Abstract
Background
Despite its benefits, a major concern regarding antipsychotic treatment is its possible impact on the brain’s structure and function. This study sought to explore the characteristics of white matter structural networks in chronic never-treated schizophrenia and those treated with clozapine or risperidone, and its potential association with cognitive function.
Methods
Diffusion tensor imaging was performed on a unique sample of 34 schizophrenia patients treated with antipsychotic monotherapy for over 5 years (17 treated with clozapine and 17 treated with risperidone), 17 never-treated schizophrenia patients with illness duration over 5 years, and 27 healthy control participants. Graph theory and network-based statistic approaches were employed.
Results
We observed a disrupted organization of white matter structural networks as well as decreased nodal and connectivity characteristics across the schizophrenia groups, mainly involving thalamus, prefrontal, and occipital regions. Alterations in nodal and connectivity characteristics were relatively milder in risperidone-treated patients than clozapine-treated patients and never-treated patients. Altered global network measures were significantly associated with cognitive performance levels. Structural connectivity as reflected by network-based statistic mediated the difference in cognitive performance levels between clozapine-treated and risperidone-treated patients.
Limitations
These results are constrained by the lack of random assignment to different types of antipsychotic treatment.
Conclusion
These findings provide insight into the white matter structural network deficits in patients with chronic schizophrenia, either being treated or untreated, and suggest white matter structural networks supporting cognitive function may benefit from antipsychotic treatment, especially in those treated with risperidone.
Keywords: schizophrenia, white matter structural networks, long-term, risperidone, clozapine
Significance Statement.
While the majority of schizophrenia patients benefit from antipsychotic treatment, mainly through a reduction of positive symptoms, about one-third of patients continue to suffer from cognitive impairments, suboptimal social functioning, and progressive brain tissue loss, suggesting a deteriorating course in schizophrenia. Despite its benefits, a major concern regarding antipsychotic treatment is its possible impact on the brain’s structure and function as well as its associations with symptom expression and cognitive impairments. This study sought to explore the characteristics of white matter structural networks in chronic schizophrenia treated with clozapine or risperidone and those never treated, and their potential associations with cognitive function. Our study revealed differential white matter structural network disturbances among those patients, which were significantly associated with cognitive performance levels. Our results suggest that white matter structural networks supporting cognitive function may benefit from antipsychotic treatment, especially in patients treated with risperidone.
Introduction
Schizophrenia is characterized by a spectrum of syndromes, including positive symptoms (e.g., hallucinations, delusions) and negative symptoms (e.g., affective flattening, social withdrawal), but also a range of cognitive deficits. Antipsychotic medication represents the gold standard of treatment given its effectiveness in reducing psychotic symptom expression and relapse rates in patients (Miyamoto et al., 2005). While the majority of schizophrenia patients experience a benefit from antipsychotic treatment, mainly through a reduction of positive symptoms, about one-third of patients continue to suffer from cognitive impairments, suboptimal social functioning, and progressive brain tissue loss (Harvey et al., 1999; Ho et al., 2003; van Haren et al., 2007; Cahn et al., 2009; Velthorst et al., 2017), suggesting a deteriorating course in schizophrenia. Despite its benefits, a major concern regarding antipsychotic treatment is its possible impact on the brain’s structure and function as well as its associations with symptom expression and cognitive impairments (Gong et al., 2016).
In the past decades, a great deal of effort has been made in the field of psychoradiology to elucidate medication-induced treatment effects on brain structures (Lui et al., 2016). Complex patterns of both grey and white matter changes following short-term antipsychotic treatment have been reported (Gong et al., 2016), supporting the idea of a robust impact of antipsychotic medications on brain structures. While such studies have been informative, their relatively short and diverse follow-up intervals leave questions about the effects of long-term antipsychotic treatment unanswered. A few long-term longitudinal studies of antipsychotic-treated patients reported progressive grey matter volume loss at the whole brain level and, more specifically, related to frontal and temporal lobe volumes (Andreasen et al., 2011; Ho et al., 2011; van Haren et al., 2011). On the other hand, increased white matter alterations have also been shown after a 5-year follow-up period (Sun et al., 2016). Given the fact that ideally the majority of patients are treated with antipsychotics from the time of diagnosis, it is difficult to differentiate between the influences of illness course and treatment. Through a direct comparison of never-treated and long-term–treated schizophrenia patients with similar illness durations, we recently reported cerebral alterations associated with the course of illness and greater white matter alterations in never-treated than antipsychotic-treated patients (Xiao et al., 2018). This suggests that long-term antipsychotic treatment does not adversely affect the brain’s structure over the longer term course of illness and may even confer some benefits (Xiao et al., 2018). The potential beneficial effects of long-term antipsychotic treatment are additionally supported by functional brain imaging data (Yao et al., 2019). However, previous studies reporting brain structure alterations in antipsychotic-treated patients were limited by very diverse antipsychotic treatments, which did not allow specific conclusions about specific effects of individual agents on white matter brain structure (Bartzokis et al., 2007, 2009; Vita et al., 2015; Leroux et al., 2018).
Clozapine and risperidone represent some of the first so-called atypical antipsychotics introduced into clinical practice and are still widely prescribed. Although both work very effectively, they rely on different pharmacological mechanisms. Clozapine has a high affinity to dopamine D4 receptors (Van Tol et al., 1991), presumably mediating its antipsychotic efficacy. Clozapine also has a high affinity to muscarinic M1–5 receptors (Meltzer and McGurk, 1999) and acts predominantly as an antagonist at M1 and M4 receptors (Bymaster et al., 2003), indicating stronger inherent anticholinergic properties than other antipsychotics. In contrast, risperidone does not have any significant affinity for D4 and M1–5 receptors (Meltzer and McGurk, 1999) but instead shows higher affinity and longer dissociation latencies at D2 receptors than other atypical antipsychotics (Kapur and Seeman, 2001). This explains its increased risk of considerably disturbing extrapyramidal side effects. Several studies have suggested that chronic exposure to clozapine or risperidone may have differential impacts on white matter structure compared with other antipsychotic agents. For instance, Leroux et al. (Leroux et al., 2018) reported different structural connectivity abnormalities in prefronto-fronto-subcortical networks in schizophrenia patients with chronic exposure to clozapine compared with patients with chronic exposure to typical antipsychotics. Bartzokis (Bartzokis et al., 2007, 2009) reported that patients treated with risperidone exhibit larger frontal volume and intracortical myelin volume than patients treated with typical antipsychotics such as fluphenazine decanoate. Regarding brain structure changes induced by clozapine or risperidone, it remains unclear whether both confer benefits or have differential impact on white matter structure in schizophrenia.
When studying differential effects of antipsychotic treatment on the brain’s structure and function, another aspect must be considered. Anticholinergic effects as well as the propensity to induce extrapyramidal side effects may lead to additional detrimental effects on cognitive performance (Green and Braff, 2001; Thornton et al., 2006), though improvement of cognitive deficits by antipsychotic treatment has also been reported in schizophrenia patients (Woodward et al., 2005). The limited available reports on risperidone and clozapine suggest that risperidone may improve working memory, reasoning, and problem-solving performance, while clozapine may support language performance better than risperidone (Houthoofd et al., 2008; Han et al., 2015). However, other studies could not corroborate these positive effects of clozapine or risperidone (Meltzer and McGurk, 1999; Ayesa-Arriola et al., 2013; Olagunju et al., 2018). The effects of long-term treatment with clozapine or risperidone on cognitive function thus remain controversial (Keefe and Harvey, 2012).
This study was designed to determine the impact of long-term monotherapy of clozapine or risperidone on the topological organization of white matter structural networks and its potential associations with cognitive function in chronic schizophrenia. We employed diffusion tensor imaging (DTI) and a graph theory approach to investigate topological alterations of white matter structural networks. The 2 patient groups were additionally compared with a never-treated group of patients with similar illness durations to control for general effects associated with antipsychotic treatment. We hypothesized that schizophrenia patients treated with different types of antipsychotic medications show differential alterations in their white matter structural connectome associated with different levels of cognitive functions.
Materials and Methods
The West China Hospital Research Ethics Committee of Sichuan University approved this study, and written informed consent was obtained from all study participants or their legal guardian before enrolment.
Participants
The study included 34 chronic, clinically stable schizophrenia patients receiving long-term (ranging from 5 to 25 years) antipsychotic monotherapy with either risperidone (n = 17) or clozapine (n = 17), 17 never-treated chronic schizophrenia patients with a similar illness duration, and also 27 healthy comparison participants of similar age (Table 1). Illness onset was determined using the Nottingham Onset Schedule (Singh et al., 2005) with information provided by patients, their family members, and their medical records. Diagnosis of schizophrenia was established using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995). Patients on long-term antipsychotic monotherapy were recruited from psychiatric community facilities. Those patients had been treated in the rehabilitation clinic early in their course of illness; thus, their records of treatment were relatively intact. Based on these medical records, a patient’s antipsychotic monotherapy with either risperidone or clozapine had to be documented consistently for at least 5 years before entry into the study. Patients with known history of poor response to other antipsychotics were excluded. Never-treated patients were recruited by a nearby community mental health-screening program designed to identify and provide psychiatric care to individuals with serious but untreated mental illness. Never-treated schizophrenia patients mostly lived in rural areas or small villages and had been cared for and sheltered by their families without medical care through the course of their illness for various reasons, such as concerns regarding family stigma, lack of understanding of the disease, and poor socioeconomic conditions. Healthy controls were recruited from the same geographical region by poster advertisement. The non-patient edition of the Structured Clinical Interview for DSM-IV Axis I Disorders was used to establish the lifetime absence of psychiatric illness in the control participants, and there was no known family history of major psychiatric illnesses in any of their first-degree relatives.
Table 1.
Demographic and Neuropsychological Data
| HC | NTSZ | CTSZ | RTSZ | P value | Post-hoc tests | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age, y | 48.93 (5.99) | 47.29 (10.07) | 49.35 (5.21) | 46.12 (5.43) | .313 | NA | |
| Duration, y | NA | 18.94 (10.76) | 24.06 (7.21) | 20.29 (6.56) | .174 | NA | |
| PANSS scores | |||||||
| Positive symptom score | NA | 22.82 (6.66) | 9.59 (9.33) | 10.29 (3.27) | <.0001 | NTSZ VS CTSZ: ****; NTSZ VS RTSZ: *** | |
| Negative symptom score | NA | 25.59 (7.71) | 21.18 (4.67) | 14.12 (3.89) | <.0001 | NTSZ VS RTSZ: ****; RTSZ VS CTSZ: ** | |
| General psychopathology symptom score | NA | 43.53 (6.68) | 27.94 (4.85) | 24.76 (4.74) | <.0001 | NTSZ VS CTSZ: ****; NTSZ VS RTSZ: **** | |
| Total score | NA | 91.94 (11.21) | 58.71 (9.33) | 49.18 (10.50) | <.0001 | NTSZ vs RTSZ: ****; NTSZ vs CTSZ: ****; RTSZ vs CTSZ: * | |
| Clozapine dosage, mg/d | NA | NA | 223.53 (97.63) | NA | NA | NA | |
| Risperidone dosage, mg/d | NA | NA | NA | 5.09 (1.33) | NA | NA | |
| Chlorpromazine equivalents, mg/d | NA | NA | 335.29 (146.44) | 508.82 (133.10) | .001 | NA | |
| BACS composite score (z score) | 0.16 (0.96) | — | −2.20 (1.33) | −0.57 (1.06) | <.0001 | HC vs CTSZ: ****; RTSZ vs CTSZ: ** | |
| n | n | n | n | ||||
| Gender | Male | 23 | 12 | 15 | 15 | .45 | NA |
| Female | 4 | 5 | 2 | 2 |
Abbreviations: BACS, Brief Assessment of Cognition in Schizophrenia; CTSZ, clozapine-treated schizophrenia patients; HC, healthy comparison participants; NTSZ, never-treated schizophrenia patients; PANSS, Positive and Negative Syndrome Scale; RTSZ, risperidone-treated schizophrenia patients.
Values are represented as the mean (SD).
*Significant group difference at P < .05; **P < .01; ***P < .001; ****P < .0001.
The following exclusion criteria applied to all participants: (1) the existence of a neurological disorder or other psychiatric disorders; (2) alcohol or substance abuse disorder according to DSM-IV; (3) history of intellectual disability; and (4) significant medical conditions or treatments for such conditions, with known impact on central nervous system function or anatomy (e.g., brain injury and hepatitis). Additionally, MR plain scans of each participant were reviewed by an experienced neuroradiologist to exclude individuals with gross brain abnormalities. Participants were all of Han ancestry and right handed.
Psychopathology ratings in patients were obtained using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1989). Chlorpromazine equivalents were calculated according to Gardner (Gardner et al., 2010). Cognitive ability was measured using the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al., 2004), a well-validated cognitive battery measuring working memory, executive functioning, processing speed, motor speed, verbal fluency, and verbal memory. The BACS composite z-score was calculated by comparing each participant’s performance on each measure with the performance of a healthy Chinese comparison group of similar age, recruited from the same geographical region. BACS testing was conducted in the consistently treated schizophrenia patients and the healthy participants. Because of their instable mental status, never-treated patients did not receive BACS testing.
Imaging Data Acquisition
MRI scans were performed using a 3-T scanner (EXCITE, General Electric, Milwaukee, WI) with an 8-channel phase array head coil. Diffusion-weighted images were acquired using a bipolar diffusion-weighted spin-echo echo planar imaging sequence (TR = 10 000 ms, TE = 70 ms, flip angle = 90°) with a 128 × 128 matrix over a field of view of 240 × 240 mm and 50 axial slices of 3-mm thickness covering the whole brain without gap. The diffusion-sensitizing gradients were applied along 15 unique directions (b = 1000 s/mm2) together with an acquisition without diffusion weighting (b = 0). High-resolution T1-weighted anatomical images were acquired for registration purposes using a volumetric 3-dimensional spoiled gradient sequence (TR = 8.5 ms, TE = 3.5 ms, TI = 400 ms, flip angle = 12°) with a 512 × 512 matrix over a field of view of 240 × 240 mm and 156 contiguous axial slices of 1-mm thickness.
Image Preprocessing and Network Construction
PANDA pipeline tool (http://www.nitrc.org/projects/panda) and FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl) were used to preprocess the imaging data. All images were first corrected for eddy current distortions and head motions by applying an affine alignment of each diffusion-weighted image to the b0 image. Then, the diffusion tensors were estimated for each voxel. The fractional anisotropy (FA) of each voxel was also calculated. After the preprocesses, tractography was performed to generate 3-dimensional streamlines characterizing neural fiber tract connectivity. Streamlines were reconstructed by seeding at every voxel inside the brain and applying the fiber assignment using continuous tracking algorithm until it turned an angle greater than 45° or reached a voxel with the FA less than 0.2.
After skull-stripping, individual T1-weighted images were co-registered to the b0 images in the DTI space and then nonlinearly transformed into the ICBM152 T1 template in the MNI space. The inverse transformations were used to warp the automated anatomical labelling atlas (Tzourio-Mazoyer et al., 2002) from the MNI space to the DTI native space. Using this procedure, we obtained 90 cortical and subcortical regions (45 for each hemisphere), each representing a node of the network. In the native diffusion space, 2 regions were considered structurally connected if at least 1 fiber bundle with 2 endpoints was located in the 2 regions. We calculated the averaged FA values between the end-nodes as its weight and obtained a weighted symmetrical anatomical 90 × 90 structural connectivity matrix for each participant. These matrices were then analyzed to yield several graph theory metrics.
Network Analysis
GRETNA (www.nitrc.org/projects/gretna/) software was used to calculate the network metrics (Wang et al., 2015). A wide range of sparsity thresholds was applied to each structural connectivity matrix. For the brain networks at each sparsity level, we calculated both global and nodal network metrics. The area under the curve reflecting the measures over the sparsity range was calculated for each network metric, providing a summarizing scalar for the topological characterization of the brain networks. Global metrics included (1) small-world parameters: clustering coefficient Cp, characteristic path length Lp, normalized clustering coefficient γ, normalized characteristic path length λ, and small-worldness σ; and (2) network efficiency parameters: local efficiency Eloc and global efficiency Eglob (Rubinov and Sporns, 2010). The nodal degree was examined in each automated anatomical labelling region. To further localize specific pairs of brain regions with altered structural connectivity in patients, we used a network-based statistic (NBS) approach (Zalesky et al., 2010). NBS is a method based on the principles underpinning traditional cluster-based thresholding of statistical parametric maps to control the family-wise error rate when mass univariate testing is performed at every connection comprising the graph.
Statistical Analysis
We employed chi-square tests, 1-way ANOVA, or Kruskal-Wallis tests to compare the demographic characteristics and clinical aspects among the groups. Shapiro-Wilk tests and homogeneity tests of variance were used to confirm that quantitative data from different groups were normally distributed and meet the criteria for homogeneity of variance. Chi-square pairwise tests following chi-square tests, Tukey-Kramer tests following ANOVA, or Dunn’s tests following Kruskal-Wallis were used for post-hoc tests procedures. Post-hoc tests were corrected for multiple comparisons using false discovery rate (FDR) approach and P values were false-positive adjusted accordingly.
To determine the group differences in terms of global network metrics and regional nodal characteristics, nonparametric permutation tests (5000 permutations) were performed using a design model of 1-way ANOVA. A value of P < .05 was considered significant. For nodal properties, the statistical significance was corrected for multiple comparisons using FDR and false-positive adjustments. Post-hoc pairwise permutation tests were conducted for measures with significant group differences. The post-hoc tests were corrected for multiple comparisons using FDR, and P values were false-positive adjusted.
To determine the significance levels of altered structural connectivity networks in the NBS analysis, we first detected the significant nonzero connections within healthy participants and patients. The NBS approach was then conducted within the connections, where a primary threshold (P = .01) was first applied to an F-statistic (1-way ANOVA analysis). This F-statistic was computed for each link to define a set of supra-threshold links among which any connected components and their size (number of edges) could then be determined. To estimate the significance of each component, the null distribution of the connected component size was empirically derived using a nonparametric permutation approach (5000 permutations).
Linear regression analysis was used to test the associations between network metrics and clinical characteristics. Regressions included clinical characteristics (PANSS total scores, BACS scores, or chlorpromazine equivalents per day) as dependent variables, with global network metrics and dummy codes for patient groups as predictors. Mediation analysis used the PROCESS macro (Preacher and Hayes, 2004) for the Statistical Package for Social Sciences (SPSS, version 23.0), with a 5000 bias-corrected bootstrap sample for significance testing. Mediation effects would be considered significant if the 95% confidence interval did not include zero.
Results
Participant Characteristics
The demographic and clinical features of the sample are listed in Table 1. The age and sex distributions did not differ among the groups. While the disease duration was similar among patient subgroups, there was a significant group difference in their PANSS scores, with higher PANSS scores in the never-treated group compared with the other 2 patient groups. Additionally, the clozapine-treated group showed higher PANSS negative symptom expression and total scores than the risperidone-treated group. Chlorpromazine equivalents per day were lower in the clozapine-treated group than that in the risperidone-treated group. Regarding groups with BACS scores available, the clozapine-treated group had the lowest BACS composite scores, indicating more severe cognitive impairment compared with both healthy controls and patients on risperidone. Though no differences were found between risperidone-treated patients and healthy controls in BACS composite scores, patients on risperidone were slower in motor speed as well as attention and processing speed (supplementary Table 1), indicating certain cognitive impairments in these patients. To control for the effects of symptom severity and antipsychotic dose, PANSS total scores and chlorpromazine equivalents were included as covariates in the statistic model that tested the differences in BACS scores between the clozapine-treated and risperidone-treated groups. The group difference in BACS scores remained significant (Table 2; supplementary Table 1).
Table 2.
Comparison Between CTSZ and RTSZ Controlled for PANSS Total Scores and Chlorpromazine Equivalents
| CTSZ | RTSZ | P valuea | |
|---|---|---|---|
| BACS composite score (z score) | −2.20 (1.33) | −0.57 (1.06) | .005 |
| Nodal degree of right thalamus | 1.47 (0.49) | 1.94 (0.56) | .023 |
| Nodal degree of right cuneus | 1.72 (0.49) | 2.18 (0.55) | .044 |
| Nodal degree of right precentral | 1.66 (0.35) | 2.07 (0.46) | .098 |
| Mean nodal degree of altered nodes | 1.58 (0.24) | 1.80 (0.31) | .191 |
| Mean connectivity of NBS network | 0.22 (0.04) | 0.27 (0.06) | .037 |
Abbreviations: CTSZ, clozapine-treated schizophrenia patients; PANSS, Positive and Negative Syndrome Scale; RTSZ, risperidone-treated schizophrenia patients.
Values are represented as the mean (SD).
aControlled for PANSS total scores and chlorpromazine equivalents per day.
Group Difference in Network Metrics
Global Topology of White Matter Structural Networks
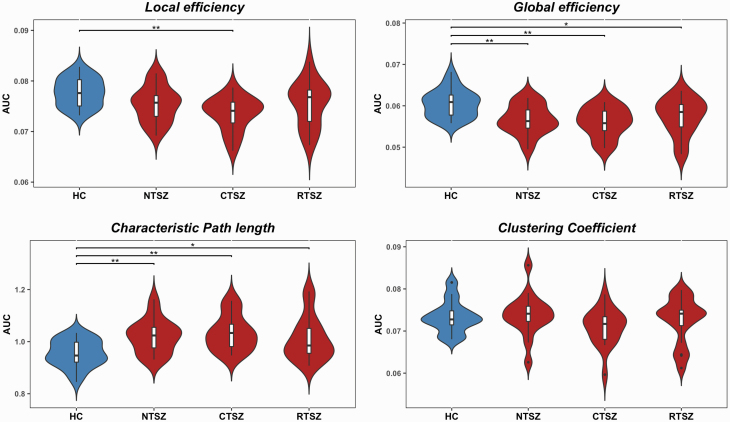
In the defined threshold range, all patient and control groups showed small-world topologic organization of the white matter structural networks, as expressed by γ > 1 and λ ≈ 1 (supplementary Figure 1). ANOVAs on the global network organization metrics revealed a significant group difference in local efficiency (F = 5.46, P = .002), global efficiency (F = 6.69, P < .001), and characteristic path length (F = 6.27, P < .001) but no differences in clustering coefficients (F = 0.835, P = .479). Post-hoc comparisons showed significantly reduced global efficiency and increased path length in all patient subgroups compared with controls. Additionally, the clozapine-treated group showed significant decreased local efficiency compared with controls (Figure 1).
Figure 1.
Group differences in global topological properties. AUC, area under curve; CTSZ, clozapine-treated schizophrenia patients; HC, healthy controls; NTSZ, never-treated schizophrenia patients; RTSZ, risperidone-treated schizophrenia patients. An asterisk designates network metrics with significant group differences. *P < .05; **P < .01.
Regional Topology of White Matter Structural Networks
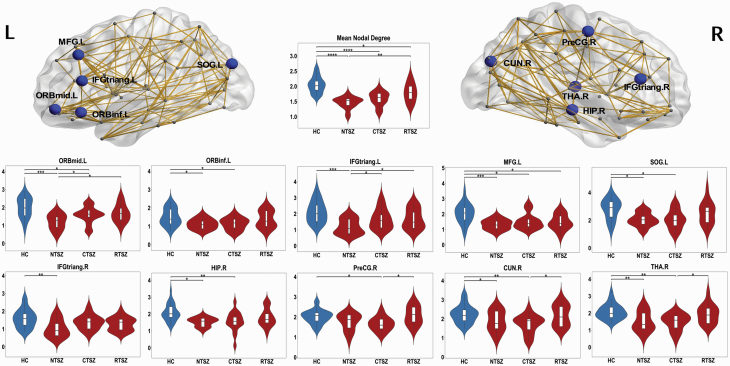
Following the discovery of altered global network organization, we further localized the regions with altered nodal metrics (nodal degree) in the patients. Figure 2 shows the results of the statistical comparisons between those regional parameters. There were significant group differences in the following regions: right thalamus, right hippocampus, bilateral inferior frontal gyrus, left inferior and middle orbitofrontal gyrus, left middle frontal gyrus, left superior occipital gyrus, and right cuneus. The post-hoc tests showed that never-treated and clozapine-treated patients had significantly reduced nodal degrees in most of these regions compared with controls, whereas the risperidone-treated group revealed relatively intact nodal metrics. The direct comparison between the clozapine-treated and risperidone-treated group revealed significant differences in right thalamus, right cuneus, and right precentral gyrus, with higher nodal degrees in the risperidone group. The group difference between the clozapine-treated and risperidone-treated groups in right thalamus and right cuneus remained significant after including the PANSS total score and chlorpromazine equivalents per day as covariates (Table 2).
Figure 2.
The distribution of brain regions with significant differences in the nodal degrees among the groups at P < .05 (corrected). The networks shown here were constructed by averaging the anatomical connection matrices of all healthy controls, with a sparsity threshold of 10%. Post-hoc tests showed that NTSZ patients and CTSZ patients had significant reduced nodal centrality in most of these regions compared with controls. Compared with the RTSZ group, the CTSZ group had significant lower values in right thalamus, right cuneus, and right precentral gyrus. CTSZ, clozapine-treated schizophrenia patients; CUN, cuneus; HC, healthy controls; HIP, hippocampus; IFGtriang, triangular part of inferior frontal gyrus; L, left; MFG, middle frontal gyrus; NTSZ, never-treated schizophrenia patients; ORBinf, orbital part of inferior frontal gyrus; ORBmid, orbital part of middle frontal gyrus; PreCG, precentral gyrus; R, right; RTSZ, risperidone-treated schizophrenia patients; SOG, superior occipital gyrus; THA, thalamus. *P < .05; **P < .01; ***P < .001.
White Matter Structural Connectivity Characteristics
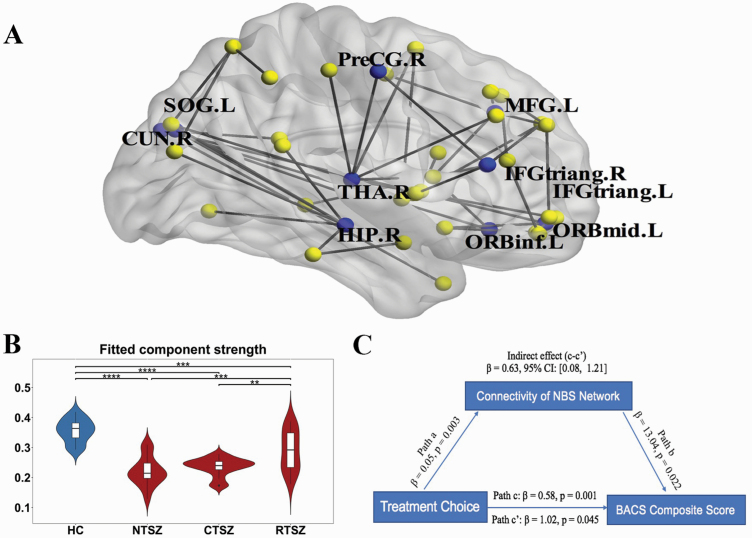
Using the NBS method, we localized a connected subnetwork of 45 connections and 41 nodes that differed among the groups (P < .001, corrected by FDR). This network primarily involved subcortical, prefrontal, and also some occipital regions (Figure 3A). Detailed information is provided in supplementary Table 2. The mean structural connectivity of the connected components was calculated by averaging the connectivity strength across all edges of the NBS network. Post-hoc tests revealed decreased values in all patient subgroups compared with HCs. The decreased pattern was more prominent in never-treated and clozapine-treated patients, as indicated by significantly lower values than in risperidone-treated patients (Figure 3B). The differences between the clozapine-treated and risperidone-treated groups remained significant after including PANSS total scores and chlorpromazine equivalents per day as covariates (Table 2).
Figure 3.
(A) The connected network with significant differences among the groups. Note that the FA-weighted white matter network was constructed using the automated anatomical labelling template. The network primarily involved the frontal and thalamus regions. (B) Differences in the mean strength of the components among the groups. Post-hoc tests revealed decreased values in all patient subgroups compared with those in HC. The decreased pattern was more prominent in NTSZ and CTSZ patients, indicated by significant lower value. (C) The structural connectivity within the NBS network significantly mediated the difference between the 2 treated groups regarding their cognitive ability level. Path c represents the variance in treatment choice associated with global cognitive ability, and Path c’ represents the association between treatment choice and cognition after taking into account the structural connectivity of the NBS network as a mediator. Path c-c’ is the mediation effect and is significant at P < .05 based on confidence intervals from bias-corrected bootstrapping of 5000 samples. CTSZ, clozapine-treated schizophrenia patients; CUN, cuneus; HC, healthy controls; HIP, hippocampus; IFGtriang, triangular part of inferior frontal gyrus; L, left; MFG, middle frontal gyrus; NTSZ, never-treated schizophrenia patients; ORBinf, orbital part of inferior frontal gyrus; ORBmid, orbital part of middle frontal gyrus; PreCG, precentral gyrus; R, right; RTSZ, risperidone-treated schizophrenia patients; SOG, superior occipital gyrus; THA, thalamus. **P < .01; ***P < .001; ****P < .0001. For detailed information regarding the white matter connections in the significant NBS components, see supplementary Table 2.
Network Metrics and Cognition
Path length, global efficiency, and local efficiency predicted cognitive ability across all groups, including healthy controls (Lp: standardized β = − 0.355, P = .002; Eglob: β = 0.366, P = .001; Eloc: β = 0.297, P = .011). In contrast, clustering coefficients and local efficiency measures did not significantly predict cognitive ability (Cp: β = 0.072, P = .500). Linear regressions on BACS scores were performed for the mean degrees of the nodes with a significant group difference and the mean structural connectivity of the NBS-connected component showing that both measures were positively related to cognitive ability (β = 0.389, P = .002; β = 0.433, P = .005). Restricting the approach to only treated patients confirmed the associations between altered network metrics and cognitive ability (supplementary Table 3). In contrast, no significant correlations were found between network metrics and PANSS total scores, and between network metrics and chlorpromazine equivalents per day, in the group of treated patients (supplementary Table 3).
Given the observed differences in structural connectivity of the NBS network and cognitive ability between the treated groups on the one hand and the positive association between their structural connectivity and cognitive ability on the other hand, we tested whether the structural connectivity of the NBS network mediated the group differences in cognitive ability between the 2 groups of treated patients. A significant mediation role of the NBS network (β = 0.63; 95% CI [0.08, 1.21]) was revealed (Figure 3C).
Discussion
This study examined, to our knowledge for the first time, differences within the white matter structural connectome in chronic schizophrenia, including patients on long-term antipsychotic monotherapy with either risperidone or clozapine, and compared them with chronically ill never-treated patients. Our findings suggest that there is a general disruption in the organizations of the white matter structural networks as well as decreased nodal and connectivity characteristics across all schizophrenia groups, mainly involving thalamus, prefrontal, and occipital regions. More specifically, we found that alterations in nodal and connectivity characteristics were more prominent in never-treated and clozapine-treated patients than in risperidone-treated patients. Regarding the behavioral relevance of these alterations, we found that greater white matter alterations were associated with more severe impairments in cognitive function. Follow-up analyses further suggested structural connectivity of the NBS network mediated the difference in cognitive ability between the groups of treated patients.
The brain is a complex interconnected system with various important topological attributes, such as small-worldness, high efficiency at low wiring costs, and highly interconnected hubs (Bullmore and Sporns, 2009; Sporns, 2011). Graph theoretical analysis can provide a powerful framework for characterizing the topological attributes of brain networks. Through graph theoretical analysis, our present findings suggest schizophrenia leads to increased characteristic path lengths and decreased global efficiency, indicating decreased ability for parallel information processing in the brain. This observation is in accordance with previous studies reporting disruptions of fundamental organizational properties associated with schizophrenia, thus supporting the model of schizophrenia as a dysconnectivity syndrome (van den Heuvel and Fornito, 2014; Narr and Leaver, 2015; Cea-Cañas et al., 2019). Regarding regional topology, we revealed changes mainly in the thalamo-prefrontal circuits, where dopaminergic transmission is highly relevant. Imbalances of dopaminergic transmission in these regions have been postulated as core disease mechanisms of schizophrenia. In line with this, structural changes within thalamo-prefrontal circuits have also been consistently reported from schizophrenia patients (Gong et al., 2016). Though occipital regions do not receive prominent dopaminergic innervation, studies have reported illness-related alterations in these regions as well (Maller et al., 2017; Zhao et al., 2018). Additionally, previous DTI studies have reported reduced FA in the thalamic radiations, corpus callosum, fronto-occipital fasciculus, and some prefrontal-subcortical bundles (Yao et al., 2013; Klauser et al., 2017; Molina et al., 2017; Leroux et al., 2018). Together, our findings support a model of topological properties being relevant to the widely reported structural changes and abnormal FA of fiber bundles in schizophrenia.
While changes in brain structure, with evidence of progression over time, have been well documented in schizophrenia, the impact of antipsychotic treatment on such changes remains controversial. Both increased (Reis Marques et al., 2014) and decreased FA (Wang et al., 2013; Szeszko et al., 2014) as well as no evidence for whiter matter changes (Kraguljac et al., 2019) after short-term (6–12 weeks) antipsychotic treatment have been reported. Clinical studies on long-term antipsychotic treatment, including our previous studies (Xiao et al., 2018; Yao et al., 2019), suggest that antipsychotic medications may have beneficial effects on the brain’s structure and function. In contrast, studies on non-human primate models have suggested adverse effects of antipsychotics by reporting associations between chronic exposure to antipsychotics with reduced brain volume and glial cell numbers (Dorph-Petersen et al., 2005; Konopaske et al., 2007). Notably, the choice of antipsychotic treatment may contribute to these controversial findings. Several studies have reported differential effects of typical and atypical antipsychotics on the brain’s structure (Bartzokis et al., 2007, 2009; Vita et al., 2015; Leroux et al., 2018). Our present finding that patients on long-term risperidone monotherapy showed fewer deficits within their white matter structural networks than never-treated patients is consistent with the hypothesis that risperidone has the capacity to restore dysconnectivity and normalize topological parameters after short-term treatment (Hu et al., 2016; Kraguljac et al., 2016; Nelson et al., 2018; Zong et al., 2019). Risperidone may thus have neuroprotective effects on white matter structural networks when administered as a long-term treatment.
In patients on long-term clozapine monotherapy, the impairments in white matter structural networks were similar to those in the group of never-treated patients, suggesting limited effects of long-term clozapine administration on white matter structural networks in this group. This finding is in line with a previous report showing limited effects of clozapine on brain structure (Guma et al., 2018) and a continuous reduction in global brain volume during mid-term (6–9 months) clozapine treatment in schizophrenia patients (Ahmed et al., 2015). Confirming our first hypothesis, we found greater alterations in nodal and connectivity characteristics in clozapine-treated than risperidone-treated patients. One possible explanation for these differential effects might be the different pharmacological mechanisms of the 2 drugs, with stronger inherent anticholinergic properties of clozapine than risperidone. On the other hand, patients in the clozapine-treated group were probably more impaired than patients in the risperidone-treated group as reflected by higher PANSS negative scores and lower BACS scores. To this regard, it is worth noting that clozapine has a unique therapeutic profile with proven clinical effectiveness even in treatment-resistant schizophrenia. However, chlorpromazine equivalents per day were considerably lower in the clozapine-treated group compared with the risperidone-treated group, which could suggest less efficient antipsychotic treatment in the clozapine group. To control for the prescribing bias, patients with a known history of poor response to other antipsychotics were excluded from this study. Although not initially hypothesized, the difference between the clozapine group and the risperidone group remained significant when controlling for variable of no interest (e.g., PANSS total score and chlorpromazine equivalent per day), supporting the idea that the observed difference in structural connectome is independent of symptom expression and antipsychotic dosage. Notably, as an affordable drug with high efficacy, clozapine has been widely prescribed to schizophrenia patients even as first-line treatment (Liu and Li, 2003), especially in less affluent and rural areas of China, where most of our participants were recruited. Although we cannot completely rule out the impacts of the prescribing bias, which may need to be addressed in future studies, our findings add useful information to the controversial debate on effects of antipsychotic medication on brain structure during long-term treatment and may have both clinical relevance and theoretical implications.
In contrast to the lack of association between white matter structural alterations on the one hand and symptom expression on the PANSS and antipsychotic dosages on the other hand, we observed significant associations between the structural network connectome and cognitive performance. This is in accordance with accumulating evidence suggesting the optimal organization of brain networks underlies cognitive ability (Park and Friston, 2013). Although cognition is not a formal part of the current diagnostic criteria for schizophrenia, significant cognitive impairments across many domains have been documented in schizophrenia patients and are recognized as a major cause of poor functioning. Longitudinal studies covering more than 10 years have revealed both overall stability as well as a significant decline of cognitive functioning in schizophrenia (Hoff et al., 2005; Øie et al., 2010). One possibility underlying this discrepancy of findings could be the differences in the characteristics of the included samples and the nature of the medication used (Townsend and Norman, 2004). In this study, we found different levels of cognitive function between patients with chronic risperidone treatment and those with chronic clozapine treatment. Moreover, the difference in cognitive ability was significantly mediated by the integrity of white matter structural networks depending on the choice of antipsychotic treatment. This constellation of findings supports the model that white matter structural networks supporting cognitive function may benefit from antipsychotic treatment, especially in those treated with risperidone.
This study has several limitations. First, this is a cross-sectional non-randomized study. The brain structural connectome and cognition differences between the groups as well as their relationship with treatment history are constrained by the lack of random assignment to different types of antipsychotic treatment. Though patients with a history of poor response to other antipsychotics were excluded, the impacts of the prescribing bias that more impaired patients were more likely to receive clozapine treatment cannot be completely rule out. A longitudinal study with a randomized design extending for over 5 years is a challenge, since it is ethically not feasible to demand participants to stick to 1 particular antipsychotic drug for such a long-term course. Thus, our cross-sectional approach may be regarded as a proxy to study the effects of long-term course of antipsychotic treatment. Nonetheless, this issue needs to be considered when interpreting our findings. Second, due to the unstable psychosis status of never-treated patients, cognitive evaluations were not conducted in these patients. The relationship between cognitive impairments and deficits of white matter structural networks in never-treated schizophrenia should be further evaluated. Third, we did not control for the usage of non-antipsychotic medications, which was selected based on individual clinical preference. Fourth, the sample size was relatively small with a predominance of males. This bias limits the generalization of our findings to female patients. Lastly, our findings are limited by the intrinsic nature of DTI, which relies on water diffusion as an indirect marker of white matter microstructure and has yet been unable to resolve complex fiber architectures.
In conclusion, by studying a sample of chronically ill patients with schizophrenia on 3 different choices of antipsychotic treatment, that is, clozapine, risperidone, or none, this study revealed differential alterations in the structural connectome of those patients and a mediating role of white matter structural connectivity on cognitive ability in the 2 groups of patients on long-term medication. Our results provide insight into the white matter structural network deficits in chronic schizophrenia patients receiving clozapine and risperidone monotherapy and suggest that white matter structural networks supporting cognitive function may benefit from antipsychotic treatment, especially in those treated with risperidone.
Supplementary Material
Acknowledgments
We thank all the participants for their cooperation.
This study was supported by the National Natural Science Foundation of China (grants 81671664, 81761128023, 81901702, and 81901705); grants from the Humboldt Foundation (Ref 3.5-1206715-CHN-BES, Ref 3.5-CHN-1207072-HFST-P); Science-Technology Support Plan Projects of Sichuan Province (No. 2020YFS0117); and 1.3.5 project for disciplines of excellence, Post-Doctor Research Project, West China Hospital, Sichuan University (grants 2018HXBH05, ZYJC18020, and ZYYC08001).
Interest Statement: None.
References
- Ahmed M, Cannon DM, Scanlon C, Holleran L, Schmidt H, McFarland J, Langan C, McCarthy P, Barker GJ, Hallahan B, McDonald C (2015) Progressive brain atrophy and cortical thinning in schizophrenia after commencing clozapine treatment. Neuropsychopharmacology 40:2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC (2011) Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayesa-Arriola R, Rodríguez-Sánchez JM, Pérez-Iglesias R, Roiz-Santiáñez R, Martínez-García O, Sánchez-Moreno J, Tabarés-Seisdedos R, Vázquez-Barquero JL, Crespo-Facorro B (2013) Long-term (3-year) neurocognitive effectiveness of antipsychotic medications in first-episode non-affective psychosis: a randomized comparison of haloperidol, olanzapine, and risperidone. Psychopharmacology (Berl) 227:615–625. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, Lieu C, Altshuler LL, Mintz J (2007) Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophr Res 93:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Stewart SB, Oluwadara B, Lucas AJ, Pantages J, Pratt E, Sherin JE, Altshuler LL, Mintz J, Gitlin MJ, Subotnik KL, Nuechterlein KH (2009) In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res 113:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, Mckinzie DL (2003) Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 27:1125–1143. [DOI] [PubMed] [Google Scholar]
- Cahn W, Rais M, Stigter FP, van Haren NE, Caspers E, Hulshoff Pol HE, Xu Z, Schnack HG, Kahn RS (2009) Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol 19:147–151. [DOI] [PubMed] [Google Scholar]
- Cea-Cañas B, de Luis R, Lubeiro A, Gomez-Pilar J, Sotelo E, Del Valle P, Gómez-García M, Alonso-Sánchez A, Molina V (2019) Structural connectivity in schizophrenia and bipolar disorder: effects of chronicity and antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry 92:369–377. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA (2005) The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology 30:1649–1661. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J (1995) Structured clinical interview for DSM-IV axis I disorders, patient edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatry Institute. [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ (2010) International consensus study of antipsychotic dosing. Am J Psychiatry 167:686–693. [DOI] [PubMed] [Google Scholar]
- Gong Q, Lui S, Sweeney JA (2016) A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am J Psychiatry 173:232–243. [DOI] [PubMed] [Google Scholar]
- Green MF, Braff DL (2001) Translating the basic and clinical cognitive neuroscience of schizophrenia to drug development and clinical trials of antipsychotic medications. Biol Psychiatry 49:374–384. [DOI] [PubMed] [Google Scholar]
- Guma E, Rocchetti J, Devenyi GA, Tanti A, Mathieu A, Lerch JP, Elgbeili G, Courcot B, Mechawar N, Chakravarty MM, Giros B (2018) Regional brain volume changes following chronic antipsychotic administration are mediated by the dopamine D2 receptor. Neuroimage 176:226–238. [DOI] [PubMed] [Google Scholar]
- Han M, Zhang XY, Chen DC, Tan YL, Song CS, Yu YH, Huang XF (2015) Cognitive differences in schizophrenia on long-term treatments with clozapine, risperidone and typical antipsychotics. Int Clin Psychopharmacol 30:89–95. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, Davidson M, Davis KL (1999) Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry 45:32–40. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M (2003) Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 60:585–594. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011) Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE (2005) Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res 78:27–34. [DOI] [PubMed] [Google Scholar]
- Houthoofd SA, Morrens M, Sabbe BG (2008) Cognitive and psychomotor effects of risperidone in schizophrenia and schizoaffective disorder. Clin Ther 30:1565–1589. [DOI] [PubMed] [Google Scholar]
- Hu M, Zong X, Zheng J, Mann JJ, Li Z, Pantazatos SP, Li Y, Liao Y, He Y, Zhou J, Sang D, Zhao H, Tang J, Chen H, Lv L, Chen X (2016) Risperidone-induced topological alterations of anatomical brain network in first-episode drug-naive schizophrenia patients: a longitudinal diffusion tensor imaging study. Psychol Med 46:2549–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Seeman P (2001) Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry 158:360–369. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP (1989) The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl 7:59–67. [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L (2004) The Brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 68:283–297. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD (2012) Cognitive impairment in schizophrenia. Handb Exp Pharmacol 213:11–37. [DOI] [PubMed] [Google Scholar]
- Klauser P, Baker ST, Cropley VL, Bousman C, Fornito A, Cocchi L, Fullerton JM, Rasser P, Schall U, Henskens F, Michie PT, Loughland C, Catts SV, Mowry B, Weickert TW, Shannon Weickert C, Carr V, Lenroot R, Pantelis C, Zalesky A (2017) White matter disruptions in schizophrenia are spatially widespread and topologically converge on brain network hubs. Schizophr Bull 43:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA (2007) Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology 32:1216–1223. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Hadley JA, Visscher K, Knight D, ver Hoef L, Falola B, Lahti AC (2016) Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin 10:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Anthony T, Skidmore FM, Marstrander J, Morgan CJ, Reid MA, White DM, Jindal RD, Melas Skefos NH, Lahti AC (2019) Micro- and macrostructural white matter integrity in never-treated and currently unmedicated patients with schizophrenia and effects of short-term antipsychotic treatment. Biol Psychiatry Cogn Neurosci Neuroimaging 4:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux E, Vandevelde A, Tréhout M, Dollfus S (2018) Abnormalities of fronto-subcortical pathways in schizophrenia and the differential impacts of antipsychotic treatment: a DTI-based tractography study. Psychiatry Res Neuroimaging 280:22–29. [DOI] [PubMed] [Google Scholar]
- Liu T, Li C (2003) The comparison of use of antipsychotics in first episode patients with schizophrenia (in Chinese). Medical Journal of Chinese People Health 15:289–290. [Google Scholar]
- Lui S, Zhou XJ, Sweeney JA, Gong Q (2016) Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology 281:357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Anderson RJ, Thomson RH, Daskalakis ZJ, Rosenfeld JV, Fitzgerald PB (2017) Occipital bending in schizophrenia. Aust N Z J Psychiatry 51:32–41. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25:233–255. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10:79–104. [DOI] [PubMed] [Google Scholar]
- Molina V, Lubeiro A, Soto O, Rodriguez M, Álvarez A, Hernández R, de Luis-García R (2017) Alterations in prefrontal connectivity in schizophrenia assessed using diffusion magnetic resonance imaging. Prog Neuropsychopharmacol Biol Psychiatry 76:107–115. [DOI] [PubMed] [Google Scholar]
- Narr KL, Leaver AM (2015) Connectome and schizophrenia. Curr Opin Psychiatry 28:229–235. [DOI] [PubMed] [Google Scholar]
- Nelson EA, White DM, Kraguljac NV, Lahti AC (2018) Gyrification connectomes in unmedicated patients with schizophrenia and following a short course of antipsychotic drug treatment. Front Psychiatry 9:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øie M, Sundet K, Rund BR (2010) Neurocognitive decline in early-onset schizophrenia compared with ADHD and normal controls: evidence from a 13-year follow-up study. Schizophr Bull 36:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagunju AT, Clark SR, Baune BT (2018) Clozapine and psychosocial function in schizophrenia: a systematic review and meta-analysis. CNS Drugs 32:1011–1023. [DOI] [PubMed] [Google Scholar]
- Park HJ, Friston K (2013) Structural and functional brain networks: from connections to cognition. Science 342:1238411. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF (2004) SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 36:717–731. [DOI] [PubMed] [Google Scholar]
- Reis Marques T, Taylor H, Chaddock C, Dell’acqua F, Handley R, Reinders AA, Mondelli V, Bonaccorso S, Diforti M, Simmons A, David AS, Murray RM, Pariante CM, Kapur S, Dazzan P (2014) White matter integrity as a predictor of response to treatment in first episode psychosis. Brain 137:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Singh SP, Cooper JE, Fisher HL, Tarrant CJ, Lloyd T, Banjo J, Corfe S, Jones P (2005) Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS). Schizophr Res 80:117–130. [DOI] [PubMed] [Google Scholar]
- Sporns O. (2011) The human connectome: a complex network. Ann N Y Acad Sci 1224:109–125. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen Y, Lee R, Bezerianos A, Collinson SL, Sim K (2016) Disruption of brain anatomical networks in schizophrenia: a longitudinal, diffusion tensor imaging based study. Schizophr Res 171:149–157. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, Malhotra AK (2014) White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology 39:1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AE, Van Snellenberg JX, Sepehry AA, Honer W (2006) The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. J Psychopharmacol 20: 335–346. [DOI] [PubMed] [Google Scholar]
- Townsend LA, Norman RM (2004) Course of cognitive functioning in first episode schizophrenia spectrum disorders. Expert Rev Neurother 4:61–68. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Fornito A (2014) Brain networks in schizophrenia. Neuropsychol Rev 24:32–48. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, Evans AC, Kahn RS (2007) Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology 32:2057–2066. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS (2011) Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry 68:871–880. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O (1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350:610–614. [DOI] [PubMed] [Google Scholar]
- Velthorst E, Fett AJ, Reichenberg A, Perlman G, van Os J, Bromet EJ, Kotov R (2017) The 20-year longitudinal trajectories of social functioning in individuals with psychotic disorders. Am J Psychiatry 174:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Barlati S, Sacchetti E (2015) The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry 78:403–412. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Xia M, Liao X, Evans A, He Y (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Cheung C, Deng W, Li M, Huang C, Ma X, Wang Y, Jiang L, Sham PC, Collier DA, Gong Q, Chua SE, McAlonan GM, Li T (2013) White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol Med 43:2301–2309. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH (2005) A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 8:457–472. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Sun H, Shi S, Jiang D, Tao B, Zhao Y, Zhang W, Gong Q, Sweeney JA, Lui S (2018) White matter abnormalities in never-treated patients with long-term schizophrenia. Am J Psychiatry 175:1129–1136. [DOI] [PubMed] [Google Scholar]
- Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, Gong QY (2013) White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 45:100–106. [DOI] [PubMed] [Google Scholar]
- Yao L, Li F, Liu J, Liao W, Li X, Li M, Meng Y, Liang S, Zhang C, Yang X, Wang Q, Ma X, Guo W, Sweeney JA, Gong Q, Lui S, Deng W, Li T (2019) Functional brain networks in never-treated and treated long-term Ill schizophrenia patients. Neuropsychopharmacology 44, 1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010) Network-based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207. [DOI] [PubMed] [Google Scholar]
- Zhao C, Zhu J, Liu X, Pu C, Lai Y, Chen L, Yu X, Hong N (2018) Structural and functional brain abnormalities in schizophrenia: a cross-sectional study at different stages of the disease. Prog Neuropsychopharmacol Biol Psychiatry 83:27–32. [DOI] [PubMed] [Google Scholar]
- Zong X, Hu M, Pantazatos SP, Mann JJ, Wang G, Liao Y, Liu ZC, Liao W, Yao T, Li Z, He Y, Lv L, Sang D, Tang J, Chen H, Zheng J, Chen X (2019) A dissociation in effects of risperidone monotherapy on functional and anatomical connectivity within the default mode network. Schizophrenia Bull 45:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.