Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
Recipient telomere length is independently associated with overall survival and NRM after transplantation for MDS.
The effect of telomere length on NRM was most prominent after higher intensity conditioning and in patients with severe acute graft-versus-host disease.
Abstract
Allogeneic hematopoietic stem cell transplantation is the only potentially curative treatment for patients with myelodysplastic syndrome (MDS), but long-term survival is limited by the risk of transplant-related complications. Short telomere length, mediated by inherited or acquired factors, impairs cellular response to genotoxic and replicative stress and could identify patients at higher risk for toxicity after transplantation. We measured relative telomere length in pretransplant recipient blood samples in 1514 MDS patients and evaluated the association of telomere length with MDS disease characteristics and transplantation outcomes. Shorter telomere length was significantly associated with older age, male sex, somatic mutations that impair the DNA damage response, and more severe pretransplant cytopenias, but not with bone marrow blast count, MDS treatment history, or history of prior cancer therapy. Among 1267 patients ≥40 years old, telomere length in the shortest quartile was associated with inferior survival (P < .001) because of a high risk of nonrelapse mortality (NRM; P = .001) after adjusting for significant clinical and genetic variables. The adverse impact of shorter telomeres on NRM was independent of recipient comorbidities and was observed selectively among patients receiving more intensive conditioning, including myeloablative regimens and higher dose melphalan-based reduced-intensity regimens. The effect of shorter telomeres on NRM was prominent among patients who developed severe acute graft-versus-host disease, suggesting that short telomere length may limit regenerative potential of mucosal tissues after acute injury. MDS patients with shorter telomere length, who have inferior survival driven by excess toxicity, could be considered for strategies focused on minimizing toxic effects of transplantation.
Visual Abstract
Introduction
The only curative treatment for patients with myelodysplastic syndrome (MDS) is allogeneic hematopoietic stem cell transplantation.1 MDS genetic characteristics measured before transplantation improve standard clinical prognostic models, primarily by identifying patients with poor survival because of an elevated risk of relapse.2-5 In contrast, estimating the risk of nonrelapse mortality (NRM) relies on clinical ascertainment of patient comorbidities and indicators of end-organ dysfunction.6-8 Patients in whom comorbidity indices predict an elevated risk of treatment-induced toxicity are either transplanted with reduced-intensity conditioning (RIC) regimens or directed to nontransplant therapeutic approaches. Identification of patients most at risk of toxicity will improve selection of conditioning regimens and enable prioritization of patients most likely to benefit from transplantation.1
In rare cases, germline genetic factors have been shown to influence the risk of transplant toxicity. Patients with dyskeratosis congenita, an inherited telomere biology disorder associated with premature telomere attrition, bone marrow failure, and a predisposition to MDS, have a high rate of NRM.9-11 This effect was observed early after conditioning with radiation and DNA alkylating agents, suggesting that short telomere length may restrict tissue regenerative capacity in the setting of genotoxic or replicative stress. Moreover, in patients without documented telomere biology disorders, shorter blood telomere length has been linked to increased toxicity after chemotherapy for solid tumors and adverse outcomes after allogeneic stem cell transplantation in patients with severe aplastic anemia and in a cohort of younger patients (median age 31) with a variety of malignant and nonmalignant hematologic diseases.12-14
Although several studies have suggested that MDS patients have shorter telomeres compared with healthy controls,15-17 the effect of telomere length on transplantation outcomes in MDS has not been systematically assessed. We therefore evaluated the effect of telomere length on survival after transplantation in a cohort of MDS patients with comprehensive annotation of clinical, genetic, and transplantation variables.
Methods
Patients
We previously described a cohort of 1514 MDS patients who were enrolled in The Center for International Blood and Marrow Transplant Research (CIBMTR) repository and research database who had banked whole peripheral blood DNA.2 All the patients provided written informed consent to participate in the CIBMTR research database and repository. Clinical outcome data were refreshed for the current analysis and the updated median follow-up time for censored patients was 5.0 years. Peripheral blood counts and bone marrow blast counts were measured at time of transplantation. This study was conducted with the approval of the institutional review board at the Dana-Farber Cancer Institute.
Telomere length measurement
Aliquots of whole blood in anticoagulant citrate dextrose solution were collected and stored in a uniform manner according to the protocol of the CIBMTR research sample repository. DNA was extracted using a single method (Qiagen DNA Blood Mini Kit) in 1 laboratory (LabCorp) in a single batch using an automated, plate-based method. Samples were maintained in Tris EDTA buffer and uniformly subjected to a total of 2 freeze-thaw cycles before real-time quantitative polymerase chain reaction (qPCR) analysis. Relative leukocyte telomere length was measured in triplicate using qPCR with the following primers: Tel1: 5′-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT-3′, Tel2: 5′-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA-3′. 36B4u: 5′-CAGCAAGTGGGAAGGTGTAATCC-3′, 36B4d: 5′-CCCATTCTATCATCAACGGGTACAA-3′. The relative telomere to single (T/S) copy reference gene (36B4) ratio for each sample was calculated by subtracting the T/S ratio of a calibrator DNA from the T/S ratio of each sample. Quality control samples were interspersed throughout the plates in order to assess interplate and intraplate variability of cycle threshold values. Samples were stored, processed, and analyzed uniformly in a single laboratory.
Telomere measurement was successful in 1508 of 1514 patients and the mean coefficient of variation of triplicates among all samples was 0.37% for the telomere assay and 0.26% for the 36B4 assay. The mean coefficient of variation of T/S ratios for the 2 quality control samples were 8.7% and 8.3%. The intraclass correlation of the assay was 0.82 for 1 year, 0.75 for 3 years, and 0.58 for 10 years.
For patients age 40 and older, recursive partitioning models were used to select quantiles of telomere length based on association with overall survival. Quantiles considered were multiples of 5 from 5% to 45%. Based on these models, patients were grouped into 3 groups: (1) shortest telomere length (first quartile), (2) intermediate telomere length (second and third quartiles), or (3) longest telomere length (fourth quartile), where quartiles were internally defined in the cohort.
Genetic studies
As previously reported, genetic profiling was performed on whole peripheral blood and included targeted sequencing of 129 genes, selected based on their known or suspected involvement in the pathogenesis of myeloid malignancies.2
Curation of conditioning regimen intensity
Conditioning intensities were classified based on chemotherapy regimen and/or total body irradiation dose as myeloablative, nonmyeloablative, or reduced intensity.18,19
Statistical analyses
The Wilcoxon or Kruskal-Wallis rank-sum test was used to assess a location shift in the distribution of continuous variables between 2 or 3 groups, respectively. For associations with ordered categorical variables, Cuzick’s rank-sum test was used for continuous variables; Kruskal-Wallis or Jonckheere-Terpstra tests were used for singly or doubly ordered contingency tables, respectively. Descriptive statistics (proportions, medians, etc) were reported with 95% confidence interval (CI) or range. Chi-square and Fisher’s exact tests were used to test for association between pairs of categorical variables. Odds ratios and 95% CIs were calculated for binary outcomes from contingency tables or logistic regression models. All P values were 2-sided, and adjustments for multiple hypothesis testing were performed where indicated.
Overall survival (OS) was defined as time from transplant until death from any cause. Subjects not confirmed dead were censored at the time last known to be alive. Differences in survival curves were assessed using log-rank tests. NRM was defined as death without relapse. NRM, with relapse as a competing risk, was assessed with the use of Gray’s test. For relapse, death without relapse was considered a competing risk.
Univariate and multivariable analyses of OS were performed using Cox regression. OS estimates were calculated using the method of Kaplan-Meier and reported with 95% CIs based on Greenwood’s formula. Forward and backward stepwise variable selection was used to generate a multivariable OS model integrating clinical and genetic features. Variables used in selection procedure included modifiable clinical, nonmodifiable clinical, and genetic features (supplemental Table 1 on the Blood Web site). Hazard ratios with 95% CIs and Wald P values were reported for covariates in multivariable Cox models. An additional multivariable OS model was generated with hematopoietic cell transplantation-specific comorbidity index (HCT-CI) score forced on top of the variables selected by the initial approach (supplemental Tables 2 and 3). Multivariable models for competing risks of relapse and NRM were generated using the Fine and Gray method.
Results
Telomere length and MDS characteristics
We measured relative leukocyte telomere length in peripheral blood samples obtained uniformly just before transplantation in an otherwise unselected cohort of 1514 MDS patients. As expected, female patients had significantly longer telomere length than male patients (P < .001) (Table 1), and increasing patient age was associated with shorter blood telomere length (Figure 1A). However, the association between age and telomere length was only evident among younger patients, with the greatest effect in those younger than age 10 years (P < .001) and those 10 to 39 years old (P < .001). There was no significant association between age and blood telomere length among older MDS patients 40 to 77 years old (Figure 1B). Because MDS in patients age 40 and older is genetically and clinically distinct from MDS in younger individuals,2 and older patients account for most transplants for MDS, we report here the association between telomere length and MDS characteristics in patients age 40 and older (n = 1267) (Table 1). Transplant outcomes by telomere quartiles in patients younger than age 40 years are presented in supplemental Figure 1.
Table 1.
Characteristics of patients age 40 y or older and association with telomere length quartiles
| Telomere length quartile | ||||
|---|---|---|---|---|
| Shortest, n = 317 | Intermediate (2nd and 3rd), n = 633 | Longest, n = 317 | P | |
| Patient-related variable | ||||
| Age at transplantation, median (range), y | 61 (40-77) | 61 (40-77) | 60 (40-76) | .12* |
| Female, n (%) | 99 (31) | 251 (40) | 146 (46) | <.001† |
| Karnofsky performance status score <90, n (%) | 99 (31) | 196 (31) | 87 (27) | .11† |
| Disease-related variable | ||||
| Hemoglobin, median (IQR), g/dL | 9.0 (8.0-10.4) | 9.6 (8.2-11.4) | 9.8 (8.4-11.5) | <.001* |
| Platelet count, median (IQR), ×109/L | 53 (20-99) | 83 (34-160) | 96 (50-159) | <.001* |
| Absolute neutrophil count, median (IQR), ×109/L | 0.9 (0.4-1.7) | 1.2 (0.6-2.3) | 1.6 (0.7-2.8) | <.001* |
| Bone marrow blasts, median (IQR), % | 3 (1-7) | 3 (1-5) | 3 (1-5) | .27* |
| IPSS-R cytogenetic risk group, n (%) | .62‡ | |||
| Very good | 3 (1) | 3 (0) | 3 (1) | |
| Good | 116 (37) | 249 (39) | 126 (40) | |
| Intermediate | 51 (16) | 93 (15) | 48 (15) | |
| Poor | 52 (16) | 105 (17) | 45 (14) | |
| Very poor | 29 (9) | 56 (9) | 27 (9) | |
| Unknown | 66 (21) | 127 (20) | 68 (21) | |
| Prior MDS-directed therapy, n (%) | 186 (59) | 424 (67) | 201 (63) | .08† |
| Therapy-related MDS, n (%) | 79 (25) | 122 (19) | 68 (21) | .29† |
| Time from diagnosis to HSCT, median (IQR), mo | 9.5 (5.5-23.7) | 8.2 (5.4-15.5) | 8.4 (5.4-16.5) | .13* |
| Transplantation-related variable | ||||
| Conditioning regimen, n (%) | .70† | |||
| Myeloablative | 140 (44) | 291 (46) | 151 (48) | |
| Reduced intensity | 145 (46) | 274 (43) | 136 (43) | |
| Nonmyeloablative | 28 (9) | 64 (10) | 29 (9) | |
| Missing | 4 (1) | 4 (1) | 1 (0) | |
| Donor type, n (%) | .39† | |||
| Matched, related | 35 (11) | 84 (13) | 46 (15) | |
| Matched, unrelated | 188 (59) | 380 (60) | 187 (59) | |
| Mismatched | 71 (22) | 113 (18) | 58 (18) | |
| Cord blood | 23 (7) | 56 (9) | 26 (8) | |
| Graft type, n (%) | .73† | |||
| Bone marrow | 41 (13) | 78 (12) | 38 (12) | |
| Peripheral blood stem cells | 251 (79) | 497 (79) | 253 (80) | |
| Cord blood | 22 (7) | 54 (9) | 25 (8) | |
| Other | 3 (1) | 4 (1) | 1 (0) | |
| In vivo T-cell depletion, n (%) | .38† | |||
| No | 178 (56) | 356 (56) | 162 (51) | |
| Yes | 129 (41) | 248 (39) | 136 (43) | |
| Missing | 10 (3) | 29 (5) | 19 (6) | |
| GVHD prophylaxis, n (%) | .99† | |||
| Tacrolimus-based | 245 (77) | 502 (79) | 242 (76) | |
| Cyclosporine A-based | 39 (12) | 80 (13) | 41 (13) | |
| Other | 10 (3) | 18 (3) | 9 (3) | |
| CD34 selection | 8 (3) | 13 (2) | 8 (3) | |
| Ex vivo T-cell depletion | 5 (2) | 7 (1) | 4 (1) | |
| Cyclophosphamide-based | 4 (1) | 8 (1) | 5 (2) | |
| None reported | 6 (2) | 5 (1) | 8 (3) | |
Peripheral blood counts and bone marrow blast counts at time of transplantation.
HSCT, hematopoietic stem cell transplantation; IQR, interquartile range.
Cuzick’s trend test.
Kruskal-Wallis trend test.
Jonckheere-Terpstra test.
Figure 1.
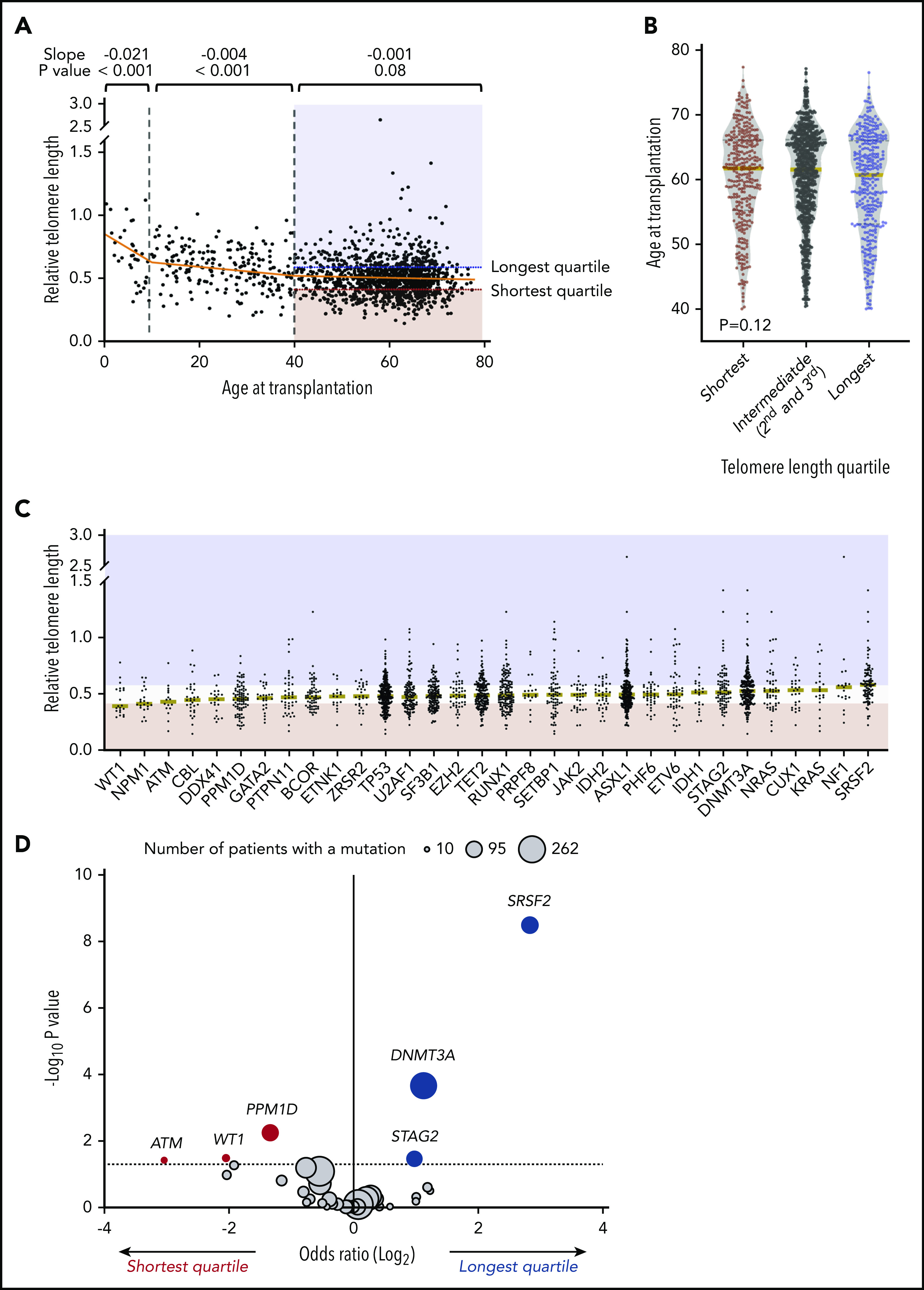
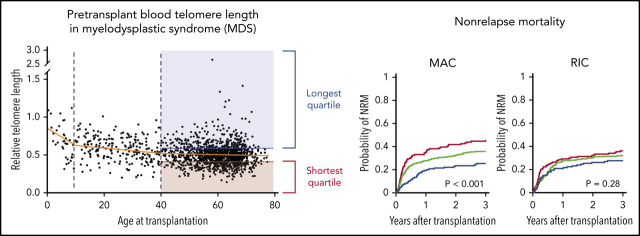
Association of telomere length with age and somatic mutations. (A) Association between recipient telomere length and age at transplantation. Linear regression lines for age groups 0 to 10, 10 to 40, and 40 to 77 years are shown in orange. Red and blue areas highlight the telomere lengths in the shortest quartile and the longest telomere length groups, respectively. (B) Violin plots for distribution of age at transplantation in telomere length groups in patients age 40 or older. Yellow lines indicate median ages at transplantation. (C) Distribution of telomere lengths according to somatic gene mutations in patients age 40 or older. Yellow lines represent the median telomere lengths and genes are ordered from shortest to longest by median telomere length. Areas in red and blue reflect telomere lengths in the shortest and the longest quartile telomere length groups, respectively. Genes with more than 15 mutated patients shown. (D) Association between somatic mutations and the shortest or the longest quartile telomere length groups in patients age 40 years or older. The x-axis depicts the magnitude of association (log2 odds ratio), and the y-axis shows the negative log of the nominal P value (-log10 P). The dashed line represents the threshold of significance (.05) for nominal P values. Each circle represents a mutated gene and the size of the circle reflects the number of patients with mutation. Genes with a mutation in at least 10 patients are shown.
To determine whether telomere length correlated with disease-related factors, we first analyzed the association between telomere length groups and components of the Revised International Prognostic Scoring System (IPSS-R). Shorter blood telomere length was associated with more severe peripheral cytopenias, including lower hemoglobin (median 9.0 vs 9.6 vs 9.8 g/dL, P < .001), lower platelet count (median 53 vs 83 vs 96 × 109/L, P < .001), and lower absolute neutrophil count (median 0.9 vs 1.2 vs 1.6 × 109/L, P < .001). The bone marrow blast count (P = .27) and distribution of IPSS-R cytogenetic risk groups (P = .62) were similar across telomere length groups.
Because exposure to antineoplastic therapy has been associated with accelerated telomere attrition,20-27 we next determined whether telomere length differences in the cohort were explained by treatment history. Telomere length was similar in patients who had received prior MDS treatment with DNA methyltransferase inhibitors (n = 713), chemotherapy (n = 38), or DNA methyltransferase inhibitors and chemotherapy (n = 60), compared with those who had not received MDS-directed treatment (n = 361). Further, we observed no association between telomere length and the time interval between MDS diagnosis and transplantation (P = .13) (Table 1). Last, the distribution of blood telomere length was similar in patients who had previously received treatment with chemotherapy or radiation for a non-myeloid cancer (therapy-related MDS) compared with those with primary MDS (P = .29).
Independent of the IPSS-R, somatic gene mutations in MDS have been closely linked to clinical phenotype and transplantation outcomes.2,28-30 We therefore analyzed the association between MDS mutation status and blood telomere length (Figure 1C-D; supplemental Table 4). Mutations in 3 genes, SRSF2 (P < .001), DNA methyltransferase 3A (DNMT3A; P < .001), and STAG2 (P = .04), were significantly more common in those with the longest quartile blood telomere length than in those with the shortest quartile telomere length, whereas 3 gene mutations were significantly more common in patients with the shortest telomere length: PPM1D (P = .01), WT1 (P = .03), and ATM (P = .04). Differences in telomere length according to somatic mutation status were not explained by differences in age or sex. Further, there was no consistent relationship between telomere length and MDS clone size across different gene mutations, as inferred by variant allele fraction, suggesting that differences in telomere length were not generally explained by overall disease burden (supplemental Figure 2). There was no association between telomere length and the proportion of neutrophils in the white blood cell differential (supplemental Figure 3), indicating that measured whole blood telomere length was not explained primarily by leukocyte constituency.
Clinical outcomes according to telomere length
To determine whether recipient telomere length was associated with clinical outcomes after transplantation, we evaluated OS and rates of relapse and NRM. In multivariable analysis, intermediate and short blood telomere length were independently associated with inferior overall survival compared with the longest telomere quartile (hazard ratio for death in shortest vs longest, 1.49; 95% CI, 1.22-1.83; P < .001; intermediate vs longest, 1.34; 95% CI, 1.12-1.60; P = .001) (Figure 2A). Other clinical and genetic characteristics previously reported to be associated with survival remained significant in the model, including recipient age, Karnofsky performance score <90, very high IPSS-R risk category, presence of TP53, JAK2 V617F, or RAS/tyrosine kinase pathway mutations, and cord blood grafts.2,31 Shorter and intermediate telomere length, transplantation before year 2008, and JAK2 V617F mutations were associated with a higher rate of NRM but not a higher rate of relapse (Figure 2B). TP53 mutations and very high IPSS-R risk category were associated with a higher rate of relapse but not a higher rate of NRM (Figure 2C). HCT-CI score, which was forced into the model, was not independently associated with NRM or relapse. Complete results of the multivariable Cox model for OS and the Fine-Gray models for the rates of relapse and NRM are provided in supplemental Table 3.
Figure 2.
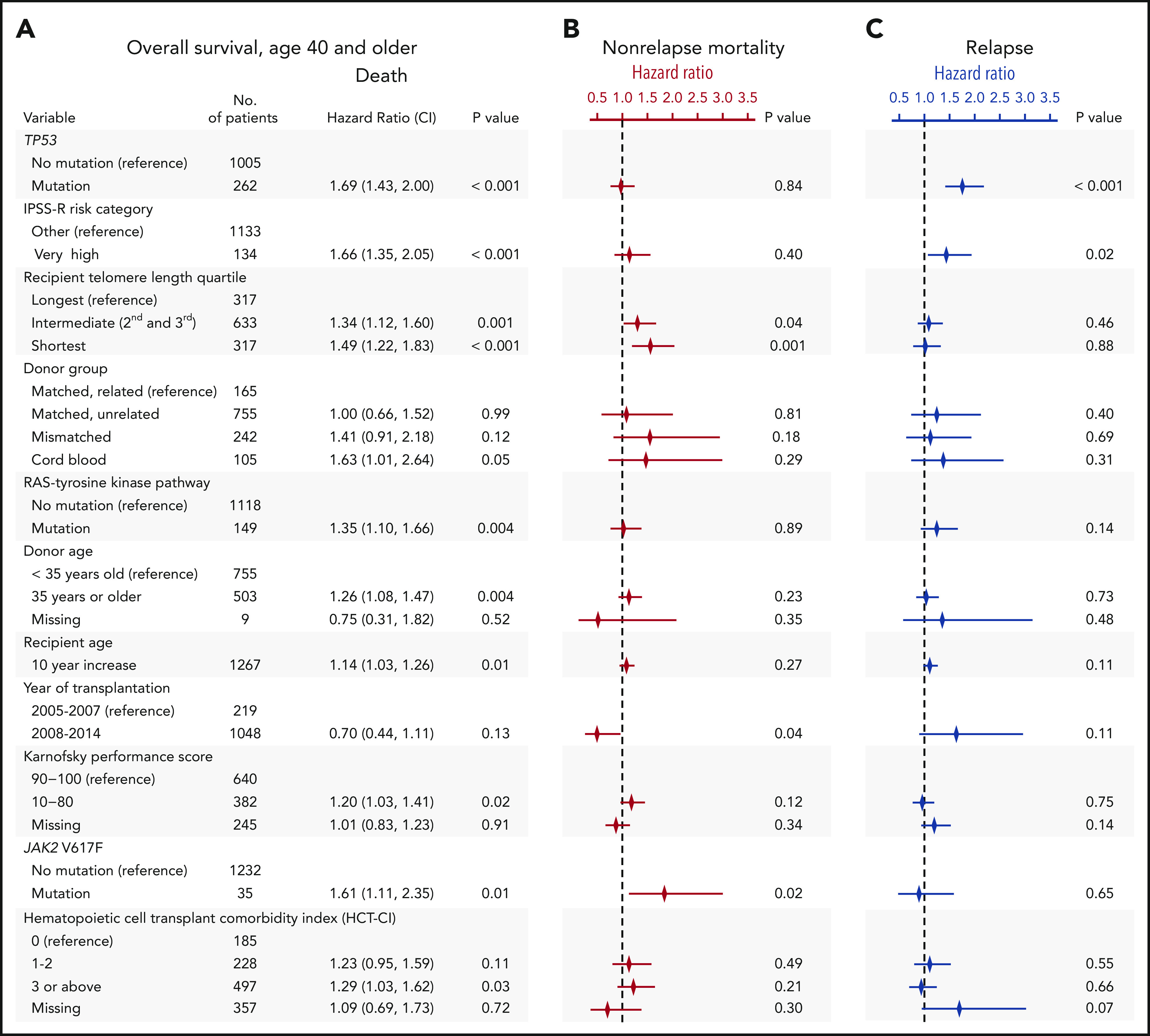
Multivariable models for overall survival, nonrelapse mortality and relapse. (A) Stepwise multivariable Cox regression model for overall survival in patients age 40 years or older. Variables shown are those that significantly contributed to the model, with the exception of HCT-CI score, which was forced into the model. A complete list of variables offered for selection in the model is shown in supplemental Table 1. Multivariable models without HCT-CI are presented in supplemental Table 2. P values for overall significance: <.001 for recipient telomere length, <.001 for donor group, .16 for HCT-CI score. (B-C) Results of Fine-Gray models for nonrelapse mortality and relapse, respectively. The symbols represent the hazard ratios for nonrelapse mortality (in red) and relapse (in blue) and lines show 95% confidence intervals. Complete tables are shown in supplemental Table 3.
Outcomes according to conditioning intensity
Patients in the longest telomere length quartile had superior overall survival irrespective of conditioning intensity (Figure 3A-B). However, the specific risk of NRM is closely linked to conditioning regimen intensity.19,32,33 Therefore, we asked whether the effect of telomere length on NRM was different in patients who received myeloablative regimens compared with those who received RIC regimens. In patients who received myeloablative conditioning (MAC), shorter telomere length was associated with increased cumulative incidence of NRM (P < .001) (Figure 3A). Patients with shortest quartile telomere length had higher NRM than those with intermediate or long telomere length (supplemental Figure 4). There was no difference in the cumulative incidence of relapse based on telomere length (P = .54).
Figure 3.
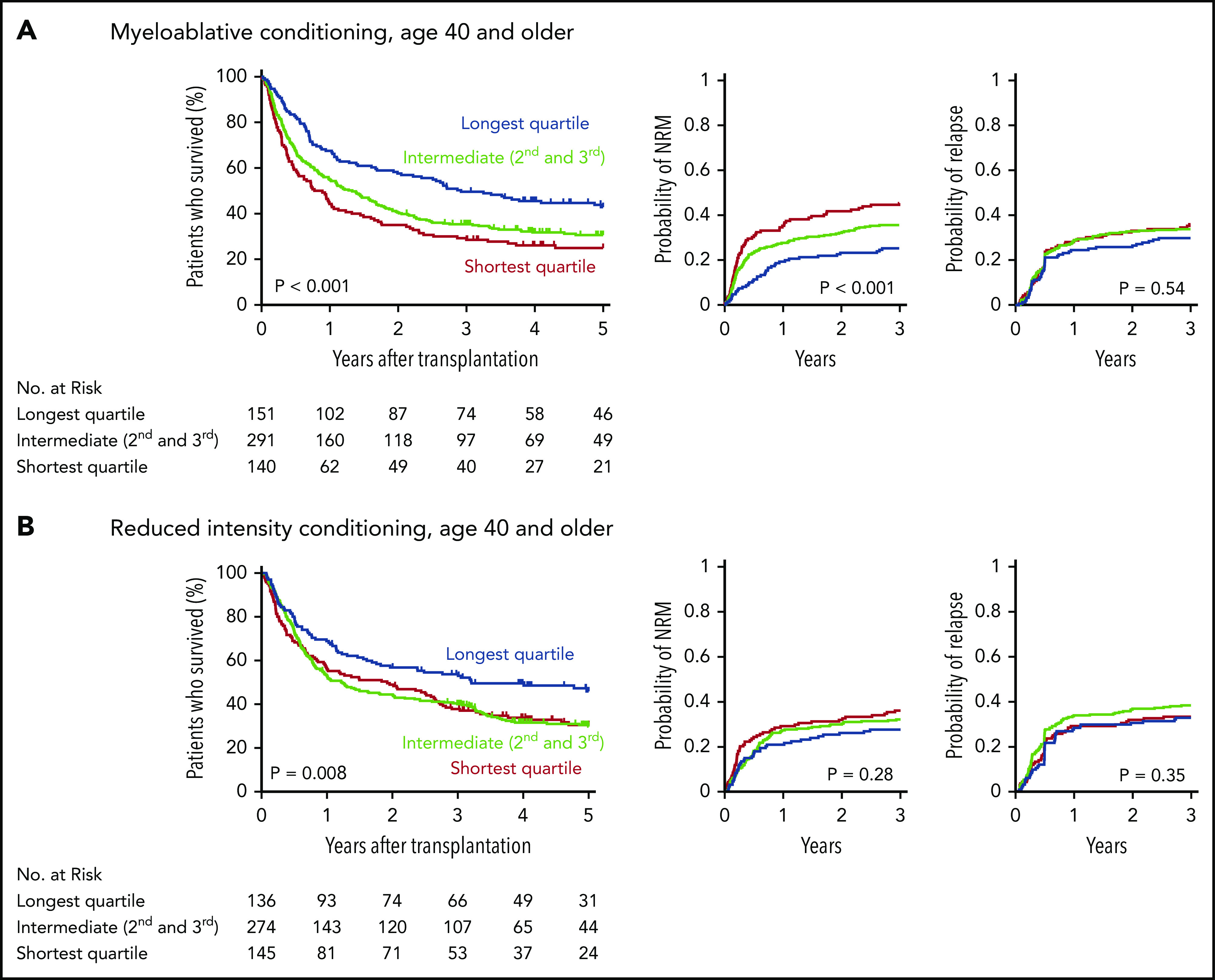
Outcomes after transplantation by conditioning intensity. Shown are the Kaplan-Meier curves for overall survival and cumulative incidence curves for nonrelapse mortality and relapse based on telomere length group in patients who received myeloablative (A) or reduced-intensity (B) conditioning regimens. Telomere length groups are indicated by color: the shortest quartile (red), second and third quartiles (green), and the longest quartile (blue). Patient characteristics by telomere length quartiles in patients who received myeloablative and reduced-intensity conditioning, respectively, are shown in supplemental Tables 10 and 11.
In patients who received RIC regimens, telomere length was not significantly associated with NRM (P = .28) or relapse (P = .35) (Figure 3B; supplemental Figure 4). However, analysis of outcomes based on the dichotomous classification of regimens as either of high or reduced intensity fail to account for regimen heterogeneity. Specifically, fludarabine/melphalan-based RIC regimens are similar to MAC regimens, having a reduced risk of relapse and increased risk of NRM compared with lower intensity fludarabine/busulfan-based RIC regimens.19,34 We hypothesized that patients with shorter telomere length have increased NRM with higher intensity fludarabine/melphalan-based conditioning compared with those who received other RIC regimens. We therefore compared clinical outcomes in each telomere length group based on RIC regimen intensity (Figure 4A-C; supplemental Table 5). As expected, the cumulative incidence of relapse was lower after fludarabine/melphalan-based RIC regimens than after other RIC regimens in all 3 telomere length groups (shortest, P = .008; intermediate, P < .001; longest, P = .001; Figure 4C). In contrast, the cumulative incidence of NRM was significantly increased in patients receiving fludarabine/melphalan-based RIC regimens compared with those receiving other RIC regimens in those with shorter telomeres (3-year cumulative incidence-NRM 51% vs 28%, P = .002), but not in those with intermediate (3 year cumulative incidence-NRM 36% vs 29%, P = .15) or longer telomere length (3 year cumulative incidence-NRM 33% vs 23%, P = .15) (Figure 4B). Primary causes of death based on telomere length group and conditioning intensity are listed in supplemental Table 6.
Figure 4.
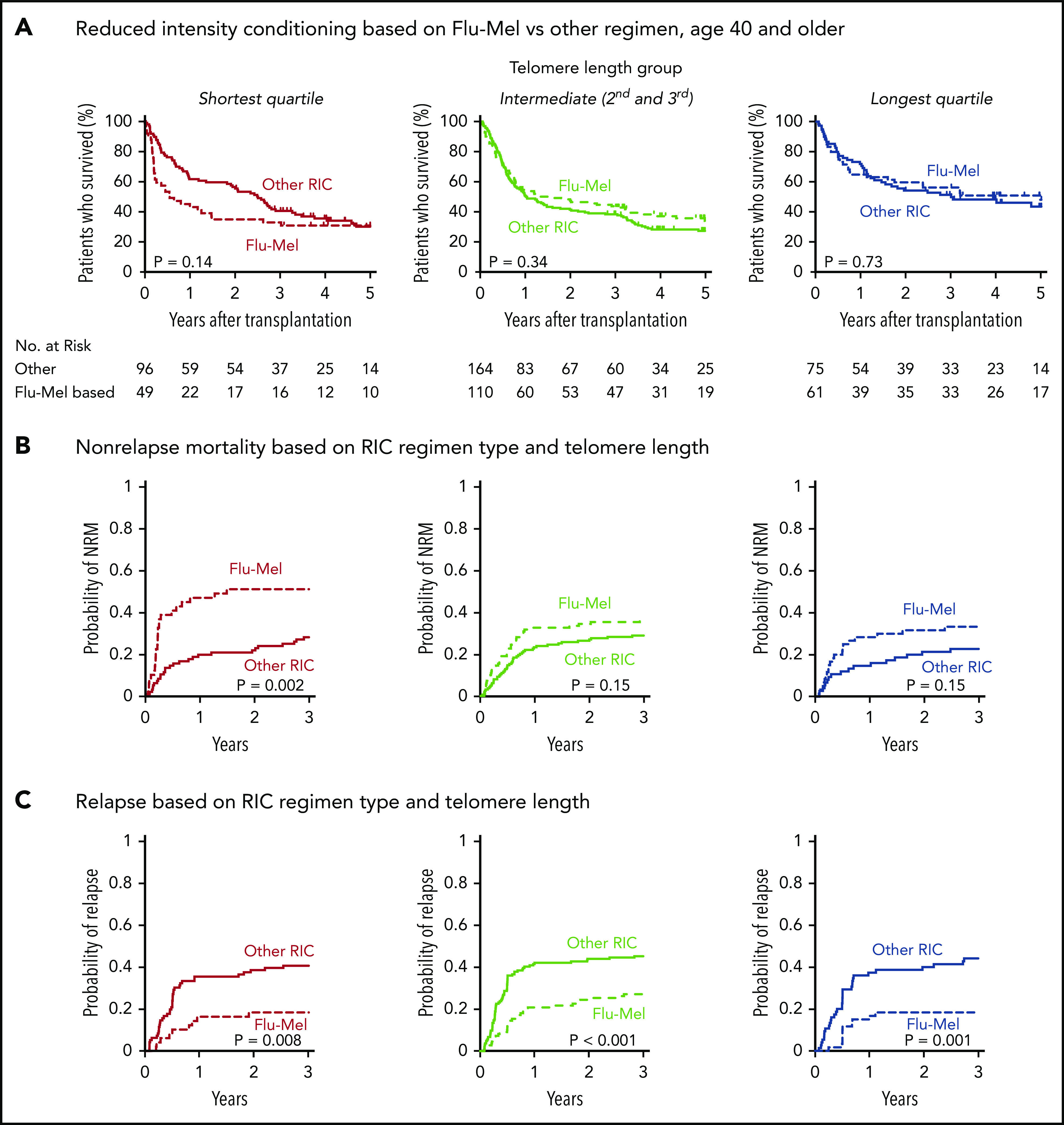
Outcomes based on fludarabine-melphalan vs other RIC regimen. Shown are Kaplan-Meier curves for overall survival and cumulative incidence (A) for nonrelapse mortality (B) and relapse based on telomere length and reduced-intensity regimen type (C). Fludarabine/melphalan (Flu-Mel)-based regimens are indicated by dashed lines; other RIC regimens are indicated by solid lines. Telomere length groups are indicated by color: the shortest quartile (red), second and third quartiles (green), and the longest quartile (blue). The distribution of melphalan doses is presented in supplemental Figure 7. Outcomes by telomere length quartiles in RIC regimen groups are presented in supplemental Figure 8.
Telomere length and recipient comorbidities
The risk of NRM after transplantation is associated with poor performance status and the presence of comorbid medical conditions.1,8,31 The proportion of patients with Karnofsky performance status score <90 was similar across all 3 telomere length groups (P = .11)(Table 1), indicating that differences in telomere length were not reflective of global differences in overall health. Detailed annotation of organ-specific comorbidities based on the HCT-CI, was available in 72% (910/1267) of patients (supplemental Figure 5). HCT-CI score ranges from 0 to 26, where 0 is the absence of comorbidities and higher score is associated with inferior outcomes.
MDS patients with longer telomere length had lower HCT-CI scores than those with intermediate and shorter telomere length (P = .005) (Figure 5A). This effect was driven by a significant association between shorter telomere length and an increased frequency of pulmonary dysfunction (odds ratio shortest vs longest, 1.90; false discovery rate [FDR]-corrected P = .003), hepatic dysfunction (odds ratio shortest vs longest, 3.80; FDR-corrected P = .003), and infection before transplantation (odds ratio shortest vs longest, 4.46; FDR-corrected P = .003). There were no other differences across the HCT-CI component comorbidities according to telomere length (Figure 5B; supplemental Table 7).
Figure 5.
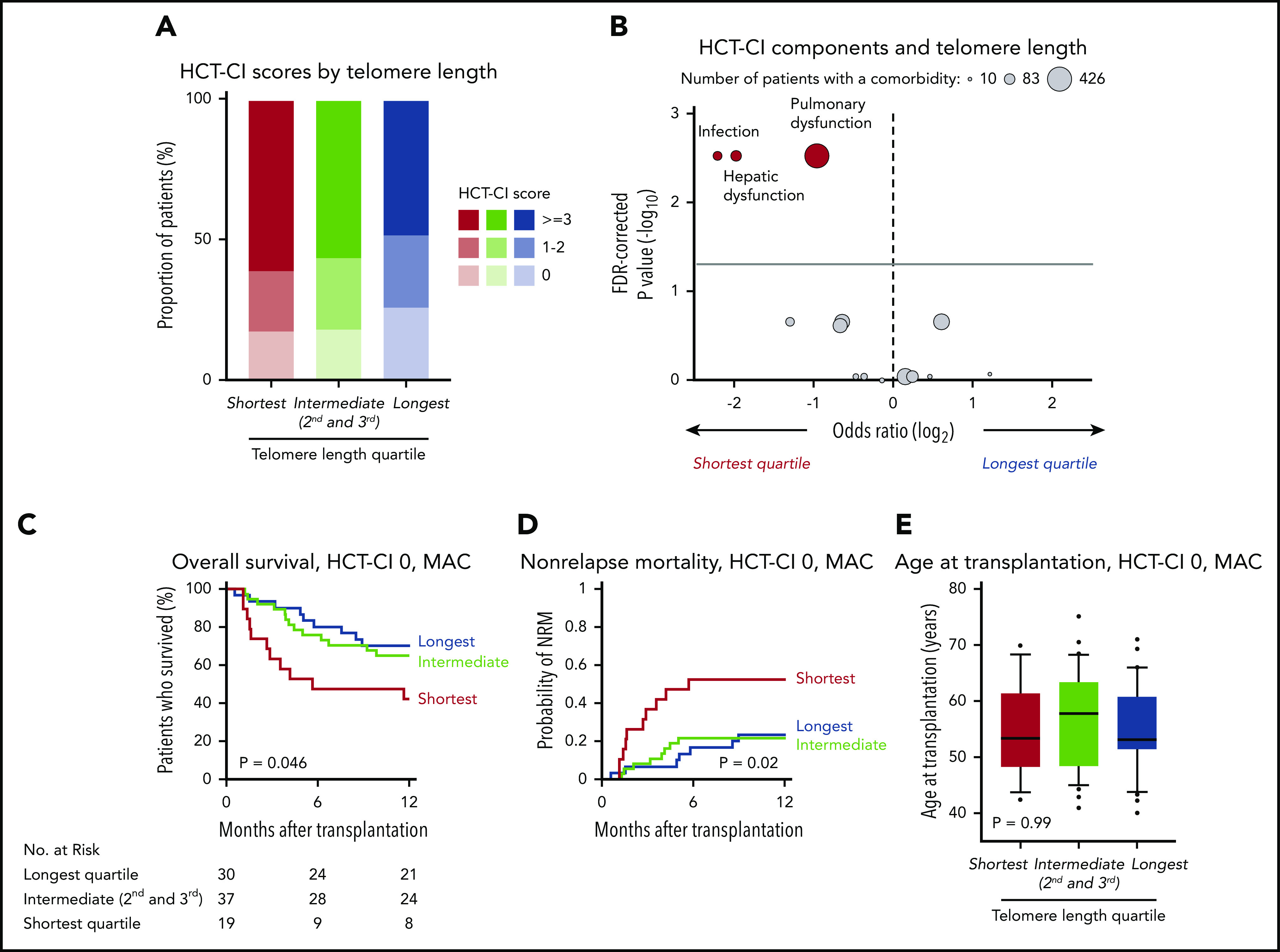
Telomere length and comorbidities. (A) Distribution of HCT-CI scores according to telomere length groups. (B) Association between each HCT-CI component comorbidity and the shortest or the longest telomere length group in patients age 40 years or older. The x-axis depicts the magnitude of association (log2 odds ratio), and the y-axis shows the negative log of the P values corrected for multiple hypothesis testing (-log10 P). The solid line represents the threshold of significance (0.05) for FDR-corrected P values. Each circle represents 1 comorbidity; the size of the circle reflects the number of patients with a comorbidity. (C-E) Overall survival, cumulative incidence of nonrelapse mortality, and age at transplantation, respectively, by telomere length quartiles in patients with HCT-CI score 0 who received myeloablative conditioning.
To determine whether the association between telomere length and NRM was evident in patients without documented comorbidities, we analyzed outcomes in 86 patients with HCT-CI score 0 who received myeloablative conditioning (Figure 5C-D). Shorter telomere length was associated with inferior survival (OS at 1 year, 42% vs 65% vs 70%, P = .046), with OS differences attributable to a marked increase in early NRM (1-year cumulative incidence-NRM, 53% vs 22% vs 23%, P = .02). There were no significant differences in disease characteristics or transplant variables based on telomere length in patients with HCT-SCI score 0 who received MAC (Figure 5E; supplemental Table 8).
Telomere length and acute graft-versus-host disease (GVHD) mortality
GVHD is a major cause of transplant-related mortality. In this MDS cohort, the cumulative incidence of severe (grade 3 or 4) acute GVHD (aGVHD) at day 100 after transplantation was 19%, and among the 265 patients that developed severe aGVHD, survival was poor compared with those who did not develop severe aGVHD (5-year OS, 25% vs 51%). Because severe acute gastrointestinal GVHD has been shown to drive rapid telomere attrition in enterocytes,35 we hypothesized that patients with shorter baseline telomere length that develop severe aGVHD have worse outcomes than those with longer telomeres. Indeed, even though there was no significant difference in the cumulative incidence of severe aGVHD across telomere length groups (Figure 6A), OS after developing severe aGVHD was significantly worse in patients with shorter telomere length than in those with intermediate or longer telomere length (Figure 6B), owing to a high rate of early NRM (Figure 6C). In contrast, there was no significant difference in the cumulative incidence of chronic GVHD or in the rate of NRM after onset of chronic GVHD between telomere length groups (supplemental Figure 6). Detailed description of GVHD prophylaxis is presented in supplemental Table 9.
Figure 6.
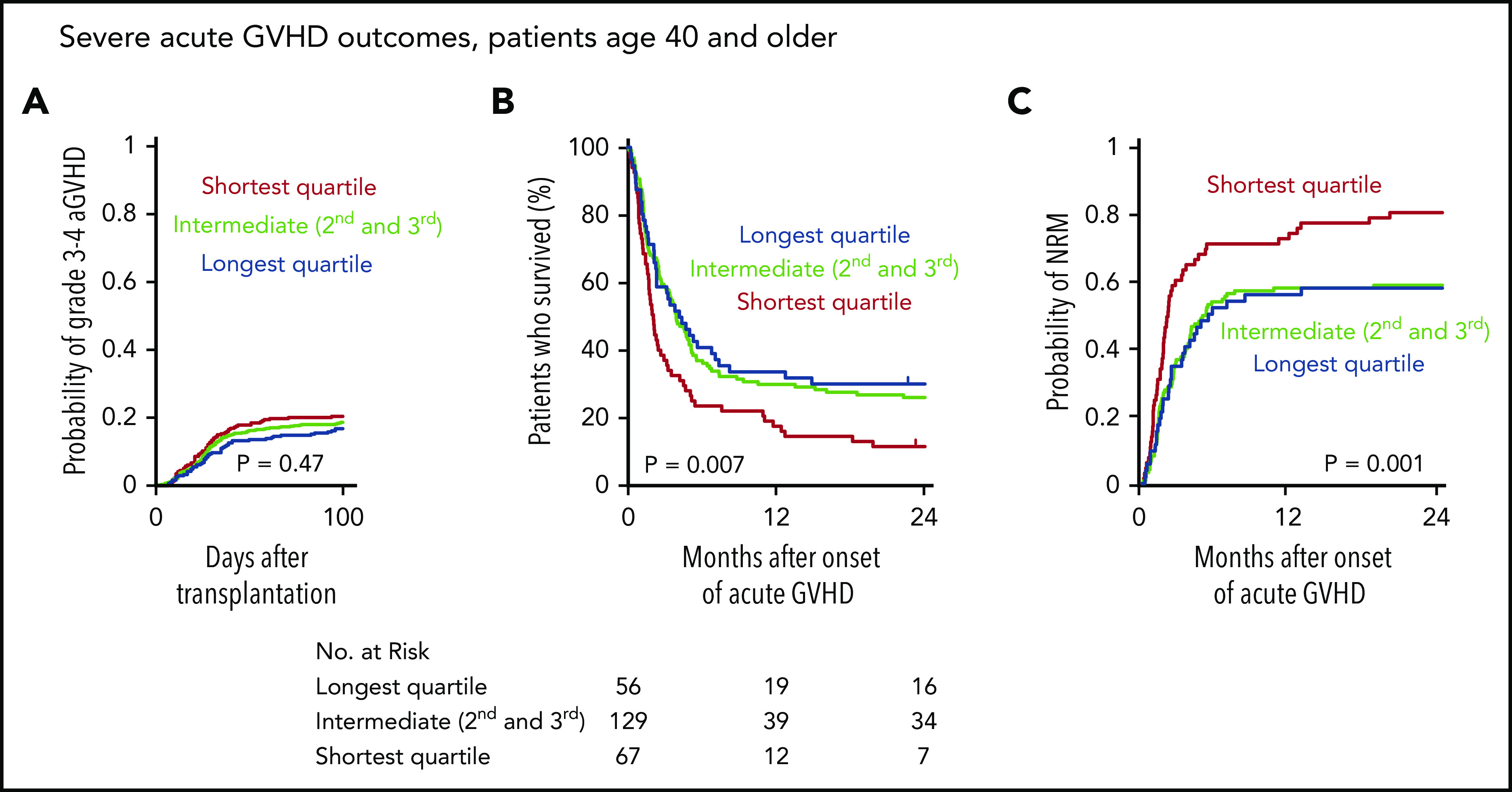
Telomere length and severe acute graft-versus-host disease outcomes. (A) Cumulative incidence of grade 3-4 acute GVHD after transplantation by telomere length quartiles; death without grade 3-4 acute GVHD was used as a competing risk. (B-C) Overall survival and cumulative incidence of nonrelapse mortality, respectively, according to telomere length quartiles after onset of grade 3-4 acute GVHD in patients age 40 or older.
Discussion
Predicting the risk of NRM after transplantation is central to clinical decision making. Current risk models, such as the HCT-CI and the European Group for Blood and Marrow Transplantation score, require integration of clinically ascertained patient comorbidities and capture only a subset of NRM risk. In this study, we paired telomere length analysis with comprehensive clinical annotation in a large, registry-based cohort of MDS patients who received allogeneic stem cell transplantation to study the association of telomere length with outcomes. We found that recipient blood telomere length was a significant predictor of survival and NRM after transplantation among patients age 40 or older with MDS, independent of established clinical factors such as age, somatic mutations, hematologic parameters, MDS treatment history, performance status, comorbidity index, and donor-recipient HLA matching.
Long telomere length was associated with superior OS independent of conditioning intensity. The effect of shorter telomere length on the risk of NRM was observed predominantly in patients receiving myeloablative conditioning regimens. When viewed as a whole, there was no effect of shorter telomere length on NRM risk among patients who received RIC. However, recent registry-level analyses have shown that RIC regimens containing higher dose melphalan may effectively be more similar to myeloablative than other RIC regimens based on their effect on relapse and NRM.19,34,36 Consistent with registry-level reports that show increased NRM risk in patients receiving higher dose melphalan-based RIC regimens, we found that patients with shorter telomeres who received melphalan-based RIC regimens had an elevated risk of early NRM compared with those receiving lower intensity busulfan-based RIC regimens. Overall, these results indicate that shorter telomere length highlights a group of patients in whom higher intensity regimens should be used with caution. If validated in a prospective analysis, measurement of pretransplantation recipient telomere length may provide a rational basis for more precise prediction of NRM risk that could influence conditioning regimen selection.
In this retrospective study, we could not define a direct causal link between short telomere length and specific cause of NRM. However, we found that the risk of death in remission among patients who develop severe acute GVHD was significantly elevated in those with shorter pretransplant telomere length compared with those having longer telomere length. Because telomere length influences tissue regenerative capacity after replicative stress, sites of GVHD related inflammatory injury may be susceptible to restricted telomere length. It has been shown that severe acute intestinal GVHD causes epithelial injury that drives increased cellular turnover and rapid telomere shortening in enterocytes, approaching 200-fold more than at steady state.35 Most patients with severe aGVHD have gastrointestinal involvement37 and gastrointestinal manifestations are most strongly associated with NRM risk in patients with severe aGVHD.38 To the extent that our measurement reflects differences in constitutional telomere length,39 our results suggest that longer baseline telomeres may buffer against replicative exhaustion that limits competence for regeneration in the setting of acute inflammatory injury.
A high HCT-CI score has been associated with poor outcomes after transplantation for MDS, but pretransplant comorbidities not completely explain NRM risk.5,40,41 The association between telomere length and NRM in this study was independent of HCT-CI in our multivariable model and evident in patients with HCT-CI score 0, suggesting that telomere length reflects a biological risk that is distinct from manifest comorbidities. Among HCT-CI component comorbidities, we did find that shorter telomere length was significantly associated with pulmonary and hepatic dysfunction and pretransplant infection history. We note that this mirrors the spectrum of end-organ manifestations of constitutional telomere biology disorders.42-45 This raises the possibility that some patients with short telomeres in the cohort have unrecognized germline determinants of telomere length, or that globally short telomeres could have end-organ consequences even in the absence of a germline risk allele.
Telomere length did not appear to be a proxy for other MDS- or patient-associated variables that influence toxicity outcomes. For example, the history of disease-modifying treatment, time interval between diagnosis and transplantation, and history of cytotoxic treatment of nonmyeloid cancers were not associated with differences in telomere length. These results indicate that telomere length heterogeneity in MDS patients was not primarily related to replication history or cyclic regeneration after myelosuppressive therapy. However, we did observe an association between shorter telomere length and deeper cytopenias. These data are consistent with a mouse model of telomerase deficiency in which short telomeres were associated with impaired granulocyte-macrophage colony-forming unit activity and decreased long-term hematopoietic repopulation capacity of bone marrow cells.46 Telomere length may thus modulate MDS phenotype by incurring reduced hematopoietic competency, leading to more severe cytopenias.
Distinct sets of MDS-associated somatic gene mutations were significantly associated with longer or shorter telomere length, further suggesting a possible link between telomere biology and MDS pathogenesis. Critically short telomeres trigger cellular senescence via activation of the DNA damage response (DDR) pathway. Mutations in genes that are required for sensing DNA damage (ATM) or enacting the DDR (PPM1D, TP53) could therefore enable selective survival of MDS clones by impairing senescence or apoptosis induced by telomere attrition. In this setting, constitutional or acquired variation in hematopoietic telomere length could alter the baseline fitness of the HSC compartment and favor expansion of DDR-deficient clones. In contrast, loss of de novo or maintenance DNMT activity via concurrent deletion of Dnmt3a and Dnmt3b or Dnmt1, respectively, has been shown to cause telomere elongation in mouse embryonic stem cells.47 In this model, loss of DNMT activity was associated with markedly reduced methylation of subtelomeric DNA, suggesting that DNMT3A mutations may cause clone-intrinsic acquisition of an aberrant telomere lengthening phenotype. Definitive evaluation of the interaction between MDS gene mutations and inherited or acquired differences in telomere length will require purification of MDS clonal cells and comparison with constitutional baseline samples.
Translation of our results to clinical practice will be enhanced by prospective validation using flow-fluorescent in-situ hybridization (flow-FISH), which affords specific benefits over qPCR but is infeasible for studying large retrospective cohorts. Specifically, flow-FISH enables comparison with age-matched population controls,48,49 measurement of telomere length in different leukocyte subsets, and more accurate distinction of differences in the shortest and longest decile of telomere lengths.50 We directly addressed potential technical variability of qPCR by processing and analyzing all samples in a uniform manner, and the potential vulnerability of qPCR to aneuploidy by using a reference gene located on chromosome 12q, which is not subject to recurrent copy number alteration in MDS.51
In summary, our findings show that telomere length measured in MDS patients at the time of transplantation is a strong predictor of NRM independent of age, disease characteristics, and clinical comorbidities. Patients with shorter telomere length, who have inferior survival driven by excess toxicity without an effect on relapse, could be considered for strategies focused on minimizing toxic effects of transplantation.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by the Aplastic Anemia & MDS International Foundation (R.C.L.), National Institutes of Health, National Cancer Institute grants K08CA204734 (R.C.L.) and 5P30 CA006516 (R.R. and D.N.), the Jock and Bunny Adams Research and Education Endowment (J.H.A.), the Jim and Lois Champy Fund (R.C.L. and D.P.S.), the Anna Fuller Fund (R.C.L), the Sigrid Juselius Foundation (M.M.), the Maud Kuistila Memorial Foundation (M.M.), the Väre Foundation for Pediatric Cancer Research (M.M.), and the Orion Research Foundation (M.M.). Dana-Farber/Harvard Cancer Center (DF/HCC) Genotyping and Genetics for Populations Sciences is partly supported by the National Institutes of Health, National Cancer Institute grant 5P30CA006516-54 (M.M.C., E.H.O., and I.D.). The Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases (W.S., T.W., S.R.S., and Z.-H.H.); grant/cooperative agreement 4U10HL069294 from the National Institutes of Health, National Heart, Lung and Blood Institute and National Cancer Institute; Health Resources and Services Administration contract HHSH250201200016C; and grants N00014-18-1-2850 and N0014-19-1-2888 from the Office of Naval Research. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration or any other agency of the US Government.
Footnotes
Presented in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 3 December 2018.
Deidentified individual participant data are available indefinitely. Proposals for access should be sent to inforequest@mcw.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.M., S.A., and R.C.L. designed the study, analyzed data, and wrote the manuscript; J.G.G., M.M.C., E.H.O., and I.D.V. measured telomere lengths; C.R.R. analyzed data and contributed to the research discussion; D.N., T.W., and R.R. curated clinical data, conceived the statistical plan, and performed statistical analysis; W.S., S.R.S., and Z.-H.H. curated clinical data, clinical data collection and quality assurance, and contributed to research discussion; J.H.A., C.C., C.J.G., and D.P.S. interpreted data and contributed to research discussion; and all authors reviewed the manuscript during its preparation and approved the submission.
Conflict-of-interest disclosure: R.C.L. has received research funding from Jazz Pharmaceuticals and MedImmune and consulting fees from Takeda Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: R. Coleman Lindsley, Dana-Farber Cancer Institute, 450 Brookline Ave – DA-530C, Boston, MA 02215; e-mail: coleman_lindsley@dfci.harvard.edu.
REFERENCES
- 1.de Witte T, Bowen D, Robin M, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129(13):1753-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bejar R, Stevenson KE, Caughey B, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32(25):2691-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Della Porta MG, Gallì A, Bacigalupo A, et al. Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2016;34(30):3627-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizato T, Nannya Y, Atsuta Y, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood. 2017;129(17):2347-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405-411. [DOI] [PubMed] [Google Scholar]
- 8.Gagelmann N, Eikema D-J, Stelljes M, et al. Optimized EBMT transplant-specific risk score in myelodysplastic syndromes after allogeneic stem-cell transplantation. Haematologica. 2019;104(5):929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fioredda F, Iacobelli S, Korthof ET, et al. Outcome of haematopoietic stem cell transplantation in dyskeratosis congenita. Br J Haematol. 2018;183(1):110-118. [DOI] [PubMed] [Google Scholar]
- 10.Barbaro P, Vedi A. Survival after hematopoietic stem cell transplant in patients with dyskeratosis congenita: systematic review of the literature. Biol Blood Marrow Transplant. 2016;22(7):1152-1158. [DOI] [PubMed] [Google Scholar]
- 11.Gadalla SM, Sales-Bonfim C, Carreras J, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with dyskeratosis congenita. Biol Blood Marrow Transplant. 2013;19(8):1238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg MB, Lincz LF, Adler K, Scorgie FE, Ackland SP, Sakoff JA. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. Br J Cancer. 2012;107(9):1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, McReynolds LJ, Dagnall C, et al. Pre-transplant short telomeres are associated with high mortality risk after unrelated donor haematopoietic cell transplant for severe aplastic anaemia. Br J Haematol. 2020;188(2):309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peffault de Latour R, Calado RT, Busson M, et al. Age-adjusted recipient pretransplantation telomere length and treatment-related mortality after hematopoietic stem cell transplantation. Blood. 2012;120(16):3353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boultwood J, Fidler C, Kusec R, et al. Telomere length in myelodysplastic syndromes. Am J Hematol. 1997;56(4):266-271. [DOI] [PubMed] [Google Scholar]
- 16.Rollison DE, Epling-Burnette PK, Park JY, et al. Telomere length in myelodysplastic syndromes. Leuk Lymphoma. 2011;52(8):1528-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rigolin GM, Porta MD, Bugli AM, et al. Flow cytometric detection of accelerated telomere shortening in myelodysplastic syndromes: correlations with aetiological and clinical-biological findings. Eur J Haematol. 2004;73(5):351-358. [DOI] [PubMed] [Google Scholar]
- 18.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eapen M, Brazauskas R, Hemmer M, et al. Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic syndrome: conditioning regimen intensity. Blood Adv. 2018;2(16):2095-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benitez-Buelga C, Sanchez-Barroso L, Gallardo M, et al. Impact of chemotherapy on telomere length in sporadic and familial breast cancer patients. Breast Cancer Res Treat. 2015;149(2):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelhardt M, Ozkaynak MF, Drullinsky P, et al. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia. 1998;12(1):13-24. [DOI] [PubMed] [Google Scholar]
- 22.Schröder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;84(10):1348-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szyper-Kravitz M, Uziel O, Shapiro H, et al. Granulocyte colony-stimulating factor administration upregulates telomerase activity in CD34+ haematopoietic cells and may prevent telomere attrition after chemotherapy. Br J Haematol. 2003;120(2):329-336. [DOI] [PubMed] [Google Scholar]
- 24.Lee J-J, Nam C-E, Cho S-H, Park KS, Chung IJ, Kim HJ. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann Hematol. 2003;82(8):492-495. [DOI] [PubMed] [Google Scholar]
- 25.Unryn BM, Hao D, Glück S, Riabowol KT. Acceleration of telomere loss by chemotherapy is greater in older patients with locally advanced head and neck cancer. Clin Cancer Res. 2006;12(21):6345-6350. [DOI] [PubMed] [Google Scholar]
- 26.Yoon SY, Sung HJ, Park KH, et al. Telomere length shortening of peripheral blood mononuclear cells in solid-cancer patients undergoing standard-dose chemotherapy might be correlated with good treatment response and neutropenia severity. Acta Haematol. 2007;118(1):30-37. [DOI] [PubMed] [Google Scholar]
- 27.Diker-Cohen T, Uziel O, Szyper-Kravitz M, Shapira H, Natur A, Lahav M. The effect of chemotherapy on telomere dynamics: clinical results and possible mechanisms. Leuk Lymphoma. 2013;54(9):2023-2029. [DOI] [PubMed] [Google Scholar]
- 28.Papaemmanuil E, Gerstung M, Malcovati L, et al. ; Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium . Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616-3627, quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28(2):241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaffer BC, Ahn KW, Hu Z-H, et al. Scoring system prognostic of outcome in patients undergoing allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. J Clin Oncol. 2016;34(16):1864-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kröger N, Iacobelli S, Franke G-N, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35(19):2157-2164. [DOI] [PubMed] [Google Scholar]
- 34.Jain T, Alahdab F, Firwana B, Sonbol MB, Almader-Douglas D, Palmer J. Choosing a reduced-intensity conditioning regimen for allogeneic stem cell transplantation, fludarabine/busulfan versus fludarabine melphalan: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2019;25(4):728-733. [DOI] [PubMed] [Google Scholar]
- 35.Hummel S, Ventura Ferreira MS, Heudobler D, et al. Telomere shortening in enterocytes of patients with uncontrolled acute intestinal graft-versus-host disease. Blood. 2015;126(22):2518-2521. [DOI] [PubMed] [Google Scholar]
- 36.Kekre N, Marquez-Malaver FJ, Cabrero M, et al. Fludarabine/busulfan versus fludarabine/melphalan conditioning in patients undergoing reduced-intensity conditioning hematopoietic stem cell transplantation for lymphoma. Biol Blood Marrow Transplant. 2016;22(10):1808-1815. [DOI] [PubMed] [Google Scholar]
- 37.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naymagon S, Naymagon L, Wong S-Y, et al. Acute graft-versus-host disease of the gut: considerations for the gastroenterologist. Nat Rev Gastroenterol Hepatol. 2017;14(12):711-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults [published correction appears in Nat Commun. 2013;4:1976]. Nat Commun. 2013;4(1):1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Della Porta MG, Alessandrino EP, Bacigalupo A, et al. ; Gruppo Italiano Trapianto di Midollo Osseo . Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood. 2014;123(15):2333-2342. [DOI] [PubMed] [Google Scholar]
- 41.Carré M, Porcher R, Finke J, et al. Role of age and hematopoietic cell transplantation-specific comorbidity index in myelodysplastic patients undergoing an allotransplant: a retrospective study from the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2020;26(3):451-457. [DOI] [PubMed] [Google Scholar]
- 42.Armanios MY, Chen JJ-L, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317-1326. [DOI] [PubMed] [Google Scholar]
- 43.Kapuria D, Ben-Yakov G, Ortolano R, et al. The spectrum of hepatic involvement in patients with telomere disease. Hepatology. 2019;69(6):2579-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner CL, Hanumanthu VS, Talbot CCJ Jr., et al. Short telomere syndromes cause a primary T cell immunodeficiency. J Clin Invest. 2018;128(12):5222-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12(12):753-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samper E, Fernández P, Eguía R, et al. Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood. 2002;99(8):2767-2775. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalo S, Jaco I, Fraga MF, et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol. 2006;8(4):416-424. [DOI] [PubMed] [Google Scholar]
- 48.Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97(3):353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110(5):1439-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Savage SA, Alsaggaf R, et al. Telomere length calibration from qPCR measurement: limitations of current method. Cells. 2018;7(11):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012;730(1-2):59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.