Abstract
Idiopathic Pulmonary Fibrosis (IPF) is a chronically progressive interstitial lung that affects over 3 M people worldwide and rising in incidence. With a median survival of 2–3 years, IPF is consequently associated with high morbidity, mortality, and healthcare burden. Although two antifibrotic therapies, pirfenidone and nintedanib, are approved for human use, these agents reduce the rate of decline of pulmonary function but are not curative and do not reverse established fibrosis. In this review, we discuss the prevailing epithelial injury hypothesis, wherein pathogenic airway epithelial cell-state changes known as Epithelial Mesenchymal Transition (EMT) promotes the expansion of myofibroblast populations. Myofibroblasts are principal components of extracellular matrix production that result in airspace loss and mortality. We review the epigenetic transition driving EMT, a process produced by changes in histone acetylation regulating mesenchymal gene expression programs. This mechanistic work has focused on the central role of bromodomain-containing protein 4 in mediating EMT and myofibroblast transition and initial preclinical work has provided evidence of efficacy. As nanomedicine presents a promising approach to enhancing the efficacy of such anti-IPF agents, we then focus on the state of nanomedicine formulations for inhalable delivery in the treatment of pulmonary diseases, including liposomes, polymeric nanoparticles (NPs), inorganic NPs, and exosomes. These nanoscale agents potentially provide unique properties to existing pulmonary therapeutics, including controlled release, reduced systemic toxicity, and combination delivery. NP-based approaches for pulmonary delivery thus offer substantial promise to modify epigenetic regulators of EMT and advance treatments for IPF.
Keywords: fibrosis, epigenetics (MeSH), nanomedicine, epithelial mesenchymal transformation, bromodomain-containing protein 4, lung, aerosol - therapeutic
Introduction
The Interstitial Lung Diseases (ILDs) are a heterogeneous group of parenchymal diseases with various mixtures of fibrosis and inflammation. Idiopathic Pulmonary Fibrosis (IPF) is the most common and aggressive ILD subtype. IPF is a lethal diagnosis that leads to progressive dyspnea, reduced exercise capacity, and has a median life expectancy of 2–3 years after diagnosis (Raghu et al., 2011). Although there is substantial variability in case definition, comprehensive studies indicate that IPF affects over 3 M people worldwide and the incidence is increasing, particularly in aging populations (Hutchinson et al., 2015).
Mechanistically, it is thought that environmental insults (e.g. cigarette smoking, silicates and burning biofuels) in a genetically predisposed population trigger a chronic epithelial injury-repair response, disrupting alveolar stem cell populations and leading to epithelial growth factor (EGF) stimulation. In response to these factors, epithelial cells undergo cell-state changes to acquire mesenchymal characteristics and promote quiescent fibroblasts to transition into active extracellular matrix (ECM) production. Myofibroblasts perpetuate epithelial injury and further promote ECM deposition resulting in obliteration of alveolar spaces (Torr et al., 2015). Because myofibroblast numbers are inversely related to survival in human IPF (King et al., 2001), and IPF fibroblasts cause lung fibrosis in humanized mice (Geng et al., 2019), therapies reducing myofibroblast activity have emerged as a focus of recent therapeutic approaches.
Several studies have implicated the atypical histone acetyltransferase (HAT) known as bromodomain-containing protein 4 (BRD4) in the EMT and transforming growth factor (TGF)-induced myofibroblast transdifferentiation. In combination with preclinical studies in vivo that have observed small molecule BRD4 inhibitors interfere with fibrosis in mouse models of IPF, including bleomycin model (Tang et al., 2013), radiation induced fibrosis (Wang et al., 2018) and TGF-β-induced fibrosis (Tian et al., 2016), BRD4 pathway is a mechanistically relevant and a biologically important mediator of inducible lung fibrosis. However, BRD4 is implicated in a variety of essential cellular functions, including chromatin organization, DNA-damage repair, and post-mitotic gene expression (Dey, 2003; Devaiah et al., 2016b). A major challenge with advancing epigenetic regulators for the treatment of IPF is how to provide controlled delivery to myofibroblastic foci.
In this review, we describe the mechanistic pathways how epithelial inflammation/injury promotes EMT and myofibroblast expansion and the evidence that BRD4 is an epigenetic regulator of EMT and myofibroblast transdifferentiation. Finally, we propose how nanotechnology can advance therapeutics for IPF and ILD. Encapsulation in nanoparticles (NPs) enables selective drug delivery via well-accepted aerosol administration while enhancing overall pharmacokinetics. We propose that nanomedicine can advance the therapeutics of IPF by enhancing therapeutic efficacy and reducing systemic effects, thereby facilitating the development of the next generation of therapeutics for pulmonary fibrosis treatment.
The Role and Regulation of Fibrosis
The Injury-Inflammation-Fibrosis Paradigm
Fibrosis is defined as pathological state in which tissue and organ function is hindered due to excessive scar formation during the process of wound healing, often associated with a chronic inflammatory state (Wilson and Wynn, 2009). The fibrogenic response usually consists of several phases: 1) activation of the initial response due to organ injury, 2) stimulation of fibroblasts by inflammatory mediators, and 3) deposition of ECM components, resulting in permanent scarring (Mack, 2018).
In the second phase, after the initial injury, inflammatory and profibrogenic cytokines are released such as those from T helper type (Th)-2 cells (IL-4, IL-5, IL-10 and IL-13) or Th-1 cells (IL-2, IFN-γ and TNF). Interleukin (IL)-4 is notable in several studies that the deletion of IL-4 reduces fibrosis in the lung (2). Encompassing a wide range of biological and immunosuppressive activities, IL-4 can activate B cells, induce differentiation of naive CD4+ T cells, contribute to the development of M2-type monocytes or macrophages, and inhibit expression of several proinflammatory mediators (e.g., TNF-α; IL-1, 6, 8,12) (Zhu, 2015). Notably, in bleomycin-induced lung fibrosis, IL-4 was found to suppress inflammation, but was profibrotic. Another important profibrotic cytokine is IL-13 (Karo-Atar et al., 2016). IL-13 has the ability to directly and indirectly activate fibroblasts along with tumor necrosis factor (TNF)-α and IL-4, to produce an upregulation of IL-13Ra2 on macrophages, allowing IL-13 to bind and induce the release of TGFβ (Mack, 2018).
Contrary to the effects of Th-2 cytokines, Th-1 cytokines can be both anti- and profibrotic. Classically, the adaptive immune response is triggered when IL-12 interacts with interferon gamma (IFN-γ); it is also important to note that macrophages can also express IFN-γ in a response to microbial stimuli (Zhu, 2015). Once, activated, IFN-γ and IL-12 combine with TGFβ to induce collagen (COL) degradation and for ECM remodeling. In contrast, Th-1 polarizing cytokines IL-6, IL-1β, and TNF-α, are released from monocytes triggering a latent transcription factors such as STAT3 and STAT1, leading to fibroblast migration and recruitment, promoting proliferation and differentiation into myofibroblasts (Mack, 2018).
Myofibroblasts
Myofibroblasts are not normally present in substantial numbers in normal adult tissues but their population rapidly expands in response to injury-repair. In normal conditions, tissue fibroblasts have little secretory activity. However, upon injury, fibroblasts actively produce ECM components [collagen (COL) and fibronectin (FN)] causing a cascade of cytokine release, resulting in chemotaxis of inflammatory cells (Hinz et al., 2007). ECM remodeling triggers local fibroblasts to produce contractile stress fibers made out of cytoplasmic actins, transforming them into “proto-myofibroblasts.” In the presence of high ECM stress, TGFβ and FN splice products causing the phosphorylation of focal adhesion kinases (Phan, 2012), the proto-myofibroblast assumes a stable phenotype characterized by α-SMA replacing actin in the stress fibers (Hinz et al., 2007). Activated myofibroblasts are characterized by a spindle-like morphology with intracytoplasmic stress fibers, a contractile phenotype, expression of α-smooth muscle actin (α-SMA), transgelin (TAGLN/SM22), COL and hyaluronans. Expression of these contractile fibers by myofibroblasts enables wound contraction, and results in wound closure.
When the tissue repair process is completed, myofibroblasts disappear through apoptosis, a process driven by ECM softening (stress release) or pro-apoptotic factors such as IL-1β, fibroblast growth factor 1, and prostaglandin E2. However, under pathological conditions, such as pulmonary fibrosis, cirrhosis or hypertrophic scar, myofibroblasts persist in fibroblastic foci, producing excess scar and interfering with normal organ function. Myofibroblasts are derived from multiple mesenchymal types. In the lung, myofibroblasts are thought to derive from locally activated tissue fibroblasts, vascular pericytes or recruitment of bone-marrow derived fibrocytes (Hinz et al., 2007; Rock et al., 2011; Phan, 2012). Alternatively, it has been shown that injured epithelial cells can express mesenchymal intermediate filaments (vimentin) and α-SMA through a process of EMT, producing a myofibroblast-like phenotype (Radisky et al., 2007). In cancer, a substantial population of myofibroblasts are thought to arise from EMT. However, because myofibroblast populations are distinct in different organs, the contributions of EMT directly to the lung myofibroblast population is controversial (Rock et al., 2011).
Pulmonary Fibrosis and Epithelial Mesenchymal Transition
Epithelial Response to Acute and Chronic Lung Injury
Lining the airways and the respiratory compartments, epithelial cells, are exposed to a variety of environmental challenges such as microbial agents, toxic components and particulates leading to inflammation and fibrosis. The airways and distal epithelial cells comprise of a physical and chemical barrier to maintain lung function. Activated airway epithelial cells can modulate their direct barrier function through the release of a variety of defense mechanisms, such as the release of defensins and mucins, as well as by modulating fluid transport (Fehrenbach, 2001). Epithelial cells also have the ability to release multiple mediators and adhesion molecules that can mediate and activate migratory inflammatory and immunological cells. Additionally, epithelial cells interact with other resident cells, such as fibroblasts along with the ECM. The mechanism by which epithelial cells respond to different types of lung injury, acute or chronic, varies drastically from the inflammatory response to fibrosis.
Acute lung injury or acute respiratory distress syndrome (ARDS) can be caused by a variety of factors; pneumonia, trauma, sepsis, aspiration, post-lung transplant, or certain viral infections (Johnson and Matthay, 2010). Alveolar epithelial cells are key for alveolar homeostasis and aid in the proper alveolar fluid clearance through the direction of epithelial sodium channel (ENaC). When acute injury occurs, permeability of the alveolar-capillary membrane is caused by a disruption in the ENaC either through hypoxia such that the reoxygenation and β-agonists reverse ENaC or transcription factors and inflammation decrease the number of ENaC molecules (Lutter and Spiteri, 2005). When this occurs, fluid enters the alveoli and proinflammatory cytokines (IL-6 and IL-8) are released causing the release of proinflammatory cells such as neutrophils and macrophages. If not treated, this cascade develops into hypercytokinemia releasing IL-1 and TNF-α which will eventually lead to impaired fibrotic repair and lung failure (Johnson and Matthay, 2010).
Differing form acute lung injury, environmental exposures of particulates and chemicals is a much more common cause of chronic epithelial damage. Particulate matter generated from cigarette smoking, diesel emissions, biofuel exposures (including burn pit exposures) contain mixtures of metal, dusta, byproducts of hydrocarbon combustion induce oxidative cellular and DNA damage. These environmental exposures induce alveolar injury and clinical exacerbations in patients with existing chronic airway disease, including IPF, asthma and COPD (Winterbottom et al., 2018). Although the initial mechanism of action is similar in that there is damage to the alveolar wall that causes recruitment of proinflammatory cells, the gradual injury of the epithelium allows time for airway remodeling (Mercer et al., 2006). In asthma and COPD, epithelial cells are exposed to a range of inflammatory mediators over a prolonged period affecting the epithelial cell integrity and metabolism (Athanazio, 2012). Changes in cellular composition of the epithelium can cause goblet cell hyperplasia and pathophysiological of the mucous glands, affecting the function of the airway mucosa (Lutter and Spiteri, 2005).
Epithelial Mesenchymal Transition and the Myofibroblast Expansion
EMT is a multi-step, reversible process observed in several biological contexts: embryonic development (type I EMT), malignant transformation (Type III EMT), and in normal tissues undergoing injury response (Type II EMT). For the purposes of this review, we will focus primarily on type II EMT. Type II EMT begins when oxidative or physical injury to the resting differentiated epithelial cell triggers a phenotypic change characterized by loss of apical-basal polarity and loss of intercellular tight adheren junctions (Kalluri, 2009; Hill et al., 2019). The cells de-differentiate and become motile, enabling the cells to repopulate the injured epithelial surface. EMT can be initiated by the local production of signaling factors; most notable are TGF-β, fibroblast growth factors, EGFs, and the Wnt/β-catenin signaling pathways (Ijaz et al., 2014). These pleotropic signaling factors induce the expression of mesenchymal transcription factors such as Snail, Slug, and Twist whose actions repress epithelial genes (E-cadherin) and stimulate mesenchymal genes (N-cadherin, α-SMA, vimentin and fibronectin) (Hill et al., 2019). Consequently, the apical-basal polarity will switch to a front-back polarity in which the tight adherens junctions turn into N-cadherin junctions and vimentin stress fibers, promoting motility.
Systems-level studies have led to the conclusion that EMT is a multi-step, dynamic process produced by cooperative effects of master transcription factors in response to EGF and ECM cues. Time series RNA profiles have identified cliques of master transcription factors (such as ETS2, HNF4a and JUNB) that cooperate to control partial-EMT state, as summarized in Table 1. These transcription factors control cell type by producing the formation of superenhancers that control mesenchymal gene-regulatory networks. Superenhancers are clusters of functional enrichers enriched in RNA Polymerase, Mediator and acetylated histones that control cell-type specification genes (Whyte et al., 2013). Of relevance here, superenhancers are stabilized by the presence of BRD4 (Shin, 2018). In studies using siRNA-mediated gene knockdowns and small-molecule BRD4 inhibitors, TGF-β-induced partial EMT was reversed. Overall, this data demonstrates that BRD4 maintains epigenetic memory of TGFβ stimulation and the EMT phenotype. While EMT is traditionally defined as the conversion of epithelial cells into mesenchymal-like cells, recent studies expanded this cellular reprogramming process to mediate changes in epithelial morphology, motility, and gene expression (Lamouille et al., 2014).
TABLE 1.
A summary of factors and genes in myofibroblast expansion.
| Factors | Representative genes/Pathway | Action |
|---|---|---|
| Fibroblast and transforming growth factor signals | FGFs, EGFs, and wnt/β-catenin pathway | Initiates EMT |
| Changes in ECM stiffness (COL, MMP deposition) | Integrin activation | Transition from proto- to myofibroblast |
| Mesenchymal transcription factors | Snail, slug, and twist | Reprogram epigenome: repress epithelial genes and stimulate mesenchymal genes |
| Master transcription factors | ETS2, HNF4a, JUNB, NFκB | Control partial-EMT state |
| Superenhancers | BRD4 | Control expression of gene regulatory networks specifying cell identity |
Epithelial Mesenchymal Transition and Myofibroblast Expansion: The “Epithelial Injury” Hypothesis
Under normal conditions, the differentiated epithelial phenotype and the mucosal barrier function is maintained through interactions with the basal lamina and supporting subepithelial fibroblasts, known as the epithelial mesenchymal trophic unit (Evans et al., 1999). Upon exposure to environmental oxidants or insults, distal epithelial injury stimulates activation of the unfolded protein response, and release of fibrogenic growth factors. The consequent EMT transition results in secretion of alternative splice variant of ECM proteins, including FN. Changes in ECM composition (stress) are an important signal for pulmonary mesenchymal cell populations to acquire myofibroblast properties (Tomasek et al., 2002).
Myofibroblasts release inflammatory/profibrotic mediators that perpetuate epithelial injury and further promote ECM deposition (Zhang et al., 1994; Torr et al., 2015). The effects of TGF-β and fibrogenic cytokines further stimulate prosurvival signaling in the myofibroblast population, leading to persistence, resistance to apoptosis and migratory invasiveness (Horowitz et al., 2006). With myofibroblast persistence, deposition of ECM stiffens the lungs, reducing normal elastic properties and pulmonary function. Not only is this cell type primarily responsible for producing ECM and interstitial fibrosis (Hinz et al., 2007; Rock et al., 2011; Hung et al., 2013), these cells form the pathognomonic fibroblastic foci of human IPF (Hinz et al., 2007; Thannickal, 2012). Clinically in IPF, increased myofibroblast numbers are inversely related to survival (King et al., 2001). Therapeutics targeting the myofibroblast foci are therefore an exciting approach for treatment of ILDs.
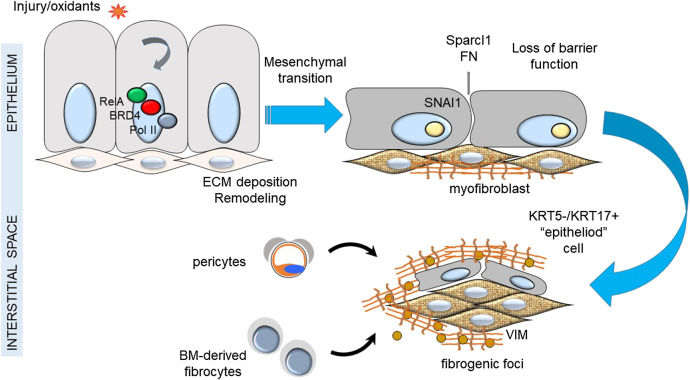
In IPF, depletion of alveolar epithelial stem cell populations from mitochondrial dysfunction results in tonic oxidative stress, alveolar cell dysfunction, impaired renewal, senescence and production of profibrotic factors. Compensatory alveolar stress response promotes EMT and myofibroblast transdifferentiation (Kulkarni et al., 2016). Although epithelial cells appear not to be a major source of lung myofibroblasts in IPF, this is not to say that epithelial transition is not a major driver of fibroblastic foci. Injured epithelial cells are a source of TGFβ and COL secretion that trigger myofibroblast formation (Table 1). Recent single cell sequencing studies have identified a keratin (KRT)5−/KRT17+ pathologic, ECM-producing epithelial cell population that was highly enriched in IPF lungs (Habermann et al., 2020). Interestingly these epithelial cells exhibited EMT signatures. This finding supports the prevailing epithelial injury hypothesis, illustrated in Figure 1.
FIGURE 1.
Epithelial injury hypothesis in IPF. Shown is a schematic view of the relationship between injury of the epithelial surface, epithelial-mesenchymal transition and formation of fibrogenic foci.
New Linkages of Epithelial Innate Signaling on Fibrosis Programs
In addition to environmental oxidant exposure, an emerging body of work has linked respiratory virus infections with epithelial injury and remodeling. Airway remodeling is a collective term that refers to structural changes in the airways resulting in enhanced collagen deposition in the subepithelial basement membrane (lamina reticularis), disruption of the epithelial barrier, epithelial cell-state change (mucous metaplasia and/or mesenchymal transition), and smooth muscle hypertrophy (Bergeron et al., 2010). Collectively, this process narrows the small airways, producing obstruction and reduced lung compliance accounting for enhanced morbidity and mortality (Hough et al., 2020). Respiratory viruses, such as Rhinovirus and Respiratory Syncytial Virus are major triggers of remodeling and chronic lung disease (Jartti and Gern, 2017). These agents primarily replicate in airway epithelial cells producing innate immune response-driven remodeling (Brasier, 2019; Brasier, 2020).
More recently, COVID-19 ARDS has been observed to be presaged by a hyper-inflammatory state characterized by the presence of circulating inflammatory and pro-fibrotic cytokines including IL-6, IL-7, GM-CSF, MCP, and MIP1α (Ruan et al., 2020). In COVID-19 infections, circulating ECM components (hyaluronans, type III pro-collagen and laminin) are associated with increased with disease severity (Ding et al., 2020). These data suggest that COVID-19 also induces ECM remodeling, a process important in myofibroblast transdifferentiation. In survivors, radiographic evidence of pulmonary scarring (Shi et al., 2020) and functional defects in lung function (diffusion capacity) are commonly seen (Mo et al., 2020; Shi et al., 2020; Wang et al., 2020). In fatal cases, increases in the TGF-ECM networks, including upregulated TGFBR2, COL isoforms, and FN (12) are seen, and pulmonary fibrosis is found upon an autopsy (Zhang T. et al., 2020). These data suggest that fibrosis and impaired pulmonary function is a sequelae of severe COVID-19-ARDS infections. Although the relationship of post-COVID-19 lung scarring/fibrosis with chronic progressive, ILD is not known due to the short-term follow-up, it is significant that post-infectious pulmonary fibrosis is also a known outcome in survivors of SARS, an infection with the closely related virus, SARS-CoV (Hui et al., 2005). We interpret these studies to indicate that viral epithelial infections may be an important modulator of airway fibrosis.
Mechanisms How Innate Inflammation Produces Epithelial Mesenchymal Transition
Chronic oxidative stress induced by innate signaling triggers adaptive cell-state transitions of the normal epithelium to activate the EMT program. High throughput RNA-seq studies of normal human airway cells have discovered that the core regulatory network of ∼3,000 TGFβ-induced genes, substantially overlaps with the NFκB network (Tian et al., 2015). Using selective inducible knockouts in bronchiolar-derived basal cell progenitors, it was found that NFκB signaling drives the transition to a committed mesenchymal phenotype (Tian et al., 2018c). These findings led to the discovery that tonic (or repetitive) NFκB signaling in the airway produces EMT in vivo (Tian et al., 2017a). Repetitive AEs provoked by exposure to TLR3 agonists activates SNAI expression, EMT, remodeling and expansion of myofibroblast population(s).
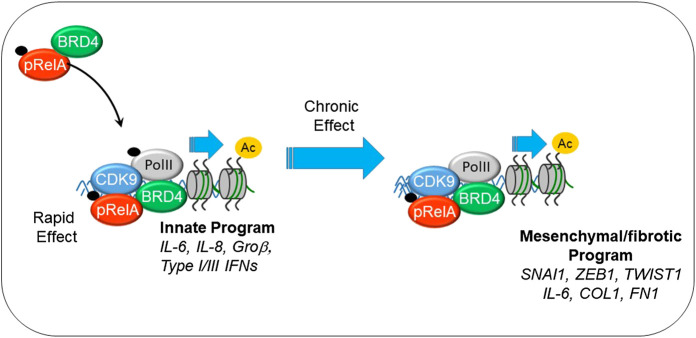
Virus-induced NFκB complexes with the coactivator bromodomain containing (BRD4), a multifunctional protein with intrinsic RNA polymerase kinase (Huang, 2009; Devaiah et al., 2012) and atypical HAT (Huang, 2009; Devaiah et al., 2016a) activity. Through its sequence specific DNA-binding activity, NFκB redistributes BRD4 to innate inflammatory genes as well as the core EMT regulators SNAI1, ZEB1 and TWIST1 (Brown et al., 2014; Tian et al., 2016), as schematically shown in Figure 2. NFκB -dependent activation of the core EMT regulators results in expression of COL and FN, key ECM regulators of myofibroblast expansion, airway remodeling and expansion of the subepithelial basement membrane. Supporting this chromatin remodeling mechanism, recent analyses using transposase-accessible-next generation sequencing (ATAC-Seq) in highly differentiated lower airway epithelial cells infected with Respiratory Syncytial Virus demonstrate that the TGFβ-FN-JUN pathways controlling EMT are activated by chromatin decondensation at their proximal promoters (Xu et al., 2020).
FIGURE 2.
Mechanisms how innate signaling couples to EMT. Shown is the interaction between phosphorylated 65 kDa NFκB subunit (pRelA), BRD4. Initially this chromatin remodeling complex activates innate response genes important in viral restriction. With chronic stimulation, the complex directly activates SNAI, COL, FN, and other components of EMT.
Although these fundamental studies of the NFκB -BRD4 pathway were initially developed in vitro, evidence that NFκB mediates mesenchymal transition, myofibroblast expansion, and pulmonary fibrosis has been developed in in vivo (Tian et al., 2016). In mice, acute TLR3 activation induces CXCL8/IL-8 expression and neutrophilia, but chronic TLR3 activation produces airway epithelial mesenchymal transition, expansion of the myofibroblast population, and fibrosis (Tian et al., 2016). Activation of the BRD4 kinase and atypical HAT activity in the airway mucosa has been demonstrated in response to TLR3 activation (Tian et al., 2017a) and viral replication (Tian et al., 2017b; Tian et al., 2018d). Here, the unique, BRD4-dependent histone acetylation on Lys 122 is induced by virus or allergen, and this induction is NFκB-dependent. Viral and allergen-induced NFκB -BRD4 complex formation was been demonstrated through proximity ligation assays, a technique to detect molecular interactions between the two proteins. Finally, using highly selective BRD4 inhibitors, it was found that inhibition of BRD4 HAT activity interferes with remodeling, airway hyperreactivity and myofibroblast transition in vivo (Tian et al., 2018a). These studies validate the central relevant of the NFκB -BRD4 pathway in airway remodeling.
Histone Modifications Underly Epithelial Mesenchymal Transition
In recent years, epigenetics has become the forefront as a major mechanism underlying fibrosis. The term epigenetics refers to altering gene expression without altering the DNA sequence. Extensive research aided by deep sequencing technology, has identified histone codes to be of interest in EMT. In eukaryotic cells, DNA is condensed in chromatin with the nucleosome, a major part of chromatin, comprised of four different histones to form an octamer (H3, H4, H2A, and H2B) (O’Reilly, 2017). Although histones are globular in nature, they have a “tails” that can possess multiple histone modifications, eight of which are known. Histone tail modifications include methylation, phosphorylation, acetylation, ubiquitination, sumoylation, citrullination, and ADP-ribosylation (O’Reilly, 2017). Additionally, histone modifications are mediated by a family of enzymes: Histone Deacetylases (HDACs), and HATs. HDACs remove acetyl groups on N terminal lysines, whereas HATs catalyze their addition.
First discovered in hepatic fibrosis, HDACs were shown to enhance the migratory and ECM producing properties of tissue myofibroblasts (de Ruijter et al., 2003). This idea was expanded in pulmonary fibrosis, where HDACs were extensively studied for their role in COL secretion. Inhibition of HDAC induced hyperacetylation in H3 and H4 tails. In idiopathic pulmonary fibroblasts, elevated expression of all class I (HDAC 1, 2, 3, and 8), class IIa (HDAC 4, 5, 7, 9) and class IIb (HDAC 6 and 10) HDACs were discovered to be in both tissue and isolated myofibroblasts (de Ruijter et al., 2003). During hypoxia, another regulator of EMT, cross-talk between chromatin modifiers HDAC3 and WDR5 regulates repression of epithelial genes and activation of mesenchymal genes (Lin and Wu, 2020). The histone mark histone 3 lysine 4 acetylation (H3K4Ac) was shown to bind promoters of several EMT marker genes (e.g., CDH1 and VIM) (Wang J. Q. et al., 2019). Additionally, both H3K4me3 (activator) and H3K27me3 (repressor) were observed in the EMT promoters (Lin and Wu, 2020). This is of much interest as the presence of activation and repressor histone marks allows EMT markers to cycle from expressed to silenced in response to dynamic changes in the extracellular environment. Furthermore, studies using chromatin immunoprecipitation with anti-H3K4Ac antibodies and whole genome sequencing identified new EMT marker genes, glioma-associated oncogene homolog 1 (GLI1) and smoothened (SMO), that are regulated by HDAC3 for in migration and invasion (Wang J. Q. et al., 2019). Although there has been great progress in the use of HDAC-inhibitors demonstrating anti-fibrotic activity in mechanistic studies, the advancement of HDAC inhibitors (HDACi) as therapeutics for IPF has been challenging, discussed below.
The ubiquitious HAT, P300, regulates hundreds of transcription factors such as TGF-β1 and can cause the hyperacetylation of H4 on the COL promoter. In response to changes in cell substrate stiffness, activated P300 accumulates in the nucleus, enhancing Smad dependent transcription (Dou et al., 2018). Smad2/3 binds directly to gene promoters to induce the transcription of profibrotic molecules, including α-SMA, COLI and tissue inhibitor of metalloprotein, inducing myofibroblast activation and ECM deposition (O’Reilly, 2017).
A body of work has shown that transdifferentiation, anti-apoptotic activity, ECM production and migration of epithelial-like mesenchymal cells is are mediated by BRD4 (Devaiah et al., 2016a). BRD4 is a member of a family of bromodomain and extraterminal (BET) proteins, including BRD2, BRD3 and BRDt that share two tandem bromodomains (BDs) and an extra-terminal domain, from which the family was named (Taniguchi, 2016). These paralogs are thought to have arisen during a distal evolutionary duplication event. Although the functions of the BET isoforms are tissue specific and pleiotropic, BRD4 activity has been implicated in cell cycle regulation DNA repair, innate inflammation (Brasier et al., 2011; Taniguchi, 2016), and cell-state transition (Chang et al., 2016; Tian et al., 2017a); all processes that play important roles in lung diseases.
Recent work has identified a coenzyme A -dependent atypical histone acetyl transferase activity (HAT) (Devaiah et al., 2016a) and N terminal kinase activity directed for the COOH terminus of RNA Polymerase II (Devaiah et al., 2012). Through these activities, BRD4 plays a central role in the maintenance of cell-type specifying superenhancers, controlling mesenchymal gene regulatory programs by transcriptional elongation discussed above. Small molecule inhibitors and siRNA mediated knockdown experiments have shown that BRD4 plays a central role in myofibroblast transdifferentation, mediating TGF-β-induced NOX4-αSMA expression, contractility and motility as well (Ijaz et al., 2017). In this way BRD4 mediates both EMT and fibroblast-myofibroblast transdifferentiation.
Deoxyribonucleic Acid Methylation Regulates Epithelial Mesenchymal Transition
Environmental particulate matter emitted by traffic-related air pollution; polyaromatic hydrocarbons, black carbon, ozone (O3), and nitrogen oxides (NOx), have been shown to be associated with changes in DNA methylation (DNAm). DNAm, one of the most well-known mechanisms of epigenetic gene regulation, is described as the attachment of methyl groups to DNA, commonly at the fifth carbon of cytosines, leading to the formation of 5-methylcytosine (5-mC) (Moen et al., 2015). Methylation on the genome predominantly occurs at C-G dinucleotides (CpGs), resulting in the initiation of transcription, elongation efficiency, and alternative splicing.
Tumor hypoxia, a known inducer of EMT through hypoxia-inducible factor-1α (HIF-1α), can directly dysregulate the activity of ten-eleven translocation DNA demethylases. A decrease in DNA demethylation triggers the accumulation of DNA methylation driven by DNA methyltransferases (DNMT) to affect genes encoding cell adhesion functions that are involved in EMT. It has been suggested that DNA methyltransferase 1 associated protein 1 (DNMT1) can directly interact with Snail and suppress E-cadherin in epithelial cells and subsequent activation of epithelial–mesenchymal transition or cell death (Espada et al., 2011). Studies have shown that traffic-related air pollution and smoking can cause increased DNMT1 protein accumulation leading to misexpression of genes to cause cancer.
Therapeutic Strategies for Pulmonary Fibrosis
Glucocorticoids
Glucocorticoids such as cortisone, hydrocortisone, and prednisone, have historically been used to treat lung inflammation. These drugs act by decreasing inflammation both, directly through the glucocorticoid receptor and indirect mechanisms (Barnes, 2009). However, all potent glucocorticosteroids cause side effects such as glaucoma, high blood pressure, psychological effects, osteopenia, and acne (Newman, 2018). Previously, it was thought that pulmonary fibrosis was a product of immune activity. Therefore, corticosteroids were combined with to azathioprine or cyclophosphamide and a mucolytic/antioxidant, N-acetylcysteine. These studies were stopped due to negative effects in varying patient groups and are no longer used (Newman, 2018).
Although inhaled corticosteroids have been effective for several lung diseases such as cystic fibrosis, non-specific interstitial pneumonia (NSIP) and inflammatory diseases such as asthma and COPD, however, it is not effective with IPF (Mapel et al., 1996).
In 2014, oral nintedanib, was a breakthrough therapy approved by the FDA. Nintedanib is a broad spectrum small-molecule inhibitor of receptor tyrosine kinases that reduces fibroblast proliferation and activation. Nintedanib has been subjected to a number of randomized controlled clinical trials. These studies have shown that nintedanib slows the decrease in forced vital capacity (FVC) from IPF and other progressive fibrosing ILDs (Richeldi et al., 2014; Mazzei et al., 2015; Flaherty et al., 2019). Pirfenidone was also licensed in the same year. Pirfenidone is a small molecule with antifibrotic, anti-inflammatory and antioxidant effects that inhibits TGF-β-stimulated collagen production. Several prospective studies have found that pirfenidone also slows the loss of FVC in IPF and improves progression-free survival (Spagnolo et al., 2010). Although these medications represent a substantial advancement for the treatment of IPF, they neither reverse established fibrosis nor are curative.
Histone Deacetylase Inhibitors
Trichostatin A (TSA), a non-selective HDACi, has been shown to attenuate pulmonary fibrosis in several models. Bleomycin-induced pulmonary fibrosis (IPF), is characterized by progressive fibrosis leading to end-stage lung disease and respiratory failure. In the bleomycin model, TSA prevented WIF-1 loss, an important Wnt signaling regulator, resulting in a reduction of collagen overexpression through b-catenin (Yoon et al., 2019). TSA has also been shown to block TGF-β mediated ECM production in corneal fibroblasts. In a separate study, epigenetic defects in COX-2 expression were restored by HDAC inhibition, protecting from pulmonary fibrogenesis (Coward et al., 2009). Although there are currently many ongoing studies with the use of TSA and the reduction of fibrosis in vivo with mice and rats, there has yet to be any significant human clinical trials with this drug related to pulmonary fibrosis. A major limitation of HDACi is the lack of isoform specificity and concerns with long-term toxicity (Royce and Karagiannis, 2014), including vascular calcification (Kwon and Kim, 2017).
Bromodomain-Containing Protein 4 Inhibitors
A number of studies have implicated BRD4 as a potential therapeutic target for modifying epigenetic responses in lung disease. It has been shown that inhibition of BRD4 blocks EMT (Chang et al., 2016) and fibroblast-myofibroblast transdifferentiation in vitro (Ijaz et al., 2017). Highly selective BRD4 inhibitors have the ability to block allergen, viral, and growth factor induced remodeling and fibrosis in small animal models in vivo (Tian et al., 2016; Tian et al., 2018d; Zhao et al., 2019). Moreover, inhibition of BRD4 interferes with inflammation-induced pericyte-myofibroblast transition in vivo and leakage of plasma proteins into the alveolar space (Zhao et al., 2019). Finally, BRD4 activation in myofibroblasts from humans with IPF, and small molecule BRD4 inhibitors can prevent interstitial fibrosis in the bleomycin lung injury model (Tang et al., 2013). Radiation induced lung fibrosis (Wang et al., 2018) and fibrosis mediated by TGFβ administration (Tian et al., 2016).
The medicinal chemistry of BRD4 inhibitors has been rapidly advancing (Liu et al., 2018; Tian et al., 2018b; Brasier and Zhou, 2020; Liu et al., 2020). Recently, highly selective inhibitors of BRD4 that interact with distinct regions of the protein have been developed (Liu et al., 2020). These molecules have demonstrated efficacy and exhibit promising medicinal properties (Liu et al., 2020). However, systemic administration of BRD4 inhibitors may be problematic due to its ubiquitous expression and role in chromatin organization, cell stress and innate responses.
We contend that the advancement of epigenetic inhibitors into well-tolerated therapy for chronic lung disease will depend on advancement of strategies that can selectively deliver these agents to the diseased tissue. In the subsequent section, we will review the promise of aerosolized delivery of nanomedicines for addressing this problem.
Nanomedicine
NP formulations can provide a wide variety of benefits for the delivery of existing active pharmaceutical ingredients (APIs)—that is, the efficacious molecules identified previously for their anti-fibrotic properties. This includes dramatically improved API solubility, enhanced cellular internalization, controlled or stimuli-responsive release of the API, and enhanced selectivity through targeting, ultimately increasing therapeutic efficacy while reducing toxic side effects (Peer et al., 2007; Alshamsan, 2010; Menon et al., 2013; Patel et al., 2013; Guo, 2014; Menon et al., 2014; Pradhan, 2014; Wang, 2016; Kong, 2017; Song, 2017; Yang, 2018). The peripheral reactive groups of many NPs also provide the opportunity for ligand conjugation and active targeting strategies (Gu et al., 2007; Bugno et al., 2015). Additionally, NPs can provide specific benefits for pulmonary delivery. For example, NPs have been found to facilitate the solubility of APIs in certain pulmonary aerosolization solvents, such as perfluorooctyl bromide and fluorocarbons (Hsu et al., 2003; Lehmler et al., 2008). NPs are also readily internalized by vascular endothelial cells, providing accumulated quantities substantially greater than their micro-sized counterparts (typically >1 µm in diameter) (Foster et al., 2001; Davda and Labhasetwar, 2002). However, despite these potential advantages, EMT-targeting NPs have so far been primarily utilized for anti-cancer strategies, and those targeting EMT in lung fibrosis currently remain limited in the literature. This is the case even as EMT becomes increasingly implicated as an important pathway for pulmonary fibrosis (Willis and Borok, 2007; Naguchi et al., 2014), with key EMT-markers, such as TGF-β (Burgess et al., 2005; Fernandez and Eickelberg, 2012) and Snail (Wettstein et al., 2013), identified as promising targets for anti-fibrotic therapies.
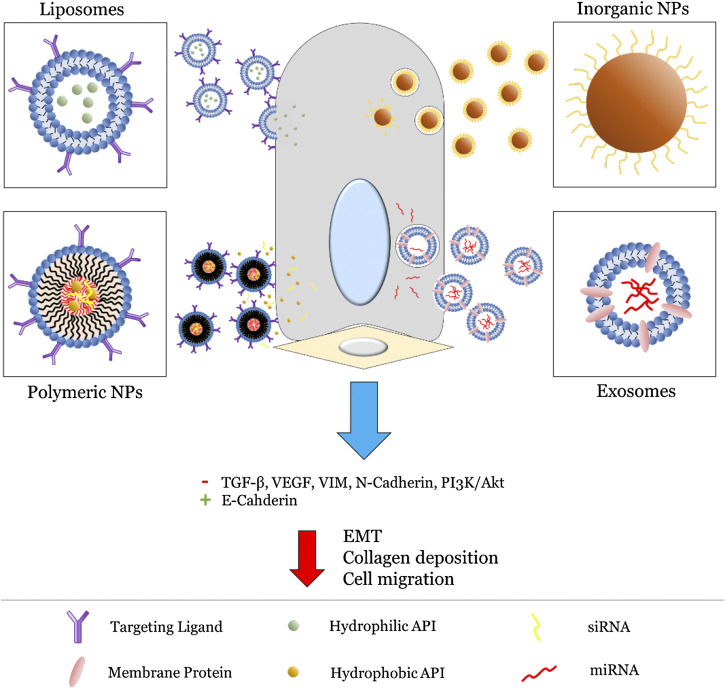
In the following sections, we review a selection of nanomedicines formulated for pulmonary delivery organized by NP type, with a scope limited to those specifically utilizing aerosolic delivery methods. A general overview of these strategies is presented in Figure 3. Nanomedicines targeting EMT-mediated pulmonary fibrosis are especially focused on, with the goal of highlighting current shortcomings in this area of research. To this end, promising directions and potential considerations for future NP formulations are also identified at the end.
FIGURE 3.
General strategies utilized for NP (liposomes, polymeric NPs, inorganic NPs, and exosomes) targeting the EMT pathway. For liposomes and polymeric NPs, this primarily involves active targeting via surface-bound ligands (typically proteins) followed by release of an encapsulated API. On the other hand, inorganic NPs and exosomes involve innate inhibition via their intrinsic physiochemical properties. Altogether, this leads to inhibition of key EMT biomarkers and subsequent reduction in fibrosis.
Liposomes
Liposomes are a promising nanocarrier for pulmonary drug delivery as they are primarily composed of natural or synthetic phospholipids either native to or compatible with lung tissues, such as phosphatidylcholine and egg phosphatidylethanolamine (Gao et al., 2013). These nanoformulations are also capable of including additional surfactants and cholesterol. Liposomes are comprised of spontaneously-formed lipid bilayer vesicles with diameters ranging from 30 nm to several micrometers, though those between 50 and 450 nm are favored for therapeutic delivery (Etheridge et al., 2013; Gao et al., 2013). Multiple forms of vesicles exist, including multilamellar vesicles (containing lamellar phase bilayers), small and large unilamellar liposomes (containing a single lipid bilayer), cochleate vesicles, and multivesicular liposomes (one vesicle containing multiple smaller vesicles). Liposomes can also be classified by preparation method, including reverse-phase evaporation and vesicle extradition (Bozzuto and Molinari, 2015). In general, liposomes consist of a hydrophobic shell encapsulating a hydrophilic solvent. This property enables loading of both lipophilic and hydrophilic APIs with relative ease. Further structural modifications provide additional benefits, such as poly (ethylene glycol) (PEG) or deoxyribonucleic acid (DNA) anchoring to embedded cholesterol for a “stealth effect” or as biosensors (Hosta-Rigau et al., 2013), or the addition of fatty acyl chains to extend transition temperature and increase stability (Bitounis et al., 2012). Circulation time in vivo can also be extended by including hydrophilic carbohydrates, e.g., monosialoganglioside (GM1), and polymers, e.g., PEG (Gabizon and Papahadjopoulos, 1988).
Aerosolized liposomes are well represented in the literature for pulmonary delivery, with promising results against multiple cancer models (including lung, renal, breast, and colon) (Knight et al., 1999; Koshkina et al., 2000; Skubitz and Anderson, 2000; Koshkina et al., 2001; Verschraegan et al., 2004), respiratory tract infections (RTIs) (Conley et al., 1997), immunodeficiency (Ten et al., 2002), pulmonary aspergillosis (Allen et al., 1994), cystic fibrosis (Davies et al., 2014), as a method of immunosuppression (Letsou et al., 1999), and as a delivery vehicle of insulin (Huang and Wang, 2006). Aerosolized liposomes have also been shown to be effectively delivered by multiple common clinical methods, including air-jet, ultrasonic, vibrating-mesh, and continuous-flow nebulizers (Waldrep et al., 1994; Elhissi and Taylor, 2005; Elhissi et al., 2007), and are clinically safe in human patients (Thomas et al., 1991; Vidgren et al., 1995; Gilbert et al., 1997; Waldrep et al., 1997). This demonstrates the high biocompatibility and adaptability of liposomes for pulmonary delivery. In fact, a liposomal formulation is the only currently approved NP formulation of aerosolized drug for pulmonary delivery. This comes in the form of Arikayce, an amikacin liposome nebulized suspension antibiotic for mycobacterium avium complex (MAC) lung disease, developed by Insmed and approved in 2018 (Cipolla et al., 2013; Insmed, 2020). Other notable clinical trials are summarized in Table 2.
TABLE 2.
A summary of current clinical trials for aerosolized liposomes.
| NP | APIs | Target disease | Phase | Status | Sponsor | Identifier |
|---|---|---|---|---|---|---|
| 9-Nitro-20 (S)-Camptothecin (L9NC) liposomes | N/A | Non-small-cell lung cancer | II | Completed in 2007 | University of New Mexico | NCT00250120 |
| 9-Nitro-20 (S)-Camptothecin (L9NC) liposomes | N/A | Metastatic endometrial cancer | II | Completed in 2007 | University of New Mexico | NCT00249990 |
| AmBisome | N/A | ABPA | II | Completed in 2019 | Poitiers university hospital | NCT02273661 |
| AmBisome | Intraconazole | ABPA | III | Recruiting | Poitiers university hospital | NCT03656081 |
| AmBisome | N/A | Lung transplant infections | II | Completed in 2014 | University health network, toronto | NCT01254708 |
| AmBisome | N/A | Lung transplant infections | III | Completed in 2007 | University of Pittsburgh | NCT00177710 |
| Arikayce | N/A | Cystic fibrosis | I/II | Completed in 2008 | Insmed incorporated | NCT00777296 |
| Arikayce | N/A | Cystic fibrosis | I/II | Completed in 2009 | Insmed incorporated | NCT00558844 |
| Arikayce | N/A | Cystic fibrosis | II | Completed in 2010 | Insmed incorporated | NCT03905642 |
| Arikayce | Mycobacterial multi-drug regimen | Mycobacterium abscessus lung disease | II | Completed in 2019 | Kevin winthrop | NCT03038178 |
| Arikayce | Mycobacterial multi-drug regimen | NTM + MAC | III | Completed in 2018 | Insmed incorporated | NCT02628600 |
| Bupivacaine liposomes | N/A | Coronary heart disease | III | Completed in 2020 | Kathirvel subramaniam | NCT03270514 |
| Cisplatin liposome | N/A | Osteosarcoma metastatic | I/II | Completed in 2008 | Insmed incorporated | NCT00102531 |
| Cyclosporin liposome | N/A | Bronchiolitis obliterans syndrome | II/III | Completed in 2014 | Pari Pharma GmbH | NCT01334892 |
| Cyclosporin liposome | Tacrolimus + mycophenolate mofetil prednisone + rampamycin | Lung transplant rejection | I/II | Completed in 2015 | University of Maryland, college Park | NCT01650545 |
| Pulmaquin | N/A | Non-cystic fibrosis bronchiectasis | III | Completed in 2016 | Aradigm corporation | NCT02104245 |
| Pulmaquin | N/A | Non-cystic fibrosis bronchiectasis | III | Completed in 2016 | Aradigm corporation | NCT01515007 |
AmBisome, amphotericin B liposome; ABPA, allergic bronchopulmonary aspergillosis; NTM, nontuberculous mycobacterial lung infection; Pulmaquin, ciprofloxacin liposome.
Despite the available aerosolized liposome formulations for pulmonary delivery, comparatively few utilize an EMT-targeted approach. These are summarized in Table 3. The majority of these formulations utilize APIs loaded into the liposome vesicle, including PGE2 (also known as dinoprostone, an oxytocic drug used in pregnancies for smooth muscle contradictions) (Ivanova et al., 2013), paclitaxel (an anti-cancer cytoskeletal drug that targets tubulin) (Zhou et al., 2016; Jin et al., 2019), and simvastatin (a lipid-lowering drug typically used for heart defects) (Jin et al., 2019). One formulation, however, used hyaluronic acid (a ligand of CD44) embedded in the liposome bilayer as both the API and a targeting moiety for overexpressed CD44 (Pandolfi et al., 2019). In all cases, biomarkers of the EMT pathway were subsequently inhibited by the API, including TGF-β, TNF, and VEGF. This led to significant inhibition of EMT, reducing either inflammation and fibrosis of the lungs (Ivanova et al., 2013; Zhou et al., 2016; Pandolfi et al., 2019) or tumor metastasis (Jin et al., 2019). Additionally, the liposomes improved the delivery efficiency through improved cellular entry, tissue retention of API, and selectivity endowed from a surface-decorated ligand.
TABLE 3.
A summary of current erosolized liposome formulations targeting EMT-markers in the literature.
| NP | API(s) | Disease | Model(s) | Effect | Ref |
|---|---|---|---|---|---|
| Liposome | PGE2 | Idiopathic pulmonary fibrosis (IPF) | IPF mice | Inhibition of TGFB-initiated pathways and TNF, restricted inflammation and fibrosis | Ivanova et al. (2013) |
| Liposome | Paclitaxel | Pulmonary fibrosis (PF) | PF mice | Inhibition of TGF-β1, reduced collagen levels and alveolar thickness | Zhou et al. (2016) |
| Liposome | Simvastatin + paclitaxel | Lung cancer | A549T mice | Inhibition of EMT and metastasis | Jin et al. (2019) |
| DPPE liposome | Hyaluronic acid | CTD-ILD + BOS | A549 + Calu-3 + THP-1 cells | Inhibition of VEGF mRNA | Pandolfi et al. (2019) |
DPPE, 1,2-Bis(diphenylphosphino)ethane; CTD-ILD, connective tissue disease-associated interstitial lung disease; BOS, Bohring-Opitz Syndrome.
Even with the broad array of available aerosolized liposomes and initial promising results for EMT-targeted delivery, challenges remain if these NPs are to be further pursued. For one, liposome stability when aerosolized remains a leading concern. When passed through a nebulizer, significant loss of encapsulated API can occur from mechanical-induced fragmentation of vesicles (Taylor et al., 1990). Strategies exist to mitigate this, such as reducing vesicle size by extruding the liposomes through a membrane filter, but it remains a concern for any potential formulation. Additionally, liposomes face several biological challenges for drug delivery. This includes preferential uptake by the reticuloendothelial system (RES) and opsonization (Poste et al., 1976; Senior, 1986), high clearance of large liposomes (Oku and Namba, 1994; Ulrich, 2002), the “accelerated blood clearance” (ABC) phenomenon if PEGylated liposomes are administered repeatedly (Ishida et al., 2006; Dams et al., 2000), and potential triggering of the innate immune response (Szebeni, 2005; Szebeni and Moghimi, 2009). While the unique anatomy and physiology of the lungs could attenuate some of these concerns, they should still be kept in mind when designing a liposome aerosol. For this reason, efforts have increasingly turned away from simpler formulations like liposomes and into more complex, and robust, NP systems.
Polymeric Nanoparticles
Polymeric NPs, which have increasingly become more explored as pulmonary drug delivery vehicles in the literature, are one such system. While liposomes are primarily composed of phospholipids with the addition of surfactants and cholesterol, polymeric NPs are instead composed of one or more synthetic polymer repeating chains linked together. The most common of these polymers utilized for pulmonary delivery include poly (lactic acid) (PLA), poly (ɛ-caprolactone) (PCL), poly (lactic-co-glycolic acid) (PLGA), chitosan, alginate, and gelatin bases (Menon et al., 2014). While not naturally derived, these polymers are biodegradable, which mitigates some risk of toxicity (Beck-Broichsitter et al., 2012c; Beck-Broichsitter et al., 2014b). Additionally, the nature of polymer chemistry (or polymerization), wherein monomer molecules are consecutively bonded together through step-growth or chain-growth synthesis techniques, affords greater control and diversity for polymeric NPs than comparable liposomal formulations (Kricheldorf, 1991; Hickey et al., 2015). This ability to finetune molecular weight (MW), chemical composition, and chain structure provides a wide diversity of potential NPs (as detailed later in this review).
The choice of polymer, polymer ratio, or polymer functional groups can also significantly change polymeric NP properties. For example, the rate of in vivo biodegradability can vary based on composition (pure vs hybrid NPs) (Singh et al., 2010), type of polymer (Anderson and Shive, 2012), or MW (Mohammad and Reineke, 2013), or even determine the mechanism of biodegradation itself (hydrolysis vs oxidative degradation, for instance) (Ulbricht et al., 2014). Adding another polymeric coating can also change how a polymeric NP interacts physiologically. This includes enhancing tissue penetration (for example, adding a dense PEG coating to infiltrate farther into therapeutically unsusceptible tissues like the brain or through mucus coatings) (Hong et al., 2009; Tang et al., 2009; Nance et al., 2012) and clearance (such as being susceptible to or avoiding the ABC phenomenon) (Alexis et al., 2008; Ishihara et al., 2010; Saadati et al., 2013). Other fine-tunable properties include lipophilicity and drug loading (Johnstone and Lippard, 2013), circulation time and interaction with vascular proteins (Sheng et al., 2009; Sunoqrot et al., 2014; Yang et al., 2014), NP targeting and tissue accumulation (Park et al., 2007), and NP shelf-life.
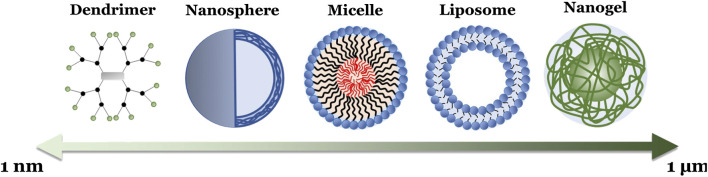
In addition to polymer choice, polymeric NPs can also be formulated with a diverse range of molecular architectures that further influence their behaviors (Menon et al., 2014), including linear (single chain) polymers, hyperbranched (multiple end group) polymers (including dendrimers), micelles, and crosslinked emulsions (Box 1; Figure 4). As such, polymeric NPs typically range from 1 to 1,000 nm in diameter, exist in a variety of morphologies (e.g., spheres, vesicles, rods, tubules, and lamellae), and consist of homopolymers and various copolymers. Of note for drug delivery are amphiphilic copolymers. These polymer chains, consisting of a lipophilic polymer (such as PLA or PCL) conjugated to a hydrophilic polymer (e.g., PEG), spontaneously form a variety of complex morphologies in aqueous solvents due to hydrophobic interactions (Forster and Antonietti, 1999; Letchford and Burt, 2007). Altering the properties of these copolymers, such as constituent chain length, hydrophilic-lipophilic balance (HLB), and charge, can even further alter a NP’s properties, including hydrodynamic size (Wittgren et al., 1996), shell thickness (Stubenrauch et al., 2006), and exposed peripheral functional groups (Urbani et al., 2008; Pearson et al., 2016). This in turn allows further finetuning of drug delivery characteristics, such as API solubility, loading capacity, and release profiles (Yan et al., 2011; Glavas et al., 2015). Altogether, this endows polymeric NPs with advantages similar to liposomes (e.g., easy encapsulation and high loading of lipophilic APIs, API protection, enhanced half-life and lowered blood clearance, sustained and controlled release, and the availability of readily modifiable surface end groups for targeting and stealth strategies) while also providing researchers with a more adaptable NP platform for various delivery needs.
BOX 1. Various polymeric nanoparticle formulations.
Linear Polymer-based: A single-chain polymeric NP composed of only one polymer type, which is the simplest form of polymeric NP. Can be broadly defined as a solid colloidal particle consisting of a uniform macromolecular component, which typically forms a three-dimensional nanosphere (though can also include nanocapsules, cubes, and plates) encapsulating therapeutic agents (or, in the case of a surface modification such as PEGylation, extend as a directly conjugated chain off the original molecule) (Chan et al., 2010; Bolhassani et al., 2014). As mentioned, these are typically synthesized using either step-growth (mostly via condensation polymerization) or chain-growth (mostly via addition polymerization such as radical, ionic, ring-opening, or living polymerizations) techniques (Kricheldorf, 1991). The polymer is then dispersed in an organic phase and cross-linked to form a spherical structure. A linear polymer can also be attached to an API through precise post-polymerization modifications of semi-telechelic chains, such as chemical ligation of specific antibodies (cysteine, tyrosine, arginine, and histidine), enzyme-mediated ligation, in situ polymerization initiated by an API, or through recombinant gene technology (Ekladious et al., 2019). Nanosphere diameters can range from 20–200 nm while single chain modifications can be even smaller, with some of the smallest only 1–10 nm in diameter (Bolhassani et al., 2014; Kroger and Paulussea, 2018).
Hyperbranched Polymers: Polymeric NPs composed of a hyperbranched structure, wherein exponentially increasing numbers of “branch-like” polymer chains extend from a central core to form a surrounding shell. Dendrimers are the most notable example. These complex highly branched monodisperse polymeric NPs form a spherical morphology, including a central core, a hyperbranched body, and peripheral reactive functional groups, the number of which are doubled with an increase of each dendrimer generation (for example, a generation 0 (G0) poly (amidoamine) (PAMAM) dendrimer has a theoretical four surface groups, a G1 would have 8, a G2 would have 16.) (Shi et al., 2006; Hsu et al., 2016; Franiak-Pietryga et al., 2018). Dendrimers can be synthesized through several techniques, including divergent and convergent synthesis, (Holister et al., 2003; Killops et al., 2008; Franc and Kakkar, 2009), as well as click chemistry, such as Diels-Alder (Franc and Kakkar, 2009), azide-alkyne (Franc and Kakkar, 2008), and thiol-ene reactions (Killops et al., 2008). This allows for tight control of the number of branches and results in a homologous NP with predictable properties (Landmark et al., 2008; Yang et al., 2012; Bugno et al., 2016). Unlike other polymeric NPs, the most common dendrimers for delivery are PAMAM and poly (propylene polybenzy isocyanate) (PPI) dendrimers, with other examples including poly (l-lysine), polyester, and polyether dendrimers (Kesharwani et al., 2014). Diameters can range anywhere from 1–sub-100 nm with increasing generation, but tend to favor smaller sizes of ≤20 nm for delivery (Franiak-Pietryga et al., 2018).
Micelles: NP core/shell structures, composed of amphiphilic block copolymer subunits, that self-assemble into uniform morphologies when exposed to aqueous solvents (Croy and Kwon, 2006; Zhang et al., 2014). This leads to the formation of a hydrophobic core surrounded by a hydrophilic corona, with or without peripheral reactive functional groups [that can then be further modified with polymer coatings (e.g. PEGylation) or conjugated to ligands (Choi et al., 2003)]. Differences in copolymer composition, length, and solvent interaction can further alter micelle characteristics, including size and shape, leading to the formation of spheres, vesicles, rods, tubules, or lamellae (Zhang et al., 2014). The copolymer subunits themselves are synthesized similarly to linear polymers. This can involve either site selective chain-growth polymerization utilizing an existing chain as the initiator (de Espinosa and Meier, 2011), limited step-growth polymerization with two polymer species (Delaittre et al., 2009), or click chemistry between two available functional groups [such as an azide and an alkyne in the presence of cationic Cu(I)] (Li et al., 2010; Engler et al., 2013). Spherical micelles can range from 10 to 100 nm in diameter, while polymersomes dimensions can range from 50 nm to 5 µm (Croy and Kwon, 2006; Zhang et al., 2014; Zhang and Zhang, 2017).
Emulsions: Three-dimensional networks of crosslinked hydrophilic or amphiphilic polymers, including emulsions, nanogels, and hydrogels (Kabanov and Vinogradov, 2009; Chacko et al., 2012; Kousalova and Etrych, 2018). These networks form either through physical interactions, such as hydrophobic effects, or through chemical interactions, such as hydrogen bonding, electrostatic and ionic interactions, or the formation of chemical bonds (Kabanov and Vinogradov, 2009). This generates an extensive “swollen” polymer mesh that displays the properties of both a conventional NP system and a hydrogel. This includes being highly absorbent (Hoshino et al., 2012), possessing upper diameters far larger than other polymeric NPs, an extensive capacity for API loading and release (Chen et al., 2010), viscoelasticity (Mamaghani et al., 2015), and temperature dependent phase transitions (Hoshino et al., 2012). Depending on the base network, emulsions can range widely from 10 to a few thousands of nanometers in size (Kabanov and Vinogradov, 2009).
FIGURE 4.
Schematic illustration of the different polymeric NP formulations, with their relative sizes, summarized in Box 1.
The extensive variety of polymeric NPs has given rise to a broad list of aerosolized formulations in the literature. This includes NPs tested against lung tumors (orthotopic and small cell) (Bhattarai et al., 2007; Rosiere et al., 2016; Qiao et al., 2017; Yan et al., 2017; Elbatanony et al., 2020; Vaidya et al., 2020), type 2 diabetes (Shrestha et al., 2015), biofilms and lung infections (Cheow et al., 2010; Casciaro et al., 2019), pneumonia (Shah et al., 2013), tuberculosis (Pandey et al., 2003; Costa-Guoveia et al., 2017), asthma (Yoo et al., 2013), and other respiratory diseases (Beck-Broichsitter et al., 2010; Kolte et al., 2017). The majority of these make use of nanospheres, including PLGA, poly (ethylenimine) (PEI), PCL, and porous silicon (PSi) nanospheres, and micelles, including PLGA-co-polyspermine (PSPE) and folate-PEG-dextran copolymers. Much like liposomes, polymeric NPs have also already been extensively tested with common pulmonary delivery methods, like vibrating-mesh (Beck-Broichsitter et al., 2012a; Beck-Broichsitter et al., 2012b; Beck-Broichsitter et al., 2014a), air-jet (Beck-Broichsitter et al., 2012b), and ultrasonic nebulizers (Beck-Broichsitter et al., 2012b). Special attention has been placed on developing dry powder formulations, such as through spray freeze drying, which enhances the stability of sensitive polymer NPs and allows for finer control of delivered particles (Cheow et al., 2011; D’Addio et al., 2013; Ali and Lamprecht, 2014). However, despite these current advances, many polymeric NP erosolization studies remain in pre-clinical in vitro stages. This is most visible for clinical trials, with no currently reported studies utilizing a polymeric NP aerosol. While a promising and robust alternative to liposomes, this demonstrates that polymeric NP aerosols are still in their infancy, with no sign of an approved product in the near future.
Despite this, polymer NPs are comparatively well represented when it comes to EMT-mediated pulmonary delivery, with currently studied formulations including emulsions, nanospheres, and micelles. These are summarized in Table 4. Like with liposomes, the majority of these utilize APIs loaded into a lipophilic core, including pirfenidone (a drug used to treat pulmonary fibrosis) (Singh et al., 2019), MDB5 (a novel form of Hedgehog (Hh) inhibitor GDC-0449) (Kumar et al., 2019), salinomycin (an antibiotic with anti-cancer properties) (Sousa et al., 2019), and doxorubicin (an anthracycline chemotherapeutic) (Fang et al., 2014; Seifi-Najmi et al., 2016). Multiple formulations also make use of siRNA targeting genes along the EMT pathway (Fang et al., 2014; Seifi-Najmi et al., 2016; Li et al., 2019; Suresh et al., 2019), including one directly conjugated to PEI to form a polyplex (Ding et al., 2018; Wang Y. et al., 2019), as well as an inhibitor of CXCR4 (Suresh et al., 2019), a chemokine receptor involved in the promotion of tumor migration and EMT (Lin et al., 2018). These all resulted in the inhibition of key EMT-biomarkers, including PAI-1, TGF-β, VEGF, Hh, Vimentin, and N-Cadherin. This led to decreased collagen deposition (Wang Y. et al., 2019; Kumar et al., 2019; Li et al., 2019), cell migration and metastasis (Fang et al., 2014; Seifi-Najmi et al., 2016; Sousa et al., 2019), and tumor growth (Suresh et al., 2019). Polymeric NPs increased lung penetration and retention, provided stability by a polymer-siRNA conjugate, and facilitated codelivery further enhanced API efficacies.
TABLE 4.
A summary of current erosolized polymeric NP formulations targeting EMT-markers in the literature.
| NP | API(s) | Disease | Model(s) | Effects | Ref |
|---|---|---|---|---|---|
| Poly (ethylenimine) (PEI-C) polyplex | siPAI-1 | Pulmonary fibrosis (PF) | PF mice | Inhibition of PAI-1 and TGF-β, decreased collagen deposition in lungs | Ding et al. (2018), Wang Y. et al. (2019) |
| Perfluorocarbon (PFC) nanoemulsion | CXCR4 antagonist + anti-STAT3 siRNA | Lung metastasis | 4T1.Luc mice | Inhibition of VEGF, decreased tumor growth and increased survival | Li et al. (2019) |
| Poly (ɛ-caprolactone) (PCL) | Pirfenidone | Pulmonary fibrosis (PF) | A549 cells | Inhibition of TGF- β1 mRNA and EMT | Singh et al. (2019) |
| PEG-PCC-g-DC micelles | MDB5 | Liver fibrosis | CBDL mice | Inhibition of hh activity, HSC activation, and decreased collagen deposition | Kumar et al. (2019) |
| Pluronic F127 (PM) micelles | Salinomycin (SAL) | Lung cancer | A549 cells | Inhibition of VIM and EMT, reduced cell migration | Sousa et al. (2019) |
| PEG-PLL-PLLeu Polypetide micelles | ZEB1 siRNA + doxorubicine | Non-small cell lung cancer | H460 mice | Inhibition of EMT and metastasis | Fang et al. (2014) |
| CMD-chitosan nanoparticles | siRNA + doxorubicin | Lung cancer | A549 cells | Inhibition of EMT markers, reduced cell migration | Seifi-Najmi et al. (2016) |
| EGFR-conjugated gelatin nanoparticles | siRNA | Non-small cell lung cancer | H820 + H1975 cells | Inhibition of Vimentin, N-Cadherin, and EMT | Suresh et al. (2019) |
While a quickly growing subfield in the aerosol delivery of NPs, concerns remain for polymeric NPs that must be addressed before they can properly compete with liposomes. Perhaps the largest of these is induced aggregation. When passed through a nebulizer, polymeric NPs, especially those with high surface hydrophobicity, can exhibit extensive aggregation within aerosol droplets (Dailey et al., 2003b). This effect can be further pronounced depending on the specific nebulizer used—for instance, NPs administered through jet nebulizers appear more susceptible to aggregation than those passed through ultrasonic nebulizers under the same conditions (Dailey et al., 2003b). Polymer NP variation can also have a profound effect. Charge, for example, influences the stability of polymeric NPs in erosol solvents, with anionic formulations being more stable than cationic (Dailey et al., 2003a). Additionally, non-biodegradable polymer nanospheres, such as polystyrene, have been implicated in the promotion of pulmonary inflammation (Dailey et al., 2006). Though current evidence suggests that biodegradable polymeric NPs avoid this same inflammatory response, care will still need to be taken in preventing potentially irritating or toxic doses. Finally, polymeric NPs can suffer from some of the same biological limitations as liposomes, including susceptibility to opsonization and enhanced clearance (Owens and Peppas, 2006), the ABC phenomenon (Ishihara et al., 2009; Saadati et al., 2013), and concerns over long term accumulation and toxicity in organs like the liver and brain (Raghnaill et al., 2014). Despite these potential disadvantages, the broad possibilities of polymeric NPs cause them to remain a tantalizing prospective for future erosol delivery against EMT-mediated fibrosis. Especially as other, more conventional NPs lag even further behind in this area of study.
Inorganic Nanoparticles
Although a popular option for other routes and diseases, inorganic NPs have so far rarely been used for pulmonary fibrosis therapy. One contributing factor may be that, unlike liposomes and polymeric NPs, these NPs are composed of inorganic materials that are not readily present or biocompatible in lung tissues. For drug delivery, this often includes gold (Au), silver (Ag), iron (Fe), and zinc (Zn), and to a lesser extent titanium (Ti) and silicon (Si), either in their pure form or as a compound (e.g., oxides, hydroxides, borohydrides, chlorides, sulfides, and sulfates) (Mody et al., 2010). Inorganic NPs typically consist of a spherical, inorganic, electrostatically charged core functionalized with targeting ligands or other moieties. Other, less common shapes include diamond, octagonal, rods, and thin sheets (Xia et al., 2008; Kinnear et al., 2017). The most common formulation strategy begins with the nucleation of metal ion complexes in a colloidal solution using a reducing agent (Mody et al., 2010). The inorganic NP can then undergo different forms of surface chemistry. This can include ligand exchange reactions, wherein a inorganic NP is conjugated to any number of biomolecules (including DNA, RNA, proteins, and polymers such as PEG) utilizing weak binding between carboxyl groups and the NP surface (Kanninen et al., 2008), or ligand removal of existing surface features, such as through temperature elevation, ablation, or electrochemical etching (Niu and Li, 2014; Lu et al., 2018). This can introduce functionality to the inorganic NP, like ligand-mediated active targeting, enhance biocompatibility and stability, or facilitate the loading of APIs. Reported inorganic NPs have a wide range of sizes, though nanomedicine formulations tend to be within the range of 1–100 nm (Mody et al., 2010).
Despite the current lack of prominent aerosolized inorganic NPs being studied in the literature, they possess certain properties that makes prospective formulations appealing, particularly in targeting the EMT pathway. One intrinsic advantage of many inorganic NPs is an innate therapeutic inhibition of the EMT process. Even unmodified inorganic NPs have been found to inhibit cell proliferation, reduce expression of key EMT markers such as Snail, N-Cadherin, and Vimentin, and overall suppress oncogenic and inflammatory signaling pathways (Arvizo et al., 2013; Xiong et al., 2019). Additionally, inorganic NPs can also be used for plasmonic photothermal therapy (PPTT), wherein infiltrated cells can be locally heated by an irradiated laser (using a specific plasmon-resonant absorption wavelength) without damaging surrounding tissues (Huang et al., 2008). While primarily utilized in anti-cancer therapies, this technique has also been found to cause inhibition of the EMT pathway (Wu et al., 2018). Other advantages include excellent stability, wide availability, ease of use, and the straightforward scale-up for large scale production.
Consistent with their lack of aerosolized formulations in the literature, inorganic NPs have been used less frequently than other NPs to target EMT-mediated fibrosis. Current examples are nevertheless summarized in Table 5. Unlike other formulations, the majority of these to do not involve additional APIs loaded into the metallic core. Instead, the intrinsic EMT inhibition of various metals were utilized, including those of Zn (Wahab et al., 2016; Peng et al., 2018), Si (Peng et al., 2018), Au (Kaushik et al., 2016), Ti (Li et al., 2018a), and Ag (Sunil Gowda et al., 2018). Two APIs that were used, however, include cold plasma (a partially ionized gas (that is externally discharged using an air plasma device with antibacterial and wound healing properties) (Kaushik et al., 2016) and gallic acid [an antioxidant capable of inhibiting glioma cell metastasis and radiation-induced reactive oxygen species (ROS)] (Sunil Gowda et al., 2018). Both were added as promising novel inhibitors of EMT markers (specifically PI3K and vimentin, N-cadherin, and Snail-1, respectively) to enhance the existing anti-fibrotic efficacy of their inorganic NPs. In general, common EMT markers targeted by inorganic NPs included N-cadherin, E-cadherin, and TGF-β (Wahab et al., 2016; Li et al., 2018b; Peng et al., 2018). This in turn led to significant inhibition of EMT, reducing cell migration, tumor growth, and treatment-induced radiation toxicity and resistance. Thus, unlike other NP formulations which only promoted the delivery of APIs, the inorganic NPs here provided key inhibition themselves, in addition to other advantages like enhanced stability and cell penetration and accumulation.
TABLE 5.
A summary of current erosolized inorganic NP formulations targeting EMT-markers in the literature.
| NP | API(s) | Disease | Model(s) | Effect | Ref. |
|---|---|---|---|---|---|
| ZnO2 and SiO2 NPs | N/A | Fibrosis | LX-2 cells | Inhibition of N-Cadherin and EMT, elevation of E-Cadherin | Peng et al. (2018) |
| PEG-coated Au NPs | Cold plasma | Lung cancer | T98G + A549 cells | Inhibition of PI3K/AKT axis and EMT | Kaushik et al. (2016) |
| TiO2 NPs | N/A | Lung cancer | A549 cells | Inhibition of TGF-β and EMT, reduced cell migration | Li et al. (2018a) |
| ZnO nanostructures | N/A | Lung cancer | H460 + MRC5 cells | Inhibition of N-Cadherin and EMT | Wahab et al. (2016) |
| Ag NPs | Gallic acid | Lung cancer | A549 cells | Inhibition of Vimentin, N-Cadherin, and Snail-1 | Sunil Gowda et al., (2018) |
Despite the unique and promising advantages provided by inorganic NPs, however, significant disadvantages remain which challenge their application for pulmonary fibrosis treatment. Perhaps the most significant is localized toxicity. As previously mentioned, inorganic NPs are composed of metals which are not naturally found in significant quantities in the lungs. Exposure to these NPs (at industrial and biomedical levels) has been found to cause acute pulmonary inflammation (Cho et al., 2010; Cho et al., 2012a; Cho et al., 2012b) and oxidative stress (Limbach et al., 2007). Specifically, exposure to common inorganic NPs led to elevated neutrophil and eosinophil recruitment and infiltration into lung tissues (Cho et al., 2010; Cho et al., 2012a), the extent of which appears dependent on the relationship between zeta potential and solubility (Cho et al., 2012b). This includes for carbon (C), cerium (Ce), Ti, Si, nickel (Ni), Zn, and Cu NPs, especially in their pure or oxide forms. However, other studies have demonstrated that biomedical quantities of inorganic NPs are no more toxic than naturally occuring dusts and other aerosols (Veranth et al., 2007). In either case, pulmonary toxicity will remain a concern for any potential aerosol formulation. Additionally, inorganic NPs in general can exihibit elevated systemic clearance by the kidneys and the reticuloendothelial system (RES) in the liver, spleen, and lungs, penetration through vascular endothelium and the blood brain barrier (BBB), and potentially toxic tissue accumulation (Li and Chen, 2011). Finally, due to the current lack of relevant examples in the literature, the potential for aerosolized delivery of inorganic NPs is largely unknown currently. However, the existing use of aerosol vapor deposition for inorganic NP films (Palgrave and Parkin, 2006) and other naturally occurring erosols (Quadros and Marr, 2012) both suggest its feasibility. For these reasons, future treatments for pulmonary fibrosis may lie in formulations that are closer in design, not farther, to naturally occurring lung vesicles.
Exosomes
Exosomes represent perhaps the most novel formulations currently being explored for pulmonary delivery. A type of membrane-bound extracellular vesicle (EV), small vesicles naturally derived from pro- and eukaryotic cells, exosomes are a more homogenous population of EVs—sharing a similar spherical shape and sizes that range from ∼50–150 nm—that are uniquely rich in cholesterol and diacylglycerol (Ellis and Kuehn, 2010; Silverman and Reiner, 2011; Abels and Breakefield, 2016). They are generated when the plasma membrane of a cell “buds” inwardly to form an intermediate endosome-vesicle known as the multivesicular bodies. These multivesicular bodies are then either degraded by lysosomes or fuse with the plasma membrane to be released as exosomes, which in turn intersperses endosomal surface proteins into the exosome bilayer (Fevrier and Raposo, 2004; Colombo et al., 2014). This helps differentiate exosomes as well as provides unique characteristics depending on the origin cell (Thery et al., 2002). However, there is still some debate on the exact definition, and there is considerable overlap in size and morphology between exosomes and the two other categories of EVs: apoptotic bodies and micro vesicles. Additionally, the exact biological functions of exosomes remain somewhat unclear, though they are known to act as miRNA transporters between cells with important regulatory effects for intracellular mRNA activity (Valadi et al., 2007). Other suggested functions include elimination of undegraded endosomal and lysosomal proteins and membranes, regulation of T cell activity, and antigen transfer to dendritic cells (Thery et al., 2002).
As delivery vehicles, exosomes share many similarities with liposomes, including similar size and morphology (as lipid bilayers) and the ability to encapsulate both hydrophilic and lipophilic APIs (Johnsen et al., 2014; van der Meel et al., 2014). However, the complex composition of surface proteins on exosomes provides additional benefits, which most notably includes enhanced organotropism—that is, exosomes are capable of efficient targeting and uptake by specific recipient cells related to the surface proteins on the original plasma membrane (Antimisiaris et al., 2018). Additionally, the unique natural lipid composition of exosomes makes them highly biocompatible, while selection of autologous exosomes can further prevent any adverse patient immune responses (Antimisiaris et al., 2018).
Examples of aerosolized or inhaled exosomes, though limited, do exist in the literature, including as a potential therapy for tuberculosis (Cheng and Schorey, 2013), lung inflammation (Zhang et al., 2018), pulmonary fibrosis (Dinh et al., 2020), and the general delivery of therapeutic miRNA (Zhang et al., 2017). Overwhelmingly, the exosomes used were derived from alveolar macrophages (Cheng and Schorey, 2013; Zhang et al., 2017; Zhang et al., 2018), though one study did utilize exosomes derived from mesenchymal stem cells instead (Dinh et al., 2020). This same study also utilized a compressed air nebulizer for successful aerosol delivery (Dinh et al., 2020), suggesting that clinically relevant nebulizer systems could be viable for a future exosome formulation. However, comprehensive studies characterizing the physical properties of aerosolized exosomes still appear to be missing. Interestingly, despite the overall current lack of studies on the efficacy and patient safety of aerosolized exosome formulations, several clinical studies have recently surfaced due to the coronavirus disease 2019 (COVID-19) pandemic. These are summarized in Table 6.
TABLE 6.
A summary of current clinical trials for erosolized exosomes.
| NP | APIs | Target disease | Phase | Status | Sponsor | Identifier |
|---|---|---|---|---|---|---|
| CSTC-derived exosomes | N/A | Coronavirus + pneumonia | I | Active | TC ericyes university | NCT04389385 |
| MPC-derived exosomes | N/A | Drug resistance | I/II | Recruiting | Ruijin hospital | NCT04544215 |
| MSC-derived exosomes | N/A | Coronavirus | I | Completed in 2020 | Ruijin hospital | NCT04276987 |
| MSC-derived exosomes | N/A | Healthy tolerance | I | Recruiting | Ruijin hospital | NCT04313647 |
| MSC-derived exosomes | N/A | ARDS | I/II | Not yet recruiting | Ruijin hospital | NCT04602104 |
CSTC, COVID-19 specific T cell; MPC, mesenchymal stem/progenitor cell; MSC, mesenchymal stem cell; ARDS, acute respiratory distress syndrome.
Similarly, the use of exosomes for EMT-targeting appears to still be in its infancy, with no current examples in the literature of erosolized exosomes specific for the pathway. However, examples of non-aerosolized formulations do exist. This includes for intrauterine adhesion (Yao et al., 2019), liver fibrosis (Li et al., 2012), acute lung injury (ALI) (Xiao et al., 2020), and cancer [including colorectal (Zhang X. et al., 2020), liver (Chen et al., 2018), thyroid (Hardin et al., 2018), and lung (Zhao et al., 2018)]. In the majority of these studies, MSC-derived exosomes were used [including from bone marrow (Yao et al., 2019) and umbilical cord (Li et al., 2012; Zhao et al., 2018)], though others involved exosomes derived directly from carcinomas (Chen et al., 2018; Zhang X. et al., 2020) or stem-like cells (Hardin et al., 2018). Like inorganic NPs, most of the exosomes involved no additional API and instead relied on the intrinsic EMT-inhibition of the derived surface proteins. However, one exception did utilize the additional delivery of an exosomal miRNA (Zhang X. et al., 2020). In all instances, EMT inhibition was achieved through the regulation of key biomarkers, including VIM, TGF-β, Smad2/3, FSP-1, E-Cadherin, CK19, NF-κB, ERK, JNK, and the Akt and hedgehog pathways. This highlights the inherent advantages to using an exosome formulation directly cultured from EMT-associated cells, with the potential for similar results for exosomes derived from fibrotic lung epithelial cells.
Even with the unique advantages provided by exosomes, concerns remain for any future erosol formulations that must be addressed. For one, exosomes have also been linked to the promotion of EMT for a variety of diseases, notably for cancers [including renal (Wang L. et al., 2019), esophageal (Min et al., 2018), pancreatic (Li et al., 2018b), breast (Wang H. et al., 2019), and lung (He et al., 2019)]. Care will need to be taken in selecting a model exosome for fibrosis therapy which does not induce further long-term tissue injury, especially given the unknown physiological functions of many EVs. Additionally, the exosomal surface can quickly become a disadvantage for the design of more robust formulations or combination therapies—due to the complex nature of surface proteins and the current lack of synthetic alternatives to natural biogenesis, the controlled modification of exosomes with other NP strategies, such as PEGylation, is difficult if not impossible (Antimisiaris et al., 2018). This has led to increased interest in engineered exosomes, so called “EV-mimetics,” in recent years as one potential solution to limited natural yields and to facilitate more varied chemistry. Finally, exosomes in general suffer several therapeutic limitations, including rapid in vivo blood clearance, methods which result in low drug loading and retention, and difficult and costly synthesis strategies with limited yields (Antimisiaris et al., 2018).
Other Directions and Considerations
Although promising NP formulations for targeting EMT-mediated pulmonary fibrosis already exist, there are additional directions which could be pursued for further benefits. For example, current therapies largely rely on the passive delivery of APIs (whether drugs or genetic material) or the active targeting of the encapsulated moiety itself. Instead, ligands, especially antibodies and synthetic peptides, could be conjugated to the surface of NP formulations to facilitate active targeting. These sequences could be derived from a multitude of EMT-related axis and phenotypes. For example, a ligand utilizing a passive targeting approach could target an overexpressed protein characteristic of fibrosis, such as fibronectin (Torikata et al., 1985), while a ligand inhibitor utilizing a more active targeting approach could instead target a receptor along the EMT pathway itself, such as TGF-β family receptors, tyrosine kinase receptors, and numerous integrins (Stone et al., 2016). Similar targeting strategies have already demonstrated success in anti-cancer NP formulations, many of which could easily be translated to target the EMT pathway (Shargh et al., 2015; Jeong et al., 2018).
Additionally, more focus could be placed on developing combination therapies. Drug molecules (anti-inflammatory, fibrotic, and oxidation), genetic material (siRNA), and artificial proteins (antibodies and peptides) could all be combined utilizing a robust NP platform. For example, protein-NP conjugates have demonstrated the ability to significantly increase the potential efficacy of existing therapeutic formulations (Jeong et al., 2020). This could lead to the development of more adaptable therapies capable of overcoming the efficacy and toxicity limitations of other, more conventional therapies. This has become even more important in recent years as increased awareness of genetic variation in patients, including for pulmonary fibrosis, has brought into question the past rationale of a “one-size-fits-all” therapeutic (Kaur et al., 2017). Again, this approach has already found promising success in the development of anti-cancer formulations (Nam et al., 2019).
Despite the many promising advantages of nanomedicines, the treatment of EMT-mediated pulmonary fibrosis provides unique challenges for NP therapeutics. For one, NPs have been linked to the facilitation of EMT and lung fibrosis themselves. Though most frequently caused by industrial pollutants such as cerium oxide, silver, and silica NPs (Ma et al., 2017; Wang et al., 2017; Gliga et al., 2018), the elevated infiltration and retention of other NP formulations can still toe the line between beneficial and toxic and must be carefully analyzed (Fattal et al., 2014). Inorganic NPs in particular run the risk of becoming acutely toxic to local tissues, but polymeric NPs and even liposomes and SLNPs can still lead to lung damage if not safely absorbed and eventually cleared.
The ability of NP formulations to be properly administered using common pulmonary delivery methods, such as through nebulizers, pressurized metered-dose inhalers, and dry powder inhales (DPIs), can also be a limiting factor (Dailey et al., 2003b). Aggregation, especially, becomes a frequent concern when NPs are aerosolized through fine pores. Liposomes, micelles, and other spontaneously assembled NPs, which rely on maintaining specific concentrations or solvent conditions, can also become unstable when subjected to the erosolization process. Despite this, however, numerous NP formulations have already demonstrated successful aerosolization, including liposomes (Zaru et al., 2009; Arora et al., 2010), polymeric NPs (Doan and Oliver, 2009; Ungaro et al., 2009; Beck-Broichsitter et al., 2010), and even Cu/Zn NPs (Langenback et al., 1999). This indicates that the aerosolization of these novel formulations is certainly a surmountable obstacle worth investigating.
Conclusion
In conclusion, the IPF has substantial morbidity and mortality for which no curative treatments are available. In this chapter, we have reviewed the prevailing epithelial injury hypothesis. In this model, epithelial injury-induced EMT drives the expansion of pathogenic myofibroblast populations. Myofibroblasts are principal components of ECM production that result in airspace loss and mortality. We review the substantial mechanistic understanding of EMT, and the recent work demonstrating that reversible histone modifications are an important process regulating gene expression programs controlling mesenchymal transition. This mechanistic work has focused on the central role of BRD4 in mediating EMT and myofibroblast transition and initial preclinical work has provided evidence of efficacy in multiple models of fibrosis, including bleomycin, radiation, TGF-β induced and viral induced remodeling. Advancing epigenetic regulators as therapeutics will be challenging. Additionally, we review the current state of nanomedicine with a focus on formulations that hold promise for erosol delivery and EMT-targeting, including liposomes, polymeric NPs, inorganic NPs, and exosomes. These agents can provide a host of benefits, including controlled release for enhanced therapeutic efficacy, reduced systemic toxicity, and intrinsic inhibitory effects against common EMT biomarkers. Many of these NP formulations have already demonstrated success as erosolized formulations in both the lab and clinic. With further development, NP-based approaches for pulmonary delivery offer the substantial promise to modify epigenetic regulators of EMT and advance treatments for IPF further than current strategies allow. This could open up a new field of study centered on the EMT pathway which combines new physiological understandings and state-of-the-art advances in nanoparticle delivery.
Author Contributions
Writing: MS, AD, and MP. Editing: MS, AD, MP, SH, and AB.
Funding
This work was supported through grants from the National Institute of Allergy and Infectious Diseases (NIAID 2P01AI062885 (AB), NCATS UL1TR002373 (AB)), Falk Q24 Catalyst Award (AB and SH), and the National Science Foundation (NSF DMR-1808251 (SH)).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Ksenija Bernau, Nathan Sandbo and Sandro Mecozzi of the University of Wisconsin-Madison for helpful discussions.
References
- Abels E. R., Breakefield X. O. (2016). Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 36, 301312 10.1007/s10571-016-0366-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis F., Pridgen E., Molnar L. K., Farokhzad O. C. (2008). Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 5, 505–515. 10.1021/mp800051m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. E., Lamprecht A. (2014). Spray freeze drying for dry powder inhalation of nanoparticles. Eur. J. Pharm. Biopharm. 87, 510–517. 10.1016/j.ejpb.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Allen S. D., Sorensen K. N., Neial M. J., Durrant C., Proffit R. T. (1994). Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-lipsomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. J. Antimicrob. Chemother. 34, 1001–1013. 10.1093/jac/34.6.1001 [DOI] [PubMed] [Google Scholar]
- Alshamsan A., Haddadi A., Hamdy S., Samuel J., El-Kadi A. O. S., Uludağ H., et al. (2010). STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol. Pharm. 7, 1643–1654. 10.1021/mp100067u [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Shive M. S. (2012). Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 64, 72–82. 10.1016/j.addr.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Antimisiaris S. G., Mourtas S., Marazioti A. (2018). Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 10 (4), 218 10.3390/pharmaceutics10040218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D., K. Goyal A., R. Paliwal S., Khurana B., P. Vyas S. (2010). Oral mucosal immunization: recent advancement and future prospects. Curr. Immunol. Rev. 6, 234–259. 10.2174/157339510791823637 [DOI] [Google Scholar]
- Arvizo R. R., Saha S., Wang E., Robertson J. D., Bhattacharya R., Mukherjee P. (2013). Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. Unit. States Am. 110, 6700–6705. 10.1073/pnas.1214547110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanazio R. (2012). Airway disease: similarities and differences between asthma, COPD and bronchiectasis. Clinics 67 (11), 1335–1343. 10.6061/clinics/2012(11)19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. (2009). How corticosteroids control inflammation: quintiles prize lecture 2005. Br. J. Pharmacol. 148 (3), 245–254. 10.1038/sj.bjp.0706736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Broichsitter M., Gauss J., Gessler T., Seeger W., Kissel T., Schmehl T. (2010). Pulmonary targeting with biodegradable salbutamol-loaded nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 23, 47–57. 10.1089/jamp.2009.0759 [DOI] [PubMed] [Google Scholar]
- Beck-Broichsitter M., Kleimann P., Gessler T., Seeger W., Kissel T., Schmehl T. (2012a). Nebulization performance of biodegradable sildenafil-loaded nanoparticles using the Aeroneb Pro: formulation aspects and nanoparticle stability to nebulization. Int. J. Pharm. 422, 398–408. 10.1016/j.ijpharm.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Beck-Broichsitter M., Kleimann P., Schmehl T., Betz T., Bakowsky U., Kissel T., et al. (2012b). Impact of lyoprotectants for the stabilization of biodegradable nanoparticles on the performance of air-jet, ultrasonic, and vibrating-mesh nebulizers. Eur. J. Pharm. Biopharm. 82, 272–280. 10.1016/j.ejpb.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Beck-Broichsitter M., Schmehl T., Gessler T., Seeger W., Kissel T. (2012c). Development of a biodegradable nanoparticle platform for sildenafil: formulation optimization by factorial design analysis combined with application of charge-modified branched polyesters. J. Contr. Release 157, 469–477. 10.1016/j.jconrel.2011.09.058 [DOI] [PubMed] [Google Scholar]
- Beck-Broichsitter M., Knuedeler M.-C., Oesterheld N., Seeger W., Schmehl T. (2014a). Boosting the aerodynamic properties of vibrating-mesh nebulized polymeric nanosuspensions. Int. J. Pharm. 459, 23–29. 10.1016/j.ijpharm.2013.11.040 [DOI] [PubMed] [Google Scholar]
- Beck-Broichsitter M., Ruppert C., Schmehl T., Günther A., Seeger W. (2014b). Biophysical inhibition of synthetic vs. naturally-derived pulmonary surfactant preparations by polymeric nanoparticles. Biochim. Biophys. Acta 1838, 474–481. 10.1016/j.bbamem.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Bergeron C., Tulic M. K., Hamid Q. (2010). Airway remodelling in asthma: from benchside to clinical practice. Can. Respir. J. J. Can. Thorac. Soc. 17 (4), e85–e93. 10.1155/2010/318029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai S. R., Kim S. Y., Jang K. Y., Yi H. K., Lee Y. H., Bhattarai N., et al. (2007). Amphiphilic triblock copolymer poly(p-dioxanone-co-L-lactide)-block-poly(ethylene glycol), enhancement of gene expression and inhibition of lung metastasis by aerosol delivery. Gene Ther. 14, 476–483. 10.1038/sj.gt.3302876 [DOI] [PubMed] [Google Scholar]
- Bitounis D., Fanciullino R., Iliadis A., Ciccolini J. (2012). Optimizing druggability through liposomal formulations: new approaches to an old concept. ISRN Pharm. 2012 738432 10.5402/2012/738432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhassani A., Javanzad S., Saleh T., Hashemi M., Aghasadeghi M. R., Sadat S. M. (2014). Polymeric nanoparticles. Hum. Vaccines Immunother. 10, 321–332. 10.4161/hv.26796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzuto G., Molinari A. (2015). Liposomes as nanomedical devices. Int. J. Nanomedicine 10, 975–999. 10.2147/ijn.s68861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A. R. (2019). Mechanisms how mucosal innate immunity affects progression of allergic airway disease. Expet Rev. Respir. Med. 13 (4), 349–356. 10.1080/17476348.2019.1578211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A. R. (2020). RSV reprograms the CDK9BRD4 chromatin remodeling complex to couple innate inflammation to airway remodeling. Viruses 12 (4), 472 10.3390/v12040472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A. R., Tian B., Jamaluddin M., Kalita M. K., Garofalo R. P., Lu M. (2011). RelA Ser276 phosphorylation-coupled Lys310 acetylation controls transcriptional elongation of inflammatory cytokines in respiratory syncytial virus infection. J. Virol. 85 (22), 11752–11769. 10.1128/JVI.05360-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A. R., Zhou J. (2020). Validation of the epigenetic reader bromodomain-containing protein 4 (BRD4) as a therapeutic target for treatment of airway remodeling. Drug Discov. Today 25 (1), 126–132. 10.1016/j.drudis.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. D., Lin C. Y., Duan Q., Griffin G., Federation A. J., Paranal R. M., et al. (2014). NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 56 (2), 219–231. 10.1016/j.molcel.2014.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugno J., Hsu H.-j., Hong S. (2015). Recent advances in targeted drug delivery approaches using dendritic polymers. Biomater. Sci. 3, 1025–1034. 10.1039/c4bm00351a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugno J., Hsu H.-J., Pearson R. M., Noh H., Hong S. (2016). Size and surface charge of engineered poly(amidoamine) dendrimers modulate tumor accumulation and penetration: a model study using multicellular tumor spheroids. Mol. Pharm. 13, 2155–2163. 10.1021/acs.molpharmaceut.5b00946 [DOI] [PubMed] [Google Scholar]
- Burgess H. A., Daugherty L. E., Thatcher T. H., Lakatos H. F., Ray D. M., Redonnet M., et al. (2005). PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 288, L1146–L1153. 10.1152/ajplung.00383.2004 [DOI] [PubMed] [Google Scholar]
- Casciaro B., d’Angelo I., Zhang X., Loffredo M. R., Conte G., Cappiello F., et al. (2019). Poly(lactide-co-glycolide) nanoparticles for prolonged therapeutic efficacy of esculentin-1a-derived antimicrobial peptides against Pseudomonas aeruginosa lung infection: in Vitro and in Vivo studies. Biomacromolecules 20, 1876–1888. 10.1021/acs.biomac.8b01829 [DOI] [PubMed] [Google Scholar]
- Chacko R. T., Ventura J., Zhuang J., Thayumanavan S. (2012). Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 64, 836–851. 10.1016/j.addr.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. M., Valencia P. M., Zhang L., Langer R., Farokhzad O. C. (2010). Polymeric nanoparticles for drug delivery. Cancer Nanotechnol. 624, 163–175. 10.1007/978-1-60761-609-2_11 [DOI] [PubMed] [Google Scholar]
- Chang H., Liu Y., Xue M., Liu H., Du S., Zhang L., et al. (2016). Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic Acids Res. 44 (6), 2514–2527. 10.1093/nar/gkw126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Guo P., He Y., Chen Z., Chen L., Luo Y., et al. (2018). HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 9, 513 10.1038/s41419-018-0534-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zheng X., Qian H., Mao Z., Ding D., Jiang X. (2010). Hollow Core−Porous shell structure poly(acrylic acid) nanogels with a superhigh capacity of drug loading. ACS Appl. Mater. Interfaces 2, 3532–3538. 10.1021/am100709d [DOI] [PubMed] [Google Scholar]
- Cheng Y., Schorey J. S. (2013). Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur. J. Immunol. 43, 3279–3290. 10.1002/eji.201343727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheow W. S., Chang M. W., Hadinoto K. (2010). Antibacterial efficacy of inhalable antibiotic-encapsulated biodegradable polymeric nanoparticles against E. coli biofilm cells. J. Biomed. Nanotechnol. 6, 391–403. 10.1166/jbn.2010.1116 [DOI] [PubMed] [Google Scholar]
- Cheow W. S., Ng M. L. L., Kho K., Hadinoto K. (2011). Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: effect of freeze-drying adjuvants. Int. J. Pharm. 404, 289–300. 10.1016/j.ijpharm.2010.11.021 [DOI] [PubMed] [Google Scholar]
- Cho W.-S., Duffin R., Poland C. A., Duschl A., Oostingh G. J., MacNee W., et al. (2012a). Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ionsin vitroandin vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 6, 22–35. 10.3109/17435390.2011.552810 [DOI] [PubMed] [Google Scholar]
- Cho W.-S., Duffin R., Thielbeer F., Bradley M., Megson I. L., MacNee W., et al. (2012b). Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles. Toxicol. Sci. 126, 469–477. 10.1093/toxsci/kfs006 [DOI] [PubMed] [Google Scholar]
- Cho W.-S., Duffin R., Poland C. A., Howie S. E. M., MacNee W., Bradley M., et al. (2010). Metal oxide nanoparticles induce unique inflammatory footprints in the lung: important implications for nanoparticle testing. Environ. Health Perspect. 118, 1699–1706. 10.1289/ehp.1002201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. W., Kim W. S., Kim J. H. (2003). Surface modification of functional nanoparticles for controlled drug delivery. J. Dispersion Sci. Technol. 24, 475–487. 10.1081/dis-120021803 [DOI] [Google Scholar]
- Cipolla D., Gonda I., Chan H.-K. (2013). Liposomal formulations for inhalation. Ther. Deliv. 4, 1047–1072. 10.4155/tde.13.71 [DOI] [PubMed] [Google Scholar]
- Colombo M., Raposo G., Théry C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Conley J., Yang H., Wilson T., Blasetti K., Di Ninno V., Schnell G., et al. (1997). Aerosol delivery of liposome-encapsulated ciprofloxacin: aerosol characterization and efficacy against Francisella tularensis infection in mice. Antimicrob. Agents Chemother. 41, 1288–1292. 10.1128/aac.41.6.1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Guoveia J., Pancani E., Jouny S., Machelart A., Delorme V., Salzano G., et al. (2017). Combination therapy for tuberculosis treatment: pulmonary administration of ethionamide and booster co-loaded nanoparticles. Nature 7, 5390 10.1038/s41598-017-05453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward W. R., Watts K., Feghali-Bostwick C. A., Knox A., Pang L. (2009). Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. MCB (Mol. Cell. Biol.) 29 (15), 4325–4339. 10.1128/mcb.01776-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy S., Kwon G. (2006). Polymeric micelles for drug delivery. Curr. Pharm. Des. 12, 4669–4684. 10.2174/138161206779026245 [DOI] [PubMed] [Google Scholar]
- D’Addio S. M., Chan J. G., Kwok P. C., Benson B. R., Prud’homme R. K., Chan H. K. (2013). Aerosol delivery of nanoparticles in uniform mannitol carriers formulated by ultrasonic spray freeze drying. Pharm. Res. 30, 2891–2901. 10.1007/s11095-013-1120-6 [DOI] [PubMed] [Google Scholar]
- Dailey L. A., Jekel N., Fink L., Gessler T., Schmehl T., Wittmar M., et al. (2006). Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol. Appl. Pharmacol. 215, 100–108. 10.1016/j.taap.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Dailey L. A., Kleeman E., Wittmar M., Gessler T., Schmehl T., Roberts C., et al. (2003a). Surfactant-free, biodegradable nanoparticles for aerosol therapy based on the branched polyesters, DEAPA-PVAL-g-PLGA. Pharmacuetical Res. 20, 2011–2020. 10.1023/b:pham.0000008051.94834.10 [DOI] [PubMed] [Google Scholar]
- Dailey L. A., Schmehl T., Gessler T., Wittmar M., Grimminger F., Seeger W., et al. (2003b). Nebulization of biodegradable nanoparticles: impact of nebulizer technology and nanoparticle characteristics on aerosol features. J. Contr. Release 86, 131–144. 10.1016/s0168-3659(02)00370-x [DOI] [PubMed] [Google Scholar]
- Dams E. T., Laverman P., Oyen W. J., Storm G., Scherphof G. L., van Der Meer J. W., et al. (2000). Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Therapeut. 292, 1071–1079. [PubMed] [Google Scholar]
- Davda J., Labhasetwar V. (2002). Characterization of nanoparticle uptake by endothelial cells. Int. J. Pharm. 233, 51–59. 10.1016/s0378-5173(01)00923-1 [DOI] [PubMed] [Google Scholar]
- Davies L. A., Nunez-Alonso G. A., McLachlan G., Hyde S. C., Gill D. R. (2014). Aerosol delivery of DNA/liposomes to the lung for cystic fibrosis gene therapy. Hum. Gene Ther. Clin. Dev. 25, 97–107. 10.1089/humc.2014.019 [DOI] [PubMed] [Google Scholar]
- de Espinosa L. M., Meier M. A. R. (2011). Synthesis of star- and block-copolymers using ADMET: head-to-tail selectivity during step-growth polymerization. Chem. Commun. 47, 1908–1910. 10.1039/c0cc04161k [DOI] [PubMed] [Google Scholar]
- Delaittre G., Dire C., Rieger J., Putaux J.-L., Charleux B. (2009). Formation of polymer vesicles by simultaneous chain growth and self-assembly of amphiphilic block copolymers. Chem. Commun. 15, 2887–2889. 10.1039/b903040a [DOI] [PubMed] [Google Scholar]
- de Ruijter A. J. M. d., Gennip A. H. v., Caron H. N., Kemp S., Kuilenburg A. B. P. v. (2003). Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370 (Pt 3), 737–749. 10.1042/BJ20021321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah B. N., Case-Borden C., Gegonne A., Hsu C. H., Chen Q., Meerzaman D., et al. (2016a). BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat. Struct. Mol. Biol. 23 (6), 540–548. 10.1038/nsmb.3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah B. N., Gegonne A., Singer D. S. (2016b). Bromodomain 4: a cellular Swiss army knife. J. Leukoc. Biol. 100 (4), 679–686. 10.1189/jlb.2RI0616-250R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah B. N., Lewis B. A., Cherman N., Hewitt M. C., Albrecht B. K., Robey P. G., et al. (2012). BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc. Natl. Acad. Sci. U. S. A. 109 (18), 6927–6932. 10.1073/pnas.1120422109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A., Chitsaz F., Abbasi A., Misteli T., Ozato K. (2003). The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. U. S. A. 100 (15), 8758–8763. 10.1073/pnas.1433065100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Zhu C., Yu F., Wu P., Chen G., Ullah A., et al. (2018). Pulmonary delivery of polyplexes for combined PAI-1 gene silencing and CXCR4 inhibition to treat lung fibrosis. Nanomed. Nanotechnol. Biol. Med. 14, 1765–1776. 10.1016/j.nano.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Ding M., Zhang Q., Li Q., Wu T., Huang Y.-z. (2020). Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir. Med. 167, 105981 10.1016/j.rmed.2020.105981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh P.-U. C., Paudel D., Brochu H., Popowski K. D., Gracieux M. C., Coresand J., et al. (2020). Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 11, 1064 10.1038/s41467-020-14344-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan T. V. P., Olivier J. C. (2009). Preparation of rifampicin-loaded PLGA microspheres for lung delivery as aerosol by premix membrane homogenization. Int. J. Pharm. 382, 61–66. 10.1016/j.ijpharm.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Dou C., Dou C., Liu Z., Liu Z., Tu K., Tu K., et al. (2018). P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology 154 (8), 2209–2221.e14. 10.1053/j.gastro.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekladious I., Colson Y. L., Grinstaff M. W. (2019). Polymer-drug conjugate therapeutics: advances, insights and prospects. Nat. Rev. Drug Discov. 18, 273–294. 10.1038/s41573-018-0005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbatanony R. S., Parvathaneni V., Kulkarni N. S., Shukla S. K., Chauhan G., Kunda N. K., et al. (2020). Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—development and in-vitro efficacy. Drug Deliv. Transl. Res. 1–17. 10.1007/s13346-020-00802-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhissi A., Faizi M., Naji W., Gill H., Taylor K. (2007). Physical stability and aerosol properties of liposomes delivered using an air-jet nebulizer and a novel micropump device with large mesh apertures. Int. J. Pharm. 334, 62–70. 10.1016/j.ijpharm.2006.10.022 [DOI] [PubMed] [Google Scholar]
- Elhissi A. M. A., Taylor K. M. G. (2005). Delivery of liposomes generated from proliposomes using air-jet, ultrasonic, and vibrating-mesh nebulisers. J. Drug Deliv. Sci. Technol. 15, 261–265. 10.1016/s1773-2247(05)50047-9 [DOI] [Google Scholar]
- Ellis T. N., Kuehn M. J. (2010). Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94. 10.1128/mmbr.00031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. C., Lee H., Hammond P. T. (2013). Highly efficient “grafting onto” a polypeptide backbone by “click chemistry”. Angew. Chem. Int. Ed. 48 (49), 9334–9338. 10.1002/anie.200904070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada J., Peinado H., Lopez-Serra L., Setién F., Lopez-Serra P., Portela A., et al. (2011). Regulation of SNAIL1 and E-cadherin function by DNMT1 in a DNA methylation-independent context. Nucleic Acids Res. 39 (21), 9194–9205. 10.1093/nar/gkr658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge M. L., Campbell S. A., Erdman A. G., Haynes C. L., Wolf S. M., McCullough J. (2013). The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 9, 1–14. 10.1016/j.nano.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Van Winkle L. S., Fanucchi M. V., Plopper C. G. (1999). The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am. J. Respir. Cell Mol. Biol. 21 (6), 655–657. 10.1165/ajrcmb.21.6.3807 [DOI] [PubMed] [Google Scholar]
- Fang S., Wu L., Li M., Yi H., Gao G., Sheng Z., et al. (2014). ZEB1 knockdown mediated using polypeptide cationic micelles inhibits metastasis and effects sensitization to a chemotherapeutic drug for cancer therapy. Nanoscale 6, 10084–10094. 10.1039/c4nr01518e [DOI] [PubMed] [Google Scholar]
- Fattal E., Grabowski N., Mura S., Vergnaud J., Tsapis N., Hillaireau H. (2014). Lung toxicity of biodegradable nanoparticles. J. Biomed. Nanotechnol. 10, 2852–2864. 10.1166/jbn.2014.1939 [DOI] [PubMed] [Google Scholar]
- Fehrenbach H. (2001). Alveolar epithelial type II cell: defender of the alveolus revisited. Respir. Res. 2 (1), 33–46. 10.1186/rr36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez I. E., Eickelberg O. (2012). The impact of TGF-β on lung fibrosis. Proc. Am. Thorac. Soc. 9, 111–116. 10.1513/pats.201203-023aw [DOI] [PubMed] [Google Scholar]
- Fevrier B., Raposo G. (2004). Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 16, 415–421. 10.1016/j.ceb.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Flaherty K. R., Wells A. U., Cottin V., Devaraj A., Walsh S. L. F., Inoue Y., et al. (2019). Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 381 (18), 1718–1727. 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- Foster K. A., Yazdanian M., Audus K. L. (2001). Microparticulate uptake mechanisms of in-vitro cell culture models of the respiratory epithelium. J. Pharm. Pharmacol. 53, 57–66. 10.1211/0022357011775190 [DOI] [PubMed] [Google Scholar]
- Forster S., Antonietti M. (1999). Amphiphilic block copolymers in structure‐controlled nanomaterial hybrids. Adv. Mater. 10, 195–217. [DOI] [Google Scholar]
- Franc G., Kakkar A. (2008). Dendrimer design using CuI-catalyzed alkyne-azide “click-chemistry”. Chem. Commun. 42, 5267–5276. 10.1039/b809870k [DOI] [PubMed] [Google Scholar]
- Franc G., Kakkar A. K. (2009). Diels-alder “click” chemistry in designing dendritic macromolecules. Chem. Eur J. 15, 5630–5639. 10.1002/chem.200900252 [DOI] [PubMed] [Google Scholar]
- Franiak-Pietryga I., Ziemba B., Messmer B., Skowronska-Krawcyzk D. (2018). “Dendrimers as drug nanocarriers: the future of gene therapy and targeted therapies in cancer,” in Dendrimers—fundamentals and applications. Editor Simonescu C. M. (London, UK: IntechOpen; ). [Google Scholar]
- Gabizon A., Papahadjopoulos D. (1988). Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. U. S. A. 85, 6949–6953. 10.1073/pnas.85.18.6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Hu C.-M. J., Fang R. H., Zhang L. (2013). Liposome-like nanostructures for drug delivery. J. Mater. Chem. B 1 (48), 6569–6585. 10.1039/c3tb21238f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Liu X., Liang J., Habiel D. M., Vrishika K., Coelho A. L., et al. (2019). PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis. JCI Insight 4 (6), e125326 10.1172/jci.insight.125326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert B. E., Knight C., Alvarez F. G., Waldrep J. C., Rodarte J. R., Knight V., et al. (1997). Tolerance of volunteers to cyclosporine A-dilauroylphosphatidylcholine liposome aerosol. Am. J. Respir. Crit. Care Med. 156, 1789–1793. 10.1164/ajrccm.156.6.9702101 [DOI] [PubMed] [Google Scholar]
- Glavas L., Odelius K., Albertsson A. C. (2015). Tuning loading and release by modification of micelle core crystallinity and preparation. Polym. Adv. Technol. 26, 880–888. 10.1002/pat.3524 [DOI] [Google Scholar]
- Gliga A. R., Bucchianico S. D., Lindvall J., Fadeel B., Karlsson H. (2018). RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Nature 8, 6668 10.1038/s41598-018-25085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F. X., Karnik R., Wang A. Z., Alexis F., Levy-Nissenbaum E., Hong S., et al. (2007). Targeted nanoparticles for cancer therapy. Nano Today 2, 14–21. 10.1016/s1748-0132(07)70083-x [DOI] [Google Scholar]
- Guo L., Yan D. D., Yang D., Li Y., Wang X., Zalewski O., et al. (2014). Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 8, 5670–5681. 10.1021/nn5002112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann A. C., Gutierrez A. J., Bui L. T., Yahn S. L., Winters N. I., Calvi C. L., et al. (2020). Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 6 (28), eaba1972 10.1126/sciadv.aba1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin H., Helein H., Meyer K., Robertson S., Zhang R., Zhong W., et al. (2018). Thyroid cancer stem-like cell exosomes: regulation of EMT via transfer of lncRNAs. Lab. Invest. 98, 1133–1142. 10.1038/s41374-018-0065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Li Z., Yu Y., Zeng Q., Cheng Y., Ji W., et al. (2019). Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp. Cell Res. 379, 203–213. 10.1016/j.yexcr.2019.03.035 [DOI] [PubMed] [Google Scholar]
- Hickey J. W., Santos J. L., Williford J.-M., Mao H.-Q. (2015). Control of polymeric nanoparticle size to improve therapeutic delivery. J. Contr. Release 219, 536–547. 10.1016/j.jconrel.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Jones M., Davies D., Wang Y. (2019). Epithelial-mesenchymal transition contributes to pulmonary fibrosis via aberrant epithelial/fibroblastic cross-talk. J. Lung Health Dis. 3 (2), 31–35. 10.29245/2689-999x/2019/2.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B., Phan S. H., Thannickal V. J., Galli A., Bochaton-Piallat M. L., Gabbiani G. (2007). The myofibroblast: one function, multiple origins. Am. J. Pathol. 170 (176), 1807–1816. 10.2353/ajpath.2007.070112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holister P., Roman Vas C., Harper T. (2003). Dendrimers: technology white papers. Madrid, Spain: Científica. [Google Scholar]
- Hong S., Rattan R., Majoros I. J., Mullen D. G., Peters J. L., Shi X., et al. (2009). The role of ganglioside GM1in cellular internalization mechanisms of poly(amidoamine) dendrimers. Bioconjugate Chem. 20, 1503–1513. 10.1021/bc900029k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. C., Cui Z., Moore T. A., Meier T. R., Reddy R. C., Toews G. B., et al. (2006). Constitutive activation of prosurvival signaling in alveolar mesenchymal cells isolated from patients with nonresolving acute respiratory distress syndrome. Am. J. Physiol. Lung Cell Mol. Physiol. 290 (3), L415–L425. 10.1152/ajplung.00276.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Imamura K., Yue M., Inoue G., Miura Y. (2012). Reversible absorption of CO2 triggered by phase transition of amine-containing micro- and nanogel particles. J. Am. Chem. Soc. 134, 18177–18180. 10.1021/ja3080192 [DOI] [PubMed] [Google Scholar]
- Hosta-Rigau L., Zhang Y., Teo B. M., Postma A., Städler B., Hosta-Rigau L., et al. (2013). Cholesterol - a biological compound as a building block in bionanotechnology. Nanoscale 5, 89–109. 10.1039/c2nr32923a [DOI] [PubMed] [Google Scholar]
- Hough K. P., Curtiss M. L., Blain T. J., Liu R.-M., Trevor J., Deshane J. S., et al. (2020). Airway remodeling in asthma. Front. Med. 7, 191 10.3389/fmed.2020.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.-H., Jay M., Bummer P. M., Lehmler H. J. (2003). Chemical stability of esters of nicotinic acid intended for pulmonary administration by liquid ventilation. Pharm. Res. 20, 918–925. 10.1023/a:1023899505837 [DOI] [PubMed] [Google Scholar]
- Hsu H.-J., Bugno J., Lee S.-r., Hong S. (2016). Dendrimer-based nanocarriers: a versatile platform for drug delivery. WIREs Nanomed. Nanobiotechnol. 9, e1409 10.1002/wnan.1409 [DOI] [PubMed] [Google Scholar]
- Huang B., Yang X.-D., Zhou M.-M., Ozato K., Chen L.-F. (2009). Brd4 coactivates transcriptional activation of NF-κB via specific binding to acetylated RelA. MCB (Mol. Cell. Biol.) 29 (5), 1375–1387. 10.1128/mcb.01365-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Jain P. K., El-Sayed I. H., El-Sayed M. A. (2008). Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Laser Med. Sci. 23 (3), 217–228. 10.1007/s10103-007-0470-x [DOI] [PubMed] [Google Scholar]
- Huang Y.-Y., Wang C.-H. (2006). Pulmonary delivery of insulin by liposomal carriers. J. Contr. Release 113, 9–14. 10.1016/j.jconrel.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Hui D. S., Joynt G. M., Wong K. T., Gomersall C. D., Li T. S., Antonio G., et al. (2005). Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 60 (5), 401–409. 10.1136/thx.2004.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C., Linn G., Chow Y.-H., Kobayashi A., Mittelsteadt K., Altemeier W. A., et al. (2013). Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 188 (7), 820–830. 10.1164/rccm.201212-2297OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J., Fogarty A., Hubbard R., McKeever T. (2015). Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur. Respir. J. 46 (3), 795–806. 10.1183/09031936.00185114 [DOI] [PubMed] [Google Scholar]
- Ijaz T., Jamaluddin M., Zhao Y., Zhang Y., Jay J., Finnerty C. C., et al. (2017). Coordinate activities of BRD4 and CDK9 in the transcriptional elongation complex are required for TGFβ-induced Nox4 expression and myofibroblast transdifferentiation Cell Death Dis. 8 (2), e2606 10.1038/cddis.2016.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz T., Pazdrak K., Kalita M., Konig R., Choudhary S., Tian B., et al. (2014). Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ. J. 7 (1), 13 10.1186/1939-4551-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insmed (2020). Arikayce. Available at: https://www.arikayce.com/about-arikayce/ (Accessed January 1, 2020).
- Ishida T., Ichihara M., Wang X., Yamamoto K., Kimura J., Majima E., et al. (2006). Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Contr. Release 112, 12–25. 10.1016/j.jconrel.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Ishihara T., Maeda T., Sakamoto H., Takasaki N., Shigyo M., Ishida T., et al. (2010). Evasion of the accelerated blood clearance phenomenon by coating of nanoparticles with various hydrophilic polymers. Biomacromolecules 11, 2700–2706. 10.1021/bm100754e [DOI] [PubMed] [Google Scholar]
- Ishihara T., Takeda M., Sakamoto H., Kimoto A., Kobayashi C., Takasaki N., et al. (2009). Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm. Res. 26, 2270–2279. 10.1007/s11095-009-9943-x [DOI] [PubMed] [Google Scholar]
- Ivanova V., Garbuzenko O. B., Reuhl K. R., Reimer D. C., Pozharov V. P., Minko T. (2013). Inhalation treatment of pulmonary fibrosis by liposomal prostaglandin E2. Eur. J. Pharm. Biopharm. 84, 335–344. 10.1016/j.ejpb.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jartti T., Gern J. E. (2017). Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 140 (4), 895–906. 10.1016/j.jaci.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W.-j., Bu J., Han Y., Drelich A. J., Nair A., Král P., et al. (2020). Nanoparticle conjugation stabilizes and multimerizes β-hairpin peptides to effectively target PD-1/PD-L1 β-sheet-rich interfaces. J. Am. Chem. Soc. 142, 1832–1837. 10.1021/jacs.9b10160 [DOI] [PubMed] [Google Scholar]
- Jeong W., Bu J., Kubiatowicz L. J., Chen S. S., Kim Y., Hong S. (2018). Peptide–nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg. 5, 38 10.1186/s40580-018-0170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., He Y., Zhao P., Hu Y., Tao J., Chen J., et al. (2019). Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics 9, 265–278. 10.7150/thno.27246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen K. B., Gudbergsson J. M., Skov M. N., Pilgaard L., Moos T., Duroux M. (2014). A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta 1846, 75–87. 10.1016/j.bbcan.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Johnson E. R., Matthay M. A. (2010). Acute lung injury: epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 23 (4), 243–252. 10.1089/jamp.2009.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T. C., Lippard S. J. (2013). The effect of ligand lipophilicity on the nanoparticle encapsulation of Pt(IV) prodrugs. Inorg. Chem. 52, 9915–9920. 10.1021/ic4010642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanov A. V., Vinogradov S. V. (2009). Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew. Chem. Int. Ed. 48, 5418–5429. 10.1002/anie.200900441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., Weinberg R. A. (2009). The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119 (6), 1420–1428. 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen P., Johans C., Merta J., Kontturi K. (2008). Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid Interface Sci. 318, 88–95. 10.1016/j.jcis.2007.09.069 [DOI] [PubMed] [Google Scholar]
- Karo-Atar D., Bordowitz A., Wand O., Pasmanik-Chor M., Fernandez I. E., Itan M., et al. (2016). A protective role for IL-13 receptor α 1 in bleomycin-induced pulmonary injury and repair. Mucosal Immunol. 9 (1), 240–253. 10.1038/mi.2015.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A., Mathai S. K., Schwartz D. (2017). Genetics in idiopathic pulmonary fibrosis pathogenesis, prognosis, and treatment. Front. Med. 4, 1–8. 10.3389/fmed.2017.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N. K., Kaushik N., Yoo K. C., Uddin N., Kim J. S., Lee S. J., et al. (2016). Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signaling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials 87, 118–130. 10.1016/j.biomaterials.2016.02.014 [DOI] [PubMed] [Google Scholar]
- Kesharwani P., Jain K., Jain N. K. (2014). Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 39, 268–307. 10.1016/j.progpolymsci.2013.07.005 [DOI] [Google Scholar]
- Killops K. L., Campos L. M., Hawker C. J. (2008). Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene “click” chemistry. J. Am. Chem. Soc. 130, 5062–5064. 10.1021/ja8006325 [DOI] [PubMed] [Google Scholar]
- King T. E., Jr., Schwarz M. I., Brown K., Tooze J. A., Colby T. V., Waldron J. A., Jr., et al. (2001). Idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 164 (6), 1025–1032. 10.1164/ajrccm.164.6.2001056 [DOI] [PubMed] [Google Scholar]
- Kinnear C., Moore T. L., Rodriguez-Lorenzo L., Rothen-Rutishauser B., Petri-Fink A. (2017). Form follows function: nanoparticle shape and its implications for nanomedicine. Chem. Rev. 117, 11476–11521. 10.1021/acs.chemrev.7b00194 [DOI] [PubMed] [Google Scholar]
- Knight V., Koshkina N. V., Waldrep J. C., Giovanella B. C., Gilbert B. E. (1999). Anticancer exffect of 9-nitrocamptothecin liposome aerosol on human cancer xenografts in nude mice. Canc. Chemother. Pharmacol. 44, 177–186. 10.1007/s002800050965 [DOI] [PubMed] [Google Scholar]
- Kolte A., Patil S., Lesimple P., Hanrahan J. W., Misra A. (2017). PEGylated composite nanoparticles of PLGA and polyethylenimine for safe and efficient delivery of pDNA to lungs. Int. J. Pharm. 524, 382–396. 10.1016/j.ijpharm.2017.03.094 [DOI] [PubMed] [Google Scholar]
- Kong M., Tang J., Qiao Q., Wu T., Qi Y., Tan S., et al. (2017). Biodegradable hollow mesoporous silica nanoparticles for regulating tumor microenvironment and enhancing antitumor efficiency. Theranostics 7, 3276–3292. 10.7150/thno.19987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshkina N. V., Kleinerman E. S., Waidrep C., Jia S. F., Worth L. L., Gilbert B. E., et al. (2000). 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin. Canc. Res. 6, 2876–2880. 10.1111/j.1749-6632.2000.tb07033.x [DOI] [PubMed] [Google Scholar]
- Koshkina N. V., Waldrep J. C., Roberts L. E., Golunski E., Melton S., Knight V. (2001). Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin. Canc. Res. 7, 3258–3262. [PubMed] [Google Scholar]
- Kousalova J., Etrych T. (2018). Polymeric nanogels as drug delivery systems. Physiol. Res. 67, S305–S317. 10.33549/physiolres.933979 [DOI] [PubMed] [Google Scholar]
- Kricheldorf H. R. (1991). Handbook of polymer synthesis. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Kroger A. P. P., Paulusse J. M. J. (2018). Single-chain polymer nanoparticles in controlled drug delivery and targeted imaging. J. Contr. Release 286, 326–347. 10.1016/j.jconrel.2018.07.041 [DOI] [PubMed] [Google Scholar]
- Kulkarni T., de Andrade J., Zhou Y., Luckhardt T., Thannickal V. J. (2016). Alveolar epithelial disintegrity in pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 311 (2), L185–L191. 10.1152/ajplung.00115.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Dong Y., Kumar V., Almawash S., Mahato R. I. (2019). The use of micelles to deliver potential hedgehog pathway inhibitor for the treatment of liver fibrosis. Theranostics 9, 7537–7555. 10.7150/thno.38913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D.-H., Kim Y.-K., Kook H. (2017). New aspects of vascular calcification: histone deacetylases and beyond. J. Kor. Med. Sci. 32, 1738–1748. 10.3346/jkms.2017.32.11.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S., Xu J., Derynck R. (2014). Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15 (13), 178–196. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmark K. J., DiMaggio S., Ward J., Kelly C., Vogt S., Hong S., et al. (2008). Synthesis, characterization, and in Vitro testing of superparamagnetic iron oxide nanoparticles targeted using folic acid-conjugated dendrimers. ACS Nano. 2, 773–783. 10.1021/nn800034w [DOI] [PubMed] [Google Scholar]
- Langenback E. G., Davis J. M., Robbins C., Sahgal N., Perry R. J., Simon S. R. (1999). Improved pulmonary distribution of recombinant human Cu/Zn superoxide dismutase, using a modified ultrasonic nebulizer. Pediatr. Pulmonol. 27, 124–129. [DOI] [PubMed] [Google Scholar]
- Lehmler H., Xu L., Vyas S., Ojogun V., Knutson B., Ludewig G. (2008). Synthesis, physicochemical properties and in vitro cytotoxicity of nicotinic acid ester prodrugs intended for pulmonary delivery using perfluorooctyl bromide as vehiclefluorooctyl bromide as vehicle. Int. J. Pharm. 353, 35–44. 10.1016/j.ijpharm.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchford K., Burt H. (2007). A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 65, 259–269. 10.1016/j.ejpb.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Letsou G. V., Safi H. J., Reardon M. J., Ergenoglu M., Li Z., Klonaris C. N., et al. (1999). Pharmacokinetics of liposomal aerosolized cyclosporine A for pulmonary immunosuppression. Ann. Thorac. Surg. 68, 2044–2048. 10.1016/s0003-4975(99)01183-2 [DOI] [PubMed] [Google Scholar]
- Li T., Yan Y., Wang B., Qian H., Zhang X., Shen L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cell. Dev. 22, 845 10.1089/scd.2012.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Song L., Hu X., Liu C., Shi J., Wang H., et al. (2018a). Inhibition of epithelial-mesenchymal transition and tissue regeneration by waterborne titanium dioxide nanoparticles. ACS Appl. Mater. Interfaces 10, 3449–3458. 10.1021/acsami.7b18986 [DOI] [PubMed] [Google Scholar]
- Li Z., Jiang P., Li J., Peng M., Zhao X., Zhang X., et al. (2018b). Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene 37, 3822–3838. 10.1038/s41388-018-0237-9 [DOI] [PubMed] [Google Scholar]
- Li X., Yuan W., Gu S., Ren J. (2010). Synthesis and self-assembly of tunable thermosensitive chitosan amphiphilic copolymers by click chemistry. Mater. Lett. 64, 2663–2666. 10.1016/j.matlet.2010.08.077 [DOI] [Google Scholar]
- Li Y.-F., Chen C. (2011). Fate and toxicity of metallic and metal‐containing nanoparticles for biomedical applications. Small 7 (21), 2965–2980. 10.1002/smll.201101059 [DOI] [PubMed] [Google Scholar]
- Li Z., Chen G., Ding L., Wang Y., Zhu C., Wang K., et al. (2019). Increased survival by pulmonary treatment of established lung metastases with dual STAT3/CXCR4 inhibition by siRNA nanoemulsions. Mol. Ther. 27, 2100–2110. 10.1016/j.ymthe.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach L. K., Wick P., Manser P., Grass R. N., Bruinink A., Stark W. J. (2007). Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 41, 4158–4163. 10.1021/es062629t [DOI] [PubMed] [Google Scholar]
- Lin Y., Ma Q., Li L., Wang H. (2018). The CXCL12-CXCR4 axis promotes migration, invasiveness, and EMT in human papillary thyroid carcinoma B-CPAP cells via NF-κB signaling. Biochem. Cell. Biol. 96, 619–626. 10.1139/bcb-2017-0074 [DOI] [PubMed] [Google Scholar]
- Lin Y.-T., Wu K.-J. (2020). Epigenetic regulation of epithelial-mesenchymal transition: focusing on hypoxia and TGF-β signaling. J. Biomed. Sci. 27 (1), 39 10.1186/s12929-020-00632-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Chen H., Wang P., Li Y., Wold E. A., Leonard P. G., et al. (2020). Discovery of orally bioavailable chromone derivatives as potent and selective BRD4 inhibitors: scaffold hopping, optimization, and pharmacological evaluation. J. Med. Chem. 63, 5242 10.1021/acs.jmedchem.0c00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Tian B., Chen H., Wang P., Brasier A. R., Zhou J. (2018). Discovery of potent and selective BRD4 inhibitors capable of blocking TLR3-induced acute airway inflammation. Eur. J. Med. Chem. 151, 450–461. 10.1016/j.ejmech.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Lou B., Zou S., Kobayashi H., Liu J., Xiao L., et al. (2018). Robust removal of ligands from noble metal nanoparticles by electrochemical strategies. ACS Catal. 8, 8484–8492. 10.1021/acscatal.8b01627 [DOI] [Google Scholar]
- Lutter R., Spiteri M. (2005). Current perspectives in epithelial cell injury and repair: consequences for epithelial functions. Eur. Respir. Rev. 14, 126–130. 10.1183/09059180.05.00009701 [DOI] [Google Scholar]
- Ma J., Bishoff B., Mercer R. R., Barger M., Schwegler-Berry D., Castranova V. (2017). Role of epithelial-mesenchymal transition (EMT) and fibroblast function in cerium oxide nanoparticles-induced lung fibrosis. Toxicol. Appl. Pharmacol. 323, 16–25. 10.1016/j.taap.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M. (2018). Inflammation and fibrosis. Matrix Biol. 68-69, 106–121. 10.1016/j.matbio.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Mamaghani P. Y., Kaffashi B., Salehi R., Davaran S. (2015). Synthesis, characterization, and viscoelastic behavior of thermothickening poly (N-Isopropylacrylamide-Methacrylicacide-Vinylpyrrolidone) nanogels as an injectable biocompatible drug carrier. Int. J. Polym. Mater. Polym. Biomater. 64, 55–63. 10.1080/00914037.2014.886236 [DOI] [Google Scholar]
- Mapel D. W., Samet J. M., Coultas D. B. (1996). Corticosteroids and the treatment of idiopathic pulmonary fibrosis. Chest 110 (4), 1058–1067. 10.1378/chest.110.4.1058 [DOI] [PubMed] [Google Scholar]
- Mazzei M. E., Richeldi L., Collard H. R. (2015). Nintedanib in the treatment of idiopathic pulmonary fibrosis. Ther. Adv. Respr. 9 (3), 121–129. 10.1177/1753465815579365 [DOI] [PubMed] [Google Scholar]
- Menon J. U., Ravikumar P., Pise A., Gyawali D., Hsia C. C. W., Nguyen K. T. (2014). Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater. 10, 2643–2652. 10.1016/j.actbio.2014.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon J. U., Wadajkar A. S., Xie Z., Nguyen K. T. (2013). “Nanomaterials for management of lung disorders and drug delivery,” in Nanomaterials in Drug Delivery, Imaging, and Tissue Engineering. Editor Tiwari A. (Hoboken, NJ: John Wiley & Sons, Inc.), 167–202. [Google Scholar]
- Mercer B., Lemaître V., Powell C., D’Armiento J. (2006). The epithelial cell in lung Health and emphysema pathogenesis. Curr Respir Med Rev. 2 (2), 101–142. 10.2174/157339806776843085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Sun X., Yang X., Zhu H., Liu J., Wang Y., et al. (2018). Exosomes derived from irradiated esophageal carcinoma-infiltrating T cells promote metastasis by inducing the epithelial-mesenchymal transition in esophageal cancer cells. Pathol. Oncol. Res. 24, 11–18. 10.1007/s12253-016-0185-z [DOI] [PubMed] [Google Scholar]
- Mo X., Jian W., Su Z., Chen M., Peng H., Peng P., et al. (2020). Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 55, 2001217 10.1183/13993003.01217-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody V., Siwale R., Singh A., Mody H. (2010). Introduction to metallic nanoparticles. J Pharm Bioall Sci 2, 282–289. 10.4103/0975-7406.72127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen E. L., Mariani C. J., Zullow H., Jeff-Eke M., Litwin E., Nikitas J. N. (2015). New themes in the biological functions of 5-methylcytosine and 5-hydroxymethylcytosine. Immunol. Rev. 263 (1), 36–49. 10.1111/imr.12242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad A. K., Reineke J. J. (2013). Quantitative detection of PLGA nanoparticle degradation in tissues following intravenous administration. Mol. Pharm. 10, 2183–2189. 10.1021/mp300559v [DOI] [PubMed] [Google Scholar]
- Naguchi S., Yamauchi Y., Takizawa H. (2014). Novel therapeutic strategies for fibrotic lung disease: a review with a focus on epithelial-mesenchymal transition. Recent Pat. Inflamm. Allergy Drug Discov. 8, 9–18. 10.2174/1872213x07666131229131451 [DOI] [PubMed] [Google Scholar]
- Nam J., Son S., Park K. S., Zou W., Shea L. D., Moon J. J. (2019). Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 4, 398–414. 10.1038/s41578-019-0108-1 [DOI] [Google Scholar]
- Nance E. A., Woodworth G. F., Sailor K. A., Shih T.-Y., Xu Q., Swaminatha G., et al. (2012). A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 4, 149ra119 10.1126/scitranslmed.3003594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. P. (2018). Delivering drugs to the lungs: the history of repurposing in the treatment. Adv. Drug Deliv. Rev. 133, 135–118. 10.1016/j.addr.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Niu Z., Li Y. (2014). Removal and utilization of capping agents in nanocatalysis. Chem. Mater. 26, 72–83. 10.1021/cm4022479 [DOI] [Google Scholar]
- Oku N., Namba Y. (1994). Long-circulating liposomes. Crit. Rev. Ther. Drug Carrier Syst. 11, 231–270. 10.4155/tde-2018-0035 [DOI] [PubMed] [Google Scholar]
- O’Reilly S. (2017). Epigenetics in fibrosis. Mol. Aspect. Med. 54, 89–102. 10.1016/j.mam.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Owens D. E., Peppas N. A. (2006). Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. Jouurnal Pharm. 307, 93–102. 10.1016/j.ijpharm.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Palgrave R. G., Parkin I. P. (2006). Aerosol assisted chemical vapor deposition using nanoparticle precursors: a route to nanocomposite thin films. J. Am. Chem. Soc. 128, 1587–1597. 10.1021/ja055563v [DOI] [PubMed] [Google Scholar]
- Pandey R., Sharma A., Zahoor A., Sharma S., Khuller G. K., Prasad B. (2003). Poly (dl-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 52, 981–986. 10.1093/jac/dkg477 [DOI] [PubMed] [Google Scholar]
- Pandolfi L., Frangipane V., Bocca C., Marengo A., Tarro Genta E., Bozzini S., et al. (2019). Hyaluronic acid-decorated liposomes as innovative targeted delivery system for lung fibrotic cells. Molecules 24, 3291 10.3390/molecules24183291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Kim J.-H., Nam Y. S., Lee S., Nam H. Y., Kim K., et al. (2007). Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J. Contr. Release 122, 305–314. 10.1016/j.jconrel.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Patel P., Soni T., Thakkar V., Gandhi T. (2013). Nanoparticle as an emerging tool in pulmonary drug delivery system. Micro Nanosys. 5, 288–302. 10.2174/18764029113059990002 [DOI] [Google Scholar]
- Pearson R. M., Sen S., Hsu H.-j., Pasko M., Gaske M., Král P., et al. (2016). Tuning the selectivity of dendron micelles through variations of the poly(ethylene glycol) corona. ACS Nano. 10 (7), 6905–6914. 10.1021/acsnano.6b02708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D., Karp J. M., Hong S., Farokhzad O. C., Margalit R., Langer R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760. 10.1038/nnano.2007.387 [DOI] [PubMed] [Google Scholar]
- Peng F., Tee J. K., Setyawati M. I., Ding X., Yeo H. L. A., Tan Y. L., et al. (2018). Inorganic nanomaterials as highly efficient inhibitors of cellular hepatic fibrosis. ACS Appl. Mater. Interfaces 10, 31938–31946. 10.1021/acsami.8b10527 [DOI] [PubMed] [Google Scholar]
- Phan S. H. (2012). Genesis of the myofibroblast in lung injury and fibrosis. Proc. Am. Thorac. Soc. 9 (3), 148–152. 10.1513/pats.201201-011aw [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Papahadjopoulos D., Vail W. J. (1976). Chapter 4 lipid vesicles as carriers for introducing biologically active materials into cells. Methods Cell Biol. 14, 33–71. 10.1016/s0091-679x(08)60468-9 [DOI] [PubMed] [Google Scholar]
- Pradhan P., Qin H., Leleux J. A., Gwak D., Sakamaki I., Kwak L. W., et al. (2014). The effect of combined IL10 siRNA and CpG ODN as pathogen-mimicking microparticles on Th1/Th2 cytokine balance in dendritic cells and protective immunity against B cell lymphoma. Biomaterials 35, 5491–5504. 10.1016/j.biomaterials.2014.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J.-B., Jang Y., Fan Q.-Q., Chang S.-H., Xing L., Cui P.-F., et al. (2017). Aerosol delivery of biocompatible dihydroergotamine-loaded PLGA-PSPE polymeric micelles for efficient lung cancer therapy. Polym. Chem. 8, 1540–1554. 10.1039/c7py00024c [DOI] [Google Scholar]
- Quadros M. E., Marr L. C. (2012). Environmental and human Health risks of aerosolized silver nanoparticles. J. Air Waste Manag. Assoc. 60, 770–781. 10.3155/1047-3289.60.7.770 [DOI] [PubMed] [Google Scholar]
- Radisky D. C., Kenny P. A., Bissell M. J. (2007). Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J. Cell. Biochem. 101(4), 830–839. 10.1002/jcb.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghnaill M. N., Bramini M., Ye D., Couraud P.-O., Romero I. A., Weksler B., et al. (2014). Paracrine signalling of inflammatory cytokines from an in vitro blood brain barrier model upon exposure to polymeric nanoparticles. Analyst 139, 923–930. 10.1039/c3an01621h [DOI] [PubMed] [Google Scholar]
- Raghu G., Collard H. R., Egan J. J., Martinez F. J., Behr J., Brown K. K., et al. (2011). An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183 (6), 788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L., du Bois R. M., Raghu G., Azuma A., Brown K. K., Costabel U., et al. (2014). Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370 (22), 2071–2082. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- Rock J. R., Barkauskas C. E., Cronce M. J., Xue Y., Harris J. R., Liang J., et al. (2011). Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. U. S. A. 108 (52), E1475–E1483. 10.1073/pnas.1117988108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiere R., Van Woensel M., Mathieu V., Langer I., Mathivet T., Vermeersch M., et al. (2016). Development and evaluation of well-tolerated and tumor-penetrating polymeric micelle-based dry powders for inhaled anti-cancer chemotherapy. Int. J. Pharm. 501, 148–159. 10.1016/j.ijpharm.2016.01.073 [DOI] [PubMed] [Google Scholar]
- Royce S. G., Karagiannis T. C. (2014). Histone deacetylases and their inhibitors. Curr. Opin. Allergy Clin. Immunol. 14 (1), 44–48. 10.1097/ACI.0000000000000029 [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. (2020). Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46 (5), 846–848. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadati R., Dadashzadeh S., Abbasian Z., Soleimanjahi H. (2013). Accelerated blood clearance of PEGylated PLGA nanoparticles following repeated injections: effects of polymer dose, PEG coating, and encapsulated anticancer drug. Pharm. Res. 30, 985–995. 10.1007/s11095-012-0934-y [DOI] [PubMed] [Google Scholar]
- Seifi-Najmi M., Hajivalili M., Safaralizadeh R., Sadreddini S., Esmaeili S., Razavi R., et al. (2016). SiRNA/DOX lodeded chitosan based nanoparticles: development, Characterization and in vitro evaluation on A549 lung cancer cell line. Cell Mol Biol (Noisy-le-grand) 62, 87. [PubMed] [Google Scholar]
- Senior J. H. (1986). Fate and behavior of liposomes in vivo: a review of controlling factors. Crit. Rev. Ther. Drug Carrier Syst. 3, 123–193. [Google Scholar]
- Shah P. N., Lin L. Y., Smolen J. A., Tagaev J. A., Gunsten S. P., Han D. S., et al. (2013). Synthesis, characterization, and in vivo efficacy of shell cross-linked nanoparticle formulations carrying silver antimicrobials as aerosolized therapeutics. ACS Nano. 7, 4977–4987. 10.1021/nn400322f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargh V. H., Hondermarck H., Liang M. (2015). Antibody-targeted biodegradable nanoparticles for cancer therapy. Futur. Med. 11, 63–79. 10.2217/nnm.15.186 [DOI] [PubMed] [Google Scholar]
- Sheng Y., Liu C., Yuan Y., Tao X., Yang F., Shan X., et al. (2009). Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials 30, 2340–2348. 10.1016/j.biomaterials.2008.12.070 [DOI] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. (2020). Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 20 (4), 425–434. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Bi X., Ganser T. R., Hong S., Myc L. A., Desai A., et al. (2006). HPLC analysis of functionalized poly(amidoamine) dendrimers and the interaction between a folate-dendrimer conjugate and folate binding protein. Analyst 131, 842–848. 10.1039/b602546c [DOI] [PubMed] [Google Scholar]
- Shin H. Y. (2018). Targeting super-enhancers for disease treatment and diagnosis. Mol Cells 41 (6), 506–514. 10.14348/molcells.2018.2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha N., Shahbazi M.-A., Araújo F., Mäkilä E., Raula J., Kauppinen E. I., et al. (2015). Multistage pH-responsive mucoadhesive nanocarriers prepared by aerosol flow reactor technology: a controlled dual protein-drug delivery system. Biomaterials 68, 9–20. 10.1016/j.biomaterials.2015.07.045 [DOI] [PubMed] [Google Scholar]
- Silverman J. M., Reiner N. E. (2011). Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 13, 1–9. 10.1111/j.1462-5822.2010.01537.x [DOI] [PubMed] [Google Scholar]
- Singh A., Kulshrestha R., Pandey A., Kaushik M., Dinda A. K. (2019). “Efficacy of MePEG PCL diblock copolymeric nanoparticles and PCLNanoparticles for drug delivery in lung fibrosis,” in American thoracic society 2019 international conference, Dallas, TX, May 17–22, 2019. [Google Scholar]
- Singh N. K., Purkayastha B. D., Roy J. K., Banik R. M., Yashpal M., Singh G., et al. (2010). Nanoparticle-induced controlled biodegradation and its mechanism in poly(ε-caprolactone). ACS Appl. Mater. Interfaces 2, 69–81. 10.1021/am900584r [DOI] [PubMed] [Google Scholar]
- Skubitz K. M., Anderson P. M. (2000). Inhalational interleukin-2 liposomes for pulmonary metastases: a phase I clinical trial. Anti Canc. Drugs 11, 555–563. 10.1097/00001813-200008000-00006 [DOI] [PubMed] [Google Scholar]
- Song Q., Yin Y., Shang L., Wu T., Zhang D., Kong M., et al. (2017). Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 17, 6366–6375. 10.1021/acs.nanolett.7b03186 [DOI] [PubMed] [Google Scholar]
- Sousa C., Gouveia L., Kreutzer B., Silva-Lima B., Maphasa R., Dube A., et al. (2019). Polymeric micellar formulation enhances antimicrobial and anticancer properties of salinomycin. Pharm. Res. 36, 83 10.1007/s11095-019-2615-6 [DOI] [PubMed] [Google Scholar]
- Spagnolo P., Del Giovane C., Luppi F., Cerri S., Balduzzi S., Walters E. H., et al. (2010). Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst. Rev. 9, CD003134 10.1002/14651858.CD003134.pub2 [DOI] [PubMed] [Google Scholar]
- Stone R. C., Pastar I., Ojeh N., Chen V., Liu S., Garzon K. I., et al. (2016). Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 365, 495–506. 10.1007/s00441-016-2464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch K., Moitzi C., Fritz G., Glatter O., Trimmel G., Stelzer F. (2006). Precise tuning of micelle, core, and shell size by the composition of amphiphilic block copolymers derived from ROMP investigated by DLS and SAXS. Macromolecules 39, 5865–5874. 10.1021/ma060451p [DOI] [Google Scholar]
- Sunil Gowda S. N., Rajasowmiya S., Vadivel V., Banu Devi S., Celestin Jerald A., Marimuthu S., et al. (2018). Gallic acid-coated sliver nanoparticle alters the expression of radiation-induced epithelial-mesenchymal transition in non-small lung cancer cells. Toxicol. Vitro 52, 170–177. 10.1016/j.tiv.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Sunoqrot S., Bugno J., Lantvit D., Burdette J. E., Hong S. (2014). Prolonged blood circulation and enhanced tumor accumulation of folate-targeted dendrimer-polymer hybrid nanoparticles. J. Contr. Release 191, 115–122. 10.1016/j.jconrel.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh D., Zambre A., Mukherjee S., Ghoshdastidar S., Jiang Y., Joshi T., et al. (2019). Silencing AXL by covalent siRNA-gelatin-antibody nanoconjugate inactivates mTOR/EMT pathway and stimulates p53 for TKI sensitization in NSCLC. Nanomed. Nanotechnol. Biol. Med. 20, 102007 10.1016/j.nano.2019.04.010 [DOI] [PubMed] [Google Scholar]
- Szebeni J. (2005). Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology 216, 106–121. 10.1016/j.tox.2005.07.023 [DOI] [PubMed] [Google Scholar]
- Szebeni J., Moghimi S. M. (2009). Liposome triggering of innate immune responses: a perspective on benefits and adverse reactions. J. Liposome Res. 19, 85–90. 10.1080/08982100902792855 [DOI] [PubMed] [Google Scholar]
- Tang B. C., Dawson M., Lai S. K., Wang Y.-Y., Suk J. S., Yang M., et al. (2009). Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc. Natl. Acad. Sci. U. S. A. 106, 19268–19273. 10.1073/pnas.0905998106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Peng R., Phillips J. E., Deguzman J., Ren Y., Apparsundaram S., et al. (2013). Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. Am. J. Pathol. 183 (2), 470–479. 10.1016/j.ajpath.2013.04.020 [DOI] [PubMed] [Google Scholar]
- Taniguchi Y. (2016). The bromodomain and extra-terminal domain (BET) family: functional anatomy of BET paralogous proteins. IJMS 17 (11), 1849 10.3390/ijms17111849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. M. G., Taylor G., Kellaway I. W., Stevens J. (1990). The stability of liposomes to nebulisation. Int. J. Pharm. 58, 57–61. 10.1016/0378-5173(90)90287-e [DOI] [Google Scholar]
- Ten R. M., Anderson P. M., Zein N. N., Temesgen Z., Clawson M. L., Weiss W. (2002). Interleukin-2 liposomes for primary immune deficiency using the aerosol route. Int. Immunopharm. 2, 333–344. 10.1016/s1567-5769(01)00143-6 [DOI] [PubMed] [Google Scholar]
- Thannickal V. J. (2012). Mechanisms of pulmonary fibrosis: role of activated myofibroblasts and NADPH oxidase. Fibrogenesis Tissue Repair 5 (1), S23 10.1186/1755-1536-5-s1-s23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C., Zitvogel L., Amigorena S. (2002). Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579. 10.1038/nri855 [DOI] [PubMed] [Google Scholar]
- Thomas D. A., Myers M. A., Wichert B., Schreier H., Gonzalez-Rothi R. J. (1991). Acute effects of liposome aerosol inhalation on pulmonary function in healthy human volunteers. Chest 99, 1268–1270. 10.1378/chest.99.5.1268 [DOI] [PubMed] [Google Scholar]
- Tian B., Li X., Kalita M., Widen S. G., Yang J., Bhavnani S. K., et al. (2015). Analysis of the TGFβ-induced program in primary airway epithelial cells shows essential role of NF-κB/RelA signaling network in type II epithelial mesenchymal transition. BMC Genom. 16 (1), 529 10.1186/s12864-015-1707-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Liu Z., Litvinov J., Maroto R., Jamaluddin M., Rytting E., et al. (2018a). Efficacy of novel highly specific bromodomain-containing protein 4 inhibitors in innate inflammation-driven airway remodeling. Am. J. Respir. Cell Mol. Biol., 60, 68 10.1165/rcmb.2017-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Liu Z., Yang J., Sun H., Zhao Y., Wakamiya M., et al. (2018b). Selective antagonists of the bronchiolar epithelial NF-κB-Bromodomain-Containing protein 4 pathway in viral-induced airway inflammation Cell Rep. 23 (4), 1138–1151. 10.1016/j.celrep.2018.03.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Widen S. G., Yang J., Wood T. G., Kudlicki A., Zhao Y., et al. (Forthcoming 2018c). NF&B is a master transcription factor mediating partial-to fully committed epithelial-mesenchymal transition. J. Biol. Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Yang J., Zhao Y., Ivanciuc T., Sun H., Wakamiya M., et al. (2018d). Central role of the NF-κB pathway in theScgb1a1-expressing epithelium in mediating respiratory syncytial virus-induced airway inflammation. J. Virol. 92 (11), e00441 10.1128/JVI.00441-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Patrikeev I., Ochoa L., Vargas G., Belanger K. K., Litvinov J., et al. (2017a). NF-κB mediates mesenchymal transition, remodeling, and pulmonary fibrosis in response to chronic inflammation by viral RNA patterns. Am. J. Respir. Cell Mol. Biol. 56 (4), 506–520. 10.1165/rcmb.2016-0259OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Yang J., Zhao Y., Ivanciuc T., Sun H., Garofalo R. P., et al. (2017b). Bromodomain containing 4 (BRD4) couples NFκB/RelA with airway inflammation and the IRF-RIG-I amplification loop in respiratory syncytial virus infection. J. Virol. 91, e00007–e000017. 10.1128/JVI.00007-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Zhao Y., Sun H., Zhang Y., Yang J., Brasier A. R. (2016). BRD4 mediates NF-κB-dependent epithelial-mesenchymal transition and pulmonary fibrosis via transcriptional elongation. Am. J. Physiol. Lung Cell Mol. Physiol. 311 (6), L1183–L1201. 10.1152/ajplung.00224.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3 (5), 349–363. 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- Torikata C., Villiger B., Kuhn C., McDonald J. A. (1985). Ultrastructural distribution of fibronectin in normal and fibrotic human lung. Lab. Invest. 52, 399–408. [PubMed] [Google Scholar]
- Torr E. E., Ngam C. R., Bernau K., Tomasini-Johansson B., Acton B., Sandbo N. (2015). Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J. Biol. Chem. 290 (11), 6951–6961. 10.1074/jbc.M114.606186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht J., Jordan R., Luxenhofer R. (2014). On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 35, 4848–4861. 10.1016/j.biomaterials.2014.02.029 [DOI] [PubMed] [Google Scholar]
- Ulrich A. S. (2002). Biophysical aspects of using liposomes as delivery vehicles. Biosci. Rep. 22, 129–150. 10.1023/a:1020178304031 [DOI] [PubMed] [Google Scholar]
- Ungaro F., d’Emmanuele di Villa Bianca R., Giovino C., Miro A., Sorrentino R., Quaglia F., et al. (2009). Insulin-loaded PLGA/cyclodextrin large porous particles with improved aerosolization properties: in vivo deposition and hypoglycaemic activity after delivery to rat lungs. J. Contr. Release 135, 25–34. 10.1016/j.jconrel.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Urbani C. N., Bell C. A., Lonsdale D., Whittaker M. R., Monteiro M. J. (2008). Self-Assembly of amphiphilic polymeric dendrimers synthesized with selective degradable linkages. Macromolecules 41, 76–86. 10.1021/ma701993w [DOI] [Google Scholar]
- Vaidya B., Kulkarni N. S., Shukla S. K., Parvathaneni V., Chauhan G., Damon J. K., et al. (2020). Development of inhalable quinacrine loaded bovine serum albumin modified cationic nanoparticles: repurposing quinacrine for lung cancer therapeutics. Int. J. Pharm. 577, 118995 10.1016/j.ijpharm.2019.118995 [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- van der Meel R., Fens M. H. A. M., Vader P., van Solinge W. W., Eniola-Adefeso O., Schiffelers R. M. (2014). Extracellular vesicles as drug delivery systems: lessons from the liposome field. J. Contr. Release 195, 72–85. 10.1016/j.jconrel.2014.07.049 [DOI] [PubMed] [Google Scholar]
- Veranth J. M., Kaser E. G., Veranth M. M., Koch M., Yost G. S. (2007). Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part. Fibre Toxicol. 4, 1–18. 10.1186/1743-8977-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschraegan C. F., Gilbert B. E., Loyer E., Huaringa A., Walsh G., Newman R. A., et al. (2004). Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-Camptothecin in patients with advanced pulmonary malignancies. Clin. Canc. Res. 10, 2319–2326. 10.1158/1078-0432.ccr-0929-3 [DOI] [PubMed] [Google Scholar]
- Vidgren M., Waldrep J. C., Arppe J., Black M., Rodarte J. A., Cole W., et al. (1995). A study of 99mtechnetium-labelled beclomethasone dipropionate dilauroylphosphatidylcholine liposome aerosol in normal volunteers. Int. J. Pharm. 115, 209–216. 10.1016/0378-5173(94)00265-7 [DOI] [Google Scholar]
- Wahab R., Kaushik N., Khan F., Kaushik N. K., Choi E. H., Musarrat J., et al. (2016). Self-styled ZnO nanostructures promotes the cancer cell damage and supresses the epithelial phenotype of glioblastoma. Sci. Rep. 6, 19950 10.1038/srep19950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrep J. C., Gilbert B. E., Knight C. M., Black M. B., Scherer P. W., Knight V., et al. (1997). Pulmonary delivery of beclomethasone liposome aerosol in volunteers. Chest 111, 316–323. 10.1378/chest.111.2.316 [DOI] [PubMed] [Google Scholar]
- Waldrep J. C., Keyhani K., Black M., Knight V. (1994). Operating characteristics of 18 different continuous-flow jet nebulizers with beclomethasone dipropionate liposome aerosol. Chest 105, 106–110. 10.1378/chest.105.1.106 [DOI] [PubMed] [Google Scholar]
- Wang D., Wang T., Lu J., Yu H., Jiao S., Feng B., et al. (2016). Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 16, 5503–5513. 10.1021/acs.nanolett.6b01994 [DOI] [PubMed] [Google Scholar]
- Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S., et al. (2020). Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit. Care 24 (1), 188 10.1186/s13054-020-02895-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wei H., Wang J., Li L., Chen A., Li Z. (2019). MicroRNA-181d-5p-Containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol. Ther. Nucleic Acids 19, 654–667. 10.1016/j.omtn.2019.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhou F., Li Z., Mei H., Wang Y., Ma H., et al. (2018). Pharmacological targeting of BET proteins attenuates radiation-induced lung fibrosis. Sci. Rep. 8 (1), 998 10.1038/s41598-018-19343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Q., Yan F. Q., Wang L. H., Yin W. J., Chang T. Y., Liu J. P., et al. (2019). Identification of new hypoxia‐regulated epithelial‐mesenchymal transition marker genes labeled by H3K4 acetylation. Genes, Chromosomes, and Cancer, 59 (52), 73–83. 10.1002/gcc.22802 [DOI] [PubMed] [Google Scholar]
- Wang L., Yang G., Zhao D., Wang J., Bai Y., Peng Q., et al. (2019). CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol. Cancer 18, 86 10.1186/s12943-019-0997-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ding L., Li Z., Chen G., Sun M., Oupicky D. (2019). Treatment of acute lung injury and early- and late-stage pulmonary fibrosis with combination emulsion siRNA polyplexes. J. Contr. Release 314, 12–24. 10.1016/j.jconrel.2019.10.030 [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang C., Liu S., He W., Wang L., Gan J., et al. (2017). Specifically formed corona on silica nanoparticles enhances transforming growth factor β1 activity in triggering lung fibrosis. ACS Nano. 11, 1659–1672. 10.1021/acsnano.6b07461 [DOI] [PubMed] [Google Scholar]
- Wettstein G., Bellaye P. S., Kolb M., Hammann A., Crestani B., Soler P., et al. (2013). Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. Faseb. J. Am. Soc. Exp. Biol. 27, 1549–1560. 10.1096/fj.12-220053 [DOI] [PubMed] [Google Scholar]
- Whyte W. A., Orlando D. A., Hnisz D., Abraham B. J., Lin C. Y., Kagey M. H., et al. (2013). Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153 (2), 307–319. 10.1016/j.cell.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis B. C., Borok Z. (2007). TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L525–L534. 10.1152/ajplung.00163.2007 [DOI] [PubMed] [Google Scholar]
- Wilson M. S., Wynn T. A. (2009). Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2 (2), 103–121. 10.1038/mi.2008.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbottom C. J., Shah R. J., Patterson K. C., Kreider M. E., Panettieri R. A., Jr., Rivera-Lebron B., et al. (2018). Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest 153 (5), 1221–1228. 10.1016/j.chest.2017.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittgren B., Wahlund K.-G., Dérand H., Wesslén B. (1996). Size characterization of a charged amphiphilic copolymer in solutions of different salts and salt concentrations using flow field-flow fractionation. Langmuir 12, 5999–6005. 10.1021/la960435t [DOI] [Google Scholar]
- Wu Y., Ali M. R. K., Dong B., Han T., Chen K., Chen J., et al. (2018). Gold nanorod photothermal therapy alters cell junctions and actin network in inhibiting cancer cell collective migration. ACS Nano 12, 9279–9290. 10.1021/acsnano.8b04128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Xiong Y., Lim B., Skrabalak S. E. (2008). Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103. 10.1002/anie.200802248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., He W., Guan W., Hou F., Yan P., Xu J., et al. (2020). Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 11, 863 10.1038/s41419-020-03034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Arvizo R. R., Saha S., Robertson D. J., McMeekin S., Bhattacharya R., et al. (2019). Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Cell Stress 3, 267–279. 10.18632/oncotarget.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Qiao D., Mann M., Garofalo R. P., Brasier A. R. (2020). Respiratory syncytial virus infection induces chromatin remodeling to activate growth factor and extracellular matrix secretion pathways. Viruses 12 (8), 804 10.3390/v12080804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Ye Z., Chen M., Liu Z., Xiao Y., Zhang Y., et al. (2011). Fine tuning micellar core-forming block of poly(ethylene glycol)-block-poly(ε-caprolactone) amphiphilic copolymers based on chemical modification for the solubilization and delivery of doxorubicin. Biomacromolecules 12, 2562–2572. 10.1021/bm200375x [DOI] [PubMed] [Google Scholar]
- Yan Y., Zhou K., Xiong H., Miller J. B., Motea E. A., Boothman D. A., et al. (2017). Aerosol delivery of stabilized polyester-siRNA nanoparticles to silence gene expression in orthotopic lung tumors. Biomaterials 118, 84–93. 10.1016/j.biomaterials.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Xu L., Xu J., Zhang R., Song G., Chao Y., et al. (2018). Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 18, 2475–2484. 10.1021/acs.nanolett.8b00040 [DOI] [PubMed] [Google Scholar]
- Yang W., Liu S., Bai T., Keefe A. J., Zhang L., Ella-Menye J.-R., et al. (2014). Poly(carboxybetaine) nanomaterials enable long circulation and prevent polymer-specific antibody production. Nano Today 9, 10–16. 10.1016/j.nantod.2014.02.004 [DOI] [Google Scholar]
- Yang Y., Sunoqrot S., Stowell C., Ji J., Lee C.-W., Kim J. W., et al. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 13, 2154–2162. 10.1021/bm300545b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Chen R., Wang G., Zhang Y., Liu F. (2019). Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 10, 1–17. 10.1186/s13287-019-1332-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D., Guk K., Kim H., Khang G., Wu D., Lee D. (2013). Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int. J. Pharm. 450, 87–94. 10.1016/j.ijpharm.2013.04.028 [DOI] [PubMed] [Google Scholar]
- Yoon S., Kang G., Eom G. (2019). HDAC inhibitors: therapeutic potential in fibrosis-associated human diseases. IJMS, 20, 1329 10.3390/ijms20061329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaru M., Sinico C., De Logu A., Caddeo C., Lai F., Manca M. L., et al. (2009). Rifampicin-loaded liposomes for the passive targeting to alveolar macrophages:in vitroandin vivoevaluation. J. Liposome Res. 19, 68–76. 10.1080/08982100802610835 [DOI] [PubMed] [Google Scholar]
- Zhang D., Lee H., Wang X., Rai A., Groot M., Jin Y. (2018). Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol. Ther. 26, 2119–2130. 10.1016/j.ymthe.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Lee H., Zhu Z., Minhas J. K., Jin Y. (2017). Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo . Am. J. Physiol. Lung Cell Mol. Physiol. 312, L110–L121. 10.1152/ajplung.00423.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Rekhter M. D., Gordon D., Phan S. H. (1994). Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am. J. Pathol. 145(1), 114–125. [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Sun L. X., Feng R. E. (2020). Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019. Zhonghua Jiehe He Huxi Zazhi 43 (6), 496–502. 10.3760/cma.j.cn112147-20200311-00312 [DOI] [PubMed] [Google Scholar]
- Zhang X., Bai J., Yin H., Long L., Zheng Z., Wang Q., et al. (2020). Exosomal miR‐1255b‐5p targets human telomerase reverse transcriptase in colorectal cancer cells to suppress epithelial‐to‐mesenchymal transition. Mol. Oncol. 14, 2589–2068. 10.1002/1878-0261.12765 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang X.-y., Zhang P.-y. (2017). Polymersomes in nanomedicine—a review. Cnano 13, 124–129. 10.2174/1573413712666161018144519 [DOI] [Google Scholar]
- Zhang Y., Huang Y., Li S. (2014). Polymeric micelles: nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 15, 862–871. 10.1208/s12249-014-0113-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Wu X., Qian M., Song Y., Wu D., Zhang W. (2018). Knockdown of TGF-β1 expression in human umbilical cord mesenchymal stem cells reverts their exosome-mediated EMT promoting effect on lung cancer cells. Canc. Lett. 428, 34–44. 10.1016/j.canlet.2018.04.026 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Tian B., Sun H., Zhang J., Zhang Y., Ivannikov M., et al. (2019). Pharmacoproteomics reveal novel protective activity of bromodomain containing 4 inhibitors on vascular homeostasis in TLR3-mediated airway remodeling. Journal of Proteomics 205, 103415 10.1016/j.jprot.2019.103415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhu W. P., Cai X. J., Chen M. (2016). Atomized paclitaxel liposome inhalation treatment of bleomycin-induced pulmonary fibrosis in rats. Genet. Mol. Res. 15, 1–11. 10.4238/gmr.15027309 [DOI] [PubMed] [Google Scholar]
- Zhu J. (2015). T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine, 75 (71), 14–24. 10.1016/j.cyto.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]