Abstract
A left ventricle pseudoaneurysm (LV PSA) is defined as a free wall rupture of the left ventricle contained by the adjacent pericardial tissue. This rare complication is most commonly encountered following myocardial infarction, trauma, or infection. Surgery is typically warranted to avoid progression to spontaneous rupture, which may potentially lead to cardiac tamponade and death. Cardiac magnetic resonance imaging is the modality of choice to characterize left ventricle morphology and function. Accurate distinction between a pseudoaneurysm and a true aneurysm is crucial, since management and prognosis are significantly different between these 2 entities. We present a case of a 63-year-old male heart transplant recipient, admitted for suspicion of acute cellular rejection, with an unexpected finding of a LV PSA.
Keywords: Left ventricle pseudoaneurysm, Cardiac magnetic resonance, Acute cellular rejection, Heart transplant, Myocardial infarction, Left ventricular aneurysm
Introduction
Left ventricle pseudoaneurysm (LV PSA) is defined as an outpouching formed by myocardial free wall rupture, with the extravasated contents contained by the adjacent pericardium and scar tissue [1], [2], [3], [4], [5]. LV PSAs are rare entities, most commonly attributed to free wall rupture of myocardial tissue due to infarction. Other causes include cardiac infection, cardiac surgery, and trauma. LV PSAs are seen after myocardial infarction in 0.2%-0.3% of cases, most commonly after large infarcts in elderly and male patients. The prognosis of an untreated cardiac pseudoaneurysm is poor, with high rupture rates, particularly in the early period following myocardial infarction [2]. By definition, pseudoaneurysms do not contain all layers of myocardial tissue, as opposed to true aneurysms, and must be differentiated for appropriate management.
Clinical findings are usually nonspecific, such as chest pain, congestive heart failure, thromboembolic events, and arrhythmias. Sudden death is the least frequent presentation. A new to-and-fro murmur and electrocardiogram abnormalities are frequently seen [2]. Since the presentation is often nonspecific, a high degree of clinical suspicion is necessary to diagnose LV PSA.
Diagnostic imaging is crucial to establish the diagnosis and guide appropriate treatment. Cardiac magnetic resonance (MR) and computed tomography are mainstays in anatomic characterization and differentiation from other etiologies such as a true left ventricular aneurysm. There are numerous imaging features described in the literature to assist in the accurate diagnosis and differentiation of LV PSA [4].
We present a case of a 63-year-old male with a prior orthotropic heart transplant admitted for suspicion of acute rejection, with an unexpected finding of a LV PSA.
Case Report
A 63-year-old male heart transplant recipient was admitted as a transfer from an outside hospital for suspicion of acute cellular rejection. The patient initially presented with general malaise, nausea, and vomiting for a week. At the outside hospital, he was found to have diabetic ketoacidosis, for which treatment with insulin infusion was instituted, and the patient ultimately developed anuric renal failure. Episodes of severe bradycardia were also reported, which did not require intervention and resolved with correction of acidosis and electrolyte abnormalities. The patient was then transferred for escalation of care and suspicion of acute cellular rejection. At admission, he reported substance abuse and noncompliance with immunosuppressor medications. Besides a heart transplant ten years before presentation, his past medical history also included coronary artery disease, diabetes mellitus, dyslipidemia, hypertension, and chronic kidney disease.
Initial workup with echocardiography reported moderate to severe left ventricle hypertrophy. It also showed an outpouching at the apical segment of the left ventricle, which was connected to its cavity by a narrow neck. Color flow Doppler demonstrated a bidirectional shunt through the discontinuity at the apical segment of the left ventricle, further suggesting free wall rupture with pseudoaneurysm formation (Fig. 1A, B). A small amount of fluid and clot were noted in the pericardium without clear signs of cardiac tamponade (Fig. 2A, B).
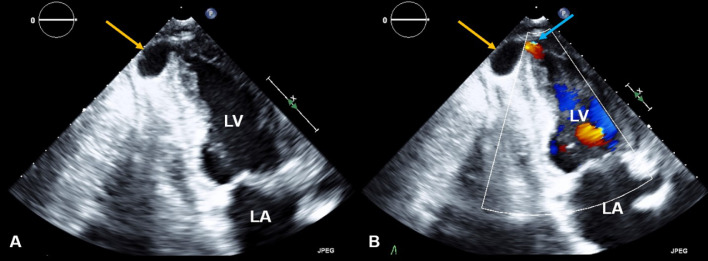
Fig. 1.
LV PSA on echocardiography. Echocardiography apical 2-chamber views 2D mode (A) and color flow Doppler (B) shows an apical pseudoaneurysm (yellow arrows) with color flow present (blue arrow). There is moderate to severe left ventricle hypertrophy with normal ventricular size.
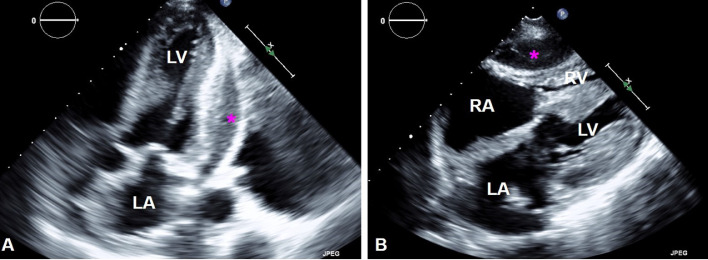
Fig. 2.
Pericardial fluid on echocardiography. Apical 2-chamber view (A) shows a small amount of pericardial fluid (pink stars). Subcostal view (B) shows the pericardial effusion exerting compression over the right ventricle.
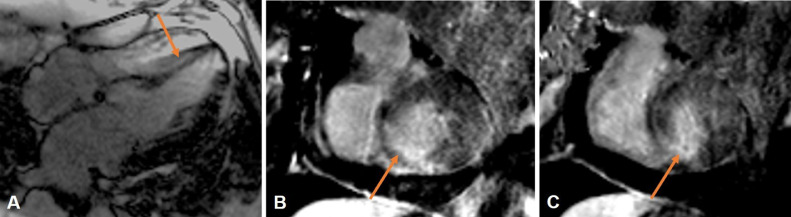
Further assessment with cardiac MR again demonstrated a large left ventricular apical outpouching with active extravasation through a narrow myocardial opening. The neck's maximal width was 7 mm, and the outpouching sac measured 48 mm in maximal diameter, with a neck to sac ratio of less than 0.5 (0.15), which is strongly suggestive of a pseudoaneurysm (Fig. 3A, B). Additionally, cardiac MR images demonstrated discontinuity of the myocardial wall, with greater than 50% decrease in the aneurysm sac wall thickness measured at 1 cm from the aneurysmal neck, another characteristic that supports LV PSA diagnosis. Moderate left ventricular hypertrophy was noted along with mild enlargement of the left ventricle and preservation of systolic function (ejection fraction of 61%). Moderate pericardial effusion was confirmed with mass effect upon the free wall of the right ventricle (Fig. 4A, B). Late gadolinium-enhanced images showed subendocardial enhancement of the mid to basal inferolateral septal wall, involving greater than 50% myocardial thickness, associated with myocardial thinning, reflecting scar from remote infarct and nonviability (Fig. 5A-C).
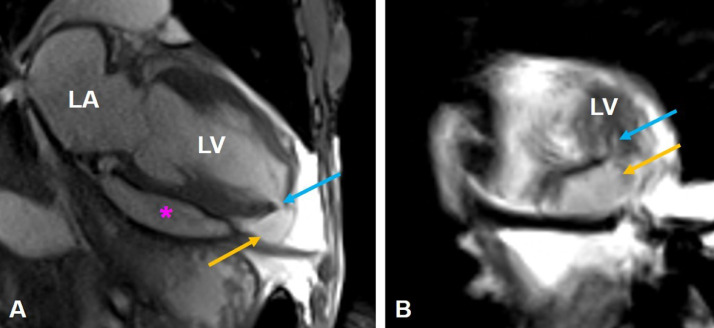
Fig. 3.
LV PSA on cardiac MR. Long-axis 2-chamber SSFP (A) shows an apical left ventricular pseudoaneurysm arising from the inferior aspect of the left ventricular apex (yellow arrows). The neck of the pseudoaneurysm (blue arrows) measured approximately 7 mm. The pseudoaneurysm sac measured approximately 2.5 × 4.8 cm. There is moderate pericardial effusion (pink star).
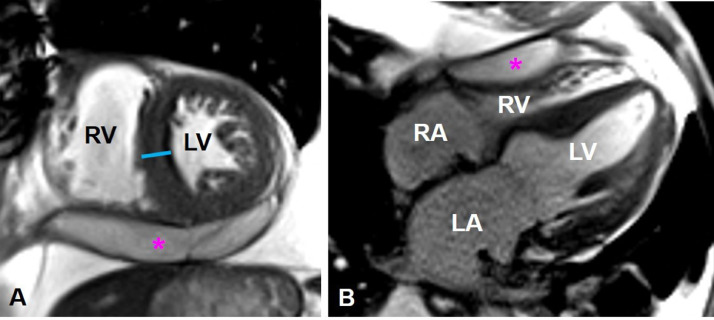
Fig. 4.
Left ventricular myocardial hypertrophy and pericardial effusion. Cardiac MR short-axis (A) and long-axis 4-chamber SSFP (B) show moderate left ventricle hypertrophy (blue line). There is moderate pericardial effusion (pink star) exerting mass effect upon the free wall of the right ventricle.
Fig. 5.
Late gadolinium enhancement. Cardiac MR late gadolinium-enhanced long-axis 4-chamber (A), and short-axis views (B, C) demonstrate myocardial thinning and late gadolinium enhancement of the basal to the mid inferoseptal wall of the left ventricle.
The patient was previously admitted for acute cellular rejection grade 2 R 1 month prior to this presentation. At that time, echocardiography depicted left ventricular hypertrophy with reduced function without additional morphologic abnormalities. He was treated with thymoglobulin for four days, and repeated biopsy showed improvement of his rejection to grade 1 R. At the current admission, repeated right ventricle biopsy was negative for acute cellular rejection (0R), despite continued intermittent refusal of medications and treatment modalities.
After a multidisciplinary meeting and thorough discussion of the patient's clinical and radiologic findings, the exact etiology of the patient's LV PSA remained uncertain. Cardiac MR demonstrated signs of previous myocardial infarction, including myocardial thinning and late gadolinium enhancement of the basal and mid inferoseptal wall, however there was no clear evidence of extension to the left ventricular apex. The cardiothoracic surgery team decided not to proceed with correction of the left ventricle pseudoaneurysm given the patient's high-risk clinical status, including history of acute graft rejection, thrombocytopenia, and reduced functional capacity. During admission, the patient's renal function and functional status progressively deteriorated, and he was ultimately referred to palliative care.
Discussion
LV PSA is defined as a free rupture of the myocardial wall contained by pericardial adhesion. This rare pathology is a complication that follows myocardial injury, most commonly myocardial infarction [1], [2], [3], [4], [5]. Clinically, findings are nonspecific, ranging from mild dyspnea to heart failure, and sudden cardiac death. Untreated pseudoaneurysms have a high risk of rupture, which remains even several years after diagnosis [4,6].
Various imaging modalities have been used to assess LV PSA. Historically, angiography was considered the modality of choice; however, currently, noninvasive techniques are preferred in most cases. On chest radiographs, a mass or abnormal contour of the heart may be seen, although the most common finding reported is simply heart enlargement [6]. Peripheral calcification of the pseudoaneurysm sac may be identified at later stages. On transthoracic echocardiography, LVA PSA usually presents as a focal outpouching with a narrow neck connecting the saccular pseudoaneurysm to the ventricular cavity. Color Doppler may aid in diagnosis by depicting aliasing and to-and-fro flow through the neck of the pseudoaneurysm. Additional findings may include pericardial effusion with variable degrees of echogenicity reflecting blood products and thrombosis [5,7,8].
Computed tomography (CT) provides excellent spatial resolution with accurate identification and morphologic assessment of the pseudoaneurysm sac. Additional findings may include pericardial effusion with variable attenuation values, chest parenchymal abnormalities characteristic of heart failure, and thromboembolic events [9].
Cardiac MR imaging is a valuable non-invasive technique that allows anatomic and functional characterization of the left ventricle. LV PSA typically shows loss of epicardial fat signal at its orifice. Cine cardiac MR may demonstrate myocardial wall dyskinesia and blood flow turbulence in the cardiac chambers and through the myocardial opening. Delayed gadolinium enhancement images may indicate late enhancement of pericardial tissue adjacent to the pseudoaneurysm sac in addition to the expected findings related to the patient's baseline etiology (eg, myocardial infarct, trauma, infection). Cardiac MR also presents an advantage in the assessment of thrombus, a commonly encountered complication of LV PSAs [3], [4], [5].
The primary differential diagnosis of LV PSAs is a true left ventricle aneurysm (LVA). In most LV PSA cases, surgery is warranted to avoid catastrophic outcomes such as spontaneous rupture with progression to pericardial tamponade and death. In contrast, true aneurysms have a better prognosis and may be treated conservatively in most cases [1], [2], [3], [4], [5], [6]. Thus, accurate characterization and differentiation between these two entities are crucial for appropriate management and prognosis.
Previous studies have described distinct imaging features to differentiate between true and false aneurysms of the left ventricle. One of the first documented imaging features described was localization and morphology. True LVAs typically have a wide neck, are often apical, may contain thrombus, and are rarely associated with pericardial enhancement. Pseudoaneurysms classically have a narrow neck, are most commonly inferior or lateral in location, often contain thrombus, and are commonly associated with pericardial enhancement. Some studies suggest an orifice-pseudoaneurysm diameter ratio of <0.5 indicates a pseudoaneurysm rather than a true aneurysm, although these findings are controversial in the literature. Additionally, a recent study reported that a more than 50% decrease in aneurysm sac wall thickness measured at 1 cm from the aneurysmal neck is a sensitive and specific marker to diagnose LV PSA [4]. Despite these distinct characteristics, the differentiation between these two entities often presents a challenge to radiologists and cardiologists.
Surgical treatment is typically warranted in acute cases of large and symptomatic LV PSAs. The benefits of surgery outweigh the risks of rupture in almost all cases. Previous studies suggest that conservative management can be considered in asymptomatic patients with small aneurysms (less than 3 cm of dimension) or increased surgical risk [10,11].
Conclusions
In this case report, we demonstrated an atypical case of an apical LV PSA in a heart transplant recipient without a definitive finding of an underlying apical myocardial infarction. Although an accurate distinction between false and true aneurysms is crucial for appropriate management, this differentiation is often challenging clinically and radiologically. This case report describes the essential features of LV PSAs in different imaging modalities and highlights the importance of vigilance even in atypical clinical presentations.
Patient Consent Statement
The authors obtained written informed consent from the patient for submission of this manuscript for publication.
Footnotes
Funding: Candace M Howard is supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 5U54GM115428 . The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Juliana Sitta, Email: jsitta@umc.edu.
Candace M. Howard, Email: chowardclaudio@umc.edu.
References
- 1.Erdim R, Yildirimturk O, Polat B, Aytekin S, Demiroglu C, Aytekin V. Left ventricular pseudoaneurysm complicating inferior myocardial infarction: a case report. Int J Angiol. 2011;20(2):107–110. doi: 10.1055/s-0031-1279681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csapo K, Voith L, Szuk T, Edes I, Kereiakes DJ. Postinfarction left ventricular pseudoaneurysm. Clin Cardiol. 1997;20(10):898–903. doi: 10.1002/clc.4960201021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konen E, Merchant N, Gutierrez C, Provost Y, Mickleborough L, Paul NS. True versus false left ventricular aneurysm: differentiation with MR imaging–initial experience. Radiology. 2005;236(1):65–70. doi: 10.1148/radiol.2361031699. Epub 2005 Jun 13. PMID: 15955851. [DOI] [PubMed] [Google Scholar]
- 4.Ballard DH, Jokerst C, Raptis CA, Pilgram TK, Woodard PK. Myocardial cut-off sign is a sensitive and specific cardiac computed tomography and magnetic resonance imaging sign to distinguish left ventricular pseudoaneurysms from true aneurysms. J Thorac Imaging. 2020 doi: 10.1097/RTI.0000000000000525. Epub ahead of print. PMID:PMCID: PMC7666661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SL, Gropler RJ, Harris KM. Distinguishing left ventricular aneurysm from pseudoaneurysm. A review of the literature. Chest. 1997;111(5):1403–1409. doi: 10.1378/chest.111.5.1403. PMID: 9149600. [DOI] [PubMed] [Google Scholar]
- 6.Meng X, Yang YK, Yang KQ, Zhang Y, Lu PP, Fan P. Vol. 96. May 2017. p. e6793. (Clinical Characteristics and Outcomes of Left Ventricular Pseudoaneurysm: A Retrospective Study in a Single-Center of China. Medicine (Baltimore)). PMID: 28471977PMCID: PMC5419923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. Sep 1998;32(3):557–561. doi: 10.1016/s0735-1097(98)00290-3. PMID: 9741493. [DOI] [PubMed] [Google Scholar]
- 8.Catherwood E, Mintz GS, Kotler MN, Parry WR, Segal BL. Two-dimensional echocardiographic recognition of left ventricular pseudoaneurysm. Circulation. Aug 1980;62(2):294–303. doi: 10.1161/01.cir.62.2.294. PMID: 7397972. [DOI] [PubMed] [Google Scholar]
- 9.Hirose H, Matsunaga I, Strong MD., 3rd Left ventricular pseudoaneurysm found by CT scan. Open Cardiovasc Med J. 2008;2:26–27. doi: 10.2174/1874192400802010026. Epub 2008 Apr 7. PMID: 18949094PMCID: PMC2570575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alapati L, Chitwood WR, Cahill J, Mehra S, Movahed A. Left ventricular pseudoaneurysm: a case report and review of the literature. World J Clin Cases. 2014;2(4):90–93. doi: 10.12998/wjcc.v2.i4.90. PMID: 24749118; PMCID: PMC3985042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulten EA, Blankstein R. Pseudoaneurysms of the heart. Circulation. 2012;125(15):1920–1925. doi: 10.1161/CIRCULATIONAHA.111.043984. PMID: 22508841. [DOI] [PubMed] [Google Scholar]