Abstract
Background and aims
There is a growing demand for health and social care services to provide technology‐mediated interventions that promote the health and well‐being of older people with health or care needs and of their informal carers. The objectives of this study were to scope and review the nature and extent of prior intervention studies involving ambient assisted living technology‐mediated interventions for older people and their informal carers, and how and in what ways (if any) the goals and aims of these interventions reflected the domains of the World Health Organization framework for healthy ageing.
Methods
We conducted a scoping review. Data were collected between June and October 2018 with an updated search in October 2020. A total of 85 articles were eligible for inclusion.
Results
Nine categories described the aims and content of the included studies. The healthy ageing domain “Ability to meet basic needs” was mirrored in four categories, whereas “Ability to contribute to society” was not addressed at all.
Conclusion
The ways in which domains of healthy ageing are mirrored suggest that there is an emphasis on individual factors and individual responsibility, and a lack of attention given to broader, environmental factors affecting healthy ageing. Only a few of the studies used a dyadic approach when assessing health outcomes concerning older people and their informal carers.
Keywords: aged, aged 80 and over, ambient assisted living technology, caregivers, health services for the aged, healthy aging
The objectives of this study were to scope and review the nature and extent of prior intervention studies involving Ambient Assisted Living (AAL) technology‐mediated interventions for older people and their informal carers, and how and in what ways (if any) the goals and aims of these interventions reflected the domains of the WHO framework for healthy ageing (WHO, 2015).The ways in which domains of healthy ageing are mirrored suggest there is an emphasis on individual factors and individual responsibility, and a lack of attention given to broader, environmental factors affecting healthy ageing.
1. INTRODUCTION
Globally, there is a growing demand for the provision of health and social care services to older people (ie, 65 years and older, 1 ) with health or care needs and their informal carers via the use of technology‐mediated interventions to enhance their health and well‐being. 2 , 3 More older people are benefitting from increased longevity as both life expectancy and healthy life expectancy (HALE) have increased by over 8% globally between 2000 and 2016. 4 However, there is an increased risk of developing long‐lasting health or care needs due to the ageing process, social isolation, chronic illness, or disability. When experiencing such needs older people may rely on care and support from informal carers, that is, a person who provides (usually) unpaid care outside a professional or formal framework. 5 The term “informal carer,” often used in Europe is synonymous with the North American term “family caregiver.” 6
Within the OECD countries, approximately 13% of people aged 50 and over report providing informal care at least once a week. The proportion of people aged 50 and over providing informal care is close to 20% in the Czech Republic, Austria, Belgium, the United Kingdom, France, and Germany, and less than 10% in Portugal, Sweden, Poland, the United States, Ireland, and Greece. 7
Without support, informal caring can adversely affect the carer's health and well‐being. 8 The caregiving relationship is, by definition, made up of two people, a dyad. 9 Previous research indicates the importance of including both members of the dyad in interventions to promote their health and well‐being. 10 In this study, this is referred to as a dyadic approach.
Older people and their informal carers are, to an increasing extent, offered support through ambient assisted living (AAL) technology‐mediated interventions. AAL technologies are defined as information and communication technologies (ICT), stand‐alone assistive devices, and smart home technologies which enable individuals to stay active longer, remain socially connected and live independently into old age. 11 Examples from the literature are fall detectors, activity recognition systems, mobile and wearable sensors, intelligent houses, cameras, robots designed for company and service, ICT‐solutions for support, health‐care or social contacts. 12 , 13
Previous reviews in the field have been based on type of technology or type of disease. Gagnon‐Roy et al 14 identified four types of technology for people with dementia, namely: monitoring technology, tracking technology, smart homes, and cognitive orthoses. Dietlein et al 15 focused on gaming technology for people with dementia, stating that the overall effectiveness of these games is unclear. Zhang and Kaufman 16 pointed to inconsistency in the evidence regarding the actual impact of gaming for older people. Robbins et al 17 provided a more comprehensive review of the field using the concept of active ageing and digital elements as inclusion criteria for the research under review.
AAL technology‐mediated interventions are promoted to enhance the health and well‐being of older people and their informal carers. However, health and well‐being are concepts that, throughout history, have held a variety of meanings. 18 In this study, we use the framework of “healthy ageing” presented by the World Health Organization (WHO) in the first World report on ageing and health. 1 The framework offers a holistic view of ageing and health, capturing the complex dynamics of internal determinants, socioeconomic factors, and broader environmental determinants of health. 1 This framework differs from the biomedical perspective, which tends to reduce the ageing process to a process of decline. 19 According to the WHO 1 framework, it is possible to have a health condition and still enjoy good health. Rather than regarding healthy ageing as a threshold state of functioning, it should be seen as a process relevant for all older people regardless of chronic illness or disability. 20 Previous work at EU‐level has mainly focused on active ageing and prolonged working life, with the risk of over‐emphasizing activity as a reflection of middle‐age perspectives and thereby making it potentially coercive to older people. 21
Healthy ageing is defined as the process of developing and maintaining functional ability that enables well‐being in older age. Functional ability is made up of intrinsic capacity (all physical and mental capacities a person can draw on) and environmental characteristics. 1 Healthy ageing is based on a life‐course perspective, starting at birth, and considers the exposures, opportunities and barriers encountered and the resources a person comes across throughout their life. 1 The framework has a rights‐based approach founded on international human rights law. The goal is to build and maintain one's functional ability. Functional ability can be divided into five key domains. These are (a) the ability to meet basic needs, (b) the ability to learn, grow, and make decisions, (c) the ability to be mobile, (d) the ability to build and maintain relationships, and (e) the ability to contribute. By optimizing functional ability within the five key domains, which are strongly interconnected, older people are enabled to do the things they value. 22
Since its publication, the WHO 1 framework has emerged as an important conceptualization of healthy ageing. In 2020, the 73rd World Health Assembly endorsed the proposal for a Decade of Healthy ageing (2020‐2030). This review contributes to the current knowledge around the promotion and maintenance of healthy ageing among older people and their informal carers supported by the rapidly emerging field of technology‐mediated interventions. We adopt a dual focus on older people and their informal carers, recognizing the importance of both perspectives. To the best of our knowledge, there is currently a lack of review studies offering a theoretical perspective on interventions using AAL technology and how they mirror a healthy ageing for older people and their informal carers.
The primary aim of this scoping review is to describe the nature and extent of empirical studies concerned with AAL technology‐mediated interventions for older people and their informal carers. The review was guided by the following questions:
What types of AAL technology‐based interventions for older people with health or care needs and their informal carers currently exist within the empirical literature?
In what contexts and how (if at all) have these interventions been implemented and assessed for health outcomes among older people and their informal carers?
In what ways (if any) and to what extent (if at all) do the aims/goals of these interventions mirror the five domains of functional ability within the WHO's healthy ageing framework?
2. METHODS
This review follows Arksey and O'Malley's six‐step framework 2 and the PRISMA Extension for Scoping Reviews (PRISMA‐ScR). 23 The first step was Identifying research questions, as outlined above. The second step was Identifying relevant studies. To identify empirical studies that addressed the central research questions, the search strategy was based on four clusters: (a) AAL‐technology, (b) Chronic conditions, (c) Older people, and (d) Informal carers (Table 1).
TABLE 1.
Search clusters and search terms
| AAL‐technology | Older people | Chronic disease | Informal carer |
|---|---|---|---|
| assistive technology OR e‐health OR m‐health OR assistive robot* OR service robot* OR telecare OR telemedicine OR health information technology OR internet health intervention OR gerontechnology OR welfare technology OR telehealth OR AI OR the internet of medical things OR app OR applications OR GPS OR electronic tracking OR medicine dispensing robot* OR medicine dispenser OR smartphone OR device use OR communication technology OR ICT OR health technology assessment OR web‐based healthcare robot* OR smart home OR location device OR tracking device OR ambient assisted living OR voice assistant OR virtual reality oR augmented reality OR telemonitoring OR reminder systems OR mobile health OR self‐help device |
older person OR older patient OR elderly OR aging in place OR senior citizen OR senior person OR senior patient OR aging society OR older user OR aging OR aged OR aging population OR geriatric |
Dementia OR chronic disease OR heart failure OR Chronic obstructive pulmonary disease OR Diabetes Mellitus type II OR chronic illness OR longstanding chronic illness OR stroke OR chronic conditions OR long‐term condition OR cognitive impairment OR cancer |
working carer OR unpaid carer OR family care support OR family carer OR municipal care OR family caregiver OR caregiver OR home care OR next of kin OR carer OR informal carer |
Note: Limits: NOT review*, protocol*, willingness to use. Dates: January 1, 2013 to September 30, 2018; updated to include October 1, 2018 to October 31, 2020. Age: ≥65. Language: English.
We combined the clusters with Boolean operators AND and OR to identify studies with a dual focus on older people and carers combined or with a single focus on older people. In a similar way, we searched both with and without the cluster for chronic conditions to ensure that we captured studies focusing on broader health or care needs as well, not only limited to the presence of chronic disease. Five electronic databases were searched: PubMed, CINAHL, Web of Science, PsychInfo, and Scopus. A further 17 scientific journals were hand‐searched via Browzine. The searches were conducted between June and October 2018 and updated in November 2020.
The third step was study selection. To be included in the review, studies needed to be published within the last 8 years to capture recent empirical literature and be written in English. Studies including older participants aged ≥65 with health or care needs were included. Studies were also included when 85% or more of the participants were within the set age, or where it was possible to extract results for participants aged ≥65. For studies where no age inclusion criteria and no given age range of the participant were described, inclusion was based on participants' mean age and SD.
While the scoping review method itself allowed for the inclusion of grey literature, a decision was made to only include published articles that assessed health outcomes for the older care recipient her/himself as well as for both the older care recipient and his/her informal carer. Studies that solely included health outcomes for informal carers of older people were excluded to delimit the scope. With regard to the method for selecting health outcomes, the authors referred to Wilson and Cleary's conceptual model which includes five core levels to capture the interrelationships between biomedical outcomes and societal factors for health. 24 According to their model, health outcomes can be divided into (a) biological and physiological factors, (b) physical, psychosocial, emotional, and psychological symptoms, (c) various domains of functioning, (d) subjective ratings of general health, and (e) overall quality of life (QoL). This review included all studies that assessed health outcomes as defined by Wilson and Cleary. 24 Finally, articles were excluded if the technology was solely a working tool for professional staff in the context of a hospital or specialist care settings (Figure 1).
FIGURE 1.
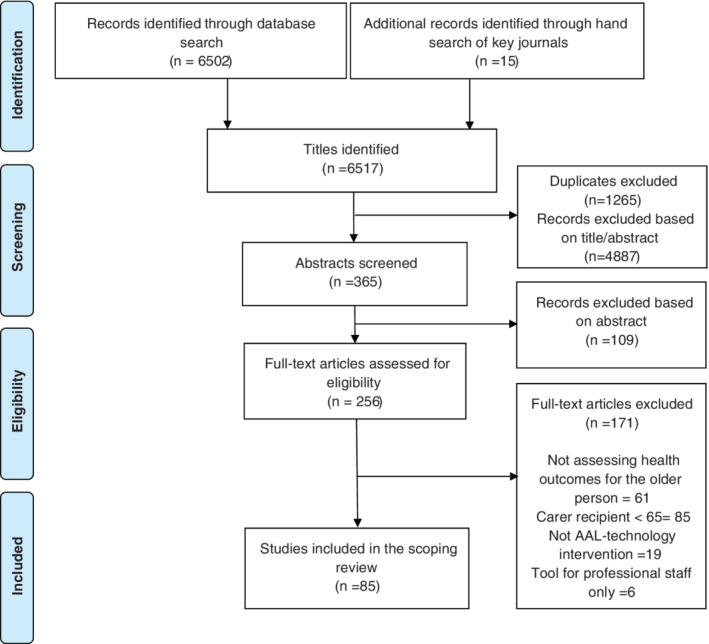
PRISMA flow diagram of identification, screening, eligibility assessment, and inclusion of studies
The fourth step, charting the data, was conducted using a descriptive‐analytical framework 25 for collecting the following information: author, year of publication and place, participants, age‐range and setting, intervention content and aims, methodology employed, outcome measures employed and for whom (older person or older person and informal carer), and summary of important results. The results were discussed and continuously updated in an iterative process.
The fifth step, collating, summarizing, and reporting results, comprised three phases. In the first phase, each study was labeled and categorized based on the purpose of the intervention. The labels were scrutinized, so the process was not linear, but went back and forth between label and category in discussion with the co‐authors. This process generated a map, contributing to the understanding of the current breadth and depth of the field. In the second phase of analysis, the five domains of functional ability were used as a critical lens to analyse if and how the categories corresponded to the WHO 1 framework for healthy ageing. The aims and goals of the interventions were compared to the definitions and core concepts of the domains. In the final phase, the studies were analyzed to identify if and to what extent (if at all) a dyadic approach was present in the design and/or implementation of the included intervention studies.
3. PROTOCOL AND REGISTRATION
This study had no pre‐published or registered protocol before commencement.
4. RESULTS
A total of 85 studies were included, 36% (n = 31) from Europe, 32% (n = 27) from North America, 18% (n = 15) from Asia, 9% (n = 8) from Oceania, 4% (n = 3) from South America, and 1% (n = 1) were cross‐national. In the ensuing results section, first, the core categories of interventions are presented (research questions I and II). Second, the results concerning the extent to which (if at all) these core categories of interventions reflect the domains of functionality within the WHO 1 framework for healthy ageing are reported (research question III).
4.1. Categories of interventions
The analysis resulted in nine categories of interventions, describing the characteristics and goals of the interventions. See Table 2, and for more details of included intervention studies; Supporting Information.
TABLE 2.
Intervention categories and included intervention studies, assessing health outcomes, and inclusion of informal carers
| Inclusion of informal carers | ||||
|---|---|---|---|---|
| Intervention categories | Number of articles | Studies assessing health outcomes for the older participants only | Studies including informal carers to varying extents | Studies in which also informal carers were assessed for health outcomes |
|
Exercise to improve physical fitness |
15 | (n = 12) 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 | (n = 2) 38 , 39 | 42 |
|
Activities for social engagement, comfort, or well‐being |
15 | (n = 9) 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 | (n = 5) 50 , 51 , 52 , Van der 53 , 54 | 57 |
| Support for daily needs and activities | 14 | (n = 7) 56 , 57 , 58 , 59 , 60 , 61 , 62 | (n = 2) 63 , 64 | (n = 5) 65 , 66 , 67 , 68 , 69 |
|
Monitoring symptoms for self‐care |
11 | (n = 5) 70 , 71 , 72 , 73 , 74 | (n = 5) 75 , 76 , 77 , 78 , 79 | 82 |
|
Education to support self‐efficacy and social inclusion |
8 | (n = 6) 81 , 82 , 83 , 84 , 85 , 86 | (n = 2) 87 , 88 | |
|
Training and maintenance of cognitive ability |
8 | (n = 5) 89 , 90 , 91 , 92 , 93 | (n = 2) 94 , 95 | 98 |
| Supervision for increased safety | 7 | (n = 2) 97 , 98 | (n = 2) 99 , 100 | (n = 3) 101 , 102 , 103 |
|
Exercise to regain physical functions |
5 | (n = 5) 104 , 105 , 106 , 107 , 108 | ||
| Receiving therapy from a distance | 2 | 111 | 112 | |
| Total: 85 | Total: 52 | Total: 21 | Total: 12 | |
4.2. Exercise to improve physical fitness
18% (n = 15) of the studies described interventions promoting physical fitness among older participants. 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 The interventions were aimed at preventing falls or further decline among older people due to frailty, sarcopenia, multiple chronic conditions, or cancer by means of improving balance, strength, and physical capacity. The interventions used gaming technology with motion capture cameras, 28 , 29 , 31 , 32 , 33 , 34 , 35 , 37 , 38 virtual reality software 39 a web‐based platform, 30 telehealth and videoconference 26 , 40 an app on a tablet, 27 and robot‐mediated exercise. 36
The studies lasted from 4 weeks to 12 months. All but one study 39 used a quantitative design. Of these, seven were RCTs. 29 , 30 , 31 , 32 , 34 , 35 , 36 One study used strong control, that is, comparing the intervention to group‐based exercise 35 whilst Hong et al 30 and Gomes et al 29 provided education and guidance to all participants but did not offer actual exercise to the control group. Jorgensen 32 used insoles as a placebo treatment and Lauzé et al 34 compared intervention to the regular routine. All of the studies except McEwen et al 39 and Lafaro et al 40 showed improvements when assessing balance and strength, thereby potentially reducing the risk of falling. McEwen et al 39 included family members in interviews in order to include their views on changes regarding physical activity and daily life activities of their older relatives. Chao et al 28 suggested that volunteer family or friends might lead the exercise due to limited resources.
Only Lafaro et al 40 assessed health outcomes for informal carers, showing gradual improvement in distress levels.
4.3. Activities for social engagement, comfort or well‐being
18% (n = 15) offered interventions focusing on activities for social engagement, entertainment or comfort ( 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 54 , 55 ; Van der 53 ). The interventions aimed at increasing older people's QoL through encouraging communication and offering positive experiences and thereby relieving and managing psychogeriatric symptoms such as behavioral and psychological symptoms in dementia (BPSD) or depression.
Interventions used robotic pets, 41 , 43 , 45 , 48 , 49 , 50 social robots, 44 tablets for apps or videos, 46 , 47 , 54 videoconferences (Van der 53 ), virtual reality technology, 42 , 51 , 52 and light therapy. 55
Study duration was 4 months or shorter, except Chu et al 44 who followed up after 5 years. Five studies used mixed methods, 42 , 43 , 50 , 51 , 52 the others were quantitative of which three were RCTs. 45 , 48 , 49 In the study by Van der Ploeg et al, 53 families provided comfort via videoconference. Gustafsson et al 50 and Moyle et al 51 interviewed family members concerning perceived effects for the older person during the intervention.
Main outcome measures among older participants within this category were indicative of improved well‐being, 44 , 46 alternatively stable or enhanced QoL derived from positive social engagement, 43 , 50 increased comfort and reductions in the use of psychoactive and pain medications, 49 fewer neuropsychiatric symptoms such as decreased agitation ( 47 , 54 ; Van der 53 ), less anxiety, 41 reduced levels of apathy, 42 , 52 and enhanced sleep. 55 Vahia et al 54 highlighted the scope for using tablets for video chats with family members as a non‐pharmacological intervention.
In 7% (n = 1) of the studies in this category, informal carers were also assessed for health outcomes. Sekiguchi et al 55 assessed the burden of care and found a decrease in five of 17 cases.
4.4. Support for daily needs and activities
16% (n = 14) of the studies aimed to support basic daily needs and functions such as nutrition, mobility, medication intake, self‐care, and hearing. 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 The technology used consisted of motion sensors, 63 medication dispensers, 66 , 67 hearing aids, 56 , 57 and tablet or mobile apps, 58 , 65 , 68 robots, 59 , 61 a scooter, 60 a smart walker, 62 and electric light wires. 64
The studies were from 3 weeks to 4 years in duration. Three studies were case reports, 57 , 66 , 67 one RCT 69 and one qualitative. 61 Results reported were an increase in medication adherence 58 , 66 , 67 and improvements or maintenance in activity performance and participation among older participants. 59 , 60 , 62 , 65 , 69 For the studies focusing on hearing impairment, McInerney and Walden 57 reported positive results with fewer communication breakdowns among the older participants, while in the study by Jupiter, 56 the older participants disliked or simply forgot to use the technology. Tchalla et al 64 showed a reduction in the prevalence of indoor falls, while Pripfl et al 61 did not show any changes according to the “Falls efficacy scale” as a result of the low usability of the technology. Informal carers took an active role in assisting the older care recipients in the use of the technology in the study by Lindhardt and Nielsen. 68
Dupuy et al 63 assessed the burden for professional caregivers and found that the burden increased more during the study period for the control group compared to the technology‐equipped group. Dupuy et al 63 considered the technology to have a greater potential for informal carers. In their research, Tchalla et al 64 and Obayashi et al 59 considered that the interventions had the potential to relieve stress for informal carers.
But the researchers did not assess carer burden as an outcome. Health outcomes were assessed for informal carers in 36% (n = 5) of the interventions. Carer burden was found to decrease in three studies. 65 , 66 , 67 In Lindhardt and Nielsen, 68 informal carers reported a reduction in worry and relief from tasks, both of which improved their relationship with their next‐of‐kin. Mortenson et al 69 reported how burden decreased for informal carers in the identified activity perceived as problematic, but this did not extend to their overall burden.
4.5. Monitoring symptoms for self‐care
13% (n = 11) of the studies reported interventions aimed at increasing the knowledge and self‐management skills of older people living with chronic diseases and thereby helping to decrease the use of health‐care resources and improve QoL among older people. 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 Interventions offered comprised programs of symptom monitoring and testing devices for collecting and sending data to health‐care professionals. The assessment was received through various channels, some combining several channels in different phases.
The channels used were videoconference 76 , 77 , 78 , 80 telephone 70 , 75 a digital health diary 73 apps with text 71 or message functions. 74 , 79 Some of the programs generated automatic feedback and risk assessment via algorithms, 71 , 72 whilst in others, data were audited directly by health care staff. 70 , 77 , 79 , 80
The studies ranged from 3 months to 3 years in duration. One study included qualitative results, 71 all others were quantitative, of which five were RCTs. 70 , 74 , 75 , 77 , 79 In the controlled studies, the control group received care according to the usual routine with face‐to‐face meetings with health‐care staff. In Dario et al's 75 study, relatives were contacted following alarms triggered by blood glucose levels among older participants. In Nouryan et al 77 and Shah et al, 78 informal carers assisted and received feedback on the health status of their older care recipient. Villani et al 79 provided health‐related information to both the older person and their informal carer, and in Maresca et al, 76 a neuropsychologist provided support for both patient and caregiver.
Results showed an overall decrease in the use of health‐care by older participants. 70 , 75 , 78 , 79 Nouryan et al 79 described no difference in the use of health‐care but reported improved outcomes of QoL among the older subjects. Maresca et al 76 reported improvements in emotional status and hematochemical values, Persson et al 73 showed significant improvement in general HRQoL, while Dario et al 75 reported no clinical significance in the improvement of older participants' QoL. Sun et al 74 reported improved levels of blood glucose for the participants. Göransson et al 71 described an increase in self‐care ability, but later a decrease in sense of security at follow up compared to at the end of intervention.
9% (n = 1) of the studies also assessed health outcomes for informal carers of older participants. De Cola et al 80 reported a significant reduction in the Caregiver Burden Inventory.
4.6. Education to support self‐efficacy and social inclusion
9% (n = 8) of the included studies described interventions framed around education, digital and health literacy, promoting social inclusion, healthy lifestyle and self‐efficacy for managing chronic disease among older people. 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 The interventions used teleconference, 81 videoconference, 88 software programs for PC, 83 , 86 , 87 web‐based programs, 84 , 85 and digital tracking tools. 82 The interventions lasted from 6 weeks to 22 months. The designs were all quantitative apart from Mullins et al, 85 who used mixed methods. Czaja et al 83 and Ferreira et al 87 conducted RCTs.
Health outcomes among older participants included decreased fatigue, 81 improved well‐being and physical and mental health 82 , 83 , 84 , 86 , 88 decrease in loneliness 85 but no effect on depressive symptoms. 88 There were indications of improvements in QoL. 87 Tsai et al 88 included family bi‐weekly appointments for a videoconference with the older person. Upton et al 86 suggested that the technology should be introduced to family members encouraging videocalls. None of the studies assessed health outcomes for the older participants' informal carers.
4.7. Training and maintenance of cognitive ability
9% (n = 8) of included studies described interventions aiming to improve or preserve cognitive skills and functions and reduce depressive symptoms among older people. 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 The interventions used mobile, web‐based or virtual reality gaming software, 89 , 90 , 91 , 92 , 95 wearable and monitoring sensors, 94 a web‐based app, 93 and an exercise robot. 96 The system in Lazarou et al 94 also included a caregiver interface for sharing information. The studies lasted up to 4 months. Half of the studies were RCTs. 89 , 91 , 92 , 93
Health outcomes among older participants were improvement in depression and Mini‐Mental State Examination (MMSE) scores, 89 in ADL functioning and sleep 94 global cognition and executive functioning 90 and in executive functioning and verbal memory. 91 In the study conducted by Merilampi et al, 95 no significant improvements in older participants' cognitive skills were shown. However, there were improvements in general well‐being and recreation. Merilampi et al 95 also discussed the potential for the intervention to increase social interaction with family members. Calabrò et al 96 showed a significant improvement in the attention process and executive functioning among older participants. In Robert et al, 93 there were indications of steady results in cognitive performance, suggesting less or slowed deterioration. Park et al 92 showed improvements in memory, but otherwise, no differences compared to the control group who used a conventional computer‐based training program. 13% (n = 1) assessed health outcomes for older participants' informal carers. Calabrò et al 96 reported a decrease in caregiver burden post‐intervention.
4.8. Supervision for increased safety
8% (n = 7) of included studies described interventions aiming to increase safety and prevent further decline of older participants through surveillance systems and active or passive alarm devices. 97 , 98 , 99 , 100 , 101 , 102 , 103 These interventions were mediated through video cameras, 97 , 101 passive monitors and sensors, 98 , 99 , 102 wearable or built‐in alarms, and digital tracking devices. 100 , 103
Interventions lasted from 2 months up to 1 year. Two studies were qualitative 101 , 103 while the others used various quantitative methods, none of them being RCT. Rohne et al 100 discussed the possibility that relatives could be alarm recipients and, as a result, there would be a potential for increased contact between older participants and their informal carers and thereby a reduced need for formal sources of care. The relatives in the study wanted to be included but did not want to be responsible 24 hours a day. Finch et al 99 described families or friends being contacted when the alarm‐center discovered a need for a further check‐up. Results showed a decrease in falls rates, 97 a reduction in the use of health‐care, 99 and a feeling of safety for the older person and their informal carer. 100 , 101 , 103
43% (n = 3) also reported health outcomes for informal carers. Lexis et al 102 assessed the burden on informal carers, (only 16 informal carers responded out of a total of 53 carer participants) and results pointed to a significant reduction in burden. Akerlind et al 101 and Watson et al 103 reported qualitative outcomes in favor of the mental health and well‐being of the informal carers.
4.9. Exercise to regain physical functions
6% (n = 5) of the studies described interventions for rehabilitation after a stroke among older participants, which aimed at improving the function of the arms and hands, from sitting to standing, or gait training. 104 , 105 , 106 , 107 , 108 The technology included exoskeletons, 105 , 106 motion sensors and virtual reality 104 or smartphones with motion sensors, 107 and robot‐assisted therapy. 108
The study designs were case reports, one of which was a case control study. 105 Informal carers were not involved in any assistance capacity to aid compliance with using the rehabilitation technology. Outcomes for the older subjects showed improvements in controlling movements and strength. None of the studies included secondary health outcomes for the informal carer.
4.10. Receiving therapy from a distance
Interventions offering treatment for dementia or post‐traumatic stress disorder (PTSD) symptoms among older people made up the smallest category, constituting only 2% (n = 2) of the included studies. The interventions were mediated through videoconference. 109 , 110 Kim et al 110 included informal carers as participants in the meetings. Both studies were quantitative cohort studies.
The PTSD symptoms among older participants decreased significantly, QoL increased, as did self‐efficacy. 109 Treatment of dementia symptoms showed no difference in outcomes compared to the control group, suggesting that therapy from a distance worked equally well as face‐to‐face sessions. 110 None of the studies assessed health outcomes for the informal carers of older participants.
5. CORRESPONDENCE BETWEEN CATEGORIES OF INTERVENTION AND DOMAINS OF FUNCTIONAL ABILITY ACCORDING TO THE WHO FRAMEWORK
Table 3 presents the correspondence between categories of intervention and domains of functional ability according to the WHO 1 model for healthy ageing.
TABLE 3.
Correspondence between categories of interventions and domains of functional ability
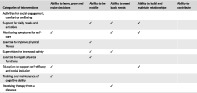
| Categories of interventions | Ability to learn, grow and make decisions | Ability to be mobile | Ability to meet basic needs | Ability to build and maintain relationships | Ability to contribute |
|---|---|---|---|---|---|
| Activities for social engagement, comfort or wellbeing | |||||
| Support for daily needs and activities | ✓ | ✓ | ✓ | ||
| Monitoring symptoms for self‐care | ✓ | ✓ | ✓ | ||
| Exercise to improve physical fitness | ✓ | ||||
| Supervision for increased safety | ✓ | ✓ | |||
| Exercise to regain physical functions | ✓ | ||||
| Education to support self‐efficacy and social inclusion | ✓ | ✓ | |||
| Training and maintenance of cognitive ability | ✓ | ||||
| Receiving therapy from a distance | ✓ |
5.1. Ability to learn, grow and make decisions
The domain is about being able to learn and apply knowledge, engaging in problem‐solving, personal development and having the ability to make choices. 1 The interventions in Monitoring symptoms for self‐care promoted health literacy and increased participation for the older person and their informal carers through education and platforms for consultation. The interventions further aimed to increase self‐management of chronic disease, thus reflecting this domain. Education to support self‐efficacy reflected the domain, since the interventions had the explicit aims of educating and enhancing self‐efficacy, promoting problem solving and the application of knowledge among older people. Finally, Training and maintenance of cognitive ability offered interventions for lifelong learning and growing, despite cognitive impairment.
5.2. Ability to be mobile
This domain refers to movement in all its forms, powered by one's own body, a vehicle or mobility supported by assistive devices. 1 The categories Exercise to improve physical fitness and Exercise to regain physical function corresponded with this domain since the interventions focused on improving balance, strength, and muscle control and thereby supporting and improving physical capacity among older people. The outcomes “Timed Up and Go” and “Falls efficacy scale” were recurrent and reflected the focus of the domain in improving the ability to get around. In the category Support for daily needs and activities, several interventions reflected the core concept of older people moving around safely and efficiently, with powered scooters or guided by increased lighting. Tchalla et al 64 and Dupuy et al 63 addressed fall prevention as did studies in the category Supervision for increased safety by optimizing the environment and the ability for older people to move around safely.
5.3. Ability to meet basic needs
Ability to meet basic needs means being able to afford an adequate diet, clothing, suitable housing, health care and long‐term services, and support to minimize the impact of economic shocks and enjoying security and safety. 1 Interventions in Supervision for increased safety were framed around safety for both the older person and their informal carer, using technology to detect whether an older person had fallen or wandered away, for example. The interventions in this category as well as in Support for daily needs and activities might contribute to enabling older people to return home after hospital visits, thus avoiding admission to residential care. Therefore, the categories reflect the domain that states that a basic need for older people might be to remain in their homes and communities. The category of Monitoring symptoms for self‐care reflects the domain in that it allows older people access to specialist health care at home, as exemplified in the work conducted by Persson et al, 73 in a similar way to the two interventions in Receiving therapy from a distance, which also allowed access to health care for both the older people and their informal carers.
5.4. Ability to build and maintain relationships
This domain represents the social network, from family members to more formal relationships within the community. 1 Several interventions in Education to support self‐efficacy and social inclusion mirror this domain since they focused on interventions affecting isolation and loneliness among older people. In the category Monitoring symptoms for self‐care, there were groups for elder peer support and it can thus be seen to mirror the domain with regard to how social relationships are viewed as a source of support. Support for daily needs and activities reported attempts to decrease isolation and promote communication among older people in two studies 56 , 57 and Pettersson et al 60 encouraged participation in the wider community.
5.5. Ability to contribute
The fifth domain is about volunteering, working, mentoring, or providing care or support. None of the categories reflected this domain when looking at the aims and goals of the interventions.
6. DISCUSSION
To summarize, the results provided a map of nine intervention categories. Most studies were found to be in the categories focusing on physical capacity and function, on managing the symptoms of dementia and cognitive impairment, on supporting functioning in daily life and on self‐caring with a chronic disease.
6.1. The dyadic approach in interventions using AAL‐technology
Previous research indicates that dyadic interventions have a positive impact on health and well‐being, not least in terms of aiding the ability to build and maintain social networks. 111 , 112 14% (n = 12) of the studies assessed health outcomes for both the older person and their informal carers in accordance with a dyadic approach. 10 Interventions aimed at relieving carer burden, using various assessment measures such as the Caregiver burden inventory, 80 the Caregiver Assistive Technology Outcome measure, 69 as well as using qualitative approaches focusing on their experiences as carers. 101 In the majority of studies where informal carers were included, their situation or health and well‐being was of subordinate interest. 86% (n = 73) of all studies in this review focused solely on the older person in the assessment of health outcomes, though 21 of these studies included informal carers to varying extents. The informal carers received alarms and provided help and support as a resource potentially replacing or alleviating professional staff. 75 , 77 , 78 Previous work confirms the viewing of informal carers as resources. 113 Cottam 114 argues that current health systems fail to recognize the role of the relationship between the informal carer and their next‐of‐kin, purporting that we even lack a language for this approach in public policy.
Based on this review, we argue that future AAL technology‐mediated technology intervention studies could usefully consider adopting and expanding a dyadic approach, thereby promoting a reciprocal and sustainable healthy ageing for both older people and their informal carers.
6.2. Domains of healthy ageing reflected in interventions
Overall, the WHO framework for healthy ageing 1 proved to be a useful tool for critically appraising the current state of the field. The domains “Ability to meet basic needs,” “Ability to learn, grow and make decisions,” “Ability to build and maintain relationships,” and “Ability to be mobile," were indeed mirrored by several interventions categories.
In the category Supervision for increased safety, interventions were framed around safety, thus reflecting the domain “Ability to meet basic needs.” However, even though monitoring and surveillance were commonly promoted as positive for safety and independence, there is a risk that these systems become a form of coercion and an unwelcome intrusion into the lives of older people. 115 Further, in this particular domain, the environment plays a crucial part in terms of poor social policies, inequality in health and social care systems, and meager politics. 1 These broader environmental factors, as described by the framework, were not given any attention in the studies. By focusing on the individual, digital health interventions tend to reduce health problems to the individual level, missing the broader social, cultural, and political dimensions of ill health. 116 It can be argued that socioeconomic factors are central to people's health and well‐being. Inequality implies that not only is having enough to make ends meet important, but so too is what we have relative to others. Thus, the lower our social position, the worse our health. 117
The domain “Ability to learn, grow and make decisions” is considered key to older people's sense of control. 1 However, this could also be viewed as an expression of how the field reflects the dominant political discourse, which emphasizes the individual's responsibility. 118 In this discourse, older people are expected to be entrepreneurial in achieving and maintaining good health, and there tends to be a focus on shifting the responsibility for care from the clinician to the patient. 116 The idea of older people in need of education and knowledge could also be a sign of ageism, whereby older people are seen as incapable and placed in an increasingly asymmetrical power relation to professionals. 119
The domain “Ability to be mobile” was mirrored in four categories. Worth highlighting are the studies focusing on improving balance and reducing “fear of falling.” The concept “fear of falling” is recognized as a health problem for older people, with consequences such as loss of health‐related QoL due to cutting down or avoiding activities, decreased participation and depression. 120 The WHO 1 states that the consequences of a decline in this domain extend beyond the individual and can affect all other domains of functional ability.
The domain “Ability to build and maintain relationships” was only mirrored in three categories, suggesting that the field, to a large extent, fails to address issues of social exclusion. The importance of relationships and connections for health are well established in the framework 1 as well as in the literature, see for instance Cottam 114 and Carstensen et al. 121
The domain “The ability to contribute” was not mirrored at all, suggesting a gap in the research field. Previous research shows that older people are involved in voluntary work, but opportunities may be conditioned due to age‐related negative perceptions within the organizations. 122 There is, though, a risk that viewing older people's engagement in voluntary work only as investment in their health and not based on their sense of citizenship may diminish their contribution to society. 119
The intervention categories in this review are largely in agreement with those described by Robbins et al., 17 which we referred to in the introduction. However, while Robbins et al 17 used the concept of “active ageing” and digital elements as an inclusion criterion, this review explored the field more broadly using the WHO 1 framework as an interpretative theoretical lens. We thereby offer a basis for a critical discussion of where the current emphasis lies in the field of interventions using AAL‐technology for older people and their informal carers, and where it might look in the future to meet the goals of healthy ageing.
We acknowledge that the WHO's five domains do not offer measures or criteria for healthy ageing “per se.” According to the Global Strategy and Action Plan on Ageing and Health (2017), there is a need to improve evaluation and measurement so as to better understand and act on healthy ageing. 123 Bosch‐Farre et al, 124 for instance, suggest a model for measuring the prevalence of active and healthy ageing. However, this aspect lies outside the primary aim and scope of this review.
6.3. Study strengths and limitations
Our results should be viewed against several study strengths and limitations. Searching the topic was problematic due to a large number of terms for technology employed by researchers. The searches were restricted to articles reporting health outcomes, thus excluding purely technical reports and technology evaluations. Updated searches, including grey literature, might produce another picture, and this needs to be taken into account when assessing the validity of the results.
In the search process, several articles were excluded as they had mixed samples of younger and older participants without differentiating results. One possible reason for such mixed samples is that many studies were diagnosis‐specific rather than focusing on age. It is, therefore, possible that the mapping of intervention categories was subsequently affected.
When adopting a dual focus on both the older person and the informal carer, there is arguably a risk of less sharpness in the analysis. However, we would argue that informal carers of older people remain marginalized in health and social care as well as in AAL technology‐mediated research. The WHO 1 framework clearly highlights the importance of promoting the rights of both older people and their informal carers. This review has identified and discussed aspects of the role of informal carers within the context of a dyadic approach that might, with a single focus on older people, have remained largely invisible to health science research. Finally, this study is unique in how it uses the framework to highlight which WHO domains of healthy ageing are interpretively present in the current field.
7. CONCLUSIONS AND IMPLICATIONS FOR RESEARCH, POLICY, AND PRACTICE
Our scoping review found that the WHO 1 framework is indeed mirrored to varying extents within the empirical literature included here. Further, findings indicate that the interventions tended to focus on an increased level of individual responsibility and also to operate at the level of the individual. These findings are relevant for policymakers when developing technology‐mediated health strategies. These findings can also be useful for health and social care professionals attempting to navigate through a growing field of interventions concerned with health promotion for older people using technology‐mediated interventions. We suggest that future research should devote greater attention to interventions addressing broader environmental factors for both older people and their informal carers, such as affordable access to safe outdoor environments, culture, healthy foods, and supporting networks and, finally, that it should adopt a dyadic approach to technology‐mediated health research.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Maria Nilsson, Stefan Andersson, Elizabeth Hanson, Lennart Magnusson
Data curation: Maria Nilsson, Stefan Andersson, Elizabeth Hanson
Formal analysis: Maria Nilsson, Stefan Andersson, Lennart Magnusson, & Elizabeth Hansson
Methodology: Maria Nilsson, Stefan Andersson, & Elizabeth Hanson
Supervision: Stefan Andersson, Lennart Magnusson, & Elizabeth Hansson
Validation: Maria Nilsson, Stefan Andersson, & Elizabeth Hanson
Visualization: Maria Nilsson & Stefan Andersson
Writing ‐ original draft preparation: Maria Nilsson & Stefan Andersson, Elizabeth Hanson
Writing ‐ review and editing: Maria Nilsson, Stefan Andersson, Lennart Magnusson, & Elizabeth Hansson
All authors have read and approved the final version of the manuscript.
Maria Nilsson, as the corresponding author, confirms having full access to all of the data and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
The lead author, Maria Nilsson, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENT
The study was accomplished while M.N. was affiliated with the Sustainable Care Research programme, led by the University of Sheffield, United Kingdom.
Nilsson MY, Andersson S, Magnusson L, Hanson E. Ambient assisted living technology‐mediated interventions for older people and their informal carers in the context of healthy ageing: A scoping review. Health Sci Rep. 2020;4:e225 10.1002/hsr2.225
Funding information Linnéuniversitetet; The Swedish Family Care Competence Centre
DATA AVAILABILITY STATEMENT
The authors confirm that all the data reported in this manuscript are derived from public domain sources, including PubMed, CINAHL, Web of Science, PsychInfo, and Scopus.
REFERENCES
- 1. Gorman M. Development and the rights of older people In: Randel J, ed. The Ageing and Development Report: Poverty, Independence and the World's Older People. London: Earthscan Publications; 1999:3‐21. [Google Scholar]
- 2. European Comission . Transformation of Health and Care in the Digital Single Market; 2019. https://ec.europa.eu/digital-single-market/en/european-policy-ehealth
- 3. WHO . Draft global strategy on digital health 2020–2024. Geneva: World Health Organization; 2020. https://www.who.int/docs/default‐source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf?sfvrsn=f112ede5_38. [Google Scholar]
- 4. WHO . World health statistics 2020. Monitoring health for the SDGs Sustainable development goals. Geneva: World Health Organization; 2020b. https://apps.who.int/iris/bitstream/handle/10665/332070/9789240005105-eng.pdf. [Google Scholar]
- 5. Eurocarers . About Carers; 2020. https://eurocarers.org/about-carers/
- 6. Eifert EK, Adams R, Dudley W, Perko M. Family caregiver identity: a literature review. Am J Health Educ. 2015;46(6):357‐367. 10.1080/19325037.2015.1099482. [DOI] [Google Scholar]
- 7. OECD . Health at a Glance. Paris, France: OECD Indicators; 2019. https://www.oecd-ilibrary.org/sites/a80d9f62-en/index.html?itemId=/content/component/a80d9f62-en. [Google Scholar]
- 8. Erlingsson C, Magnusson L, Hanson E. Family Caregivers' health in connection with providing care. Qual Health Res. 2012;22(5):640‐655. 10.1177/1049732311431247. [DOI] [PubMed] [Google Scholar]
- 9. Lyons KS, Zarit SH, Sayer AG, Whitlatch CJ. Caregiving as a dyadic process: perspectives from caregiver and receiver. J Gerontol B Psychol Sci Soc Sci. 2002;57(3):195‐204. [DOI] [PubMed] [Google Scholar]
- 10. Moon H, Adams K. The effectiveness of dyadic interventions for people with dementia and their caregivers. Dementia (London, England). 2012;12(6):821‐839. [DOI] [PubMed] [Google Scholar]
- 11. Blackman S, Matlo C, Bobrovitskiy C, et al. Ambient assisted living Technologies for Aging Well: a scoping review. J Intell Syst. 2016;25(1):55‐69. 10.1515/jisys-2014-0136. [DOI] [Google Scholar]
- 12. Iancu I, Iancu B. Elderly in the digital era. Theoretical perspectives on assistive technologies. Technologies. 2017;5(3):13 10.3390/technologies5030060. [DOI] [Google Scholar]
- 13. Singh D, Kropf J, Hanke S, Holzinger A. Ambient assisted living technologies from the perspectives of older people and professionals In: Holzinger A, Kieseberg P, Tjoa A, Weippl E, eds. Machine Learning and Knowledge Extraction. Vol 10410 Cham: Springer; 2017. 10.1007/978-3-319-66808-6_17. [DOI] [Google Scholar]
- 14. Gagnon‐Roy M, Bourget A, Stocco S, Courchesne A‐CL, Kuhne N, Provencher V. Assistive technology addressing safety issues in dementia: a scoping review. Am J Occup Ther. 2017;71(5):1‐10. 10.5014/ajot.2017.025817. [DOI] [PubMed] [Google Scholar]
- 15. Dietlein C, Eichberg S, Fleiner T, Zijlstra W. Feasibility and effects of serious games for people with dementia: a systematic review and recommendations for future research. Geron. 2018;17(1):1‐17. 10.4017/gt.2018.17.1.001.00. [DOI] [Google Scholar]
- 16. Zhang F, Kaufman D. Cognitive benefits of older adults' digital gameplay: a critical review. Geron. 2016;15(1):3‐16. 10.4017/gt.2016.15.1.002.00. [DOI] [Google Scholar]
- 17. Robbins TD, Lim Choi Keung SN, Arvanitis TN. E‐health for active ageing: a systematic review. Maturitas. 2018;114:34‐40. 10.1016/j.maturitas.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 18. Blaxter M. Health. 2nd ed. Cambridge: Polity; 2010. [Google Scholar]
- 19. Nilsson M. Våra äldre: Om konstruktioner av äldre i offentligheten. Linköping: Linköpings universitet; 2008. [Google Scholar]
- 20. Beard JR, Officer A, de Carvalho IA, et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145‐2154. 10.1016/s0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foster L, Walker A. Active and successful aging: a European policy perspective. Gerontologist. 2015;55(1):83‐90. 10.1093/geront/gnu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vega WA, Angel JL, Gutiérrez Robledo LMF, Markides KS. Contextualising Health and Aging in the Americas Effects of Space, Time and Place. Cham: Springer International Publishing; 2019. [Google Scholar]
- 23. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 24. Wilson IB, Cleary PD. Linking clinical variables with health‐related quality of life – a conceptual model of patient outcomes. JAMA. 1995;273(1):59‐65. 10.1001/jama.273.1.59. [DOI] [PubMed] [Google Scholar]
- 25. Pawson R. Evidence‐based policy: in search of a method. Evaluation. 2002;8(2):157‐181. 10.1177/1358902002008002512. [DOI] [Google Scholar]
- 26. Arena SK, Wilson CM, Peterson E. Targeted population health utilizing direct referral to home‐based older person upstreaming prevention physical therapy from a community‐based senior center. Cardiopulm Phys Therapy J. 2020;31(1):11‐21. 10.1097/CPT.0000000000000131. [DOI] [Google Scholar]
- 27. Bean JF, Brown L, DeAngelis TR, et al. The rehabilitation enhancing aging through connected health prehabilitation trial. Arch Phys Med Rehabil. 2019;100(11):1999‐2005. 10.1016/j.apmr.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 28. Chao Y‐Y, Scherer YK, Montgomery CA, Wu Y‐W, Lucke KT. Physical and psychosocial effects of Wii fit Exergames use in assisted living residents: a pilot study. Clin Nurs Res. 2015;24(6):589‐603. 10.1177/1054773814562880. [DOI] [PubMed] [Google Scholar]
- 29. Gomes GCV, Simões MDS, Lin SM, et al. Feasibility, safety, acceptability, and functional outcomes of playing Nintendo Wii fit plus(TM) for frail older adults: a randomised feasibility clinical trial. Maturitas. 2018;118:20‐28. 10.1016/j.maturitas.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 30. Hong J, Kong HJ, Yoon HJ. Web‐based Telepresence exercise program for community‐dwelling elderly women with a high risk of falling: randomized controlled trial. J Med Internet Res. 2018;20(5):e132 10.2196/mhealth.9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeon S, Kim J. Effects of augmented‐reality‐based exercise on muscle parameters, physical performance, and exercise self‐efficacy for older adults. Int J Environ Res Public Health. 2020;17(9):3260 10.3390/ijerph17093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jorgensen MG, Laessoe U, Hendriksen C, Nielsen OB, Aagaard P. Efficacy of Nintendo Wii training on mechanical leg muscle function and postural balance in community‐dwelling older adults: a randomised controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68(7):845‐852. 10.1093/gerona/gls222. [DOI] [PubMed] [Google Scholar]
- 33. Kamińska MS, Miller A, Rotter I, Szylińska A, Grochans E. The effectiveness of virtual reality training in reducing the risk of falls among elderly people. Clin Interv Aging. 2018;13:2329‐2338. 10.2147/cia.S183502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lauzé M, Martel DD, Agnoux A, et al. Feasibility, acceptability and effects of a home‐based exercise program using a gerontechnology on physical capacities after a minor injury in community‐living older adults: a pilot study. J Nutr Health Aging. 2018;22(1):16‐25. 10.1007/s12603-017-0938-8. [DOI] [PubMed] [Google Scholar]
- 35. Martel D, Lauzé M, Agnoux A, et al. Comparing the effects of a home‐based exercise program using a gerontechnology to a community‐based group exercise program on functional capacities in older adults after a minor injury. Exp Gerontol. 2018;108:41‐47. 10.1016/j.exger.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 36. Ozaki K, Kondo I, Hirano S, et al. Training with a balance exercise assist robot is more effective than conventional training for frail older adults. Geriatr Gerontol Int. 2017;17(11):1982‐1990. 10.1111/ggi.13009. [DOI] [PubMed] [Google Scholar]
- 37. Soares AV, Borges Júnior NG, Hounsell MS, Marcelino E, Rossito GM, Sagawa Júnior Y. A serious game developed for physical rehabilitation of frail elderly. Eur Res Telemed. 2016;5(2):45‐53. 10.1016/j.eurtel.2016.05.003. [DOI] [Google Scholar]
- 38. Chao YY, Scherer YK, Wu YW, Lucke KT, Montgomery CA. The feasibility of an intervention combining self‐efficacy theory and Wii fit exergames in assisted living residents: a pilot study. Geriatr Nurs. 2013;34(5):377‐382. 10.1016/j.gerinurse.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 39. McEwen D, Taillon‐Hobson A, Bilodeau M, Sveistrup H, Finestone H. Two‐week virtual reality training for dementia: single case feasibility study. J Rehabil Res Dev. 2014;51(7):1069‐1076. 10.1682/jrrd.2013.10.0231. [DOI] [PubMed] [Google Scholar]
- 40. Lafaro KJ, Raz DJ, Kim JY, et al. Pilot study of a telehealth perioperative physical activity intervention for older adults with cancer and their caregivers. Support Care Cancer. 2020;28(8):3867‐3876. 10.1007/s00520-019-05230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bemelmans R, Gelderblom GJ, Jonker P, de Witte L. Effectiveness of robot Paro in intramural psychogeriatric care: a multicenter quasi‐experimental study. J Am Med Dir Assoc. 2015;16(11):946‐950. 10.1016/j.jamda.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 42. Brimelow RE, Dawe B, Dissanayaka N. Preliminary research: virtual reality in residential aged care to reduce apathy and improve mood. Cyberpsychol Behav Soc Netw. 2020;23(3):165‐170. 10.1089/cyber.2019.0286. [DOI] [PubMed] [Google Scholar]
- 43. Chen S‐C, Moyle W, Jones C, Petsky H. A social robot intervention on depression, loneliness, and quality of life for Taiwanese older adults in long‐term care. Int Psychogeriatr. 2020;32(8):981‐991. 10.1017/S1041610220000459. [DOI] [PubMed] [Google Scholar]
- 44. Chu M‐T, Khosla R, Khaksar SMS, Nguyen K. Service innovation through social robot engagement to improve dementia care quality. Assist Technol. 2017;29(1):8‐18. 10.1080/10400435.2016.1171807. [DOI] [PubMed] [Google Scholar]
- 45. Jøranson N, Pedersen I, Rokstad AMM, Ihlebæk C. Change in quality of life in older people with dementia participating in Paro‐activity: a cluster‐randomised controlled trial. J Adv Nurs. 2016;72(12):3020‐3033. 10.1111/jan.13076. [DOI] [PubMed] [Google Scholar]
- 46. Leng FY, Yeo D, George S, Barr C. Comparison of iPad applications with traditional activities using person‐centred care approach: impact on well‐being for persons with dementia. Dementia. 2014;13(2):265‐273. 10.1177/1471301213494514. [DOI] [PubMed] [Google Scholar]
- 47. Loi SM, Mazur A, Huppert D, Hoy B, Swan J, Lautenschlager NT. A pilot study using "apps" as a novel strategy for the management of challenging behaviors seen in people living in residential care. Int Psychogeriatr. 2017;29(4):637‐643. 10.1017/s1041610216002039. [DOI] [PubMed] [Google Scholar]
- 48. Moyle W, Jones CJ, Murfield JE, et al. Use of a robotic seal as a therapeutic tool to improve dementia symptoms: a cluster‐randomized controlled trial. J Am Med Dir Assoc. 2017;18(9):766‐773. 10.1016/j.jamda.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 49. Petersen S, Houston S, Qin H, Tague C, Studley J. The utilisation of robotic pets in dementia care. J Alzheimers Dis. 2017;55(2):569‐574. 10.3233/JAD-160703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gustafsson C, Svanberg C, Müllersdorf M. Using a robotic cat in dementia care. J Gerontol Nurs. 2015;41(10):46‐56. 10.3928/00989134-20150806-44. [DOI] [PubMed] [Google Scholar]
- 51. Moyle W, Jones C, Dwan T, Petrovich T. Effectiveness of a virtual reality forest on people with dementia: a mixed methods pilot study. Gerontologist. 2018;58(3):478‐487. 10.1093/geront/gnw270. [DOI] [PubMed] [Google Scholar]
- 52. Saredakis D, Keage HAD, Corlis M, Loetscher T. Using virtual reality to improve apathy in residential aged care: mixed methods study. J Med Internet Res. 2020;22(6):15 10.2196/17632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van der Ploeg ES, Eppingstall B, O'Connor DW. Internet video chat (Skype) family conversations as a treatment of agitation in nursing home residents with dementia. Int Psychogeriatr. 2016;28(4):697‐698. 10.1017/s1041610215001854. [DOI] [PubMed] [Google Scholar]
- 54. Vahia IV, Kamat R, Vang C, et al. Use of tablet devices in the management of agitation among inpatients with dementia: an open‐label study. Am J Geriatr Psychiatry. 2017;25(8):860‐864. 10.1016/j.jagp.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 55. Sekiguchi H, Iritani S, Fujita K. Bright light therapy for sleep disturbance in dementia is most effective for mild to moderate Alzheimer's type dementia: a case series. Psychogeriatrics. 2017;17(5):275‐281. 10.1111/psyg.12233. [DOI] [PubMed] [Google Scholar]
- 56. Jupiter T. Does hearing assistive technology provide benefit to nursing home residents with dementia? A Pilot Study. J Acad Rehab Audiol. 2016;49:34‐39. [Google Scholar]
- 57. McInerney M, Walden P. Evaluating the use of an assistive listening device for communication efficiency using the Diapix task: a pilot study. Folia Phoniatr Logop. 2013;65(1):25‐31. 10.1159/000350490. [DOI] [PubMed] [Google Scholar]
- 58. Mertens A, Brandl C, Miron‐Shatz T, et al. A mobile application improves therapy‐adherence rates in elderly patients undergoing rehabilitation: a crossover design study comparing documentation via iPad with paper‐based control. Medicine (Baltimore). 2016;95(36):e4446 10.1097/md.0000000000004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Obayashi K, Kodate N, Masuyama S. Measuring the impact of age, gender and dementia on communication‐robot interventions in residential care homes. Geriatr Gerontol Int. 2020;20(4):373‐378. 10.1111/ggi.13890. [DOI] [PubMed] [Google Scholar]
- 60. Pettersson I, Hagberg L, Fredriksson C, Hermansson LN. The effect of powered scooters on activity, participation and quality of life in elderly users. Disabil Rehabil Assist Technol. 2016;11(7):558‐563. 10.3109/17483107.2015.1027301. [DOI] [PubMed] [Google Scholar]
- 61. Pripfl J, Kortner T, Batko‐Klein D, Hebesberger D, Weninger M, Gisinger C. Social service robots to support independent living: experiences from a field trial. Z Gerontol Geriatr. 2016;49(4):282‐287. 10.1007/s00391-016-1067-4. [DOI] [PubMed] [Google Scholar]
- 62. Werner C, Geravand M, Korondi PZ, Peer A, Bauer JM, Hauer K. Evaluating the sit‐to‐stand transfer assistance from a smart walker in older adults with motor impairments. Geriatr Gerontol Int. 2020;20(4):312‐316. 10.1111/ggi.13874. [DOI] [PubMed] [Google Scholar]
- 63. Dupuy L, Froger C, Consel C, Sauzéon H. Everyday functioning benefits from an assisted living platform amongst frail older adults and their caregivers. Frontiers in Aging Neuroscience. 2017;9:302 10.3389/fnagi.2017.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tchalla AE, Lachal F, Cardinaud N, et al. Preventing and managing indoor falls with home‐based technologies in mild and moderate Alzheimer's disease patients: pilot study in a community dwelling. Dement Geriatr Cogn Disord. 2013;36(3–4):251‐261. 10.1159/000351863. [DOI] [PubMed] [Google Scholar]
- 65. Ferreira Santana R, da Silva Soares T, Bruno dos Santos CT, Serra Hercules AB, da Costa Lindolpho M, Moreira Boechat YE. Tele‐care in post‐discharge follow‐up of elderly people with dementia and their caregivers: quasi‐experimental. Braz J Nurs. 2020;19(2):1‐4. 10.17665/1676-4285.20206359. [DOI] [Google Scholar]
- 66. Kamimura T, Ito H. Glycemic control in a 79‐year‐old female with mild cognitive impairment using a medication reminder device: a case report. Int Psychogeriatr. 2014;26(6):1045‐1048. 10.1017/S1041610213002408. [DOI] [PubMed] [Google Scholar]
- 67. Kamimura T. Older adults with Alzheimer's disease who have used an automatic medication dispenser for 3 or more years. Clin Gerontol. 2019;42(1):127‐133. 10.1080/07317115.2017.1347594. [DOI] [PubMed] [Google Scholar]
- 68. Lindhardt T, Nielsen MH. Older patients' use of technology for a post‐discharge nutritional intervention – a mixed‐methods feasibility study. Int J Med Inform. 2017;97:312‐321. 10.1016/j.ijmedinf.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 69. Mortenson WB, Demers L, Fuhrer MJ, Jutai JW, Lenker J, DeRuyter F. Effects of an assistive technology intervention on older adults with disabilities and their informal caregivers: an exploratory randomised controlled trial. Am J Phys Med Rehabil. 2013;92(4):297‐306. 10.1097/PHM.0b013e31827d65bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gellis ZD, Kenaley BL, Ten Have T. Integrated telehealth care for chronic illness and depression in geriatric home care patients: the integrated Telehealth education and activation of mood (I‐TEAM) study. J Am Geriatr Soc. 2014;62(5):889‐895. 10.1111/jgs.12776. [DOI] [PubMed] [Google Scholar]
- 71. Göransson C, Wengström Y, Ziegert K, Langius‐Eklöf A, Blomberg K. Self‐care ability and sense of security among older persons when using an app as a tool for support. Scand J Caring Sci. 2020;34(3):772‐781. 10.1111/scs.12782. [DOI] [PubMed] [Google Scholar]
- 72. Kao DP, Lindenfeld J, Macaulay D, et al. Impact of a telehealth and care management program on all‐cause mortality and health‐care utilisation in patients with heart failure. Telemed e‐Health. 2016;22(1):2‐11. 10.1089/tmj.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Persson HL, Lyth J, Lind L. The health diary telemonitoring and hospital‐based home care improve quality of life among elderly multimorbid COPD and chronic heart failure subjects. Int J Chron Obstruct Pulmon Dis. 2020;15:527‐541. 10.2147/copd.S236192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sun C, Sun L, Xi S, et al. Mobile phone–based telemedicine practice in older chinese patients with type 2 diabetes mellitus: randomized controlled trial. JMIR Mhealth. 2019;7(1):e10664 10.2196/10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dario C, Toffanin R, Calcaterra F, et al. Telemonitoring of type 2 diabetes mellitus in Italy. Telemed J E Health. 2017;23(2):143‐152. 10.1089/tmj.2015.0224. [DOI] [PubMed] [Google Scholar]
- 76. Maresca G, De Cola MC, Caliri S, et al. Moving towards novel multidisciplinary approaches for improving elderly quality of life: the emerging role of telemedicine in Sicily. J Telemed Telecare. 2019;25(5):318‐324. 10.1177/1357633x17753057. [DOI] [PubMed] [Google Scholar]
- 77. Nouryan CN, Morahan S, Pecinka K, et al. Home telemonitoring of community‐dwelling heart failure patients after home care discharge. Telemed e‐Health. 2018;8:447‐454. 10.1089/tmj.2018.0099. [DOI] [PubMed] [Google Scholar]
- 78. Shah MN, Wasserman EB, Wang H, et al. High‐intensity telemedicine decreases emergency department use by senior living community residents. Telemed J E Health. 2016;22(3):251‐258. 10.1089/tmj.2015.0103. [DOI] [PubMed] [Google Scholar]
- 79. Villani A, Malfatto G, Compare A, et al. Clinical and psychological telemonitoring and telecare of high risk heart failure patients. J Telemed Telecare. 2014;20(8):468‐475. 10.1177/1357633x14555644. [DOI] [PubMed] [Google Scholar]
- 80. De Cola MC, De Luca R, Bramanti A, Berte F, Bramanti P, Calabro RS. Tele‐health services for the elderly: a novel southern Italy family needs‐oriented model. J Telemed Telecare. 2016;22(6):356‐362. 10.1177/1357633x15604290. [DOI] [PubMed] [Google Scholar]
- 81. Boehm N, Muehlberg H, Stube JE. Managing Poststroke fatigue using Telehealth: a case report. Am J Occup Ther. 2015;69(6):1‐7. 10.5014/ajot.2015.016170. [DOI] [PubMed] [Google Scholar]
- 82. Castro Sweet C, Chiguluri V, Gumpina R, et al. Outcomes of a digital health program with human coaching for diabetes risk reduction in a Medicare population. J Aging Health. 2017;30(5):692‐710. 10.1177/0898264316688791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Czaja S, Boot W, Charness N, Rogers W, Sharit J. Improving social support for older adults through technology: findings from the PRISM randomized controlled trial. Gerontologist. 2018;58(3):467‐477. 10.1093/geront/gnw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. García‐Camacha A, García‐Camacha I, Martínez‐Andrés M, Notario‐Pacheco B, Rodríguez‐Martín B. Pilot testing the effectiveness of the healthy ageing supported by internet and community programme for promoting healthy lifestyles for people over 65 years of age. Scand J Caring Sci. 2020;34(3):636‐647. 10.1111/scs.12765. [DOI] [PubMed] [Google Scholar]
- 85. Mullins LB, Skemp L, Reed D, Emerson M. Internet programming to reduce loneliness and social isolation in aging. Res Gerontol Nurs. 2020;13(5):233‐242. 10.3928/19404921-20200320-01. [DOI] [PubMed] [Google Scholar]
- 86. Upton J, Kramarz A, Supey M, Gousse Y. The use of IT'S never too late technology (iN2L) in enhancing well‐being among the elderly in a residential setting. J Women Aging. 2020;32(4):481‐487. 10.1080/08952841.2020.1781508. [DOI] [PubMed] [Google Scholar]
- 87. Ferreira S, Torres A, Mealha Ó, Veloso A. Training effects on older adults in information and communication technologies considering psychosocial variables. Educ Gerontol. 2015;41(7):482‐493. 10.1080/03601277.2014.994351. [DOI] [Google Scholar]
- 88. Tsai H‐H, Cheng C‐Y, Shieh W‐Y, Chang Y‐C. Effects of a smartphone‐based videoconferencing program for older nursing home residents on depression, loneliness, and quality of life: a quasi‐experimental study. BMC Geriatr. 2020;20(1):1‐11. 10.1186/s12877-020-1426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. De Luca R, Bramanti A, De Cola MC, et al. Cognitive training for patients with dementia living in a sicilian nursing home: a novel web‐based approach. Neurol Sci. 2016;37(10):1685‐1691. 10.1007/s10072-016-2659-x. [DOI] [PubMed] [Google Scholar]
- 90. Gamito P, Oliveira J, Alves C, Santos N, Coelho C, Brito R. Virtual reality‐based cognitive stimulation to improve cognitive functioning in community elderly: a controlled study. Cyberpsychol Behav Soc Netw. 2020;23(3):150‐156. 10.1089/cyber.2019.0271. [DOI] [PubMed] [Google Scholar]
- 91. Liao YY, Tseng HY, Lin YJ, Wang CJ, Hsu WC. Using virtual reality‐based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment. Eur J Phys Rehabil Med. 2020;56(1):47‐57. 10.23736/s1973-9087.19.05899-4. [DOI] [PubMed] [Google Scholar]
- 92. Park E, Yun BJ, Min YS, et al. Effects of a mixed reality‐based cognitive training system compared to a conventional computer‐assisted cognitive training system on mild cognitive impairment: a pilot study. Cogn Behav Neurol. 2019;32(3):172‐178. 10.1097/wnn.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 93. Robert P, Manera V, Derreumaux A, et al. Efficacy of a Web App for Cognitive Training (MeMo) regarding cognitive and behavioral performance in people with neurocognitive disorders: randomized controlled trial. J Med Internet Res. 2020;22(3):e17167 10.2196/17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lazarou I, Karakostas A, Stavropoulos TG, et al. A novel and intelligent home monitoring system for care support of elders with cognitive impairment. J Alzheimers Dis. 2016;54(4):1561‐1591. 10.3233/JAD-160348. [DOI] [PubMed] [Google Scholar]
- 95. Merilampi S, Sirkka A, Leino M, Koivisto A, Finn E. Cognitive mobile games for memory impaired older adults. J Assist Technol. 2014;8(4):207‐223. 10.1108/JAT-12-2013-0033. [DOI] [Google Scholar]
- 96. Calabrò R, Luca R, Leo A, Balletta T, Marra A, Bramanti P. Lokomat training in vascular dementia: motor improvement and beyond! Aging Clin Exp Res. 2015;27(6):935‐937. 10.1007/s40520-015-0343-2. [DOI] [PubMed] [Google Scholar]
- 97. Bayen E, Jacquemot J, Netscher G, Agrawal P, Tabb Noyce L, Bayen A. Reduction in fall rate in dementia managed care through video incident review: pilot study. J Med Internet Res. 2017;19(10):e339 10.2196/jmir.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Damant J, Knapp M, Watters S, Freddolino P, Ellis M, King D. The impact of ICT services on perceptions of the quality of life of older people. J Assist Technol. 2013;7(1):5‐21. 10.1108/17549451311313183. [DOI] [Google Scholar]
- 99. Finch M, Griffin K, Pacala JT. Reduced healthcare use and apparent savings with passive home monitoring technology: a pilot study. J Am Geriatr Soc. 2017;65(6):1301‐1305. 10.1111/jgs.14892. [DOI] [PubMed] [Google Scholar]
- 100. Rohne M, Boysen ES, Ausen D. Wearable and mobile technology for safe and active living. Stud Health Technol Inform. 2017;237:133‐139. [PubMed] [Google Scholar]
- 101. Akerlind C, Martin L, Gustafsson C. eHomecare and safety: the experiences of older patients and their relatives. Geriatr Nurs. 2018;39(2):178‐185. 10.1016/j.gerinurse.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 102. Lexis M, Everink I, Van Der Heide L, Spreeuwenberg M, Willems C, De Witte L. Activity monitoring technology to support homecare delivery to frail and psychogeriatric elderly persons living at home alone. Technol Disabil. 2013;25(3):189‐197. 10.3233/TAD-130377. [DOI] [Google Scholar]
- 103. Watson P, Bearpark T, Ling J. The impact of rapid response and telecare services on elderly and vulnerable residents. Health Soc Care Community. 2020. 10.1111/hsc.13123. [DOI] [PubMed] [Google Scholar]
- 104. Fluet GG, Merians AS, Qiu Q, et al. Robotic/virtual reality intervention program individualised to meet the specific sensorimotor impairments of an individual patient: a case study. Int J Disab Hum Dev. 2014;13(3):401‐407. 10.1515/ijdhd-2014-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hirano S, Eiichi S, Tanabe S, et al. The features of gait exercise assist robot: precise assist control and enriched feedback. NeuroRehabilitation. 2017;41(1):77‐84. 10.3233/NRE-171459. [DOI] [PubMed] [Google Scholar]
- 106. Kasai R, Takeda S. The effect of a hybrid assistive limb® on sit‐to‐stand and standing patterns of stroke patients. J Phys Ther Sci. 2016;28(6):1786‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lawson S, Ziying T, Jinjuan F. Supporting stroke motor recovery through a mobile application: a pilot study. Am J Occup Ther. 2017;71(3):1‐5. 10.5014/ajot.2017.025023. [DOI] [PubMed] [Google Scholar]
- 108. Ostadabbas S, Housley SN, Sebkhi N, et al. Tongue‐controlled robotic rehabilitation: a feasibility study in people with stroke. J Rehabil Res Dev. 2016;53(6):989‐1006. 10.1682/jrrd.2015.06.0122. [DOI] [PubMed] [Google Scholar]
- 109. Knaevelsrud C, Bottche M, Pietrzak RH, Freyberger HJ, Renneberg B, Kuwert P. Integrative testimonial therapy: an Internet‐based, therapist‐assisted therapy for German elderly survivors of the World War II with posttraumatic stress symptoms. J Nerv Ment Dis. 2014;202(9):651‐658. 10.1097/nmd.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 110. Kim H, Jhoo JH, Jang JW. The effect of telemedicine on cognitive decline in patients with dementia. J Telemed Telecare. 2017;23(1):149‐154. 10.1177/1357633x15615049. [DOI] [PubMed] [Google Scholar]
- 111. Allen RS. The legacy project intervention to enhance meaningful family interactions: case examples. Clin Gerontol. 2009;32(2):164‐176. 10.1080/07317110802677005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Scherrer KS, Ingersoll‐Dayton B, Spencer B. Constructing couples' stories: narrative practice insights from a dyadic dementia intervention. Clin Soc Work J. 2014;42(1):90‐100. 10.1007/s10615-013-0440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Takter M. Vem är den enskilde i ett gemensamt hem. Malmö Högskola: Malmö; 2017. [Google Scholar]
- 114. Cottam H. Radical Help: how we Can Remake the Relationships between us and Revolutionise the Welfare State. London: Virago; 2018. [Google Scholar]
- 115. Mort M, Roberts C, Callén B. Ageing with telecare: care or coercion in austerity? Sociol Health Illn. 2013;35(6):799‐812. 10.1111/j.1467-9566.2012.01530.x. [DOI] [PubMed] [Google Scholar]
- 116. Lupton D. Digital Health: Critical and Cross‐Disciplinary Perspectives. Milton Park, Abingdon: Routledge; 2018. [Google Scholar]
- 117. Marmot M. The Health Gap: The Challenge of an Unequal World. London: Bloomsbury; 2016. [DOI] [PubMed] [Google Scholar]
- 118. Schou J, Hjelholt M. Digitalising the welfare state: citizenship discourses in Danish digitalisation strategies from 2002 to 2015. Crit Pol Stud. 2017;13:1‐20. 10.1080/19460171.2017.1333441. [DOI] [Google Scholar]
- 119. Jönson H, Harnett T. Socialt Arbete Med äldre. Stockholm: Natur & Kultur; 2015. [Google Scholar]
- 120. Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37(1):19‐24. 10.1093/ageing/afm169. [DOI] [PubMed] [Google Scholar]
- 121. Carstensen G, Rosberg B, Mc Kee KJ, Aberg AC. Before evening falls: perspectives of a good old age and healthy ageing among oldest‐old Swedish men. Arch Gerontol Geriatr. 2019;82:35‐44. 10.1016/j.archger.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 122. Principi A, Jensen PH, Lamura G. Active Ageing: Voluntary Work by Older People in Europe. Bristol, England: Policy Press; 2014. [Google Scholar]
- 123. WHO . Global Strategy and Action Plan on Ageing and Health. Geneva: World Health Organisation; 2017. [Google Scholar]
- 124. Bosch‐Farre C, Garre‐Olmo J, Bonmati‐Tomas A, et al. Prevalence and related factors of active and healthy ageing in Europe according to two models: results from the survey of health, ageing and retirement in Europe (SHARE). PLoS One. 2018;13(10):19 10.1371/journal.pone.0206353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The authors confirm that all the data reported in this manuscript are derived from public domain sources, including PubMed, CINAHL, Web of Science, PsychInfo, and Scopus.


