Highlights
-
•
EZH2 is considered as a direct target of miR-506-3p.
-
•
MiR-506-3p overexpression decreased the level of β-catenin.
-
•
MiR-506-3p increases response to PARP inhibitors and cisplatin in serous ovarian cancer by targeting EZH2/β-catenin pathway.
Keywords: miR-506-3p, PARP inhibitors, Chemo-resistance, Ovarian cancer
Abstract
Chemo-resistance is an important barrier to effective treatment of ovarian cancer. Poly (ADP-ribose) polymerase (PARP) inhibitors are currently promising targeted drugs used to treat BRCA-mutant ovarian cancer. Ovarian cancer patients with BRCA 1/2 mutations appear to benefit better from PARP inhibitors and chemotherapy. Understanding the mechanisms underlying PARP inhibitors and chemotherapy resistance is urgently needed. There is increasing evidence that microRNAs (miRNAs) are involved in drug resistance. MiR-506-3p is an effective inhibitor of the epithelial-to-mesenchymal transition (EMT), and can enhance chemotherapy and olaparib response in high-grade serous ovarian cancer (HGS-OvCa). Enhancer of Zeste Homolog 2 (EZH2) is considered as a direct target of miR-506-3p. The silencing of EZH2 mimics the inhibitory effects of miR-506-3p on chemo-resistance and olaparib response. Rescue of EZH2 prevented the functions of miR-506-3p. Moreover, EZH2 activates the β-catenin pathway. MiR-506-3p overexpression decreased the level of β-catenin, and the sensitivity to olaparib and cisplatin mediated by miR-506-3p was partially reversed by regulating β-catenin expression in ovarian cancer. Our results suggest that miR-506-3p increases response to PARP inhibitors and cisplatin in serous ovarian cancer by targeting EZH2/β-catenin signal pathway, which opens the possibility of using miR-506-3p overexpression as a potential therapeutic for ovarian cancer.
Introduction
Ovarian cancer remains the commonest cause of gynecological cancer-related death, with 21,750 new cases and 13,940 deaths in the United States estimated for 2020 [1]. About 70% of the deaths are from advanced high-grade serous ovarian cancer (HGS-OvCa) [2]. Although cytoreductive surgery followed by platinum-based chemotherapy have been widely performed, the 5-year-survival rate of those patients is still unsatisfactory [3]. Recently, Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors are currently promising targeted durgs as first-line maintenance treatment of ovarian cancer and maintenance treatment of platinum-sensitive recurrent ovarian cancer. PARP is a pivotal enzyme that repairs DNA single-strand breaks using the base excision repair pathway. HGS-OvCa frequently contains TP53 mutations and alterations of BRCA1/2. There are some defects in the homologous recombination repair (HRR) pathway in 51% of HGS-OvCa [4]. Various PARP inhibitors (PARPi) have been developed and tested in clinical trials [5], [6], [7]. The U.S. Food and Drug Administration approved olaparib in 2014 for ovarian cancer patients with BRCA1/2 mutations [8]. The use of olaparib maintenance therapy provides substantial benefits of progression-free survival (PFS) for women newly diagnosed with advanced ovarian cancer and BRCA1/2 mutations, delaying PFS for nearly 3 years compared with placebo [9]. However, not all BRCA-deficient tumors respond to PARPi treatment, and those tumors from that response to PARPi treatment will eventually acquire resistance [5, 8, 10]. Therefore, there is an urgent need to better understand the underlying mechanisms of PARPi and chemotherapy resistance in ovarian cancer and more effective therapeutic approaches to improve the prognosis of ovarian cancer patients.
MicroRNAs (miRNAs) are a class of small noncoding RNAs (∼22 nt) that can be used as a post-transcriptional regulator. MiRNAs can combine with the 3′-untranslated region (3′-UTR) of target genes and ultimately alter mRNA degradation or protein translation inhibition [11]. In the past few years, miRNAs has been shown to be involved in the regulation of various pathological processes during tumor initiation and progression [12]. Recently, emerging evidence has demonstrated that miRNAs play important roles in regulating drug resistance [13].
MiR-506, located on chromosome Xq27.3, was recently discovered to play a key role in regulating cell proliferation, differentiation, migration and invasion [14]. Dysregulation of miR-506-3p has been demonstrated in various types of cancers, including serous ovarian cancer. MiR-506-3p increased the expression of E-cadherin and prevented TGF β-induced epithelial-to-mesenchymal transition (EMT) by targeting SNAI2 in ovarian cancer [15, 16]. By directly targeting the CDK4/6-FOXM1 axis of ovarian cancer, miR-506-3p inhibits proliferation and induces senescence [17]. Elevated miR-506-3p expression was statistically associated with longer PFS and overall survival (OS) in ovarian cancer patients. Notably, miR-506-3p expression is also significantly correlated with response to platinum treatment in the INT-Milan database [18]. Our previous studies showed that miR-506-3p could increase response to chemotherapy and PARPi through regulation of RAD51-homologous recombination in serous ovarian cancers. However, apart from RAD51, we could not completely rule out the possibility that other targets of miR-506-3p mediate the drug-sensitivity.
The enhancer of zeste homolog 2 (EZH2), a critical transcriptional factor in tumorigenesis and neoplastic development, is also identified as a direct target of miR-506-3p [19]. EZH2 inhibition promotes EMT in ovarian cancer cells. A retrospective analysis indicated that EZH2 was associated with poor prognosis in head-and-neck squamous cell carcinoma via regulating the EMT and chemo-sensitivity [19]. Inhibition of EZH2 can reverse chemotherapy drug TMZ chemo-sensitivity in glioblastoma [20]. More importantly, the addition of an EZH2 inhibitor also sensitizes the BRCA-mutant breast cells to PARPi [21]. Although EZH2 plays an essential part in the development of drug resistance, it is unclear that whether miR-506-3p can increase the response of serous ovarian cancer to chemotherapy and PARPi by regulating EZH2.
In this article, we report the interaction between miR-506-3p and EZH2, which regulates drug-resistance and the response to PARPi in ovarian cancer. Our results provide an experimental basis for investigating miR-506-3p/EZH2 axis as a potential PARPi response regulator in ovarian cancer.
Materials and methods
Ovarian cancer patients and data collection
The study was approved by the Institute Research Ethics Committee of Tianjin Medical University General Hospital. Data of 92 HGS-OvCa cases were collected from the department of obstetrics and gynecology of Tianjin Medical University General Hospital. All patients received a combination of surgery and platinum-based chemotherapy. All patients signed informed consent. The ovarian tissue taken from patients was preserved in 4% PFA and made paraffin-embedded sections.
Cell cultures of human ovarian cancer cells
Human ovarian cancer cell lines (HeyA8, SKOV3) and cervical cancer cells (HeLa) were purchased from the American Type Culture Collection (ATCC, USA). All cell lines were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) in a humidified atmosphere containing 5% CO2 at 37 °C.
MiRNA transfection
MiR-506-3p mimic and negative control were obtained from Guangzhou RiboBio Co., Ltd (RiboBio, China). The cells were incubated at 2 × 105 per well in 6-well plates and allowed to attach for at least 16 h. MiR-506-3p mimic or miR-ctrl was transfected with LipofectamineRNAiMAX (Invitrogen, USA) at a final concentration of 20 nM. Total RNA and protein were extracted 48 h after transfection.
Real-time RT-PCR analysis
Total RNA was isolated from the ovarian cancer cell lines and cleaned using an RNeasy Mini kit (Qiagen, Duesseldorf, Germany). cDNA synthesis was performed with a TaqMan Reverse Transcription kit (Applied Biosystems, CA, USA). The qRT-PCR was performed with the 7500 Real-Time PCR system. The relative mRNA levels of each sample were determined by the Ct method with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western blot analysis
Primary β-actin antibody (rabbit), EZH2 antibody (rabbit) and β-catenin antibody (rabbit) were obtained from Cell Signaling Technology (Cell Signaling, USA). Briefly, 30 μg of whole-cell lysate from each sample was loaded on a 10% polyacrylamide gel for electrophoresis. The membrane was blocked in 5% non-fat milk in 1 × Tris-buffered saline solution (pH 7.4) containing 0.05% Tween-20 and detected by primary antibodies at 1/1000 dilution (for β-actin, EZH2, and β-catenin). The secondary antibodies were used at 1/1000 dilution. ECL Western Blotting Substrate from Solarbio (Solarbio, China) was used to visualize the proteins.
Luciferase reporter assay
The 3′-UTR of EZH2 containing the predicted miR-506-3p binding site was amplified separately from normal fetal genomic DNA by PCR using the following specific primers: EZH2 forward: GGCGAGCTCCATCTGCTACCTCCTCCC; reverse: GGCTCTAGAGATTCAACAAGGACAAGTTC. The PCR product was cloned in the correct direction into the pmirGLO-control vector between the SacI and XbaI sites. The common miR-506-3p binding sites in the 3′-UTR of EZH2 were deleted by PCR with a QuikChange II XL site-directed mutagenesis kit (Stratagene, USA). All clones were verified by DNA sequencing. For the luciferase reporter assay, the HeLa cells in 24-well plates were transfected with a triplicate repeat of pmirGLO reporter plasmid (0.5 μg) or pmirGLO- EZH2–3′-UTR and a miR-506-3p mimic, or miR-ctrl (50 nM) and Lipofectamine 3000 (1μL, Invitrogen, USA). According to the manufacturer's instructions, cells were subjected to lysis 48 h after transfection, and luciferase activities were determined as for a dual-luciferase assay reporter system (Promega, USA).
Gene silencing with small interfering RNA (siRNA)
HeyA8 and SKOV3 cells were transfected with siRNA (Genepharma, China) to block the expression of EZH2. Then, 2 × 105 cells per well were seeded in a 6-well plate, until the cells were 60−80% confluent. Prepare the following solutions: Solution A: for each transfection, 5ul of EZH2 siRNA duplex was diluted in 245ul of Opti-MEM ⓇReduced-Serum Medium (Life Technologies, USA); Solution B: for each transfection, 5ul of LipofectamineRNAiMAX Transfection Reagent (Invitrogen, USA) was diluted in 245ul of Opti-MEM ⓇReduced-Serum Medium (Life Technologies, USA). Solution A and B were mixed using a pipette, incubated at room temperature for 20 min. For each transfection, add 1.5 mL of siRNA Transfection Medium to each tube containing the siRNA Transfection Reagent mixture. The mixture was laid onto the washed cells and incubated for 48 h at 37 °C in a CO2 incubator. Aspirate the medium and replace it with fresh medium for CCK8 assay and Western blot analysis.
CCK8 assay
Following transfection, cell proliferation was assessed using CCK8 assay (DOJINDO, Japan), according to the manufacturer's instructions. Forty-eight hours after transfection with 20 nM miR-506-3p, or EZH2, or 50uM si-EZH2, or β-catenin or coculture with 30uM β-catenin inhibitor FH535 (Selleck, USA), 1.3 × 103 cells per well were seeded in 96-well plates and treated with a titration of cisplatin (DOJINDO, Japan) or olaparib (Selleck, USA). The medium and drug were supplemented at day 3 for cisplatin or olaparib treatment. After incubation for 5 (cisplatin) or 7 (olaparib) days, cell viability was estimated and surviving fractions were calculated. Cell survival was calculated by normalizing the absorbance to that of untreated controls.
Colony-formation assay
Forty-eight hours after transfection with β-catenin or coculture with 30uM β-catenin inhibitor FH535, cells were harvested. 800 transfected cells per well were seeded into a 6-well plate) and treated with cisplatin for 2 days or olaparib for 7 days, and then recovered for 10–14 days, during which time the surviving cells produced colonies of proliferating cells. Colony formation was quantified by staining the cells with 0.1% crystal violet and counting viable colonies containing >50 cells.
Immunohistochemical (IHC) staining
IHC staining was performed on tumor tissues from the mouse orthotopic model and tissue microarrays that included samples from 92 HGS-OvCa patients, both of which were assembled or our previous study [15]. Rabbit EZH2 antibody (1:50, Cell Signaling, USA), rabbit β-catenin antibody (1:100, Cell Signaling, USA), and goat anti-rabbit IgG/HRP antibody (1:100, Solarbio, China) were used. EZH2-positive was defined as cells that were immunoreactive in the nucleus, whereas, β-catenin positive cells were defined as those with immunoreactivity in membrane (mainly) and cytoplasm. The positive cells were quantified using a scoring system of 0–12, multiplied by the intensity of the signal (0, no signal; 1, weak signal; 2, intermediate signal; and 3, strong signal), and classified by the percentage of positive cells (0, <5%; 1, 5−25%; 2, 25−50%; 3, 50−75%; and 4, >75%). In our previous study, miRNA in situ hybridization has been performed, and low and high expression of miR-506–3p was defined as scores of <6 and≥6, respectively [15].
Statistical analysis
Data were presented as means ± SDs. Student‘s t-test was performed for determining the significance values using Statistical Product and Service Solutions software version 21.0 (SPSS, IL, USA). A p value<0.05 was considered to be statistically significant (*p<0.05, **p<0.01, ***p<0.001).
Results
Effect of mir-506-3p on EZH2 levels in human high-grade serous ovarian carcinoma
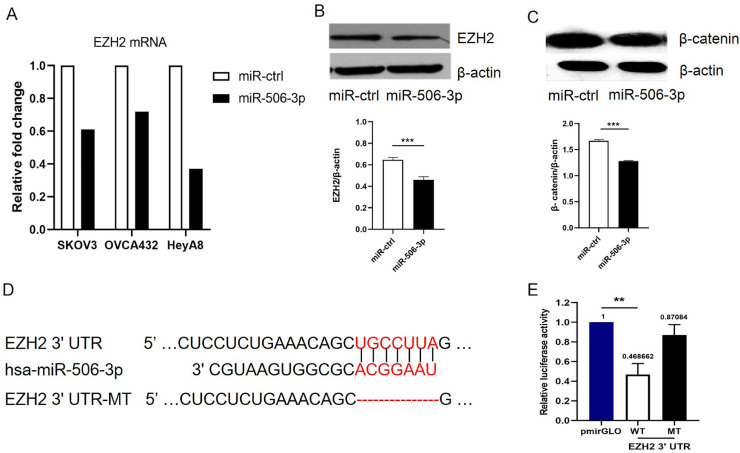
First, we examined the miR-506-3p expression level in three ovarian cancer cells to identify the genes potentially regulated by miR-506-3p. We overexpressed miR-506-3p in HeyA8 cells with relatively low miR-506-3p levels, SKOV3 cells, OVCA432 cells, and performed microarray analysis (GEO serial number: GSE50850). The data revealed that EZH2 mRNA was decreased by 3-fold in HeyA8 cells, 1.6-fold in SKOV3 cells, and 1.4-fold in OVCA432 cells after miR-506-3p overexpression (Fig. 1A) . Western blotting analysis confirmed this result in HeyA8 cells, suggesting that miR-506-3p overexpression substantially reduced EZH2 protein level (Fig. 1B).
Fig. 1.
Targeting of EZH2 by miR-506-3p.
(A) Microarray analysis of EZH2 mRNA after overexpression of miR-506-3p for 48 h in SKOV3, OVCA432 and HeyA8 cell lines. EZH2 expression levels in miR-506-3p treatments are normalized to those in miR-ctrl control treatment cells. (B-C) HeyA8 cell lines were transfected with miR-506–3p mimics or miR-ctrl for 48 h; Western blot analysis showed the changes of EZH2 and β-catenin level. The expression levels of EZH2 and β-catenin were examined with Image J. (D) TargetScan predicted that the EZH2 3′-UTR has a miR-506-3p binding site, which is highly conserved in different species. (E) Luciferase reporter assay showed that miR-506-3p directly targets the EZH2 3′-UTR. HeLa cells were co-transfected with EZH2 3′-UTR-luciferase reporter, wild-type or mutant, and miR-506-3p mimic or miR-ctrl for 48 h before analysis. Firefly luciferase activity of the reporter was normalized to the internal Renilla luciferase activity. 3′-UTR = 3′-untranslated region. MT = mutant; WT = wild-type.
EZH2 as a direct target of miR-506-3p
The miRNA target prediction algorithm TargetScan 6.0 predicted that the 3′-UTR of EZH2 mRNA contains a putative miR-506-3p binding site (Fig. 1D). To determine whether miR-506-3p regulates EZH2 by binding to its 3′-UTR, we cloned the EZH2 3′-UTR into the pmirGLO luciferase reporter vector and transfected either this vector (pmirGLO EZH2-3′-UTR) or parental luciferase expression vector, together with miR-506-3p mimic or miR-ctrl, into HeLa cells. Co-transfection of pmirGLO-EZH2-3′-UTR and miR-506–3p mimic caused a 53.1% reduction in luciferase activity compared to miR-ctrl (p = 0.001, Fig. 1E), indicating that miR-506-3p directly targets EZH2. In order to further confirm that miR-506-3p specifically regulates EZH2, we generated a mutant construct, mirGLO-EZH2-3′-UTR-MT, whose sequence complementary to the seed sequence of miR-506-3p on the EZH2 3′-UTR has been deleted. Then, we transfected cells with the mutant construct and either miR-506-3p mimic or miR-ctrl. Deletion of the miR-506-3p binding site from the EZH2 3′-UTR eliminated the effect of miR-506-3p on luciferase activity (Fig. 1E). Taken together, these results confirmed that miR-506-3p specifically targets the 3′-UTR of EZH2, thus inhibiting EZH2 gene expression.
MiR-506-3p impacts sensitivity to a PARPi or cisplatin in ovarian cancer by EZH2
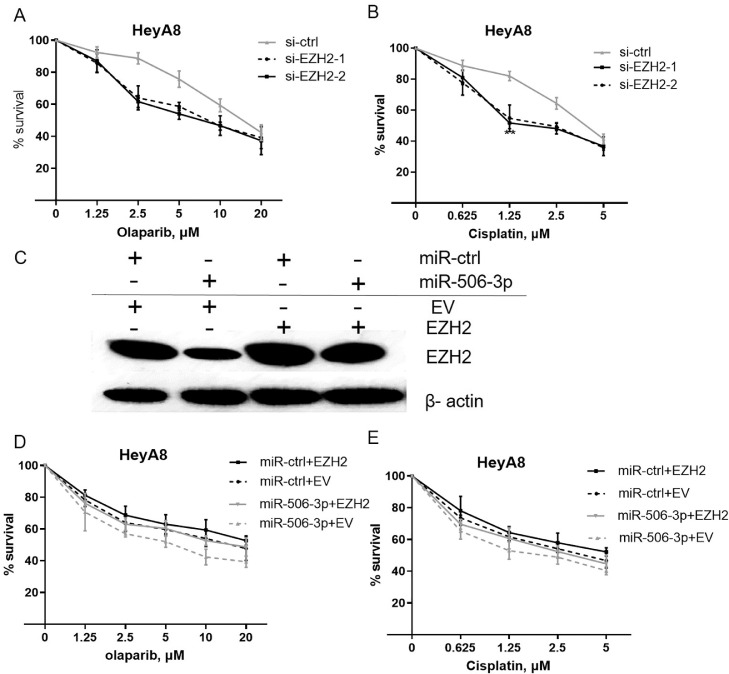
EZH2 plays an important role in mediating multiple tumor resistance. Our previous studies suggest that miR-506-3p overexpression sensitize ovarian cancer cells to a commercially available PARPi (olaparib) or cisplatin. Next, we carried out loss-of-function and rescue experiments to further prove that EZH2 participate in miR-506-3p-mediated cellular sensitivity to olaparib and cisplatin in ovarian cancer cells. Silencing of EZH2 by transfecting siRNA led to significant decrease in the proliferative capacity of ovarian cancer cells treated with olaparib or cisplatin (survival percent for 10 μM olaparib treatment, si-ctrl vs si-EZH2–1 and si-EZH2–2: 59.33 vs 46.33 and 46.67, p = 0.009, p = 0.040, respectively; survival percent for 1.25 μM cisplatin treatment, si-ctrl vs si-EZH2-1 and si-EZH2–2: 82.00 vs 54.67 and 51.67, p = 0.014, p = 0.001) (Fig. 2, A-B). Furthermore, by overexpressing EZH2 without its 3′-UTR, the effect of miR-506-3p on olaparib and cisplatin sensitivity could be completely rescued (Fig. 2, C–E), indicating that miR-506-3p–mediated sensitivity to olaparib and cisplatin is mainly a result of EZH2 expression inhibition.
Fig. 2.
EZH2 and miR-506–3p–induced increases in PARPi/cisplatin sensitivity in ovarian cancer cells.
(A,B) HeyA8 cells were transfected with 50 nM si-ctrl or si-EZH2. After 48 h, cells were reseeded for olaparib (A) or cisplatin (B) sensitivity assay. Cell viability was assessed by CCK8 assay. (C–E) HeyA8 cells were co-transfected with EZH2 without the 3′-UTR or empty vector (EV) together with 20 nM miR-506-3p or miR-ctrl. After 24 h, cells were harvested for western blot analysis (C) or reseeded for olaparib (D) or cisplatin (E) sensitivity assay.
EZH2 regulates the Wnt/β-catenin pathway
Cancer cells acquire essential characteristics for invasion, and metastatic dissemination through the process of EMT, resulting in the increase of chemo-resistance. Wnt/β-catenin, one of the most important signaling pathways involved in EMT, is also associated with cisplatin resistance. An analysis of miRNAs and signal pathways between cisplatin sensitive (A2780) and cisplatin resistant (A2780/CP70) ovarian cancer cell lines revealed changes in Wnt/β-catenin pathway [22]. To explore the relationship between miR-506-3p and the Wnt/β-catenin pathway, we transfected miR-506-3p or miR-ctrl to HeyA8 cells. The western blot result showed that miR-506-3p significantly reduced β-catenin expression, indicating miR-506-3p may regulate the Wnt/β-catenin pathway (Fig. 1C).
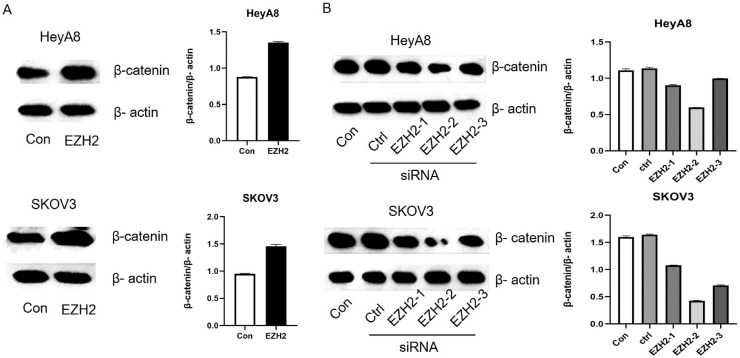
MiR-506-3p overexpression decreases EZH2 and β-catenin levels, and studies by Changet al. and Jang et al. suggest that EZH2 activates the Wnt/β-catenin pathway [23, 24]. Subsequently, we overexpressed EZH2 in HeyA8 and SKOV3 cells in order to explore the mechanism between EZH2 and the Wnt/β-catenin pathway. As expected, EZH2–transfected HeyA8 and SKOV3 cells had higher expression level of β-catenin than controls (Fig. 3A). Conversely, EZH2 siRNA transfection reduced β-catenin expression (Fig. 3B). This suggests that EZH2 regulates the Wnt/β-catenin pathway.
Fig. 3.
EZH2 regulates β-catenin signal pathway.
(A) Two ovarian cancer cell lines were transfected with EZH2 without the 3′-UTR or empty vector for 48 h, Western blot analysis showed the changes of β-catenin level. (B) Knockdown of EZH2 by si-EZH2-1, si-EZH2-2 or si-EZH2-3 compared with si-ctrl transfection in HeyA8 and SKOV3 cells, β-catenin expression by Western blot are shown.
The effect of β-catenin on sensitivity to a PARPi or cisplatin in ovarian cancer
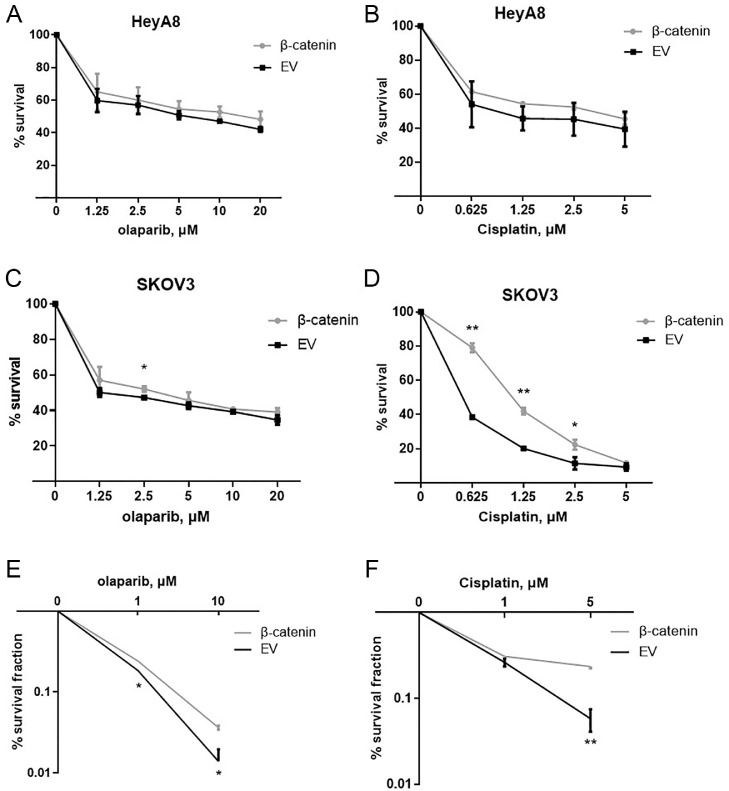
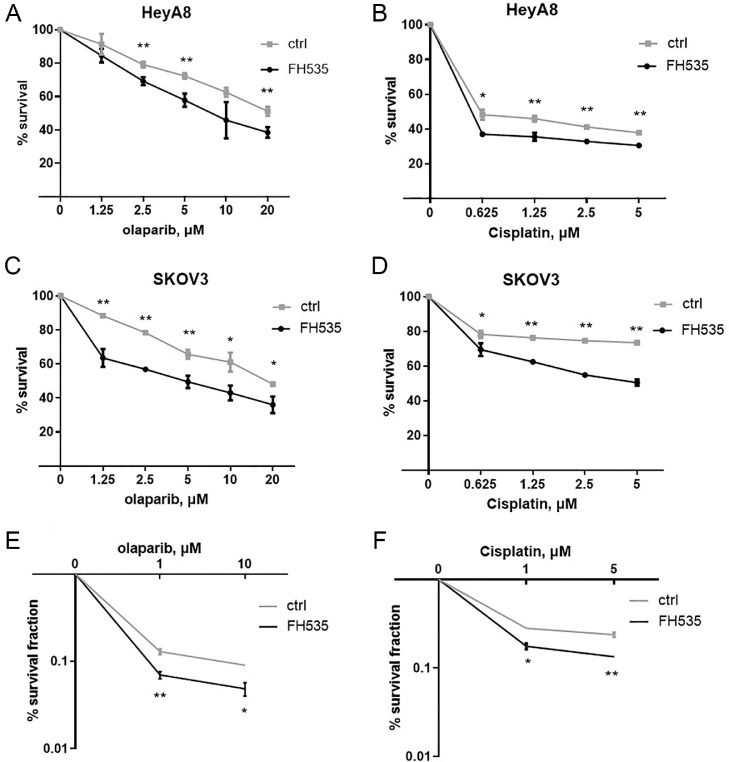
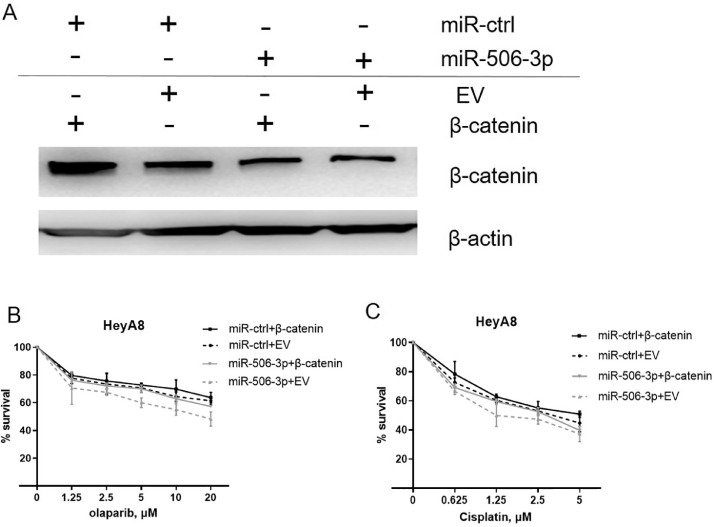
To further assess the effect of β-catenin on olaparib and cisplatin sensitivity, we overexpressed β-catenin in HeyA8 and SKOV3 cells. β-catenin transfection induced resistance to olaparib and cisplatin in HeyA8 and SKOV3 cells (Fig. 4, A–F). This β-catenin induced resistance to both olaparib and cisplatin has been confirmed in a clonogenic cell-survival assay (Fig. 4, E and F). In contrast, β-catenin inhibitor FH535 treated HeyA8 and SKOV3 cells were more sensitive to olaparib or cisplatin than controls (Fig. 5, A–F). However, the effect of miR-506-3p on olaparib and cisplatin sensitivity was partially rescued by overexpressing β-catenin (Fig. 6, A–C), suggesting that miR-506-3p affect the sensitivity to olaparib and cisplatin by inhibiting β-catenin signal.
Fig. 4.
β-catenin overexpression decreases sensitivity to a PARPi or cisplatin in ovarian cancer cells.
HeyA8 and SKOV3 cells were transfected with either β-catenin without the 3′-UTR or EV. Cell viability was assessed by CCK8 assay (A–D) and clonogenic cell-survival assay (E,F).
Fig. 5.
β-catenin knockdown increases sensitivity to a PARPi or cisplatin in ovarian cancer cells.
HeyA8 and SKOV3 cells were transfected with either β-catenin inhibitor FH535 or ctrl. Cell viability was assayed by CCK8 assay (A–D) and clonogenic cell-survival assay (E,F).
Fig. 6.
β-catenin and miR-506–3p–induced increases in PARPi/cisplatin sensitivity in ovarian cancer cells.
(A-C) HeyA8 cells were co-transfected with β-catenin without the 3′-UTR or EV together with 20 nM miR-506-3p or miR-ctrl. After 24 h, cells were harvested for Western blot analysis (A) or reseeded for olaparib (B) or cisplatin (C) sensitivity assay.
EZH2 and β-catenin downregulation mediated by miR-506-3p result in a greater sensitivity to a PARPi and cisplatin in vivo
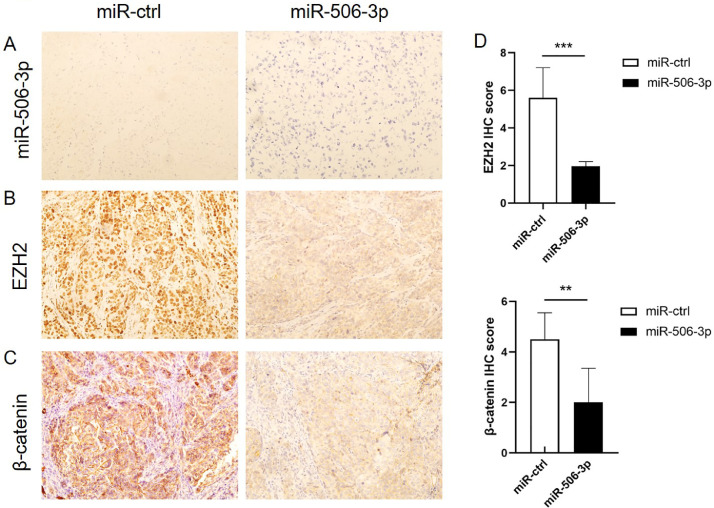
Our previous research revealed that delivery of miR-506-3p incorporated in DOPC nanoliposomes inhibited tumor growth and remarkably enhanced the effect of olaparib and cisplatin in orthotopic ovarian cancer mouse models [18]. Further, EZH2 and β-catenin expression in samples of HeyA8-ip1 tumors from control and miR-506-3p–treated mice were examined by IHC staining. Consistent with in vitro results, miR-506-3p–treated tumors showed lower EZH2 and β-catenin expression compared to control (p<0.01, p<0.01) (Fig. 7, A–D). These data suggest a greater sensitivity to PARPi and cisplatin upon EZH2 and β-catenin downregulation mediated by miR-506-3p in vivo.
Fig. 7.
MiR-506-3p, EZH2 and β-catenin expression in an orthotopic mouse model of ovarian cancer.
(A) MiR-506–3p expression in HeyA8-ip1 tumors from control and miR-506-3p treated mice was assessed by miRNA in situ hybridization. (B-D) Samples of HeyA8-ip1 tumors from control and miR-506-3p treated mice were subjected to IHC staining for EZH2 and β-catenin; Expression of EZH2 and β-catenin protein was calculated as IHC staining scores.
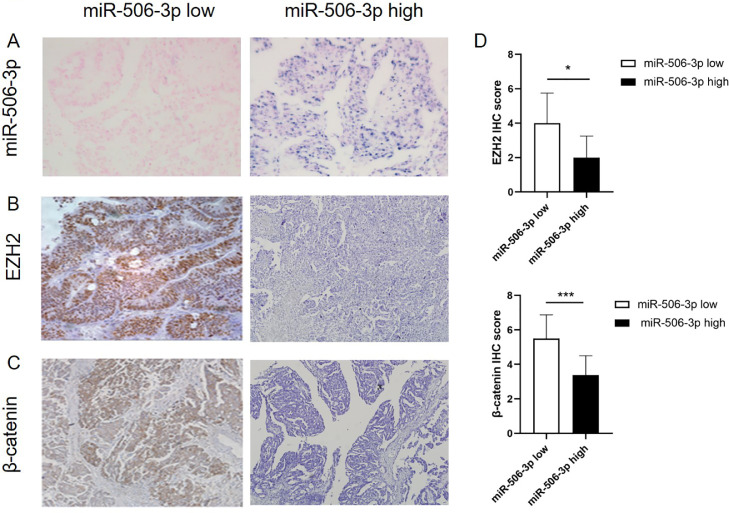
Moreover, we collected clinical and pathological data from 92 HGS-OvCa patients and made tissue microarray (TMA). The expression of EZH2 and β-catenin was detected by IHC and the expression of miR-506-3p was detected by miRNA in situ hybridization to explore the relationship between miR-506-3p expression and clinicopathological parameters, patient survival, cisplatin sensitivity, EZH2 and β-catenin expression. The results suggest that miR-506-3p is inversely associated with the expression of EZH2 and β-catenin in ovarian cancer patients (Fig. 8, A–D). It is also confirmed by clinical cases that miR-506-3p is closely related to EZH2 and β-catenin expression, prognosis and chemo-sensitivity of patients with ovarian cancer.
Fig. 8.
MiR-506-3p, EZH2 and β-catenin expression in 92 ovarian cancer patients.
(A) MiR-506–3p expression in ovarian tumors from 92 patients was assessed by miRNA in situ hybridization. (B–D) Samples of ovarian tumors from 92 patients were subjected to IHC staining for EZH2 and β-catenin; Expression of EZH2 and β-catenin protein was calculated as IHC staining scores.
Discussion
Intense studies have shown that miRNAs are critical regulators underlying drug resistance in cancer cells. In this study, we showed that miR-506-3p increases the response to PARPi and chemotherapy in serous ovarian cancer by directly targeting EZH2/β-catenin.
PARPi trap PARP on DNA at sites of single-strand breaks, thereby stopping the repair of the single-strand breaks and producing double-strand breaks which cannot be repaired accurately in tumors with defects in HRR, such as tumors with a mutation in BRCA1/2 [25]. Olaparib is a PARPi, expected to be used to treat BRCA-deficient ovarian tumors in patients [26, 27]. It has been extensively studied as a monotherapy and in combination with various chemotherapy drugs traditionally used in ovarian cancer patients. A study evaluated patients who used olaparib as a maintenance therapy for recurrent ovarian cancer with or without BRCA 1/2 mutations, and found that patients with BRCA mutations had a longer PFS than patients without the mutations [28, 29]. The SOLO 1/2/3 studies also used olaparib as a maintenance monotherapy for patients with BRCA1/2 mutations and have undergone platinum-based chemotherapy as a first-line treatment [9,30,31]. Compared with placebo, the use of maintenance therapy with olaparib in newly diagnosed advanced ovarian cancer and a BRCA1/2 mutation could delaying PFS for nearly 3 years [9]. Overall, the results from clinical trials with PARPi suggest gynecologic cancer patients with BRCA 1/2 mutations appear to benefit the most from chemotherapy supplemented with PARPi [32]. Therefore, further research should be conducted to study the relationship between combination therapy including PARPi and conventional chemotherapy.
Numerous evidences have confirmed that miR-506-3p serves as an exact tumor suppressor, inhibiting cell proliferation, invasion and migration of ovarian cancer cells [15], [16], [17]. More importantly, miR-506-3p could also be a promising prognostic indicator and target for epithelial ovarian cancer prevention and treatment [16]. Through two clinically annotated genomics datasets (TCGA and Bagnoli) analysis, high level of miR-506-3p expression was associated with better response to therapy and longer survival in ovarian cancer patients. Here, we discovered the tumor-suppressive effect of miR-506-3p in drug-resistance, thereby broadening the knowledge of the functions of miR-506-3p in ovarian cancer.
MiR-506-3p exhibits anti-tumor effects in ovarian cancer via regulating multiple targeting genes, including CDH2, SNAI2, Vimentin, CDK4, CDK6, and RAD51. For instance, miR-506-3p inhibits cell migration and invasion by targeting SNAI2, and suppresses proliferation and induces senescence by directly targeting the CDK4/6-FOXM1 axis in ovarian cancer. In our study, we demonstrated that EZH2 was a direct target of miR-506-3p. EZH2 is a catalytic subunit of polycomb repressor complex 2 (PRC2), which mediates gene silencing through methyltransferase activity and is involved in the determination of cell lineages. It has been revealed that EZH2 plays a key role in the development of multidrug resistance. Previously, miR-138 has been reported to increase the chemo-sensitivity of osteosarcoma cells to cisplatin by targeting EZH2, and overexpression of EZH2 partially eliminates the inhibitory effect of miR-138 plus cisplatin treatment [33]. MiR-126 also increased chemo-sensitivity in drug-resistant gastric cancer cells by targeting EZH2 [34]. EZH2 inhibition reverses chemotherapy drug temozolomide chemo-sensitivity in glioblastoma [20]. Moreover, the addition of an EZH2 inhibitor also sensitizes the BRCA-mutant breast cells to PARPi. In accordance with this observation, we found that silencing of EZH2 in two ovarian cancer cell lines mimicked the inhibitory effects of miR-506-3p on chemo-resistance and PARPi, and restoration of EZH2 blocked the functions of miR-506-3p, indicating that the carcinogenic functions of EZH2 in drug-resistance of ovarian cancer cells.
Next, further investigation was performed to determine the downstream signaling of miR-506-3p/EZH2 to more fully explain the role miR-506-3p in ovarian cancer drug-resistance. The Wnt/β-catenin pathway is one of the crucial signaling pathways thought to be involved in EMT [35]. Cancer cells acquire essential characteristics for invasion, and metastatic dissemination through the process of EMT, resulting in the increase of chemo-resistance. The Wnt/β-catenin pathway plays a vital role in cisplatin resistance through interaction with MARK, PI3K/AKT, apoptotic pathway, integrin pathway, and regulation of downstream target genes [36], [37], [38]. Emerging evidences have revealed that aberrant activation of β-catenin has been shown to ovarian cancer proliferation and chemo-resistance. An analysis of miRNAs and signal pathways between cisplatin sensitive (A2780), and cisplatin resistant (A2780/CP70) ovarian cancer cell lines revealed changes in Wnt/β-catenin pathway [22]. Elevated β-catenin activity contributes to carboplatin resistance in A2780/CP70 ovarian cancer cells [39]. Conversely, inhibition of β-catenin sensitizes ovarian cancer cells to chemotherapy [40]. Besides, a PARP inhibitor XAV939 could block the β-catenin pathway by stabilizing Axin [41]. PARP-1 inhibition might even augment cisplatin cytotoxicity in cervical cancer cells by inhibiting β-catenin signaling pathway [42]. The studies implicate that β-catenin signaling pathway is closely related to PARPi. Our studies confirmed that miR-506-3p overexpression decreased β-catenin level, and the sensitivity to olaparib and cisplatin mediated by miR-506-3p was partially reversed by regulating β-catenin expression in ovarian cancer cells. Rescue experiment results show that miR-506-3p can inhibit the expression of β-catenin to regulate the sensitivity to olaparib and cisplatin. Meanwhile, we observed that EZH2 could activate the β-catenin pathway in two ovarian cancer cells, which is consistent with previous studies [23, 24, 43]. Specimens of orthotopic mouse model and clinical cases also confirmed the augmentation of sensitivity to olaparib and cisplatin by miR-506-3p through regulation of EZH2/β-catenin in ovarian cancer. MiR-506-3p may be a potential target for reversing the resistance of olaparib and cisplatin in serous ovarian cancer. However, the understanding of the underlying mechanisms of these miRNAs is still superficial, especially in ovarian cancer. Apart from miR-506-3p/RAD51 pathway reported in our previous study and miR-506-3p/EZH2/β-catenin pathway in this study, we could not completely rule out the possibility that other targets of miR-506-3p mediate the drug-sensitivity. A study reported a crosstalk between FOXM1 and the Wnt/β-catenin pathway in glioma [44]. FOXM1 promotes the nuclear localization of β-catenin, and the subcellular localization of β-catenin determines its transcriptional activity [45]. Thus, it remains unclear that whether miR-506-3p influence the response of cisplatin by directly or indirectly regulating the transcription factors EZH2 and FOXM1 and the Wnt/β-catenin pathway. Although a single miR-506-3p could be used as a prognostic biomarker for ovarian cancer with considerable sensitivity and specificity, future researches focusing complex regulatory mechanism and clinical application are still required.
Conclusions
In conclusion, our findings indicate that miR-506-3p acts as a critical regulator in the sensitivity to olaparib and cisplatin in serous ovarian cancer by decreasing EZH2 and β-catenin expression, and provide potent evidence that targeting the miR-506-3p/EZH2/β-catenin axis may benefit ovarian cancer patients in combination with PARPi or chemotherapy.
CRediT authorship contribution statement
Yue Sun: Investigation, Formal analysis, Writing - original draft. Jing Wu: Investigation. Xiaoying Dong: Investigation, Methodology. Jingzi Zhang: Investigation. Chao Meng: Investigation. Guoyan Liu: Conceptualization, Funding acquisition, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors report no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grant number 81472761 to G.L.).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100987.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penelope M.W., Susan J.J., Team Head, Group Leader, Gynaecological Cancers Group a b, Cancer causes, b CGa Epidemiology of epithelial ovarian cancer. Best Prac. Res. Clin. Obstet. Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z.C., Birkbak N.J., Culhane A.C. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin. Cancer Res. 2012;18(20):5806–5815. doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonnenblick A., de Azambuja E., Azim H.A., Jr., Piccart M. An update on PARP inhibitors–moving to the adjuvant setting. Nat. Rev. Clin. Oncol. 2015;12(1):27–41. doi: 10.1038/nrclinonc.2014.163. [DOI] [PubMed] [Google Scholar]
- 6.Tutt A., Robson M., Garber J.E., Domchek S.M., Carmichael J. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. The Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman B., Shapira-Frommer R., Schmutzler R.K., Audeh M.W., Domchek S.M. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledermann A.J., El-Khouly Fatima. PARP inhibitors in ovarian cancer: clinical evidence for informed treatment decisions. Br. J. Cancer. 2015;113:S10–S16. doi: 10.1038/bjc.2015.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore K., Colombo N., Scambia G. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018;27(26):2495–2505. doi: 10.1056/NEJMoa1810858. 379. [DOI] [PubMed] [Google Scholar]
- 10.Previs R.A., Sood A.K., Mills G.B., Westin S.N. The rise of genomic profiling in ovarian cancer. Expert Rev. Mol. Diagn. 2016;16(12):1337–1351. doi: 10.1080/14737159.2016.1259069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farazi T.A., Hoell J.I., Morozov P., Tuschl T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013;774(2):1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacFarlane L.-.A., Murphy P.R. MicroRNA: biogenesis, function and role in cancer. Curr. Genomics. 2010;11(7):537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Ju J., Ni B., Wang H. The emerging role of miR-506 in cancer. Oncotarget. 2015;7(38):62778–62788. doi: 10.18632/oncotarget.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D., Sun Y., Hu L. Integrated analyses identify a master microrna regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell. 2013;23:186–199. doi: 10.1016/j.ccr.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y., Hu L., Zheng H. MiR-506 inhibits multiple targets in the epithelial-to-mesenchymal transition network and is associated with good prognosis in epithelial ovarian cancer. J. Pathol. 2015;235(1):25–36. doi: 10.1002/path.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G., Sun Y., Ji P. MiR-506 suppresses proliferation and induces senescence by directly targeting the CDK4/6-FOXM1 axis in ovarian cancer. J. Pathol. 2014;233(3):308–318. doi: 10.1002/path.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G., Da Y., Rajesha R. Augmentation of response to chemotherapy by microRNA-506 through regulation of RAD51 in serous ovarian cancers. J. Natl. Cancer Inst. 2015;107(7):djv108. doi: 10.1093/jnci/djv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardenas H., Zhao J., Vieth E. EZH2 inhibition promotes epithelial-to-mesenchymal transition in ovarian cancer cells. Oncotarget. 2016;7:84453–84467. doi: 10.18632/oncotarget.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan T.Y., Wang H., Xiang P., Liu Y.W., Qi S.T. Inhibition of EZH2 reverses chemotherapeutic drug TMZ chemosensitivity in Glioblastoma. Int. J. Clin. Exp. Pathol. 2014;7(10):6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi H., Du Y., Nakai K. EZH2 contributes to the response to PARP inhibitors through its PARP-mediated poly-ADP ribosylation in breast cancer. Oncogene. 2018;37(2):208–217. doi: 10.1038/onc.2017.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S.R., Kumar A.R., Shah P.P., Rai S.N., Kakar S.S. MicroRNA signature of cis-platin resistant vs. cis-platin sensitive ovarian cancer cell lines. J. Ovarian Res. 2011;4:17. doi: 10.1186/1757-2215-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang C.J., Yang J.Y., Xia W., et al. EZH2 Promotes Expansion of Breast Tumor Initiating Cells through Activation of RAF1-β-Catenin Signaling. 2011, 19(1):86–100. [DOI] [PMC free article] [PubMed]
- 24.Jung H.Y., Jun S., Lee M. PAF and EZH2 Induce Wnt/β-catenin signaling hyperactivation. Mol. Cell. 2013;52(2):193–205. doi: 10.1016/j.molcel.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljungman Mats. Targeting the DNA damage response in cancer. Chem. Rev. 2009;109(7):2929–2950. doi: 10.1021/cr900047g. [DOI] [PubMed] [Google Scholar]
- 26.Menear K.A., Adcock C., Boulter R. 4-[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J. Med. Chem. 2008;51(20):6581–6591. doi: 10.1021/jm8001263. [DOI] [PubMed] [Google Scholar]
- 27.Hall M., Shaw H. Emerging treatment options for recurrent ovarian cancer: the potential role of olaparib. Oncotargets Ther. 2013;6:1197–1206. doi: 10.2147/OTT.S30748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledermann J.A., Harter P., Gourley C., Friedlander M., Matulonis U. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer (SOC) and a BRCA mutation (BRCAm) J. Clin. Oncol. 2013;31(15_suppl):5505. [Google Scholar]
- 29.Ledermann J., Harter P., Gourley C. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 30.Pujade-Lauraine E., Ledermann J.A., Penson R.T. Treatment with olaparib monotherapy in the maintenance setting significantly improves progression-free survival in patients with platinum-sensitive relapsed ovarian cancer: results from the phase III SOLO2 study. Gynecol. Oncol. 2017;145:219–220. [Google Scholar]
- 31.Penson R.T., Valencia R.V., Cibula D. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 Mutation (SOLO3): a randomized phase III trial. Clin. Oncol. 2020;38(11):1164–1174. doi: 10.1200/JCO.19.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapiezynski J., Taratula O., Rodriguez-Rodriguez L., Minko T. Precision targeted therapy of ovarian cancer. J. Control Rel. 2016;243:250–268. doi: 10.1016/j.jconrel.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Z., Tang J., Wang J. MiR-138 Acts as a tumor suppressor by targeting EZH2 and enhances cisplatin-induced apoptosis in osteosarcoma cells. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P., Li Z., Liu H. MicroRNA-126 increases chemosensitivity in drug-resistant gastric cancer cells by targeting EZH2. Biochem. Bioph. Res. Commun. 2016;479(1):91–96. doi: 10.1016/j.bbrc.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 35.Arend R., Londoño A., Straughn J., Buchsbaum D. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol. Oncol. 2013;131(3):772–779. doi: 10.1016/j.ygyno.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y., Shen N., Wang Z. Sorafenib sensitizes hepatocellular carcinoma cell to cisplatin via suppression of Wnt/β-catenin signaling. Mol. Cell. Biochem. 2013;381(1–2):139–144. doi: 10.1007/s11010-013-1695-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Zhang G., Zhang H. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/p-catenin/Snail signaling pathway. Eur. J. Pharmacol. 2014;723:156–166. doi: 10.1016/j.ejphar.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Gao Y., Liu Z., Zhang X. Inhibition of cytoplasmic GSK-3β increases cisplatin resistance through activation of Wnt/β-catenin signaling in A549/DDP cells. Cancer Lett. 2013:336. doi: 10.1016/j.canlet.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Elevated β-catenin activity contributes to carboplatin resistance in A2780cp ovarian cancer cells. Biochem. Biophys. Res. Commun. 2015;468(1-2):173–178. doi: 10.1016/j.bbrc.2015.10.138. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C., Zhang Z., Zhang S., Wang W., Hu P. Targeting of Wnt/β-catenin by anthelmintic drug pyrvinium enhances sensitivity of ovarian cancer cells to chemotherapy. Med. Sci. Monit. 2017;23:266–275. doi: 10.12659/MSM.901667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao R., Tania C., Song S. Inhibition of tankyrases induces axin stabilization and blocks wnt signalling in breast cancer cells. PLoS One. 2012;7(11):e48670. doi: 10.1371/journal.pone.0048670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann M., Kumar S., Chauhan S.S., Bhatla N., Kumar L. Correction: PARP-1 inhibitor modulate β-catenin signaling to enhance cisplatin sensitivity in cancer cervix. Oncotarget. 2019;10(46):4802. doi: 10.18632/oncotarget.27101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L., Wu Y., Wu Y. The inhibition of EZH2 ameliorates osteoarthritis development through the Wnt/β-catenin pathway. Sci. Rep. 2016;6:29176. doi: 10.1038/srep29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong A., Huang S. FoxM1 and Wnt/? Catenin Signaling in glioma stem cells. Cancer Res. 2012;72(22):5658–5662. doi: 10.1158/0008-5472.CAN-12-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang N., Wei P., Gong A. FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.