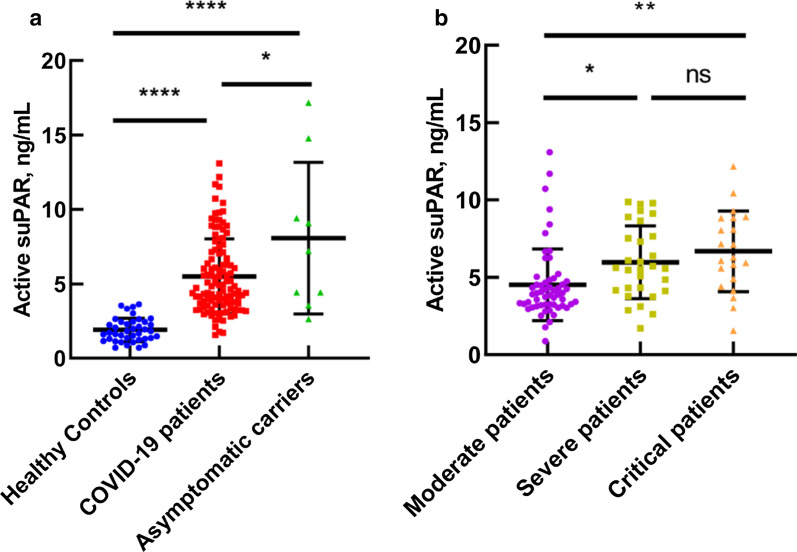
We read with great interest the recent study by Rovina et al., who found that the elevation of soluble urokinase plasminogen activator receptor (suPAR) plasma levels in coronavirus disease 2019 (COVID-19) patients, and suggested the suPAR can be an early predictor of severe respiratory failure [1]. There are three different suPAR forms (suPAR DI-III, suPAR DI, and suPAR DII-III) in circulation, in which suPAR DI-III is defined as the active form of suPAR by its capability of binding to uPA [2]. In addition, the uPA/uPAR system as a therapeutic target has been proposed to reduce mortality of COVID-19 [3]; therefore, further evaluation of the active form of suPAR plasma levels in different symptom types of COVID-19 patients and asymptomatic carriers could still provide important indications for required early admission and treatment.
In our study, we found that active suPAR levels in all COVID-19 patients were significantly higher than in healthy controls (5.51 ± 2.53 ng/mL vs 1.97 ± 0.78 ng/mL, p < 0.0001) using a ELISA assay modified from our previously reported method [4] where the active suPAR was captured by a uPAR ligand and measured using a polyclonal anti-uPAR antibody. Strikingly, the active suPAR levels in asymptomatic carriers (8.08 ± 4.81 ng/mL) are not only significantly higher than those in healthy controls (p < 0.0001) but also slightly higher than those in COVID-19 patients (p = 0.0278) (Table 1, Fig. 1a). Even though more data needs to be collected and the background of these patients are not clear, this is a significant research direction to pursue. If asymptomatic carriers could be identified and quarantined in an early stage, it will prevent them from increasing the disease transmission to an uncontrollable manner.
Table 1.
The active suPAR levels in plasma are associated correlated with high-sensitivity C-reaction protein (hs-CRP), neutrophil/leukocyte ratio, and lymphocyte counts
| Patient type | Number of samples | Active suPAR (ng/mL) | hs-CRP (mg/L) | Neutrophil/leukocyte ratio (%) | Lymphocyte (×109/L) |
|---|---|---|---|---|---|
| Moderate | 57 | 4.57 ± 2.35 | 3.01 ± 3.67 | 59.98 ± 9.34 | 1.62 ± 0.60 |
| Severe | 30 | 5.97 ± 2.31 | 38.79 ± 57.81 | 64.60 ± 11.23 | 0.97 ± 0.35 |
| Critical | 21 | 6.68 ± 2.54 | 135.39 ± 93.88 | 87.49 ± 9.53 | 0.66 ± 0.37 |
| Asymptomatic | 9 | 8.08 ± 4.81 | 1.29 ± 1.14 | 65.78 ± 9.30 | 1.57 ± 0.49 |
Fig. 1.
Level of plasma active suPAR in different classifications. a Active suPAR level in COVID-19 patients and asymptomatic carriers, compared with active suPAR level in healthy controls. b Level of active suPAR between three different COVID-19 classifications including moderate, severe, and critical
Patients involved three types of symptoms: moderate, severe, and critical in our study (Table 1). Our data show that active suPAR levels increase as the disease worsens (Fig. 1b). Moreover, correlation analyses demonstrated that active suPAR levels are positively correlated with high-sensitivity C-reaction protein (hs-CRP), neutrophil/leukocyte ratio, and lymphocyte counts (Table 1).
Therefore, taken together with the results from Rovina et al., these results demonstrated that the active suPAR as a COVID-19 prognostic biomarker may assist in the early triage of SARS-CoV-2-infected persons to prevent virus transmission. Further studies are needed to see whether the elevation of suPAR plasma levels in COVID-19 patients is due to the enhanced over-expression of uPAR or due to their increased shedding from the cell surface.
Acknowledgement
Not applicable.
Abbreviations
- COVID-19
Coronavirus disease 2019
- ELISA
Enzyme-linked immunosorbent assay
- hs-CRP
High-sensitivity C-reaction protein
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- suPAR
Soluble urokinase plasminogen activator receptor
- uPA
Urokinase plasminogen activator
Authors’ contributions
All authors had full access to all of the data in the study and take responsibility for the integrity of the data. LJ has written the letter. The authors read and approved the final manuscript.
Funding
This study is financially supported by grants from the National Key R&D Program of China (2017YFE0103200), Natural Science Foundation of China (82070142, 31670739, 22077016), Natural Science Foundation of Fujian Province (2018J01897 and 2018J01729), and Fuzhou Municipal Science and Technology Program (2020-XG-021).
Availability of data and materials
The dataset supporting the conclusion of this article is available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Fuzhou pulmonary hospital of Fujian. All subjects provided informed written consent documents in accordance with the Declaration of Helsinki before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingxiang Huang and Linlin Li have contributed equally to this work
Contributor Information
Mingdong Huang, Email: hmd_lab@fzu.edu.cn.
Longguang Jiang, Email: jianglg@fzu.edu.cn.
References
- 1.Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):187. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L, et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science (New York, NY) 2006;311(5761):656–659. doi: 10.1126/science.1121143. [DOI] [PubMed] [Google Scholar]
- 3.D’Alonzo D, De Fenza M, Pavone V. COVID-19 and pneumonia: a role for the uPA/uPAR system. Drug Discov Today. 2020 doi: 10.1016/j.drudis.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X, Xu M, Huang H, Mazar A, Iqbal Z, Yuan C, Huang M. An ELISA method detecting the active form of suPAR. Talanta. 2016;160:205–210. doi: 10.1016/j.talanta.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 2002;2002(99):1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrowski SR, Piironen T, Høyer-Hansen G, Gerstoft J, Pedersen KB, Ullum H. High plasma levels of intact and cleaved soluble urokinase receptor reflect immune activation and are independent predictors of mortality in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39:23–31. doi: 10.1097/01.qai.0000157950.02076.a6. [DOI] [PubMed] [Google Scholar]
- 7.Azam TU, Shadid HR, Blakely P, O’Hayer P, Berlin H, Pan M, et al. Soluble urokinase receptor (SuPAR) in COVID-19-related AKI. J Am Soc Nephrol. 2020 doi: 10.1681/ASN.2020060829. [DOI] [PMC free article] [PubMed] [Google Scholar]