Abstract
Background
Depression is one of the most common forms of mental illness and also a leading cause of disability worldwide. Developing novel antidepressant targets beyond the monoaminergic systems is now popular and necessary. LIM kinases, including LIM domain kinase 1 and 2 (LIMK1/2), play a key role in actin and microtubule dynamics through phosphorylating cofilin. Since depression is associated with atrophy of neurons and reduced connectivity, here we speculate that LIMK1/2 may play a role in the pathogenesis of depression.
Methods
In this study, the chronic unpredictable mild stress (CUMS), chronic restraint stress (CRS), and chronic social defeat stress (CSDS) models of depression, various behavioral tests, stereotactic injection, western blotting, and immunofluorescence methods were adopted.
Results
CUMS, CRS, and CSDS all significantly enhanced the phosphorylation levels of LIMK1 and LIMK2 in the medial prefrontal cortex (mPFC) but not the hippocampus of mice. Administration of fluoxetine, the most commonly used selective serotonin reuptake inhibitor in clinical practice, fully reversed the effects of CUMS, CRS, and CSDS on LIMK1 and LIMK2 in the mPFC. Moreover, pharmacological inhibition of LIMK1 and LIMK2 in the mPFC by LIMKi 3 infusions notably prevented the pro-depressant effects of CUMS, CRS, and CSDS in mice.
Conclusions
In summary, these results suggest that LIMK1/2 in the mPFC has a role in chronic stress-induced depressive-like effects in mice and could be a novel pharmacological target for developing antidepressants.
Keywords: chronic restraint stress, chronic social defeat stress, chronic unpredictable mild stress, cofilin, depression, fluoxetine, LIMK1/2, medial prefrontal cortex
Significance Statement.
It is now popular and necessary to develop novel antidepressant targets beyond the monoaminergic systems. LIMK1/2 plays a key role in actin and microtubule dynamics through phosphorylating Cofilin. This study mainly investigated the correlation between the LIMK-Cofilin system in the mPFC and depression. It was found that both chronic stress and fluoxetine were able to significantly regulate the phosphorylation of LIMK1, LIMK2, and Cofilin in the mPFC but not the hippocampus, and inhibiting LIMK1/2 in the mPFC led to antidepressant-like effects in mice. Therefore, LIMK1/2 in the mPFC is a potential participant underlying the pathophysiology of depression and could be a novel antidepressant target. Moreover, this study provides support for the potential use of LIMKi 3 treatment strategies against chronic stress-related mental disorders.
Introduction
Depression is one of the most common forms of mental illness and also a leading cause of disability worldwide, afflicting an estimated 12%–15% of the population at some point in life (Kessler et al., 1994). Stress, emotional trauma, and environmental and genetic factors have all been implicated in precipitating depression (Duman et al., 1997, 1999; Nestler et al., 2002). Currently, not all patients respond adequately to available medications, which also produce side effects and require weeks or months to achieve a therapeutic response (Dale et al., 2015; Boku et al., 2018). This is partly attributable to the fact that the pathophysiology underlying depression has been difficult to access.
During the development of the central nervous system, neurons establish functional neuronal networks by extending axons and dendrites, which coordinate an elaborate and complex pattern of synaptic connections. Development of neuronal networks is a multistep process consisting of primary neurite formation, axon and dendrite specification, neurite extension and arborization, and spine formation and maturation (Scott and Luo, 2001; Whitford et al., 2002; Jan and Jan, 2010). Defects in neurite formation and morphogenesis can cause neurological disorders, and it has been demonstrated that depression is associated with atrophy of neurons and reduced connectivity (Duman and Aghajanian, 2012; Qiao et al., 2016; Duman et al., 2019). Clinical brain imaging and postmortem studies have demonstrated structural and functional alterations of several limbic and cortical regions in patients with major depressive disorder, including the hippocampus and medial prefrontal cortex (mPFC) (Redlich et al., 2018). Altered connectivity of these regions could contribute to the symptoms of depression, in part via reduced function of the hippocampus and mPFC (e.g., decreased reaction time and cognitive function).
LIM kinases are serine/threonine kinases that play a key role in actin and microtubules dynamics and comprise 2 members: LIM domain kinase 1 and 2 (LIMK1/2) (Cuberos et al., 2015). LIMKs have been shown to be effectors of Rho GTPase pathways in different types of cells, including neurons (Cuberos et al., 2015). Once activated, LIMKs phosphorylate members of the actin depolymerization factor/Cofilin family and inactivate them, preventing these actin depolymerization factors from severing filamentous actin and allowing accumulation of actin microfilaments (Meng et al., 2004; Bernard, 2007; Cuberos et al., 2015). Cofilin is mainly controlled by LIMKs and slingshot phosphatases through phosphorylation (inactivation) and dephosphorylation (activation), respectively (Meng et al., 2004; Bernard, 2007; Cuberos et al., 2015). Cofilin modulates actin dynamics in a complex and concentration-dependent manner. When in a molar ratio to actin, cofilin severs filaments and accelerates the release of actin monomers from the pointed ends of actin filaments, thereby promoting F-actin disassembly (Andrianantoandro and Pollard, 2006; Bernstein and Bamburg, 2010; Hild et al., 2014). At higher concentrations, such as in dendritic spine during long-term potentiation, cofilin has been shown to increase the polymerization by nucleating new actin filaments (Andrianantoandro and Pollard, 2006; Bernstein and Bamburg, 2010; Hild et al., 2014). Also, the effect of LIMK1 on neurite outgrowth may depend on the duration of its expression. In mice, transient overexpression of LIMK1 accelerates axon formation, whereas long-term overexpression of LIMK1 leads to growth cone collapse and axon retractation (Rosso et al., 2004). By analyzing this information collectively, here we assume that LIMK1/2 in the hippocampus and mPFC may play a role in the pathophysiology of depression.
Materials And Methods
Animals
Male adult C57BL/6J mice (8 weeks old) were bought from the Experimental Animal Center of Medical College, Nantong University, and used in all experiments. The mice were housed under standard laboratory conditions (room temperature 24°C ± 1°C, 12-h-light/-dark cycle, humidity set to 55% ± 10%) for 1 week before use. All mice were provided with regular chow and water ad libitum, except during food or water deprivation stress. For behavioral assays, each experimental group consisted of 10 mice. For biochemical assays, each experimental group consisted of 5 or 6 mice. The experimental procedures involving the care and use of mice were conducted in accordance with the ARRIVE guidelines and approved by the Animal Welfare Committee of Nantong University (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Materials
LIMKi 3 (also named BMS-5, catalog no. T4598) and fluoxetine (catalog no. T0450L) were purchased from Targetmol (Boston, MA). The doses for LIMKi 3 (100 or 200 μM/mouse) and fluoxetine (20 mg/kg) were chosen based on previous reports (Lunardi et al., 2018; Song et al., 2018; Zhang et al., 2019). LIMKi 3 was stereotaxically given into the mPFC region of C57BL/6J mice, and the vehicle used was artificial cerebral spinal fluid containing 1% dimethyl sulfoxide. Fluoxetine was i.p. given in a volume of 10 mL/kg with 0.9% saline as the vehicle.
Chronic Unpredictable Mild Stress (CUMS)
The procedures for CUMS were established according to published reports (Huang et al., 2019; Zhang et al., 2019). All groups of mice were housed in the same room. In brief, except the non-stressed control mice (5/cage), C57BL/6J mice were individually housed and subjected to a stress paradigm once per day over a period of 8 weeks: continuous illumination during the dark cycle, wet bedding for 24 hours, 45° tilting for 12 hours, food or water deprivation for 12 hours, restraint stress for 2 hours, 4°C cold stress for 1 hour, and rotation on a shaker for 1 hour. The order of stressors was randomly scheduled for each week over the 8-week period. Administration of vehicle/fluoxetine/LIMKi 3 was performed daily during the final 2 weeks. Forced swim test (FST), tail suspension test (TST), and sucrose preference test were used together to assay the depressive-like behaviors of mice.
Chronic Restraint Stress (CRS)
The procedures for CRS were established according to published reports (Zain et al., 2019; Leem et al., 2020). All groups of mice were housed in the same room. Briefly, except the non-stressed control mice (5/cage), C57BL/6J mice were individually housed and received 3 h/d (from 9:00 am to 12:00 am) of restraint stress for 8 weeks using 50-mL conical transparent plastic tubes (containing vent holes at one end), which can effectively immobilize mice. Administration of vehicle/fluoxetine/LIMKi 3 was performed daily during the last 2 weeks. FST, TST, and sucrose preference test were performed together to assay the depressive-like behaviors of mice.
Chronic Social Defeat Stress (CSDS)
The procedures for CSDS were established according to published reports (Henderson et al., 2017; Colyn et al., 2019). Adequate numbers of aggressive male CD1 mice (over 50 weeks old) were screened to ensure the defeat stress before the experiments. All groups of mice were housed in the same room. Briefly, except the non-stressed control mice (5/cage), C57BL/6J mice were individually placed as “invaders” into aggressive CD1 mouse cages daily for 5–10 minutes. During this exposure, C57BL/6J mice were attacked and showed avoidance, fear, and compliance behaviors. After that, C57BL/6J mice and CD1 mice were separated by plastic dividers with holes for the next 24 hours during which the stressful sensory cues from the aggressors persisted. The stress period lasted for 10 days, and then administration of vehicle/fluoxetine/LIMKi 3 was performed daily for another 2 weeks. Finally, FST, TST, sucrose preference test, and social interaction test were performed together to assay the depressive-like behaviors of mice.
Forced Swim Test (FST)
The FST was established as we frequently described (Song et al., 2018; Zhang et al., 2019; Wang et al., 2020). This test was performed using plastic cylinders (20-cm diameter, 45-cm height). Before the test, the cylinders were filled with 15 cm of water (25°C ± 1°C), and the test mice were individually placed in the cylinders. The test time was 6 minutes, and the immobility time of each mouse was scored over the last 4 minutes by an investigator blind to the groups. For each trial, the water was replaced.
TST
The TST was established as we frequently described (Song et al., 2018; Zhang et al., 2019; Wang et al., 2020). The test mice were individually suspended 70 cm above the floor with their immobility time recorded for 6 minutes by an investigator blinded to the groups. Adhesive tape was used to fasten the mice (1 cm from the tail tip).
Sucrose Preference Test
The sucrose preference test was established as we frequently described (Song et al., 2018; Zhang et al., 2019; Wang et al., 2020). This test lasts for 4 days. During the first 2 days, all test mice (including non-stressed) were individually exposed to 2 bottles containing pure water and 1% sucrose solution, respectively. On the third day, both the food and 2 bottles were deprived for 18 hours. On the fourth day, the test lasted for 6 hours, with the 2 bottles weighed before and after the test period. The sucrose preference was measured as a percentage of the consumed sucrose solution relative to the total amount of liquid intake.
Social Interaction Test
The social interaction test comprises 2 phases and was established as we frequently described (Song et al., 2018; Xu et al., 2018). In the first phase (“target absent”), the test mice were individually placed into an open-field apparatus (75 × 75 × 30 cm) for 5 minutes and allowed to explore a wire-mesh cage placed within the predefined interaction zone (25 × 15 cm). In the second phase (“target present”), the mice were individually returned to the apparatus for 5 minutes but with an unfamiliar male CD1 in the cage. The durations in the interaction zone for each mouse were recorded by an investigator blind to the groups. The apparatus was cleaned after each trial to remove the olfactory cues.
Stereotactic Infusions
In this study, LIMKi 3 was infused into the mPFC region of C57BL/6J mice. In brief, each mouse was anesthetized with 0.5% pentobarbital sodium and fixed in a stereotaxic frame (Stoelting, Dale, IL). LIMKi 3 of 100 or 200 μM was bilaterally infused into each mouse using osmotic minipumps (RWD, China) at a rate of 0.5 µL/min (1 µL/each side, 2 µL/mouse). The cannulas (RWD) were bilaterally implanted into the mPFC region of each mouse, and the incisions were sutured. The mice were allowed to recover for 3 days before further use. The following coordinates were adopted: anteroposterior = + 2.0 mm, mediolateral = ± 0.3 mm, dorsoventral = + 1.4 mm.
Western-Blotting Analysis
After the behavioral tests, the test mice were killed. The hippocampus and mPFC tissues were rapidly dissected and homogenized in NP-40 lysis buffer (Beyotime, China) and mixed with an equal volume of 5× loading buffer. The protein mixtures were resolved in 10% SDS-PAGE gels and then transferred to nitrocellulose membranes, followed by blocking with 5% nonfat milk for 1 hour at room temperature. After overnight incubation with primary antibodies to pLIMK1 (Thr508; 1:500; Immunoway, Plano, TX; catalog no. YP0161), LIMK1 (1:1000; Immunoway; catalog no. YT2562), pLIMK2 (Thr505; 1:500; Immunoway; catalog no. YP0162), LIMK2 (1:1000; Immunoway; catalog no. YT2566), pCofilin (Ser3; 1:500; Cell Signaling, Danvers, MA; catalog no. 3313S), Cofilin (1:1000; Cell Signaling; catalog no. 3318S), and β-actin (1:5000; Abcam, Cambridge, UK; catalog no. ab8227) at 4°C, the membranes were washed 3 times in Tris Buffered saline Tween and incubated with IR-Dye 680-labeled secondary antibodies (1:5000) for 2 hours at room temperature. After Tris Buffered saline Tween washing for another 3 times, the bands were detected using the Odyssey CLx system (LI-COR, Lincoln, NE).
Immunofluorescence
This method was done as we previously described (Song et al., 2018; Zhang et al., 2019; Wang et al., 2020). After the behavioral tests, the test mice were anesthetized and perfused transcardially with 0.1 M phosphate buffer containing 4% paraformaldehyde. The brains were separated, post-fixed, and dehydrated in 30% sucrose solution. Afterwards, 25 µm of mPFC sections was collected using a freezing microtome (Leica, Germany). Briefly, the sections were treated as follows: 1, incubation in 0.3% Triton X-100 for 30 minutes; 2, incubation in 3% bovine serum albumin for 30 minutes; 3, incubation with rabbit primary antibody to pLIMK1/2 (Thr508/505; 1:100; Immunoway; catalog no. YP0591) overnight at 4°C; 4, washing; 5, incubation with fluorescein isothiocyanate-conjugated anti-rabbit secondary antibody (1:50; Thermo Fisher, Waltham, MA) for 2 hours at room temperature; 6, washing; 7, 4',6-diamidino-2-phenylindole incubation for 10 minutes; 8, washing; 9, coverslipping and observation. Subsequent fluorescence analyses were conducted using the Image J software (NIH, Bethesda, MD).
Statistical Analysis
Statistical analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago, IL). The results were expressed as means ± SEM and evaluated using t test or 2-way ANOVA, as appropriate. For 2-way ANOVAs, Bonferroni post hoc tests were used to assess isolated comparisons. P < .05 was considered significant.
Results
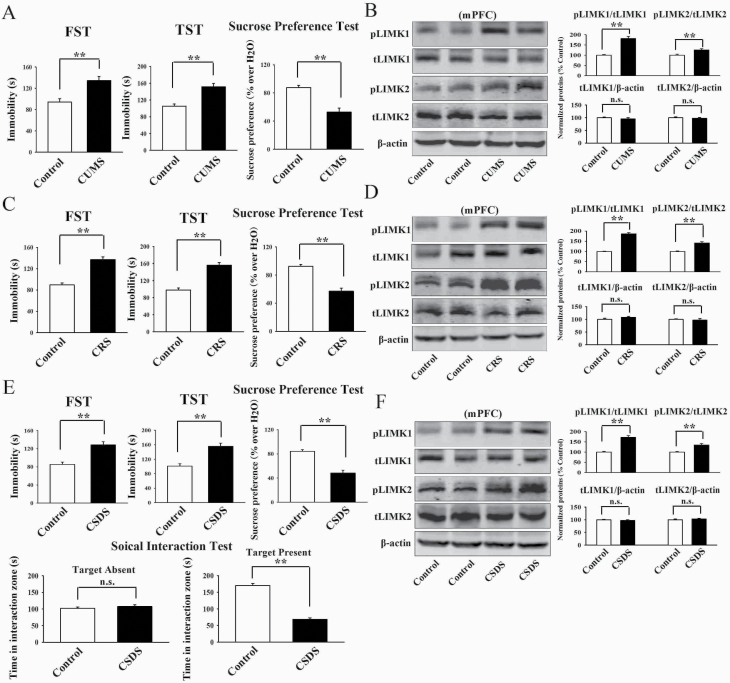
Chronic stress significantly enhanced the phosphorylation levels of LIMK1 and LIMK2 in the mPFC but not the hippocampus.
As the first step of this study, the CUMS model of depression was established. The C57BL/6J mice subjected to CUMS showed significantly more immobility in the FST (t = 5.258 > t0.01, 18 = 2.878, P < .01) and TST (t = 4.437 > t0.01, 18 = 2.878, P < .01) as well as less sucrose preference (t = 5.365 > t0.01, 18 = 2.878, P < .01) than the control mice (n = 10; Figure 1A), representing a depressive-like behavior. Then western blotting was used to explore the effects of CUMS on the expression of pLIMK1, total LIMK1, pLIMK2, and total LIMK2 in both the hippocampus and mPFC regions. It was found that CUMS significantly increased the expression of pLIMK1 (t = 6.952 > t0.01, 8 = 3.355, P < .01) and pLIMK2 (t = 3.867 > t0.01, 8 = 3.355, P < .01) in the mPFC but not the hippocampus (n = 5) (Figure 1B; supplementary Figure 1A). In contrast, the expression of total LIMK1 and LIMK2 in the hippocampus and mPFC remained unchanged among the 2 groups (n = 5) (Figure 1B; supplementary Figure 1A).
Figure 1.
Chronic stress significantly promoted the phosphorylation levels of LIMK1 and LIMK2 in the medial prefrontal cortex (mPFC) of mice. (A, C, and E) 8 weeks of chronic unpredictable mild stress (CUMS), 8 weeks of chronic restraint stress (CRS), and 10 days of chronic social defeat stress (CSDS) all induced notable depressive-like behaviors in mice, as revealed by the forced swim test (FST), tail suspension test (TST), sucrose preference test, and social interaction test (n = 10). (B, D, and F) Representative western blotting images confirm that the CUMS-treated, CRS-treated, and CSDS-treated mice all displayed significantly more expression of pLIMK1 and pLIM2 in the mPFC than the control mice (n = 5). In contrast, CUMS, CRS, and CSDS did not affect the expression of total LIMK1 and LIMK2 in the mPFC of mice (n = 5). All results are expressed as the means ± SEM; **P < .01; n.s., no significance. The comparisons were made using t test.
Afterwards, the CRS model of depression was established. Like CUMS, the C57BL/6J mice subjected to CRS also displayed significantly more immobility in the FST (t = 6.194 > t0.01, 18 = 2.878, P < .01) and TST (t = 5.466 > t0.01, 18 = 2.878, P < .01) as well as less sucrose preference (t = 5.083 > t0.01, 18 = 2.878, P < .01) than the control mice (n = 10; Figure 1C), representing a depressive-like behavior. Figure 1D and supplementary Figure 1B showed that CRS fully enhanced the levels of pLIMK1 (t = 7.275 > t0.01, 8 = 3.355, P < .01) and pLIMK2 (t = 4.407 > t0.01, 8 = 3.355, P < .01) in the mPFC but not the hippocampus (n = 5), while the levels of total LIMK1 and LIMK2 in the hippocampus and mPFC remained unchanged after CRS (n = 5).
Moreover, the CSDS model of depression was established. It was found that the CSDS-treated mice exhibited significantly more immobility in the FST (t = 5.964 > t0.01, 18 = 2.878, P < .01) and TST (t = 4.772 > t0.01, 18 = 2.878, P < .01) as well as less sucrose preference (t = 5.786 > t0.01, 18 = 2.878, P < .01) and social interaction (t = 9.093 > t0.01, 18 = 2.878, P < .01) than the control mice (n = 10; Figure 1E), proving the success and effectiveness of this model. Although it has been reported that mice subjected to CSDS have “susceptible” and “resilient” phenotypes (Jiang et al., 2018; Labonté et al., 2019), in this study, the majority of mice that received CSDS became depressed, while no mice were fully resistant to CSDS. Similarly, Figure 1F and supplementary Figure 1C showed that CSDS notably promoted the expression of pLIMK1 (t = 6.658 > t0.01, 8 = 3.355, P < .01) and pLIMK2 (t = 4.115 > t0.01, 8 = 3.355, P < .01) in the mPFC but not the hippocampus (n = 5), while the expression of total LIMK1 and LIMK2 in the hippocampus and mPFC remained unchanged after CSDS (n = 5).
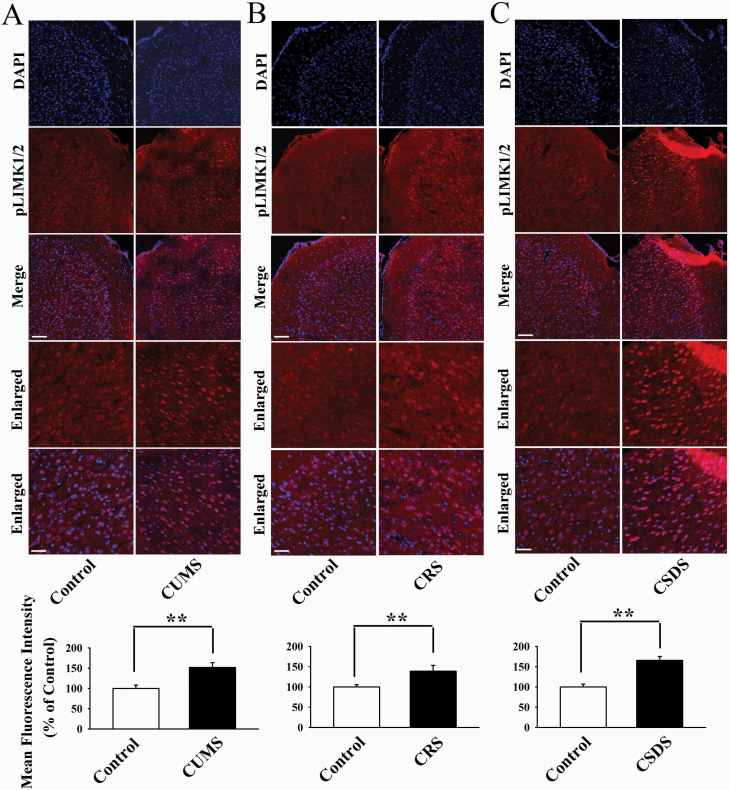
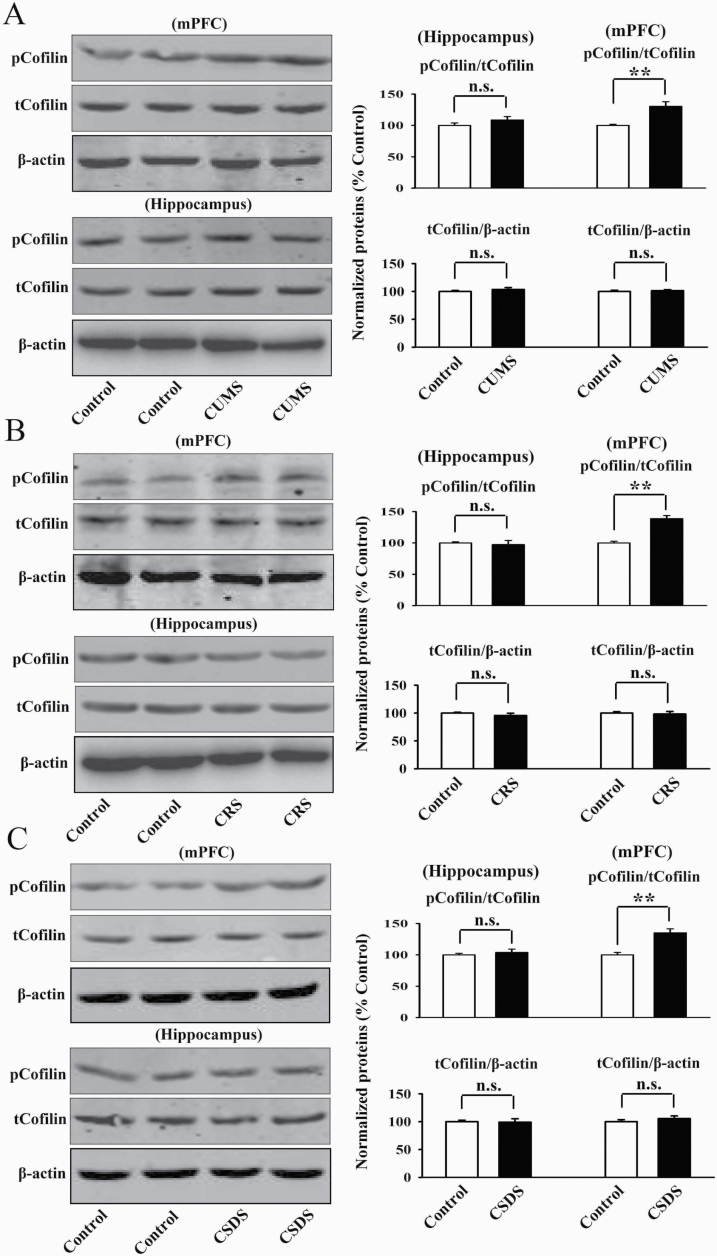
Furthermore, immunofluorescence was performed to assess the effects of chronic stress on neuronal distribution of pLIMK1 and pLIMK2 in the mPFC. As shown in Figure 2, CUMS (t = 5.958 > t0.01, 8 = 3.355, P < .01), CRS (t = 6.443 > t0.01, 8 = 3.355, P < .01), and CSDS (t = 6.906 > t0.01, 8 = 3.355, P < .01) all significantly increased the neuronal distribution of pLIMK1 and pLIMK2 in the mPFC (n = 5) in parallel with the above western blotting results. In addition, we detected Cofilin, the key downstream molecule of LIMK1/2 in the hippocampus and mPFC after chronic stress. As shown in Figure 3, CUMS (t = 4.916 > t0.01, 8 = 3.355, P < .01), CRS (t = 6.329 > t0.01, 8 = 3.355, P < .01), and CSDS (t = 5.548 > t0.01, 8 = 3.355, P < .01) all notably enhanced the pCofilin expression in the mPFC but not the hippocampus (n = 5), with total cofilin in the 2 regions not affected (n = 5). Taken together, these data suggest that the LIMK-Cofilin signaling in the mPFC may be involved in the pathogenesis of depression.
Figure 2.
Representative immunofluorescence images that show the effects of chronic unpredictable mild stress (CUMS) (A), chronic restraint stress (CRS) (B), and chronic social defeat stress (CSDS) (C) on neuronal distribution of pLIMK1 and pLIMK2 in the medial prefrontal cortex (mPFC) of mice (n = 5). The scale bar is 150 µm for representative images and 75 µm for enlarged images, respectively. Related analyses reveal that the 3 types of stress all significantly enhanced the neuronal distribution of pLIMK1 and pLIMK2 in the mPFC (n = 5). All results are expressed as the means ± SEM; **P < .01. The comparisons were made using t test.
Figure 3.
Chronic stress fully increased the phosphorylation level of Cofilin in the medial prefrontal cortex (mPFC) of mice. Representative western blotting images indicate that the chronic unpredictable mild stress (CUMS)-treated (A), chronic restraint stress (CRS)-treated (B), and chronic social defeat stress (CSDS)-treated (C) mice all exhibited significantly higher level of pCofilin in the mPFC but not the hippocampus than the control mice (n = 5). The level of total Cofilin in both the mPFC and hippocampus remained unchanged among all groups (n = 5). All results are expressed as the means ± SEM; **P < .01; n.s., no significance. The comparisons were made using t test.
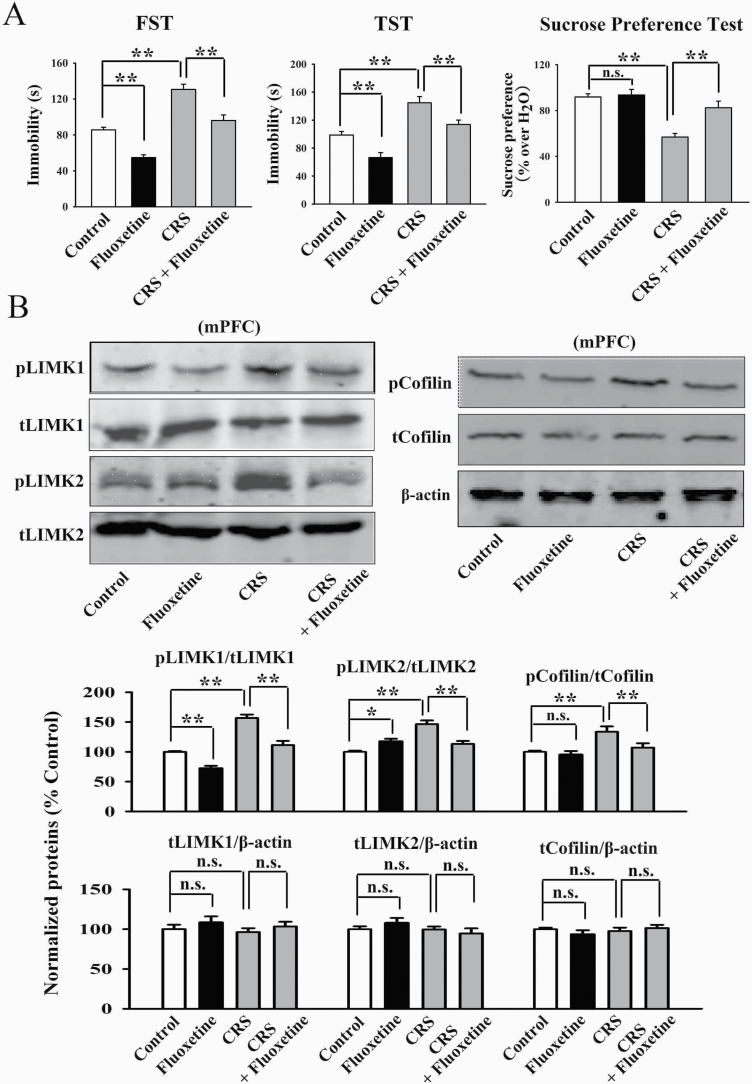
Administration of Fluoxetine Fully Reversed Chronic Stress-Induced Effects on LIMK1 and LIMK2 in the mPFC
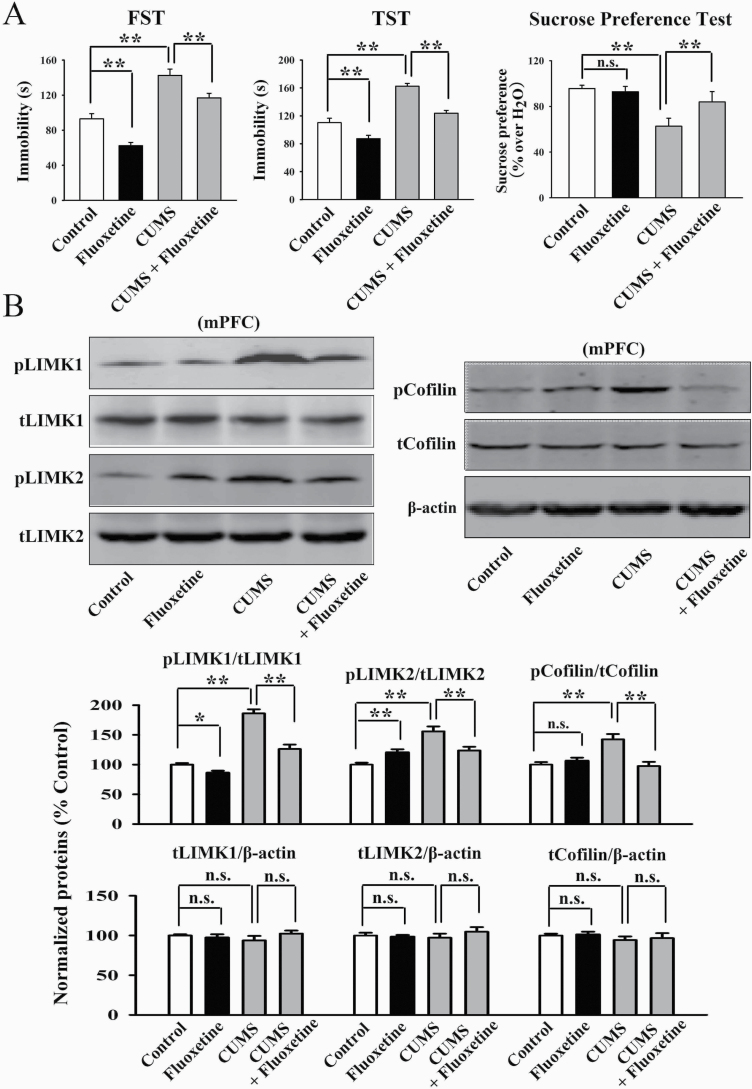
Fluoxetine is the most commonly used antidepressant in clinical practice as a selective serotonin reuptake inhibitor. Although fluoxetine increases the extracellular 5-HT concentration relatively rapidly, it always takes weeks to months of administration to have antidepressant effects, so there may be other antidepressant targets. Here, we investigated the possible role of LIMK1/2 in the mPFC. As shown in Figure 4A, the antidepressant-like actions of fluoxetine were confirmed in the CUMS model of depression, as the (fluoxetine + CUMS)-treated mice showed significantly less immobility in the FST [ANOVA: CUMS, F(1, 36) = 31.125, P < .01; fluoxetine, F(1, 36) = 23.576, P < .01; interaction, F(1, 36) = 26.304, P < .01] and TST [ANOVA: CUMS, F(1, 36) = 28.878, P < .01; fluoxetine, F(1, 36) = 19.345, P < .01; interaction, F(1, 36) = 22.661, P < .01] as well as more sucrose preference [ANOVA: CUMS, F(1, 36) = 23.048, P < .01; fluoxetine, F(1, 36) = 17.598, P < .01; interaction, F(1, 36) = 15.226, P < .01] than those of the CUMS-treated mice (n = 10). Subsequent western blotting results revealed that fluoxetine treatment fully reversed the promoting effects of CUMS on the expression of pLIMK1 [ANOVA: CUMS, F(1, 16) = 33.363, P < .01; fluoxetine, F(1, 16) = 20.682, P < .01; interaction, F(1, 16) = 26.819, P < .01], pLIMK2 [ANOVA: CUMS, F(1, 16) = 25.149, P < .01; fluoxetine, F(1, 16) = 14.055, P < .01; interaction, F(1, 16) = 17.283, P < .01] and pCofilin [ANOVA: CUMS, F(1, 16) = 21.072, P < .01; fluoxetine, F(1, 16) = 13.823, P < .01; interaction, F(1, 16) = 18.661, P < .01] in the mPFC (n = 5, Figure 4B). The expression of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 5, Figure 4B). Moreover, our immunofluorescence results were consistent with the western blotting results, indicating that fluoxetine treatment notably ameliorated the effects of CUMS on neuronal distribution of pLIMK1 and pLIMK2 in the mPFC [ANOVA: CUMS, F(1, 16) = 28.441, P < .01; fluoxetine, F(1, 16) = 22.405, P < .01; interaction, F(1, 16) = 18.772, P < .01] (n = 5, supplementary Figure 2).
Figure 4.
Repeated fluoxetine administration restored the promoting effects of chronic unpredictable mild stress (CUMS) on the LIMK-Cofilin signaling in the medial prefrontal cortex (mPFC) of mice. (A) Fluoxetine produced antidepressant-like actions in the CUMS model of depression, as revealed by the forced swim test (FST), tail suspension test (TST), and sucrose preference test (n = 10). (B) Representative western blotting images indicate that the (CUMS + fluoxetine)-treated mice had significantly less expression of pLIMK1, pLIMK2, and pCofilin in the mPFC than the CUMS-treated mice (n = 5). The levels of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 5). All results are expressed as the means ± SEM; *P < .05, **P < .01; n.s., no significance. The comparisons were made by 2-way ANOVA followed by Bonferroni’s test.
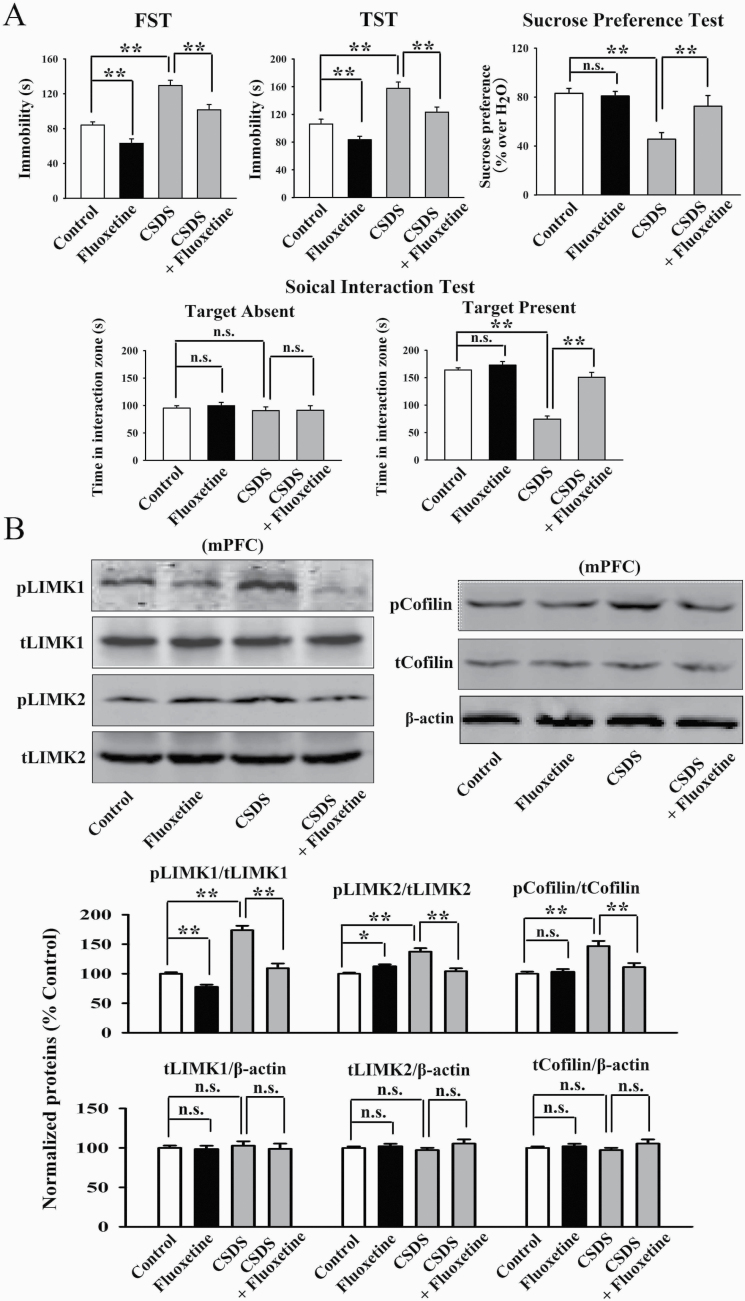
Furthermore, the CRS and CSDS models of depression were performed. Figure 5A confirms the effects of fluoxetine in the CRS model, as the (fluoxetine + CRS)-treated mice displayed significantly less immobility in the FST [ANOVA: CRS, F(1, 36) = 25.835, P < .01; fluoxetine, F(1, 36) = 16.441, P < .01; interaction, F(1, 36) = 20.103, P < .01] and TST [ANOVA: CRS, F(1, 36) = 33.705, P < .01; fluoxetine, F(1, 36) = 21.606, P < .01; interaction, F(1, 36) = 26.525, P < .01] as well as more sucrose preference [ANOVA: CRS, F(1, 36) = 24.368, P < .01; fluoxetine, F(1, 36) = 16.927, P < .01; interaction, F(1, 36) = 14.074, P < .01] than those of the CRS-treated mice (n = 10). Figure 6A confirms the effects of fluoxetine in the CSDS model, as the (fluoxetine + CSDS)-treated mice exhibited significantly less immobility in the FST [ANOVA: CSDS, F(1, 36) = 30.169, P < .01; fluoxetine, F(1, 36) = 18.495, P < .01; interaction, F(1, 36) = 25.126, P < .01] and TST [ANOVA: CSDS, F(1, 36) = 34.088, P < .01; fluoxetine, F(1, 36) = 22.465, P < .01; interaction, F(1, 36) = 19.775, P < .01] as well as more sucrose preference [ANOVA: CSDS, F(1, 36) = 26.537, P < .01; fluoxetine, F(1, 36) = 14.806, P < .01; interaction, F(1, 36) = 20.119, P < .01] and social interaction [ANOVA: CSDS, F(1, 36) = 39.182, P < .01; fluoxetine, F(1, 36) = 28.734, P < .01; interaction, F(1, 36) = 32.236, P < .01] than the CSDS-treated mice (n = 10). Figure 5B shows that fluoxetine administration significantly restored the enhancing effects of CRS on the levels of pLIMK1 [ANOVA: CRS, F(1, 16) = 26.834, P < .01; fluoxetine, F(1, 16) = 23.606, P < .01; interaction, F(1, 16) = 16.075, P < .01], pLIMK2 [ANOVA: CRS, F(1, 16) = 19.637, P < .01; fluoxetine, F(1, 16) = 14.445, P < .01; interaction, F(1, 16) = 11.872, P < .01] and pCofilin [ANOVA: CRS, F(1, 16) = 17.808, P < .01; fluoxetine, F(1, 16) = 12.678, P < .01; interaction, F(1, 16) = 14.006, P < .01] in the mPFC (n = 5). Figure 6B shows that fluoxetine administration also antagonized the increasing effects of CSDS on the levels of pLIMK1 [ANOVA: CSDS, F(1, 16) = 34.642, P < .01; fluoxetine, F(1, 16) = 29.062, P < .01; interaction, F(1, 16) = 23.164, P < .01], pLIMK2 [ANOVA: CSDS, F(1, 16) = 20.046, P < .01; fluoxetine, F(1, 16) = 13.943, P < .01; interaction, F(1, 16) = 16.779, P < .01], and pCofilin [ANOVA: CSDS, F(1, 16) = 25.073, P < .01; fluoxetine, F(1, 16) = 21.911, P < .01; interaction, F(1, 16) = 15.807, P < .01] in the mPFC (n = 5). The levels of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 5, Figures 5B and 6B). In addition, supplementary Figures 3 and 4 indicate that fluoxetine administration notably ameliorated the effects of CRS [ANOVA: CRS, F(1, 16) = 23.159, P < .01; fluoxetine, F(1, 16) = 15.763, P < .01; interaction, F(1, 16) = 19.448, P < .01] and CSDS [ANOVA: CSDS, F(1, 16) = 29.282, P < .01; fluoxetine, F(1, 16) = 21.994, P < .01; interaction, F(1, 16) = 24.265, P < .01] on neuronal distribution of pLIMK1 and pLIMK2 in the mPFC, respectively (n = 5). In summary, the antidepressant efficacy of fluoxetine involves the LIMK-Cofilin signaling in the mPFC.
Figure 5.
Repeated fluoxetine administration restored the promoting effects of chronic restraint stress (CRS) on the LIMK-Cofilin signaling in the medial prefrontal cortex (mPFC) of mice. (A) Fluoxetine produced antidepressant-like actions in the CRS model of depression, as revealed by the forced swim test (FST), tail suspension test (TST), and sucrose preference test (n = 10). (B) Representative western blotting images indicate that the (CRS + fluoxetine)-treated mice had significantly less expression of pLIMK1, pLIMK2, and pCofilin in the mPFC than those of the CRS-treated mice (n = 5). The levels of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 5). All results are expressed as the means ± SEM; *P < .05, **P < .01; n.s., no significance. The comparisons were made by 2-way ANOVA followed by Bonferroni’s test.
Figure 6.
Repeated fluoxetine administration reversed the promoting effects of chronic social defeat stress (CSDS) on the LIMK-Cofilin signaling in the medial prefrontal cortex (mPFC) of mice. (A) Fluoxetine produced antidepressant-like actions in the CSDS model of depression, as revealed by the forced swim test (FST), tail suspension test (TST), sucrose preference test, and social interaction test (n = 10). (B) Representative western blotting images indicate that the (CSDS + fluoxetine)-treated mice had significantly less expression of pLIMK1, pLIMK2, and pCofilin in the mPFC than the CSDS-treated mice (n = 5). The levels of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 5). All results are expressed as the means ± SEM; *P < .05, **P < .01; n.s., no significance. The comparisons were made by 2-way ANOVA followed by Bonferroni’s test.
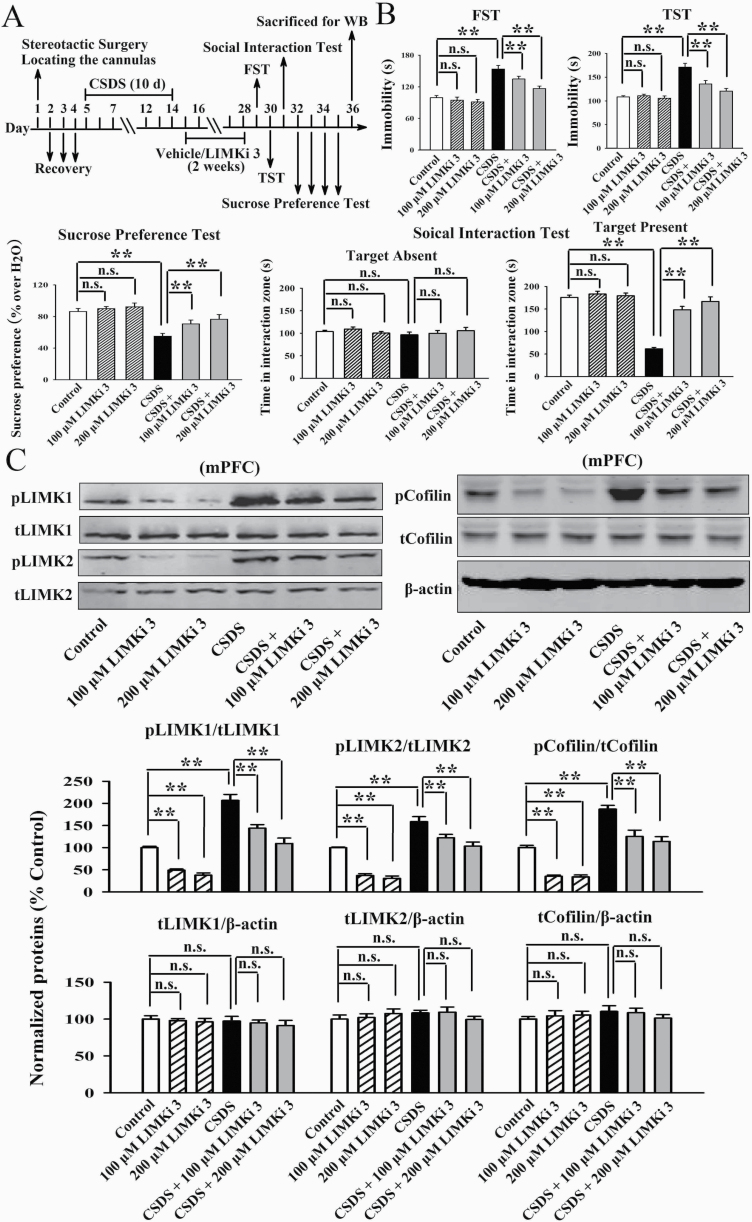
Inhibition of LIMK1 and LIMK2 in the mPFC Prevented Chronic Stress-Induced Depressive-Like Symptoms in Mice
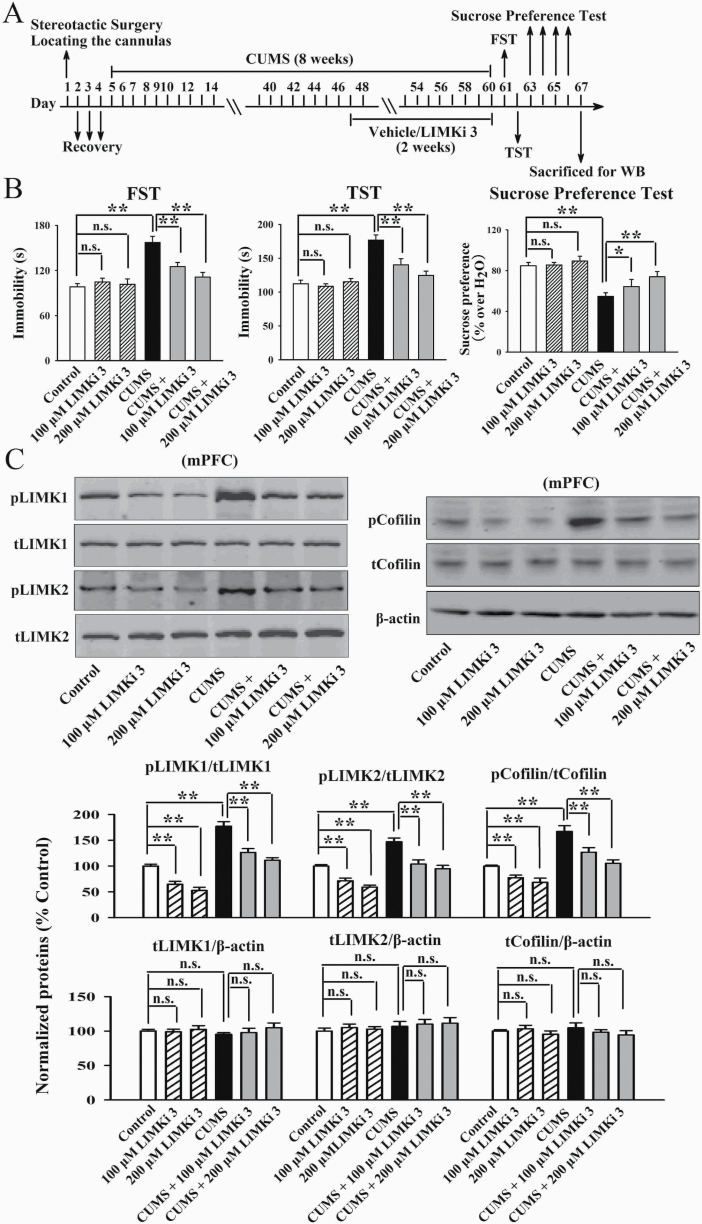
LIMKi 3 is a potent LIMK inhibitor with IC50s of 7 nM and 8 nM for LIMK1 and LIMK2, respectively. Therefore, LIMKi 3 was used to assess whether inhibition of LIMK1 and LIMK2 in the mPFC prevents chronic stress-induced depressive-like behaviors in mice. As shown in Figure 7B, infusion of LIMKi 3 into the mPFC significantly attenuated the CUMS-induced depressive-like behaviors, as both the (CUMS + 100 μM LIMKi 3)-treated and (CUMS + 200 μM LIMKi 3)-treated mice displayed notably less immobility in the FST [ANOVA: CUMS, F(1, 54) = 31.225, P < .01; LIMKi 3, F(2, 54) = 17.331, P < .01; interaction, F(2, 54) = 25.208, P < .01] and TST [ANOVA: CUMS, F(1, 54) = 35.739, P < .01; LIMKi 3, F(2, 54) = 24.813, P < .01; interaction, F(2, 54) = 19.642, P < .01] as well as more sucrose preference [ANOVA: CUMS, F(1, 54) = 27.207, P < .01; LIMKi 3, F(2, 54) = 18.237, P < .01; interaction, F(2, 54) = 15.022, P < .01] than those of the CUMS-treated mice (n = 10). LIMKi 3 treatment alone did not influence the behaviors of naïve control mice (n = 10). Subsequent western blotting results indicate that in parallel with the behavioral results, LIMKi 3 treatment fully blocked the promoting effects of CUMS on the expression of pLIMK1 [ANOVA: CUMS, F(1, 30) = 36.915, P < .01; LIMKi 3, F(2, 30) = 24.507, P < .01; interaction, F(2, 30) = 27.077, P < .01], pLIMK2 [ANOVA: CUMS, F(1, 30) = 24.007, P < .01; LIMKi 3, F(2, 30) = 18.825, P < .01; interaction, F(2, 30) = 17.024, P < .01], and pCofilin [ANOVA: CUMS, F(1, 30) = 30.904, P < .01; LIMKi 3, F(2, 30) = 25.099, P < .01; interaction, F(2, 30) = 19.335, P < .01] in the mPFC (n = 6, Figure 7C). The expression of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 6, Figure 7C).
Figure 7.
Infusion of LIMKi 3 into the medial prefrontal cortex (mPFC) produced antidepressant-like effects in the chronic unpredictable mild stress (CUMS) model of depression. (A) Schematic timeline of the experimental procedures. (B) LIMKi 3 fully prevented the CUMS-induced depressive-like behaviors in mice, as revealed by the forced swim test (FST), tail suspension test (TST), and sucrose preference test (n = 10). (C) Both the (CUMS + 100 μM LIMKi 3)-treated and (CUMS + 200 μM LIMKi 3)-treated mice had significantly lower levels of pLIMK1, pLIMK2, and pCofilin in the mPFC than the CUMS-treated mice (n = 6). The expression of total LIMK1, LIMK2, and Cofilin in the mPFC remain unchanged among all groups (n = 6). All results are expressed as the means ± SEM; *P < .05, **P < .01; n.s., no significance. The comparisons were made by 2-way ANOVA followed by Bonferroni’s test.
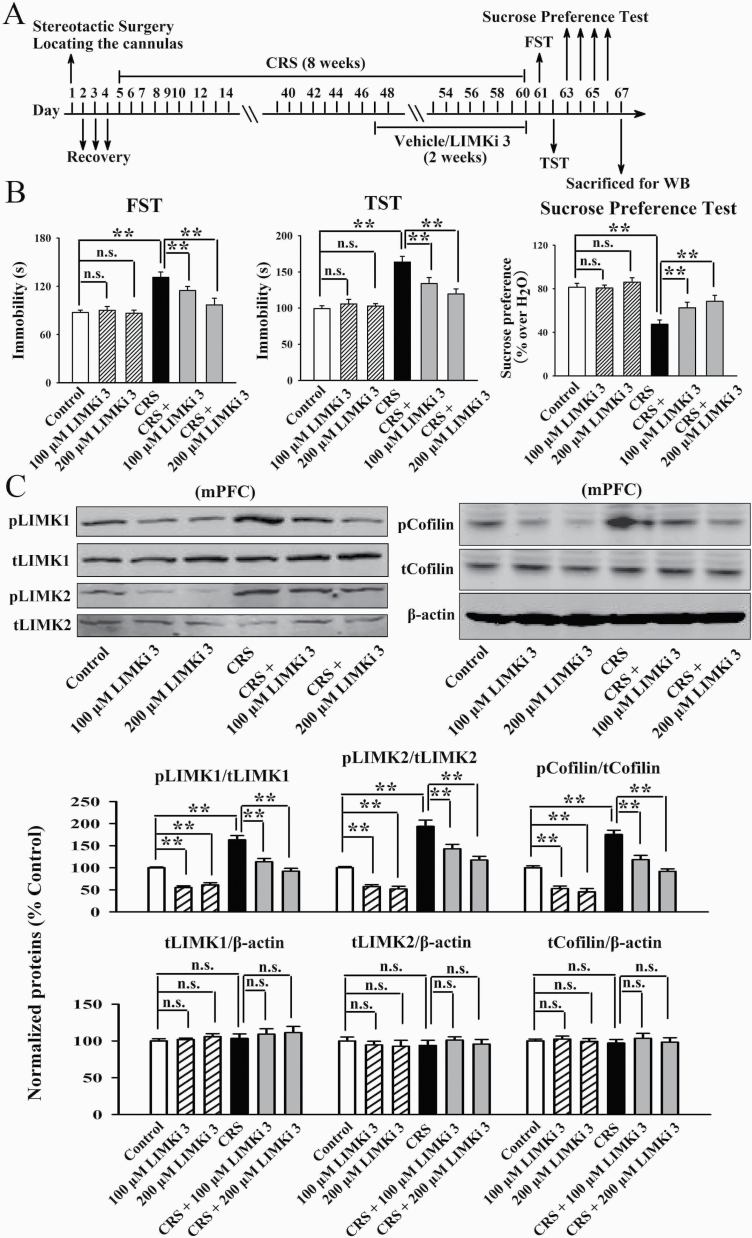
Figure 8B illustrates the CRS data. Both the (CRS + 100 μM LIMKi 3)-treated and (CRS + 200 μM LIMKi 3)-treated mice displayed notably less immobility in the FST [ANOVA: CRS, F(1, 54) = 29.657, P < .01; LIMKi 3, F(2, 54) = 23.645, P < .01; interaction, F(2, 54) = 16.073, P < .01] and TST [ANOVA: CRS, F(1, 54) = 34.102, P < .01; LIMKi 3, F(2, 54) = 22.612, P < .01; interaction, F(2, 54) = 26.198, P < .01] as well as more sucrose preference [ANOVA: CRS, F(1, 54) = 28.664, P < .01; LIMKi 3, F(2, 54) = 19.308, P < .01; interaction, F(2, 54) = 17.884, P < .01] than the CRS-treated mice (n = 10). Figure 9B illustrates the CSDS data. Both the (CSDS + 100 μM LIMKi 3)-treated and (CSDS + 200 μM LIMKi 3)-treated mice displayed notably less immobility in the FST [ANOVA: CSDS, F(1, 54) = 33.901, P < .01; LIMKi 3, F(2, 54) = 16.508, P < .01; interaction, F(2, 54) = 25.199, P < .01] and TST [ANOVA: CSDS, F(1, 54) = 35.202, P < .01; LIMKi 3, F(2, 54) = 21.701, P < .01; interaction, F(2, 54) = 28.195, P < .01] as well as more sucrose preference [ANOVA: CSDS, F(1, 54) = 27.468, P < .01; LIMKi 3, F(2, 54) = 15.382, P < .01; interaction, F(2, 54) = 19.028, P < .01] and social interaction [ANOVA: CSDS, F(1, 54) = 41.025, P < .01; LIMKi 3, F(2, 54) = 26.809, P < .01; interaction, F(2, 54) = 32.117, P < .01] than the CSDS-treated mice (n = 10). Thus, LIMKi 3 treatment also produced significant antidepressant-like effects in the CRS and CSDS models of depression. Moreover, Figures 8C and 9C show that LIMKi 3 treatment significantly prevented the enhancing effects of CRS and CSDS on the levels of pLIMK1 [ANOVA for CRS: CRS, F(1, 30) = 29.053, P < .01; LIMKi 3, F(2, 30) = 18.729, P < .01; interaction, F(2, 30) = 23.438, P < .01. ANOVA for CSDS: CSDS, F(1, 30) = 40.301, P < .01; LIMKi 3, F(2, 30) = 33.671, P < .01; interaction, F(2, 30) = 25.179, P < .01], pLIMK2 [ANOVA for CRS: CRS, F(1, 30) = 37.135, P < .01; LIMKi 3, F(2, 30) = 15.606, P < .01; interaction, F(2, 30) = 26.745, P < .01. ANOVA for CSDS: CSDS, F(1, 30) = 27.005, P < .01; LIMKi 3, F(2, 30) = 16.815, P < .01; interaction, F(2, 30) = 21.901, P < .01], and pCofilin [ANOVA for CRS: CRS, F(1, 30) = 32.119, P < .01; LIMKi 3, F(2, 30) = 23.743, P < .01; interaction, F(2, 30) = 17.689, P < .01. ANOVA for CSDS: CSDS, F(1, 30) = 34.404, P < .01; LIMKi 3, F(2, 30) = 26.599, P < .01; interaction, F(2, 30) = 19.239, P < .01] in the mPFC (n = 6), with total LIMK1, LIMK2, and Cofilin not influenced (n = 6). Collectively, pharmacological inhibition of LIMK1 and LIMK2 in the mPFC exhibits antidepressant-like actions in mice.
Figure 8.
Infusion of LIMKi 3 into the medial prefrontal cortex (mPFC) produced antidepressant-like effects in the chronic restraint stress (CRS) model of depression. (A) Schematic timeline of the experimental procedures. (B) LIMKi 3 significantly attenuated the CRS-induced depressive-like behaviors in mice, as revealed by the forced swim test (FST), tail suspension test (TST), and sucrose preference test (n = 10). (C) Both the (CRS + 100 μM LIMKi 3)-treated and (CRS + 200 μM LIMKi 3)-treated mice had significantly lower levels of pLIMK1, pLIMK2, and pCofilin in the mPFC than the CRS-treated mice (n = 6). The expression of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 6). All results are expressed as the means ± SEM; **P < .01; n.s., no significance. The comparisons were made by 2-way ANOVA followed by Bonferroni’s test.
Figure 9.
Infusion of LIMKi 3 into the medial prefrontal cortex (mPFC) produced antidepressant-like effects in the chronic social defeat stress (CSDS) model of depression. (A) Schematic timeline of the experimental procedures. (B) LIMKi 3 notably blocked the CSDS-induced depressive-like behaviors in mice, as revealed by the forced swim test (FST), tail suspension test (TST), sucrose preference test, and social interaction test (n = 10). (C) Both the (CSDS + 100 μM LIMKi 3)-treated and (CSDS + 200 μM LIMKi 3)-treated mice had significantly lower levels of pLIMK1, pLIMK2, and pCofilin in the mPFC than those of the CSDS-treated mice (n = 6). The expression of total LIMK1, LIMK2, and Cofilin in the mPFC remained unchanged among all groups (n = 6). All results are expressed as the means ± SEM; **P < .01; n.s., no significance. The comparisons were made by 2-way ANOVA followed by Bonferroni’s test.
Discussion
This study mainly investigated the correlation between the LIMK-Cofilin system in the mPFC and depression. It was found that both chronic stress and fluoxetine were able to significantly regulate the phosphorylation of LIMK1, LIMK2, and Cofilin in the mPFC but not the hippocampus, and inhibiting LIMK1/2 in the mPFC led to antidepressant-like effects in mice. As to how chronic stress affects the activities of LIMK-Cofilin in the mPFC and why chronic stress does not influence LIMK-Cofilin in the hippocampus, currently it remains unclear and further studies are required. In addition to the hippocampus and mPFC, some other brain regions including amygdale, hypothalamus, and nucleus accumbens are also closely correlated with depression (Nestler et al., 2002; Willner et al., 2013). Would the LIMK-Cofilin system in the amygdala, hypothalamus, and nucleus accumbens be implicated in depression? Further in-depth studies are ongoing in our group.
LIMK proteins have been shown to be involved in many neuronal disorders, such as intellectual disability, Williams-Beuren syndrome, schizophrenia, Alzheimer’s disease, and Parkinson’s disease (Cuberos et al., 2015). To our knowledge, our study has provided the first comprehensive evidence directly supporting the role of LIMK proteins in depression. It is worth mentioning that 2 previous reports demonstrated some phenomena contrary to our findings. In 2018, Fan et al. (Fan et al., 2018) reported that 5 weeks of CUMS increased the expression of miR-134 and decreased the LIMK1-Cofilin signaling in the ventromedial prefrontal cortex of rats. In 2019, Lu et al. (Lu et al., 2019) reported that 3 weeks of CUMS downregulated the expression of both pCofilin and Cofilin in the hippocampus. It is currently difficult to clarify these contradictions. However, we have noticed another phenomenon reported in Rosso et al. (Rosso et al., 2004) showing that transient overexpression of LIMK1 accelerates axon formation, whereas long-term overexpression of LIMK1 leads to growth cone collapse and axon retraction, which could support our findings. Rosso et al. (Rosso et al., 2004) explains that long-term overexpression of LIMK1 leads to large accumulation of F-actin present in the growth cones of neurons, which affects process extension by preventing microtubule advance and decreasing the membrane vesicle content of neuritic tips. Alternatively, a decrease in actin turnover may allow myosin to generate sufficient contractile force to produce axon retraction. According to Rosso et al. (Rosso et al., 2004), we speculate that in this study, 8 weeks of CUMS induced a long-term overexpression of LIMK1/2 in the mPFC, which then produced neuronal atrophy. Besides, there are some other studies indicating that abnormal LIMK1-Cofilin signaling in either direction could lead to the disruption of cytoskeleton dynamics, causing synaptic dysfunction and behavioral deficits associated with mental disorders (Duffney et al., 2013, 2015; Yan et al., 2016; Pyronneau et al., 2017).
It is very interesting and meaningful to demonstrate that LIMKi 3 (BMS-5) administration prevented chronic stress, suggesting a novel antidepressant candidate. As 3 models of depression were used together in this study, the conclusion that LIMKi 3 has antidepressant-like actions should be reliable and plausible. LIMKi 3 is chemically named N-[5-[2-(2, 6-dichlorophenyl)-5-(difluoromethyl)pyrazol-3-yl]-1,3-thiazol-2-yl]-2-methylpropanamide, possessing no hydrophilic groups and should therefore be liposoluble and able to penetrate the blood-brain barrier. For the clinical application potentials of LIMKi 3, so far there are few related studies. For example, Petrilli et al. (Petrilli et al., 2014) has reported that LIMKi 3 had beneficial efficacy against neurofibromatosis type 2 through inhibition of LIMKs. Park et al. (Park et al., 2014) has shown that LIMKi 3 treatment significantly increased adhesion and decreased migration and invasion in mesenchymal glioblastoma multiforme cells, providing a novel way to target the invasive machinery in glioblastoma multiforme. Our findings extend the knowledge of the pharmacological effects and therapeutic potentials of LIMKi 3.
Another notable phenomenon is that the LIMK-Cofilin signaling in the mPFC is involved in the antidepressant efficacy of fluoxetine, the most commonly used selective serotonin reuptake inhibitor. Before this study, many other studies have explored the antidepressant mechanisms of fluoxetine and reported various pharmacological targets including Aquaporin 4, ΔFosB, peroxisome proliferators-activated receptor α, salt-inducible kinase 2, and so on (Kong et al., 2009; Vialou et al., 2015; Song et al., 2018; Jiang et al., 2019). However, among these proteins, it remains unknown which is the primary antidepressant target for fluoxetine. Here, our findings offer another candidate. On the other hand, fluoxetine may be a multiple-targeting drug. It will be interesting and meaningful to clarify the cellular and biological link between LIMK-Cofilin and other reported targets of fluoxetine, which will be done in the future using network pharmacology. Moreover, if the LIMK-Cofilin system in the mPFC is also necessary for the effects of other well-known antidepressants such as venlafaxine, paroxetine, and mirtazapine, it can be an exact antidepressant target.
In conclusion, the present study identifies LIMK1/2 in the mPFC as a potential participant underlying the pathophysiology of depression and provides support for the potential use of LIMKi 3 treatment strategies against chronic stress-related mental disorders.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (no. 81873795), a grant from the Natural Science Foundation of Jiangsu Province (no. 18KJB310013), and 2 grants from the Science and Technology Projects of Nantong City (nos. MS12018076 and JC2018009).
Statement of Interest
None.
References
- Andrianantoandro E, Pollard TD (2006) Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell 24:13–23. [DOI] [PubMed] [Google Scholar]
- Bernard O. (2007) Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol 39:1071–1076. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR (2010) ADF/cofilin: a functional node in cell biology. Trends Cell Biol 20:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, Toda H, Hishimoto A (2018) Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci 72:3–12. [DOI] [PubMed] [Google Scholar]
- Colyn L, Venzala E, Marco S, Perez-Otaño I, Tordera RM (2019) Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behav Brain Res 373:112079. [DOI] [PubMed] [Google Scholar]
- Cuberos H, Vallée B, Vourc’h P, Tastet J, Andres CR, Bénédetti H (2015) Roles of LIM kinases in central nervous system function and dysfunction. FEBS Lett 589:3795–3806. [DOI] [PubMed] [Google Scholar]
- Dale E, Bang-Andersen B, Sánchez C (2015) Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol 95:81–97. [DOI] [PubMed] [Google Scholar]
- Duffney LJ, Wei J, Cheng J, Liu W, Smith KR, Kittler JT, Yan Z (2013) Shank3 deficiency induces NMDA receptor hypofunction via an actin-dependent mechanism. J Neurosci 33:15767–15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, Ma K, Dietz DM, Kajiwara Y, Buxbaum JD, Yan Z (2015) Autism-like deficits in Shank3-deficient mice are rescued by targeting actin regulators. Cell Rep 11:1400–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ (1997) A molecular and cellular theory of depression. Arch Gen Psychiatry 54:597–606. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J (1999) Neural plasticity to stress and antidepressant treatment. Biol Psychiatry 46:1181–1189. [DOI] [PubMed] [Google Scholar]
- Duman RS, Sanacora G, Krystal JH (2019) Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102:75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Zhu X, Song Q, Wang P, Liu Z, Yu SY (2018) MiR-134 modulates chronic stress-induced structural plasticity and depression-like behaviors via downregulation of Limk1/cofilin signaling in rats. Neuropharmacology 131:364–376. [DOI] [PubMed] [Google Scholar]
- Henderson F, Vialou V, El Mestikawy S, Fabre V (2017) Effects of social defeat stress on sleep in mice. Front Behav Neurosci 11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild G, Kalmár L, Kardos R, Nyitrai M, Bugyi B (2014) The other side of the coin: functional and structural versatility of ADF/cofilins. Eur J Cell Biol 93:238–251. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Chen XR, Han QQ, Wang J, Pilot A, Yu R, Liu Q, Li B, Wu GC, Wang YQ, Yu J (2019) The protective effects of Ghrelin/GHSR on hippocampal neurogenesis in CUMS mice. Neuropharmacology 155:31–43. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY (2010) Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci 11:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Wang H, Wang JL, Wang YJ, Zhu Q, Wang CN, Song L, Gao TT, Wang Y, Meng GL, Wu F, Ling Y, Zhang W, Li JX (2019) Hippocampal salt-inducible kinase 2 plays a role in depression via the CREB-regulated transcription coactivator 1-cAMP response element binding-brain-derived neurotrophic factor pathway. Biol Psychiatry 85:650–666. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lin WJ, Sadahiro M, Labonté B, Menard C, Pfau ML, Tamminga CA, Turecki G, Nestler EJ, Russo SJ, Salton SR (2018) VGF function in depression and antidepressant efficacy. Mol Psychiatry 23:1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS (1994) Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, Hu G (2009) Requirement of AQP4 for antidepressive efficiency of fluoxetine: implication in adult hippocampal neurogenesis. Neuropsychopharmacology 34:1263–1276. [DOI] [PubMed] [Google Scholar]
- Labonté B, Jeong YH, Parise E, Issler O, Fatma M, Engmann O, Cho KA, Neve R, Nestler EJ, Koo JW (2019) Gadd45b mediates depressive-like role through DNA demethylation. Sci Rep 9:4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem YH, Yoon SS, Jo SA (2020) Imipramine ameliorates depressive symptoms by blocking differential alteration of dendritic spine structure in amygdala and prefrontal cortex of chronic stress-induced mice. Biomol Ther (Seoul) 28:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sun G, Yang F, Guan Z, Zhang Z, Zhao J, Liu Y, Chu L, Pei L (2019) Baicalin regulates depression behavior in mice exposed to chronic mild stress via the Rac/LIMK/cofilin pathway. Biomed Pharmacother 116:109054. [DOI] [PubMed] [Google Scholar]
- Lunardi P, Sachser RM, Sierra RO, Pedraza LK, Medina C, de la Fuente V, Romano A, Quillfeldt JA, de Oliveira Alvares L (2018) Effects of hippocampal LIMK inhibition on memory acquisition, consolidation, retrieval, reconsolidation, and extinction. Mol Neurobiol 55:958–967. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015) Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172:3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Takahashi H, Meng J, Zhang Y, Lu G, Asrar S, Nakamura T, Jia Z (2004) Regulation of ADF/cofilin phosphorylation and synaptic function by LIM-kinase. Neuropharmacology 47:746–754. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25. [DOI] [PubMed] [Google Scholar]
- Park JB, Agnihotri S, Golbourn B, Bertrand KC, Luck A, Sabha N, Smith CA, Byron S, Zadeh G, Croul S, Berens M, Rutka JT (2014) Transcriptional profiling of GBM invasion genes identifies effective inhibitors of the LIM kinase-Cofilin pathway. Oncotarget 5:9382–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli A, Copik A, Posadas M, Chang LS, Welling DB, Giovannini M, Fernández-Valle C (2014) LIM domain kinases as potential therapeutic targets for neurofibromatosis type 2. Oncogene 33:3571–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyronneau A, He Q, Hwang JY, Porch M, Contractor A, Zukin RS (2017) Aberrant Rac1-cofilin signaling mediates defects in dendritic spines, synaptic function, and sensory perception in fragile X syndrome. Sci Signal 10:eaan0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM (2016) Dendritic spines in depression: what we learned from animal models. Neural Plast 2016:8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, et al. (2018) The limbic system in youth depression: brain structural and functional alterations in adolescent in-patients with severe depression. Neuropsychopharmacology 43:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S, Bollati F, Bisbal M, Peretti D, Sumi T, Nakamura T, Quiroga S, Ferreira A, Cáceres A (2004) LIMK1 regulates Golgi dynamics, traffic of Golgi-derived vesicles, and process extension in primary cultured neurons. Mol Biol Cell 15:3433–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EK, Luo L (2001) How do dendrites take their shape? Nat Neurosci 4:359–365. [DOI] [PubMed] [Google Scholar]
- Song L, Wang H, Wang YJ, Wang JL, Zhu Q, Wu F, Zhang W, Jiang B (2018) Hippocampal PPARα is a novel therapeutic target for depression and mediates the antidepressant actions of fluoxetine in mice. Br J Pharmacol 175:2968–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Thibault M, Kaska S, Cooper S, Gajewski P, Eagle A, Mazei-Robison M, Nestler EJ, Robison AJ (2015) Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 99:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CN, Gong SN, Guan W, Wang JL, Gao TT, Wang Y, Sun F, Jiang B (2020) Hippocampal overexpression of chordin protects against the chronic social defeat stress-induced depressive-like effects in mice. Brain Res Bull 158:31–39. [DOI] [PubMed] [Google Scholar]
- Whitford KL, Dijkhuizen P, Polleux F, Ghosh A (2002) Molecular control of cortical dendrite development. Annu Rev Neurosci 25:127–149. [DOI] [PubMed] [Google Scholar]
- Willner P, Scheel-Krüger J, Belzung C (2013) The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 37:2331–2371. [DOI] [PubMed] [Google Scholar]
- Xu D, Sun Y, Wang C, Wang H, Wang Y, Zhao W, Bao G, Wang F, Cui Z, Jiang B (2018) Hippocampal mTOR signaling is required for the antidepressant effects of paroxetine. Neuropharmacology 128:181–195. [DOI] [PubMed] [Google Scholar]
- Yan Z, Kim E, Datta D, Lewis DA, Soderling SH (2016) Synaptic actin dysregulation, a convergent mechanism of mental disorders? J Neurosci 36:11411–11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain MA, Pandy V, Majeed ABA, Wong WF, Mohamed Z (2019) Chronic restraint stress impairs sociability but not social recognition and spatial memoryin C57BL/6J mice. Exp Anim 68:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Gao TT, Wang Y, Wang JL, Guan W, Wang YJ, Wang CN, Liu JF, Jiang B (2019) Andrographolide exerts significant antidepressant-like effects involving the hippocampal BDNF system in mice. Int J Neuropsychopharmacol 22:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SS, Tian YH, Jin SJ, Wang WC, Zhao JX, Si XM, Zhang L, Xu H, Jin JY (2019) Isoflurane produces antidepressant effects inducing BDNF-TrkB signaling in CUMS mice. Psychopharmacology (Berl) 236:3301–3315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.