Abstract
Working group 2 (WG2) of the Asia Partnership Conference of Regenerative Medicine has discussed eligibility of mesenchymal stromal cells (MSCs) as starting cells for the manufacture of cell therapy products, and comparability before and after changes in their manufacturing process. Asian countries and regions have their own regulations on the quality of starting cells, and these regulations are not harmonized. As cell therapy products are being developed across countries and regions, we propose a risk-based approach based on donor location, window period of virus test, and additional virus tests on the master cell bank to fill the gaps in regulation while controlling the risk of viral contamination. Moreover, a standard procedure of comparability assessment after changes in the manufacturing process of MSC-based products does not exist. The WG2 discussed points of comparability assessment specifically for MSC-based products considering the similarities and differences with parallel assessments for protein and polypeptide products, which are within the scope of the International Council for Harmonization Q5E guideline. We also summarize possible characterization procedures for MSC-based products and report our discussion on stability evaluations under accelerated and stress conditions for comparability assessment of cell therapy products.
Keywords: APACRM, FIRM, MSC, Regenerative medicine, Regulation
Abbreviations: APACRM, Asia Partnership Conference of Regenerative Medicine; ECDC, European center for disease prevention and control; FIRM, Forum for innovative regenerative medicine; ICH, International council for harmonization; MSC, Mesenchymal stromal cell; WG2, Working group 2
Highlights
-
•
Regulations in Cell therapy and Regenerative medicine (WG2 white paper).
-
•
A risk-based approach for starting cells to fill gaps in regulation.
-
•
Controlling the risk of viral contamination for global development.
-
•
Characterization of MSC-based products for comparability assessment.
-
•
Stability of MSC-based products under accelerated and stress conditions.
1. Asia Partnership Conference of Regenerative Medicine
The Forum for Innovative Regenerative Medicine (FIRM) is a leading industry association for regenerative medicine, including cellular and gene therapy in Japan. FIRM is dedicated to improving human health through global collaborations in regenerative medicine. As part of this endeavor, FIRM has organized the Asia Partnership Conference of Regenerative Medicine (APACRM), an annual conference on regulatory harmonization for regenerative medicine among Asian countries and regions, since 2018.
The purpose of APACRM is to promote regulatory harmonization among Asian countries and regions to develop an ideal environment for the clinical application of regenerative medicine. Its goal is to establish a realistic platform for an optimized regulatory framework in Asia. This international discussion platform serves as a base for the development of common scientific understandings on the nature of regenerative medicine through dialogues between the industry and health authorities to identify common challenges in participating countries and regions, and to share sound scientific approaches/regulations for ensuring the safety, efficacy and quality of regenerative medicines. It is also expected to help maximizing opportunities to expedite the distributions of those advanced therapeutic products in Asian markets. These activities are further expected to contribute to the establishment schemes, through regulatory harmonization in the field of regenerative medicine. FIRM has taken a role of as the secretariat of APACRM and has closely collaborated with industrial groups such as the China Medicinal Biotech Association (CMBA) in China, Association of Biotechnology Led Enterprises (ABLE) in India, Council for Advanced Regenerative Medicine (CARM) in Korea, Singapore Association of Pharmaceuticals Industries (SAPI) in Singapore, and Biotechnology and Pharmaceutical Industries Promotion Office (BPIPO) in Taiwan. The activities of FIRM in APACRM has been supported not only by the Ministry of Health, Labour and Welfare (MHLW) and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, but also by other Asian health authorities, including the Central Drugs Standard Control Organization (CDSCO) in India, Ministry of Food and Drug Safety (MFDS) in Korea, Health Sciences Authority (HSA) in Singapore, and Center for Drug Evaluation (CDE) in Taiwan.
At the second APACRM in 2019, industrial cell therapy professionals and reviewers at health authorities throughout Asia discussed acceptance criteria for mesenchymal stromal cells (MSCs), acceptance of foreign non-clinical studies/clinical studies, and donor eligibility/raw materials. APACRM working group 2 (WG2) recommendations on eligibility of starting materials (cells) and comparability of cell therapy products before and after changes in their manufacturing process have been established based on the outcome of the APACRM round-table discussion in 2019. APACRM WG2 comprised 25 industrial professionals from the Asia region with a wide range of backgrounds (listed in Table 1), all working in cell therapy manufacturing and development companies. Although the third APACRM was canceled due to the COVID-19 pandemic, this white paper summarizes the discussions in APACRM WG2, including points to be considered for eligibility of MSCs as starting materials, comparability of MSC-based products before and after changes in their manufacturing process.
Table 1.
Member of APACRM WG2.
| Country and region | Industrial Group | Member | Affiliation |
|---|---|---|---|
| China | CMBA | Wenbin Liao | Baylx, Inc. |
| Shuai Liu | BOE Regenerative Medicine Technology Co.,Ltd | ||
| Xin Jin | |||
| Xiang Zhao | Shanghai Haixin Biotech Co., Ltd | ||
| India | ABLE | Pawan Kumar Gupta | Stempeutics Research Pvt. Ltd. |
| Japan | FIRM | Toshimitsu Tanaka (WG2 lead and topic lead for starting material) | Astellas Pharma Inc. |
| Mayu Mikami (topic lead for comparability: stability) | |||
| Koji Takakura (WG2 secretary) | |||
| Uichi Koshimizu | Daiichi Sankyo Co., Ltd. | ||
| Kahori Yokota (sub-topic lead for starting material) | |||
| Hidetaka Ohara | Sumitomo Dainippon Pharma Co., Ltd. | ||
| Shinobu Kuwae | Takeda Pharmaceutical Co., Ltd. | ||
| Republic of Korea | CARM | Hyun-Sook Park | CEFO Co., Ltd. |
| Sunray Lee | |||
| Seunghee Lee | Kangstem Biotech | ||
| Eun Kyung Chung | SCM Lifescience | ||
| Dong Sik Ham | |||
| Yong Bo Kim | |||
| Bryan Choi | Strategic Center for Regenerative Medicine (SCRM) | ||
| Sung Min Park | Synex | ||
| Jooyoun Lee | Xcell Therapeutics | ||
| Taiwan | BPIPO | Shing-Mou Lee (topic lead for comparability: characterization) | EMO BIOMEDICINE CORP. |
| Ling-Mei Wang | Steminent Biotherapeutics Inc. | ||
| Shiaw-Min Hwang | U-Neuron Biomedical Inc. | ||
| Ryan Chang |
CMBA: China Medicinal Biotech Association.
ABLE: Association of Biotechnology Led Enterprises.
FIRM: Forum for Innovative Regenerative Medicine.
CARM: The Council for Advanced Regenerative Medicine.
BPIPO: Biotechnology and Pharmaceutical Industries Promotion Office.
2. Discussion in working group 2
APACRM WG2 focused on two items: eligibility of cells as starting materials for cellular therapy products and comparability of cell therapy products subject to changes in their manufacturing process. As the characteristics of cell types used for cellular therapy are broad, allogeneic MSC products were selected as a model for our discussion.
Many Asian countries and regions have their own regulations regarding the quality of starting cells, depending on healthcare history, governance of remedy, and state of infectious disorders. Asian regulations regarding the eligibility of starting cells in China, India, Japan, Korea, and Taiwan were summarized, while benchmarking relevant regulations from the US and EU. Although some gaps in regulatory requirements among countries and regions were identified, we suppose that cellular therapy products will generally be developed across countries and regions. Therefore, we discussed a risk-based approach to reconcile the regulatory gaps to enable global development from scientific and risk perspectives.
Although the International Council for Harmonization (ICH) of Technical Requirements for Pharmaceuticals for Human Use has published the Q5E document on the Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process, the scope of that guideline is largely proteins and polypeptides. We reviewed each chapter of the ICH Q5E to identify different points to be compared between proteins/polypeptides and cells, and we identified many principles that can be applied to cellular therapy products. However, we concluded that the characterization of physicochemical properties, biological activities, immunochemical/immunoreactive properties, purity/impurities, and contaminants of cellular therapy products differ substantially from those of protein or polypeptide products; thus, further discussion on characterization items for cells is necessary. In addition, stability studies under the accelerated and stressful conditions of ICH Q5E are useful to provide insight into potential product differences in the degradation pathways of protein and polypeptide products. However, its applicability to cellular therapy products should be further discussed.
3. Starting cells
3.1. Regulation of eligibility of starting cells
We reviewed guidelines for eligibility on starting cells in China, India, Japan, Korea, and Taiwan, where WG2 members are located as of July 2020. In addition, the US and EU guidelines were reviewed as benchmarks. Table 2 summarizes the regulations on the donor screening test, and Table 3 summarizes the regulations on donor interviews, informed consent, site for collection, window period, traceability, and legitimacy for starting cells.
Table 2.
Requirement of donor screening test.
| Country/region | Guideline | Donor screening test |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV | HBV | HCV | HTLV (Type 1/2) | EBV | PVB 19 |
CMV | WNV | Treponema pallidum | Chlamydia trachomatis | Neisseria gonorrhoeae | TSE/CJD | ||
| China | The requirements about the cell source and donors in China. Quality control of stem cell preparations and guidelines for pre-clinical research (for trial implementation). |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| India | National guidelines for stem cell research (issued by Indian council of medical research – department of biotechnology). Draft guidance document for regulatory approval of SCCP (issued by Central Drugs Standard Control Organization). |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Japan | Standards for biological materials (Standard for Human Cell/Tissue materials). Quality and safety assurance for drugs and medical devices manufactured using human (allogeneic) somatic stem cells. |
✓ | ✓ | ✓ | ✓ | ✓1 | ✓ | ✓1 | ✓1 | ✓ | ✓ | ✓ | ✓ |
| Korea | Guideline on eligibility determination for donors of cell therapy products. | ✓ | ✓ | ✓ | ✓2 | ✓2 | ✓ | ✓3 | ✓3 | ||||
| Taiwan | Standard for Human cell therapy product donor suitability. | ✓ | ✓ | ✓ | ✓4 | ✓4 | ✓ | ✓5 | ✓5 | ✓ | |||
| US | Guidance for industry, Eligibility determination for donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps) (21 CFR part 1271). Use of nucleic acid tests to reduce the risk of transmission of west nile virus from living donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps). |
✓ | ✓ | ✓ | ✓4 | ✓4 | ✓ | ✓ | ✓1 | ✓1 | ✓ | ||
| EU | Directive 2006/17/EC (Directive 2004/23/EC). | ✓ | ✓ | ✓ | ✓6 | ✓1 | ✓1 | ✓ | ✓ | ||||
As needed.
To be tested for leukocyte-rich tissue.
To be performed only on germ cells or embryo-derived cells.
For viable, leukocyte-rich cells or tissue.
Reproductive tissue.
HTLV-I antibody testing must be performed for donors living in, or originating from, high-prevalence areas or with sexual partners originating from those areas or where the donor's parents originate from those areas.
Table 3.
Requirement of donor interview, informed consent, site for collection, window period, traceability and legitimacy.
| Country/region | Guideline | Negation of infectious diseases by interview | Informed Consent for commercial use |
Appropriate site for collection (staff, facility, procedure etc.) | Window period consideration | Traceability of cell collection and preparation | Legitimacy of Source Material |
|---|---|---|---|---|---|---|---|
| China | The requirements about the cell source and donors in China. Quality control of stem cell preparations and guidelines for pre-clinical research (for trial implementation). |
✓ | ✓1 | ✓ | ✓ | ||
| India | National guidelines for stem cell research. Draft guidance document for regulatory approval of SCCP. |
✓ | ✓ | ✓ | |||
| Japan | Standards for biological materials (Standard for Human Cell/Tissue Materials). Quality and safety assurance for drugs and medical devices manufactured using human (allogeneic) somatic stem cells. |
✓ | ✓ | ✓ | ✓ | ✓ | |
| Korea | Guideline on eligibility determination for donors of cell therapy products. | ✓ | ✓ | ✓2 | ✓ | ✓2 | |
| Taiwan | Standard for Human cell therapy product donor suitability. | ✓ | ✓ | ✓ | ✓3 | ✓ | ✓ |
| US | Guidance for industry, eligibility determination for donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps) (21 CFR part 1271). Use of nucleic acid tests to reduce the risk of transmission of west nile virus from living donors of human cells, tissues, and cellular and tissue-based products (HCT/Ps). |
✓4 | ✓ | ✓ | ✓5 | ✓6 | |
| EU | Directive 2006/17/EC (Directive 2004/23/EC) | ✓ | ✓ | ✓ | ✓7 | ✓ |
Currently, commercial (or payable) application of stem cells (MSCs) in clinical treatment is strictly forbidden, so the “informed consent” is limited to clinical research, not for commercial use.
Advanced Regenerative Medicine and Advanced Bio Act.
The window period is considered in the beginning donor screening stage.
Zika: Guidance for industry donor screening recommendations to reduce the risk of transmission of Zika virus by human cells, tissues, and cellular and tissue-based products.
Anonymous semen donors only.
CFR1271/55(d).
If samples from a living donor undergo serology testing and are also tested by NAT for HIV, HBV, and HCV, retesting after a time interval is not required.
As shown in Table 2, all countries and regions require donor screening tests for human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). Tests for other viruses such as Human T-cell Leukemia Virus (HTLV), Epstein–Barr Virus (EBV), Human Parvovirus B19 (PVB19), West Nile Virus (WNV), and bacteria such as Treponema pallidum (syphilis), Chlamydia trachomatis, and Neisseria gonorrhoeae depend on the country/region and type of cells or tissues. In Japan, Taiwan, the US, and the EU, a donor screening test for transmissible spongiform encephalopathy (TSE)/Creutzfeldt-Jakob disease (CJD) disease is also required.
3.2. Risk-based approach
Each country or region has its own requirements for the quality of starting cells based on the history of healthcare, governance of remedy, and situation of infectious disorders in each region. However, cellular therapy products are commonly developed in a different country or region from where the starting cells are procured. In these circumstances, the starting cells should also comply with the regulations of the country or region of development, although these regulations may not always agree with those of the country or region of procurement. To help reconcile the gaps in the regulations from risk-based perspectives and to facilitate the development of cellular therapy products across the globe, some of the important points to be considered are illustrated below using a case study.
3.2.1. Case study
Bone marrow or adipose tissue was procured from Country (or Region) A. The donor screening test for HIV, HBV, HCV, and T. pallidum was performed once by Nucleic Acid Amplification Test (NAT) just before the collection of bone marrow or adipose tissue from a donor. The bone marrow or adipose tissue was then transported to Country (or Region) B for the development of MSC therapy.
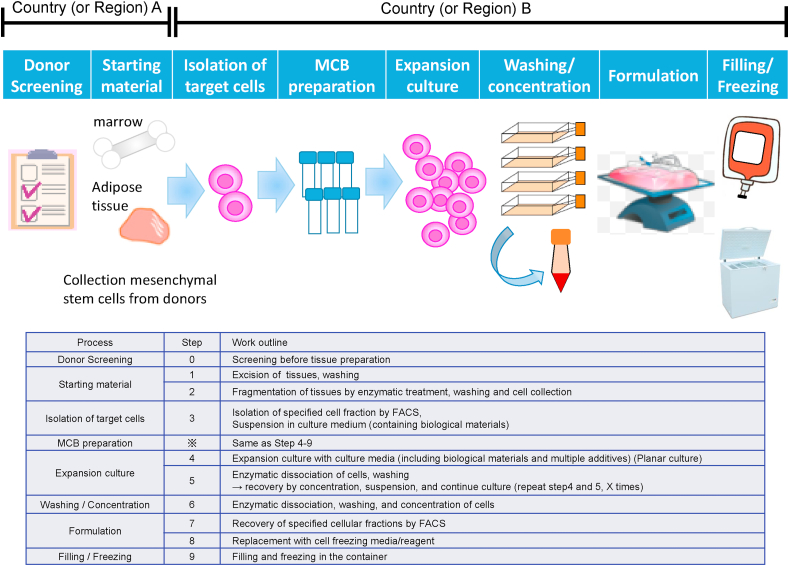
The manufacturing scheme for the developing product is shown in Fig. 1. The steps of “donor screening” and “starting material” in this figure were performed in Country (or Region) A, and the steps from “isolation of target cells” to “freezing” were performed in Country (or Region) B. In this case study, development is conducted in Country (or Region) B; thus, the regulatory requirements of Country B cover the full spectrum of MSC product manufacturing, including the donor screening step. The regulations of country B require a donor screening test for HTLV and a second donor screening test considering a window period when bone marrow or adipose tissue is procedure. The requirements for procurement of starting cells between Country (or Region) A and B in this case study are summarized in Table 4.
Fig. 1.
Manufacturing scheme for the developing product.
Table 4.
Comparison of requirement for donor screening between Country (or Region) A and B.
| Country (or Region) | Required donor screening test | Window period consideration |
|---|---|---|
| A | HIV, HBV, HCV, Treponema pallidum. | No |
| B | HIV, HBV, HCV, HTLV, Treponema pallidum. | Yes |
The WG2 discussed how we can develop MSC products using bone marrow adipose tissue (starting cells) from a donor in the absence of any of the tests required by these regulations. Based on our discussion, we provided some examples of risk assessment for the quality and safety of the final product in the instance of missing tests on the donor (as specified in the case study).
3.2.2. Donor location
In this case study, the HTLV test was required for donor screening in Country (or Region) B, but not in Country (or Region) A. This may be due to the high prevalence of HTLV infection in Country (or Region) B.
The European Center for Disease Prevention and Control (ECDC) issued a technical report on the geographical distribution of areas with a high prevalence of HTLV-1 infection [1]. This report summarizes prevalence studies performed worldwide and shows maps indicating the prevalence of HTLV-1 infection in different countries and regions. For Asia, countries or regions with high prevalence are Japan and Taiwan, and those with low prevalence include India and Korea. Although China has a low prevalence of HTLV-1 overall, certain areas show a high prevalence. Therefore, the risk of HTLV infection from the starting cells procured in Country (or Region) A could be considered low if it is a low prevalence in this country (or region).
In cases where a specific virus test on a donor is missing, the infection risk of missing the virus test on starting cells could be assessed based on the prevalence of the virus in that country or region.
3.2.3. Window period of the PCR test and serologic test
In this case study, a donor screening test was performed once under the regulation of Country (or Region) A. However, Country (or Region) B requires a second donor screening test in consideration of a window period.
EU Directive 2006/17/EC (Directive 2004/23/EC) mentions that samples from a living donor should undergo serology tests and NAT but retesting after a time interval is not required. This is because the window period of the serology test is longer than that of the NAT. In the WHO technical report [2], the window period of NAT for HIV, HBV, and HCV is 4, 17, and 3 days, respectively. In contrast, the window period of serological tests (antigen enzyme immunoassay/chemiluminescence immunoassay) for HIV, HBV, and HCV is 14, 42, and 9 days, respectively. Thus, the risk of false negatives due to the window period is lower in NAT than in the serological test, and this risk can be minimized by a combination of serological test and NAT. Furthermore, the window period of NAT is reduced by improvements in the assay method. In 1996, the window period of NAT for HIV, HBV, and HCV was 11, 34, and 23 days, respectively [3]. As the WHO technical report was issued in 2017, the window period of HIV, HBV, and HCV was shortened by 7, 17, and 20 days, respectively, from 1996 to 2017.
Under the circumstances that the second donor screening test is not performed in consideration of the window period, the possibility that a virus infection slipped through a donor screening test if a recent NAT was used can be considered to be low.
3.2.4. Additional virus test
An additional virus test is one option to mitigate the risk of virus contamination when NAT for a specific virus is missed during donor screening. To this end, the tropism and viral receptors for each virus were investigated, as shown in Table 5.
Table 5.
Tropism and viral receptor for each virus.
| Virus | Tropism | Viral receptor |
|---|---|---|
| CMV | Vascular endothelial cells, DCs, MΦ, smooth muscle, retinal pigment epithelial cells, trophoblast cells, hepatocytes, and brain cells. In cultured cells, the growth of CMV is limited to fibroblasts [4]. There have been cases in which CMV proliferated during culture of MSC [5] |
Heparan sulfate glycosaminoglycans, integrins, epidermal growth factor receptors |
| HTLV-1 | Wide range of cells expressing GLUT-1 [6] | GLUT-1 |
| WNV | Various primary cells, cell lines in culture Monocytes, MΦ, DCs, endothelial cells, and neurons in humans and mice [7] |
Not identified |
| HIV | CD4+ T cells, MΦ [8] | CD4, CXCR4, CCR5 |
| HBV | Hepatocytes | Sodium taurocholate cotransporting polypeptide (NTCP) [9] |
| HCV | Hepatocytes | Heparan sulfate proteoglycan, CD81, Scavenger Receptor class B type I (SR-B1), claudin-1 (CLDN-1) [10] |
HIV infects CD4 (cluster of differentiation 4) T cells and macrophages (Mφ) using CD4, CXCR4 (C-X-C chemokine receptor type 4), or CCR5(C–C chemokine receptor type 5) as receptors. HBV and HCV infect hepatocytes only. HIV, HBV, and HCV have not been reported to infect or proliferate in MSCs.
However, CMV has been reported to proliferate during MSC culture. CMV can also infect various cell types such as vascular endothelial cells, dendritic cells (DCs), Mφ, smooth muscle cells, retinal pigment epithelial cells, trophoblast cells, hepatocytes, and brain cells. HTLV-1 infects various cell types expressing its receptor GLUT-1 (glucose transporter 1), including MSCs. Although it has been reported that WNV replicates in various types of primary cells and in immortalized cell lines, infection of MSCs has not been reported.
These findings suggest that CMV and HTLV-1 infect MSCs and proliferate in it, and that additional tests on the master cell bank (MCB) or final products of MSCs to detect these viruses is reasonable.
There is no evidence that additional HIV, HBV, HCV, and WNV tests on MSCs of MCB or final product can appropriately detect propagation of these viruses through the culturing process. However, NAT can detect these viruses in culture medium, assuming that none of these viruses are retained inside the MSC due to a lack of infectivity. In addition, HIV, HBV, and HCV should be tested at donor screening in all guidelines, as shown in Table 2. Therefore, infection with HIV, HBV, and HCV may be sufficiently controlled by a donor screening test.
In the case study, the infection risk of HTLV-1 in starting cells is considered low if procurement is taken place in low prevalent Country (or Region) A, as discussed in the section on Donor location. However, if an additional virus test on HTLV-1 is performed on MCB or the final product, the risk of HTLV-1 infection in patients can be further reduced. In case a recent NAT is used for a donor screening test, possibility that a virus infection slipped can be considered to be low even if a second donor screening test in consideration of a window period is not performed. Furthermore, additional tests for CMV, HTLV-1, and WNV on MCB or final product of MSC may be considered good risk mitigation, compensating for the lack of a second donor screening test.
4. Comparability
4.1. Characterization, a case study of MSC-based products
Basic characterization of MSCs based on the International Society for Cell & Gene Therapy (ISCT) position statement announced in 2006 (minimal criteria) defines MSCs as being plastic adherent, expressing CD73, CD90, CD105, and lacking expression of CD11b, CD14, CD19, CD34, CD45, human leukocyte antigen DR isotype (HLA-DR), and capable of in vitro differentiation into adipocytes, chondrocytes, and osteoblast lineages [11]. With increasing scientific evidence and recent clinical studies, MSCs are known for their homing, immunomodulatory, and regenerative properties that are induced upon interaction with host microenvironmental factors [12]. The MSC-specific surface markers and tri-lineage differentiation capability, as described above, are commonly used to identify MSCs, rather than the mechanism of action (MOA) for intended clinical use. In the APACRM WG2 meetings, we discussed routinely used physicochemical assays, biological characterization assays, and potency assays of MSCs for comparability assessment. The advantages and disadvantages of certain characterization assays are listed and summarized in Table 6. Our discussion focused on cost- and time-effectiveness, accessibility, reproducibility, and suitability of the assays for comparability studies. The recommendation level of each assay was given based on the discussion and opinions collected from WG2 members.
Table 6.
Characterization Test for MSC-based Product for comparability assessment.
| MSC product characterization | Method/Read-out parameter | Advantages | Disadvantages | Recommendation level |
|---|---|---|---|---|
| Viability (fresh & post-thaw) | Hemocytometer/viable cell count. | Simple, rapid, cheap. |
|
Low + |
| Automated cell-counting instruments/viable cell count. | High precision and high sample throughput. |
|
High +++ |
|
| Flow Cytometry-based Assay/viable, apoptotic, and necrotic cell count. |
|
|
High +++ |
|
| Senescence | Beta-galactosidase assay/Senescence cell count. | Can detect senescent cells in the MSCs after long term in vitro expansion. |
|
Middle ++ |
| Growth rate | Cell counting/Population doubling time (PDT). |
|
|
High +++ |
| Functional Characterization | ||||
| Immunosuppression (MSC-mediated immunosuppression) | Machine learning Morphological profiling method/IFN-γ-stimulated MSCs [13]. |
|
need special software and devices | High +++ |
| Immunosuppression (MSC-mediated Inhibition of T-cell activation) | Flow Cytometry-based Assay/Expression level of PDL-1 surface protein (%) (IFN-γ-stimulated & resting MSCs). |
|
|
High +++ |
| Flow Cytometry-based Assay/Expression level of IDO intracellular Protein (%) (IFN-γ-stimulated & resting MSCs). |
|
|
High +++ |
|
| Quantitative Flow Cytometry-based Assay/IDO immunosuppressive protein binding value (IPBv) [15,17] (IFN-γ-stimulated & resting MSCs). |
|
|
High +++ |
|
| Immunosuppression Inhibition of PBMC/T cell Proliferation |
Cell proliferation assay/Suppression rate. |
|
|
High +++ |
While physicochemical characteristics include surface markers, secreted substances, and the expression profiles of genes involved in the function of MSCs, assays of these characteristics do not report on whether the product is biologically active and potent. The ISCT MSC committee recommends characterizing MSCs by a matrix of functional assays to demonstrate secretion of trophic factors, modulation of immune cells, and other relevant properties, including promoting angiogenesis for the intended MOA [8]. Different starting materials and manufacturing processes result in similar but different MSC-based products. As in biosimilars development, functional characterization is required to demonstrate the comparability of an MSC product to innovator products and to previous lots of the same product. Potency measurements that reflect relevant biological properties of cellular products can also serve as a measure of comparability between production lots. Both quantitative and robust functional/biological matrix assays are highly recommended by WG2 members to demonstrate product potency.
4.1.1. Viability and senescence
As a living cellular product administered into the human body, MSCs should be able to sense signals from the targeted area and be able to migrate to the area in response through contact-dependent and contact-independent interactions with targeted cells. To guarantee consistent quality of MSC-based products, manufacturers should ensure that the product is living, healthy, and functional.
The basic characteristic of cellular products is viability. Trypan blue exclusion is the most popular method for viability testing. However, the test can only distinguish between live and dead cells. To ensure the functionality of cryopreserved cellular products after thawing, manufacturers need to know how many dysfunctional apoptotic or senescent cells are in the product. The flow cytometry-based method with Annexin V/propidium iodide (PI) staining can be used for apoptosis analysis of cellular products.
The morphology and size of MSCs also provides valuable information for quality monitoring [13]. Senescent cells with enlarged and irregular shapes may be observed in MSC cultures after long-term expansion in vitro. The presence of senescent cells in therapeutic MSC batches may reduce cell viability, differentiation potential, and trophic capabilities. The detection of senescence-associated beta-galactosidase (SA-b-gal) activity is the most contemporary standard for the evaluation of cell senescence [14].
The population doubling time (PDT) or cumulative population doubling number at each passage can be calculated to evaluate the impact of cryopreservation on the replicative capability and functionality of thawed MSCs. Several published studies have demonstrated that replicatively exhausted MSCs display poor functionality despite exhibiting high viability [[15], [16], [17]].
4.1.2. Inhibition of the PBMC/T-cell proliferation assay
Regarding characterization of the immune functions of MSCs, a biological assay to address the inhibition of peripheral blood mononuclear cell (PBMC) proliferation/activation is frequently used to determine the immunosuppressive capability of MSCs [13,15]. This assay directly measures the effect of MSCs on activated T cell proliferation. The interaction between MSCs and responder PBMC is bidirectional. This complex interaction occurs via contact-dependent and contact–independent mechanisms of effector (MSCs) and responder cells (T cells) [15].
Variations in the responses of MSCs and PBMC interaction result in differences among individual PBMC responder cells. PBMC donors largely determine variations in the outcome of MSC and PBMC interactions. To control the variation in the immunosuppression assay, interferon-γ (IFN-γ)-stimulated MSCs are commonly recommended to mimic an inflammatory environment of disease in vivo that MSCs are likely to encounter. IFN-γ can also be used as a surrogate for the action of activated PBMCs on MSCs in a function assay [15].
4.1.3. Measurement of activation markers, secreted proteins, and gene expression
Indoleamine 2,3-dioxygenase (IDO) is a key mediator through which MSCs inhibit T cell proliferation. The ISCT MSC committee recommends immunological characterization of MSCs by measuring IDO induction and function as a potent readout of MSC functionality against T cell activity. The committee also recommends using resting MSCs or other reference materials as internal controls [18].
Expression of immune-related genes, such as IDO, C-X-C motif chemokine ligand 10 (CXCL10), and CXCL9, can be measured by quantitative polymerase chain reaction (qPCR) array or enzyme-linked immunosorbent assay (ELISA). Increased expression of surface markers such as programmed death ligand 1 (PDL-1), intercellular adhesion molecule 1 (ICAM-1), HLA-DR, or intracellular protein of IDO that are involved in the immunomodulatory function of MSCs can be analyzed by the flow cytometry-based method. However, the flow cytometry readout is usually limited to the ratio (%) of positive or negative cells in the sample. Thus, it is difficult to evaluate small changes in the comparability study. A modified quantitative flow cytometry-based assay standardized by the fluorescence intensity of beads was developed to measure IDO in IFN-γ-stimulated MSCs [19]. This assay provides a quantitative measurement of the intracellular IDO expression level of primed MSCs. As recommended by the ISCT MSC committee, this assay uses resting MSCs as an internal control, and a cell line as reference material to monitor the whole process of operation. This assay has already been applied to comparability and stability studies of therapeutic MSC products during manufacturing [20].
4.2. Stability
Stability data, including those generated from accelerated or stress conditions, are considered to provide an insight into potential differences in post-change products as per ICH Q5E. Although the scope of ICH Q5E is proteins and polypeptides, its general principles are also applicable to cell therapy products. Since any modification to the process/formulation may affect the stability of post-change products, a real-time/real-temperature stability study needs to be initiated for appropriate comparability assessment. However, the question of whether tests under accelerated or stress conditions provide any value for comparability assessment of cell therapy products remains unanswered.
The optimal conditions required for accelerated and stress stability are unknown; especially concerning the typical real-time storage conditions of cell therapy products, the vapor phase of liquid nitrogen (<-135 °C). Although the conditions should be carefully selected on a case-by-case basis per ICH Q5C, the members of APACRM WG2 have generally agreed that −80 °C and −20 °C are the typical temperatures for accelerated and stress storage conditions, respectively.
One main reason for assessing the stability of process/formulation changes is to detect any subtle differences that might not be captured by a characterization study per ICH Q5E. Furthermore, the stability under accelerated/stress conditions can provide information to elucidate the degradation pathway of pre- and post-change products. Although proteins and polypeptide products are considered to degrade to a certain point/amount during real-time/real-temperature storage, cells stored under the vapor phase of liquid nitrogen are generally considered not to degrade. Since liquid water does not exist below −130 °C, life-associated reactions simply stop; under these conditions, only crystalline/glassy states exist, and these show viscosities so high that diffusion is insignificant over less than geological time spans [21]. Therefore, it may not be relevant to evaluate the degradation pathway of cells during storage in the vapor phase of liquid nitrogen. Alternatively, Mazur also reported that the challenge for the cells is not being stored at very low temperatures, but rather to traverse an intermediate zone of temperature (from −15 °C to −60 °C) twice, once during freezing and another during thawing [21]. Thus, characterization of cells after cryopreservation and thawing might be more relevant than evaluating their degradation pathway. In addition, degradation of cells may be very complex (compared with proteins and polypeptides), and the degradation pathway may not be helpful in distinguishing the difference between pre- and post-change products.
A few members of WG2 have performed accelerated and stress condition stability assays for their cell therapy products. Lower viability was observed, as expected, and some products showed decreased potency as well. The European Medicines Agency (EMA) stated in their questions and answers on comparability considerations for Advanced Therapy Medicinal Products that “for cell therapy products which have relatively long shelf life, it is more reasonable to focus on dedicated stability studies under accelerated or stress conditions that can be of value to identify possible differences” [22]. Although WG2 members did not have a specific idea what would be the ‘value’ from the experience, taking into account the EMA statement, the necessity for accelerated/stress condition stability data for a comparability assessment needs to be evaluated on a case-by-case basis, and it may assist in understanding pre- and post-change products in some cases.
5. Conclusion & future directions
Each country or region has its own requirements for the quality of starting cells based on the history of healthcare, governance of remedy, and situation of infectious disorders. The risk-based approach can give assistance to reconcile the gaps in the regulations to facilitate the development of cellular therapy products across the globe. A standard procedure of comparability assessment after changes in the manufacturing process for MSC-based products does not exist. We proposed characterization test item for MSC-based product and points to be considered for stability study for comparability assessment. A strategy of risk-based approach and comparability assessment depends on biological and physicochemical feature of developed product, our insights in this white paper could be reference.
Conflict of interest
The authors have no conflict of interest, financial or otherwise.
Acknowledgement
The authors are grateful to Dr. Masayuki Nomura, Chair of International Affairs Committee at FIRM, for supporting WG2.
The authors would like to thank Dr. Yoji Sato, Head of Division of Cell-Based Therapeutics Products at the Japan National Institute of Health Sciences, for discussions on starting cells and comparability for cellular therapy products with WG2.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Toshimitsu Tanaka, Email: toshimitsu.tanaka@astellas.com.
Shing-Mou Lee, Email: smlee@emobio.com.
Mayu Mikami, Email: mayu.mikami@astellas.com.
Kahori Yokota, Email: yokota.kahori.d4@daiichisankyo.co.jp.
Koji Takakura, Email: koji.takakura@astellas.com.
References
- 1.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2015. Geographical distribution of areas with a high prevalence of HTLV-1 infection. [Google Scholar]
- 2.WHO technical report series 1004; WHO expert committee on biological standardization. Sixty-seventh report; 2017. [Google Scholar]
- 3.Schreiber G.B., Busch M.P., Kleinman S.H., Korelitz J.J. The risk of transfusion-transmitted viral infection. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 4.Revello M.G., Gerna G. Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications. Rev Med Viol. 2010;20:136–155. doi: 10.1002/rmv.645. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K., Otabe K., Shimizu N., Komori K., Mizuno M., Katano H. High-sensitivity virus and mycoplasma screening test reveals high prevalence of parvovirus B19 infection in human synovial tissues and bone marrow. Stem Cell Res Ther. 2018;9:80. doi: 10.1186/s13287-018-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodorigues E.S., Macedo M.D., Pinto M.T., Orellana M.D., Rocha M.C., Jr., Magalhaes D.A.R. HTLV-1 infects human mesenchymal stromal cell in vitro and modifies their phenotypic characteristics. Virology. 2014;449:190–199. doi: 10.1016/j.virol.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Brinton M.A. Replication cycle and molecular biology of the West Nile virus. Viruses. 2014;6:13–53. doi: 10.3390/v6010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyanagi Y. Outline of the HIV Replication and its cellular factors: the track of an invader in cell. Uirusu. 2005;55:251–258. doi: 10.2222/jsv.55.251. [DOI] [PubMed] [Google Scholar]
- 9.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1 doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathalie Alazard-Dany N., Solène Denolly S., Boston B., Cosset F.L. Overview of HCV life cycle with a special focus on current and possible future antiviral targets. Viruses. 2019;11:30. doi: 10.3390/v11010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominici M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I. Mesenchymal stem versus stromal cells: international society for cell & gene therapy (ISCT) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Marklein R.A., Klinker M., Drake K.A., Polikowsky H.G., Lessey-Morillon E.C., Bauer S.R. Morphological profiling using machine learning reveals emergent subpopulations of interferon-γ-stimulated mesenchymal stromal cells that predict immunosuppression. Cytotherapy. 2019;21:17–31. doi: 10.1016/j.jcyt.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhai W., Yong D., El-Jawhari J.J., Cuthbert R., McGonagle D., Naing M.W. Identification of senescent cells in multipotent mesenchymal stromal cell cultures: current methods and future directions. Cytotherapy. 2019;21:803–819. doi: 10.1016/j.jcyt.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Chinnadurai R., Rajan D., Qayed M., Arafat D., Garcia M., Liu Y. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018;22:2504–2517. doi: 10.1016/j.celrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loisel S., Dulong J., Menard C., Renoud M.L., Meziere N., Isabelle B. Brief Report: proteasomal indoleamine 2,3-dioxygenase degradation reduces the immunosuppressive potential of clinical grade-mesenchymal stromal cells undergoing replicative senescence. Stem Cell. 2017;35:1431–1436. doi: 10.1002/stem.2580. [DOI] [PubMed] [Google Scholar]
- 17.Sepulveda J.C., Tome M., Fernandez M.E., Delgado M., Campisi J., Bernad A. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cell. 2014;32:1865–1877. doi: 10.1002/stem.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galipeau J., Krampera M., Barrett J., Dazzi F., Deans R.J., Debruijn J. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S.-M., Lai W.-L., Yu S.-N., Chen H.-H., Shen P.-C., Lin S.-H. Developing a flow cytometry-based quantitative Ido assay to measure immune potency of mesenchymal stromal cells product for phase 1 clinical trial. Cytotherapy. 2019;21:S37–S38. [Google Scholar]
- 20.Lee S.-M., Yu S.-N., Chen H.-H., Lai W.-L., Shen P.-C., Lin S.-H. ACTO annual meeting Poster. 2019. Predicting immunosuppressive capacity of mesenchymal stromal cell based product by indoleamine 2,3-dioxygenase quantification. [Google Scholar]
- 21.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247 doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 22.EMA/CAT/499821/2019 committee for advanced therapies (CAT), questions and answers comparability considerations for advanced therapy medicinal products. ATMP); 6 December 2019. [Google Scholar]