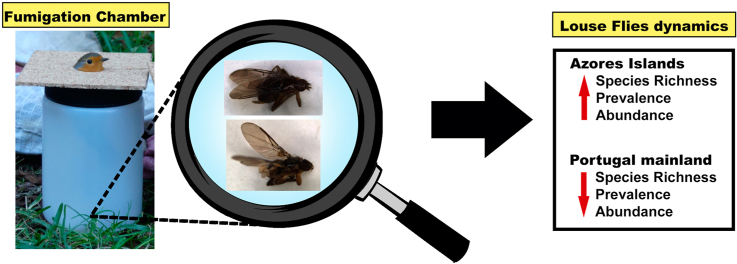
Abstract
Hippoboscid flies, also known as louse flies, are obligate blood-feeders ectoparasites of birds and mammals. By studying louse fly parasites of four Passeriformes species, Eurasian blackbird (Turdus merula), Eurasian blackcap (Sylvia atricapilla), common chaffinch (Fringilla coelebs) and European robin (Erithacus rubecula), with dissimilar time of colonization of Azores islands, we tested whether: (i) island host populations have lower parasite richness than the mainland one; (ii) island host populations undergo higher parasite prevalence, mean intensities and mean abundance than the mainland one; (iii) island parasite diversity are composed exclusively by specific parasites and (iv) parasite richness is positively correlated with the island area and proximity to the continent. For these purposes, 775 birds were sampled for presence of louse flies, by modified fumigation chamber method, from Azores Islands (São Miguel, Terceira and Flores) and Portugal mainland. Insular and mainland parasite assemblages were statistically compared. We record for the first time to Azores, Ornithomya fringillina and Icosta minor from Portugal mainland. Louse flies had highest prevalence and abundance from Azores Islands compared to those observed in mainland birds, especially blackbirds. The insular parasite diversity of Azores blackbirds, blackcaps and chaffinches was richer than the one observed in mainland population. None of the hippoboscid flies observed on the islands and mainland were host-specific. Thus, our findings provide an upgrade of parasite island syndromes knowledge, in the context of the ectoparasites, namely to the hippoboscid flies case.
Keywords: Hippoboscid flies, Louse flies, Wild birds, Ectoparasite assemblage, Azores, Portugal
Graphical abstract
Highlights
-
•
We investigate the louse flies of four Passeriformes species from Macaronesia.
-
•
Louse flies of Azores blackbirds had highest prevalence than in mainland birds.
-
•
Insular parasite diversity was richer than the one observed in mainland population.
-
•
Hippoboscid flies observed on the islands and mainland were not host-specific.
-
•
Ornithomya fringillina recorded for the first time to Azores.
1. Introduction
Over the last two decades, the insular diversity of parasites have been the focus of biogeographical studies, to understand which factors are involved in species’ range expansion (Losos and Ricklefs, 2010; Moyer et al., 2002; Poulin, 2004). Studies on parasite traits found that the parasite ability of establishment during the host expansion is crucial. Parasites may have a successful establishment, or instead be absence from the new area by “missing the boat” (parasites do not present from the founding hosts that colonize a new region) or “drowning on arrival” (parasites do arrive with hosts, but fail the establishment) (MacLeod et al., 2010; Paterson et al., 2003). Not least are the ecological features of the host, such as: population size, geographic range and migration events. For example, seabirds with trans-oceanic dispersal movements, larger population and geographical range, may explain the high ectoparasites diversity (Gómez-Díaz et al., 2012; Hughes and Page, 2007). Additionally, environmental parameters can determine ectoparasite species distributions. Birds in arid regions have fewer ectoparasitic lice than birds in humid regions (Moyer et al., 2002), but arid conditions provide a climatic refuge from the competitively superior species (Malenke et al., 2011).
The insular vertebrates populations often undergo a series of changes (morphometric, life-history, behavioural, physiological and genetic) as result of isolation, phenomenon known as insular syndrome (Blondel, 2000). This concept has been adapted to insular communities of parasites, originating the parasite island syndromes. Nieberding et al. (2006) studied the colonization patterns of the Mediterranean Islands by Heligmosomoides polygyrus (Dujardin, 1845), a specific nematode of rodents and recorded a significant loss of genetic diversity and an ecological niche enlargement following colonization, as result of founder effect. Additionally, Pérez-Rodríguez et al. (2013) studied the haemoparasites in the Macaronesia and reported: (i) impoverishment of insular haemosporidians assemblage; (ii) lower prevalence of parasites in the island populations compared with mainland and (iii) reduced host specialization on islands. The authors attributed these results to: reduced availability of appropriate vectors on islands, sequential founder population bottlenecks and migratory traits of birds. Recently, the parasite island syndromes were studied to Azorean communities of ectoparasites, namely to chewing lice by Literák et al. (2015) and found: (i) fewer chewing lice species in the Azores birds; (ii) higher louse prevalence from insular birds and (iii) only chewing lice host specific in the Azores. These authors suggested that the findings can be correlated with migratory and ecological traits of birds and chewing lice features.
Island area and his distance from the mainland source population are key factors to the island syndrome (Blondel, 2000; Losos and Ricklefs, 2010). However, these factors shown contradictory results to parasite island syndromes; while Nieberding et al. (2006) and Pérez-Rodríguez et al. (2013) reported a decreasing parasite richness with increasing island distance to the continent, Ishtiaq et al. (2010), Literák et al. (2015) did not recorded this effect.
Hippoboscid flies, known as louse flies or keds, are obligate blood-feeders ectoparasites of domestic and wild birds and mammals. The members of the Hippoboscidae family are larviparous, with larval development occurring in uterus, where they are nourished by milk glands; when fully developed, pre-pupae are deposited or released in proximity to the host, such as birds’ roost, nests or the hair of mammals, and immediately begin to darken and form the puparium, i.e., the last larval instar (Hutson, 1984; Maa and Peterson, 1987). Adult louse fly are dorsoventrally flattened, with a depressed head and a hind pair of wings, although few species having vestigial or no wings (Reeves and Lloyd, 2019).
Louse flies are known to act as vectors of infectious agents, including arbovirus, bacteria, avian and mammalian trypanosomes, hemosporidian blood protozoa and helminths (Baker, 1967; Halos et al., 2004; Rani et al., 2011), and serve as disseminators of lice and mites, which have with them a phoretic relationship (Hill et al., 1967; Keirans, 1975). Additionally, Gancz et al. (2004) and Farajollahi et al. (2005) suspected the vector competence of louse fly in the transmission of West Nile Virus.
Worldwide, approximately 213 louse fly species are known, of which 30 have been recorded in Europe (Dick, 2006; Pape et al., 2015). The Portuguese hippoboscid fauna is composed of 10 species on the mainland territory, 4 species from Azores Islands and 3 from Madeira Island (Carles–Tolrá and Báez, 2002; Oslejskova et al., 2020; Smit, 2008, 2010). However, considering the few studies directed to louse fly species research, we believe that this list is still far from complete.
The main goal of this study was to characterize the diversity of hippoboscid flies infesting four species of passerines, Eurasian blackbird Turdus merula Linnaeus, 1758, Eurasian blackcap, Sylvia atricapilla (Linnaeus, 1758), common chaffinch Fringilla coelebs Linnaeus, 1758 and European robin Erithacus rubecula (Linnaeus, 1758) from Azores Islands, and compare it with the diversity found on the same species in mainland Portugal. A comparison of louse flies in hosts originating from the mainland and islands allowed to test the predictions derived from parasite island syndromes and island biogeography theory: (i) whether island host populations have lower parasite richness than the mainland one; (ii) whether island host populations have higher parasite prevalence, mean intensities and mean abundance than the mainland one; (iii) whether island parasite diversity are composed exclusively by specific parasites; and (iv) whether parasite richness are positively correlated with the island area and proximity to the continent.
2. Material and methods
2.1. Study area
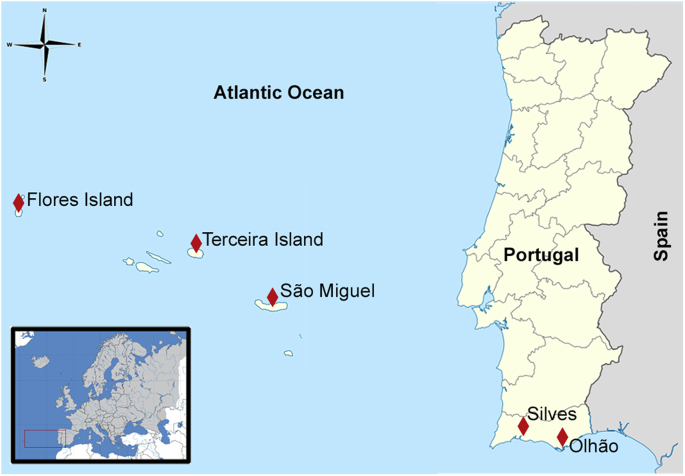
Portugal is situated in the southwest of the European continent, covers 92,090 km2 and comprises a continental part and two Macaronesian archipelagos constituted by volcanic islands and islets located in North Atlantic, Azores archipelago and Madeira archipelago. Azores (36°55’ and 39°43′N and 25°01’ and 31°07′W) is located approximately about 1600 km from Europe and 1900 km from North America and consists of nine islands geographically clustered into three groups: Eastern group, with São Miguel (area: 747 km2; distance to mainland: 1364 km) and Santa Maria Islands; Central group, constituted by Faial, Pico, São Jorge, Terceira (area: 403 km2; distance to mainland: 1519 km) and Graciosa Islands and Western group, with Flores (area: 141 km2; distance to mainland: 1839 km) and Corvo Islands (França et al., 2003; Pacheco et al., 2013).
This study took place in the south mainland Portugal, specifically at Silves and Olhão, and in three islands of the Azores archipelago, namely São Miguel, Terceira and Flores (Fig. 1). The selection of the places was determined by the occurrence of the studied bird species, and hence, the three islands representing locations with different areas and distance to mainland.
Fig. 1.
Map of the mainland Portugal and Azores Islands with the geographic distribution of the study areas (Silves, Olhão, São Miguel Island, Terceira Island and Flores Island).
2.2. Field sampling
Live birds of the species T. merula (subspecies: T. m. merula Linnaeus, 1758 from mainland and T. m. azorensis Hartert, 1905 from Azores), S. atricapilla (subspecies: S. a. atricapilla (Linnaeus, 1758) from mainland and S. a. gularis Alexander, 1898 from Azores), F. coelebs (subspecies: F. c. balearica von Jordans, 1923 from mainland and F. c. moreletti Pucheran, 1859 from Azores) and E. rubecula were captured randomly with mist nets, during October–December of two consecutive years (2018–2019). The time of the year was determined by the studied bird species abundance, namely in the case of robin and chaffinch that are more common in mainland Portugal during winter migration. Due to the patchy distribution and abundance of sampled bird species, individuals were captured at 2–3 sites on each island, to increase capture rates and to avoid repeated captures of the same individuals. Each bird was individualized with metal ring, sexed and aged (juveniles: <1 year old; adults: >1 year old), based on plumage features (Demongin, 2016).
Birds were sampled for presence of louse flies using a modified fumigation chamber method from Clayton and Drown (2001), where birds' bodies were exposed to chloroform, for 5 min and bird's heads underwent visual examination (Visnak and Dumbacher, 1999). This is a standard practice performed by numerous bird banders throughout the world, especially for the study of avian chewing lice (Sychra et al., 2008; Literák et al., 2015). All birds were released after examination at the site of capture.
2.3. Arthropods collection
The hippoboscid flies were placed individually into small tubes containing 70% ethanol, until further processing at Entomology Laboratory at Faculty of Sciences, University of Lisbon. Each louse fly was examined under a Stereo Microscope Olympus SZX7, and identified using the following dichotomous keys: Hill (1962), Maa (1966, 1969), Hutson (1984), Maa and Peterson (1987) and Petersen et al. (2007). The systematics and nomenclature rules followed Maa and Peterson (1987). Chewing lice and mites found in phoretic association with louse flies, were identified using specific identification keys (Gustafsson and Bush, 2017; Mironov et al., 2005). Images were acquired on Zeiss Stereo LUMAR stereoscope V12, equipped with a Zeiss Axiocam 503 color 3 MP, controlled with the Axiovision 4.9.1 software and digitally processed using ImageJ 1.52p software (Schindelin et al., 2012).
2.4. Statistical data analyses
Observed louse fly species richness was compared with estimated species using a rarefaction analyses with 100 randomization models, which were extrapolated to a total of 500 samples using bias-corrected formula for Chao1 and Chao2 which included the upper limit to be considered as a rare or infrequent species (R = 2). Chao2 estimator, an asymptotic species richness Chao (1987), was chosen as the best estimator. Additionally, the Shannon index of diversity was used to estimate diversity. These analysis were performed using EstimateS 9.1.0 software (Colwell, 2013).
Hippoboscid flies species shared between islands and mainland populations were compared to test different prevalence, mean abundance and intensity. Firstly, prevalence, abundance and intensity of each louse fly was estimated. Due to the low prevalence of louse flies on chaffinch, robin and blackcap, these birds were excluded from the others statistical analyses. Statistical differences in the geographical patterns of louse fly species were assessed with Fisher's Exact Test and bootstrap 2-sample t-test with 1000 replications, to detect associations in louse fly prevalence (%), and in intensity and abundance, respectively. Blackbird's age, gender and year of fieldwork were used as co-factors that could produce variation in louse fly prevalence between islands and mainland. Posteriorly, a Chi-square test adjusted using a post hoc test, Bonferroni correction, and Kruskal-Wallis with Dunn's post hoc test (adjusted using the Bonferroni correction) was used to detect the statistical differences of louse fly prevalence only among islands populations of blackbirds.
Sample size varied depending on the variable in analyses, since not all data from all individuals were collected.
General statistical analyses were done with the software Quantitative Parasitology 3.0 (Reiczigel et al., 2019). The analysis of post hoc tests was done using IBM®SPSSS®Statistics Version 26 (IBM Corp, 2019).
3. Results
3.1. Louse flies richness
Three species of louse fly were found: Ornithoica turdi (Olivier, 1811) (Fig. 2A and B), Ornithomya fringillina Curtis, 1836 (Fig. 2C and D) and Icosta minor (Bigot, 1858) (Fig. 2E and F).
Fig. 2.
Photos of three species of hippoboscid fly and their wings collected from Passeriformes species: (A and B) Ornithoica turdi, (C and D) Ornithomya fringillina and (E and F) Icosta minor. Scale bar: 1 mm.
Considering the four host species together, 2 louse fly species were recorded in Azores Islands, O. turdi and O. fringillina and 2 in mainland Portugal, O. turdi and I. minor. The observed richness of louse flies for each host species was: 2 – O. turdi and O. fringillina – and 1 – O. turdi – species in blackbirds from Azores Islands and mainland Portugal, respectively; 2 – O. turdi and O. fringillina – in blackcap and chaffinch from Azores Islands; and 1 species in robin from Azores Islands – O. turdi – and mainland Portugal– I. minor.
The results of the rarefaction analyses and Shannon index of diversity were summarized in Table 1. The observed richness (Sobs) coincided with asymptotic species richness (Sest) for all analyses. Asymptotic species richness reaching the asymptote in the sample numbers of: 26 and 49 for blackbirds from Azores and mainland, respectively; 119 for blackcap from Azores; 117 for chaffinch from Azores; 23 and 29 for robin from Azores and mainland, respectively; 88 and 209 birds of any species from Azores and mainland, respectively.
Table 1.
Number of birds analysed (n) and respective observed richness of species (Sobs), asymptotic richness of species (Sest), the best estimator of richness (Chao2) and Shannon index of diversity for the louse fly assemblages of each bird species from Azores and mainland Portugal.
| n | Sobs | Sest | Chao2 (CI 95%) | Shannon index ± SD | |
|---|---|---|---|---|---|
| Blackbird | |||||
| Azores | 180 | 2 | 2 | 2.00 (2.00–2.32) | 0.4 ± 0.11 |
| Mainland | 60 | 1 | 1 | 1.00 (1.00–1.80) | 0 |
| Blackcap | |||||
| Azores | 181 | 2 | 2 | 2.00 (2.00–2.66) | 0.65 ± 0.06 |
| Chaffinch | |||||
| Azores | 180 | 2 | 2 | 2.00 (2.00–2.6) | 0.63 ± 0.07 |
| Robin | |||||
| Azores | 25 | 1 | 1 | 1.00 (1.00–2.15) | 0 |
| Mainland | 29 | 1 | 1 | 1.00 (1.00–3.60) | 0 |
| Total | |||||
| Azores | 566 | 2 | 2 | 2.00 (2.00–3.14) | 0.46 ± 20.1 |
| Mainland | 209 | 2 | 2 | 2.00 (2.00–3.84) | 0.5 ± 0 |
The highest species diversity (±SD) was observed in the community of Azorean blackbirds (0.4 ± 0.11) compared with mainland; Independently of the species, mainland birds (0.5 ± 0) shown high louse flies diversity than Azores (0.46 ± 0.1).
Observed parasite richness was not correlated with the island area and their distance to mainland.
3.2. Prevalence of louse flies
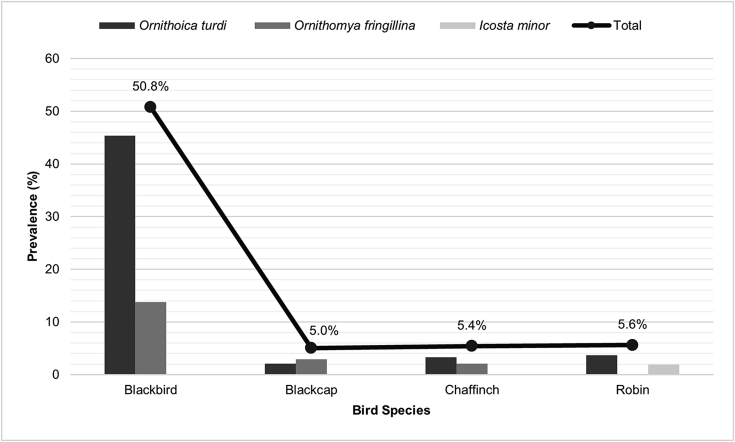
Overall, 19.4% of the 775 individuals analysed for this study were infected by at least one hippoboscid fly species. The highest prevalence of flies was found in blackbirds (50.8%), followed by robins (5.6%), chaffinches (5.4%), and blackcaps (5.0%) (Fig. 3; Table S1 in supplementary material). O. turdi was the most representative species (16.0%), followed by O. fringillina (5.8%) and I. minor founded only in a bird species (0.1%). The highest prevalence of infestation by O. turdi was found in blackbirds (45.4%), followed by robins (3.7%), chaffinches (3.3%) and blackcaps (2.1%). For the case of O. fringillina, the highest prevalence was found in blackbirds (13.8%), followed by blackcaps (2.9%) and chaffinches (2.1%). Finally, I. minor was only found in a robin (1.9%). Among the infested birds per species, 102 (42.5%) blackbirds were infested by only one fly species, whereas 20 (8.3%) carried a double infestation (O. turdi and O. fringillina), therefore only single infestations were recorded in blackcaps, chaffinches and robins.
Fig. 3.
Prevalence (%) of hippoboscid fly species found on blackbirds, blackcaps, chaffinches and robins from the Azores Islands and mainland Portugal.
3.3. Louse fly infestation and location
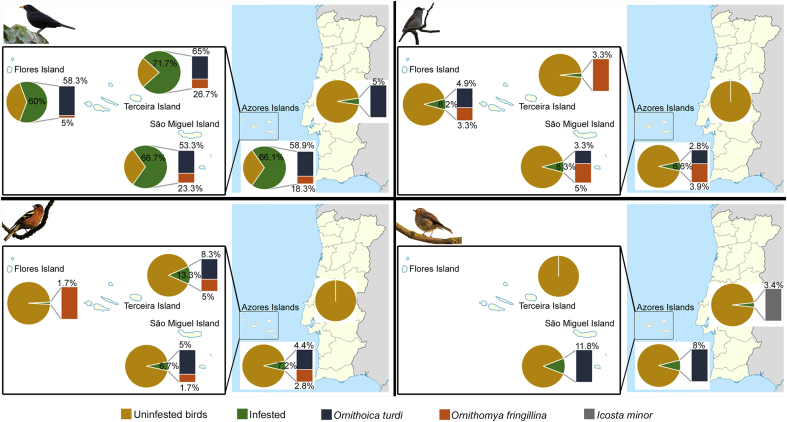
Louse fly prevalence changed according location of fieldwork (Fig. 4; Table S2 in supplementary material). Overall louse flies prevalence was much higher in Azorean blackbirds, blackcap and chaffinch (66.1%, 6.6% and 7.2%, respectively) than mainland specimens (5%, 0% and 0%) (Fisher test; blackbirds: p < 0.001, blackcap and chaffinch: p < 0.05).
Fig. 4.
Map showing the prevalence (represented by different color; yellow color represent the prevalence of uninfested birds) of hippoboscid fly species in blackbirds, blackcaps, chaffinches and robins in the each of the sampled Azorean Islands (São Miguel, Terceira and Flores) and the mainland Portugal. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Considering each louse fly species, only blackbirds exhibit prevalence differences between Azores Islands and mainland. Ornithoica turdi were found on 58.3%, 65%, 53.3% and 5% of blackbirds from Flores, Terceira, São Miguel and mainland, respectively. These differences in the prevalence differ statistically between each Azores Islands and mainland (Fisher test; p < 0.001). For the case of O. fringillina, were only found in Flores, Terceira and São Miguel blackbirds, 5%, 26.7% and 23.3%, respectively. A single Icosta minor was recorded from mainland robin.
When comparing the prevalence of O. turdi and O. fringillina only among islands, infestation rates revealed not to be statistically different to O. turdi (ꭓ2 = 1.698; p > 0.05), while O. fringillina prevalence was statistically different among Azores Islands (ꭓ2 = 10.909; p < 0.01). Ornithomya fringillina of blackbirs from Flores Island differ statistically to Terceira (Kruskal-Wallis test: p < 0.01) and São Miguel Island (Kruskal-Wallis test: p < 0.05).
Overall, the mean abundances of hippoboscid flies were higher in the Azores birds than on the mainland (Table S2 in supplementary material). Ornithoica turdi, the only shared species among blackbird populations, was statistically more abundance in islands than mainland.
3.4. Phoresy
Overall, 4 (1.4%) and 47 (16.4%) of 286 louse fly carried chewing lice and mites, respectively. Summarized in Table 2, 51 phoretic cases, involve hippoboscid flies of the species O. fringillina (10) and O. turdi (41). Ornithomya fringillina bearing phoretic lice of the Guimaraesiella amsel (Fig. 5A) were only collected on blackbirds species (10.8%); Mites of the family Epidermoptidae were found attached to O. turdi on blackbirds (Fig. 5B) (18.8%) and O. fringillina on blackbirds (13.5%) and a blackcap (14.3%).
Table 2.
Prevalence of phoresy of chewing lice and mites on louse flies from blackbirds and blackcap.
| Bird–Hippoboscid fly associations |
|||
|---|---|---|---|
| Blackbird |
Blackcap |
||
| Ornithoica turdi | Ornithomya fringillina | Ornithomya fringillina | |
| Number of flies | 218 | 37 | 7 |
| % chewing lice on hippoboscid flies | 0 | 10.8 | 0 |
| % mites on hippoboscid flies | 18.8 | 13.5 | 14.3 |
Fig. 5.
Photos of phoretic association of Guimaraesiella amsel on Ornithomya fringillina (A) and Epidermoptidae mites on Ornithoica turdi (B). Scale bar: 1 mm.
4. Discussion
Considering the records published by Carles–Tolrá and Báez (2002) and Smit (2010) our work contributed for this topic by reporting the following new records for the Azores Islands and mainland Portugal: (i) Ornithoica turdi from Flores and Terceira Island; (ii) Ornithomya fringillina from Flores, Terceira and São Miguel Island; and (iii) Icosta minor from mainland Portugal.
The diversity of hippoboscid flies found in Azores blackbirds, blackcap and chaffinch was richer than the one observed in mainland population of the same species. In the case of blackcap and chaffinch, the mainland diversity was clearly impoverished, without presence of ectoparasites. Contrariwise, when consider all hosts species together, the richness of louse flies was similar among Azores and mainland, with two species at each location. Both results do not support the one of the assumptions of the parasite island syndromes postulated by Paterson et al. (2003) that host–parasite associations are compromised on islands, resulting in lower numbers of species, as recorded from Macaronesia on: blackcap chewing lice (Literák et al., 2015); and blackcap and trumpeter finches haemosporidians (Barrientos et al., 2014; Pérez-Rodríguez et al., 2013). Our result suggest that hippoboscid species do not fail the establishment to the new area by “missing the boat” or “drowning on arrival” (MacLeod et al., 2010; Paterson et al., 1999), but instead had a successful establishment on Azores Islands. The louse fly species identified by us are generalist parasites, parasitizing mainly small birds, including various Passeriformes species, as observed by Oboňa et al. (2019) and can rely on other host species to colonize and thrive in the islands, this result was to be expected. Although the parasites infesting mainland birds are also not host-specific, I. minor was only found in the mainland. According to the hosts geographical range, we suspected that the European robin, acquired I. minor through host-switching events between unrelated hosts as previously suggested by Paterson et al. (2003).
Overall, louse flies do not display host-specificity and so, we could not confirm the one the parasite island syndromes hypothesis advanced by Pérez-Rodríguez et al. (2013), that the island parasites was not host-specific. Furthermore, we found an uncorrelation among parasite richness and island area and their distance from the mainland. These uncorrelation do not support the basic principle of the theory of island biogeography, that biggest island and islands that are located near the putative source of colonizers, have greater species richness, but may be attributed to do not host-specificity of louse flies (Losos and Ricklefs, 2010).
The general observed louse flies prevalence in islands was significant higher compared with mainland birds; Azorean blackbirds showed prevalence and mean abundance significantly highest than to those observed in mainland Portugal populations; blackcaps and chaffinches only presented hippoboscid flies in the Azores; no statistical differences were observed for the robins. Mainland prevalence's of louse fly species were very similar with findings from other European countries (Table 3). Ornithoica turdi and I. minor were not found in blackbirds, blackcap, chaffinch and robin from Czech Republic, Germany, Slovakia and Finland. Furthermore, O. fringillina was reported in: 3.8% and 0.2% blackcaps from Czech Republic and Germany, respectively; 3.1% chaffinches from Finland; and 33.3% and 10% robins from Czech Republic and Finland, respectively.
Table 3.
Reports of Hippoboscidae species in wild Passeriformes species from Europe.
| Location | Host Species | Louse fly | n | Prevalence (%) | Literature source |
|---|---|---|---|---|---|
| Czech Republic | Blackbird | – | 12 | – | Sychra et al. (2008) |
| Blackcap | O. fringillina | 78 | 3.8 | ||
| Robin | O. fringillina | 15 | 33.3 | ||
| Blackbird | – | 21 | – | Sychra et al. (2011) | |
| Blackcap | – | 114 | – | ||
| Robin | – | 281 | – | ||
| Chaffinch | – | 31 | – | ||
| Germany | Blackbird | - | 42 | – | Labitzke and Jentzsch (2019) |
| Blackcap | O. fringillina | 1595 | 0.2 | ||
| Chaffinch | – | 4 | – | ||
| Robin | – | 235 | – | ||
| Slovakia | Blackbird | – | 2 | – | Bush et al. (2018) |
| Blackcap | – | 1 | – | ||
| Chaffinch | – | 4 | – | ||
| Robin | – | 2 | – | ||
| Finland | Chaffinch | O. fringillina | 162 | 3.1 | Sorjonen (1971) |
| Robin | O. fringillina | 19 | 10,5 |
The statistically differences verified between mainland and Azorean birds are in accordance with the results of blackcap chewing lice (Literák et al., 2015). Conversely, Pérez-Rodríguez et al. (2013) reported lower prevalence of haemoparasites in the island populations and explain this, with the absence or reduced availability of appropriate vectors. In the case of ectoparasites, such as louse flies, due to direct transmission routes, without any interference of vectors, the transmission is more efficient (Sychra et al., 2008). Thus, some hypotheses may account for the scarcity of avian hippoboscid flies in mainland and high prevalence in Azores Islands such as: (i) island birds, where predation risk is either absent or negligible, takes less time in the nest sanitation and consequently could be exposed to more louse flies, attracted by faecal volatile components (Ibáñez-Álamo et al., 2016). Nest sanitation has been considered a behavioural adaptation to arthropod control and reduction of predator attraction, mainly by rejection of faeces over the side of the nest, removal of the faecal sacs of the young and frequent renovation of the nest lining material (Bucher, 1988; Petit et al., 1989); (ii) the risk of parasite infestation seems to be host density dependent (Begon et al., 1996). High host densities in islands could account for the high parasite prevalence in islands (Dobson, 1988). Although Lynch and Baker (1993) report a chaffinch density in Azores Islands (5–10 birds/ha) fairly higher than that from mainland (1 bird/ha) and our observations over the last two years of fieldwork (unpublished data) are in agree with this observations, we do not have recent data on birds density in Azores Islands and mainland, to confirm these hypothesis; (iii) Abiotic factors, namely the absolute minimum temperature (°C) and/or total precipitation (mm) showed to be more favourable in Azores Islands (Table S3 in supplementary material) for the high parasites’ prevalence. Senar et al. (1994) found that the best time of the year for collecting adult louse flies is during the period May–October, partly due to abiotic factors; (iv) the large flow of migratory birds specimens in mainland at the time of year when the sampling was carried out, could represent a low prevalence of parasites. Birds migration offers an adaptive advantage “in terms of reduced risks of parasitism by moving to areas that harbour lower densities of conspecifics” (Sychra et al., 2011); while, Cork et al. (2001) suggested that birds with higher parasite loads may die in the early stages of the migration, never reach to the wintering and/or nesting site. Moreover, Sychra et al. (2008, 2011) and Hutson (1981) only found hippoboscid flies post-breeding migration from Czech Republic and Britain birds, respectively.
Our data are partially in accordance with the results of Barrientos et al. (2014) and Literák et al. (2015), for chewing lice in the Canary islands and Azores islands, respectively, who observed that parasitological parameters do not have significant differences among island populations. However, our results for O. fringillina in blackbirds show a lower prevalence on the Flores Island compared to the other two islands. Considering the low host-specificity of this louse fly species, mainly found in Passeriformes, but also in other bird orders, this result was unexpected (Oboňa et al., 2019).
Looking at Azores birds, we report a general fairly high prevalence of louse flies from blackbirds compared with blackcap, chaffinch and robins. Using the premise of the classical theory of island biogeography and the analogy postulated by Kuris et al. (1980), which predict that hosts, can be viewed as islands for parasite colonization. Thus, it will be expectable that larger host species, provide more space for parasites. Although our results do not showed highest parasite richness on Azorean blackbirds, the larger-bodied species of our study, we believe that the same principle could be apply to the prevalence of parasites, as had been shown by Corbet (1956). Moreover, as louse flies hide between the feathers to escape the preening activity of birds, Tella et al. (1998) suggested that louse flies may exhibit a positive correlation with feathers size.
Furthermore, louse flies are known to provide a ride on another less mobile organism, such as mites and chewing lice. Our results corroborate the Keirans (1975) and Philips and Fain (1991) findings, that phoresy is more common for Ischnoceran lice and skin mites (Epidermoptidae) and appears to be exceptionally rare amongst Amblyceran lice and feather mites, respectively. Guimaraesiella amsel and Epidermoptidae mites represent new records to the Azores fauna. However, phoretic association with louse flies, O. fringillina and O. turdi, have already been recorded by Bartlow et al. (2016) and Philips and Fain (1991). Thus, despite the phoresy being a non-transversal behaviour to all mites and chewing lice, some species can use this behaviour as a dispersal mechanism, shaping their distribution and abundance.
5. Conclusions
Our findings resulted in the recording of 1 species of louse fly new to the fauna of Azores: Ornithomya fringillina; and a new species to the fauna of mainland Portugal, Icosta minor. Our results point out that Macaronesian birds, especially blackbirds, have higher louse flies load and mean abundance when compared with their mainland counterparts. Moreover, these results do not support the idea of parasite island syndromes (low richness, frequent host-switching and reduced specialization) for the blackcaps haemosporidians from Macaronesia. The parasite parameters changes between island and mainland bird species may be partially interpreted as the likely outcome of host and abiotic factors. Considering that hippoboscid flies are obligates blood-feeders ectoparasites of birds and do not depend of any vectors, our study adds a new host-parasite interaction perspective to the parasite island syndromes concept. Thus, we are aware that to better understand the parasite island syndromes on the Azores Islands is still necessary to proceed with an extensive acquisition of knowledge on bird–parasite interactions.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
We would like to thank Silvio Gonçalves and Clara Rego for the permission to capture birds on the Aldeia da Cuada – Lajes das Flores and Centro Experimental de Agricultura Biológica – Lagoa das Sete Cidades, respectively. To Instituto da Conservação da Natureza e das Florestas, I.P., Direção Regional do Ambiente dos Açores and Direção Regional da Ciência e Tecnologia dos Açores for their kindly authorization to capture birds and collect samples from the mainland Portugal (Ref. 160/2018/CAPT and 907/2019/CAPT) and the Azores islands (Ref. 83/2018/DRA and 82/2019/DRA; 37/DRCT/2018 and 44/2019/DRCT). To thank the technical imaging support of Faculty of Sciences of the University of Lisbon's Microscopy Facility which is a node of the Portuguese Platform of BioImaging, reference PPBI-POCI-01-0145-FEDER-022122. This study was financially supported by the National Science and Technology Foundation of the Ministry of Science, Technology and Higher Education, Portugal (FCT/MCTES: UIDP/50017/2020+UIDB/50017/2020 to CESAM – Centro de Estudos do Ambiente e do Mar; UIDB/00276/2020 to CIISA – Centro de Investigação Interdisciplinar em Sanidade Animal; and individual PhD grant PD/BD/127919/2016 to André Tomás). Likewise, we thank to the anonymous reviewers for the helpful comments and suggestions that have contributed to the improvement of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2020.12.004.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Baker J.R. A review of the role played by the hippoboscidae (Diptera) as vectors of endoparasites. J. Parasitol. 1967;53:412–418. doi: 10.2307/3276603. [DOI] [PubMed] [Google Scholar]
- Barrientos R., Valera F., Barbosa A., Carrillo C.M., Moreno E. Biogeography of haemo- and ectoparasites of an arid-land bird, the Trumpeter finch. J. Arid Environ. 2014;106:11–17. doi: 10.1016/j.jaridenv.2014.03.005. [DOI] [Google Scholar]
- Bartlow A.W., Villa S.M., Thompson M.W., Bush S.E. Walk or ride? Phoretic behaviour of amblyceran and ischnoceran lice. Int. J. Parasitol. 2016;46:221–227. doi: 10.1016/j.ijpara.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Begon M., Harper J.L., Townsend C.R. 3nd ed. Blackwell Scientific Publications; Sunderland: 1996. Ecology: Individuals, Populations and Communities. [Google Scholar]
- Blondel J. Evolution and ecology of birds on islands: trends and prospects. Vie Milieu. 2000;50:205–220. [Google Scholar]
- Bucher E.H. Do birds use biological control against nest parasites? Parasitol. Today Off. 1988;4:1–3. doi: 10.1016/0169-4758(88)90045-2. [DOI] [PubMed] [Google Scholar]
- Bush S.E., Gustafsson D.R., Clayton D.H. New records of ectoparasites from passerine birds in the High Tatras of Slovakia. Oecol. Montana. 2018;27:43–45. [Google Scholar]
- Carles–Tolrá M., Báez M. Hippoboscidae. In: Carles-Tolrá M., editor. Catálogo de Los Díptera de España, Portugal y Andorra (Insecta) Sociedad Entomológica Aragonesa (SEA); Zaragoza: 2002. p. 208. [Google Scholar]
- Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43:783. doi: 10.2307/2531532. [DOI] [PubMed] [Google Scholar]
- Clayton D.H., Drown D.M. Critical evaluation of five methods for quantifying chewing lice (Insecta: phthiraptera) J. Parasitol. 2001;87:1291–1300. doi: 10.1645/0022-3395(2001)087[1291:CEOFMF]2.0.CO;2. 10.1645/0022-3395(2001)087[1291:CEOFMF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Colwell R.K. EstimateS: statistical estimation of species richness and shared species from samples. 2013. http://purl.oclc.org/estimates Available at: Version 9.1.
- Corbet G.B. The life-history and host-relations of a hippoboscid fly Ornithomyia fringillina Curtis. J. Anim. Ecol. 1956;25:403–420. doi: 10.2307/1934. [DOI] [Google Scholar]
- Cork S.C., Csörgö T., Scebba S., Lövei G. Association of Veterinary Teachers and Research Workers. Current Topics in Veterinary Science. 55 Th Annual Conference. vol. 70. 2001. The prevalence of nematodes parasites in transcontinental songbirds; pp. 1–53. Scarborough, UK, 9th-12th April, 2001. Abstracts. Research in Veterinary Science, (Suppl A), 20. [Google Scholar]
- Demongin L. Laurent Demongin; 2016. Identification guide to birds in the hand, Beauregard-Vendon. [Google Scholar]
- Dick C. Dep. Zool. F. Museum Nat. Hist; Chicago: 2006. Checklist of world Hippoboscidae (Diptera: Hippoboscoidea) [Google Scholar]
- Dobson A.P. Restoring island ecosystems: the potential of parasites to control introduced mammals. Conserv. Biol. 1988;2:31–39. [Google Scholar]
- Farajollahi A., Crans W.J., Nickerson D., Bryant P., Wolf B., Glaser A., Andreadis T.G. Detection of West nile virus RNA from the louse fly Icosta americana (Diptera: Hippoboscidae) J. Am. Mosq. Contr. Assoc. 2005;21:474–476. doi: 10.2987/8756-971x(2006)21[474:downvr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- França Z., Virgilio Cruz José, Nunes J.C., Forjaz V.H. Geologia dos Açores: Uma perspectiva actual. Açoreana. 2003;10:11–140. [Google Scholar]
- Gancz A.Y., Barker I.K., Lindsay R., Dibernardo A., McKeever K., Hunter B. West Nile virus outbreak in North American owls, ontario, 2002. Emerg. Infect. Dis. 2004;10:2135–2142. doi: 10.3201/eid1012.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Díaz E., Morris-Pocock J.A., González-Solís J., McCoy K.D. Trans-oceanic host dispersal explains high seabird tick diversity on Cape Verde islands. Biol. Lett. 2012;8:616–619. doi: 10.1098/rsbl.2012.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson D.R., Bush S.E. Morphological revision of the hyperdiverse Brueelia-complex (Insecta: Phthiraptera: Ischnocera: Philopteridae) with new taxa, checklists and generic key. Zootaxa. 2017;4313:1–443. doi: 10.11646/zootaxa.4313.1.1. [DOI] [Google Scholar]
- Halos L., Jamal T., Maillard R., Girard B., Guillot J., Chomel B., Vayssier-Taussat M., Boulouis H.-J. Role of Hippoboscidae flies as potential vectors of Bartonella spp. infecting wild and domestic ruminants. Appl. Environ. Microbiol. 2004;70:6302–6305. doi: 10.1128/AEM.70.10.6302-6305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D.S. Revision of the British species of Ornithomya Latreille (Diptera: hippoboscidae) Proc. R. Ent. Soc. Lond. (B) 1962;31:11–18. doi: 10.1111/j.1365-3113.1962.tb01163.x. [DOI] [Google Scholar]
- Hill D.S., Wilson N., Corbet G.B. Mites associated with British species of Ornithomya (Diptera: Hippoboscidae) J. Med. Entomol. 1967;4:102–122. doi: 10.1093/jmedent/4.2.102. [DOI] [PubMed] [Google Scholar]
- Hughes J., Page R.D.M. Comparative tests of ectoparasite species richness in seabirds. BMC Evol. Biol. 2007;7:1–21. doi: 10.1186/1471-2148-7-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson A.M. The population of the louse‐fly, Crataerina pallida (Diptera, Hippoboscidae) on the European swift, Apus apus (Aves, Apodidae) J. Zool. 1981;194:305–316. doi: 10.1111/j.1469-7998.1981.tb04583.x. [DOI] [Google Scholar]
- Hutson A.M. Keds, flat-flies and bat-flies: Diptera, Hippoboscidae and Nycteribiidae. Handbooks identif. Br. Insects. 1984;10:1–40. [Google Scholar]
- Ibáñez-Álamo J.D., Ruiz-Raya F., Rodríguez L., Soler M. Fecal sacs attract insects to the nest and provoke an activation of the immune system of nestlings. Front. Zool. 2016;13:1–9. doi: 10.1186/s12983-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp . 2019. IBM®SPSS®Statistics for Windows. [Google Scholar]
- Ishtiaq F., Clegg S.M., Phillimore A.B., Black R.A., Owens I.P.F., Sheldon B.C. Biogeographical patterns of blood parasite lineage diversity in avian hosts from southern Melanesian islands. J. Biogeogr. 2010;37:120–132. doi: 10.1111/j.1365-2699.2009.02189.x. [DOI] [Google Scholar]
- Keirans J.E. A review of the phoretic relationship between mallophaga (Phthiraptera: Insecta) and Hippoboscidae (Diptera: Insecta) J. Med. Entomol. 1975;12:71–76. doi: 10.1093/jmedent/12.1.71. [DOI] [PubMed] [Google Scholar]
- Kuris A.M., Blaustein A.R., Alio J.J. Hosts as islands. Am. Nat. 1980;116:570–586. [Google Scholar]
- Labitzke V., Jentzsch M. Louse fly records during bird ringing at the Helmestausee Berga-Kelbra (Diptera, Hippoboscidae) Vogelwarte. 2019;57:81–89. [Google Scholar]
- Literák I., Sychra O., Resendes R., Rodrígues P. Chewing lice in azorean blackcaps (Sylvia atricapilla): a contribution to parasite island syndromes. J. Parasitol. 2015;101:252–254. doi: 10.1645/14-601.1. [DOI] [PubMed] [Google Scholar]
- Losos J.B., Ricklefs R.E. Princeton University Press; 2010. The Theory of Island Biogeography Revisited. [Google Scholar]
- Lynch A., Baker A.J. A population memetics approach to cultural evolution in chaffinch Song: meme diversity within populations. Am. Nat. 1993;141:597–620. doi: 10.1086/285493. [DOI] [PubMed] [Google Scholar]
- Maa T. The genus Ornithoica rondani (Diptera: Hippoboscidae) Pac. Insects Monogr. 1966;10:10–124. [Google Scholar]
- Maa T. Revision of Icosta (= Lynchia auctt .) with erection of a related genus Phthona (Diptera: hippoboscidae) Pac. Insects Monogr. 1969;20:25–203. [Google Scholar]
- Maa T., Peterson B. Hippoboscidae. In: McAlpine J., editor. Manual of Neartic Diptera - Volume 2. Agriculture Canada Monograph No. 28; 1987. pp. 1271–1281. [Google Scholar]
- MacLeod C.J., Paterson A.M., Tompkins D.M., Duncan R.P. Parasites lost - do invaders miss the boat or drown on arrival? Ecol. Lett. 2010;13:516–527. doi: 10.1111/j.1461-0248.2010.01446.x. [DOI] [PubMed] [Google Scholar]
- Malenke J.R., Newbold N., Clayton D.H. Condition-specific competition governs the geographic distribution and diversity of ectoparasites. Am. Nat. 2011;177:522–534. doi: 10.1086/658176. [DOI] [PubMed] [Google Scholar]
- Mironov S.V., Bochkov A.V., Fain A. Phylogeny and evolution of parasitism in feather mites of the families Epidermoptidae and Dermationidae (Acari: Analgoidea) Zool. Anz. 2005;243:155–179. doi: 10.1016/j.jcz.2004.10.001. [DOI] [Google Scholar]
- Moyer B.R., Drown D.M., Clayton D.H. Low humidity reduces ectoparasite pressure: implications for host life history evolution. Oikos. 2002;97:223–228. doi: 10.1034/j.1600-0706.2002.970208.x. [DOI] [Google Scholar]
- Nieberding C., Morand S., Libois R., Michaux J.R. Parasites and the island syndrome: the colonization of the western Mediterranean islands by Heligmosomoides polygyrus (Dujardin, 1845) J. Biogeogr. 2006;33:1212–1222. doi: 10.1111/j.1365-2699.2006.01503.x. [DOI] [Google Scholar]
- Oboňa J., Sychra O., Greš S., Heřman P., Manko P., Roháček J., Šestáková A., Šlapák J., Hromada M. A revised annotated checklist of louse flies (Diptera, Hippoboscidae) from Slovakia. ZooKeys. 2019:129–152. doi: 10.3897/zookeys.862.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslejskova L., Kounkova S., Gustafsson D.R., Resendes R., Rodrigues P., Literak I., Sychra O. Insect ectoparasites from wild passerine birds in the Azores Islands. Parasite. 2020;27:64. doi: 10.1051/parasite/2020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco J.M., Ferreira T., Queiroz G., Wallenstein N., Coutinho R., Cruz J.V., Pimentel A., Silva R., Gaspar J.L., Goulart C. Notas sobre a geologia do arquipélago dos Açores. In: Dias R., Araújo A., Terrinha P., Kullberg J.C., editors. vol. 2. Escolar; 2013. pp. 596–690. (Geologia de Portugal). [Google Scholar]
- Pape T., Beuk P., Pont A., Shatalkin A., Ozerov A., Woźnica A., Merz B., Bystrowski C., Raper C., Bergström C., Kehlmaier C., Clements D., Greathead D., Kameneva E., Nartshuk E., Petersen F., Weber G., Bächli G., Geller-Grimm F., Van de Weyer G., Tschorsnig H.-P., de Jong H., van Zuijlen J.-W., Vaňhara J., Roháček J., Ziegler J., Majer J., Hůrka K., Holston K., Rognes K., Greve-Jensen L., Munari L., de Meyer M., Pollet M., Speight M., Ebejer M., Martinez M., Carles-Tolrá M., Földvári M., Chvála M., Barták M., Evenhuis N., Chandler P., Cerretti P., Meier R., Rozkosny R., Prescher S., Gaimari S., Zatwarnicki T., Zeegers T., Dikow T., Korneyev V., Richter V., Michelsen V., Tanasijtshuk V., Mathis W., Hubenov Z., de Jong Y. Fauna europaea: Diptera – brachycera. Biodivers. Data J. 2015;3 doi: 10.3897/BDJ.3.e4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A.M., Palma R.L., Gray R.D. How frequently do avian lice miss the boat? Implications for coevolutionary studies. Syst. Biol. 1999;48:214–223. doi: 10.1080/106351599260544. [DOI] [Google Scholar]
- Paterson A.M., Palma R.L., Gray R.D. Drowning on arrival, missing the boat, and x-events: how likely are sorting events. In: Page R.D.M., editor. Tangled Trees: Phylogeny, Cospeciation, and Coevolution. University of Chicago Press; Chicago: 2003. pp. 287–307. [Google Scholar]
- Pérez-Rodríguez A., Ramirez A., Richardson D.S., Pérez-Tris J. Evolution of parasite island syndromes without long-term host population isolation: parasite dynamics in Macaronesian blackcaps Sylvia atricapilla. Global Ecol. Biogeogr. 2013;22:1272–1281. doi: 10.1111/geb.12084. [DOI] [Google Scholar]
- Petersen F.T., Damgaard J., Meier R. DNA taxonomy: how many DNA Sequences are needed for solving a taxonomic problem? The case of two parapatric species of louse flies (Diptera: Hippoboscidae : Ornithomya Latreille, 1802) Arthropod Syst. Phylogeny. 2007;65:119–125. [Google Scholar]
- Petit K.E., Petit L.J., Petit D.R. Fecal sac removal: do the pattern and distance of dispersal affect the chance of nest predation? Condor. 1989;91:479–482. doi: 10.2307/1368331. [DOI] [Google Scholar]
- Philips J., Fain A. Acarine symbionts of louseflies (Diptera : Hippoboscidae) Acarologia. 1991;32:377–384. [Google Scholar]
- Poulin R. Macroecological patterns of species richness in parasite assemblages. Basic Appl. Ecol. 2004;5:423–434. doi: 10.1016/j.baae.2004.08.003. [DOI] [Google Scholar]
- Rani P.A.M.A., Coleman G.T., Irwin P.J., Traub R.J. Hippobosca longipennis - a potential intermediate host of a species of Acanthocheilonema in dogs in northern India. Parasites Vectors. 2011;4:1–7. doi: 10.1186/1756-3305-4-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W.K., Lloyd J.E. Louse flies, keds, and bat flies (Hippoboscoidea) In: Mullen G.R., Durden L.A., editors. Medical and Veterinary Entomology. Elsevier; London: 2019. pp. 421–438. [Google Scholar]
- Reiczigel Jenő, Marozzi Marco, Fábián Ibolya, Rózsa Lajos. Biostatistics for Parasitologists – A Primer to Quantitative Parasitology. Trends Parasitol. 2019;35(4):277–281. doi: 10.1016/j.pt.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senar J.C., Copete J.L., Domenech J., von Walter G. Prevalence of louse-flies Diptera, Hippoboscidae parasiting a cardueline finch and its effect on body condition. Ardea. 1994;82:157–160. [Google Scholar]
- Smit J.T. Diptera (Scenopinidae, Xylomyidae, Syrphidae, Tephritidae, Hippoboscidae, Oestridae, Tachinidae) In: Borges P.A.V., Abreu C., Aguiar A.M.F., Carvalho P., Jardim R., Melo I., Oliveira P., Sérgio C., Serrano A.R.M., editors. A List of the Terrestrial Fungi, Flora and Fauna of Madeira and Selvagens Archipelagos. Direcção Regional do Ambiente da Madeira and Universidade dos Açores, Funchal and Angra do Heroísmo; 2008. pp. 328–339. [Google Scholar]
- Smit J.T. Diptera (hippoboscidae, Scenopinidae, Syrphidae, Tachinidae, Tephritidae) In: Borges P.A.V., Costa A., Cunha R., Gabriel R., Gonçalves V., Martins A., Melo I., Parente M., Raposeiro P., Rodrigues P., Santos R.S., Silva L., Vieira P., Vieira V., editors. A List of the TerrestriaL and Marine Biota from the Azores. Princípia; Cascais: 2010. pp. 233–240. [Google Scholar]
- Sorjonen J. Occurrence of three species of Ornithomya Diptera, Hippoboscidae on birds in two areas of southern Finland. Ann. Zool. Fenn. 1971;8:442–445. [Google Scholar]
- Sychra O., Literák I., Podzemný P., Benedikt V. Insect ectoparasites from wild passerine birds in Czech Republic. Parasite. 2008;15:599–604. doi: 10.1051/parasite/2008154599. [DOI] [PubMed] [Google Scholar]
- Sychra O., Literák I., Podzemný P., Harmat P., Hrabák R. Insect ectoparasites on wild birds in the Czech Republic during the pre-breeding period. Parasite. 2011;18:13–19. doi: 10.1051/parasite/2011181013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella J.L., Gajon A., Gortazar C., Osacar J.J. High host specificity of Crataerina melbae (Diptera: Hippoboscidae) in a mixed colony of birds. J. Parasitol. 1998;84:198–200. doi: 10.2307/3284563. [DOI] [PubMed] [Google Scholar]
- Visnak R.M., Dumbacher J.P. Comparison of four fumigants for removing avian lice. J. Field Ornithol. 1999;70:42–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.