Abstract
β-glucans are complex polysaccharides that are found in several plants and foods, including mushrooms. β-glucans display an array of potentially therapeutic properties. β-glucans have metabolic and gastro-intestinal effects, modulating the gut microbiome, altering lipid and glucose metabolism, reducing cholesterol, leading to their investigation as potential therapies for metabolic syndrome, obesity and diet regulation, gastrointestinal conditions such as irritable bowel, and to reduce cardiovascular and diabetes risk. β-glucans also have immune-modulating effects, leading to their investigation as adjuvant agents for cancers (solid and haematological malignancies), for immune-mediated conditions (e.g., allergic rhinitis, respiratory infections), and to enhance wound healing. The therapeutic potential of β-glucans is evidenced by the fact that two glucan isolates were licensed as drugs in Japan as immune-adjuvant therapy for cancer in 1980. Significant challenges exist to further clinical testing and translation of β-glucans. The diverse range of conditions for which β-glucans are in clinical testing underlines the incomplete understanding of the diverse mechanisms of action of β-glucans, a key knowledge gap. Furthermore, important differences appear to exist in the effects of apparently similar β-glucan preparations, which may be due to differences in sources and extraction procedures, another poorly understood issue. This review will describe the biology, potential mechanisms of action and key therapeutic targets being investigated in clinical trials of β-glucans and identify and discuss the key challenges to successful translation of this intriguing potential therapeutic.
Keywords: β-glucan, clinical trials, biomedicine, immunomodulation, metabolism
1. Introduction
The therapeutic potential of foods containing the polysaccharide beta-glucan (β-glucans) has long been known. Specifically, the medicinal properties of mushrooms—a major source β-glucans—were detailed in manuscripts from India dating back 500 years [1]. A popular mushroom known as Agaricus blazeii is native to a small area of the mountains of Brazil near Sao Paulo. More recently, apparent lower incidences of cancers, viral and bacterial-induced illnesses, and increased life spans seen in people living in a small area of the mountains of Brazil near Sao Paulo were attributed by some to the ingestion of this popular local mushroom known as Agaricus blazeii [1]. There are at least 700 species of mushrooms like A. blazeii that are considered to possess medicinal properties [2,3].
β-glucans are a key active ingredient in mushrooms and are also found in oats, barley, yeast, bacteria and algae. In microbial sources, they are a structural component and in grain sources, they are found in the endospermic and aleuronic walls [4,5,6]. Today, β-glucans are widely marketed as biologically active compounds that have the potential to improve health [7]. Of therapeutic importance, β-glucans have potentially important metabolic and gastro-intestinal effects, modulating the gut microbiome, altering lipid and glucose metabolism, reducing cholesterol, leading to their investigation as potential therapies for metabolic syndrome, obesity and diet regulation, gastrointestinal conditions such as irritable bowel, and to reduce cardiovascular and diabetes risk. β-glucans also appear to have immune-modulating effects, leading to their investigation as adjuvant agents for solid cancers and haematological malignancies, for immune-mediated conditions, such as allergic rhinitis and respiratory tract infections, and to enhance wound healing. Two glucan isolates were licensed as drugs in Japan as an immune-adjuvant therapy for cancer in 1980 [8,9].
Consequently, β-glucans are also being tested for clinical efficacy in clinical trials for a variety of conditions, including inflammatory conditions, cardiometabolic diseases, obesity and cancer. In fact, over 200 clinical trials of β-glucans are completed or in progress, suggesting their diverse biological properties might have the significant therapeutic potential [10]. The US Food and Drug Administration passed a ruling in 1997, stating that oat bran—a major source of β-glucan—was to be registered as the first cholesterol-reducing food, and recommended a dose of 3 g/day of β-glucan, this can be provided by ≤40 g oat bran or ≤60 g oatmeal [11]. However, notwithstanding this ruling, significant challenges exist to the successful clinical testing and translation of β-glucans as a therapy. Our incomplete understanding of the diverse mechanisms of action of β-glucans constitutes a key knowledge gap. A second challenge relates to the important differences that appear to exist in the effects of apparently similar β-glucan preparations, which may be due to differences in sources and extraction procedures, another poorly understood issue. For the most part, β-glucans are marketed as “natural” food (as opposed to medicinal) products, frequently comprising a complex mixture of polysaccharides and even contaminants that can potentially contribute to varied effects and outcomes. [12].
This review will describe the biology, mechanisms of action and key therapeutic targets being investigated in clinical trials of β-glucans. We will also identify and discuss the key challenges to the successful translation of this promising potential therapeutic.
2. Discovery and Characterization of β-glucans
In their seminal work on the discovery of Properdin in the complement system, Pillemer and colleagues used a compound called Zymosan [13]. Zymosan is a crude mixture of yeast cell wall materials and was used as an immune stimulator [13]. Zymosan contains polysaccharide proteins, fats, and inorganic elements [14]. Zymosan was also used for a range of applications including the promotion of survival after ionizing radiation, increasing resistance to several biological infections, inhibition of dietary-induced hypercholesterolemia and inhibition of tumour development [14,15,16,17,18].
Zymosan had an abundance of adverse side effects, including pyrexia, anaemia, pulmonary hyperplasia, and microemboli, and so in 1961 Riggi and Diluzio identified the component of Zymosan responsible for reticuloendothelial stimulation (RES) [19]. Their work determined that this stimulatory agent was a polysaccharide from the cell wall of yeast or Saccharomyces cerevisiae. This finding was contradictory to previous reports that suggested a lipid mixture in Zymosan was the active ingredient, [15,20]. They concluded that there was a 1,3 β-type linkage that was uniquely characteristic to this polysaccharide or glucan. This linkage is required for the stimulation of cells or RES. This study initiated the era of glucan research [19].
In 1969, Chihara et al. isolated 1,3 1,6 β-glucans form the mushroom Lentinus edodes. They demonstrated its ability to inhibit sarcoma in allogenic mice [20]. Since then, β-glucans has been isolated from an abundance of sources, most recently a 1,3 1,6 extract has been isolated from bananas [21]. Two glucan isolates are now licensed drugs in Japan as an immune-adjuvant therapy for cancer since 1980 [8,9].
3. β-glucan Structure
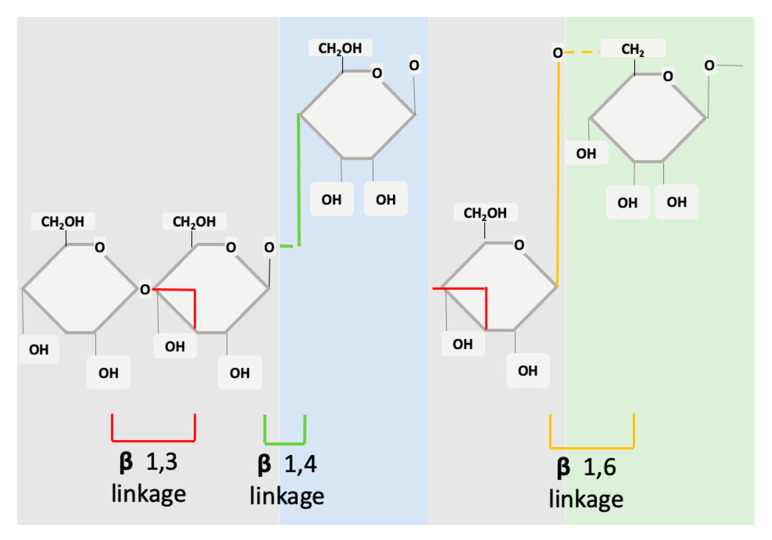
Structurally, β-glucans are comprised of glucose units linked together by several different types of beta-glycosidic linkages (Figure 1). In the basic form, the molecule is a polymer of monosaccharide residues [0]. β-glucans are composed of β-D-glucose monomer units, which are held together by glycosidic linkages at differing positions (1,3), (1,4) or (1,6). This structure can be either branched or unbranched [22]. The β-glucans source will determine if the molecule has branched structures and to what extent. The monosaccharide units interconnect at several points to form a wide variety of different branched and linear structures [23].
Figure 1.
Structure of cereal β-glucans (1,3 1,4) and non-cereal β-glucans (1,3 1,6).
The fine structure of β-glucans can vary in meaningful ways that modify its effects and mechanisms of action. A variance will occur between glycosidic linkages, molecular weight, branching, degree of polymerization, and solubility [0,23] β-glucans from different sources will have different effects or functions [24]. Borchani et al. provide a more detailed review of the structural chemistry of β-glucans [25].
When compared to other biopolymers (e.g., proteins), β-glucans have a greater structural variation and therefore have a higher capacity for biological information. This variation and flexibility underlie the potential for β-glucans to influence complicated and diverse cell pathways, activities, and signalling processes [0].
4. Sources of β-glucans
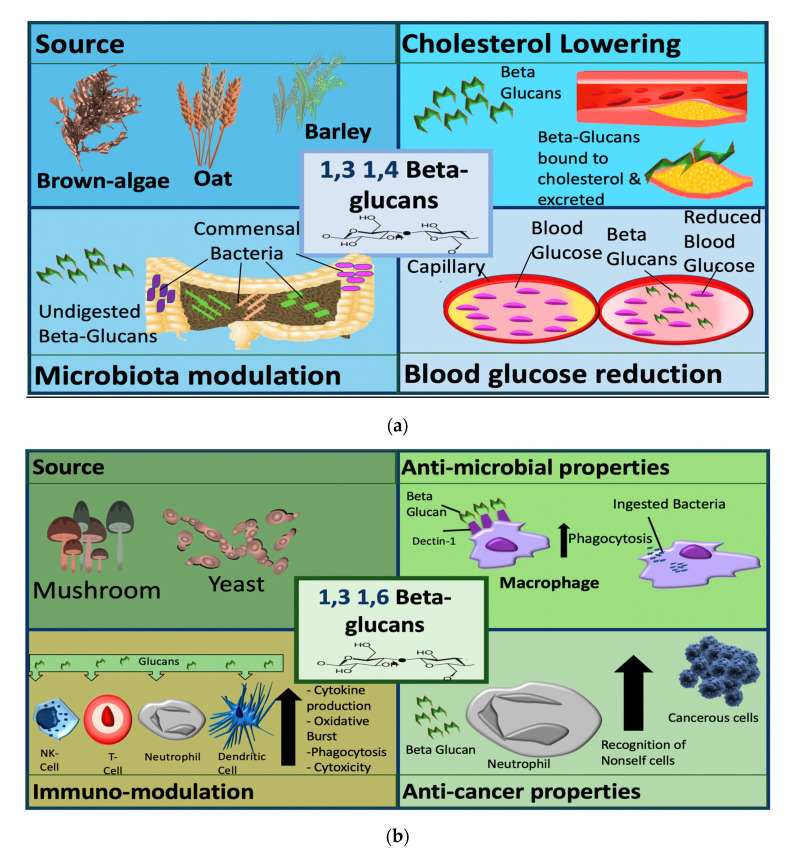
β-glucans can be divided into two sub-groups, namely cereal and non-cereal (Figure 2). Cereal or grain derived β-glucans usually have 1,3 1,4 glycosidic linkages without any 1,6 bonds or branching [26,27,28]. They are fibrous structures found in aleurone (proteins stored as granules in the cells of plant seeds), in the sub-aleurone layer and the cell wall of endospores [29]. Cereals include oat, barley, wheat and rice [29].
Figure 2.
Sources and mechanisms of β-glucans dependent on structure. In the panel (a) cereal β-glucans; in the panel (b) fungal β-glucans.
Regardless of source cereal β-glucans share similar structures, some differences include variation in 1,3 1,4 linkage ratio, molecular size, and some have large cellulose structures [30,31]. β-glucan content also varies among cereal sources—there is higher glucan content in barley then oats, the least is found in rice and wheat [32].
Non-cereal β-glucans are fibrous structures found in yeast, fungi, bacteria, and algae [33]. β-glucans originating from yeast have linear (1,3) backbones with long chains of 1,6 branching [27,34,35]. Unlike grain β-glucans, fungal β-glucans differ between species concerning the degree of branching and distribution [0]. Curdlan, a glucan isolated from Agrobacterium, contains no side branching, just a β-D glucan backbone [36]. The solubility of the molecule is reliant on 1,3 linkages. β-glucans are classified as soluble dietary fibre as the beta configuration is not digestible by enzymes in the human gastrointestinal (GI)-tract [37]. They are further classified pharmacologically as biological response modifiers (BRMs) as they influence the immune system counterparts [38]. For cereal-based β-glucan at least, higher molecular weight β-glucans appear to be more effective than lower weight molecules [39]. With this level of variance, it is not surprising that there is a range of diverse applications of β-glucans in clinical trials ranging from the alleviation of respiratory illnesses to improving fatigue and weight loss.
5. Mechanisms of Action
The mechanism of action of β-glucan can be broadly divided into two major areas, namely metabolic/GI effects and immune-modulatory effects. The molecular and structural characteristics will determine functional effects [40,41,42]. β-glucans display diverse mechanisms of action and have a defined structure-activity relationship [43].
5.1. Role of β-glucans Structure
A distinguishing characteristic of all β-glucans that is necessary for biological activity is its 1,3 backbone [44,45]. The degree and the specific profile of biological activity appear to be related to specific β-glucans structural characteristics. First, side-chain frequency and length are important, with a higher degree of branching associated with greater biological activity [46]. Structures with a branching frequency of 0.20 and 0.33 appear to be the most biologically active [45,47]. While it remains unclear precisely how differences in structure modulate the activity of β-glucans [48], sidechains length and frequency play a crucial role in the immunomodulatory activity [48,49]. The overall size of the β-glucans molecule is also important, with higher molecular weight fractions having a greater effect [48,50].
5.2. Role of β-glucan Source
β-glucans are classified by their source, into cereal and non-cereal β-glucans (Figure 2). Cereal β-glucans, which are 1,3 and 1,4 linked, mainly display metabolic activities, such as the ability to lower cholesterol and blood glucose and have been explored in clinical studies to target metabolic conditions. These 1,3 and 1,4 linked glucans appear to be recognized as dietary fibres after ingestion and elicit their metabolic effects via this mechanism. Non-cereal (predominantly fungal and yeast) β-glucans have more pronounced immunomodulatory functions and are the focus of immunomodulation and anti-cancer studies. Fungal and yeast β-glucans have a 1,3 and 1,6 linkage structure and are recognized by some receptors including dectin 1, complement receptor 3 (CR3) and toll-like receptors (TLRs) [51,52,53,54].
The pathways activated by β-glucans are not fully understood, but β-glucans appear to be recognized as pathogen-associated molecular patterns (PAMPs) and they modulate immune cell function via this mechanism. The different mechanisms of β-glucans dependent on the structure are categorized by source in Figure 2.
β-glucans from the same source can also differ in activity profile. In work carried out by the authors, we demonstrated that two β-glucan extracts from the same mushroom could have different effect profiles in an in-vitro lung injury model [55].
5.3. Cholesterol-Lowering Effects
Coronary vascular disease is directly correlated with metabolic syndrome [56]. Metabolic syndrome is characterized by a cluster of metabolic dysfunctions—including abdominal obesity, atherogenic dyslipidaemia, small LDL particles and low HDL cholesterol levels, elevated blood pressure, insulin resistance and glucose intolerance [57] (Table 1). Cholesterol plays a key role in the pathogenesis of plaque formation. While cholesterol at normal levels is beneficial to the body, higher levels leads to the accumulation of cholesterol deposits in the blood vessel walls—termed atherosclerotic plaques—which lead to blockage of arteries supplying key organs including the heart [58]. The enzyme 3-hydroxy-3-methyl glutaryl-co-enzyme A (HMG-CoA) reductase acts as a catalyst in the production of cholesterol.
Table 1.
Cereal glucans in metabolic/GI disorders.
| Areas of Research | Study Title | NCT | Design | β-glucan Type | Dose | Population |
|---|---|---|---|---|---|---|
| Microbiota and gastrointestinal health | Characterization of the Gut Microbiota Composition and Activity After Three Weeks of Chitin-glucan Supplementation | NCT03505177 | n/a | Chitin-glucan | 4.5 g/day | Healthy |
| Characterization of Chitin-glucan Fibre Fermentation in Human After a Single Administration | NCT03494491 | n/a | Chitin-glucan | 4.5 g/day | Healthy | |
| Effect of 6 Weeks Daily Consumption of a Cereal-based Juice Beverage on Gastrointestinal Health (NEWDRINK) | NCT03046667 | n/a | Barley | Drink = 1 dose per day—dose not stated | Irritable Bowel | |
| Characterization of Gut Microbiota Composition and Activity After a Daily Supplementation of 4.5 g/Day of Chitin Glucan Fibre during 3 Weeks in At-cardiometabolic Risk Volunteers (FITACHITIN) | NCT03773900 | n/a | Chitin | 1.5 g/3 times daily | Cardiometabolic Risk Abdominal Obesity | |
| β-glucan on Faecal Microflora in Polypectomised Patients | NCT00893659 | n/a | Unknown | 3 g/day | Polypectomised Patients | |
| Synbiotics and Gastrointestinal Function Related Quality of Life After Colectomy for Cancer | NCT01479907 | n/a | Unknown | 2.5 g/sachet | Colorectal Neoplasms | |
| The Effect of Oats Containing 1.4g β Glucan on Faecal Bacterial Population(s) and Plasma Cholesterol in Healthy Adults with Elevated Cholesterol Levels | NCT03450395 | n/a | Oats | 40 g of crude oats/day | Microbiome Plasma Cholesterol Prebiotic | |
| Beta-glucan Effects on Lipid Profile, Glycemia and intestinal Health (BELT) (BELT) | NCT03313713 | n/a | Unknown | 3 g/day | Atherosclerosis | |
| Healthy Effects of an Innovative Probiotic Pasta (SFLABPASTA) | NCT02236533 | n/a | Barley | Pasta once a day | Obesity, Inflammation, Dyslipidaemia, Constipation | |
| The Effectiveness of Pleuran in Treatment of Acute Gastroenteritis in Children (EPTAGE) | NCT03988257 | Phase 2 | Mushroom | 10 mg Pleuran | Diarrhoea; Acute | |
| Impact of Consumption of Beta-glucans on the Intestinal Microbiota and Glucose and Lipid Metabolism | NCT02041104 | n/a | Barley | 6 g/day | Metabolic Syndrome, Dyslipidaemia, Obesity, Abdominal, Hyperglycaemia, Hypertension | |
| Combined Nutritional Therapies for the Treatment of Ulcerative Colitis | NCT03444311 | n/a | Oat | Unknown | Colitis, Ulcerative | |
| Prebiotic Supplementation and Intestinal Barrier Function in Elderly: an RCT | NCT03336385 | n/a | Oat | Daily—dose not known | Prebiotics, Aged | |
| Chronic Cardiovascular and Gut-bacteria Effects of Phenolic Rich Oats in Adults with Above Average Blood Pressure | NCT02847312 | n/a | Oat | 60 g of Oat cake | Healthy | |
| The Effect of Hot Cereal on Digestive Health in Children | NCT02868515 | n/a | Oat | 3 g/day | Subjective Measures of Digestive, Health Post Consumption | |
| Dietary Fibres Effect on the Gut Microbiota Composition | NCT04114513 | n/a | Unknown Tate & Lyle powder | 2 increasing to 8 g per day | Microbiome, Metabolic Syndrome, Cardiovascular Risk Factor, Inflammation, Dyslipidaemias | |
| β -1,3/1,6-D-Glucan Ganoderma Lucidum on Ulcerative Colitis | NCT04029649 | Phase 2, Phase 3 | Fungal | Capsule containing 180 mg/three times daily | Ulcerative Colitis | |
| Glycemic control; Diabetes | β glucan and Acetate Production | NCT03714646 | n/a | Unknown | 12 g once | Pre-Diabetes, Obesity |
| Inulin and Acetate Production and Human Substrate Metabolism | NCT03711383 | n/a | Unknown | Unknown | Obesity, Pre-Diabetes | |
| Efficacy and Safety Study of Soluble Beta-1,3/1,6-glucan (SBG) Versus Placebo in Chronic Diabetic Foot Ulcers | NCT00804414 | Phase 3 | SBG—Yeast | Topical Application | Diabetes, Diabetic Ulcer | |
| The Glycaemic Response Elicited by β-glucans of Different Physical Properties and Form | NCT01610518 | n/a | Oat | 4 g | Type 2 Diabetes | |
| Effect of Serving Size and Addition of Sugar on the Glycaemic Response Elicited by Oatmeal (Panther) | NCT02506972 | n/a | Oat | 30 g of oats | Diabetes Mellitus | |
| Effect of Viscous Soluble Fibres on Body Weight | NCT03257449 | n/a | Oat, Barley | Unknown | Overweight and Obesity, T2DM (Type 2 Diabetes Mellitus), General Population | |
| Impact of DHA/Oat on Metabolic Health in Gestational Diabetes Mellitus | NCT03569501 | n/a | Oat | 4.05 mg/day | Gestational Diabetes Mellitus in Pregnancy | |
| Efficacy of Soluble β-1,3/1,6-Glucan Compared to Placebo on Chronic Leg Ulcers in Diabetes Patients | NCT00288392 | Phase 2 | SBG—Yeast | Unknown | Foot Ulcer | |
| Food Modification to Alter Glycaemia and Insulinaemia | NCT03706378 | n/a | Yellow Noodle—wheat | 50 g of β-glucan in 230.4 g of yellow noodle per day | Diabetes | |
| The Glycaemic Response of Local Foods Using the Continuous Glucose Monitoring System | NCT03703544 | n/a | Yellow Noodle—wheat | Unknown | Diabetes | |
| Oat β-glucan as a Supplement in Chilean Type 2 Diabetics | NCT04299763 | Phase 2 | Oat | 5 g daily with breakfast | Type2 Diabetes | |
| The Effect of Insoluble Yeast β-glucan Intake on Pre-diabetic Patients | NCT03495362 | n/a | Yeast | 500 mg capsule twice a day | Pre-diabetic | |
| Effects of (1,3), (1,6)- β-D-glucan on Insulin Sensitivity and Inflammatory Markers of the Metabolic Syndrome | NCT00403689 | n/a | Yeast | 1.5 g/daily | Overweight | |
| Intake of Beta-glucan and Postprandial Regulation of Blood Glucose Metabolism in Healthy Subjects | NCT03293693 | n/a | unknown | 0.5 g–8 g | Post Prandial Blood Glucose, Gut Microbiota, Satiety | |
| The Effect of Content of Barley Beta-glucans in Bread on Postprandial Blood Sugar (ARRS-bGL-01) | NCT03878576 | n/a | Barley | 25 g | Glycaemic Index | |
| Evaluation of Woulgan in Diabetic Foot Ulcer | NCT02631512 | Phase 4 | Woulgan-contains SBG—yeast | Gel Application | Diabetic Foot Ulcers | |
| A Study of the Effect of Oats on Post Prandial Glucose Response | NCT02651597 | n/a | Oat | Unknown | Normoglycemic, Normal Body Weight | |
| Effects of Barley on Glucose Control | NCT02367989 | n/a | Barley | 2–6 g/day | Healthy | |
| Barley and Rice Mixture Effects on Blood Glucose | NCT03387345 | n/a | Barley | Unknown | Blood Glucose, Dietary Fibre, Hunger | |
| Lipid Regulation | Effect of the Molecular Weight of Oat β-glucan on Its Ability to Lower Serum Cholesterol (Bluebird) | NCT00981981 | Phase 2 | Oat | 3–4 g/day | Hypercholesterolemia |
| Effect of Beta-glucan on Cholesterol Lowering | NCT01408719 | n/a | Barley | 3–5 g β glucan | Hypercholesterolemia | |
| The Effect of Viscous Dietary Fibres on LDL-cholesterol | NCT04133805 | n/a | Barley, Oat | Unknown | Cardiovascular Risk Factor, Hypercholesterolemia | |
| Oat and Cholesterol | NCT03911427 | n/a | Oat | Powdered sachets three times daily | Elevated LDL Cholesterol | |
| Impact of Consumption of Oats in Lipid Profile of Children and Adolescents with Dyslipidaemia | NCT01581697 | Phase 1, Phase 2 | Oat bran | 3 g with 3 meals a day | Atherosclerosis, Hypercholesterolemia | |
| Nutritional Counselling Associated with the Ingestion of Oat Bran in Hypercholesterolemic Subjects | NCT02189200 | n/a | Oat | 40 g oat bran per day | Dietary Modification | |
| Effects of Lentinula Edodes Bars on Dyslipidaemia and Oxidative Stress in Cholesterol Individuals: Randomized Study | NCT04186780 | n/a | Fungal | 2 cereal bars of Shiitake per day | Dyslipidaemias | |
| Effects of Chitin-glucan on Oxidized Low-Density Lipoprotein (LDL) | NCT01232309 | n/a | Chitin | 1.5 g–4.5 g of glucan | Cardiovascular | |
| Obesity and diet regulation | Effects of Oligofructose and Barley on Satiety and Energy Intake | NCT00776256 | n/a | Oats, Barley | 1 g/serving | Appetite, Regulation |
| β-glucan and Insulin Sensitivity in Obese Humans | NCT01393210 | n/a | Unknown | Unknown | Obesity | |
| Diet for the Maintenance of Weight Loss and Metabolic Health in Obese Postmenopausal Women (WELCOME) | NCT04136093 | n/a | Oat, Barley | 50 g of oatmeal and barley groats | Metabolic Syndrome, Diet Modification, Postmenopause | |
| Efficacy of Hydroxycinnamates and Beta-glucans as a Dietary Tool Against Obesity Pilot Study (OBHEALTH_PS) (OBHEALTH_PS) | NCT04321590 | n/a | Oat | 3 g or 5 g/day | Overweight, Obesity | |
| Dietary Fibres and Satiety in Bariatric Patients (FIBAR) | NCT03573258 | Early phase 1 | Oat | 6 g | Bariatric Surgery Candidate | |
| SATIN: Satiety Innovation. Study 2-University of Aberdeen (SATIN) | NCT02604316 | n/a | Viscofibre, Oat and Barley | 6 g for 10 days | Overweight, Obesity | |
| Effects of β-glucan on Energy Intake and Satiety | NCT02637388 | n/a | Oatwell, Powder—Oats | 4 g as part of breakfast | Obesity | |
| A Trial Comparing a Diet Including Products Aimed at Targeting Satiety (SATIN) | NCT02485743 | n/a | Unknown | Unknown | Weight, Appetite | |
| The Effect of a Breakfast Meal Containing Oat β-glucan on Food Intake at a Subsequent Meal in Normal-weight and Overweight Subjects | NCT03490851 | n/a | Oat | 2–4 g | Satiety | |
| Efficacy and Safety of Fermented Barley on Decrement of Body Fat in Obese Subjects | NCT01402128 | Phase 2, Phase 3 | Barley | 3 g/day | Overweight; Hyperlipidaemia | |
| The Effect of Dietary Fibre on Food Liking | NCT03241238 | n/a | Unknown | Unknown | Different Fermentable Fibre, Satiation | |
| Metabolic Syndrome | β-glucans and the Metabolic Syndrome—a Human Intervention Study Under BEST | NCT01317264 | n/a | Oat, Barley, Mutant Barley | 5 g/day | healthy |
| Effect of Dietary Fibre and Whole Grain on the Metabolic Syndrome | NCT01316354 | n/a | Unconfirmed | Bread with 50 g available carbohydrate | Metabolic Syndrome | |
| Pivotal Assessment of the Effects of Bioactive on Health and Wellbeing. From Human Genome to Food Industry (PATHWAY-27) | NCT02702713 | n/a | Oat (N.C), Pathway-27 website http://www.pathway27.eu/ | 3 g beta-glucan—not stated frequency | Metabolic Syndrome | |
| Metabolic Effect of New Foods Through Gut-brain Axis (CHECKMATE) | NCT01851304 | n/a | Barley | 3 g/100 g bread portion | Obesity, Overweight | |
| Effects of β-glucan From Barley and Oats on Glucose and Lipid Metabolism, and satiety (glucan) | NCT03648112 | n/a | Barley, Oats | 80 g crude flakes/day (oat or barley) | Lipid Metabolism, Glucose Metabolism, Satiety | |
| Effects of Dried Bilberry, Liquid Oats, or Their Combination After AMI (BIOAMI) | NCT03620266 | n/a | Glucanova®—Oat | Shakes containing oats 3 times daily, | Myocardial Infarction | |
| Influence of Dietary Fibre-rich Meals on Gene Expression and Postprandial Glucose and Lipid Response | NCT01005342 | n/a | Oat | 62–82 g—single intake | Hypoglycaemia, Hyperglycaemia | |
| The Effects of β-glucan Enriched Oatcake Consumption on Metabolic Disease Risk Factors | NCT02615444 | n/a | Oat | 4 g/day | Metabolic Syndrome X, Cardiovascular Diseases | |
| Canola Oil, Fibre and DHA Enhanced Clinical Trial | NCT02091583 | n/a | Barley | 3 g/day | Metabolic Syndrome | |
| Glycaemic Impact of Oatmeal Plus OatWellXF28 | NCT02818452 | n/a | Oat | 27 g–32.72 g of Oatmeal containing β-glucan | Glycaemic Responses | |
| Four-hour Glycaemic Kinetic Response Following 13C-enriched Oatmeal Breakfast Compared to Hot Corn Grits | NCT03165773 | n/a | Oat | 87 g oatmeal | Glycaemic and Insulinemic Response | |
| Portfolio 5—Multicentre Dietary Advice on Serum Lipids in Hyperlipidaemia | NCT00438425 | n/a | Oat, Barley | 9.8 g/1000 kcal | Hyperlipidaemia, Cardiovascular Disease | |
| Magnetic Resonance Imaging-Portfolio Diet Study #7 (MRIPD#7) | NCT02078635 | n/a | Oat, Barley | 9.8 g/1000 kcal | Cardiovascular Diseases, Hypercholesterolemia, Diabetes, Metabolic Syndrome, Obesity | |
| Effects of Chitin-glucan on Oxidized Low-Density Lipoprotein (LDL) | NCT01232309 | n/a | Chitin | 1.5 g–4.5 g daily | Cardiovascular | |
| Compare the Efficacy and Safety of β-glucan as Add-On to Statin in Subjects with Hyperlipidaemia. (BetAvena) | NCT03857256 | Phase 2 | CP105F, Oat β-glucan | 1.5 g, 3 g or 6 g daily, Administered 3 times daily | Hyperlipidaemias | |
| The Effect of Oral β-glucan Supplement on Appetite and Insulin Resistance in non-Alcoholic Fatty Liver Disease | NCT02178839 | n/a | Oat | 3 g daily | Non-Alcoholic Fatty Liver Disease | |
| ProAliFun_6.5_Health Effects of a Functional Pasta Enriched with Barley Beta-glucans on Healthy Subjects (ProAliFun65) | NCT02710513 | n/a | Barley | 100 g of β-glucan pasta/day | Healthy | |
| Clinical Trial to Evaluate the Addition to an Antiretroviral Treatment of a Probiotic (RECOVER) | NCT03542786 | n/a | Oat | As part of pro-biotic once a day for 6 months | HIV, Premature Aging |
The synthesis of bile acids occurs in the liver, in a series of reactions that convert hydrophobic cholesterol into a water-soluble compound, thus representing the primary pathway for cholesterol catabolism [59,60]. The formation of primary bile acids is primarily catalysed by the cytochrome P450 enzyme 7-α hydroxylase (CYP7A1) [61]. CYP7A1 regulates cholesterol synthesis as well as its excretion from the liver [62,63,64]. Bile acids are directly involved in the regulation of cholesterol [65]. Barley beta-glucan has been shown to regulate CYP7A1 and HMG-CoA and ultimately regulate cholesterol synthesis and its decomposition into bile acids. By regulating enzyme activity in the liver, cholesterol is reduced in the blood vessels [64].
β-glucans may also elicit some of their effects on cholesterol through modulation of the gut microbiota, which is a key regulator of bile acid metabolism [66]. As β-glucans are resistant to the digestion of gastric and pancreatic enzymes they are fermented in the colon by host microbiota and elicit their effects in this way. There have been reports of the correlation of changes to microbiota and reduction of total cholesterol after β-glucan administration [67]. Oat and barley β-glucans are fermented by intestinal microflora resulting in SCFA as the end products [68]. When the SCFAs are absorbed they inhibit cholesterol synthesis by limiting the activity of HMG-CoA or through the catabolism of LDL-cholesterol [64]. Other research groups have suggested that foods containing β-glucans may influence the gut microbiota with ultimate effects on bile acid signalling and SCFA signalling which regulate cholesterol metabolism [66].
Cereal β-glucans also increase faecal bile acid excretion [69]. A preclinical study in pigs demonstrated that glucans in the diet increased bile acid and cholesterol metabolism, this study also showed the potential prebiotic effect on gut microbiota [70] (please see the section below on “Effects on Gut and microbiota”).
The cholesterol-lowering effects of cereal β-glucans appear to be correlated to their viscosity. [71,72]. Highly viscous fibres such as β-glucans have demonstrated viscosity-dependent health benefits including cholesterol-lowering and improved glycaemic control [73]. A clinical trial that examined the physicochemical properties of β-glucans found that cholesterol-lowering ability was correlated to viscosity, the high-viscosity preparations displayed the strongest effect. [74].Viscous β-glucans also appear to modulate host bile acid metabolism [73]. β-glucan can bind to bile which is produced in the liver, and increase faecal excretion ultimately decreasing cholesterol build-up [75]. More specifically, the β-glucans bind the whole micelles that contain bile acids in the intestine. This prevents interaction with luminal membrane transporters that are located on the intestinal epithelium, preventing the absorption of fats and cholesterol [76,77]. This ultimately lowers systemic LDL cholesterol as a result of the reduction of de novo synthesis of bile acids from cholesterol [73].
In a clinical trial in 268 men and women with high cholesterol, oat β-glucans reduced cholesterol and triglyceride, lowering the risk of cardiovascular diseases [78]. Other studies have shown that oat β-glucans reduce systolic and diastolic blood pressure and thus improve patients’ blood pressure when administered daily. Oat β-glucans have also reduced the risk of cardiovascular disease in hypertensive patients. [77,79,80,81].
5.4. Enhancement of Glycaemic Control
Cereal derived β-glucans decrease insulin resistance [82] and reduce postprandial blood glucose concentrations [72,83,84,85,86,87,88]. Products containing oat β-glucans have been shown to decrease postprandial glucose in healthy individuals [80,89,90] and type II diabetic patients [91,92]. A recent CT has found significant correlations between oat β-glucans viscosity, glucose and insulin levels and gastric emptying responses. The study showed that oat β-glucans favourably slowed down gastric emptying and reduced glycaemic and insulinemic responses in healthy individuals [93]. This study also demonstrated that viscosity would determine the effect of the β-glucans. It is hypothesized that by forming viscous solutions and retaining water in the digestive tract stool volume is increased and homeostasis of blood glucose levels are improved [37,94].
A study by Miyamoto et al. 2018 investigated the metabolic effects of Barley flour containing varying amounts of β-glucan, in a preclinical mouse model of high-fat diet-induced obesity. Barley glucan caused appetite suppression and improved insulin sensitivity via short-chain fatty acid (SCFA) -induced production of gut hormones. High levels of β-glucan decreased fat gain which improved insulin sensitivity [95]. In pigs, 6% of oat b-glucan significantly decreased glucose concentrations and increased insulin levels. It is hypothesized that these changes were associated with gastric emptying peptide and GLP-1 [96].
In terms of mechanism of action, viscous β-glucans can form a gelatinous layer in the gut, which acts as a barrier and hinders absorption of glucose and lipids [97,98,99]. The layer acts as a filter and slows digestion and absorption, larger molecules are not filtered through and pass directly through to the intestine [100]. Other hypotheses are that the layer delays starch interaction and thus reduction in the absorption of carbohydrates and ultimate reduction of glycaemia [101].
There are some apparent controversies and conflicts in the literature regarding the glycaemic effects of β-glucans. These relate in part to the extrapolation of findings from one specific glucan preparation to other preparations from the same source, despite the fact that cultivation methods and extraction procedures will all influence the activity profile. In addition, β-glucans can establish secondary structures which can also affect activity [48]. For example, barley β-glucan has shown positive effects on glucose control levels in rats when fed diets containing barley β-glucan over twelve weeks and in another study where rats were fed diets containing barley β-glucan over six weeks [102,103]. In contrast, when barley was administered to obese rats for two weeks, there was no effect on postprandial glycaemic response. In this study, both a high concentration and a low concentration barley β-glucan was administered. The authors state that their study did not influence glucose levels. It must be highlighted that the authors used two novel barley varieties [104]. As the structure is unknown, this extract cannot be compared to other barley extracts or can conclusions be made about treatment durations as it is likely that each experiment used a different extract.
5.5. Effects on Gut and Microbiota
Oat β-glucan also acts as a prebiotic improving gastrointestinal function indirectly by enhancing the intestinal microbiota given that is a non-digestible by humans [105]. It stimulates the growth and activity of commensal bacteria in the colon [106]. The growth of normal intestinal microbiota (Lactobacilli and Bifidobacteria species) are supported by β-glucans in-vivo and in-vitro models [68]. When rats were administered oat and barley glucan, they demonstrated higher Lactobacilli and Bifidobacteria availability. These experiments showed that higher doses of glucan had a better effect and oat glucan was more effective over barley [107,108]. Other species of intestinal microbiota enhanced by β-glucans include Lactobacillus acidophilus, Lactobacillus casei and Bifobacterium spp. [109].
The regulation of various pathways and digestion is improved when the human microbiome is enriched [110], such as by prebiotic like β-glucans. The microbiota also helps to breakdown non-digestible β-glucans into SCFAs which can display biological activity [111], such as influencing hormone secretion. Specifically, the improved insulin sensitivity may result from the promotion of gut hormone secretion from enteroendocrine cells by SCFAs [95]. SCFA stimulated gut hormone secretion from enteroendocrine cells has also been shown in human studies [112].
5.6. Key Knowledge Deficits
Unfortunately, our understanding of β-glucan structure-activity and source-activity relationships remains limited. The effect of one β-glucan preparation is frequently extrapolated to all β-glucans [48], which is an over-simplification as multiple factors affect the structure and activity of each β-glucans preparation. Insufficient attention is being given in research publications to the importance of glucan source or of variations in glucan structures to their activity profile, with less than 20% of 10,000 publications relating to immunological activities of β-glucans, including structure in the title or abstract in one analysis [48]. An array of effects is being attributed to β-glucans that in reality are likely to vary substantially depending on the originating glucan source and preparation method, which both have significant effects on glucan structure and hence on activity profile.
Additional research is therefore required to fully understand and characterize the key mechanisms of bioactive and metabolic activity of β-glucans. It is critically important that research carried out in this field describes clearly the source, molecular weight and molecular structure of the specific β-glucan molecule being investigated. Methods that can be potentially employed for this purpose include structural analysis; nuclear magnetic resonance (NMR), chemolytic methods (methylation analysis), vibrational spectroscopic methods (Fourier transformed infrared spectroscopy FTIR, Raman) and Gel permeation chromatography methods (GPC) for molecular weight determination [113,114]. It is only when this is addressed will key source-structure-function relationships of β-glucans be properly established.
6. Fungal β-glucans (1,3 and 1,6 Linked)
Fungal β-glucans, such as those isolated from fungi (e.g., mushrooms) and yeast appear to have a more immune-modulating effect profile, leading to their investigation as priming or activation adjuvant agents for infectious diseases or cancers (Table 2). β-glucans from yeast have been shown to activate the immune response and initiate the inflammation process as well as improving resistance to infections and inhibiting cancer development [115]. Of importance, β-glucans do not have a direct cytotoxic effect on cancerous cells or tumours, but instead, elicit an indirect effect through the activation of immune cells [8].
Table 2.
Fungal β-glucans for immunomodulatory indications.
| Areas of Research | Study Title: | NCT | Design | β-glucan Type | Dose | Population |
|---|---|---|---|---|---|---|
| Solid cancer and Haematological malignancy | Efficacy and Safety Study of SBG vs Placebo in Head and Neck Cancer Patients Undergoing Radiation Therapy | NCT00790322 | Phase III | Soluble- β-glucan SBG, Yeast derived | Not-stated | Head and Neck Cancer |
| The Protective Effect of Soluble Beta-1,3/1,6-glucan Compared to Placebo in Oral Mucositis in Head and Neck Cancer Patients | NCT00289003 | Phase II | SBG—Yeast | Unknown | Oral Mucositis | |
| Safety of Soluble β-glucan (SBG) in Treatment of Patients with Non-Hodgkin’s Lymphoma | NCT00533728 | Phase 1 | SBG, Yeast | Unknown | Non-Hodgkin’s Lymphoma | |
| Effect of SBG in Patients with Breast Cancer | NCT00533364 | Phase 1, Phase 2 | SBG, Yeast | Unknown | Breast Cancer | |
| The Effect of β-glucan in Non-Small Cell Lung Cancer | NCT00682032 | n/a | Imucell WGP-Yeast | 1 (one) 250 mg β-glucan capsule 3 times a day for 14 days | Non-Small Cell Lung Cancer | |
| Bivalent Vaccine with Escalating Doses of the Immunological Adjuvant OPT-821, in Combination with Oral β-glucan for High-Risk Neuroblastoma | NCT00911560 | Phase 1, Phase 2 | Yeast | Oral β-glucan (40 mg/kg/day) in conjunction with vaccine | Neuroblastoma | |
| β-glucan and Monoclonal Antibody 3F8 in Treating Patients with Metastatic Neuroblastoma | NCT00492167 | Phase 1 | Yeast | In conjunction with monoclonal antibody—dose-escalation study of β-glucan | Neuroblastoma | |
| Lung Cancer Vaccine Plus Oral Dietary Supplement | NCT01829373 | Phase 1 | Vaccine plus oral beta-glucan-Yeast | Unknown | Lung Cancer | |
| β-glucan and Rituximab in Treating Young Patients with Relapsed or Progressive Lymphoma or Leukaemia, or Lymphoproliferative Disorder Related to Donor Stem Cell Transplantation | NCT00087009 | Phase 1 | Unknown | Oral β-glucan in conjunction with IV Rituximab | Leukaemia, Lymphoma, Lymphoproliferative Disorder | |
| Rituximab Plus β-glucan in Chronic Lymphocytic Leukaemia (CLL)/Small Lymphocytic Lymphoma (SLL) | NCT00290407 | Phase 2 | Imucell WGP, Yeast | 250 mg, orally (tablet), three times a day for 9 weeks | Leukaemia, Lymphocytic, Chronic, Lymphoma, Small Lymphocytic | |
| β-glucan and Monoclonal Antibody in Treating Patients with Metastatic Neuroblastoma | NCT00037011 | Phase 1 | Unknown | Oral β-glucan in conjunction with IV antibody | Neuroblastoma | |
| β-glucan in Treating Patients with Locally Advanced or Metastatic non-Small Cell Lung Cancer | NCT00857025 | Phase 1 | glucan MM-10-001, Fungal | Oral β-glucan once daily | Lung Cancer | |
| Imprime PGG, Alemtuzumab, and Rituximab in Treating Patients with High-Risk Chronic Lymphocytic Leukaemia | NCT01269385 | Phase 1, Phase 2 | PGG β-glucan, Imprime PGG, Yeast | Dose escalation study IV administration | B-cell Chronic Lymphocytic Leukaemia, Refractory Chronic Lymphocytic Leukaemia, Stage 0 Chronic Lymphocytic Leukaemia, Stage I Chronic Lymphocytic Leukaemia, Stage II Chronic Lymphocytic Leukaemia | |
| (PM-01) IMPRIME PGG® With BTH1704 and Gemcitabine for Advanced Pancreatic Cancer (PM-01) | NCT02132403 | Phase 1 | IMPRIME PGG, Yeast | Assigned doses | Pancreatic Cancer | |
| A Phase 2 Clinical Trial of Rituxan and B-glucan PGG in Relapsed Indolent Non-Hodgkin Lymphoma | NCT02086175 | Phase 2 | IMPRIME PGG, Yeast | I.V 4 mg/kg weekly for 4 weeks. | Relapsed/Refractory Indolent B Cell Non-Hodgkin Lymphoma | |
| Biological Therapy in Treating Patients with Neuroblastoma That Has Not Responded to Previous Treatment | NCT00089258 | Phase 2 | Unknown | Unknown | Neuroblastoma | |
| MucoLox Formulation to Mitigate Mucositis Symptoms in Head/Neck Cancer | NCT03461354 | Phase 2 | Unknown | Unknown | Mucositis Oral, Head and Neck Cancer | |
| Phase 2 Study of Imprime PGG & Pembrolizumab in Subjects with Adv SCCHN Who Failed Pembro Monotherapy or Experiencing SD | NCT03246685 | Phase 2 | Imprime PGG, Yeast | 4 mg/kg IV over a 2-h infusion time on Days 1, 8 and 15 of each 3-week treatment cycle. | Squamous Cell Carcinoma of the Head and Neck | |
| Study of Imprime PGG and Pembrolizumab in Advanced Melanoma and Triple-Negative Breast Cancer | NCT02981303 | Phase 2 | Imprime, PGG, Yeast | 4 mg/kg IV over a 2-h infusion time | Advanced Melanoma, Triple-Negative Breast Cancer | |
| Phase I, Dose-Escalation Study of Soluble Beta-glucan (SBG) in Patients with Advanced Solid Tumours | NCT01910597 | Phase 1 | SBG -yeast | Unknown | Advanced Solid Tumours | |
| Immunomodulation | Efficacy and Safety of Resveratrol and Carbossimetyl Beta-glucan in Treatment of Upper Airways Disease in Infancy (VIRNEO) | NCT03683108 | Phase 3, Phase 3 | Carbossimetyl β glucan, Carbossimetyl β glucan | 3 drops, 4 times a day for 1 week. | Common Cold |
| Nebulized Resveratrol Plus Carboxymethyl-β-glucan for Reducing IL-5 in Children with Allergic Rhinitis (RENIM) | NCT03349619 | Phase 4 | Carboxymethyl-β-glucan | Two sprays (100 uL/spray) three times a day for 4 weeks | Allergic Rhinitis | |
| Effects of Orally Administered Beta-glucan on Leukocyte Function in Humans (BG) | NCT01727895 | n/a | Glucan, #300, Yeast | 500 mg/day | Immunologic Deficiency Syndromes | |
| Safety and Efficacy Study of Oral XIGO Tablets to Treat Common Cold | NCT01092039 | n/a | Unknown | Unknown | Common Cold | |
| Efficacy and Safety of Imuneks 10 mg Capsules in the Prophylaxis of Cold | NCT02807220 | Phase 4 | Micro ionized β-glucan -source unknown | 10 mg—2 capsules every morning | Cold Symptoms | |
| Alleviation of Cedar Pollen Induced Allergic Symptoms by Orally Taken Superfine β-1,3-glucan | NCT00276445 | Phase 4 | Fungal | Unknown | Allergic Conjunctivitis |
Mushroom derived β-glucans are the most potent anti-tumour and immune-modulating of the β-glucans [0,45]. Mushroom β-glucans have demonstrated positive therapeutic effects in respiratory conditions, preventing recurrent respiratory tract infections in children [116], prevention of symptoms of allergic rhinitis, and upper respiratory tract infections [117,118].
β-glucans appear to alleviate allergic problems to allergens e.g., pollen via mechanisms related to the decreasing pro-inflammatory cytokines IL-6, TNF-α and increased formation of anti-oxidants [119,120]. Immune cells recognize β-glucans as PAMPs. Thus, glucan effects are elicited through pattern recognition receptors (PRRs). These include Dectin-1, Complement receptor 3 (CR3), Toll-like receptors (TLRs), Lactosylceramides, scavenger receptors [121,122,123]. Dectin-1 is the critical receptor for β-glucans [124]. TLR 2,4 and 6 co-bind to dectin-1 after glucan recognition [125]. This recognition and binding of TLR and dectin-1 modulate the release of pro-and anti-inflammatory cytokines to control the immune response [126].
The exact mechanism by which β-glucans suppress inflammatory cytokines and induce anti-inflammatory cytokines are complex and incompletely understood. In fact, non-cereal β-glucans inhibit lipopolysaccharide-induced nitric oxide and TNF-α release in-vitro. [127], and reduce the secretion of TNF-α, IL-6 in lipopolysaccharide challenged mice [128]. Stimulated monocytes isolated from glucan treated mice release less TNF-α and IL-6 after toxic stimulation [129]. Mushroom derived β-glucans reduced pro-inflammatory cytokine levels in healthy female volunteers [130].
When β-glucan binds to dectin-1, TLRs are also required in this recognition for the release of inflammatory cytokines [131,132,133]. Perhaps during injury one of these receptors is blocked, or during injury, the β-glucans bind to a separate receptor [134]. β-glucans from yeast have been shown to induce a strong immune-modulatory cytokine interleukin 1-receptor antagonist (IL-1Ra) expression. This expression is independent of the common β-glucan receptors (Dectin-1 and CR3). Smeekens et al. suggest that an unknown β-glucan receptor exists that specifically induces an Akt/P13K-dependant anti-inflammatory response [134]. Our previous work demonstrated that β-glucans from Shiitake mushroom, induced NFKβ in-vitro in the absence of injury. In the presence of LPS, the same extract significantly reduced injury [135].
Other research areas of interest about the use of β-glucans for clinical purposes were summarized in Table 3.
Table 3.
Other effects of β-glucans.
| Areas of Research | Study Title | NCT | Design | β-glucan Type | Dose | Population |
|---|---|---|---|---|---|---|
| Wound Healing | Efficacy and Safety Study of Soluble β-1,3/1,6-glucan in Thermal Burns | NCT00283426 | Phase 1 | SBG, Yeast | Unknown | Burns |
| A Randomized Comparison Study of Aquacel Ag and Glucan II as Donor Site Dressings | NCT00581217 | n/a | Glucan II, Oat | unknown | Burns | |
| Multi-Centre, Prospective, Randomized, Comparison of AWBAT™-D vs. Xeroform™ or Glucan II™ for Treatment of Donor Sites in Burn Surgery (AWBAT-D) | NCT00964470 | n/a | Glucan II, Oat | Unknown | Treatment of Donor Site Burns | |
| Efficacy of TR 987, β-1,3-1,6-D-glucan, in the Treatment of Chronic Venous Insufficiency Ulcers | NCT03154619 | Phase 2 | Glucoprime, Yeast | Gel application twice weekly | Venous Leg Ulcer | |
| Preadmission Skin Wipe Use for Surgical Site Infection Prophylaxis in Adult Orthopaedic Surgery Patients | NCT03401749 | Phase 4 | Unknown | Ingredient in wipes before surgery | Surgical Site Infection | |
| To Study the Effect of β-glucans on Wound Healing | NCT02078128 | n/a | Unknown | 30 mg/kg daily | Burns | |
| Beta-1,3/1,6-D-glucan Ganoderma Lucidum on Non-infectious and Idiopathic Uveitis | NCT04162314 | Phase 2, Phase 3 | Fungal | Capsule containing 180 mg/three times daily | Uveitis | |
| Soluble β-glucan (SBG) as Treatment for Diabetic Foot Ulcers | NCT00632008 | Phase 3 | SBG—Yeast | Topical Application twice a week | Chronic Diabetic Foot Ulcers | |
| Clinical Trial to Evaluate Papilocare® Gel Efficacy into Repairment of Cervical Lesions Caused by HPV (PAPILOCAN) | NCT04210336 | Phase 3 | Unknown | Topical application | HPV Infection, Lesion Cervix | |
| Treatment of Chronic Anal Fissure (TOCA) | NCT02158013 | n/a | Yeast | Gel application/ twice daily two weeks | Chronic anal fissure | |
| Irritation and Anal Bleeding in Patients Affected by Haemorrhoids. | NCT03569930 | Phase 4 | Unknown | Anal application Frequency, Unknown | Haemorrhoids | |
| Cognitive Performance | A Follow-up Trial of Proglucamune® in the Treatment of Protective Qi Insufficiency, a TCM Condition | NCT03782974 | n/a | Yeast | 2 tablets of proglucamune/day—200 mg of β-glucan for 8 weeks | Protective Qi Insufficiency (a Condition Term From TCM) |
| An Evaluation of Proglucamune in the Treatment of Protective Qi Insufficiency | NCT03829228 | n/a | Yeast, Fungal, Proglucamune tablet | 2–100 mg tablets per day | Protective Qi Insufficiency | |
| Investigation of How Morning Nutrition Influences Cognitive Performance | NCT03169283 | n/a | Oat (NC) | Cereal β-glucan in morning | Cognitive Performance | |
| Safety Studies | Dose Escalation Safety Study of MM-10-001 in Healthy Subjects | NCT00677027 | Phase 1 | Lentinan—Fungal | Unknown | Healthy |
7. Insights into β-Glucan Effects from Pre-Clinical Models
7.1. Bacterial Sepsis
β-glucans improved the immune response and survival of mice from influenza infection [136]. In a model of E. coli injury (intraperitoneal injection), bacterial counts in peripheral blood reached zero in mice administered β-glucans, while mortality in control animals was 100% at 24 h [137]. PGG is a commercial source of purified yeast β-glucans that has been shown to enhance bacterial clearance from blood and reduce mortality in rat intra-abdominal sepsis models [138,139,140]. PGG enhanced clearance of antibiotic-resistant S. aureus in a rat intra-abdominal infection model, potentially mediated by increasing circulating monocytes and neutrophils and increasing neutrophil oxidative microbicidal activity without generating harmful inflammatory responses. PGG treatment also had a synergistic activity with antibiotic administration to further enhance bacterial clearance and reduce infection-related mortality [141].
In a rat model of caecal ligation puncture (CLP) induced polymicrobial sepsis, β-glucan treatment attenuated pro-inflammatory cytokines TNF-α and IL-6 elevations, while increasing anti-inflammatory cytokine IL-10 concentrations and accession of cellular antioxidants ultimately protecting the cells from oxidative stress [142].
7.2. Lung Injury
β-glucans decreased lung injury severity following abdominal aortic ischemia-reperfusion in rats, reducing oxidative stress, decreasing lung permeability (reduced alveolar protein concentration and wet/dry lung ratio), reduced the systemic inflammatory response, decreased lung leukocyte infiltration, and decreased histologic evidence of lung injury [143]. β-glucans decreased lung injury severity following CLP induced sepsis [144]. β-glucan treatment decreased circulating monocytes and lymphocytes in bronchoalveolar lavage and reduced secondary lung injury as a result of CLP induced sepsis. β-glucans decreased inflammatory cytokines TNF-α, IL-1β and IL-6, and reduced the lung injury score, in a lung injury model induced by CLP [145].
7.3. Cancer Therapy
One of the most interesting applications of β-glucans is for cancer treatment, specifically as an adjuvant to enhance “conventional” cancer chemotherapeutics. β-glucans regulate complement-dependent cytotoxicity (CDC). β-glucans are recognized as PAMPs, triggering the response of immune effector cells. This will then elicit an anticancer immune response through the formation of a complex. When they enter the bloodstream, they are bound by endogenous plasma anti-β glucan antibodies (ABA). This binding activates complement and complement protein iC3b binds to the ABA, resulting in β-glucans—ABA—iC3b complex [146,147]. This complex binds to immune effector cells and activates specific aspects of innate immune function including CR3 phagocytosis. The activation and formation of this complex facilitate the direct killing of antibody-targeted tumour cells [146,147].
This mechanism was observed in both a pre-clinical model [148] and demonstrated with whole blood from healthy volunteers. The authors found that anti-cancer properties are dependent on the formation of the complex with naturally occurring ABAs [146]. In the preclinical model, mice were administered anti-tumour monoclonal antibodies (mABs) in combination with β-glucans. Results showed that the dual treatment produced significantly greater tumour regression in both mammary and hepatic tumours. The combinational treatment had a greater effect on each treatment individually. Interestingly, mice that were deficient in CR3 or serum CR3 or granulocytes did respond to treatment [148].
8. Clinical Trials of β-glucans
This review focused on the current clinical trials (CTs) registered on clinicaltrials.gov. The CTs were identified by an electronic search using the keyword “glucan”. The search was last performed on 23rd November 2020. CTs that used β-glucans for diagnostic purposes for fungal infections were excluded as were the trials using glucose polymers not specifying glucans as treatment. We identified over 200 registered clinical trials of β-glucans on clinicaltrials.gov. Trials that included measuring elevated blood β-glucan levels as a diagnostic test for fungal infections, or that used glucose polymers instead of β-glucans, are excluded from this analysis. In CTs, β-glucans have been administered orally in capsules, in food and as part of vaccines as adjuvants. Studies have shown that oral administration is as active as the injected dose [149]. The majority of studies for intervention purposes were aimed at oral administration of (cereal-derived) β-glucan for metabolic diseases (Table 1).
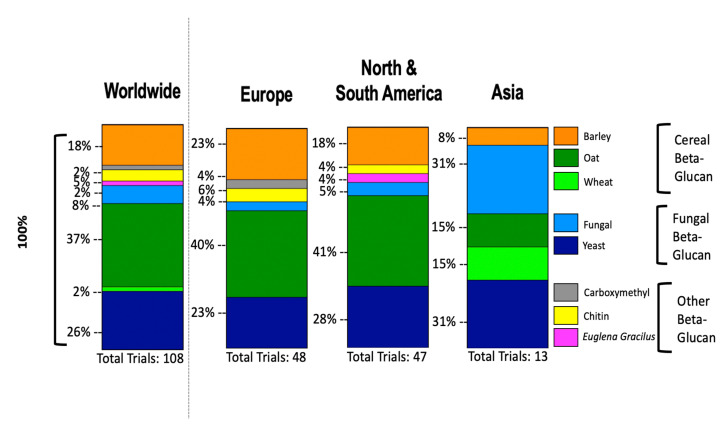
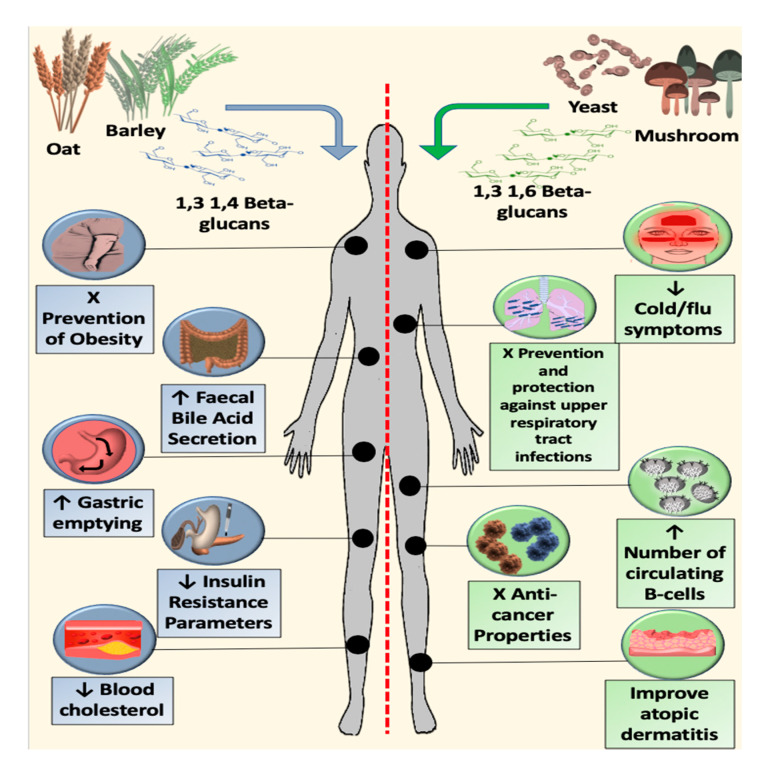
We reported the use of β-glucan by source worldwide and stratified by 3 main continents (Figure 3) and we represented an image that summarizes updated applications of β-glucan in clinical trials (Figure 4).
Figure 3.
Bar graphs displaying the percentage of beta-glucan source used worldwide (left panel), further stratified for the three continents of Europe, America and Asia (right panel). Data obtained from clinicaltrials.gov.
Figure 4.
Graphical representation of the effects of two different structures of beta-glucan in clinical trials.
9. Metabolic Effects of Cereal β-glucans
When barley containing β-glucan ranging from 3 g to 5 g was administered to 30 mildly hypercholesterolemic patients in a controlled, four-phase, crossover trial copositive effects included an increase of faecal bile acid excretion and SCFAs [150], reduction of blood cholesterol levels [151], prevention and treatment of obesity [152], reduction in visceral fat obesity [153], the influence of gastric emptying and glycaemic response [154].
In clinical studies, reported benefits of oat β-glucan include beneficial modulation of postprandial glycaemia [155] and satiety [156], improved appetite control [157], reduction of serum LDL cholesterol [74,158,159] reduction in total cholesterol [158], reduction of inflammation and oxidation hypercholesterolemic patients [160], and reduction in insulin-resistant parameters [161].
A clinical trial evaluated the safety of pre-treatment of 1,3 1,6 β-glucan in patients undergoing coronary artery bypass grafting (CABG) found the treatment was well tolerated. The measured parameters also showed that the isoenzyme creatine kinase was significantly reduced. Release of this enzyme is associated with muscle damage [162]. A study carried out in 1991 investigated the cholesterol-lowering potential of β-glucan in the diet. At six weeks, significant differences were observed for both total cholesterol and LDL in the β-glucan treatment group [163].
β-glucans were shown to reduce a 50% bile acid secretion from the small bowel in patients who were administered oat bran. This effect was not observed when glucanase enzymes were used to hydrolyse the β-glucan [164].
Patients with mild to moderate hyperlipidaemia were administered a low dosage diet of 3 g/day of β-glucans. This dose did not significantly reduce total cholesterol or LDL cholesterol [165]. Patients with elevated cholesterol given 6 g of concentrated oat glucan per day, demonstrated a significant reduction in total cholesterol or LDL cholesterol [159].
The study by Velikonja et al. 2019 (NCT02041104) aimed to determine if the consumption of 6 g/day of barley-derived β-glucans (given in bread) could modify gut microbiota composition, production of short-chain fatty acids, and improve metabolic status in patients with metabolic syndrome. The β-glucans were concentrated from barley with dry milling, sieving, and air classification technology. The barley β-glucan significantly lowered cholesterol levels. β-glucans decreased triglyceride levels and reduced subject weight. The treatment surprisingly decreased gastrointestinal bacterial diversity and richness. Test group participants also noticed an increase in stool frequency and flatulence [166].
Wang et al. investigated the physiochemical relationship between β-glucans and their ability to lower cholesterol (NCT01408719). Their objective was to determine if both the molecular weight and/or the daily dose of β-glucan affected cholesterol. Secondly, they wished to determine if genetic variations in genes (CYP7A1 and APOE) associated with cholesterol metabolism influenced the responsiveness of serum cholesterol biomarkers to β-glucan. β-glucans were obtained from barley using food processing protocols, micronizing, boiling, or toasting. The doses administered were 3 g/day or a higher dose of 5 g/day. Results demonstrated that the physicochemical properties of the β-glucan molecule affect activity. The higher molecular weight β-glucans lowered total cholesterol, while the lower molecular weight β-glucans did not. Interestingly, they showed that individuals carrying the G allele of the CYP7A1 gene were more responsive to the higher molecular weight β-glucans’ ability to lower cholesterol in comparison to the participants homozygous for the T allele [167].
Other clinical trials have investigated the effects of β-glucans indirectly. An example of this is the trial registered NCT00069524. This study investigated the anti-hyperlipidaemic effects of oyster mushrooms, which are rich in β-glucans. The trial assessed the safety and efficacy of oyster mushrooms in patients with HIV and antiretroviral treatment-induced hyperlipidaemia. Participants were administered 15 g/day of freeze-dried mushroom. However, the mushroom did not lower non-HDL cholesterol in the participants [168].
10. Immunomodulatory Effects of Fungal β-glucans
β-glucans originating from yeast or fungal sources are most widely recognized for their immunomodulatory effects, although other β-glucans may possess similar if less potent effects. Jensenak et al. 2013 investigated the potential immunomodulatory and preventative clinical effect of mushroom derived β-glucans in combination with vitamin C in children with recurrent respiratory tract infections. Although the treatment resulted in a significant reduction in respiratory morbidity as well as a reduction in the number of flu-like symptoms and infections, the β-glucan alone cannot be attributed to these effects as it was administered in combination with Vitamin C [116]. Another similar CT enrolled children with acute rhinopharyngitis and recurrent respiratory infections. Participants were administered resveratrol plus carboxymethyl-β-glucan or saline isotonic solution. The treatment significantly reduced nasal obstruction, rhinorrhoea, sneezing, cough, fever and use of medications [169]. However, the β-glucan therapy was also administered in combination therefore the positive effects observed cannot solely be attributed to β-glucan administration. In contrast, β-glucans from Shiitake mushroom did not affect immune parameters in healthy subjects, except for an increase in the number of circulating B-cells. The treatment was safe and well-tolerated [170].
Trauma patients exhibit increased susceptibility to infection, in a randomized double-blind placebo-controlled trial 38 patients trauma patients undergoing surgery were administered β-glucan of the unknown source [171]. β-glucan (50 mg/m2) was administered intravenously daily for seven days. Results showed that morbidity from sepsis was significantly greater in the placebo group (49%) compared to β-glucan group (9.5%). There was a positive correlation between β-glucan administration, immune function measured using IL-1β and decreased septicaemia.
A randomized double-blind controlled CT treated patient with severe multiple trauma with β-glucan (312–685 mg/patient) intravenously to prevent nosocomial pneumonia and sepsis. Interestingly, pneumonia occurred in 11 out of 20-patients in the control group and only two patients in the β-glucan treatment group. The mortality rate related to infection in the control group was found to be 30% and 4.8% in the treatment group. The overall mortality rate that included cerebral deaths was 42% in the control group and 23% in the treatment group [172].
A series of CTs carried out by Babineau et al., commenced with a randomized Phase I/II double-blind placebo-controlled study administering PGG β-glucan to high-risk patients undergoing major abdominal or thoracic surgery. Patients were administered 0.5 mg/kg of PGG 12 to 24 h preoperatively. There were no adverse drug reactions observed. Results demonstrated that treated patients had significantly fewer infectious complications, decreased intravenous antibiotic requirement and shorter ICU days. Molecular parameters and cytokine were not measured therefore the mechanism cannot be determined. However, measurement of leukocyte function of treated patients showed increased killing towards S. aureus and Candida albicans in vitro. These results were not statistically significant. Limitations of this study included a small study size of 30 patients [173]. The second CT carried out by Babineau and colleagues, was an interventional, multicentre, double-blind, randomized, placebo-controlled study. High-risk patients undergoing major thoracic or abdominal surgery were administered saline or PGG at increasing doses (0.1 mg/kg, 0.5 mg/kg and 1 mg/kg or 2 mg/kg). One dose was administered preoperatively, and a further three doses were administered postoperatively. Results showed that there were reductions in infection incidences among patients who received 0.5 mg/kg of PGG in comparison to the placebo group and the group administered the lowest concentration of PGG 0.1 mg/kg. Only one patient who received 0.5 mg/kg developed a severe infection. Diabetic patients who received the higher doses (0.5 mg/kg, and 1mg/kg or 2 mg/kg) had significantly lower incidences of infection in comparison to patients who received the lowest dose or placebo groups. Patients who were administered 0.5 mg/kg had fewer hospitalization days [174].
The final CT in this series enrolled 1249 patients in a multicentre, prospective, randomized double-blind placebo-controlled trial. Patients enrolled were scheduled for gastrointestinal procedures with two or more defined risk factors. Patients in treatment groups were administered PGG glucan at a dose of 0.5 mg/kg or 1.0 mg/kg preoperatively and three times postoperatively. Results showed that there was no difference in severe infections and mortality between treatment and placebo groups. In malnourished patients undergoing noncolorectal procedures, PGG reduced postoperative infection and death. Unfortunately, the study was ultimately terminated due to patients experiencing more frequent adverse reactions in the treatment group compared to control [175]. Leentjens et al. (NCT01727895) investigated the effects of orally administered β-glucan on innate immune responses in humans. Test groups received 1000 mg/day of a water-insoluble commercial β-glucan from yeast (glucan #300). For analysis, peripheral blood mononuclear cells (PBMCs) were collected and cultured. Cells were treated with various stimuli, and ELISAs were performed. A microbicidal activity assay and β-glucan detection assay were also performed. Results showed that β-glucan was not detectable in serum, and the immune response was not modulated or enhanced [176], suggesting that perhaps the dose used was sub-therapeutic.
Hala Helmi Hazzaa and colleagues in their clinical trial (NCT02402296) investigated the potential immune activation of β-glucans in dentistry. The focus of this trial was to identify a potential substance that would stimulate protective immune responses and influence mount pathways that would contribute to resolving chronic lesions observed in periodontal disease. Results showed that test groups had a higher mean of probing pocket-depth reduction, and a reduction in gingival inflammation compared to the control group. Protective healing patterns were also enhanced. The β-glucan administered was called Imurrill commercial capsules, the source unknown. Publication of this data in a peer-reviewed journal is awaited.
11. CTs Cancer Therapy
β-glucans are widely studied as a potential cancer treatment both alone and in combination with other chemotherapeutics and mAb vaccinations. Lentinan a purified polysaccharide from the mushroom Shiitake was administered to patients with advanced or recurrent stomach, colorectal and breast cancers. The extract was administered intravenously at 1 mg/day or 2 mg/day. The extract was also administered with two chemotherapeutic compounds (5-FU and Tegafur). Life span prolongation was measured and a significant effect was observed on host immune response, although this cannot be attributed to Lentinan as it was administered in combination with two other drugs [177].
In patients with myelodysplastic syndromes, Maitake mushroom extract increased neutrophil and monocyte function. Monocyte response to E. coli was also reduced. The treatment was well tolerated [178]. Maitake extract was used, thus β-glucans cannot exclusively be responsible for these effects. Another CT administered β-glucans to patients with advanced malignancies receiving chemotherapy. The patients were monitored for tolerability and to determine any effects on haematopoiesis. The treatment was well tolerated with some amelioration of the blood counts but a larger trial is required for confirmation [179].
In a Phase I CT which enrolled 20 patients with chronic lymphocytic leukaemia, monoclonal antibodies in combination with PGG glucan was administered as a treatment. In this study, the combination treatment was well tolerated and therefore administered as a treatment in the follow-on Phase II study. Monotherapy with rituximab and alemtuzumab specifically rarely achieved a complete response or a sustained response. The concept of this study was that the β-glucans would increase the cytotoxic capacity of the innate immune system when administered with the mAb. The authors stated that the study was too small to provide preliminary data, but there was some promise with this application as the addition of β-glucans did improve the duration of response of mAb [180]. In support of this concept is another CT where the same PGG glucan was administered in another mAb trial for the treatment of small-cell lung cancer (NSCLC) [181]. The combination improved efficacy improved antitumor antibody therapy and improved objective response rate (ORR) in enrolled patients. In another study, the same patient group (NSCLC) were enrolled, this time administered mAb, β-glucans and chemotherapeutics demonstrating promising results [182]. At present β-glucans formulations in these trials are being developed for the treatment of cancer in conjunction with tumour targeted antibodies. The Phase I/II trial NCT03003468 is currently underway due to be completed in September 2021. This trial will investigate PGG, the mAb pembrolizumab in patients with NSCLC. The use of β-glucans as a potential cancer treatment is promising as it seems to be well tolerated in patients. However, as the majority of these studies administer β-glucans in combination with anticancer drugs or as part of monoclonal antibody treatment, attributing any effect specifically to β-glucans will be difficult.
12. Translational Challenges and Opportunities
The wide range of variety within the β-glucan class of macromolecules may explain their lack of translation to clinical use despite their promising mechanistic effects. The diversity between β-glucan samples occurs physiochemically in regard to conformation (inter-molecular and intra-molecular forces), degree of branching, monosaccharide composition, linkage ratio and linkage type. Also to consider are co-extracted chemicals such as mannose which can dilute or even contaminate β-glucan therapeutics [183]. It is established that molecular weights have a direct effect on activity. There is variance in molecular weights between sources with extraction procedure also having influence [184,185,186,187]. β-glucans originating from the same species can also have a range of molecular weights. For example, β-glucans can have a range of molecular weight values ranging from 65–3100 × 103, 31–2700 × 103, 21–1100 × 103, and 209–487 × 103 for oat, barley, rye and wheat [185]. Ultimately, this diversity amongst samples produces inconsistent results as essentially every research group is using a different product.
A potential solution to this is to isolate β-glucan using standardized (openly reported) methodologies, from the best-understood sources (barley, oats, mushrooms) characterize the molecular effects of a carefully selected range of these β-glucan samples (low and high degree of branching, low and high molecular weight; one plant and one fungal source) and then focus on the translation of the most promising of these β-glucan variants. In this way, research groups can compare results more precisely. Another solution to variance amongst biological samples is a chemical or enzymatic modification of the samples. However, this approach may have disadvantages such as lowered activity and potential toxicity [187].
The translational approach to β-glucan is characterized by heterogeneity in application also. Specifically, routes of administration, dose, time points, populations and length of treatment all vary in preclinical and clinical applications. This approach has led to apparently conflicting findings [188].
In another study, lower doses of β-glucan increased body weight and elevated plasma glucose and triglyceride levels. In this study, the higher doses presented more beneficial effects [95]. Furthermore, β-glucans display limited weak solubility under neutral conditions and unsuitable hydrophobic/hydrophilic balance [187]. Molecular modifications of polysaccharides can increase water solubility but this, in turn, may affect biological activity, the introduction of potentially harmful chemicals. This instability will ultimately influence the route of administration.
There is no standardized method for β-glucan extraction which leads to huge variances in preparations. Most importantly, very few articles state the amount of β-glucan in each product or the method by which they determined this. Structural variability, low purity levels and unknown receptor pathways all contribute to the current limitations of β-glucan research. The physiochemical differences between preparations which include molecular weight, degree of branching, solubility, denaturing affect receptor binding. This leads to the activation of multiple pathways with no key pathways being defined.
Specific targeting of cereal-derived β-glucan (with their 1,3 1,4 branching pattern) to metabolic disorders and microbial/fungal derived β-glucan (with their 1,3 1,6 branching pattern) to immune-modulatory indications might best harness the different effect profiles of both glucan sources. Similar approaches could be taken to differing molecular weights of glucans, to best define their optimal indications within these general areas. This raises the possibility that cereal glucans may eventually be better classified as a dietary supplement while non-cereal β-glucan might find therapeutic immune-modulatory uses as an active pharmaceutical ingredient.
Finally, disease targets studies to date remain very broad and the target mechanism of action is generally not clearly defined. Focusing on elucidating the most therapeutically relevant mechanisms of action, and on developing better characterized b-glucan preparations, may lead to the successful translation of b-glucans for a number of focused metabolic and immune-modulatory indications for which the most promising evidence exists.
13. Conclusions
β-glucans are natural molecules that have significant therapeutic promise, particularly as metabolic and immune-modulatory agents [189]. Enthusiasm for their therapeutic potential is reflected in the high number of clinical trials of β-glucans that have been completed or are in progress. However, concerns around deficits in the understanding of the complex relationship between β-glucans structure and their effect profile, together with heterogeneity in the approach to clinical translation, and variations in the approaches to extracting and purifying these agents, has hampered the search for clinical indications for β-glucans. A more rigorous approach, carefully defining optimal isolation and purification procedures from key sources, then characterizing the relationship between specific variations in a β-glucans branching structure, molecular weight, and cereal/microbial source and their effect profile may be the best approach to ultimately realize the therapeutic promise of these intriguing compounds. Thus, facilitating a greater understanding of the mechanisms of action of β-glucans. This could lead to targeting a more precise clinical objective as the mechanism of action would be established.
Author Contributions
Conceptualization, E.R. and E.J.M.; original draft preparation, E.J.M., E.R. and J.G.L.; writing—review and editing, E.J.M., E.R., I.M., N.J.R. and J.G.L.; visualization, E.J.M.; supervision, J.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vetvicka V., Vannucci L., Sima P., Richter J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules. 2019;24:1251. doi: 10.3390/molecules24071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 3.Gil-Ramírez A., Soler-Rivas C. Mushrooms: Cultivation, Antioxidant Properties and Health Benefits. Nova Science Publishers; Harpak, NY, USA: 2014. The use of edible mushroom extracts as bioactive ingredients to design novel functional foods with hypocholesterolemic activities; pp. 43–74. [Google Scholar]
- 4.Reshetnikov S.V., Tan K.-K. Higher Basidiomycota as a Source of Antitumor and Immunostimulating Polysaccharides (Review) Int. J. Med. Mushrooms. 2001;3:34. doi: 10.1615/IntJMedMushr.v3.i4.80. [DOI] [Google Scholar]
- 5.Ahmad A., Kaleem M. Biopolymers for Food Design. Academic Press; Cambridge, MA, USA: 2018. β-Glucan as a Food Ingredient; pp. 351–381. [Google Scholar]
- 6.Nie S., Cui S.W., Jiang L. Bioactive Polysaccharides. Academic Press; Cambridge, MA, USA: 2018. Beta-Glucans and Their Derivatives; pp. 99–141. [DOI] [Google Scholar]
- 7.Bernstein A.M., Titgemeier B., Kirkpatrick K., Golubic M., Roizen M.F. Major Cereal Grain Fibers and Psyllium in Relation to Cardiovascular Health. Nutrients. 2013;5:1471–1487. doi: 10.3390/nu5051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q., Sheng X., Shi A., Hu H., Yang Y., Liu L., Fei L., Liu H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules. 2017;22:257. doi: 10.3390/molecules22020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novak M., Vetvicka V. β-Glucans, History, and the Present: Immunomodulatory Aspects and Mechanisms of Action. J. Immunotoxicol. 2008;5:47–57. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- 10.Yang D., Zhou Z., Zhang L.-J. Progress in Molecular Biology and Translational Science. Volume 163. Elsevier BV; Hoboken, NJ, USA: 2019. An overview of fungal glycan-based therapeutics; pp. 135–163. [DOI] [PubMed] [Google Scholar]
- 11.Vetvicka V., Větvičková J. Glucans and Cancer: Comparison of Commercially Available β-glucans–Part IV. Anticancer Res. 2018;38:1327–1333. doi: 10.21873/anticanres.12355. [DOI] [PubMed] [Google Scholar]
- 12.Kerckhoffs D.A.J.M., Hornstra G., Mensink R.P. Cholesterol-lowering effect of β-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when β-glucan is incorporated into bread and cookies. Am. J. Clin. Nutr. 2003;78:221–227. doi: 10.1093/ajcn/78.2.221. [DOI] [PubMed] [Google Scholar]
- 13.Vetvicka V., Vannucci L., Sima P. β-glucan as a new tool in vaccine development. Scand. J. Immunol. 2019;91:e12833. doi: 10.1111/sji.12833. [DOI] [PubMed] [Google Scholar]
- 14.Pillemer L., Blum L., Lepow I.H., Ross O.A., Todd E.W., Wardlaw A.C. The Properdin System and Immunity: I. Demonstration and Isolation of a New Serum Protein, Properdin, and Its Role in Immune Phenomena. Science. 1954;120:279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 15.Riggi S.J., Di Luzio N.R. Identification of a reticuloendothelial stimulating agent in zymosan. Am. J. Physiol. Content. 1961;200:297–300. doi: 10.1152/ajplegacy.1961.200.2.297. [DOI] [PubMed] [Google Scholar]
- 16.Ross O.A. The properdin system in relation to fatal bacteremia following total-body irradiation of laboratory animals. Ann. N. Y. Acad. Sci. 1956;66:274–279. doi: 10.1111/j.1749-6632.1956.tb40133.x. [DOI] [Google Scholar]
- 17.Kiser J.S., Lindh H., De Mello G.C. THE EFFECT OF VARIOUS SUBSTANCES ON RESISTANCE TO EXPERIMENTAL INFECTIONS. Ann. N. Y. Acad. Sci. 1956;66:312–328. doi: 10.1111/j.1749-6632.1956.tb40137.x. [DOI] [Google Scholar]
- 18.Old L.J., Clarke D.A., Benacerraf B., Goldsmith M. The reticuloendothelial system and the neoplastic process*. Ann. N. Y. Acad. Sci. 2006;88:264–280. doi: 10.1111/j.1749-6632.1960.tb20026.x. [DOI] [PubMed] [Google Scholar]
- 19.Di Carlo F.J., Fiore J.V. On the Composition of Zymosan. Science. 1958;127:756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- 20.Heller J.H. NONTOXIC RES STIMULATORY LIPIDS*. Ann. N. Y. Acad. Sci. 2006;88:116–121. doi: 10.1111/j.1749-6632.1960.tb20013.x. [DOI] [PubMed] [Google Scholar]
- 21.Chihara G., Maeda Y.Y., Hamuro J., Sasaki T., Fukuoka F. Inhibition of Mouse Sarcoma 180 by Polysaccharides from Lentinus edodes (Berk.) Sing. Nat. Cell Biol. 1969;222:687–688. doi: 10.1038/222687a0. [DOI] [PubMed] [Google Scholar]
- 22.Yang J., Tu J., Liu H., Wen L., Jiang G., Yang B. Identification of an immunostimulatory polysaccharide in banana. Food Chem. 2019;277:46–53. doi: 10.1016/j.foodchem.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Kaur R., Sharma M., Ji D., Xu M., Agyei D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers. 2019;8:1. doi: 10.3390/fib8010001. [DOI] [Google Scholar]
- 24.Bae I.Y., Kim H.W., Yoo H.J., Kim E.S., Lee S., Park D.Y., Lee H.G. Correlation of branching structure of mushroom β-glucan with its physiological activities. Food Res. Int. 2013;51:195–200. doi: 10.1016/j.foodres.2012.12.008. [DOI] [Google Scholar]
- 25.Bashir K.M.I., Choi J.-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017;18:1906. doi: 10.3390/ijms18091906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borchani C., Fonteyn F., Jamin G., Destain J., Willems L., Paquot M., Blecker C., Thonart P., Chema B., Fabienne F., et al. Structural Characterization, Technological Functionality and Physiological Aspects of Fungal ?-D-Glucans: A Review. Crit. Rev. Food Sci. Nutr. 2015;56:1746–1752. doi: 10.1080/10408398.2013.854733. [DOI] [PubMed] [Google Scholar]
- 27.Johansson L., Virkki L., Maunu S., Lehto M., Ekholm P., Varo P. Structural characterization of water soluble β-glucan of oat bran. Carbohydr. Polym. 2000;42:143–148. doi: 10.1016/S0144-8617(99)00157-5. [DOI] [Google Scholar]
- 28.Tohamy A.A., El-Ghor A.A., El Nahas S., Noshy M.M. β-Glucan inhibits the genotoxicity of cyclophosphamide, adriamycin and cisplatin. Mutat. Res. Toxicol. Environ. Mutagen. 2003;541:45–53. doi: 10.1016/S1383-5718(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 29.Gupta M., Abu-Ghannam N., Gallaghar E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of its By-Products. Compr. Rev. Food Sci. Food Saf. 2010;9:318–328. doi: 10.1111/j.1541-4337.2010.00112.x. [DOI] [PubMed] [Google Scholar]
- 30.Sikora P., Tosh S.M., Brummer Y., Olsson O. Identification of high β-glucan oat lines and localization and chemical characterization of their seed kernel β-glucans. Food Chem. 2013;137:83–91. doi: 10.1016/j.foodchem.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Izydorczyk M., Dexter J. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products–a Review. Food Res. Int. 2008;41:850–868. doi: 10.1016/j.foodres.2008.04.001. [DOI] [Google Scholar]
- 32.Benito-Román Ó., Alonso E., Gairola K., Cocero M. Fixed-bed extraction of β-glucan from cereals by means of pressurized hot water. J. Supercrit. Fluids. 2013;82:122–128. doi: 10.1016/j.supflu.2013.07.003. [DOI] [Google Scholar]
- 33.Mejía S.M.V., De Francisco A., Bohrer B. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020;60:3693–3704. doi: 10.1080/10408398.2019.1706444. [DOI] [PubMed] [Google Scholar]
- 34.Zhu F., Du B., Xu B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016;52:275–288. doi: 10.1016/j.foodhyd.2015.07.003. [DOI] [Google Scholar]
- 35.Man D.H., Yeon S.H., Sung H.H., Hyun W.S. Solubilization of water-insoluble β-glucan isolated from Ganoderma lucidum. J. Environ. Biol. 2008;29:237–242. [PubMed] [Google Scholar]
- 36.Nakashima A., Yamada K., Iwata O., Sugimoto R., Atsuji K., Ogawa T., Ishibashi-Ohgo N., Suzuki K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018;64:8–17. doi: 10.3177/jnsv.64.8. [DOI] [PubMed] [Google Scholar]
- 37.Zhan X., Lin C.-C., Zhang H.-T. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2012;93:525–531. doi: 10.1007/s00253-011-3740-2. [DOI] [PubMed] [Google Scholar]
- 38.Burkus Z., Temelli F. Rheological properties of barley β-glucan. Carbohydr. Polym. 2005;59:459–465. doi: 10.1016/j.carbpol.2004.10.012. [DOI] [Google Scholar]
- 39.Novak M. Glucans as Biological Response Modifiers. Endocr. Metab. Immune Disord. -Drug Targets. 2009;9:67–75. doi: 10.2174/187153009787582423. [DOI] [PubMed] [Google Scholar]
- 40.Henrion M., Francey C., Lê K.-A., Lamothe L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients. 2019;11:1729. doi: 10.3390/nu11081729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banchathanakij R., Suphantharika M. Effect of different β-glucans on the gelatinisation and retrogradation of rice starch. Food Chem. 2009;114:5–14. doi: 10.1016/j.foodchem.2008.09.016. [DOI] [Google Scholar]
- 42.Ulmius M., Önning G., Nilsson L. Solution behavior of barley β-glucan as studied with asymmetrical flow field-flow fractionation. Food Hydrocoll. 2012;26:175–180. doi: 10.1016/j.foodhyd.2011.05.004. [DOI] [Google Scholar]
- 43.Surenjav U., Zhang L., Xu X., Zhang X., Zeng F. Effects of molecular structure on antitumor activities of (1→3)-β-d-glucans from different Lentinus Edodes. Carbohydr. Polym. 2006;63:97–104. doi: 10.1016/j.carbpol.2005.08.011. [DOI] [Google Scholar]
- 44.Rieder A., Grimmer S., Kolset S.O., Michaelsen T.E., Knutsen S.H. Cereal β-glucan preparations of different weight average molecular weights induce variable cytokine secretion in human intestinal epithelial cell lines. Food Chem. 2011;128:1037–1043. doi: 10.1016/j.foodchem.2011.04.010. [DOI] [Google Scholar]
- 45.Zeković D.B., Kwiatkowski S., Vrvić M.M., JakovljeviĆ D., Moran C.A. Natural and Modified (1→3)-β-D-Glucans in Health Promotion and Disease Alleviation. Crit. Rev. Biotechnol. 2005;25:205–230. doi: 10.1080/07388550500376166. [DOI] [PubMed] [Google Scholar]
- 46.Chen J., Seviour R.J. Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycol. Res. 2007;111:635–652. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Driscoll M., Hansen R., Ding C., Cramer D.E., Yan J. Therapeutic potential of various β-glucan sources in conjunction with anti-tumor monoclonal antibody in cancer therapy. Cancer Biol. Ther. 2009;8:218–225. doi: 10.4161/cbt.8.3.7337. [DOI] [PubMed] [Google Scholar]
- 48.Soltanian S., Stuyven E., Cox E., Sorgeloos P., Bossier P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009;35:109–138. doi: 10.1080/10408410902753746. [DOI] [PubMed] [Google Scholar]
- 49.Han B., Baruah K., Cox E., Vanrompay D., Bossier P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020;11:658. doi: 10.3389/fimmu.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raa J. Immune modulation by non-digestible and non-absorbable beta-1,3/1,6-glucan. Microb. Ecol. Health Dis. 2015;26:27824. doi: 10.3402/mehd.v26.27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sletmoen M., Stokke B.T. Higher order structure of (1,3)-β-D-glucans and its influence on their biological activities and complexation abilities. Biopolym. Orig.Res. Biomol. 2008;89:310–321. doi: 10.1002/bip.20920. [DOI] [PubMed] [Google Scholar]
- 52.Brown G.D., Gordon S. A new receptor for β-glucans. Nat. Cell Biol. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 53.Chan G.C.-F., Chan W.K., Sze D.M.-Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown G.D., Gordon S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 56.Murphy E., Masterson C., Rezoagli E., O’Toole D., Major I., Stack G.D., Lynch M., Laffey J.G., Rowan N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020;732:139330. doi: 10.1016/j.scitotenv.2020.139330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ford E.S., Giles W.H., Mokdad A.H. Increasing Prevalence of the Metabolic Syndrome Among U.S. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 58.Detection E.E.P.O. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 59.Dhewantara F.X. Kusmiati Cholesterol-Lowering Effect of Beta Glucan Extracted from Saccharomyces cerevisiae in Rats. Sci. Pharm. 2016;84:153–165. doi: 10.3797/scipharm.isp.2015.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. Role of Bile Acids and Bile Acid Receptors in Metabolic Regulation. Physiol. Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 61.Staels B., Fonseca V.A. Bile Acids and Metabolic Regulation: Mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32:S237–S245. doi: 10.2337/dc09-S355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pullinger C.R., Eng C., Salen G., Shefer S., Batta A.K., Erickson S.K., Verhagen A., Rivera C.R., Mulvihill S.J., Malloy M.J., et al. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Investig. 2002;110:109–117. doi: 10.1172/JCI0215387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato K., Ohuchi A., Sook S.H., Toyomizu M., Akiba Y. Changes in mRNA expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol 7 alpha-hydroxylase in chickens. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2003;1630:96–102. doi: 10.1016/j.bbaexp.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Tong L.-T., Zhong K., Liu L., Qiu J., Guo L., Zhou X., Cao L., Zhou S. Effects of dietary wheat bran arabinoxylans on cholesterol metabolism of hypercholesterolemic hamsters. Carbohydr. Polym. 2014;112:1–5. doi: 10.1016/j.carbpol.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 65.Tong L.-T., Zhong K., Liu L., Zhou X., Qiu J., Zhou S. Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem. 2015;169:344–349. doi: 10.1016/j.foodchem.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 66.Houten S.M., Watanabe M., Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joyce S.A., Kamil A., Fleige L., Gahan C.G. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019;6:171. doi: 10.3389/fnut.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou A.L., Hergert N., Rompato G., Lefevre M. Whole Grain Oats Improve Insulin Sensitivity and Plasma Cholesterol Profile and Modify Gut Microbiota Composition in C57BL/6J Mice. J. Nutr. 2014;145:222–230. doi: 10.3945/jn.114.199778. [DOI] [PubMed] [Google Scholar]
- 69.Drzikova B., Dongowski G., Gebhardt E., Habel A. The composition of dietary fibre-rich extrudates from oat affects bile acid binding and fermentation in vitro. Food Chem. 2005;90:181–192. doi: 10.1016/j.foodchem.2004.03.041. [DOI] [Google Scholar]
- 70.Andersson K., Svedberg K., Lindholm M.W., Oste R., Hellstrand P. Oats (Avena sativa) reduce atherogenesis in LDL-receptor-deficient mice. Atherosclerosis. 2010;212:93–99. doi: 10.1016/j.atherosclerosis.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Gunness P., Michiels J., Vanhaecke L., De Smet S., Kravchuk O., Van De Meene A., Gidley M.J. Reduction in circulating bile acid and restricted diffusion across the intestinal epithelium are associated with a decrease in blood cholesterol in the presence of oat β-glucan. FASEB J. 2016;30:4227–4238. doi: 10.1096/fj.201600465R. [DOI] [PubMed] [Google Scholar]
- 72.Cheng H.-H., Lai M.-H. Fermentation of Resistant Rice Starch Produces Propionate Reducing Serum and Hepatic Cholesterol in Rats. J. Nutr. 2000;130:1991–1995. doi: 10.1093/jn/130.8.1991. [DOI] [PubMed] [Google Scholar]
- 73.Tiwari U., Cummins E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition. 2011;27:1008–1016. doi: 10.1016/j.nut.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 74.McRorie J., McKeown N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017;117:251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Wolever T.M., Tosh S.M., Gibbs A.L., Brand-Miller J., Duncan A.M., Hart V., Lamarche B., Thomson B.A., Duss R., Wood P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 2010;92:723–732. doi: 10.3945/ajcn.2010.29174. [DOI] [PubMed] [Google Scholar]
- 76.Kahlon T., Smith G., Shao Q. In vitro binding of bile acids by kidney bean (Phaseolus vulgaris), black gram (Vigna mungo), bengal gram (Cicer arietinum) and moth bean (Phaseolus aconitifolins) Food Chem. 2005;90:241–246. doi: 10.1016/j.foodchem.2004.03.046. [DOI] [Google Scholar]
- 77.Ellegård L., Andersson H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: An ileostomy study. Eur. J. Clin. Nutr. 2007;61:938–945. doi: 10.1038/sj.ejcn.1602607. [DOI] [PubMed] [Google Scholar]
- 78.Theuwissen E., Mensink R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008;94:285–292. doi: 10.1016/j.physbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Maki K.C., Shinnick F., Seeley M.A., Veith P.E., Quinn L.C., Hallissey P.J., Temer A., Davidson M. Food Products Containing Free Tall Oil-Based Phytosterols and Oat β-Glucan Lower Serum Total and LDL Cholesterol in Hypercholesterolemic Adults. J. Nutr. 2003;133:808–813. doi: 10.1093/jn/133.3.808. [DOI] [PubMed] [Google Scholar]
- 80.Keenan J.M., Pins J.J., Frazel C., Moran A., Turnquist L. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: A pilot trial. J. Fam. Pr. 2002;51:369. [PubMed] [Google Scholar]
- 81.Biörklund M., Van Rees A., Mensink R.P., Önning G., Bi M. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with β-glucans from oats or barley: A randomised dose-controlled trial. Eur. J. Clin. Nutr. 2005;59:1272–1281. doi: 10.1038/sj.ejcn.1602240. [DOI] [PubMed] [Google Scholar]
- 82.Streppel M.T., Arends L.R., Veer P.V.T., Grobbee D.E., Geleijnse J.M. Dietary Fiber and Blood Pressure. Arch. Intern. Med. 2005;165:150–156. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- 83.El Khoury D., Cuda C., Luhovyy B.L., Anderson G.H. Beta Glucan: Health Benefits in Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012;2012:851362. doi: 10.1155/2012/851362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Behall K.M., Scholfield D.J., Hallfrisch J.G., Liljeberg-Elmståhl H.G. Consumption of Both Resistant Starch and -Glucan Improves Postprandial Plasma Glucose and Insulin in Women. Diabetes Care. 2006;29:976–981. doi: 10.2337/dc05-2012. [DOI] [PubMed] [Google Scholar]
- 85.Selph S., Ginsburg A.D., Chou R. Impact of contacting study authors to obtain additional data for systematic reviews: Diagnostic accuracy studies for hepatic fibrosis. Syst. Rev. 2014;3:107. doi: 10.1186/2046-4053-3-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wood P.J., Beer M.U., Butler G.B. Evaluation of role of concentration and molecular weight of oat β-glucan in determining effect of viscosity on plasma glucose and insulin following an oral glucose load. Br. J. Nutr. 2000;84:19–23. doi: 10.1017/S0007114500001185. [DOI] [PubMed] [Google Scholar]
- 87.Hooda S., Matte J., Vasanthan T., Zijlstra R.T. Dietary purified oat β-glucan reduces peak glucose absorption and portal insulin release in portal-vein catheterized grower pigs. Livest. Sci. 2010;134:15–17. doi: 10.1016/j.livsci.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 88.Regand A., Chowdhury Z., Tosh S.M., Wolever T.M., Wood P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011;129:297–304. doi: 10.1016/j.foodchem.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 89.Dong J., Cai F., Shen R.-L., Liu Y. Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta-glucan in streptozotocin-induced diabetic mice. Food Chem. 2011;129:1066–1071. doi: 10.1016/j.foodchem.2011.05.076. [DOI] [PubMed] [Google Scholar]
- 90.Granfeldt Y., Nyberg L., Björck I. Muesli with 4 g oat β-glucans lowers glucose and insulin responses after a bread meal in healthy subjects. Eur. J. Clin. Nutr. 2007;62:600–607. doi: 10.1038/sj.ejcn.1602747. [DOI] [PubMed] [Google Scholar]
- 91.Mäkeläinen H., Anttila H., Sihvonen J., Hietanen R.-M., Tahvonen R., Salminen E., Mikola M., Sontag-Strohm T. The effect of β-glucan on the glycemic and insulin index. Eur. J. Clin. Nutr. 2006;61:779–785. doi: 10.1038/sj.ejcn.1602561. [DOI] [PubMed] [Google Scholar]
- 92.Jenkins A.L., Jenkins D.J.A., Zdravkovic U., Würsch P., Vuksan V. Depression of the glycemic index by high levels of β-glucan fiber in two functional foods tested in type 2 diabetes. Eur. J. Clin. Nutr. 2002;56:622–628. doi: 10.1038/sj.ejcn.1601367. [DOI] [PubMed] [Google Scholar]
- 93.Tapola N., Karvonen H., Niskanen L., Mikola M., Sarkkinen E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2005;15:255–261. doi: 10.1016/j.numecd.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Wolever T.M.S., Tosh S.M., Spruill S.E., Jenkins A.L., Ezatagha A., Duss R., Johnson J., Chu Y., Steinert R.E. Increasing oat β-glucan viscosity in a breakfast meal slows gastric emptying and reduces glycemic and insulinemic responses but has no effect on appetite, food intake, or plasma ghrelin and PYY responses in healthy humans: A randomized, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2019;111:319–328. doi: 10.1093/ajcn/nqz285. [DOI] [PubMed] [Google Scholar]
- 95.Gamel T.H., Abdel-Aal E.-S.M., Ames N.P., Duss R., Tosh S.M. Enzymatic extraction of beta-glucan from oat bran cereals and oat crackers and optimization of viscosity measurement. J. Cereal Sci. 2014;59:33–40. doi: 10.1016/j.jcs.2013.10.011. [DOI] [Google Scholar]
- 96.Miyamoto J., Watanabe K., Taira S., Kasubuchi M., Li X., Irie J., Itoh H., Kimura I. Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS ONE. 2018;13:e0196579. doi: 10.1371/journal.pone.0196579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hooda S., Matte J.J., Vasanthan T., Zijlstra R.T. Dietary Oat β-Glucan Reduces Peak Net Glucose Flux and Insulin Production and Modulates Plasma Incretin in Portal-Vein Catheterized Grower Pigs. J. Nutr. 2010;140:1564–1569. doi: 10.3945/jn.110.122721. [DOI] [PubMed] [Google Scholar]
- 98.King D.E. Dietary fiber, inflammation, and cardiovascular disease. Mol. Nutr. Food Res. 2005;49:594–600. doi: 10.1002/mnfr.200400112. [DOI] [PubMed] [Google Scholar]
- 99.Liatis S., Tsapogas P., Chala E., Dimosthenopoulos C., Kyriakopoulos K., Kapantais E., Katsilambros N. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type2 diabetes. Diabetes Metab. 2009;35:115–120. doi: 10.1016/j.diabet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Lobato R.V., Silva V.D.O., Andrade E.F., Orlando D.R., Zangeronimo M.G., De Sousa R.V., Pereira L.J. Metabolic effects of ?-glucans (saccharomyces cerevisae) per os administration in rats with streptozotocin-induced diabetes. Nutrición Hospitalaria. 2015;32:256–264. doi: 10.3305/nh.2015.32.1.9013. [DOI] [PubMed] [Google Scholar]
- 101.Reyna-Villasmil N., Cano C., Bermúdez V.J., Medina M.T., Souki A.J., Ambard M., Nuñez M., Ferrer M.A., Inglett G.E. Sweeteners and Beta-Glucans Improve Metabolic and Anthropometrics Variables in Well Controlled Type 2 Diabetic Patients. Am. J. Ther. 2003;10:438–443. doi: 10.1097/00045391-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 102.Battilana P., Ornstein K., Minehira K., Schwarz J.M., Acheson K., Schneiter P., Burri J., Jéquier E., Tappy L. Mechanisms of action of β-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. 2001;55:327–333. doi: 10.1038/sj.ejcn.1601160. [DOI] [PubMed] [Google Scholar]
- 103.Choi J.S., Kim H., Jung M.H., Hong S., Song J. Consumption of barley β-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010;54:1004–1013. doi: 10.1002/mnfr.200900127. [DOI] [PubMed] [Google Scholar]
- 104.Brockman D.A., Chen X., Gallaher D.D. Consumption of a high β-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur. J. Nutr. 2012;52:1743–1753. doi: 10.1007/s00394-012-0478-2. [DOI] [PubMed] [Google Scholar]
- 105.Belobrajdic D.P., Jobling S.A., Morell M.K., Taketa S., Bird A.R. Wholegrain barley β-glucan fermentation does not improve glucose tolerance in rats fed a high-fat diet. Nutr. Res. 2015;35:162–168. doi: 10.1016/j.nutres.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 106.Mälkki Y., Virtanen E. Gastrointestinal Effects of Oat Bran and Oat Gum: A Review. LWT. 2001;34:337–347. doi: 10.1006/fstl.2001.0795. [DOI] [Google Scholar]
- 107.Atanasov J., Schloermann W., Trautvetter U., Glei M. The effects of β-glucans on intestinal health. Ernahrungs Umschau. 2020;67:52–59. doi: 10.4455/eu.2020.010. [DOI] [Google Scholar]
- 108.Shen X.J., Rawls J.F., Randall T.A., Burcall L., Mpande C.N., Jenkins N., Jovov B., Abdo Z., Sandler R.S., Keku T.O. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–147. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drzikova B., Dongowski G., Gebhardt E. Dietary fibre-rich oat-based products affect serum lipids, microbiota, formation of short-chain fatty acids and steroids in rats. Br. J. Nutr. 2005;94:1012–1025. doi: 10.1079/BJN20051577. [DOI] [PubMed] [Google Scholar]
- 110.Jayachandran M., Chen J., Chung S.S.M., Xu B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018;61:101–110. doi: 10.1016/j.jnutbio.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Ryan P.M., London L.E.E., Bjorndahl T.C., Mandal R., Murphy K., Fitzgerald G.F., Shanahan F., Ross R.P., Wishart D.S., Caplice N.M., et al. Microbiome and metabolome modifying effects of several cardiovascular disease interventions in apo-E-/- mice. Microbiome. 2017;5:30. doi: 10.1186/s40168-017-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swennen K., Courtin C.M., Delcour J.A. Non-digestible Oligosaccharides with Prebiotic Properties. Crit. Rev. Food Sci. Nutr. 2006;46:459–471. doi: 10.1080/10408390500215746. [DOI] [PubMed] [Google Scholar]
- 113.Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E.K., MacDougall K., Preston T., Tedford C., Finlayson G.S., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2014;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Synytsya A., Novak M. Structural analysis of glucans. Ann. Transl. Med. 2014;2:17. doi: 10.3978/j.issn.2305-5839.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Synytsya A., Novák M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013;92:792–809. doi: 10.1016/j.carbpol.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 116.C.Ooi V., Liu V.E.C.A.F. Immunomodulation and Anti-Cancer Activity of Polysaccharide-Protein Complexes. Curr. Med. Chem. 2000;7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 117.Jesenak M., Majtan J., Rennerova Z., Kyselovic J., Banovcin P., Hrubisko M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013;15:395–399. doi: 10.1016/j.intimp.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 118.Yamauchi E., Shoji S., Nishihara M., Shimoda T., Nishima S. Contribution of Lung Fibroblast Migration in the Fibrotic Process of Airway Remodeling in Asthma. Allergol. Int. 2008;57:73–78. doi: 10.2332/allergolint.O-06-481. [DOI] [PubMed] [Google Scholar]
- 119.Fuller R., Butt H., Noakes P.S., Kenyon J., Yam T.S., Calder P.C. Influence of yeast-derived 1,3/1,6 glucopolysaccharide on circulating cytokines and chemokines with respect to upper respiratory tract infections. Nutrition. 2012;28:665–669. doi: 10.1016/j.nut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 120.Kofuji K., Aoki A., Tsubaki K., Konishi M., Isobe T., Murata Y. Antioxidant Activity ofβ-Glucan. ISRN Pharm. 2012;2012:125864. doi: 10.5402/2012/125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamada J., Hamuro J., Hatanaka H., Hamabata K., Kinoshita S. Alleviation of seasonal allergic symptoms with superfine β-1,3-glucan: A randomized study. J. Allergy Clin. Immunol. 2007;119:1119–1126. doi: 10.1016/j.jaci.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 122.Vaclav V., Josef R., Vladimir S., Lucie R.D., Vlastimil K. Placebo-driven clinical trials of transfer point glucan #300 in children with chronic respiratory problems: III. clinical findings. Am. J. Immunol. 2013;9:88–93. doi: 10.3844/ajisp.2013.88.93. [DOI] [Google Scholar]
- 123.Fang J., Wang Y., Lv X., Shen X., Ni X., Ding K. Structure of a β-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-κB signaling. Glycoconj. J. 2012;29:365–377. doi: 10.1007/s10719-012-9416-z. [DOI] [PubMed] [Google Scholar]
- 124.Dalonso N., Goldman G.H., Gern R.M.M. β-(1→3),(1→6)-Glucans: Medicinal activities, characterization, biosynthesis and new horizons. Appl. Microbiol. Biotechnol. 2015;99:7893–7906. doi: 10.1007/s00253-015-6849-x. [DOI] [PubMed] [Google Scholar]
- 125.Baert K., Sonck E., Goddeeris B.M., Devriendt B., Cox E. Cell type-specific differences in β-glucan recognition and signalling in porcine innate immune cells. Dev. Comp. Immunol. 2015;48:192–203. doi: 10.1016/j.dci.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 126.Guo Y., Fukuda T., Donai K., Kuroda K., Masuda M., Nakamura S., Yoneyama H., Isogai E. Leptospiral lipopolysaccharide stimulates the expression of toll-like receptor 2 and cytokines in pig fibroblasts. Anim. Sci. J. 2014;86:238–244. doi: 10.1111/asj.12254. [DOI] [PubMed] [Google Scholar]
- 127.Kanjan P., Sahasrabudhe N.M., De Haan B.J., De Vos P. Immune effects of β-glucan are determined by combined effects on Dectin-1, TLR2, 4 and 5. J. Funct. Foods. 2017;37:433–440. doi: 10.1016/j.jff.2017.07.061. [DOI] [Google Scholar]
- 128.Xu X., Chen P., Zhang L., Ashida H. Chain structures of glucans from Lentinus edodes and their effects on NO production from RAW 264.7 macrophages. Carbohydr. Polym. 2012;87:1855–1862. doi: 10.1016/j.carbpol.2011.10.015. [DOI] [Google Scholar]
- 129.Jedinak A., Dudhgaonkar S., Wu Q., Simon J.E., Sliva D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-κB and AP-1 signaling. Nutr. J. 2011;10:52. doi: 10.1186/1475-2891-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soltys J., Quinn M.T. Modulation of Endotoxin- and Enterotoxin-Induced Cytokine Release by In Vivo Treatment with β-(1,6)-Branched β-(1,3)-Glucan. Infect. Immun. 1999;67:244–252. doi: 10.1128/IAI.67.1.244-252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johnson E., Førland D.T., Saetre L., Bernardshaw S.V., Lyberg T., Hetland G., Sætre L. Effect of an Extract Based on the Medicinal MushroomAgaricus blazeiMurill on Release of Cytokines, Chemokines and Leukocyte Growth Factors in Human BloodEx VivoandIn Vivo. Scand. J. Immunol. 2009;69:242–250. doi: 10.1111/j.1365-3083.2008.02218.x. [DOI] [PubMed] [Google Scholar]
- 132.Brown G.D., Herre J., Williams D.L., Willment J.A., Marshall A.S.J., Gordon S. Dectin-1 Mediates the Biological Effects of β-Glucans. J. Exp. Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gantner B.N., Simmons R.M., Canavera S.J., Akira S., Underhill D.M. Collaborative Induction of Inflammatory Responses by Dectin-1 and Toll-like Receptor. J. Exp. Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herre J. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 135.Smeekens S.P., Gresnigt M.S., Becker K.L., Cheng S.-C., Netea S.A., Jacobs L., Jansen T., Van De Veerdonk F.L., Williams D.L., Joosten L.A., et al. An anti-inflammatory property of Candida albicans β-glucan: Induction of high levels of interleukin-1 receptor antagonist via a Dectin-1/CR3 independent mechanism. Cytokine. 2015;71:215–222. doi: 10.1016/j.cyto.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murphy E., Masterson C., Rezoagli E., O’Toole D., Laffey J., Major I., Stack G., Rowan N. ALUNG INJURY, SEPSIS, AND ARDS. American Thoracic Society; New York, NY, USA: 2019. Immunomodulation Properties of a Novel β-Glucan Extract from the Mushroom Lentinus Edodes in an In-Vitro Lung Injury Model; p. A2114. [Google Scholar]
- 137.Vetvicka V., Vetvickova J. Glucan supplementation enhances the immune response against an influenza challenge in mice. Ann. Transl. Med. 2015;3:22. doi: 10.3978/j.issn.2305-5839.2015.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rasmussen L.-T., Fandrem J., Seljelid R. Dynamics of Blood Components and Peritoneal Fluid during Treatment of Murine E. coli Sepsis with beta-1,3-D-polyglucose Derivatives. Scand. J. Immunol. 1990;32:333–340. doi: 10.1111/j.1365-3083.1990.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 139.Onderdonk A.B., Cisneros R.L., Hinkson P., Ostroff G. Anti-infective effect of poly-beta 1-6-glucotriosyl-beta 1-3-glucopyranose glucan in vivo. Infect. Immun. 1992;60:1642–1647. doi: 10.1128/IAI.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cisneros R.L., Gibson F.C., Tzianabos A.O. Passive transfer of poly-(1-6)-beta-glucotriosyl-(1-3)-beta-glucopyranose glucan protection against lethal infection in an animal model of intra-abdominal sepsis. Infect. Immun. 1996;64:2201–2205. doi: 10.1128/IAI.64.6.2201-2205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tzianabos A.O., Gibson F.C., Cisneros R.L., Kasper D.L. Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J. Infect. Dis. 1998;178:200–206. doi: 10.1086/515594. [DOI] [PubMed] [Google Scholar]
- 142.Liang J., Melican D., Cafro L., Palace G., Fisette L., Armstrong R., Patchen M.L. Enhanced clearance of a multiple antibiotic resistant Staphylococcus aureus in rats treated with PGG-glucan is associated with increased leukocyte counts and increased neutrophil oxidative burst activity. Int. J. Immunopharmacol. 1998;20:595–614. doi: 10.1016/S0192-0561(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 143.Senoglu N., Yuzbasioglu M.F., Aral M., Ezberci M., Kurutas E.B., Bulbuloglu E., Oksuz H., Ciragil P., Ezberci F. Protective Effects ofN-Acetylcysteine and β -Glucan Pretreatment on Oxidative Stress in Cecal Ligation and Puncture Model of Sepsis. J. Investig. Surg. 2008;21:237–243. doi: 10.1080/08941930802180136. [DOI] [PubMed] [Google Scholar]
- 144.Gülmen Ş., Kiris I., Kocyigit A., Dogus D.K., Ceylan B.G., Meteoglu I. β-Glucan Protects against Lung Injury Induced by Abdominal Aortic Ischemia-Reperfusion in Rats. J. Surg. Res. 2010;164:e325–e332. doi: 10.1016/j.jss.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 145.Babayigit H., Kucuk C., Sozuer E., Yazici C., Kose K., Akgun H. Protective effect of β-glucan on lung injury after cecal ligation and puncture in rats. Intensive Care Med. 2005;31:865–870. doi: 10.1007/s00134-005-2629-x. [DOI] [PubMed] [Google Scholar]
- 146.Bedirli A., Kerem M., Pasaoglu H., Akyurek N., Tezcaner T., Elbeg S., Memis L., Sakrak O. Beta-glucan attenuates inflammatory cytokine release and prevents acute lung injury in an experimental model of sepsis. Shock. 2007;27:397–401. doi: 10.1097/01.shk.0000245030.24235.f1. [DOI] [PubMed] [Google Scholar]
- 147.Chan A.S.H., Jonas A.B., Qiu X., Ottoson N.R., Walsh R.M., Gorden K.B., Harrison B., Maimonis P.J., Leonardo S.M., Ertelt K.E., et al. Imprime PGG-Mediated Anti-Cancer Immune Activation Requires Immune Complex Formation. PLoS ONE. 2016;11:e0165909. doi: 10.1371/journal.pone.0165909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bose N., Chan A.S.H., Eguerrero F., Maristany C.M., Eqiu X., Walsh R., Ertelt K.E., Jonas A.B., Gorden K.B., Dudney C.M., et al. Binding of Soluble Yeast β-Glucan to Human Neutrophils and Monocytes is Complement-Dependent. Front. Immunol. 2013;4:230. doi: 10.3389/fimmu.2013.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hong F., Hansen R.D., Yan J., Allendorf D.J., Baran J.T., Ostroff G.R., Ross G.D. β-Glucan Functions as an Adjuvant for Monoclonal Antibody Immunotherapy by Recruiting Tumoricidal Granulocytes as Killer Cells. Cancer Res. 2003;63:9023–9031. [PubMed] [Google Scholar]
- 150.Vetvicka V., Dvořák B., Vetvickova J., Richter J., Krizan J., Sima P., Yvin J.-C. Orally administered marine (1→3)-β-d-glucan Phycarine stimulates both humoral and cellular immunity. Int. J. Biol. Macromol. 2007;40:291–298. doi: 10.1016/j.ijbiomac.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 151.Thandapilly S.J., Ndou S.P., Wang Y., Nyachoti C.M., Ames N. Barley β-glucan increases fecal bile acid excretion and short chain fatty acid levels in mildly hypercholesterolemic individuals. Food Funct. 2018;9:3092–3096. doi: 10.1039/C8FO00157J. [DOI] [PubMed] [Google Scholar]
- 152.Wang Y., Harding S.V., Thandapilly S.J., Tosh S.M., Jones P.J.H., Ames N.P. Barley β-glucan reduces blood cholesterol levels via interrupting bile acid metabolism. Br. J. Nutr. 2017;118:822–829. doi: 10.1017/S0007114517002835. [DOI] [PubMed] [Google Scholar]
- 153.Aoe S., Ikenaga T., Noguchi H., Kohashi C., Kakumoto K., Kohda N. Effect of cooked white rice with high β-glucan barley on appetite and energy intake in healthy Japanese subjects: A randomized controlled trial. Plant Foods Hum. Nutr. 2014;69:325–330. doi: 10.1007/s11130-014-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aoe S., Ichinose Y., Kohyama N., Komae K., Takahashi A., Abe D., Yoshioka T., Yanagisawa T. Effects of high β-glucan barley on visceral fat obesity in Japanese individuals: A randomized, double-blind study. Nutrition. 2017;42:1–6. doi: 10.1016/j.nut.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 155.Thondre P.S., Shafat A., Clegg M.E. Molecular weight of barley β-glucan influences energy expenditure, gastric emptying and glycaemic response in human subjects. Br. J. Nutr. 2013;110:2173–2179. doi: 10.1017/S0007114513001682. [DOI] [PubMed] [Google Scholar]
- 156.Zaremba S.M., Gow I.F., Drummond S., McCluskey J.T., Steinert R.E. Effects of oat β-glucan consumption at breakfast on ad libitum eating, appetite, glycemia, insulinemia and GLP-1 concentrations in healthy subjects. Appetite. 2018;128:197–204. doi: 10.1016/j.appet.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 157.Pentikäinen S.P., Karhunen L., Flander L., Katina K., Meynier A., Aymard P., Vinoy S., Poutanen K. Enrichment of biscuits and juice with oat β-glucan enhances postprandial satiety. Appetite. 2014;75:150–156. doi: 10.1016/j.appet.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 158.Rebello C.J., Chu Y., Johnson W.D., Martin C.K., Han H., Bordenave N., Shi Y., O’Shea M., Greenway F.L. The role of meal viscosity and oat β-glucan characteristics in human appetite control: A randomized crossover trial. Nutr. J. 2014;13:49. doi: 10.1186/1475-2891-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Thongoun P., Pavadhgul P., Bumrungpert A., Satitvipawee P., Harjani Y., Kurilich A. Effect of oat consumption on lipid profiles in hypercholesterolemic adults. J. Med. Assoc. Thail. Chotmaihet Thangphaet. 2013;96:25. [PubMed] [Google Scholar]
- 160.Queenan K.M., Stewart M.L., Smith K.N., Thomas W., Fulcher R.G., Slavin J.L. Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr. J. 2007;6:6–8. doi: 10.1186/1475-2891-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pavadhgul P., Bumrungpert A., Harjani Y., Kurilich A. Oat porridge consumption alleviates markers of inflammation and oxidative stress in hypercholesterolemic adults. ASIA Pac. J. Clin. Nutr. 2019;28:260–265. doi: 10.6133/apjcn.201906_28(2).0008. [DOI] [PubMed] [Google Scholar]
- 162.De Souza S.R., De Oliveira G.M.M., Luiz R.R., Rosa G. Effects of oat bran and nutrition counseling on the lipid and glucose profile and anthropometric parameters of hypercholesterolemia patients. Nutrición Hospitalaria. 2016;33:123–130. doi: 10.20960/nh.40. [DOI] [PubMed] [Google Scholar]
- 163.Aarsæther E., Rydningen M., Engstad R.E., Busund R. Cardioprotective effect of pretreatment with β-glucan in coronary artery bypass grafting. Scand. Cardiovasc. J. 2006;40:298–304. doi: 10.1080/14017430600868567. [DOI] [PubMed] [Google Scholar]
- 164.Davidson M.H., Dugan L.D., Burns J.H., Bova J., Story K., Drennan K.B. The Hypocholesterolemic Effects of β-Glucan in Oatmeal and Oat Bran. JAMA. 1991;265:1833–1839. doi: 10.1001/jama.1991.03460140061027. [DOI] [PubMed] [Google Scholar]
- 165.Lia A., Hallmans G., Sandberg A.S., Sundberg B., Aman P., Andersson H. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am. J. Clin. Nutr. 1995;62:1245–1251. doi: 10.1093/ajcn/62.6.1245. [DOI] [PubMed] [Google Scholar]
- 166.Lovegrove J.A., Clohessy A., Milon H., Williams C.M. Modest doses of β-glucan do not reduce concentrations of potentially atherogenic lipoproteins. Am. J. Clin. Nutr. 2000;72:49–55. doi: 10.1093/ajcn/72.1.49. [DOI] [PubMed] [Google Scholar]
- 167.Velikonja A., Lipoglavšek L., Zorec M., Orel R., Avguštin G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe. 2019;55:67–77. doi: 10.1016/j.anaerobe.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y., Harding S.V., Eck P., Thandapilly S.J., Gamel T.H., Abdel-Aal E.-S.M., Crow G.H., Tosh S.M., Jones P.J.H., Ames N. High-Molecular-Weight β-Glucan Decreases Serum Cholesterol Differentially Based on the CYP7A1 rs3808607 Polymorphism in Mildly Hypercholesterolemic Adults. J. Nutr. 2015;146:720–727. doi: 10.3945/jn.115.223206. [DOI] [PubMed] [Google Scholar]
- 169.Abrams D.I., Couey P., Shade S.B., Kelly M.E., Kamanu-Elias N., Stamets P. Antihyperlipidemic effects of Pleurotus ostreatus (oyster mushrooms) in HIV-infected individuals taking antiretroviral therapy. BMC Complement. Altern. Med. 2011;11:60. doi: 10.1186/1472-6882-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Varricchio A.M., Capasso M., Della Volpe A., Malafronte L., Mansi N., Varricchio A., Ciprandi G. Resveratrol plus carboxymethyl-β-glucan in children with recurrent respiratory infections: A preliminary and real-life experience. Ital. J. Pediatr. 2014;40:93. doi: 10.1186/s13052-014-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gaullier J.-M., Sleboda J., Ofjord E.S., Ulvestad E., Nurminiemi M., Moe C., Albrektsen T., Gudmundsen O. Supplementation with a Soluble Beta-Glucan Exported from Shiitake Medicinal Mushroom, Lentinus edodes (Berk.) Singer Mycelium: A Crossover, Placebo-Controlled Study in Healthy Elderly. Int. J. Med. Mushrooms. 2011;13:319–326. doi: 10.1615/IntJMedMushr.v13.i4.10. [DOI] [PubMed] [Google Scholar]
- 172.Browder W., Williams D., Pretus H., Olivero G., Enrichens F., Mao P., Franchello A. Beneficial effect of enhanced macrophage function in the trauma patient. Ann. Surg. 1990;211:605–613. [PMC free article] [PubMed] [Google Scholar]
- 173.Júnior J.D.F., Júnior M.D.R.E.S., Maciel F.M., Soares A.D.M., Mendes N.F. Infection prevention in patients with severe multiple trauma with the immunomodulator beta 1-3 polyglucose (glucan) Surg. Gynecol. Obstet. 1993;177:383–388. [PubMed] [Google Scholar]
- 174.Babineau T.J., Marcello P., Swails W., Kenler A., Bistrian B., Forse R.A. Randomized Phase I/II Trial of a Macrophage-Specific Immunomodulator (PGG-Glucan) in High-Risk Surgical Patients. Ann. Surg. 1994;220:601–609. doi: 10.1097/00000658-199411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Babineau T.J., Hackford A., Kenler A., Bistrian B., Forse R.A., Fairchild P.G., Heard S., Keroack M., Caushaj P., Benotti P.N. A Phase II Multicenter, Double-blind, Randomized, Placebo-Controlled Study of Three Dosages of an Immunomodulator (PGG-Glucan) in High-Risk Surgical Patients. Arch. Surg. 1994;129:1204–1210. doi: 10.1001/archsurg.1994.01420350102014. [DOI] [PubMed] [Google Scholar]
- 176.Dellinger E.P., Babineau T.J., Bleicher P., Kaiser A.B., Seibert G.B., Postier R.G., Vogel S.B., Norman J., Kaufman D., Galandiuk S., et al. Effect of PGG-glucan on the Rate of Serious Postoperative Infection or Death Observed After High-Risk Gastrointestinal Operations. Arch. Surg. 1999;134:977–983. doi: 10.1001/archsurg.134.9.977. [DOI] [PubMed] [Google Scholar]
- 177.Leentjens J., Quintin J., Gerretsen J., Kox M., Pickkers P., Moorlag S.J. The Effects of Orally Administered Beta-Glucan on Innate Immune Responses in Humans, a Randomized Open-Label Intervention Pilot-Study. PLoS ONE. 2014;9:e108794. doi: 10.1371/journal.pone.0108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Taguchi T. Effects of lentinan on the patients with advanced or recurrent gastric, colorectal and breast cancer. Jpn. J. Cancer Chemother. 1983;10:387–393. [PubMed] [Google Scholar]
- 179.Wesa K.M., Cunningham-Rundles S., Klimek V.M., Vertosick E., Coleton M.I., Yeung K.S., Lin H., Nimer S., Cassileth B.R. Maitake mushroom extract in myelodysplastic syndromes (MDS): A phase II study. Cancer Immunol. Immunother. 2015;64:237–247. doi: 10.1007/s00262-014-1628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Weitberg A.B. A phase I/II trial of beta-(1,3)/(1,6) D-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J. Exp. Clin. Cancer Res. 2008;27:40. doi: 10.1186/1756-9966-27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zent C.S., Call T.G., Bowen D.A., Conte M.J., LaPlant B.R., Witzig T.E., Ansell S.M., Weiner G.J. Early treatment of high risk chronic lymphocytic leukemia with alemtuzumab, rituximab and poly-(1-6)-beta-glucotriosyl-(1-3)- beta-glucopyranose beta-glucan is well tolerated and achieves high complete remission rates. Leuk. Lymphoma. 2015;56:2373–2378. doi: 10.3109/10428194.2015.1016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomas M., Sadjadian P., Kollmeier J., Lowe J., Mattson P., Trout J.R., Gargano M., Patchen M.L., Walsh R., Beliveau M., et al. A randomized, open-label, multicenter, phase II study evaluating the efficacy and safety of BTH1677 (1,3–1,6 beta glucan; Imprime PGG) in combination with cetuximab and chemotherapy in patients with advanced non-small cell lung cancer. Investig. New Drugs. 2017;35:345–358. doi: 10.1007/s10637-017-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Engel-Riedel W., Schneller F., Wolf M., Schuette W., Lowe J., Mattson P., Gargano M., Patchen M.L., Huhn R., Braun A. Imprime Pgg, a Novel Immune Modulator, in the 1St-Line Treatment of Stage Iv Nsclc: Results from a Randomized, Controlled, Multicenter Phase 2 Study. Ann. Oncol. 2014;25:v1. doi: 10.1093/annonc/mdu438.35. [DOI] [Google Scholar]
- 184.Du B., Meenu M., Liu H., Xu B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019;20:4032. doi: 10.3390/ijms20164032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui W., Wood P. Relationships between structural features, molecular weight and rheological properties of cereal β-D-glucans. Hydrocolloids. 2000:159–168. doi: 10.1016/b978-044450178-3/50019-6. [DOI] [Google Scholar]
- 186.Lazaridou A., Biliaderis C. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007;46:101–118. doi: 10.1016/j.jcs.2007.05.003. [DOI] [Google Scholar]
- 187.Bohn J.A., BeMiller J.N. (1→3)-β-d-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995;28:3–14. doi: 10.1016/0144-8617(95)00076-3. [DOI] [Google Scholar]
- 188.Yuan H., Lan P., He Y., Li C., Ma X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules. 2019;25:57. doi: 10.3390/molecules25010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Castro E.D.M., Calder P.C., Roche H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2020:1901071. doi: 10.1002/mnfr.201901071. [DOI] [PMC free article] [PubMed] [Google Scholar]