Abstract
Introduction
We aimed to assess differences in breast cancer risk across benign breast disease diagnosed at prevalent or incident screens.
Materials and methods
We conducted a retrospective cohort study with data from 629,087 women participating in a long-standing population-based breast cancer screening program in Spain. Each benign breast disease was classified as non-proliferative, proliferative without atypia, or proliferative with atypia, and whether it was diagnosed in a prevalent or incident screen. We used partly conditional Cox hazard regression to estimate the adjusted hazard ratios of the risk of breast cancer.
Results
Compared with women without benign breast disease, the risk of breast cancer was significantly higher (p-value = 0.005) in women with benign breast disease diagnosed in an incident screen (aHR, 2.67; 95%CI: 2.24–3.19) than in those with benign breast disease diagnosed in a prevalent screen (aHR, 1.87; 95%CI: 1.57–2.24). The highest risk was found in women with a proliferative benign breast disease with atypia (aHR, 4.35; 95%CI: 2.09–9.08, and 3.35; 95%CI: 1.51–7.40 for those diagnosed at incident and prevalent screens, respectively), while the lowest was found in women with non-proliferative benign breast disease (aHR, 2.39; 95%CI: 1.95–2.93, and 1.63; 95%CI: 1.32–2.02 for those diagnosed at incident and prevalent screens, respectively).
Conclusion
Our study showed that the risk of breast cancer conferred by a benign breast disease differed according to type of screen (prevalent or incident). To our knowledge, this is the first study to analyse the impact of the screening type on benign breast disease prognosis.
Keywords: Breast neoplasms, Early cancer detection, Benign breast disease, Risk factors
Highlights
-
•
Breast cancer risk after a benign breast disease varied with the screening type.
-
•
Incident benign breast disease had a higher breast cancer risk than prevalent.
-
•
The risk remained increased regardless of benign breast disease subtype.
1. Introduction
Benign breast disease is associated with an increased risk of breast cancer both in the clinical setting [1,2] and in population-based screening [3]. Quantification of the increased risk according to the characteristics of each lesion is constantly under study. Benign breast disease lesions are most commonly classified as non-proliferative lesions, proliferative lesions without atypia, or proliferative lesions with atypia [[4], [5], [6]]. The risk of subsequent breast cancer is higher in proliferative lesions than in non-proliferative lesions, while the risk is highest in proliferative lesions with atypia [3,7].
Benign breast disease has been proposed as a key risk factor in several breast cancer risk prediction models [[8], [9], [10], [11]]. These models are essential for the development of personalised screening strategies designed to improve the risk-benefit balance of breast cancer screening [12,13]. Therefore, it is important to fully understand differences in breast cancer risk in women diagnosed with benign breast disease.
In population-based breast cancer screening in Spain, women are invited to undergo a mammographic examination every 2 years from the age of 50–69 years. Mammographic examinations can therefore be classified as prevalent screens, ie, women’s first participation in screening mammography, and incident screens, ie, all subsequent screening participations. The results of prevalent and incident screens differ in several screening outcomes, such as a higher detection rate and a higher recall rate at prevalent screens [14], resulting in higher sensitivity and lower specificity [15,16]. However, breast cancer diagnosed at prevalent screens has been shown to be less aggressive and to have slower growth [17,18]. Even so, no studies have evaluated the risk of breast cancer associated to bening breast disease according to type of examination (incident or prevalent). We hypothesised that benign breast disease diagnosed in a prevalent screen confer a lower risk of subsequent breast cancer than that diagnosed in incident screens, regardless of the benign breast disease subtype.
Using data from a long-standing population-based screening program in Spain, we aimed to assess differences in the risk of breast cancer after diagnosis of benign breast disease according to the screening type and histological subtype of benign breast disease.
2. Materials and methods
2.1. Study design and participants
The Spanish Breast Cancer Screening Program follows the recommendations of the European Guidelines for Quality Assurance in Breast Cancer Diagnosis [19]. At age 50 years, women are invited to undergo 2-dimensional digital screening mammography. Two projections (mediolateral-oblique and craniocaudal views) are interpreted according to the Breast Imaging Reporting and Data System (BI-RADS) [20] scale by trained breast radiologists. Women with abnormal mammographic findings are recalled for further assessments to confirm or rule out malignancy. Women without a breast cancer diagnosis are invited back for routine screening at 2 years.
We analysed data from seven centres in the Spanish Screening program, which routinely gathers information on benign breast disease diagnoses. The study population included 632,299 women who underwent at least one screening mammogram between 1994 and 2015 and who were followed up until December 2017. Due to the longitudinal nature of the study, women with breast cancer diagnosed in their first screen (n = 3,212) were excluded from the analyses as there was no time for follow-up, leaving left 629,087 women for the analysis.
2.2. Procedures
All women with screening mammograms scored BIRADS 0, 3, 4 or 5 were recalled for further assessments. If cancer could not be ruled out with non-invasive procedures when women were attending recall, core-needle or open biopsy was performed. All biopsies were examined and classified by hospital pathologists in each screening centre. All biopsies with a non-malignant classification were classified as benign breast disease. Following the criteria of Page et al. and Dupont et al. [[4], [5], [6]], we classified benign breast diseases as: non-proliferative, proliferative without atypia, and proliferative with atypia. Only women with asymptomatic benign breast disease diagnosed at screening were included in the study.
Both cancers detected at routine screening and interval cancers (those diagnosed within 24 months after a negative screening episode and before the next screening invitation) were included in the study regardless of whether they were invasive or in situ. Interval cancers were identified by merging population-based cancer registries and hospital-based cancer registries with data from screening participants. Benign breast disease identified at the same time as cancers were excluded from the benign breast disease group.
2.3. Analysis
We used the chi-squared test to compare proportions of different variables among those women without a benign breast disease diagnosis, those with a diagnosis in a prevalent screen and those with a diagnosis in an incident screen.
We calculated incident breast cancer rates using person-years at risk for women with and without a diagnosis of benign breast disease. Women without benign breast disease contributed person-years at risk from the date of the first screening mammogram until breast cancer diagnosis (screen-detected or interval cancer), benign breast disease diagnosis, or 2 years after the last mammographic examination, whichever came first. Women with benign breast disease contributed person-years at risk from the date of benign breast disease diagnosis until breast cancer diagnosis or 2 years after last screening examination, whichever occurred first.
We used a partly conditional Cox proportional hazards model to estimate the adjusted hazard ratios (aHR) and the 95% confidence intervals (95%CI) for the risk of breast cancer by screening type and benign breast disease subtype. These models are an extension of the Cox hazards model for repeated measures, which allowed us to update the changes in benign breast disease status during the study period. All analyses were adjusted for age and calendar year. We adjusted for age because women with benign breast disease diagnosed in a prevalent screen were expected to be younger than those diagnosed in incident screens. Adjustment by calendar year was included to capture possible differences in benign breast disease diagnosis techniques and classification during the study period. An interaction between screening type and benign breast disease subtype was tested; the interaction was found to be non-significant and was consequently not included in the final models (p for interaction = 0.83). Robust standard errors were used to estimate 95% confidence intervals. The proportional hazards assumption was assessed by plotting the log-minus-log of the survival function against log time for each predictive variable. The proportional hazards assumption was reasonable for all predictors.
We plotted the adjusted cumulative incidence curves by estimating the age- and calendar-year adjusted risk of cancer development of the average woman in each category with the partly conditional Cox model. Statistical tests were two-sided and all p-values <0.05 were considered statistically significant. All analyses were performed using the statistical software R version 3.5.0 (Development Core Team, 2014).
3. Results
We analysed information from 629,087 women who underwent 2,327,384 mammographic examinations between January 1994 and December 2015. During the study period, 9431 cases of breast cancer and 9184 cases of benign breast disease were diagnosed. We found no differences in the distribution of benign breast disease subtypes across incident and prevalent screens (p = 0.48) (Table 1). The proportion of breast cancer cases was significantly lower in women without benign breast disease than in those with benign breast disease (1.5% vs 2.7%, p < 0.001). Among women with a diagnosis of benign breast disease, the proportion of breast cancer cases was higher in women diagnosed in an incident screen than in those diagnosed in a prevalent screen (3.0% vs 2.4%, p = 0.07).
Table 1.
Characteristics of the study population.
| No benign breast disease N = 619,864 | Benign breast disease diagnosed in a prevalent screen N = 5,049 | Benign breast disease diagnosed in an incident screen N = 4,174 | |
|---|---|---|---|
| Age at first screen | |||
| 50-54 | 360,020 (58.1%) | 3,420 (67.7%) | 2,687 (64.4%) |
| 55-59 | 123,994 (20.0%) | 758 (15.0%) | 936 (22.4%) |
| 60-64 | 100,956 (16.3%) | 637 (12.6%) | 499 (12.0%) |
| 65-69 | 34,894 (5.6%) | 234 (4.6%) | 52 (1.2%) |
| Year of first screen | |||
| <2005 | 299,173 (48.3%) | 2,111 (41.8%) | 2,856 (68.4%) |
| 2005–2010 | 167,747 (27.1%) | 1,220 (24.2%) | 1,038 (24.9%) |
| >2010 | 152,944 (24.7%) | 1,718 (34%) | 280 (6.7%) |
| Type of benign breast disease | |||
| No benign breast disease | 619,864 (100%) | 0 (0.0%) | 0 (0.0%) |
| Non-proliferative | 0 (0.0%) | 3,948 (78.2%) | 3,282 (78.6%) |
| Proliferative without atypia | 0 (0.0%) | 877 (17.4%) | 728 (17.4%) |
| Proliferative with atypia | 0 (0.0%) | 224 (4.4%) | 164 (3.9%) |
| Breast Cancer | |||
| No | 610,680 (98.5%) | 4,928 (97.6%) | 4,048 (97%) |
| Si | 9,184 (1.5%) | 121 (2.4%) | 126 (3.0%) |
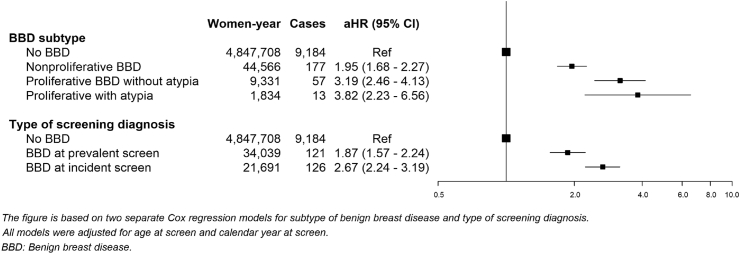
Women with benign breast disease had a higher risk of breast cancer than those without benign breast disease, regardless of the subtype of benign breast disease. The highest risk was found in women with proliferative benign breast disease with atypia (aHR, 3.82; 95%CI: 2.23–6.56), followed by those with proliferative benign breast disease without atypia (aHR, 3.19; 95%CI: 2.46–4.13) and those with non-proliferative benign breast disease (aHR, 1.95; 95%CI: 1.68–2.27) (Fig. 1). In addition, among women with benign breast disease, risk was higher in those diagnosed in an incident screen than in those diagnosed in a prevalent screen (aHR, 2.67; 95%CI: 2.24–3.19, and aHR, 1.87; 95%CI: 1.57–2.24, respectively).
Fig. 1.
Adjusted hazard ratios (aHR) of breast cancer incidence in women with benign breast disease compared with women with negative screening tests.
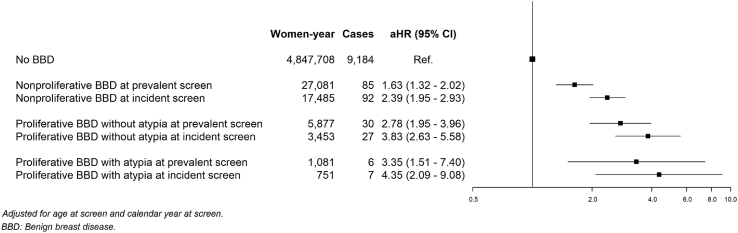
The hazard ratios associated within each combination of screening type and benign breast disease subtype are shown in Fig. 2. Across benign breast disease subtypes, those diagnosed in an incident screen conferred a higher risk than those diagnosed in prevalent screens, although not statistically significant for al subtypes. Compared with women without a benign breast disease, the highest risk was found in those women with a proliferative benign breast disease with atypia (aHR, 4.35; 95%CI: 2.09–9.08, and 3.35; 95%CI: 1.51–7.40 for those diagnosed at incident and prevalent screens, respectively, p-value for comparison; p = 0.634), followed by women with proliferative benign breast disease without atypia (aHR, 3.83; 95%CI: 2.63–5.58, and 2.78; 95%CI: 1.95–3.96 for those diagnosed at incident and prevalent screens, respectively; p-value for comparison p = 0.223). The lowest was found in women with non-proliferative benign breast disease (aHR, 2.39; 95%CI: 1.95–2.93, and 1.63; 95%CI: 1.32–2.02 for those diagnosed at incident and prevalent screens, respectively, p-value for comparison; p = 0.011).
Fig. 2.
Adjusted hazard ratios (aHR) of breast cancer incidence in women with benign breast disease compared with women with negative screening tests testing the combined effect of type of benign breast disease, and round at benign breast disease diagnosis.
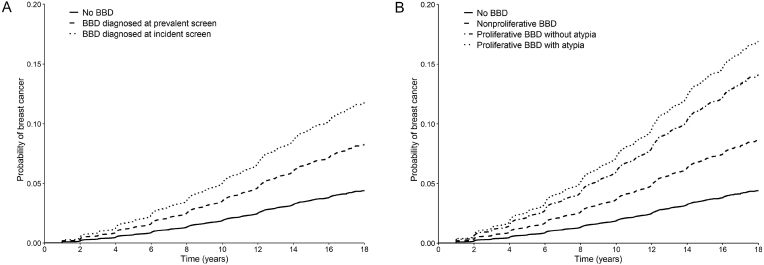
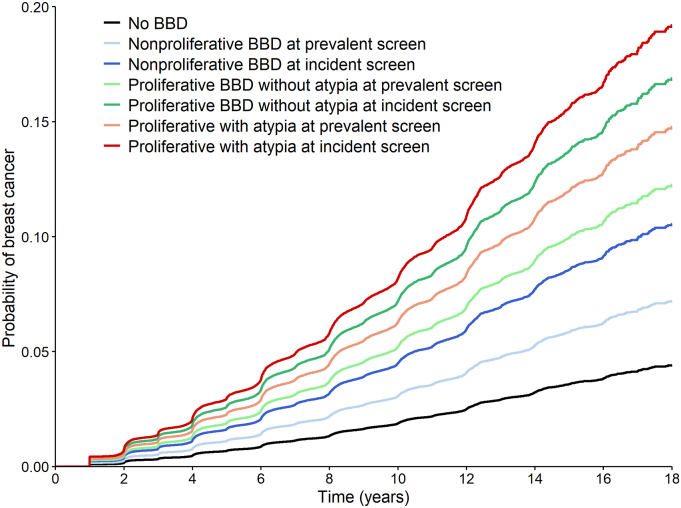
We examined the adjusted cumulative incident curves of breast cancer across the different classifications of benign breast disease and screening types. The probability of breast cancer diverged over time. The average 10-year breast cancer probability of women without a benign breast disease diagnosis was 1.9%. Among women with benign breast disease, the probability was higher in women diagnosed in an incident screen than in those diagnosed in a prevalent screen (average 10-year probability of breast cancer 4.9% vs 3.5%). Among benign breast disease subtypes, the highest probability of cancer was found in women with a proliferative benign breast disease with atypia followed by those with proliferative benign breast disease without atypia and those with a non-proliferative benign breast disease (average 10-year breast cancer probability 6.9%, 5.8% and 3.6%, respectively) (Fig. 3). The highest probability was found in women with proliferative benign breast disease diagnosed in an incident screen, with an average 10-year probability of breast cancer of 7.8% (Fig. 4).
Fig. 3.
Adjusted survival curves for breast cancer incidence based on Cox proportional hazards model for women with benign breast disease vs women with negative screening tests. Fig. 3 a. Solid line represents negative screening test group; dashed line represents benign breast disease diagnosed at prevalent round, dotted line benign breast disease diagnosed in incident round. Fig. 3 b. Solid line represents negative screening test group; dashed line represents nonproliferative benign breast disease, dashdotted line represent proliferative benign breast disease without atypia, dotted line represents proliferative benign breast disease with atypia.
Fig. 4.
Adjusted survival curves for breast cancer incidence based on Cox proportional hazards model for women with benign breast disease vs women with negative screening tests.
4. Discussion
In this study of more than 600,000 women with follow-up for more than 20 years, we found that a diagnosis of benign breast disease in an incident screen conferred a higher risk of subsequent breast cancer than diagnosis in a prevalent screen, regardless of the histological subtype. These findings highlight the importance of considering the screening type when benign breast disease was diagnosed in risk of breast cancer estimation. To our knowledge, this is the first study to include screening type in the assessment of the impact of benign breast disease on the risk of subsequent breast cancer.
Over the past decades, multiple studies have assessed the relationship between benign breast disease and the risk of breast cancer [[2], [3], [4], [5], [6]]. Particular efforts have been made to assess the association of this risk with the various benign breast disease subtypes [[4], [5], [6]]. As seen in previous reports, our study showed that women with benign breast disease had an increased risk of breast cancer [2]. Consistently, we found that the risk of breast cancer was highest among women with a proliferative benign breast disease with atypia, while proliferative benign breast disease without atypia conferred a higher risk than non-proliferative benign breast disease [3]. Although the reduced sample size for some BBD subtypes led to non-significant differences in some subgroup comparisons, we found that the difference in risk across benign breast disease subtypes remained proportional within prevalent and incident screens and was systematically higher in those BBD diagnosed in incident screens. This finding is particularly relevant since 55% of benign breast diseases are diagnosed in prevalent screens. Screening type therefore provides key information for risk prediction in benign breast disease. Unless this information is included, the risk attributed to benign breast disease diagnosed in prevalent screens could be overestimated, and that for incident screens could be underestimated.
Previous studies performed in the last decade have assessed differences in breast cancer screening outcomes, such as cancer detection rates and false positive rates, by screening type [[14], [15], [16]]. Moreover, previous authors found differences in breast cancer characteristics depending on whether the cancers were diagnosed in a prevalent or incident screen [17,18], suggesting that latent cancers diagnosed in prevalent screens have a slower growth pattern. The lower risk of breast cancer observed in women with benign breast disease diagnosed in prevalent screens might be partially explained by a slower growth pattern in prevalent benign breast disease. Age may play an important role in this effect, since women with benign breast disease diagnosed in incident screens are, on average, older than those diagnosed in a prevalent screen. However, to control for this potential confounding effect, we adjusted all analyses by age and calendar year.
The results of this study may have implications for clinical decisions on the follow-up of women with a diagnosis of benign breast disease, in which distinct follow-up strategies may be recommended depending on the benign breast disease subtype and screening type at diagnosis. The findings may also have implications for individualised risk prediction. The Breast Cancer Surveillance Consortium model [21] is being used in a large international randomised clinical trial to define the individual risk of the population targeted for breast cancer screening with a view to offering them personalised screening strategies [22]. Our findings reveal that screening type explained part of the risk associated with benign breast disease. Taking this variability into account could help to improve the discriminatory power of breast cancer risk prediction models, which is commonly moderate [11,23]. In addition, we found that women with proliferative benign breast disease diagnosed in an incident screen had a 4-fold higher risk of developing breast cancer than those without. This information, after further analysis with adjustment for other risk factors such as breast density [24], family history [25], or a risk score using information from single nucleoid polymorphisms [26], may be key to defining risk groups that could benefit from tailored screening strategies.
This study has some limitations. First, the number of cancers detected after proliferative benign breast disease with atypia was small because this subtype is uncommon, which limited our capacity to perform some subgroup analyses. Second, there is a possible bias produced by temporary changes both in the benign breast disease classification and in biopsy techniques (because fine-needle aspiration cytology has become practically obsolete). This bias is partially controlled as we adjusted our analysis by calendar year. Third, to be able to classify benign breast disease, we restricted our study to those benign breast diseases with available information on their histological subtype. Last, it was not possible to adjust the statistical analysis on other breast cancer risk factors such as breast density or familial history of breast cancer.
A strength of this study is that we analysed a large cohort of more than 600,000 women screened in a well-established population-based screening program with a 20-year follow-up. This is one of the largest cohorts analysing histopathologically confirmed benign breast disease, with nearly 10,000 diagnoses during follow-up.
In summary, our study shows that, regardless of the type of benign breast disease, women with benign breast disease diagnosed in an incident screen have a significantly higher subsequent risk of breast cancer than those with a benign breast disease diagnosed in a prevalent screen. To our knowledge, this is the first study to analyse this topic. It is important to consider this risk when developing risk-based personalised screening strategies.
Funding
This study was supported by grants from Instituto de Salud Carlos III FEDER (grant numbers: PI15/00098 and PI17/00047), and by the Research Network on Health Services in Chronic Diseases (RD12/0001/0015).
Ethics approval and consent to participate
The study was approved by the Clinic Research Ethics Committee of Hospital del Mar Medical Research Institute (2015/6189/I). The review boards of the institutions providing data granted approval for data analyses. This is an entirely register based study that used anonymised retrospective data and hence written consent was not required.
Declaration of competing interest
None declared.
Acknowledgments
The authors acknowledge the dedication and support of the entire Benign Lesion (BELE) and Individualised Risk (IRIS) Study Groups listed here in alphabetical order and grouped by institution: (a) IMIM (Hospital Del Mar Medical Research Institute), Barcelona, Spain: Rodrigo Alcantara, Andrea Burón, Xavier Castells, Laia Domingo, Javier Louro, Margarita Posso, Ana Rodríguez-Arana, Marta Román, Maria Sala, Ignasi Tusquets, Ivonne Vazquez, Mar Vernet-Tomas; (b) Corporació Sanitària Parc Taulí, Sabadell, Spain: Marisa Baré, Javier del Riego; (c) Catalan Institute of Oncology, Barcelona, Spain: Llucia Benito, Carmen Vidal (d) Hospital Santa Caterina, Girona, Spain: Joana Ferrer; (e) Catalan Institute of Oncology, Girona, Spain: Rafael Marcos-Gragera; (f) Hospital de la Santa Creu i Sant Pau, Barcelona, Spain: Judit Solà-Roca, María Jesús Quintana; (g) General Directorate of Public Health, Government of Cantabria, Spain: Mar Sánchez; (h) Principality of Asturias Health Service, Spain: Miguel Prieto; (i) Fundació Lliga per a La Investigació i Prevenció Del Cáncer, Tarragona, Spain: Francina Saladié, Jaume Galceran; (j) Hospital Clinic, Barcelona, Spain; Xavier Bargalló, Isabel Torá-Rocamora; (k) Vallés Oriental Breast Cancer Early Detection Program, Spain; Lupe Peñalva; (l) Catalonian Cancer Strategy, Barcelona, Spain: Josep Alfons Espinàs. Javier Louro is a Ph.D. candidate at the Methodology of Biomedical Research and Public Health program, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain.
References
- 1.Carter C.L., Corle D.K., Micozzi M.S., Schatzkin A., Taylor P.R. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol. 1988;128(3):467–477. doi: 10.1093/oxfordjournals.aje.a114995. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann L.C., Sellers T.A., Frost M.H., Lingle W.L., Degnim A.C., Ghosh K. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 3.Castells X., Domingo L., Corominas J.M., Tora-Rocamora I., Quintana M.J., Bare M. Breast cancer risk after diagnosis by screening mammography of nonproliferative or proliferative benign breast disease: a study from a population-based screening program. Breast Canc Res Treat. 2015;149(1):237–244. doi: 10.1007/s10549-014-3208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page D.L., Dupont W.D., Rogers L.W., Rados M.S. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55(11):2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Dupont W.D., Page D.L. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgibbons P.L., Henson D.E., Hutter R.V. Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med. 1998;122(12):1053–1055. [PubMed] [Google Scholar]
- 7.Dyrstad S.W., Yan Y., Fowler A.M., Colditz G.A. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Canc Res Treat. 2015;149(3):569–575. doi: 10.1007/s10549-014-3254-6. [DOI] [PubMed] [Google Scholar]
- 8.Tyrer J., Duffy S.W., Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 9.Gail M.H., Brinton L.A., Byar D.P., Corle D.K., Green S.B., Schairer C. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 10.Tice J.A., Cummings S.R., Smith-Bindman R., Ichikawa L., Barlow W.E., Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louro J., Posso M., Hilton Boon M., Roman M., Domingo L., Castells X. A systematic review and quality assessment of individualised breast cancer risk prediction models. Br J Canc. 2019;121(1):76–85. doi: 10.1038/s41416-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilaprinyo E., Forne C., Carles M., Sala M., Pla R., Castells X. Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0086858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onega T., Beaber E.F., Sprague B.L., Barlow W.E., Haas J.S., Tosteson A.N. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer. 2014;120(19):2955–2964. doi: 10.1002/cncr.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanks R.G., Given-Wilson R., Alison R., Jenkins J., Wallis M.G. An analysis of 11.3 million screening tests examining the association between needle biopsy rates and cancer detection rates in the English NHS Breast Cancer Screening Programme. Clin Radiol. 2019;74(5):384–389. doi: 10.1016/j.crad.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Domingo L., Hofvind S., Hubbard R.A., Roman M., Benkeser D., Sala M. Cross-national comparison of screening mammography accuracy measures in U.S., Norway, and Spain. Eur Radiol. 2016;26(8):2520–2528. doi: 10.1007/s00330-015-4074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofvind S., Geller B., Vacek P.M., Thoresen S., Skaane P. Using the European guidelines to evaluate the Norwegian breast cancer screening program. Eur J Epidemiol. 2007;22(7):447–455. doi: 10.1007/s10654-007-9137-y. [DOI] [PubMed] [Google Scholar]
- 17.Anderson T.J., Lamb J., Alexander F., Lutz W., Chetty U., Forrest A.P. Comparative pathology of prevalent and incident cancers detected by breast screening. Edinburgh Breast Screening Project. Lancet. 1986;1(8480):519–523. doi: 10.1016/s0140-6736(86)90882-2. [DOI] [PubMed] [Google Scholar]
- 18.Paci E., Ponti A., Zappa M., Patriarca S., Falini P., Delmastro G. Early diagnosis, not differential treatment, explains better survival in service screening. Eur J Canc. 2005;41(17):2728–2734. doi: 10.1016/j.ejca.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Perry N., Broeders M., de Wolf C., Tornberg S., Holland R., von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition--summary document. Ann Oncol. 2008;19(4):614–622. doi: 10.1093/annonc/mdm481. [DOI] [PubMed] [Google Scholar]
- 20.Sickles E.A., D’Orsi C.J., Bassett L.W. ACR BI-RADS® atlas, breast imaging reporting and data System. American College of Radiology; Reston, VA: 2013. ACR BI-RADS® mammography. [Google Scholar]
- 21.Tice J.A., Miglioretti D.L., Li C.S., Vachon C.M., Gard C.C., Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137–3143. doi: 10.1200/JCO.2015.60.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MyPeBS . 2017. Randomized comparison of risk-stratified versus standard breast cancer screening in European women aged 40-70 (MyPeBS) [Internet]www.brumammo.be/.../bmm-my-pebs-clinical-trial-protocol.pdf Available from: [Google Scholar]
- 23.McCarthy A.M., Guan Z., Welch M., Griffin M.E., Sippo D.A., Deng Z. Performance of breast cancer risk assessment models in a large mammography cohort. J Natl Cancer Inst. 2019;112(5):489–497. doi: 10.1093/jnci/djz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd N.F., Guo H., Martin L.J., Sun L., Stone J., Fishell E. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou A.C., Easton D.F. Risk prediction models for familial breast cancer. Future Oncol. 2006;2(2):257–274. doi: 10.2217/14796694.2.2.257. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Rice M., Tworoger S.S., Rosner B.A., Eliassen A.H., Tamimi R.M. Addition of a polygenic risk score, mammographic density, and endogenous hormones to existing breast cancer risk prediction models: a nested case-control study. PLoS Med. 2018;15(9) doi: 10.1371/journal.pmed.1002644. [DOI] [PMC free article] [PubMed] [Google Scholar]