Abstract
Introduction
Mucinous cystic neoplasms are uncommon among the tumors that develop in the retroperitoneum. We report a case of primary retroperitoneal mucinous cystadenocarcinoma with pathological considerations.
Case presentation
A 47‐year‐old woman complaining of abdominal discomfort presented at our hospital. Abdominal computed tomography and magnetic resonance imaging showed a large cystic tumor with small solid nodules located in the right retroperitoneum. The tumor was completely removed and the microscopic findings were consistent with primary retroperitoneal mucinous cystadenocarcinoma. Two years after the surgery, the patient is alive without recurrence of the tumor.
Conclusion
The microscopic findings suggested that the primary retroperitoneal mucinous cystadenocarcinoma developed from the metaplasia of the remnant coelomic epithelium. A complete tumor resection that includes the adjacent peritoneum is important to prevent local recurrence.
Keywords: coelomic epithelium, female hormone, metaplasia, mucinous cystadenocarcinoma, retroperitoneum
Abbreviations & Acronyms
- CA
cancer antigen
- CEA
carcinoembryonic antigen
- PRMC
primary retroperitoneal mucinous cystadenocarcinoma
Keynote message.
PRMC is an extremely rare tumor, and the optimal treatment is still unknown. Our pathological findings suggested that PRMC developed from the metaplasia of the remnant coelomic epithelium. A complete resection of the tumor including the adjacent peritoneum is recommended to prevent local recurrence.
Introduction
PRMC is a rare neoplasm, with only 85 cases including ours that have been reported in the literature to date. Since clinical information about PRMC is extremely limited, the genesis and treatments are still debatable. Here, we report the clinical and pathologic features of a case with PRMC.
Case presentation
A 47‐year‐old woman visited our hospital complaining of abdominal fullness which was firstly noticed 2 years ago, and had gradually progressed. A physical examination revealed a large palpable mass that was not tender in her right hemiabdomen. She was treated with a low dose of estrogen for 6 months for uterine myoma.
Contrast‐enhanced computed tomography revealed a large (16 × 11 × 20 cm) cystic tumor that was located in the right retroperitoneal space and extended into the pelvic cavity. The tumor contained enhanced solid lesions in the cystic wall. The tumor showed low‐signal intensity and high‐signal intensity on T1‐ and T2‐weighted magnetic resonance images, respectively. Diffusion‐weighted imaging demonstrated high‐signal intensity in the solid lesion. There were no findings of infiltration to the surrounding organs or metastases (Fig. 1). The serum levels of the CEA, CA19‐9, and CA125 were within the normal limits.
Fig. 1.
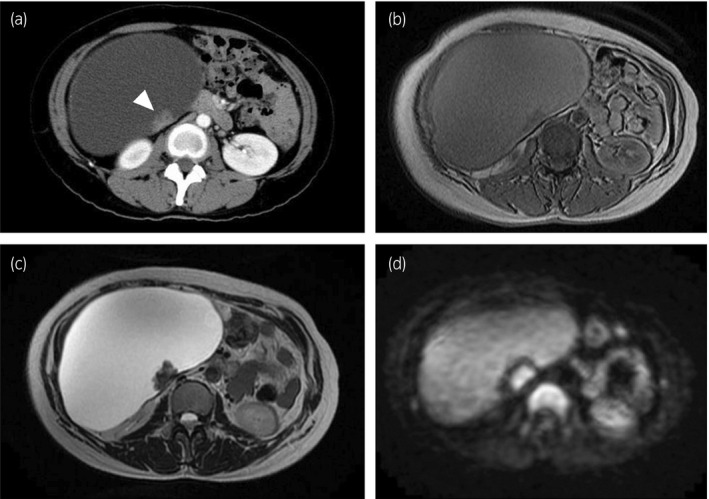
(a) Computed tomography demonstrated a large cystic tumor with enhanced solid lesion (arrowhead). Magnetic resonance imaging showed homogenous cyst on (b) T1‐weighted image and (c) T2‐weighted image. (d) Diffusion‐weighted imaging demonstrated high‐signal intensity in the solid lesion.
A midline laparotomy was performed under the diagnosis of mature teratoma because of the cystic mass including some solid components. The tumor was located in the right retroperitoneum. There was no evidence that the tumor had invaded the adjacent organs including the pancreas, intestine, and ovaries. Finally, the tumor was completely removed with the adjacent peritoneum because of the adhesion that existed. The tumor weighed 3570 g and contained a cloudy black fluid inside. The cytological examination of the fluid was negative for malignant cells. The tumor wall was smooth and had several small (1–3 cm) hard nodules (Fig. 2a).
Fig. 2.
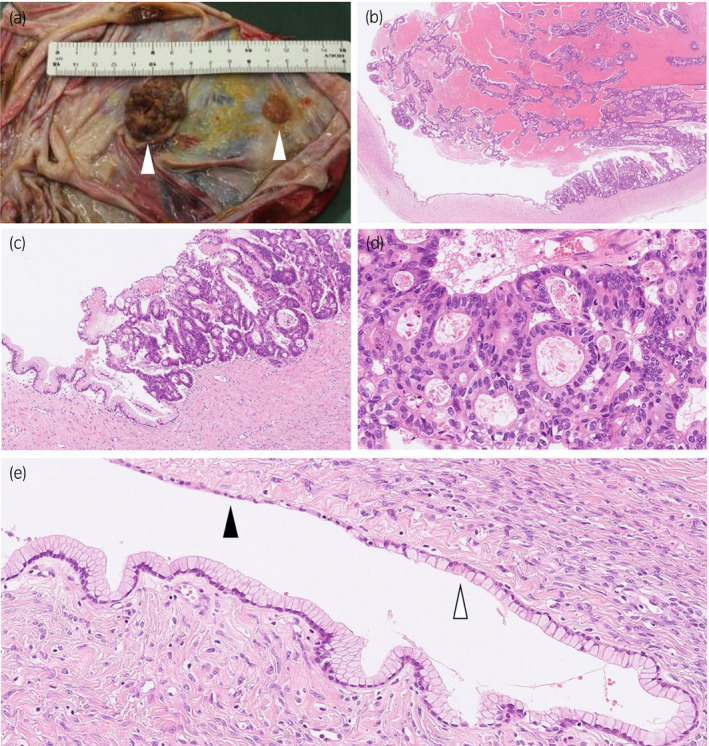
(a) Macroscopically, the wall of the unilocular cyst is smooth and has several hard nodules (arrowheads). (b) The solid nodular tumor with necrosis arises from the cyst wall (hematoxylin and eosin staining, ×18). The cyst wall is lined by a layer of mucous columnar epithelium and atypical glandular epithelial cells proliferate in a papillotubular growth fashion in the nodular area (c, ×100; d, ×400). (e) Mucinous epithelium overlying the cyst (white arrowhead) transits from thin flat mesothelial‐like cells (black arrowhead) (×200).
Pathologically, the tumor was unilocular cystic lesion with several solid nodules composed of a proliferation of atypical glandular epithelial cells in a papillotubular growth fashion (Fig. 2b–d). The cystic areas were lined by a single layer of mucous columnar epithelium that transited from the thin flat calretinin‐ and WT‐1‐immunoreactive mesothelial cells (Fig. 2e). There was an ovarian‐like stroma, which was positive for estrogen and progesterone receptors (Fig. 3), around the mucous epithelium. A diagnosis of mucinous cystadenocarcinoma was made.
Fig. 3.
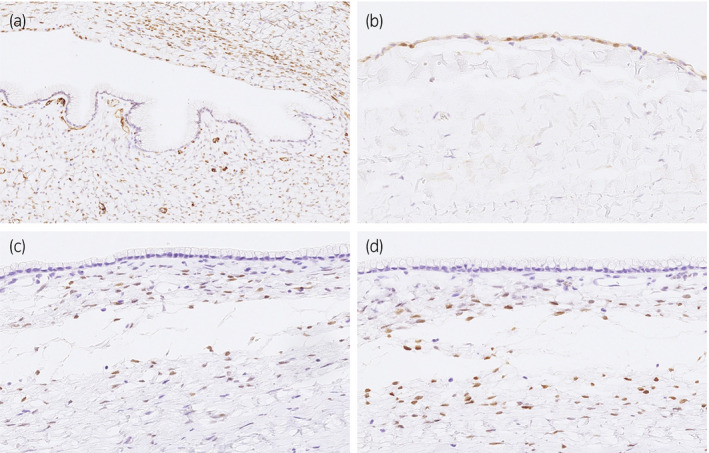
Immunohistochemical staining demonstrates that thin flat epithelium cells are positive for WT‐1 (a, ×200) and calretinin (b, ×400), representing mesothelial cells. In the cyst wall, the spindle cells beneath the mucous epithelium are positive for estrogen (c, ×400) and progesterone receptor (d, ×400), indicating an ovarian‐like stroma.
The postoperative course was uneventful, and no recurrence had developed at 2 years after the surgery.
Discussion
Primary retroperitoneal mucinous cystic tumors including PRMC are exceedingly rare neoplasms among the tumors that develop in the retroperitoneum. PRMC occurs most frequently in middle‐aged women, and the most common presentation is a palpable abdominal mass like in our case. According to a meta‐analysis of 78 patients with PRMC, recurrence after radical surgery was observed in 40% (23/57) of the patients with a median follow‐up period of 15 months. The median disease‐free survival was 15 months. 1
Computed tomography and magnetic resonance imaging were commonly performed for the diagnosis of PRMC. In our case, these imaging studies showed thin‐walled, unilocular cystic tumor with enhanced nodules. Although the presence of solid components suggested PRMC, it is difficult to differentiate PRMC from the other retroperitoneal cystic tumor such as lymphangioma, mullerian cyst, cystic mesothelioma, and mature teratoma.
Additionally, tumor markers including CEA, CA19‐9, and CA125 are elevated in some cases of PRMC. In the previous report, however, the elevation of CEA, CA19‐9, and CA125 levels was seen in only 29%, 38%, and 17% of the cases, respectively. 1 The tumor markers are not useful for diagnosis of PRMC, although the tumor markers may help to detect a recurrent tumor in cases with elevated tumor markers at the diagnosis.
Several hypotheses have been proposed about the development of PRMC including metaplasia of the remnant coelomic epithelium, 2 supernumerary ovary, 3 teratoma, 4 and intestinal duplication. 5 In recent years, the most accepted hypothesis is metaplasia of the remnant coelomic epithelium. The coelomic epithelium differentiates into the pericardial, pleural, and peritoneal mesothelium, and the mullerian duct. This hypothesis suggests that during embryonic development, a part of the coelomic epithelium cells invaginate and are trapped in the retroperitoneum. These cells then transform into mucinous epithelium and then cystadenocarcinoma may develop (Fig. 4).
Fig. 4.
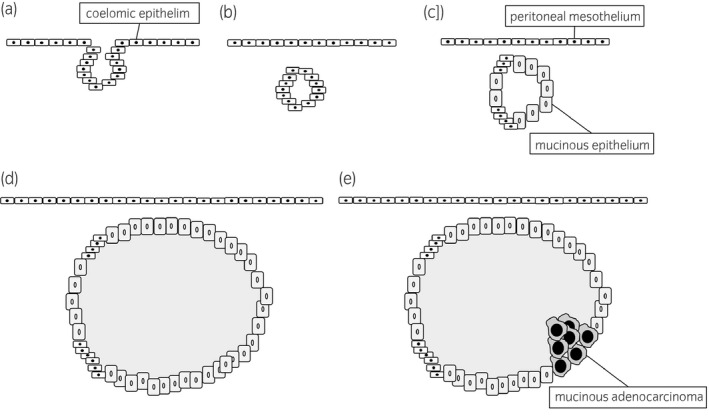
Putative mechanism of development of primary retroperitoneal cystadenocarcinoma in metaplasia of the remnant coelomic epithelium hypothesis; (a) invagination of the coelomic epithelium cells, (b) trapping in the retroperitoneum during embryonic development, (c) transformation from coelomic epithelial remnant to mucinous epithelium, formation of (d) cystadenoma or (e) cystadenocarcinoma.
In our case, the microscopic examination showed both mucinous epithelium and mesothelium in the tumor. Similar microscopic findings have been reported in previous cases, 2 , 6 and these findings strongly support the hypothesis of metaplasia of the remnant coelomic epithelium. Therefore, in order to prevent local recurrence, the peritoneum that is in contact with the tumor should be collectively removed because the remnant coelomic epithelium may still remain between PRMC and the peritoneum. In addition, the complete resection of the tumor is most important in the management of PRMC and the adjacent organs should be removed when the imaging studies or intraoperative findings suggest infiltration of PRMC, because local recurrences were reported in some cases of PRMC. 7 , 8
In some cases of PRMC, an ovarian‐like stroma has been found in the cyst wall. 9 , 10 Because PRMC has been observed in females more frequently than in males (male: 7, female: 78) and ovarian‐like stroma exist, this suggests that stimulation of female hormones may affect the development or the growth of PRMC. The present case was receiving estrogen therapy before surgery and the estrogen might have affected the enlargement of PRMC. In some cases with PRMC, a salpingo‐oophorectomy was performed to avoid stimulation of female hormones. 11 , 12 However, it is still unclear whether this procedure improves the prognosis, 13 or whether the benefits outweigh the complications, such as infertility and menopausal syndrome.
Conclusion
PRMC is an extremely rare tumor and our pathological findings supported the theory of metaplasia of the remnant coelomic epithelium. A complete resection of the tumor with the adjacent peritoneum is required to prevent local recurrence.
Conflict of interest
The authors declare no conflict of interest.
Tomisaki I, Matsuyama A, Jotatsu M, Yamamura S, Onishi R, Fujimoto N. Primary retroperitoneal mucinous cystadenocarcinoma with transition from the mesothelium. IJU Case Rep. 2020; 3: 137–140.
References
- 1. Myriokefalitaki E, Luqman I, Potdar N, Brown L, Steward W, Moss EL. Primary retroperitoneal mucinous cystadenocarcinoma (PRMCa): a systematic review of the literature and meta‐analysis. Arch Gynecol Obstet 2016; 293: 709–20. [DOI] [PubMed] [Google Scholar]
- 2. Fujii S, Konishi I, Okamura H, Mori T. Mucinous cystadenocarcinoma of the retroperitoneum: a light and electron microscopic study. Gynecol Oncol 1986; 24: 103–12. [DOI] [PubMed] [Google Scholar]
- 3. Roth LM, Ehrlich CE. Mucinous cystadenocarcinoma of the retroperitoneum. Obstet Gynecol 1977; 49: 486–8. [PubMed] [Google Scholar]
- 4. Pennell TC, Gusdon JP Jr. Retroperitoneal mucinous cystadenoma. Am J Obstet Gynecol 1989; 160: 1229–31. [DOI] [PubMed] [Google Scholar]
- 5. Abascal J, Ardaiz J, Gil P, Menendez J, Barreiro JJ, Inchausti JL. Primary retroperitoneal cyst (possible intestinal origin). Rev Esp Enferm Apar Dig 1977; 51: 819–28. [PubMed] [Google Scholar]
- 6. Tenti P, Carnevali L, Tateo S, Durola R. Primary mucinous cystoadenocarcinoma of the retroperitoneum: two cases. Gynecol Oncol 1994; 55: 308–12. [DOI] [PubMed] [Google Scholar]
- 7. Jiang H, Jin K, You Q, Fang W, Xu N. Retroperitoneal primary mucinous adenocarcinoma: a case report. Oncol Lett 2011; 2: 633–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de León DC, Pérez‐Montiel D, Chanona‐Vilchis J, Dueñas‐González A, Villavicencio‐Valencia V, Zavala‐Casas G. Primary retroperitoneal mucinous cystadenocarcinoma: report of two cases. World J Surg Oncol 2007; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamiyama H, Shimazu A, Makino Y et al Report of a case: Retroperitoneal mucinous cystadenocarcinoma with rapid progression. Int J Surg Case Rep 2015; 10: 228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tokai H, Nagata Y, Taniguchi K et al The long‐term survival in primary retroperitoneal mucinous cystadenocarcinoma: a case report. Surg Case Rep 2017; 3: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan YS, Thomas TM, Ip PP, Cheung AN. Osteoid‐forming sarcoma‐like mural nodule in a retroperitoneal mucinous cystadenocarcinoma. Histopathology 2006; 49: 201–4. [DOI] [PubMed] [Google Scholar]
- 12. Park U, Han KC, Chang HK, Huh MH. A primary mucinous cystoadenocarcinoma of the retroperitoneum. Gynecol Oncol 1991; 42: 64–7. [DOI] [PubMed] [Google Scholar]
- 13. Kessler TM, Kessler W, Neuweiler J, Nachbur BH. Treatment of a case of primary retroperitoneal mucinous cystadenocarcinoma: is adjuvant hysterectomy and bilateral salpingo‐oophorectomy justified? Am J Obstet Gynecol 2002; 187: 227–32. [DOI] [PubMed] [Google Scholar]


