Abstract
The main surgical strategy for gastrointestinal tract malignancy is en bloc resection, which consists of not only resection of the involved organs but also simultaneous resection of the surrounding or adjacent mesenteries that contain lymph vessels and nodes. After resection of the diseased organs, the defect of the gastrointestinal conduit is replaced with organs located downstream, such as the stomach and jejunum. However, esophageal and gastric reconstruction using these natural substitutes is associated with a diminished quality of life due to the loss of the reserve function, damage to the antireflux barrier, and dumping syndrome. Thus, replacement of the deficit after resection with the patient's own regenerated tissue to compensate for the lost function and tissue using regenerative medicine will be an ideal treatment. Many researchers have been trying to construct artificial organs through tissue engineering techniques; however, none have yet succeeded in growing a whole organ because of the complicated functions these organs perform, such as the processing and absorption of nutrients. While exciting results have been reported with regard to tissue engineering techniques concerning the upper gastrointestinal tract, such as the esophagus and stomach, most of these achievements have been observed in animal models, and few successful approaches in the clinical setting have been reported for the replacement of mucosal defects. We review the recent progress in regenerative medicine in relation to the upper gastrointestinal tract, such as the esophagus and stomach. We also focus on the functional capacity of regenerated tissue and its role as a culture system to recapitulate the mechanisms underlying infectious disease. With the emergence of technology such as the fabrication of decellularized constructs, organoids and cell sheet medicine, collaboration between gastrointestinal surgery and regenerative medicine is expected to help establish novel therapeutic modalities in the future.
Keywords: Regenerative medicine, Tissue engineering, The stomach, The esophagus, Organoid
Highlights
-
•
The recent progress in regenerative medicine in upper gastrointestinal tract.
1. Introduction
The accumulation of understanding about the spread of cancer cells and a continuous desire to cure patients with gastrointestinal malignancies have established the main principles of surgical strategy, so called “en bloc” resection. This consists not only of the resection of the involved organs, but also the simultaneous resection of surrounding or adjacent mesenteries that contain lymph vessels and nodes. For upper gastrointestinal malignancies, the whole esophagus or a large part of the stomach is often resected in “en bloc” strategy. After the resection of these organs, the defect of the gastrointestinal conduit is replaced with organs located downstream: the stomach is often used as a substitute after esophagectomy and the jejunum is often used after gastrectomy. However, the esophageal and gastric reconstruction using native substitutes, such as the stomach and jejunum, are associated with a diminished quality of life due to a loss of reserve function, damage of the antireflux barrier, and dumping syndrome. In addition, resection or utilization of these organs as native substitutes leads to a drastic change in food intake and the nutritional state and patients often suffer from small stomach symptoms, weight loss, and malnutrition.
Regenerative medicine is an upcoming concept involving the repair or regeneration of tissue/organ deficit caused by disease, surgical removal, and trauma using processed cells/tissues obtained from the patients themselves or other healthy donors. The regeneration of upper gastrointestinal tracts could contribute the quality of life of patients with upper gastrointestinal malignancies and could expand the possibility of general surgery. We review the recent progress in regenerative medicine, especially in relation to the upper gastrointestinal tract, such as the esophagus and stomach.
2. Tissue engineering technique
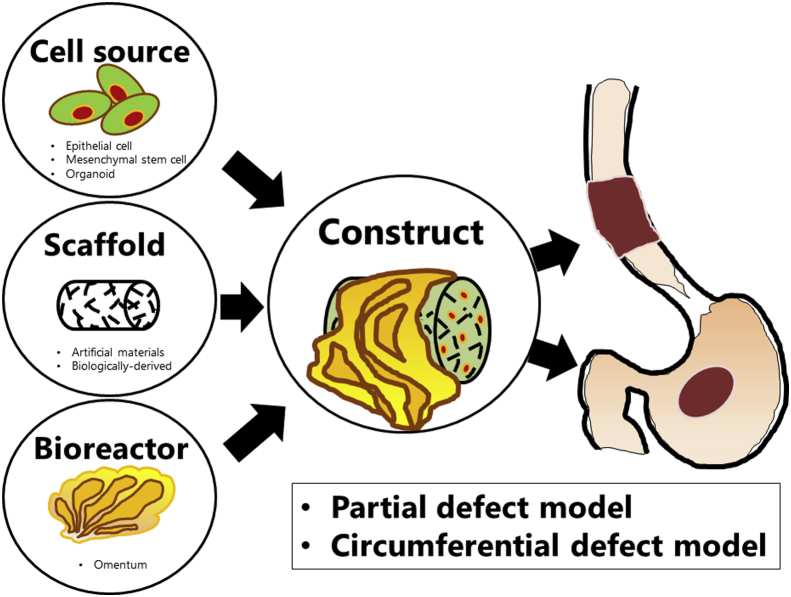
Many researchers have been trying to construct artificial organs through tissue engineering techniques. The primary elements of the construction often consist of scaffolding, cell sources and bioreactors, which are involved in several steps [1] (see Fig. 1).
Fig. 1.
The main element for tissue engineering in gastrointestinal tract. Most studies have used scaffold with cell sources and/or bioreactors in replacement for partial defect or circumferential defect animal model.
Epithelialization on the inner surface of artificial scaffolds is crucial to maintaining patency, create a protective barrier, and remodel tissues [2]. Autologous cells are often used as a cell source because immune rejection would not occur and immunosuppressive agents not needed [[3], [4], [5]]. Mesenchymal stem cells (MSCs) is also used as potential cell source for epithelial and smooth muscle regeneration [[6], [7], [8], [9]], because they are easy to obtain and have the capacity to differentiate into both epithelial cells and muscle cells.
In addition to the cellularization of implanted cells, the maintenance of the tubular configuration is important for the regeneration of the esophagus [10]. Scaffolds are used in the regeneration of esophagus to retain the organ's mechanical and bioactive properties, such as cellular attachment, proliferation and differentiation. Two types of scaffolds have been reported, one is made from artificial materials, the other is biologically derived. The ability of both types to support cellular attachment, proliferation, and differentiation has been well investigated [11]. These scaffolds are also expected to promote native tissue stem cell migration and remodeling into the site. In particular, biologically-derived acellular tissue scaffolds, such as the decellularized small intestinal submucosa (SIS), have the advantage of retaining the natural architecture of extracellular matrix (ECM), enhancing epithelial cell attachment, furthermore, they contain vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF), which promote the vascularization of an implanted graft [12,13].
Bioreactors are other important factor for supplying vascularization into tissue-engineered organs because the regenerated organ requires new blood vessels for nutrient exchange in order to maintain viability [14]. Many researchers have used a donor omentum to provide a blood supply by wrapping the regenerated organ [5,[15], [16], [17]].
3. Regeneration of the esophagus
The esophagus might be a promising gastrointestinal organ for regenerative medicine because of its relative simplicity: the esophagus has a tube-like muscular structure, the function of which is to convey the meal from the pharynx to the stomach via peristalsis. Furthermore, most of the epithelium consists of simple squamous cells and does not have any function of secretion of digestive juice.
Most reports thus far have described the regeneration of part of the esophageal wall or the replacement of the whole circumferential esophagus [10,18], however, a replacement for a fully circumferential and full wall-thick segment of the esophagus is still challenging (Table 1) [[3], [4], [5],[7], [8], [9],[15], [16], [17],[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]].
Table 1.
Animal models for regeneration of the esophagus.
| Year | Author | Animal | Portion | Cell source | Scaffold | Bioreactor | Outcome |
|---|---|---|---|---|---|---|---|
| Partial-thickness defects model | |||||||
| 2005 | Badylak SF [20] | canine | Cervical | – | UB-ECM (porcine) | – | no stenosis |
| 2006 | Ohki T [21] | canine | Chest | mucosal epithelial cell (sheet) | – | – | no stenosis |
| 2007 | Sakurai T [22] | porcine | Chest | buccal keratinocyte (injection) | – | – | no stenosis |
| 2009 | Nieponice A [23] | canine | Cervical | – | UB-ECM (porcine) | – | no stenosis |
| 2011 | Honda M [24] | canine | abdominal | adipose tissue-derived cell (injection) | – | – | no stenosis |
| 2013 | Nieponice A [25] | rodent | Cervical | bone marrow-derived cell | UB-ECM (porcine) | – | no stenosis |
| Full-thickness, partial defect model | |||||||
| 2000 | Badylak SF [26] | canine | Cervical | – | UB-ECM (porcine) | – | no stenosis |
| 2001 | Isch JA [27] | canine | Cervical | – | decellularized collagen (human) | – | no stenosis |
| 2002 | Komuro H [6] | porcine | Chest | (autologous epithelium remained) | collagen sponge | – | no stenosis |
| 2003 | Grikscheit TC [17] | rodent | Abdominal | esophageal organoid unit | biodegradable polymer tube | Omentum | unobstructed |
| 2004 | Lynen JP [28] | rabbit | Abdominal | – | polyvinylidene fluoride (PVDF) mesh | – | no stenosis |
| 2004 | Lynen JP [28] | rabbit | Abdominal | – | polygractin910 (Vicryl) mesh | – | anastomotic leakage |
| 2005 | Badylak SF [20] | canine | Cervical | – | UB-ECM (porcine) | – | stenosis |
| 2006 | Lopes MF [29] | rodent | Cervical | – | SIS-ECM (porcine) | – | no stenosis |
| 2007 | Urita Y [18] | rodent | Abdominal | – | gastric acellular matrix (rodent) | omentum | no stenosis |
| 2009 | Wei RQ [7] | canine | Cervical | oral mucosal epithelial cells | SIS-ECM (porcine) | – | no stenosis |
| 2013 | Tan B [10] | canine | Cervical | MSC | SIS-ECM (porcine) | – | no stenosis |
| 2015 | Diemer P [30] | rabbit | Abdominal | – | poly-e-caprolactone mesh | – | pseudodiverticulum |
| 2016 | Park SY [12] | rabbit | Cervical | MSC | poly-e-caprolactone mesh | – | no stenosis |
| 2017 | Okuyama H [31] | canine | Abdominal | – | In-body tissue architecture | – | no stenosis |
| Full-thickness, circumferential defect model | |||||||
| 2000 | Saito M [32] | rodent | Cervical | split-thickness skin | collagen sponge + silicone membrane | latissimus dorsi muscle | no stenosis |
| 2000 | Badylak SF [26] | canine | Cervical | – | SIS-ECM (porcine) | – | stenosis |
| 2003 | Grikscheit T [33] | rodent | Abdominal | esophageal organoid unit | biodegradable polymer tube | Omentum | stenosis |
| 2007 | Urita Y [18] | rodent | Abdominal | – | gastric acellular matrix (rodent) | omentum | asphyxation |
| 2008 | Nakase Y [19] | caninne | Chest | keratinocyte, fibroblast, smooth muscle tissue | amniotic membrane + polyglicolic acid felt | omentum | no stenosis |
| 2009 | Doede T [34] | porcine | Cervical | – | SIS-ECM (porcine) | – | stenisos |
| 2010 | Gaujoux S [35] | porcine | Cervical | – | allogenic aorta | – | no stenosis |
| 2014 | Sjoqvist S [11] | rodent | Cervical | MSC | esophageal ECM (rodent) | – | no stenosis |
| 2015 | Poghosyan T [8] | porcine | Cervical | oral mucosa, myoblast | SIS-ECM + amniotic membrane | omentum | no stenosis |
| 2017 | Catry J [36] | porcine | Abdominal | MSC | SIS-ECM (porcine) | omentum | stenosis |
| 2018 | Luc G [37] | porcine | Abdominal | – | esophageal ECM (porcine) | omentum | stenosis |
| 2019 | Takeoka Y [38] | rodent | Abdominal | dermal fibroblasts, smooth muscle cells, MSC | bio 3D printer | omentum | no stenosis |
Table 1 modified from Kanetaka K, Surg Today, 2018.
Recently, Luc et al. reported successful esophageal replacement in a large animal model [36]. They established a tissue-engineered esophagus using a decellularized porcine esophagus as a scaffold, that was recellularized with adipose tissue-derived stem cells sheets, with the omentum used as bioreactor. These constructs were transplanted in a porcine model after the resection of the full-thickness circumferential abdominal esophagus with end-to-end anastomosis. Five of 6 animals survived at the end of the 5-week follow-up period, although all these animals had postoperative complications such as stenosis and abscess formation. Histopathological analyses showed cellularization with a preserved architectural structure of the mucosal and submucosal layers; however, the muscular layer was almost absent.
More recently, two sophisticated procedures have been reported. Urbani et al. reported a two-staged bioengineering procedure for constructing a layered esophagus [38]. In the first step, a decellularized rat esophagus was seeded with human mesoangioblasts, which are known to differentiate toward smooth muscle [39], mouse fibroblasts, and murine neural crest cells. The constructs were cultured in dynamic conditions that improve muscle layer formation and differentiation and then were implanted in the omentum. These scaffolds were harvested 1 week after implantation, and seeded with rat esophageal epithelial cells in the second step. They concluded that this two-staged seeding approach allowed in vitro and in vivo smooth muscle maturation, graft neovascularization and epithelial cell engraftment.
Takeoka et al. reported on esophagus regeneration using the novel technology of bio 3D printing [37]. They created scaffold-free structures consisting of multicellular spheroids containing a mixture of cell types, including human dermal fibroblasts, esophageal smooth muscle cells and mesenchymal stem cells using the Regenova bio-3D printer with the Kensan method (Cyfuse Biomedical K.K. Japan) [40]. The constructs were transplanted using an interposition procedure with suturing between the esophagus and stomach. All animals survived and none developed complications after transplantation. A histopathological analysis revealed alpha smooth muscle actin (SMA) positive cells in the structure and the mucosal epithelium covered its inner surface; however, this was thought to be native rat epithelium extending onto the transplanted structures.
4. Regeneration of the stomach
Digestive organs, such as the stomach, the small intestine, and the colon, have highly complicated functions such as the processing and absorption of nutrients, the transport of content and the elimination of enteral waste. As there are various hurdles to regenerate these organs in their entirety, the regeneration of these organs has mainly focused on the fabrication of the epithelial component.
Because of the variation of the epithelial component and complexity of muscular layer, the regeneration of whole gastric wall of multilayered structure has not been achieved yet. The study design of regeneration of the stomach has two aspect: regeneration of epithelial lineage itself and physiological reinforcement of gastric wall. This is in clear contrast to the regeneration of the esophagus in which the regeneration of the whole esophagus or part of the esophageal wall were attempted.
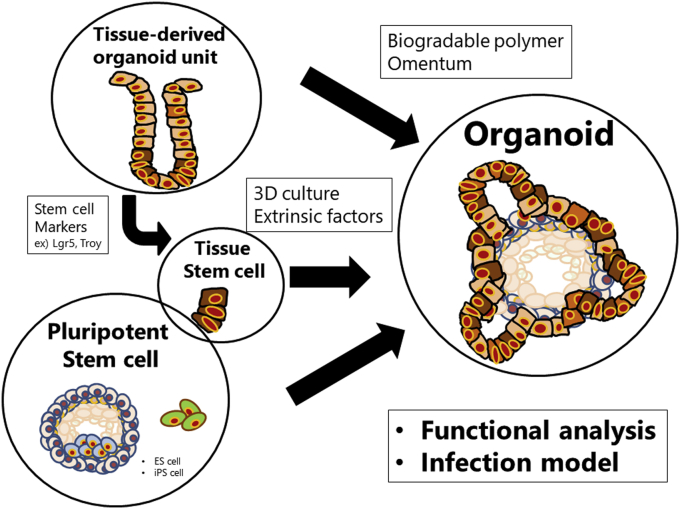
The regeneration of the epithelial lineage of the stomach is achieved from various cell origins ranging from adult cells or tissue stem cells to organoids (Table 2) [32,[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]] (see Fig. 2).
Table 2.
Regeneration of gastric mucosa.
| Year | Author | Animal | Cell source | Scaffold | Bioreactor | type of regenerated epithelium | Functional analysis |
|---|---|---|---|---|---|---|---|
| 2003 | Grikscheit T [33] | rodent | resected tissue from the antrum | biogradable polymer | omentum | mucous pit, mucous neck, parietal | – |
| 2003 | Maemura T [43] | rodent | resected tissue from the antrum | biogradable polymer | omentum | mucous, smooth muscle | – |
| 2004 | Maemura T [44] | rodent | resected tissue from the antrum | biogradable polymer | omentum | mucous, smooth muscle-like cell | – |
| 2008 | Maemura T [45] | rodent | resected tissue from the antrum | biogradable polymer | omentum | mucous, parietal, G, smooth muscle-like cell | – |
| 2009 | Sala FG [46] | swine | resected tissue from the antrum | biogradable polymer | omentum | mucous, smooth muscle | – |
| 2010 | Barker N [47] | rodent | Lgr5+ve cell | 3D culture | mucous pit, mucous neck, chief, enteroendcrine | – | – |
| 2011 | Speer AL [48] | rodent | resected tissue from the antrum | biogradable polymer | omentum | mucous, chief, parietal cell, enteroendocrine | – |
| 2010 | Stange DE [49] | rodent | Troy+ chief | 3D culture | mucous neck, chief | – | – |
| 2013 | Katano T [50] | rodent | resected tissue from the antrum | 3D culture | mucous pit, enteroendocrine | – | – |
| 2014 | McCracken KW [51] | human | iPS | 3D culture | mucous pit, enteroendocrine | H.Pylori infection | – |
| 2015 | Bartfeld S [52] | human | resected tissue from the corpus | 3D culture | mucous pit, mucous neck, chief, enteroendcrine | H.Pylori infection | – |
| 2015 | Schumacher MA [53] | rodent | resected tissue from the fundus | 3D culture | mucous pit, mucous neck, chief, parietal, D | acid secretion | |
| 2015 | Noguchi T [54] | rodent | ESC | 3D culture | mucous pit, mucous neck, chief, parietel, enteroendcrine, G | acid & pepcinogenC secretion | – |
| 2016 | Schlaermann P [55] | human | resected tissue from the corpus | 3D culture | mucous pit | H.Pylori infection | – |
| 2017 | McCracken KW [56] | human | iPS | 3D culture | mucous pit, mucous neck, chief, parietel, enteroendcrine | acid secretion | – |
Fig. 2.
The regeneration of the gastric epithelial cells. The regeneration of the epithelial lineage of the stomach is attempted from various cell origins such as tissue-derived organoid unit and pluripotent stem cells.
The evaluation has been done not only by immunohistochemistry for various epithelial marker, but also through functional analysis of these established epithelial cells. In addition, these regenerated cells were reported to be useful tool to investigate the pathological process in the infection of Helicobactor pylori (HP), which is clinically important pathogen of chronic gastritis and gastric carcinogenesis.
Another direction of the study is physiological reinforcement of gastric wall. There are several reports described bioengineered gastrointestinal sphincteric constructs [[55], [56], [57], [58]], however, regeneration of gastric wall with peristaltic movement has not been established. The regeneration therefore has been mainly focused on restoration of a partial defect of gastric wall.
4.1. Regeneration of the gastric epithelium
The gastric epithelium is anatomically divided into two major functional regions. The corpus and the antrum each have a unique repertoire of epithelial lineage: parietal, chief cells mainly reside in the corpus, and surface mucous pit and mucous neck cells in both the antrum and the corpus, with endocrine cells located throughout the whole stomach [59]. As these cells are thought to be progeny from unique stem cells, the regeneration of the gastric epithelium has been described in the context of research on the allocation of stem cells.
4.1.1. Regeneration from tissue-derived organoid unit
The first fabrication of tissue-engineered stomach (TES) was reported by Grikscheit et al., who had previously established tissue-engineered small intestine or colon from organoid units [60,61]. They developed stomach organoid units from full-thickness rat stomach, and then loaded them onto synthetic biodegradable polymers as a scaffold. The structures were implanted into the rat abdomen with wrapping by the greater omentum as a bioreactor [32]. The constructed TES appeared as a cyst with a single-lumen wrapped in omentum, and a diameter of 30–50 mm. A histopathological analysis showed the internal surface of the TES lined by epithelium, and immunohistochemistry revealed that these “neomucosa” included various cell lineages, such as mucous surface, mucous neck, and parietal cells. In addition, alpha SMA positivity was detected in the muscularis layer, indicating that the native stomach histology was maintained in the TES.
Maemura et al. generated a TES from the neonatal rat glandular stomach using the methods described by Grikscheit [41]. After construction of the TES in the abdomen, they proceeded to a second operation in which the native stomach was replaced by the TES with end-to-end anastomosis between the native esophagus and the jejunum. Six of 10 recipient rats survived for the entire study period of 12 weeks. The upper gastrointestinal tract study did not show any signs of stenosis or obstruction, and orally injected barium could pass through the TES into the connected small intestine. Histopathological studies demonstrated that the TES had continuous, well-developed neomucosal and smooth muscle layers. Interestingly, they found that the local structure was better in this replacement manner than their previous model rats for which side to side anastomosis was performed without removal of the native stomach, in which the overall structures of the submucosal and smooth muscle layers were not stratified [42]. They concluded that the movement of the luminal content was important for the development of the neomucosa and the directionality of the smooth muscle layer.
4.1.2. Regeneration from tissue stem cells
Stem cells are thought to maintain tissue renewal not only in generating clonal, multipotent stem cells in infrequent symmetric division but also in generating various epithelial lineage daughter cells in frequent asymmetric division. Recent lineage-tracing studies have inferred the existence of multipotent stem cells in the small intestine and colon [62]. Stomach epithelial cells are also fueled from these stem cell populations, as is observed in the colon, and several stem cell markers of the gastric epithelium have been reported thus far [47,[63], [64], [65]].
Barker et al. identified the orphan G protein-coupled receptor, Lgr5 as a marker of active stem cells in the small intestine and colon [64,66]. They also showed that an Lgr5-positive population was actively proliferating and has stem cell potential in the stomach [45]. These Lgr5-positive cells are located in the bottom of glands, contrary to expectation that stem cells might be located in isthmus where cellular proliferation is most predominant. They could successfully establish a long-term culture system of isolated Lgr5-positive cells in Matrigel, which contain EGF, Noggin, R-Spondin1, and which show strict dependence on Wnt3A. In this system, they found that a single Lgr5-positive population efficiently generated gastric organoids that resembled the mature antral epithelium lineage, including mucin6 and pepsinogen C positive cells. In addition, they could regenerate mucus neck cells and enteroendocrine cells when controlling the Wnt3A concentration in the culture media.
In contrast to Barker, who established gastric organoids with a pyloric lineage, Stange et al. regenerated gastric organoids with a corpus epithelial lineage [47]. They found that Troy, which is thought to be a receptor for lymphotoxin S [67], is expressed by a small subset of both chief cells and parietal cells located at the bottom of the glands of the corpus; however, only Troy-positive cells with chief cell marker were capable of generating gastric organoids and could produce all epithelial lineages present in the corpus. Furthermore, with the removal of several growth factors, such as Fgf10, Noggin and Wnt3a, the upregulation of pit cell markers was found. Their results indicated the regenerative capacity of the Troy-positive derived organoids with respect to the corpus-specific lineage. However, they did not detect the expression of markers of parietal cells, which mainly reside in the corpus.
The difficulty in developing parietal cells was also implied by Speer et al., who generated a TES from murine glandular stomach [46]. They demonstrated well-developed gastric epithelium in the TES with the presence of mucous cells, enteroendocrine cells, and chief cells. However, they only detected parietal cells in 2 out of 15 TESs, and more importantly, these two TESs had a well-developed epithelium with all four differentiated cell types.
Tissue-engineered organs have often been analyzed with respect to the RNA expression or immunofluorescence study using cell lineage-specific markers. The functional capacity of organoids was first demonstrated by Schumacher et al., who established gastric fundic-derived organoid cultures in humans [51]. They introduced two model system. Model 1 provided a stem cell-like niche in order to expand the gastric fundic stem cells. In Model 2, to obtain robust numbers of surface pit, mucous neck, and chief cells, established organoids were maintained in co-cultures with immortalized stomach mesenchymal cells. The organoids also expressed specific markers for parietal cells, H+K+-ATPase and histamine induced a significant decrease in the intraluminal pH, and omeprazole treatment could reverse it. These results indicated the functional activity and regulation of parietal cells in the TES.
4.1.3. Regeneration from pluripotent stem cells
Induced pluripotent stem (iPS) cells, which are made from human adult tissues, have remarkable characteristics, including the ability to self-renew and differentiate into all types of adult cells, such as gastric epithelial cells [68,69]. The first report describing the de novo generation of gastric mucosa from human iPS cells was by McCracken et al. [49]. They showed that the temporal manipulation of the FGF, WNT, BMP, retinoic acid and EGF signaling pathway and 3D growth could generate gastric organoids from iPS cells. Their organoids showed antral lineage cells, such as mucous secreting cells and endocrine cells. As they could not find cell types associated with the fundus/corpus, such as parietal cells, their organoids could only differentiate into the antrum/pylorus lineage and the generation of the whole region of stomach, including the corpus from the pluripotent stem cells, had not been demonstrated.
Noguchi et al. first reported the differentiation of both corpus and antral cell lineages with a functional digestive enzyme and acid secretion from pluripotent stem cells [52]. Their murine embryonic stem cell-derived stomach tissue shared a similar gene expression profile to the adult stomach. In immunohistochemistry, their organoid exhibited development of an epithelium with a glandular structure, which included chief, parietal, pit cells, and enteroendocrine cells. Moreover, they could demonstrate the functional activity of both chief and parietal cells by measuring the production of pepsinogen C from chief cells in organoid culture medium, and the reduction of the pH of the medium in response to histamine stimulation.
Efficient methods to increase parietal cell populations have reported by McCracken et al. [54]. Using various signals to direct differentiation in vitro, they generated a gastric organoid with fundic cell types via posterior foregut progenitors from human iPS cells. Their gastric organoids contained both surface mucous cells and mucous neck cells. Furthermore, these exhibited the RNA expression of both the chief cell and parietal specific transcription factor. In addition, using pH-sensitive dye with real-time confocal microscopy, they found marked decreases in luminal pH in response to histamine, which was blocked by an H2 antagonist or an H+K+-ATPase antagonist.
4.2. Helicobactor pylori infection model
The purpose of the regeneration of the stomach is not only to compensate for organ defects but also to establish an appropriate culture system for infectious diseases, such as HP (Table 2). It is very important to understand the detailed mechanisms that trigger gastric cancer initiation in patients with HP infection; however, a lack of suitable animal models has hampered the recapitulation of the exact process of carcinogenesis induced by HP. If we could obtain a primary cell culture system to assess the early effects of HP infection on healthy gastric epithelial cells, it will help to elucidate the precise mechanism of the carcinogenetic action of HP [49] [50,70,71].
Bartfeld et al. used human organoids established from human gastric corpus epithelium [50]. After confirming the presence of various cell lineages, such as mucous and chief cells, they microinjected HP into the lumen of organoids to analyze the primary response of the human epithelium to HP. In a microarray analysis, they found highly upregulated genes targeting the NFkB pathway, which is known to be activated in HP infection [[72], [73], [74]].
McCracken et al. also microinjected HP into the lumen of the epithelium of gastric organoids and measured epithelial signaling and proliferation [49]. Injected epithelium showed robust proliferation with phosphorylation of c-Met, which is target of HP virulence factor CagA, which is translocated into host cells and implicated in the process of malignant transformation [75]. These results indicated the feasibility of using gastric organoids to elucidate the initial events of human gastric disease mediated by HP.
4.3. Reinforcement of the gastric wall
In addition to the functional regeneration of epithelial lineage of the stomach, there are some reports on physiological reinforcement of a partial gastric wall with tissue engineering techniques (Table 3) [6,[76], [77], [78], [79], [80]]. After successful repair of the esophagus and trachea [81,82], Hori et al. applied tissue engineering technology to repair gastric wall defects [6]. They constructed a collagen sponge scaffold from collagen extracted from pig skin and reinforced it with polyglycolic acid felt. A partial defect in the anterior wall of the stomach in dogs was covered with collagen sponge scaffold and wrapped with omentum. At four weeks after the coverage of a stomach defect, there were no anastomotic problems and various cell types, such as neutrophils, monocytes and fibroblasts, had migrated into the site of regeneration. At sixteen weeks after the operation, the defect was replaced with a layer of firm connective tissue, and its inner surface was totally covered with the stomach mucosa. However, in their system, the luminal silicone sheet had to be removed to accelerate mucosal regeneration.
Table 3.
Reinforcement of gastric wall defect.
| Year | Author | Animal | Cell source | Scaffold | Bioreactor |
|---|---|---|---|---|---|
| 2001 | Hori Y [9] | canine | – | collagen/PGA | omentum |
| 2007 | Ueno T [73] | rodent | – | SIS-ECM (porcine) | n.d. |
| 2009 | Araki M [74] | Canine | bone marrow aspirate | PDLCL/collagen/PGA | omentum |
| 2011 | Maemura T [75] | Rodent | glandular stomach | PGA | omentum |
| 2015 | Nakatsu H [76] | Rodent | MSC | SIS-ECM (porcine) | n.d. |
| 2017 | Tanaka S [77] | Rodent | myoblast | – | omentum |
PGA, Poly glicolic acid; PDLCL, Poly(D,L-lactide) and epsilon-carprolactone.
Ueno et al. used a cell free scaffolding material, Porcine-derived small intestinal submucosa (SIS, Surgisis ES, Cook Biotech, LaFayette, Ind), for closure of gastric wall defects in rats [76]. They found no evidence of diverticular formation or shrinkage of gastric wall defect with Surgisis. A histopathological examination revealed that the grafted area was covered by the mucosa and smooth muscle and fibrosis with neovascularization was observed. They also explored the tonic contraction of muscle strips harvested from the grafted area in response to the application of carbamylcholine chloride and the amplitude in electrical field stimulation. Interestingly, the addition of MSCs to SIS resulted in the improvement of regeneration in comparison to an SIS graft alone, as it enabled the development of well-structured smooth muscle layers.
Araki et al. also established a cell-free scaffold consisting of two layers: one was an outer layer of collagen scaffold that acted as a temporary scaffold for host-tissue regeneration; the other was PDLCL, a biodegradable copolymer that act as a temporary stent for protection of the collagen scaffold from degeneration due to contact with digestive juice [77]. In addition, PDLCL could be dissolved by hydrolysis and disappeared at 4 weeks after surgery; thus, inhibition of the process of mucosal regeneration was avoided.
The use of an SIS scaffold with or without MSCs from the bone marrow of rats was examined by Nakatsu et al., with the aim of regenerating a whole-layer stomach defect [79]. A histopathological examination revealed a re-epithelialized inner lumen of the structure with a fibrous submucosal layer. More improved regeneration of well-structured smooth muscle layers in SIS grafts was detected when the graft was seeded with MSCs in comparison to when it was not seeded with MSCs. They hypothesized that the secretion of a variety of cytokines and growth factors by MSCs suppressed the local immune system, enhanced angiogenesis and stimulated mitosis and that differentiation might play an important role in the site of regeneration.
More recently, Tanaka et al. used a myoblast cell sheet without scaffold for the repair of gastric wall defects in a rat model [80]. They demonstrated that myoblast cell sheets could prevent leakage of the enteral contents. The microscopic findings showed the rapid recovery of the discontinuation. They also reported that cytokines secreted from implanted cell sheets might contribute to promoting the regeneration of the gastric defect.
5. Upper gastrointestinal tract regeneration in humans
No clinical trials of regenerative medicine have been undertaken for the stomach. In contrast, there have been several clinical trials on the reconstruction of esophageal tissue for esophageal defects (Table 4) [[83], [84], [85], [86], [87]]. Nieponice et al. used urinary bladder matrix (UBM, MatriStem, Acell, Columbia, MD, USA) in a patch esophagoplasty procedure to repair esophageal defects in four patients with various etiologies [85]. After the procedure, all of these patients had a partly restored esophageal function. However, this finding suggests the feasibility of the construct for partial rather than circumferential esophageal defects. Recently, an intriguing case was reported in which a 24-year-old patient with long gap between the pharynx and the mediastinum, due to severe infection 5 years after stabilization of the cervical spine with a metal plate, underwent placement of a metal stent that was covered by commercially available extracellular matrix [86]. Although the stents were removed after 3.5 years, 1 year after stent removal, endoscopic ultrasonography showed layered structures, such as the mucosa, submucosa and muscularis propria. Furthermore, high-resolution manometry showed peristaltic contractive movement in the neo-esophagus. At 4 years after stent removal, his weight was maintained with oral diet and he did not report any episodes of dysphagia. Indeed, “this is the first reported human case in which the esophagus was regenerated using the principles of tissue engineering: cell source, scaffold, and bioreactor” [10].
Table 4.
Human studies for regeneration of upper gastrointestinal tract.
| Year | Author | Portion | Disease | Patients' condition | Cell source | Scaffold | Bioreactor | Outcome |
|---|---|---|---|---|---|---|---|---|
| 2011 | Badylak SF [80] | chest | Barett's esophagus | circumferential EMR | – | SIS-ECM (porcine) | – | no stenosis |
| mucosal adenocarcinoma | ||||||||
| 2012 | Ohki T [81] | chest | Squamouse cell carcinoma | almost circumferential ESD | epithelial cell sheet | – | – | no stenosis |
| 2014 | Nieponice A [82] | chest | postoperative stenosis | Esophagoplasty | – | UB-ECM (porcine) | – | no stenosis |
| 2016 | Dua KS [83] | neck | post-operative severe abscess | discontinuity between pharynx and esophagus | Platelet-rich plasma | skin regenerative tissue matrix (human) | sternocleidomastoid muscle | no stenosis |
| metal stent | ||||||||
| 2017 | Yamaguchi N [84] | chest | Squamouse cell carcinoma | almost circumferential ESD | epithelial cell sheet | – | – | no stenosis |
Table 4 modified from Kanetaka K, Surg Today, 2018.
In a clinical study, successful regenerative approaches were reported for the prevention of esophageal stenosis after endoscopic mucosal resection (ESD). Endoscopic treatments such as ESD are an acceptable option for early esophageal cancer [[88], [89], [90]]; however, patients who undergo near circumferential mucosal resection often develop esophageal stricture from a scar ulcer and need to undergo frequent dilatation [91]. This severely compromises the patients' quality of life. Badylak et al. applied decullularized matrix to the ulcer after endoscopic resection for Barrett's esophagus. At four months after treatment, a histopathological analysis revealed a complete mature epithelium [83].
Ohki et al. also demonstrated the preventive effect against esophageal stricture after ESD using an autologous buccal cell sheet. They performed a clinical study of 10 patients with superficial esophageal cancer. The transplanted cell sheets composed of the patients' oral mucosa using a temperature-responsive culture dish were applied to the patients’ post-ESD esophageal ulcers [84,92,93]. They reported that autologous cell sheet transplantation reduced the re-epithelialization period and could prevent stricture after esophageal ESD.
Yamaguchi et al. launched a clinical study in order to establish a transport system for cell sheets in the 1,200 km long distance between Tokyo and Nagasaki because many centers have neither the expertise to fabricate cell sheets nor a cell processing faculty (CPF) at which to conduct the fabrication [87]. Oral mucosal tissue was harvested from patients at Nagasaki University Hospital 16 days prior to the scheduled ESD date and was shipped by air to Tokyo. Oral mucosal epithelial cell sheets produced at the CPF in Tokyo were packed in a dedicated container after ensuring the absence of infection and problems associated with survivability and quality. The fabricated cell sheets were shipped by air to Nagasaki University Hospital. ESD and the transplantation of the cell sheet was performed and the patient was observed endoscopically every one to two weeks after the procedure. No stricture was observed at the fourth postoperative week, and the post-ESD ulcer was completely epithelized. Our results indicated that even if the local hospital does not have its own cell processing center, they could perform the procedure in the clinical setting with the transportation of regenerative products.
6. Conclusion and future perspectives
Regenerative medicine has been studied in human in the field of cardiology and ophthalmology [[94], [95], [96]];however, the regeneration of digestive organs through tissue-engineering technology has mainly been studied in animal models.
There are several hurdles facing the regeneration of the whole component of the gastrointestinal tract. One is its complex multilayer structure: the epithelium of gastrointestinal tract consists of various cells with distinct function of secretion. The muscular layer has physiological movement, such as generating rhythmic peristaltic waves and contractile activity to transport, mix and grind food. The gastrointestinal tract also has complex vasculature and well-coordinated domination by the autonomic nervous system.
To regenerate this multicellular and complex construct, the integration of additional cell types such as endothelial cells and myofibroblasts might be an effective strategies [97]. Takebe et al. generated vascularized human liver by transplanting liver buds consisting of human iPS, mesenchymal stem cells and human umbilical vein endothelial cells [98]. Using cell sheet technology, Sakai et al. established the engineered hepatocyte/fibroblast sheets using layer-by-layer culture system [99,100].
The unstable quality of cells and difficulty associated with mass production are other obstacles, as the fabrication of regenerative product are still dependent of manual techniques performed by skilled workers. Therefore, to improve the prevalence of regenerative medicine in clinical practice, mass production and stabilization of product quality is an important issue to be resolved. Recently, several automated cell culture systems have been developed and these are expected to promote the popularization of regenerative medicine [101,102].
In 1913, Franz John A. Torek reported a 67-year-old female patient who underwent esophagectomy for esophageal cancer [103]. The reconstruction of gastrointestinal conduit was not performed, and the extracorporeal cervical esophagogastric tube was used in place of the whole esophagus. Despite the impairment of the esophageal function, enough nutrition was supplied via the mouth to the stomach through the rubber tube and this patient surprisingly survived 12 years. Over 100 years after this first successful surgical treatment for esophageal cancer, the U.S. Food and Drug Administration (FDA) has approved the Investigational New Drug (IND) application of Biostage, an American biotechnology company, for the Cellspan Esophageal Implant (CEI), a scaffold seeded with patients' own cells, to treat patients with esophageal cancer (https://www.biostage.com/). The CEI will be implanted into the resected esophagus, which essentially removes the entire esophagus and replaces it with native substrate. This regenerative product may be able to improve the quality of life in patients after esophagectomy and enable the regeneration of the patient's own esophagus. Although results concerning this product have not yet published, the introduction of regenerative medicine into gastrointestinal surgery in the near future is eagerly awaited.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Eguchi have no conflicts of interest or financial ties to disclose.
The laboratory which Dr. Kanetaka belong to received funding for cooperative research in cell sheet from the TERUMO Company.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Kengo Kanetaka, Email: kanetaka@nagasaki-u.ac.jp.
Susumu Eguchi, Email: sueguchi@nagasaki-u.ac.jp.
References
- 1.Kanetaka K., Kobayashi S., Eguchi S. Regenerative medicine for the esophagus. Surg Today. 2018;48:739–747. doi: 10.1007/s00595-017-1610-y. [DOI] [PubMed] [Google Scholar]
- 2.Saksena R., Gao C., Wicox M., de Mel A. Tubular organ epithelialisation. J Tissue Eng. 2016;7 doi: 10.1177/2041731416683950. 2041731416683950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komuro H., Nakamura T., Kaneko M., Nakanishi Y., Shimizu Y. Application of collagen sponge scaffold to muscular defects of the esophagus: an experimental study in piglets. J Pediatr Surg. 2002;37:1409–1413. doi: 10.1053/jpsu.2002.35402. [DOI] [PubMed] [Google Scholar]
- 4.Wei R.Q., Tan B., Tan M.Y., Luo J.C., Deng L., Chen X.H. Grafts of porcine small intestinal submucosa with cultured autologous oral mucosal epithelial cells for esophageal repair in a canine model. Exp Biol Med. 2009;234:453–461. doi: 10.3181/0901-RM-5. [DOI] [PubMed] [Google Scholar]
- 5.Poghosyan T., Sfeir R., Michaud L., Bruneval P., Domet T., Vanneaux V. Circumferential esophageal replacement using a tube-shaped tissue-engineered substitute: an experimental study in minipigs. Surgery. 2015;158:266–277. doi: 10.1016/j.surg.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Hori Y., Nakamura T., Matsumoto K., Kurokawa Y., Satomi S., Shimizu Y. Experimental study on in situ tissue engineering of the stomach by an acellular collagen sponge scaffold graft. Am Soc Artif Intern Organs J. 2001;47:206–210. doi: 10.1097/00002480-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Tan B., Wei R.Q., Tan M.Y., Luo J.C., Deng L., Chen X.H. Tissue engineered esophagus by mesenchymal stem cell seeding for esophageal repair in a canine model. J Surg Res. 2013;182:40–48. doi: 10.1016/j.jss.2012.07.054. [DOI] [PubMed] [Google Scholar]
- 8.Sjoqvist S., Jungebluth P., Lim M.L., Haag J.C., Gustafsson Y., Lemon G. Experimental orthotopic transplantation of a tissue-engineered oesophagus in rats. Nat Commun. 2014;5:3562. doi: 10.1038/ncomms4562. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Park S.Y., Choi J.W., Park J.K., Song E.H., Park S.A., Kim Y.S. Tissue-engineered artificial oesophagus patch using three-dimensionally printed polycaprolactone with mesenchymal stem cells: a preliminary report. Interact Cardiovasc Thorac Surg. 2016;22:712–717. doi: 10.1093/icvts/ivw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dua K.S., Sasikala M. Repairing the human esophagus with tissue engineering. Gastrointest Endosc. 2018;88:579–588. doi: 10.1016/j.gie.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Hussey G.S., Cramer M.C., Badylak S.F. Extracellular matrix bioscaffolds for building gastrointestinal tissue. Cell Mol Gastroenterol Hepatol. 2018;5:1–13. doi: 10.1016/j.jcmgh.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voytik-Harbin S.L., Brightman A.O., Kraine M.R., Waisner B., Badylak S.F. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478–491. [PubMed] [Google Scholar]
- 13.Hodde J.P., Record R.D., Liang H.A., Badylak S.F. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 14.Basu J., Bertram T.A. Regenerative medicine of the gastrointestinal tract. Toxicol Pathol. 2014;42:82–90. doi: 10.1177/0192623313512431. [DOI] [PubMed] [Google Scholar]
- 15.Grikscheit T., Ochoa E.R., Srinivasan A., Gaissert H., Vacanti J.P. Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. J Thorac Cardiovasc Surg. 2003;126:537–544. doi: 10.1016/s0022-5223(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 16.Urita Y., Komuro H., Chen G., Shinya M., Kaneko S., Kaneko M. Regeneration of the esophagus using gastric acellular matrix: an experimental study in a rat model. Pediatr Surg Int. 2007;23:21–26. doi: 10.1007/s00383-006-1799-0. [DOI] [PubMed] [Google Scholar]
- 17.Nakase Y., Nakamura T., Kin S., Nakashima S., Yoshikawa T., Kuriu Y. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg. 2008;136:850–859. doi: 10.1016/j.jtcvs.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Chian K.S., Leong M.F., Kono K. Regenerative medicine for oesophageal reconstruction after cancer treatment. Lancet Oncol. 2015;16:e84–92. doi: 10.1016/S1470-2045(14)70410-3. [DOI] [PubMed] [Google Scholar]
- 19.Badylak S.F., Vorp D.A., Spievack A.R., Simmons-Byrd A., Hanke J., Freytes D.O. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Ohki T., Yamato M., Murakami D., Takagi R., Yang J., Namiki H. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704–1710. doi: 10.1136/gut.2005.088518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai T., Miyazaki S., Miyata G., Satomi S., Hori Y. Autologous buccal keratinocyte implantation for the prevention of stenosis after EMR of the esophagus. Gastrointest Endosc. 2007;66:167–173. doi: 10.1016/j.gie.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 22.Nieponice A., McGrath K., Qureshi I., Beckman E.J., Luketich J.D., Gilbert T.W. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289–296. doi: 10.1016/j.gie.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Honda M., Hori Y., Nakada A., Uji M., Nishizawa Y., Yamamoto K. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest Endosc. 2011;73:777–784. doi: 10.1016/j.gie.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Nieponice A., Gilbert T.W., Johnson S.A., Turner N.J., Badylak S.F. Bone marrow-derived cells participate in the long-term remodeling in a mouse model of esophageal reconstruction. J Surg Res. 2013;182:e1–7. doi: 10.1016/j.jss.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Badylak S., Meurling S., Chen M., Spievack A., Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35:1097–1103. doi: 10.1053/jpsu.2000.7834. [DOI] [PubMed] [Google Scholar]
- 26.Isch J.A., Engum S.A., Ruble C.A., Davis M.M., Grosfeld J.L. Patch esophagoplasty using AlloDerm as a tissue scaffold. J Pediatr Surg. 2001;36:266–268. doi: 10.1053/jpsu.2001.20685. [DOI] [PubMed] [Google Scholar]
- 27.Lynen Jansen P., Klinge U., Anurov M., Titkova S., Mertens P.R., Jansen M. Surgical mesh as a scaffold for tissue regeneration in the esophagus. Eur Surg Res. 2004;36:104–111. doi: 10.1159/000076650. [DOI] [PubMed] [Google Scholar]
- 28.Lopes M.F., Cabrita A., Ilharco J., Pessa P., Paiva-Carvalho J., Pires A. Esophageal replacement in rat using porcine intestinal submucosa as a patch or a tube-shaped graft. Dis Esophagus. 2006;19:254–259. doi: 10.1111/j.1442-2050.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- 29.Diemer P., Markoew S., Le D.Q., Qvist N. Poly-epsilon-caprolactone mesh as a scaffold for in vivo tissue engineering in rabbit esophagus. Dis Esophagus. 2015;28:240–245. doi: 10.1111/dote.12172. [DOI] [PubMed] [Google Scholar]
- 30.Okuyama H., Umeda S., Takama Y., Terasawa T., Nakayama Y. Patch esophagoplasty using an in-body-tissue-engineered collagenous connective tissue membrane. J Pediatr Surg. 2018;53:223–226. doi: 10.1016/j.jpedsurg.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Saito M., Sakamoto T., Fujimaki M., Tsukada K., Honda T., Nozaki M. Experimental study of an artificial esophagus using a collagen sponge, a latissimus dorsi muscle flap, and split-thickness skin. Surg Today. 2000;30:606–613. doi: 10.1007/s005950070100. [DOI] [PubMed] [Google Scholar]
- 32.Grikscheit T., Srinivasan A., Vacanti J.P. Tissue-engineered stomach: a preliminary report of a versatile in vivo model with therapeutic potential. J Pediatr Surg. 2003;38:1305–1309. doi: 10.1016/s0022-3468(03)00386-5. [DOI] [PubMed] [Google Scholar]
- 33.Doede T., Bondartschuk M., Joerck C., Schulze E., Goernig M. Unsuccessful alloplastic esophageal replacement with porcine small intestinal submucosa. Artif Organs. 2009;33:328–333. doi: 10.1111/j.1525-1594.2009.00727.x. [DOI] [PubMed] [Google Scholar]
- 34.Gaujoux S., Le Balleur Y., Bruneval P., Larghero J., Lecourt S., Domet T. Esophageal replacement by allogenic aorta in a porcine model. Surgery. 2010;148:39–47. doi: 10.1016/j.surg.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Catry J., Luong-Nguyen M., Arakelian L., Poghosyan T., Bruneval P., Domet T. Circumferential esophageal replacement by a tissue-engineered substitute using mesenchymal stem cells: an experimental study in mini pigs. Cell Transplant. 2017;26:1831–1839. doi: 10.1177/0963689717741498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luc G., Charles G., Gronnier C., Cabau M., Kalisky C., Meulle M. Decellularized and matured esophageal scaffold for circumferential esophagus replacement: proof of concept in a pig model. Biomaterials. 2018;175:1–18. doi: 10.1016/j.biomaterials.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Takeoka Y., Matsumoto K., Taniguchi D., Tsuchiya T., Machino R., Moriyama M. Regeneration of esophagus using a scaffold-free biomimetic structure created with bio-three-dimensional printing. PloS One. 2019;14 doi: 10.1371/journal.pone.0211339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbani L., Camilli C., Phylactopoulos D.E., Crowley C., Natarajan D., Scottoni F. Multi-stage bioengineering of a layered oesophagus with in vitro expanded muscle and epithelial adult progenitors. Nat Commun. 2018;9:4286. doi: 10.1038/s41467-018-06385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellavalle A., Sampaolesi M., Tonlorenzi R., Tagliafico E., Sacchetti B., Perani L. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 40.Itoh M., Nakayama K., Noguchi R., Kamohara K., Furukawa K., Uchihashi K. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PloS One. 2015;10 doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maemura T., Shin M., Sato M., Mochizuki H., Vacanti J.P. A tissue-engineered stomach as a replacement of the native stomach. Transplantation. 2003;76:61–65. doi: 10.1097/01.TP.0000068903.63554.1B. [DOI] [PubMed] [Google Scholar]
- 42.Maemura T., Ogawa K., Shin M., Mochizuki H., Vacanti J.P. Assessment of tissue-engineered stomach derived from isolated epithelium organoid units. Transplant Proc. 2004;36:1595–1599. doi: 10.1016/j.transproceed.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Maemura T., Shin M., Kinoshita M., Majima T., Ishihara M., Saitoh D. A tissue-engineered stomach shows presence of proton pump and G-cells in a rat model, resulting in improved anemia following total gastrectomy. Artif Organs. 2008;32:234–239. doi: 10.1111/j.1525-1594.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 44.Sala F.G., Kunisaki S.M., Ochoa E.R., Vacanti J., Grikscheit T.C. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res. 2009;156:205–212. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 45.Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Speer A.L., Sala F.G., Matthews J.A., Grikscheit T.C. Murine tissue-engineered stomach demonstrates epithelial differentiation. J Surg Res. 2011;171:6–14. doi: 10.1016/j.jss.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 47.Stange D.E., Koo B.K., Huch M., Sibbel G., Basak O., Lyubimova A. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katano T., Ootani A., Mizoshita T., Tanida S., Tsukamoto H., Ozeki K. Establishment of a long-term three-dimensional primary culture of mouse glandular stomach epithelial cells within the stem cell niche. Biochem Biophys Res Commun. 2013;432:558–563. doi: 10.1016/j.bbrc.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 49.McCracken K.W., Cata E.M., Crawford C.M., Sinagoga K.L., Schumacher M., Rockich B.E. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartfeld S., Bayram T., van de Wetering M., Huch M., Begthel H., Kujala P. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126–136 e6. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schumacher M.A., Aihara E., Feng R., Engevik A., Shroyer N.F., Ottemann K.M. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593:1809–1827. doi: 10.1113/jphysiol.2014.283028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noguchi T.K., Ninomiya N., Sekine M., Komazaki S., Wang P.C., Asashima M. Generation of stomach tissue from mouse embryonic stem cells. Nat Cell Biol. 2015;17:984–993. doi: 10.1038/ncb3200. [DOI] [PubMed] [Google Scholar]
- 53.Schlaermann P., Toelle B., Berger H., Schmidt S.C., Glanemann M., Ordemann J. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65:202–213. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCracken K.W., Aihara E., Martin B., Crawford C.M., Broda T., Treguier J. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rego S.L., Zakhem E., Orlando G., Bitar K.N. Bioengineered human pyloric sphincters using autologous smooth muscle and neural progenitor cells. Tissue Eng. 2016;22:151–160. doi: 10.1089/ten.tea.2015.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasricha P.J., Ahmed I., Jankowski R.J., Micci M.A. Endoscopic injection of skeletal muscle-derived cells augments gut smooth muscle sphincter function: implications for a novel therapeutic approach. Gastrointest Endosc. 2009;70:1231–1237. doi: 10.1016/j.gie.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Rego S.L., Zakhem E., Orlando G., Bitar K.N. Bioengineering functional human sphincteric and non-sphincteric gastrointestinal smooth muscle constructs. Methods. 2016;99:128–134. doi: 10.1016/j.ymeth.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zakhem E., El Bahrawy M., Orlando G., Bitar K.N. Biomechanical properties of an implanted engineered tubular gut-sphincter complex. J Tissue Eng Regen Med. 2017;11:3398–3407. doi: 10.1002/term.2253. [DOI] [PubMed] [Google Scholar]
- 59.Mills J.C., Shivdasani R.A. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–424. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi R.S., Vacanti J.P. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant Proc. 1997;29:848–851. doi: 10.1016/s0041-1345(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 61.Grikscheit T.C., Ogilvie J.B., Ochoa E.R., Alsberg E., Mooney D., Vacanti J.P. Tissue-engineered colon exhibits function in vivo. Surgery. 2002;132:200–204. doi: 10.1067/msy.2002.125310. [DOI] [PubMed] [Google Scholar]
- 62.Barker N., van de Wetering M., Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiao X.T., Ziel J.W., McKimpson W., Madison B.B., Todisco A., Merchant J.L. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 65.Yoshioka T., Fukuda A., Araki O., Ogawa S., Hanyu Y., Matsumoto Y. Bmi1 marks gastric stem cells located in the isthmus in mice. J Pathol. 2019;248:179–190. doi: 10.1002/path.5244. [DOI] [PubMed] [Google Scholar]
- 66.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto T., Schlessinger D., Cui C.Y. Troy binding to lymphotoxin-alpha activates NF kappa B mediated transcription. Cell Cycle. 2008;7:106–111. doi: 10.4161/cc.7.1.5135. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 69.Azuma K., Yamanaka S. Recent policies that support clinical application of induced pluripotent stem cell-based regenerative therapies. Regen Ther. 2016;4:36–47. doi: 10.1016/j.reth.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertaux-Skeirik N., Feng R., Schumacher M.A., Li J., Mahe M.M., Engevik A.C. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wroblewski L.E., Piazuelo M.B., Chaturvedi R., Schumacher M., Aihara E., Feng R. Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut. 2015;64:720–730. doi: 10.1136/gutjnl-2014-307650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viala J., Chaput C., Boneca I.G., Cardona A., Girardin S.E., Moran A.P. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 73.Bartfeld S., Hess S., Bauer B., Machuy N., Ogilvie L.A., Schuchhardt J. High-throughput and single-cell imaging of NF-kappaB oscillations using monoclonal cell lines. BMC Cell Biol. 2010;11:21. doi: 10.1186/1471-2121-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrero R.L. Innate immune recognition of the extracellular mucosal pathogen, Helicobacter pylori. Mol Immunol. 2005;42:879–885. doi: 10.1016/j.molimm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Churin Y., Al-Ghoul L., Kepp O., Meyer T.F., Birchmeier W., Naumann M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249–255. doi: 10.1083/jcb.200208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ueno T., de la Fuente S.G., Abdel-Wahab O.I., Takahashi T., Gottfried M., Harris M.B. Functional evaluation of the grafted wall with porcine-derived small intestinal submucosa (SIS) to a stomach defect in rats. Surgery. 2007;142:376–383. doi: 10.1016/j.surg.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 77.Araki M., Tao H., Sato T., Nakajima N., Hyon S.H., Nagayasu T. Development of a new tissue-engineered sheet for reconstruction of the stomach. Artif Organs. 2009;33:818–826. doi: 10.1111/j.1525-1594.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 78.Maemura T., Kinoshita M., Shin M., Miyazaki H., Tsujimoto H., Ono S. Assessment of a tissue-engineered gastric wall patch in a rat model. Artif Organs. 2012;36:409–417. doi: 10.1111/j.1525-1594.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- 79.Nakatsu H., Ueno T., Oga A., Nakao M., Nishimura T., Kobayashi S. Influence of mesenchymal stem cells on stomach tissue engineering using small intestinal submucosa. J Tissue Eng Regen Med. 2015;9:296–304. doi: 10.1002/term.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka S., Kanetaka K., Fujii M., Ito S., Sakai Y., Kobayashi S. Cell sheet technology for the regeneration of gastrointestinal tissue using a novel gastric perforation rat model. Surg Today. 2017;47:114–121. doi: 10.1007/s00595-016-1360-2. [DOI] [PubMed] [Google Scholar]
- 81.Sekine T., Nakamura T., Ueda H., Matsumoto K., Yamamoto Y., Takimoto Y. Replacement of the tracheobronchial bifurcation by a newly developed Y-shaped artificial trachea. Am Soc Artif Intern Organs J. 1999;45:131–134. doi: 10.1097/00002480-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto Y., Nakamura T., Shimizu Y., Takimoto Y., Matsumoto K., Kiyotani T. Experimental replacement of the thoracic esophagus with a bioabsorbable collagen sponge scaffold supported by a silicone stent in dogs. Am Soc Artif Intern Organs J. 1999;45:311–316. doi: 10.1097/00002480-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 83.Badylak S.F., Hoppo T., Nieponice A., Gilbert T.W., Davison J.M., Jobe B.A. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng. 2011;17:1643–1650. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588 e2. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 85.Nieponice A., Ciotola F.F., Nachman F., Jobe B.A., Hoppo T., Londono R. Patch esophagoplasty: esophageal reconstruction using biologic scaffolds. Ann Thorac Surg. 2014;97:283–288. doi: 10.1016/j.athoracsur.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Dua K.S., Hogan W.J., Aadam A.A., Gasparri M. In-vivo oesophageal regeneration in a human being by use of a non-biological scaffold and extracellular matrix. Lancet. 2016;388:55–61. doi: 10.1016/S0140-6736(15)01036-3. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi N., Isomoto H., Kobayashi S., Kanai N., Kanetaka K., Sakai Y. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7:17460. doi: 10.1038/s41598-017-17663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimizu Y., Tsukagoshi H., Fujita M., Hosokawa M., Kato M., Asaka M. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387–390. doi: 10.1016/s0016-5107(02)70043-6. [DOI] [PubMed] [Google Scholar]
- 89.Oyama T., Tomori A., Hotta K., Morita S., Kominato K., Tanaka M. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 90.Katada C., Muto M., Momma K., Arima M., Tajiri H., Kanamaru C. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy. 2007;39:779–783. doi: 10.1055/s-2007-966761. [DOI] [PubMed] [Google Scholar]
- 91.Lewis J.J., Rubenstein J.H., Singal A.G., Elmunzer B.J., Kwon R.S., Piraka C.R. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett's esophagus. Gastrointest Endosc. 2011;74:753–760. doi: 10.1016/j.gie.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murakami D., Yamato M., Nishida K., Ohki T., Takagi R., Yang J. Fabrication of transplantable human oral mucosal epithelial cell sheets using temperature-responsive culture inserts without feeder layer cells. J Artif Organs. 2006;9:185–191. doi: 10.1007/s10047-006-0342-3. [DOI] [PubMed] [Google Scholar]
- 93.Takagi R., Murakami D., Kondo M., Ohki T., Sasaki R., Mizutani M. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:1253–1259. doi: 10.1016/j.gie.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 94.Sawa Y., Yoshikawa Y., Toda K., Fukushima S., Yamazaki K., Ono M. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79:991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]
- 95.Miyagawa S., Domae K., Yoshikawa Y., Fukushima S., Nakamura T., Saito A. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mandai M., Kurimoto Y., Takahashi M. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;377:792–793. doi: 10.1056/NEJMc1706274. [DOI] [PubMed] [Google Scholar]
- 97.Rahmani S., Breyner N.M., Su H.M., Verdu E.F., Didar T.F. Intestinal organoids: a new paradigm for engineering intestinal epithelium in vitro. Biomaterials. 2019;194:195–214. doi: 10.1016/j.biomaterials.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 99.Sakai Y., Yamanouchi K., Ohashi K., Koike M., Utoh R., Hasegawa H. Vascularized subcutaneous human liver tissue from engineered hepatocyte/fibroblast sheets in mice. Biomaterials. 2015;65:66–75. doi: 10.1016/j.biomaterials.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 100.Sakai Y., Koike M., Yamanouchi K., Soyama A., Hidaka M., Kuroki T. Time-dependent structural and functional characterization of subcutaneous human liver tissue. J Tissue Eng Regen Med. 2018;12:2287–2298. doi: 10.1002/term.2761. [DOI] [PubMed] [Google Scholar]
- 101.Nishimura A., Nakajima R., Takagi R., Zhou G., Suzuki D., Kiyama M. Fabrication of tissue-engineered cell sheets by automated cell culture equipment. J Tissue Eng Regen Med. 2019;13:2246–2255. doi: 10.1002/term.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kikuchi T., Kino-Oka M., Wada M., Kobayashi T., Kato M., Takeda S. A novel, flexible and automated manufacturing facility for cell-based health care products: tissue Factory. Regen Ther. 2018;9:89–99. doi: 10.1016/j.reth.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dubecz A., Schwartz S.I. Franz John A. Torek. Ann Thorac Surg. 2008;85:1497–1499. doi: 10.1016/j.athoracsur.2007.10.106. [DOI] [PubMed] [Google Scholar]