Abstract
Background/purpose
The early diagnosis of diabetes is essential for the prevention of complications. Periodontitis has been identified as the sixth complication of diabetes and chair-side screening may improve diagnosis of diabetes. This study evaluated whether gingival crevicular blood (GCB) available during routine periodontal examination can be used to screen for diabetes in Chinese patients with moderate to severe periodontitis.
Materials and methods
Finger-stick blood (FSB) and GCB were collected from patients (18 with diabetes and 42 without diabetes) during routine periodontal probing and analyzed for glucose and hemoglobin A1c (HbA1c) levels.
Results
In the diabetic group, the mean glucose levels in GCB and FSB were 12.21 ± 3.86 and 12.61 ± 4.19 mmol/L respectively, while those of the non-diabetic group were 6.14 ± 0.85 and 6.15 ± 0.87 mmol/L, respectively. The average HbA1c values of the diabetic group were 7.72% ± 1.71% and 7.89% ± 1.78% in GCB and FSB, respectively, while those of the non-diabetic group were 5.28% ± 0.31% and 5.23% ± 0.32%, respectively. Highly significant correlations were found between GCB and FSB glucose levels (r = 0.993 for the diabetic group, and r = 0.977 for the non-diabetic group) and between GCB and FSB HbA1c levels (r = 0.977 for the diabetic group, and r = 0.829 for the non-diabetic group).
Conclusion
Our study results indicate that GCB available during routine periodontal examination may be acceptable for the analyses of blood glucose and HbA1c levels. The approach is suitable for screening undiagnosed diabetes in a dental setting.
Keywords: Gingival crevicular blood, Diabetes, Blood glucose, Hemoglobin A1c, Periodontitis
Introduction
Diabetes, a pandemic disease with serious complications, is increasing in prevalence. In 2019, the International Diabetes Federation estimated that 9.3% of adults have diabetes. Type 2 diabetes accounts for most cases (approximately 90%); among patients, 79.44% live in low- and middle-income countries.1 Approximately 4.2 million people died of diabetes and its complications in 2019. One in two (50.1%) adults living with diabetes are unaware that they have the condition. In China, only 44% of patients with diabetes are diagnosed and treated in a clinic.1 Thus, there is an urgent need to screen and diagnose people with diabetes.2 Many remain undiagnosed because they have few symptoms in the early stages of type 2 diabetes, or because the symptoms that do appear are not associated with diabetes.3
Recent evidence indicates that diabetic complications such as retinopathy, cardiovascular disease and neuropathy may appear several years before the diagnosis of type 2 diabetes.4 Periodontal disease is the sixth most common complication of diabetes and interacts with diabetes in a bidirectional manner.5 The National Health and Nutrition Examination Survey (2009–2010) indicated that the prevalence of diabetes in periodontal patients was 12.5%, while the prevalence of diabetes without periodontitis is only 6.3% in the United States.6 A multivariate analysis in China suggested that participants with diabetes were 2.4-times more likely to have severe periodontitis, indicating that dentists likely encounter undiagnosed diabetes patients with periodontitis.7 Routine periodontal probing during periodontal examination produces gingival crevicular blood (GCB) in patients with moderate to severe periodontitis. This study examined whether GCB can be used to test blood glucose and hemoglobin A1c (HbA1c) values during periodontal visits as an alternative to the conventional finger-stick blood (FSB) method.
Materials and methods
Study design and patients
This study was approved by the Research Ethics Committee of Nanjing Stomatological Hospital, Medical School of Nanjing University. Informed consents were obtained from all patients before enrollment in the study. The patients were assessed for diabetic history and received periodontal examination. GCB and FSB were collected with a blood glucose test strip and in an Eppendorf tube for the HbA1c test. Glucose levels and HbA1c values of GCB and FSB were determined simultaneously in the same patient.
Patients with moderate to severe chronic periodontitis were recruited from the Department of Periodontology between March 2018 and August 2019. The diagnosis of patients was based on the clinical and radiographic criteria proposed by the American Academy of Periodontology for the classification of periodontal disease.8 Patients were eligible for this study if they met the following criteria: (1) age 20–70 years; (2) less supragingival plaque; (3) visible gingival inflammation, radiographic evidence of bone loss, clinical attachment loss: ≥3 mm, probing pocket depth: ≥ 5 mm; and (4) obvious gingival inflammation on the facial surfaces of upper anterior teeth with pronounced bleeding on probing (BOP). Patients meeting the following criteria were excluded from the study: (1) bleeding disorders; (2) severe systemic disease (type 2 diabetes excluded), such as a cardiovascular, renal, hepatic or immunological disorders; (3) any indication of antibiotic prophylaxis; and (4) drugs affecting coagulation.
Sample collection
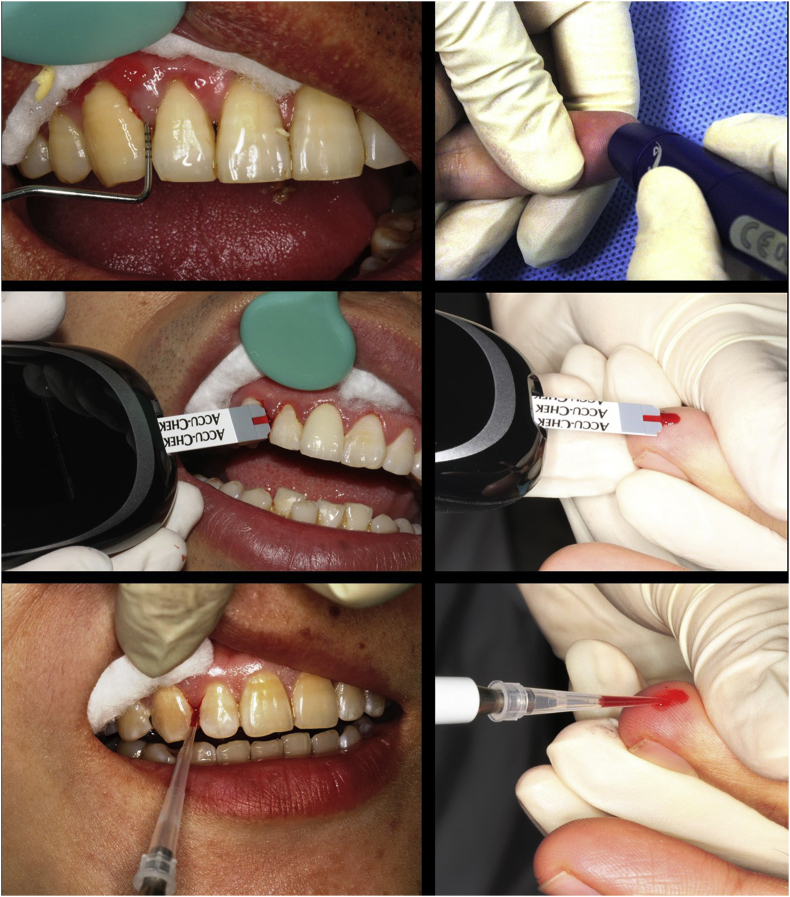
The maxillary anterior teeth were chosen as the sites of GCB acquisition as they are easily accessed. As shown in Fig. 1, contamination with saliva was prevented by using cotton rolls and air drying. BOP was observed during routine periodontal examination using a UNC-15 probe (Hu Friedy, Chicago, IL, USA). The top edge of a test strip preloaded in a glucometer (Roche Diagnostics GmbH, Mannheim, Germany) was then placed in contact with the blood oozing out from the gingival margin until the confirmation window was full and the glucometer began to count down. GCB (at least 10 μl) was then collected for HbA1c analysis using a micropipette (Eppendorf AG, Hamburg, Germany) and transferred to ethylenediaminetetraacetic acid (EDTA)-treated tubes and stored at −20 °C.
Figure 1.
Collection of gingival crevicular blood and finger-stick blood.
Immediately after the GCB sample was collected as shown in Fig. 1, the finger pad of the participant was wiped with alcohol, allowed to dry, and then punctured with a lancing device (Roche Diagnostics GmbH). The resulting FSB was drawn onto a glucose test strip, and the glucometer reading was recorded. FSB samples (at least 10 μl) were also collected in EDTA-treated tubes and stored at −20 °C for HbA1c analysis.
HbA1c analysis
HbA1c was assayed using ion-exchange high-performance liquid chromatography on a Bio-Rad D-10® autoanalyzer (Bio-Rad Laboratories, California, CA, USA).
Statistical analysis
The GCB and FSB glucose levels were considered as “random glucose”. Pearson's correlation coefficient was used to assess the correlation between glucose and HbA1c levels in GCB and FSB. Statistical analyses were performed using SPSS18.0 software (SPSS Inc., Chicago, IL, USA).
Results
A total of 60 patients (26 males and 34 females) were enrolled in the study and divided into diabetic and non-diabetic groups. Table 1 shows demographic data for the patients. The diabetic group consisted of 18 patients, including 16 already diagnosed with type 2 diabetes and two newly diagnosed patients based on the FSB HbA1c level determined in this study (FSB HbA1c > 6.5%). The non-diabetic group included 42 patients (18 males and 24 females).
Table 1.
Descriptive statistics of the participants in the study.
| Group | n | Sex |
Age in years |
Glucose level (mmol/L, mean ± SD) |
HbA1c (%, mean ± SD) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Male, n | Female, n | 20–44 | 45–70 | GCB | FSB | GCB | FSB | ||
| Diabetic | 18 | 8 | 10 | 0 | 18 | 12.21 ± 3.86 | 12.61 ± 4.19 | 7.72% ± 1.71% | 7.89% ± 1.78% |
| Non-diabetic | 42 | 18 | 24 | 26 | 16 | 6.14 ± 0.85 | 6.15 ± 0.87 | 5.28% ± 0.31% | 5.23% ± 0.32% |
| Total | 60 | 26 | 34 | 26 | 34 | 7.96 ± 3.56 | 8.09 ± 3.81 | 6.03% ± 1.58% | 6.01% ± 1.48% |
GCB: gingival crevicular blood; FSB: finger-stick blood; HbA1c: hemoglobin A1c; SD: standard deviation.
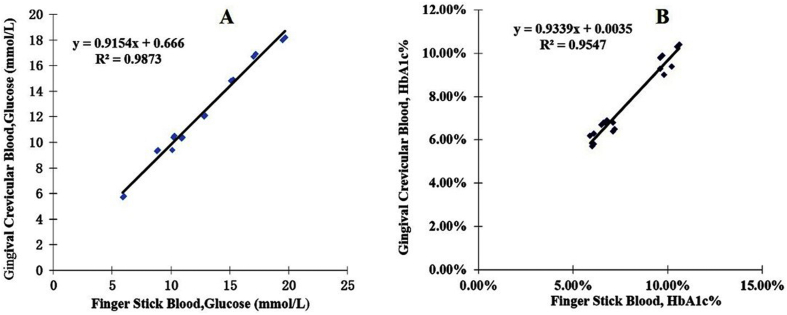
The random glucose and HbA1c values of GCB and FSB were determined simultaneously for each patient. The GCB glucose levels of diabetic patients ranged from 5.7 to 18.2 mmol/L, with an average of 12.21 ± 3.86 mmol/L. The FSB glucose levels ranged from 5.9 to 19.7 mmol/L, with an average of 12.61 ± 4.19 mmol/L. As shown in Fig. 2, the blood glucose levels assessed using GCB and FSB were nearly identical, with a correlation coefficient of 0.994 (P < 0.001). The HbA1c levels determined in GCB samples ranged from 5.7% to 10.4%, with an average of 7.72% ± 1.71%. In FSB samples, the HbA1c content ranged from 5.7% to 10.6%, with an average of 7.89% ± 1.78%. The HbA1c levels in GCB and FSB samples were highly consistent, with a correlation coefficient of 0.977 (P < 0.001; Fig. 2).
Figure 2.
Scatter plots and linear regression lines showing the relationships between blood glucose levels (A) and HbA1c values (B) determined from gingival crevicular blood and finger-stick blood in the diabetic group.
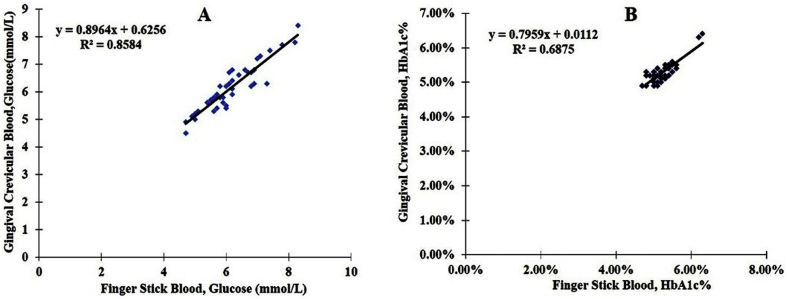
The GCB glucose levels in non-diabetic patients ranged from 4.5 to 8.4 mmol/L, with an average of 6.14 ± 0.85 mmol/L. The FSB blood glucose levels ranged from 4.7 to 8.3 mmol/L, with an average of 6.15 ± 0.87 mmol/L. The correlation coefficient between the glucose levels in GCB and FSB was 0.926 (P < 0.001; Fig. 3). The HbA1c levels in the GCB samples of non-diabetic patients ranged from 4.9% to 6.3%, with an average of 5.28% ± 0.31%. The FSB HbA1c levels ranged from 4.8% to 6.2%, with an average of 5.23% ± 0.32%. As shown in Fig. 3, the Pearson correlation coefficient between the HbA1c values of GCB and FSB was 0.829 (P < 0.001).
Figure 3.
Scatter plots and linear regression lines showing the relationships between blood glucose levels (A) and HbA1c values (B) determined from gingival crevicular blood and finger-stick blood in the non-diabetic group.
Discussion
The early detection and treatment of diabetes can reduce the burden and complications of the disease. China, India and the United States are among the countries with the greatest number of people with undiagnosed diabetes.1 Poor control of diabetes increases the risk of developing destructive periodontitis and impairs treatment outcomes. Chronic inflammatory periodontal disease considerably complicates the control of diabetes.9 The prevalence of diabetes in patients with periodontitis is nearly double that of periodontally healthy subjects.10 Dentists, and specifically periodontists, are likely to encounter undiagnosed diabetics. Therefore, screening for diabetes during dental visits presents an opportunity to improve public health.
Clinical periodontal probing is the primary method used to diagnose periodontitis. BOP under a probing force of 0.25–0.30 N around the gingival margin indicates gingival inflammation.11 The determination of blood glucose requires only 0.6 μl of blood, and HbA1c testing requires 10 μl of blood. Routine periodontal probing during periodontal examination can provide a sufficient amount of GCB to test undiagnosed patients with moderate to severe periodontitis for diabetes. This study compared the GCB glucose levels of periodontitis patients with the FSB levels. Highly significant correlations were found between GCB and FSB glucose levels in the diabetic (r = 0.994) and non-diabetic (r = 0.926) groups. The results of this study agree with those of Shetty et al. (India), Shylaja et al. (India), Parihar et al. (India), Bhavsar et al. (India) and Gupta et al. (India).12, 13, 14, 15, 16 However, in contrast to the above studies, Muller and Behbehani (Kuwait) did not find a correlation between GCB and FSB glucose levels in 46 subjects (24 with periodontitis and 22 with gingivitis; sufficient GCB was obtained in 31 patients).17 Strauss et al. (United States) reported that GCB samples were suitable for diabetes screening when adequate BOP could be obtained, and when the samples did not contact the teeth or gingival margin.18
In 2014, the American Diabetes Association recommended monitoring HbA1c levels to diagnose diabetic and prediabetic patients and to monitor glycemic control in diabetic patients. HbA1c testing is advantageous because fasting is not required, and HbA1c levels are not affected by acute perturbations (e.g., stress, diet and exercise); thus, HbA1c testing can provide an average glycemic level over a three-month period.19 For the first time, this study compared HbA1c levels in GCB and FSB samples simultaneously. Highly significant correlations in HbA1c level were found between GCB and FSB in the diabetic (r = 0.977) and non-diabetic (r = 0.829) groups. In addition, two patients in our study were found to have diabetes and referred to a physician for further treatment. Kim et al. (United States) compared HbA1c values in GCB and FSB among 29 diabetic patients and obtained a Pearson correlation coefficient of 0.98.20 Strauss et al. (United States) compared the GCB and FSB HbA1c levels of 75 patients with periodontal disease and obtained a Pearson correlation coefficient of 0.842. The authors also suggested a criterion of 6.3% for the GCB HbA1c test to screen for diabetes.21 In an examination of 408 adults with or at risk of diabetes, Strauss et al. (United States) obtained a correlation coefficient of 0.991 between GCB and FSB HbA1c levels and suggested that GCB collected during a dental visit can be used to screen for diabetes and monitor glycemic control in many at-risk patients.22
The early identification and treatment of prediabetes can interrupt the progression of the disease. A survey conducted by Rosedale et al. indicated that both periodontitis patients and dentists believe that dental visits are suitable for diabetes screening, and patients generally prefer GCB collection to FSB collection. Many patients approved of the use of GCB for diabetes screening, and most dentists agree that GCB collection is quick and easy.23 Thus, dental visits could be an important setting for diabetes screening. In addition, understanding the diabetes status of patients can help dentists make treatment decisions and optimize the oral health of patients.
In conclusion, diabetes screening during dental visits is a promising public health opportunity. For the first time, this study compared the glucose and HbA1c levels determined from GCB and FSB samples in Chinese patients with moderate to severe periodontitis. The results indicate that GCB available during routine periodontal examination can be used to estimate blood glucose and HbA1c levels. Additional studies in larger populations should be conducted to confirm the appropriateness of GCB for diabetes screening.
Declaration of competing interest
None declared.
Acknowledgments
This study was supported by the National Science Foundation of China (no. 514721115) and the Medical Science and Technology Development Foundation, Nanjing Department of Health (no. YKK18121).
References
- 1.Saeedi P., Petersohn I., Salpea P. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Dall T.M., Yang W., Gillespie K. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2019;42:1661–1668. doi: 10.2337/dc18-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle J.P., Engelgau M.M., Thompson T.J. Estimating prevalence of type 1 and type 2 diabetes in a population of African Americans with diabetes mellitus. Am J Epidemiol. 1999;149:55–63. doi: 10.1093/oxfordjournals.aje.a009728. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande A.D., Harris-Hayes M., Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossi S.G., Genco R.J. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Arora N., Papapanou P.N., Rosenbaum M., Jacobs D.R., Desvarieux M., Demmer R.T. Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from the Continuous National Health and Nutrition Examination Survey 2009-2010. J Clin Periodontol. 2014;41:643–652. doi: 10.1111/jcpe.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q., Li Z., Wang C. Prevalence and predictors for periodontitis among adults in China, 2010. Glob Health Action. 2014;7:24503. doi: 10.3402/gha.v7.24503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Casanova L., Hughes F.J., Preshaw P.M. Diabetes and periodontal disease: a two-way relationship. Br Dent J. 2014;217:433–437. doi: 10.1038/sj.bdj.2014.907. [DOI] [PubMed] [Google Scholar]
- 10.Mealey B.L., Oates T.W., American Academy of Periodontology Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 11.Heft M.W., Perelmuter S.H., Cooper B.Y., Magnusson I., Clark W.B. Relationship between gingival inflammation and painfulness of periodontal probing. J Clin Periodontol. 1991;18:213–215. doi: 10.1111/j.1600-051x.1991.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 12.Shetty N., Shankarapillai R., Mathur L.K., Manohar B., Mathur A., Jain M. Gingival crevicular blood: as a non-invasive screening tool for diabetes mellitus in dental clinics. J Indian Soc Periodontol. 2013;17:472–477. doi: 10.4103/0972-124X.118319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shylaja M.D., Punde P.A., Sam G., Khan S.N., Latheef A.A., Thorat A.J. Noninvasive technique for estimating blood glucose levels among diabetic patients. J Contemp Dent Pract. 2016;17:248–252. doi: 10.5005/jp-journals-10024-1835. [DOI] [PubMed] [Google Scholar]
- 14.Parihar S., Tripathi R., Parihar A.V., Samadi F.M., Chandra A., Bhavsar N. Estimation of gingival crevicular blood glucose level for the screening of diabetes mellitus: a simple yet reliable method. J Oral Biol Craniofac Res. 2016;6:198–203. doi: 10.1016/j.jobcr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhavsar M.V., Brahmbhatt N.A., Sahayata V., Bhavsar N.V. Gingival crevicular blood for screening of blood glucose level in patients with & without diabetes: a chair-side test. Int J Dent Hyg. 2016;14:92–97. doi: 10.1111/idh.12139. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A., Gupta N., Garg R., Jain N., Atreja G., Walia S.S. Developing a chair side, safe and non-invasive procedure for assessment of blood glucose level using gingival crevicular bleeding in dental clinics. J Nat Sci Biol Med. 2014;5:329–332. doi: 10.4103/0976-9668.136177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller H.P., Behbehani E. Screening of elevated glucose levels in gingival crevice blood using a novel, sensitive self-monitoring device. Med Princ Pract. 2004;13:361–365. doi: 10.1159/000080474. [DOI] [PubMed] [Google Scholar]
- 18.Strauss S.M., Wheeler A.J., Russell S.L. The potential use of gingival crevicular blood for measuring glucose to screen for diabetes: an examination based on characteristics of the blood collection site. J Periodontol. 2009;80:907–914. doi: 10.1902/jop.2009.080542. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes-2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J.W., Wolff R.E., Gaillard P., Wolff L.F. Gingival crevicular blood as a source to screen for diabetes control in a dental office setting. Am J Dent. 2015;28:63–67. [PubMed] [Google Scholar]
- 21.Strauss S.M., Tuthill J., Singh G. A novel intraoral diabetes screening approach in periodontal patients: results of a pilot study. J Periodontol. 2012;83:699–706. doi: 10.1902/jop.2011.110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss S.M., Rosedale M.T., Pesce M.A. The potential for glycemic control monitoring and screening for diabetes at dental visits using oral blood. Am J Public Health. 2015;105:796–801. doi: 10.2105/AJPH.2014.302357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosedale M.T., Strauss S.M. Diabetes screening at the periodontal visit: patient and provider experiences with two screening approaches. Int J Dent Hyg. 2012;10:250–258. doi: 10.1111/j.1601-5037.2011.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]