary pellicle contributes to the maintenance of one's oral health.5 The reason for this is that acquired salivary pellicle assumes the functions of lubrication,2 maintaining mineral homeostasis,3 determining the initial microbial colonizers on the tooth surface,5,24 and protecting the dental surface from acid attacks.20,24
Lubrication of the oral environment
Acquired salivary pellicle lubricates the oral environment during mastication and speech.3 It is responsible for the lubrication of tooth-to-soft-tissue contact as well as tooth-to-tooth contact.6,25 Consequently, acquired salivary pellicle could reduce the frictional coefficient between the tooth and other oral structures.6,25 It furthermore provides limited protection to the tooth surface against abrasion and attrition.6,25
Maintenance of mineral homeostasis
The saliva pellicle works with all of the saliva to maintain mineral homeostasis.3 Acquired saliva pellicle maintains the level of calcium concentration in a supersaturated status on the surface of the tooth.3,25 This prevents tooth surface dissolution.25 In addition, the presence of calcium-binding protein in the pellicle can prevent the over-precipitation of calcium-phosphate.3 The calcium-binding protein maintain the balance of hydroxyapatite crystal deposition and dissolution on the enamel surface.3 Furthermore, acquired salivary pellicle acts as a barrier layer to protect dental hard tissue against demineralization during acid challenges in the oral cavity.3
Determination of initial microbial colonizers
The compositions of salivary pellicles play an important role in determining the colonization of initially colonized bacteria.3,5 The initial colonization of microorganisms occurs with the maturation of acquired salivary pellicle.2 A mature acquired salivary pellicle provides specific receptors for bacteria to attach.17 Oral microorganisms selectively attach to acquired salivary pellicle due to the presence of specific receptors and hydrophobic components in the pellicle.17 The adhesins and lectins on the surfaces of the microorganisms bind with saccharide receptors in glycoproteins or the carbohydrate components of the pellicle.26 The initial colonizers of oral microorganisms that adhere to acquired salivary pellicle are usually Streptococcus species and Actinomyces species.3,26,27 The presence of glucosyltransferase in acquired salivary pellicle promotes the production of glucans in the microenvironment of dental biofilm.19,26,27 Streptococcus releases more glucosyltransferase to the extracellular matrix, which assists in establishing insoluble biofilms and in enhancing the colonization of other species.19,26,27 The accumulation of glucans in the extracellular matrix of biofilm promotes the colonization of Streptococcus species.26
In addition, the compositions of salivary pellicles may promote or inhibit the colonization of oral microorganisms.3 For instance, histatins and statherin can inhibit the adherence of S. mutans. On the contrary, statherin can promote the adherence of Fusobacterium nucleatum.3,28 Meanwhile, proline-rich proteins and statherin can interact with Actinomyces naeslundii. Furthermore, glycosyltransferase that S. mutans secretes into acquired salivary pellicle may induce more adherence colonization of Candida albicans.3,28 α-Amylase in the pellicle can also bind to bacteria and promote bacterial adhesion to hydroxyapatite.3,28
Protecting the dental surface from acid attacks
The anti-acid effects of salivary pellicles are supported in the literature. The acquired salivary pellicles acts as a barrier to directly impede contact between the tooth surface and the acids.29 The buffer capacity of the acquired salivary pellicle helps with neutralizing the acidity from the oral environment.30 It is a perm-selective membrane that can limit the movement of mineral ions.22 In addition, it contains calcium-binding proteins, such as mucins, statherins, histatins, and acid proline-rich proteins.22,30 The calcium-binding proteins adjust the concentration of calcium on the tooth surface to a supersaturated level to prevent the further dissolution of hydroxyapatite.22,30
Acquired salivary pellicle and oral diseases
Acquired salivary pellicle and dental caries
The effect of salivary pellicles on dental caries can be attributed to its effect on the colonization of oral microorganisms, the diffusion pathway of bacterial byproducts, and the transportation of mineral ions.1,2
Acquired salivary pellicle impacts the development of dental caries because it determines the attachment of oral microorganisms to the tooth surface.3 Dental caries is the result of the acidic byproducts of cariogenic bacteria in dental plaque biofilm.29 The composition of acquired salivary pellicle determines the initial attachment of oral microbial species to the tooth surface and the subsequent development of dental biofilm.29 This affects the cariogenic property of dental biofilm.29 Acquired salivary pellicle exerts its bactericidal or virucidal effects on oral microorganisms due to the effect of cystatins.31 Oral hygiene status, snacking habit, and types of food or beverage intake influence the composition of acquired salivary pellicle and the subsequent proliferation of bacterial colonization.12 A study found a high level of acidic proline-rich proteins, lipocalin, and cystatin in caries-free patients, compared with a high level of amylase, immunoglobulin A, and lactoferrin in caries-affected patients.31
Acquired salivary pellicle affects the diffusion of acidic products from cariogenic biofilm and therefore affects the demineralization process on the tooth surface.3 It delays the diffusion of lactic acid, mainly due to its lipid and glycolipid components.1,2 It also adsorbs part of the organic acid with its protein components.1,2 As a result, the demineralization of dental hard tissue is reduced.1,2 Acquired salivary pellicle affects the diffusion of mineral ions between the enamel and oral cavity.1,2 The protein components of the pellicle play a key role in the activity of mineral ions on the surface of the tooth.25 Statherin can bind with calcium ions from the saliva, thus maintaining the level of calcium ion in the pellicle.25 The high concentration of calcium ions in the pellicle precipitates on the demineralized enamel and thus reduces the demineralization.25 In addition, phosphorylated histatins and mucins are dominant proteins in improving the tooth's acid resistance.3 Furthermore, acquired salivary pellicle is the potent physiological inhibitor of proteolytic activity.31 The inhibition of proteolytic activity prevents the dissolution of dentine collagen and slows down the dissolution of minerals in the dentine.5,12 Additionally, acquired salivary pellicle may enhance remineralization due to its permselective ability.5,12 The porous mesh-like structure allows for the diffusion of calcium, phosphate, and fluoride ions from the oral environment to the enamel surface.5,12
Acquired salivary pellicle and dental erosion
Dental erosion is the destruction of dental hard tissue as a result of non-bacterial acid.22 Acquired salivary pellicle can protect the tooth surface from dental erosion.25,32 Acquired salivary pellicle can also prevent surface mineral loss and reduce the surface roughness of enamel when the enamel is exposed to acids.22 Carpenter et al. found that acquired salivary pellicles in patients with dental erosion present lower concentrations of statherin compared with healthy people.25 However, the protective effect of acquired salivary pellicles from dental erosion is limited. The pellicle may not protect tooth surfaces when faced with severe erosive challenges.30
Acquired salivary pellicle and periodontal disease
An acquired salivary pellicle may exert effects on the progression of periodontal disease by augmenting the bacterial adhesion on the cervical surface of the tooth and accelerating dental plaque formation.3,33 This effect may due to the change in the composition of acquired salivary pellicles on the cervical surface.34,35 The components of the pellicle on the cervical surface come from saliva and gingival crevicular fluid.34,35 The presence of gingivitis and periodontitis changes the components of the gingival crevicular fluid.34,35 A significant change in the gingival crevicular fluid is the increase in the concentration of lactoferrin and plasma proteins.3,34,35 Acquired salivary pellicles in patients with periodontal disease facilitate the attachment of proteolytic gram-negative species and increase the proportion of these species in dental biofilms.35 The enhanced proteolytic reaction may aggravate the inflammatory response of the periodontal tissue.35
Modification of acquired salivary pellicle to prevent oral diseases
Acquired salivary pellicle connects with dental hard tissue and oral environments. It is a key element in the development of several major oral diseases. Therefore, the modification of a acquired salivary pellicle may be a promising approach to preventing or intercepting the progression of oral diseases.7 A number of approaches for the modification of acquired salivary pellicles have been reported in the literature. The common approaches include modification with vegetable oils, dairy proteins, fluoride agents, plant lectins, and sugarcane.
Vegetable oils are used to enhance the anti-erosive effects and caries preventive effects of acquired salivary pellicle.18,36 These lipophilic agents can modify the composition and ultrastructure of the acquired salivary pellicle.18,36 They essentially increase the hydrophobicity of the pellicle and interact with the pellicle's internal lipophilic components.18,36 However, the scientific evidence is limited to support the efficiency of vegetable oil in preventive dentistry.18,36
Dairy proteins are another common agent used to modify acquired salivary pellicle. They increase the antimicrobial and anti-erosive effects of an acquired salivary pellicle.37,38 Cassiano et al. suggested that modifying acquired salivary pellicles using fat-free milk could modulate bacterial adhesion on the tooth surface, the mineral dissolution of dental hard tissue, and the antimicrobial properties of the acquired salivary pellicles.28 Dairy proteins influence the composition of acquired salivary pellicles by altering their lipid content and their protein profiles, as well as the molecular weight of the proteins.28,37
Fluoride agents, including acidulated phosphate fluoride and stannous fluoride, are used for acquired salivary pellicle modification.39 Fluoride agents change the affinity of the enamel surface to negative molecules with the increase of low-molecular-weight protein (S100-A9).39 Fluoride agents also improve the mineral ion concentration in pellicles by facilitating the formation of calcium fluoride in acquired salivary pellicle.39
All of these current ideas for pellicle modification focus on the impedance of oral microorganisms’ adhesion to the tooth surface or the enhancement of the acid resistance of acquired salivary pellicle. The future direction of research should involve the precise modification of the specific molecular composition and structure of acquired salivary pellicle to enhance its protective and antimicrobial functions. The personalized modification of acquired salivary pellicles may also be expected.
Summary
Acquired salivary pellicle consists of proteins, peptides, lipids, and other macro-molecules. These components contribute to the protective properties of acquired salivary pellicle. Acquired salivary pellicle is related to the development of common oral diseases, such as erosion, dental caries, and periodontal disease. The modification of acquired salivary pellicle is promising for preventing dental caries, dental erosion, and periodontal disease. Novel strategies for acquired salivary pellicle modification are in demand to improve its protective effects against oral diseases.
Declaration of completing interest
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
Acknowledgements
The authors gratefully acknowledge the financial support from the General Research Fund of Research Grants Council of Hong Kong SAR, China (No. 17100820).
References
- 1.Hannig M. The protective nature of the salivary pellicle. Int Dent J. 2002;52:417–423. [Google Scholar]
- 2.Lendenmann U., Grogan J., Oppenheim F. Saliva and dental pellicle-a review. Adv Dent Res. 2000;14:22–28. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- 3.Siqueira W., Custodio W., McDonald E. New insights into the composition and functions of the acquired enamel pellicle. J Dent Res. 2012;91:1110–1118. doi: 10.1177/0022034512462578. [DOI] [PubMed] [Google Scholar]
- 4.Nobbs A., Jenkinson H., Jakubovics N. Stick to your gums: mechanisms of oral microbial adherence. J Dent Res. 2011;90:1271–1278. doi: 10.1177/0022034511399096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen A.M.L., Belstrøm D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent Res. 2019;80:S3–S12. doi: 10.1016/j.jdent.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Hannig M., Fiebiger M., Güntzer M., Döbert A., Zimehl R., Nekrashevych Y. Protective effect of the in situ formed short-term salivary pellicle. Arch Oral Biol. 2004;49:903–910. doi: 10.1016/j.archoralbio.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Kensche A., Reich M., Kümmerer K., Hannig M., Hannig C. Lipids in preventive dentistry. Clin Oral Invest. 2013;17:669–685. doi: 10.1007/s00784-012-0835-9. [DOI] [PubMed] [Google Scholar]
- 8.Gibbins H.L., Yakubov G.E., Proctor G.B., Wilson S., Carpenter G.H. What interactions drive the salivary mucosal pellicle formation? Colloids Surf B. 2014;120:184–192. doi: 10.1016/j.colsurfb.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannig C., Hannig M., Kensche A., Carpenter G. The mucosal pellicle–An underestimated factor in oral physiology. Arch Oral Biol. 2017;80:144–152. doi: 10.1016/j.archoralbio.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Hannig M., Joiner A. Karger Publishers; 2006. The structure, function and properties of the acquired pellicle; pp. 29–64. [DOI] [PubMed] [Google Scholar]
- 11.Baek J.H., Krasieva T.B., Tang S. Optical approach to the salivary pellicle. J Biomed Optic. 2009;14:44001–44006. doi: 10.1117/1.3158994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svendsen I. Faculty of Health and Society Malmö University; 2009. In vitro and in vivo studies of salivary films at solid/liquid interfaces. Doctoral Thesis. [Google Scholar]
- 13.Zimmerman J.N., Custodio W., Hatibovic-Kofman S., Lee Y.H., Xiao Y., Siqueira W.L. Proteome and peptidome of human acquired enamel pellicle on deciduous teeth. Int J Mol Sci. 2013;14:920–934. doi: 10.3390/ijms14010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siqueira W.L., Zhang W., Helmerhorst E.J., Gygi S.P., Oppenheim F.G. Identification of protein components in in vivo human acquired enamel pellicle using LC−ESI−MS/MS. J Proteome Res. 2007;6:2152–2160. doi: 10.1021/pr060580k. [DOI] [PubMed] [Google Scholar]
- 15.Matczuk J., Żendzian-Piotrowska M., Maciejczyk M., Kurek K. Salivary lipids: a review. Adv Clin Exp Med. 2017;26:1021–1029. doi: 10.17219/acem/63030. [DOI] [PubMed] [Google Scholar]
- 16.Reich M., Hannig C., Al-Ahmad A., Bolek R., Kümmerer K. A comprehensive method for determination of fatty acids in the initial oral biofilm (pellicle) J Lipid Res. 2012;53:2226–2230. doi: 10.1194/jlr.D026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reich M., Kümmerer K., Al-Ahmad A., Hannig C. Fatty acid profile of the initial oral biofilm (pellicle): an in-situ study. Lipids. 2013;48:929–937. doi: 10.1007/s11745-013-3822-2. [DOI] [PubMed] [Google Scholar]
- 18.Ionta F.Q., Alencar CRBd, Val P.P. Effect of vegetable oils applied over acquired enamel pellicle on initial erosion. J Appl Oral Sci. 2017;25:420–426. doi: 10.1590/1678-7757-2016-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz A., Lang R., Behr J. Targeted metabolomics of pellicle and saliva in children with different caries activity. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-57531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannig M., Balz M. Protective properties of salivary pellicles from two different intraoral sites on enamel erosion. Caries Res. 2001;35:142–148. doi: 10.1159/000047446. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Ventura T.M., Cassiano LdPS., e Silva CMdS. The proteomic profile of the acquired enamel pellicle according to its location in the dental arches. Arch Oral Biol. 2017;79:20–29. doi: 10.1016/j.archoralbio.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Vukosavljevic D., Custodio W., Buzalaf M.A., Hara A.T., Siqueira W.L. Acquired pellicle as a modulator for dental erosion. Arch Oral Biol. 2014;59:631–638. doi: 10.1016/j.archoralbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Rüdiger S., Carlen A., Meurman J., Kari K., Olsson J. Dental biofilms at healthy and inflamed gingival margins. J Clin Periodontol. 2002;29:524–530. doi: 10.1034/j.1600-051x.2002.290609.x. [DOI] [PubMed] [Google Scholar]
- 24.Fábián T.K., Hermann P., Beck A., Fejérdy P., Fábián G. Salivary defense proteins: their network and role in innate and acquired oral immunity. Int J Mol Sci. 2012;13:4295–4320. doi: 10.3390/ijms13044295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter G., Cotroneo E., Moazzez R. Composition of enamel pellicle from dental erosion patients. Caries Res. 2014;48:361–367. doi: 10.1159/000356973. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira E., Napimoga M., Carneiro V. In vitro inhibition of Streptococci binding to enamel acquired pellicle by plant lectins. J Appl Microbiol. 2006;101:111–116. doi: 10.1111/j.1365-2672.2006.02910.x. [DOI] [PubMed] [Google Scholar]
- 27.Castro P., Tovar J.A., Jaramillo L. Adhesion of Streptococcus mutans to salivary proteins in caries-free and caries-susceptible individuals. Acta Odontol Latinoam. 2006;19:59–66. [PubMed] [Google Scholar]
- 28.Cassiano L.P., Ventura T.M., Silva C.M. Protein profile of the acquired enamel pellicle after rinsing with whole milk, fat-free milk, and water: an in vivo study. Caries Res. 2018;52:288–296. doi: 10.1159/000485390. [DOI] [PubMed] [Google Scholar]
- 29.Dawes C., Pedersen A.M.L., Villa A. The functions of human saliva: a review sponsored by the world workshop on oral medicine VI. Arch Oral Biol. 2015;60:863–874. doi: 10.1016/j.archoralbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Hannig M., Hannig C. The pellicle and erosion. Monogr Oral Sci. 2014;25:206–214. doi: 10.1159/000360376. [DOI] [PubMed] [Google Scholar]
- 31.Vitorino R., de Morais Guedes S., Ferreira R. Two-dimensional electrophoresis study of in vitro pellicle formation and dental caries susceptibility. Eur J Oral Sci. 2006;114:147–153. doi: 10.1111/j.1600-0722.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- 32.Stroici C., Darabă O.M., Cojocariu A.M., Bîrgăoanu A., Sachelarie L., Nazarie S. Protective role of acquired pellicle on enamel erosion. Int J Med Dent. 2012;16:272–275. [Google Scholar]
- 33.Hannig C., Spitzmüller B., Hannig M. Transaminases in the acquired pellicle. Arch Oral Biol. 2009;54:445–448. doi: 10.1016/j.archoralbio.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Rüdiger S. Institute of Odontology Department of Oral Microbiology University of Gothenburg; 2012. Studies on pellicle and early dental plaque in relation to periodontal conditions. Doctoral Thesis. [Google Scholar]
- 35.Rüdiger S.G., Dahlén G., Carlén A. Pellicle and early dental plaque in periodontitis patients before and after surgical pocket elimination. Acta Odontol Scand. 2012;70:615–621. doi: 10.3109/00016357.2011.645061. [DOI] [PubMed] [Google Scholar]
- 36.Hannig C., Kirsch J., Al-Ahmad A., Kensche A., Hannig M., Kümmerer K. Do edible oils reduce bacterial colonization of enamel in situ? Clin Oral Invest. 2013;17:649–658. doi: 10.1007/s00784-012-0734-0. [DOI] [PubMed] [Google Scholar]
- 37.Van der Mei H., White D., Kamminga-Rasker H. Influence of dentifrices and dietary components in saliva on wettability of pellicle-coated enamel in vitro and in vivo. Eur J Oral Sci. 2002;110:434–438. doi: 10.1034/j.1600-0722.2002.21341.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheaib Z., Rakmathulina E., Lussi A., Eick S. Impact of acquired pellicle modification on adhesion of early colonizers. Caries Res. 2015;49:626–632. doi: 10.1159/000442169. [DOI] [PubMed] [Google Scholar]
- 39.Masson N., Domingues R., Cury J., Leme A.P. Acidulated phosphate fluoride application changes the protein composition of human acquired enamel pellicle. Caries Res. 2013;47:251–258. doi: 10.1159/000346280. [DOI] [PubMed] [Google Scholar]