Abstract
Purpose of review:
Hypertriglyceridemia (HTG), a form of dyslipidemia characterized by elevated plasma of triglycerides (TG), is associated with an increased risk for acute pancreatitis. Moreover, HTG has recently been shown to be linked to the development of atherosclerotic cardiovascular disease (ASCVD); therefore, there is a great interest in better understanding the pathophysiology of HTG and improving its clinical management. In this review, we briefly describe TG metabolism, recent guidelines for the clinical management of HTG, and provide an overview of the current and potential new therapies for HTG.
Recent findings:
Screening patients for HTG is valuable for not only identifying patients with extreme TG elevations, who are at risk for pancreatitis, but also for managing ASCVD risk in patients with more moderate forms of HTG. Therefore, the most recent USA guidelines for cardiovascular diseases recommend using TG as a risk enhancer test, leading to a more aggressive treatment of patients with intermediate risk. Currently there are several available approaches for reducing plasma TG, which include lifestyle changes, fibrates and omega-3 fatty acid treatment. The addition of eicosapentaenoic acid (EPA) on top of statins has recently been shown to significantly reduce ASCVD events. Nevertheless, there is an unmet need for more effective treatment options. Several new therapies based on newly identified targets in TG metabolism, such as apolipoprotein C-III and angiopoietin-like 3 protein, are currently under development.
Summary:
The clinical management of HTG is important in the prevention and treatment of acute pancreatitis and also impacts on how ASCVD risk is managed. More work needs to be done to establish the mechanism for the ability of how EPA lowers ASCVD and how to best integrate it with other lipid-lowering therapies. The efficacy and safety of the novel therapies for HTG should be established soon in the ongoing late-stage clinical trials.
Keywords: cardiovascular diseases, hypertriglyceridemia, management, novel therapies, triglycerides
Introduction
Hypertriglyceridemia (HTG) is a common lipid disorder characterized by elevated levels of plasma triglycerides (TG) carried on apolipoprotein B (apoB) containing lipoproteins, which primarily include chylomicrons (CM), very low-density lipoproteins (VLDL) and remnant lipoproteins. It can develop from primary genetic disorders or more commonly from a wide variety of secondary causes, such as metabolic syndrome, obesity, iatrogenic drug use, and type 2 diabetes, all of which can disturb TG-rich lipoproteins (TRL) metabolism. The risk of acute pancreatitis is a relatively common problem, which can have significant morbidity and mortality. It typically occurs in patients with marked increases in TG, typically over 1000 mg/dL (TG>11.3 mmol/L) but can also develop at lower TG levels. Moreover, it has been shown in several Mendelian randomization and GWAS studies that genes associated with HTG are causally linked to increased atherosclerotic cardiovascular disease (ASCVD) risk [1–3]. The findings of recent multivariable Mendelian randomization analyses [4] suggest that TG-rich remnant particles have approximately the same or perhaps a greater effect on the risk of cardiovascular disease (CVD) as low-density lipoproteins (LDL), which confirms earlier work from the Copenhagen Heart Study [1]. Therefore, there has been a renewed interest in TG metabolism and recent efforts have focused on developing new treatment options for HTG, which we will cover in this review.
Metabolism of triglyceride-rich lipoproteins
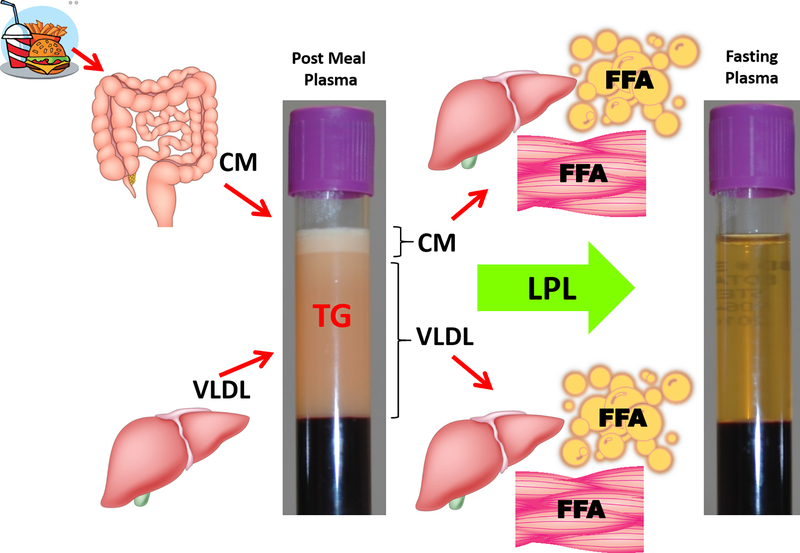
There are two main pathways by which TRL are metabolised (Fig. 1) that depend on the source of TG, namely the exogenous pathway (dietary TG source) or endogenous pathway (hepatic TG source) [5]. The exogenous pathway starts in the small intestine and ends in the liver. In this pathway dietary lipids are absorbed in the intestine and packaged into CM. CM enter the circulation by way of the lymphatics and then undergo hydrolysis by lipases that convert TG to free fatty acids (FFA) and glycerol, which then enter either peripheral tissues for energy generation or adipocytes for energy storage. CM are converted to remnant particles by this process and are mostly taken up by the liver. In the endogenous pathway, the liver synthesizes TG from glycerol and FFA that are derived from the following three main sources: adipocytes, CM remnants and dietary fat directly taken up from the intestine through the portal vein. It has been determined that the fasting hepatic de novo lipogenesis can be up to ∼10% in healthy adults and significantly up to 22% in patients with insulin resistance and nonalcoholic fatty liver disease [6]. TG are then released by the liver when they secrete VLDL into the plasma. Like CM, VLDL can then undergo lipolysis and produce remnant particles, or undergo more complete lipolysis and be converted into LDL. LDL are also removed by the liver by the LDL receptor but excess of LDL can be deposited into vessels where they can trigger atherosclerosis.
Fig. 1. Two Routes of Triglyceride-Rich Lipoproteins Metabolism: the exogenous and endogenous pathways.
The exogenous route starts with the dietary triglycerides (TG), which are absorbed in the intestine and are secreted in a form of chylomicron lipoproteins (CM) to plasma. CM once hydrolysed by the lipoprotein lipase (LPL) and depleted of TG are removed by the liver and free fatty acids (FFA) generated from the lipolysis are taken up either by the peripheral tissues for energy production or by adipocytes for storage. The endogenous route starts in the liver where TG-rich very low-density lipoproteins (VLDL) are generated. After VLDL is secreted into plasma, LPL converts them into remnant particles, which are then either removed by the liver or undergo further lipolysis and are converted to low-density lipoproteins (LDL). FFA generated from this metabolism are deposited in peripheral, muscle and/or adipose tissue.
The main enzyme involved in the lipolysis of TG on CM and VLDL is lipoprotein lipase (LPL) [5, 7]. LPL is highly regulated and for its proper function requires several positive and negative regulators to insure that FFA generated from lipolysis are being delivered to the right tissues during the fasting or fed state [5]. A crucial protein that is necessary for LPL activation is apolipoprotein C-II (apoC-II). In contrast, a closely related protein, apolipoprotein C-III (apoC-III), can inhibit LPL and appears to also interfere with hepatic uptake of remnat lipoproteins. Angiopoietin-like 3 (ANGPTL3) protein is another key inhibitor of LPL [8].
Hypertriglyceridemia
HTG is usually defined as an increase of plasma TG more than 150 mg/dL (1.7 mmol/L) in the fasting state or as >175 mg/dL (>2.0 mmol/L) in the fed state. Patients with fasting or non-fasting plasma TG between 150 – 499 mg/dL (1.7 mmol/L – 5.6 mmol/L) are usually classified as having moderate HTG, whereas in severe HTG plasma TG are ≥500 mg/dL (≥5.6 mmol/L) [9, 10].
Patients with elevated TG are most likely to have a polygenic determinant of HTG. Severe HTG usually reflects the accumulation of CM and/or CM and VLDL and their remnants. Secondary causes, such as e.g. type 2 diabetes and hypothyroidism, are also a common cause of HTG [11, 12]. It is also important to diagnose patients with Familial Chylomicronemia Syndrome (FCS), which is a rare monogenic disorder causing extremely high levels of plasma TG [13]. FCS is the third most common cause of pancreatitis with an overall associated mortality of about 5% to 6% per episode [14]. In addition, severe HTG has been shown to have many other adverse impacts on the normal life of these patients [15, 16].
Management of hypertriglyceridemia
In the 2018-MultiSociety Guideline on the Management of Blood Cholesterol, there are four general recommendations for the clinical management of HTG [9, 10]. The 1st recommendation, class I, focuses on identifying people ≥20 years old who have moderate HTG and advises to address and treat lifestyle factors, secondary factors and medications raising TG by nonpharmacological means where possible. The 2nd recommendation, class IIa, is for adults 40 – 75 years old with a moderate or severe HTG with an ASCVD risk of ≥7.5% for whom the ASCVD risk assessment should be reevaluated after lifestyle and secondary factors are addressed. If TG≥175 mg/dL (≥2.0 mmol/L) are persistent after addressing these other factors, a statin treatment (Table 1) should be considered for reducing ASCVD risk. These patients may not have a particularly elevated LDL-cholesterol (LDL-C) but appear to have a pro-atherogenic lipoprotein phenotype characterized by increased small dense LDL and remnant lipoproteins and thus may benefit from statin therapy [17, 18]. The 3rd recommendation, class IIa, is for adults 40 – 75 years old with a severe HTG and with an ASCVD risk of ≥7.5%. Most patients with severe HTG have multiple ASCVD risk factors and an enhaced risk for developing ASCVD, making it advisable to address any reversible causes of high TG and to consider the use of statin therapy. The last 4th recommendation, class IIa, is for patients with severe HTG, especially those with TG≥1000 mg/dL (≥11.3 mmol/L). To reduce their risk for acute pancreatitis, reduction of TG is necessecary and can be achieved by addressing and eliminating the underlying factors. When addressing secondary causes of HTG in patients with high risk for acute pancreatitis is not sufficiently effective, it is recommended that patients be put on a very-low-fat diet [19] (Table 1). It is generally recommended that the fat intake should be reduced to 15 to 20 g per day (10% – 15% of total daily energy intake). Carbohydrate and alcohol consumption should also be limited. If there is still a concern for acute pancreatitis, taking fibrates (Table 1) and omega-3 fatty acids (Table 2) might be beneficial for patients because although statins are known to reduce CVD events they typically only have a limited effect in reducing TG. Niacin (Table 1) is another drug that could be considered on top of statins, although it is not explicitly recommended [9] and recent studies have failed to show that niacin on top of statins reduces CVD events [20, 21].
Table 1.
Current and Potential New Therapies for Hypertriglyceridemia
| Agent | Mechanism of action and effect on lipid metabolism | Stage | Reference |
|---|---|---|---|
| Available therapies | |||
| Healthy lifestyle | Healthy low-fat diet (weight loss), physical activity, alcohol abstinence to improve the metabolism of lipids and reduce plasma TG | - | [9, 19] |
| Statins | Increase catabolism of CM remnants but have only modest effect on TG | - | [65] |
| Ezetimibe + simvastatin | Combined with statins lowers plasma cholesterol and production of TRL | - | [66] |
| Fibrates | Activate PPAR-α receptor; reduce hepatic synthesis of TRL | - | [67] |
| Niacin | Increases hepatic TRL uptake; reduces hepatic TRL production | - | [68] |
| Omega-3 PUFA | Increases clearance of TRL, inhibits hepatic TRL synthesis | - | Table 2 |
| Volanesorsen (Waylivra) | ASO against apoC-III; inhibits the production of apoC-III, reduces plasma apoC-III, TG, and hepatic TRL production, and increases TRL catabolism and HDL-C | Approved in EU only | [45, 46] |
| Therapies in development | |||
| Pemafibrate (Parmodia*) | Selective PPARα modulator; reduces plasma TG and increases HDL-C | Phase 3 | [54–57] |
| AKCEA-APOCIII-LRX | ASO against apoC-III; inhibits the production of apoC-III, reduces plasma apoC-III, TG, and hepatic TRL production, and increases TRL catabolism and HDL-C | Phase 3 planned | [50] |
| AKCEA-ANGPTL3-LRX (ISIS 703802) | ASO against ANGPTL3, inhibits the production of ANGPTL3, lowers plasma ANGPTL3 apoB-containing particles, TG, and LDL-C | Phase 2 | [49] |
| Evinacumab | mAb against ANGPTL3; lowers apoB-containing particles and plasma TG, LDL-C and HDL-C | Phase 2 | [58] |
| ARO-APOC3 | siRNA against apoC-III; inhibits the expression of APOC3 mRNA, thereby affecting the production of apoC-III, lowers plasma apoC-III and TG | Phase 1/2a | [52] |
| ARO-ANGPTL3 | siRNA against ANGPTL3; inhibits the expression of ANGPTL3 mRNA, thereby affecting the production of ANGPTL3, lowers plasma ANGPTL3, TG and LDL-C | Phase 1/2a | [52] |
| STT505/STT5058 | mAB against apoC-III; lowers circulating apoC-III and TG levels and promotes TRL clearance | Pre-clinical testing | [59] |
| D6PV (COR-003) | An apoC-II mimetic-apoC-III antagonist peptide; activates LPL and displaces apoC-III from TRL, reduces plasma apoC-II and TG | Pre-clinical testing | [64, 69] |
Under this name pemafibrate received its first global approval on 3 July 2017 for the treatment of hyperlipidemia in Japan. It is being investigated in the treatment of severe HTG in two randomized, placebo-controlled phase 3 trials [54].
ANGPTL3: angiopoietin-like 3 protein; apoB: apolipoprotein B; apoC-II: apolipoprotein C-II; apoC-III: apolipoprotein C-III; ASO: antisense oligonucleotides; CM: chylomicrons; EU: European Union; HDL-C: high-density lipoproteins cholesterol; LDL-C: low-density liporpoteins cholesterol; LPL: lipoprotein lipase; mAb: monoclonal antibodies; PPARα: peroxisome proliferator-activated receptors alfa; PUFA: polyunsaturated fatty acid; siRNA: small interfering ribonucleic acid; TG: triglycerides; TRL: triglyceride-rich lipoproteins; USA: the United States of America
Table 2.
FDA-approved Prescription Omega-3 PUFA Products
| Omega-3 PUFA type | Trade name (manufacture) | Years approved | Generic Name | Dosage |
|---|---|---|---|---|
| EPA-only | Vascepa (Amarin Pharma Inc.) | 2012 | Icosapent Ethyl | 4.0 g/day |
| EPA+DHA | Omacor/Lovaza (GlaxoSmithKline LLC.) | 2004 | Omega-3-acid ethyl esters | 4.0 g/day |
| Omtryg (Trygg Pharma, Inc.) | 2014 | Omega-3-acid ethyl esters | 4.8 g/day | |
| Epanova (AstraZeneca Pharmaceuticals LP.) | 2014 | Omega-3-carboxylic acids | 2 or 4 g/day |
PUFA: polyunsaturated fatty acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid
The recent 2019 ESC/EAS guidelines for the management of dyslipidemias [22] are similar to the 2018 AHA/ACC guidelines [9] and recommend considering the use of TG-loweing drugs in high-risk patients with TG>200 mg/dL (>2.3 mmol/L) when lifestyle measures fail to lower TG levels. Possible pharmacological interventions to consider include statins, fibrates, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and long-chain omega-3 polyunsaturated fatty acids (PUFA).
Marine-derived omega-3 fatty acids in triglyceride management
Fish oils are among the most well recognized health supplements in the USA and there has been much recent progress on their use for HTG [23]. The main active ingredient in fish oils is PUFA, i.e. eicosapentaenoic acid (EPA; 20:5 omega-3) and docosahexaenoic acid (DHA; 22:6 omega-3) [24, 25]. In normolipidemic to borderline hyperlipidemic healthy individuals, 1–5 g/day consumption of EPA/DHA supplements typically results in a variable TG reduction ranging between 4–51% [26]. In addition, intake of omega-3 PUFA decreases postprandial TG levels in both healthy subjects and patients with familial hypercholesterolemia [27, 28].
Most omega-3 PUFA clinical trials have used a mixture of EPA and DHA at various ratios, and a recent study showed that omega-3 PUFA supplements with different EPA/DHA ratios (2.3 vs. 0.3) had nearly identical effects on decreasing TG, VLDL particle number, and TRL subfractions [29]. This finding is consistent with previous studies that used purified EPA and DHA [30, 31]; however, divergent effects of EPA and DHA on other lipid paramaters, such as LDL-C and HDL-cholesterol (HDL-C), have been described [32]. Some of the proposed mechanisms of omega-3 PUFA effects on TG metabolism include decreased hepatic lipogenesis through down-regulating sterol receptor element binding protein-1c (SREBP-1c) transcription factor, increased hepatic fatty acid β-oxidation through activation of peroxisome proliferator-activated receptors alpha (PPARα), and accelerated VLDL clearance through stimulating LPL activity and apoB degradation [33–35].
The most recent recommendations from the American Heart Association concluded that omega-3 PUFA at high doses of 4 g/d (>3 g/d total EPA+DHA) are effective in reducing TG in hyperlipidemic individuals [36]. There are two major types of prescription highly purified omega-3 PUFA: a mixture of EPA and DHA, such as Omacor/Lovaza, Omtryg, and Epanova, and EPA only formulation, such as Vascepa and Epadel (approved only in Japan) (Table 2). The first randomized clinical trial showing the CVD benefit of omega-3 PUFA when added to statins was the JELIS study. In this study, highly purified EPA ethyl ester (Epadel; 1.8 g/day) reduced the risk of major coronary events in hypercholesterolemic patients on a statin compared to those receiving statin monotherapy [37]. More recently, the CHERRY study that used Epadel (1.8 g/day) showed that addition of EPA to high dose statin treatment significantly reduced coronary plaque volume, suggesting that EPA therapy may reduce the residual risk that remains in secondary prevention patients being treated with statins [38]. The RESPECT-EPA study (UMIN ID No.: UMIN000012069) is currently examining the use of Epadel (1.8 g/day) on the incidence of CVD events and is expected to be reported in 2022.
The REDUCE-IT study is the most recently completed large ASCVD outcome study of an EPA-only prescription (Vascepa) (Table 3). At a high dose of 4 g/day, it was found to lower TG from baseline by 45% and double the reduction in ASCVD events for patients on statins [39]. In contrast, a low dose of prescription omega-3 PUFA mixture (1 g/day) failed to show benefit in over 40,000 patients with respect to primary prevention in both ASCEND and VITAL studies [40, 41]. Another higher dose (4 g/day) study called STRENGTH that used an omega-3 PUFA mixture of EPA and DHA (Epanova) has been halted because of a lack of any apparent ASCVD benefits in patients with mixed dyslipidemia [42]. Thus, at this point it remains unclear whether the lack of CVD health benefits from some omega-3 PUFA clinical trials may be attributable to a low dose or to a low ratio of EPA to DHA. DHA, but not EPA, has been shown to increase total LDL-C levels and small dense LDL [43, 44]. In addition, it does not appear that the benefit from EPA in the REDUCE-IT trial was due to TG lowering and may be due to some other mechanism, since the observed benefits were similar across baseline TG levels.
Table 3.
Recent CVD Outcome Clinical Trials Using Prescription Omega-3 PUFA Products
| Omega-3 PUFA type | Trial (Country; Completion date) | No. Age (years) | Duration | Formulation Dose | Major findings |
|---|---|---|---|---|---|
| EPA-only | CHERRY (Japan; 2013) | 193; 57–78 | 6–8 months | Ethyl icosapentate (Epadel) 1.8 g/day | Addition of middle-dose EPA to high-dose statin reduced coronary plaque volume compared to statin therapy alone. |
| REDUCE-IT (USA; 2019) | 8179; ≥45 | 5 years | Icosapent Ethyl; (Vascepa) 4 g/day | High-dose EPA lowered ischemic event rates beyond statin therapy in at-risk patients with hypertriglyceridemia. | |
| RESPECT-EPA (Japan; 2022) | 3900; 20–79 | 5 years | Ethyl icosapentate (Epadel) 1.8 g/day | N/A (ongoing study) | |
| EPA+DHA | ASCEND (UK; 2018) | 15,480; ≥40 | 7.4 years | Omega-3-acid ethyl esters (Lovaza) 1.0 g/day | Low-dose EPA+DHA showed no difference in the risk of vascular events compared to placebo in patients with diabetes without CVD |
| VITAL (USA; 2019) | 25,871; Men ≥50 Women ≥55 | 5.3 years | Omega-3-acid ethyl esters (Omacor) 1.0 g/day | Low-dose EPA+DHA did not lower major CVD events incidence and cancer compared to placebo in a racially and ethnically diverse population. | |
| STENGHTH (USA, 2020) | 13,086; 18–99 (>40 if diabetes) | 5 years | Omega-3-acid ethyl esters (Epanova) 4.0 g/day | High-dose EPA+DHA failed to show apparent benefit in at-risk patients with mixed dyslipidemia, resulting in trial discontinuity. |
PUFA: polyunsaturated fatty acid; EPA: eicosapentaenoic Acid; DHA: docosahexaenoic acid; CVD: cardiovascular disease; CHERRY: Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography; REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial; RESPECT-EPA: Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid; ASCEND: A Study of Cardiovascular Events in Diabetes; VITAL: VITamin D and OmegA-3 TriaL; STENGHTH: A Long-Term Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh Cardiovascular Risk PatienTs With Hypertriglyceridemia; N/A: not available.
Therapies for severe hypertriglyceridemia
A newly approved drug for the treatment of FCS that is currently available in the European Union is Waylivra (Volanesorsen) from Akcea Therapeutics [45]. Waylivra is an antisense oligonucleotide (ASO) inhibitor of apoC-III designed to reduce the production of apoC-III [46]. In the APPROACH trial, the mean TG levels decreased by 77% in Waylivra-treated patients versus an 18% increase in patients on placebo and the levels of apoC-III decreased by an average of 84% from baseline after three months in the active treatment group [46]. It has been shown that patients with FCS may develop significant spontaneous fluctuations in platelet blood count leading to thrombocytopenia or thrombocytosis [47]. Results from the Waylivra trial showed that the drug can lead to clinically significant thrombocytopenia in humans [46], which was one of the main reasons that the drug was not approved in the US.
There are also several other therapies under development that show promise for severe HTG [12, 48] (Table 1). Akcea Therapeutics has two emerging ASO drugs named AKCEA-APOCIII-LRx and AKCEA-ANGPTL3-LRx that are targetted against apoC-III and ANGPTL3, respectively. AKCEA-APOCIII-LRx is a second-generation ASO that contains a triantennary N-acetylgalactosamine (GalNAc3) moiety targeted to hepatocytes to enhance drug’s potency [49], thereby also potentially limiting its off target toxicity like thrombocytopenia. Results from a Phase 1/2 clinical trial of AKCEA-APOCIII-LRx [50] showed significant and dose-dependent reductions in apoC-III up to 84% after six weeks of treatment and TG reductions as much as 71% from baseline. Moreover, it was demonstrated that AKCEA-APOCIII-LRx had at least 15 times higher potency in reducing plasma apoC-III and TG than Waylivra, a non-GalNAc ASO. This allowed the use of much lower doses of AKCEA-APOCIII-LRx that improved its safety and tolerability [51], and subjects did not have significant declines in their platelet count [50]. In regard to ASO treatment for ANGTPL3, six weeks of treatment with AKCEA-ANGPTL3-LRx [49] resulted in a robust, dose-dependent reductions of ANGPTL3, TG, and apoC-III up to 84.5%, 63% and 59%, respectively in a Phase 1/2 study [49].
ARO-APOC3 and ARO-ANGPTL3, which are under a development by Arrowhead Pharmaceuticals, are small interfering ribonucleic acid (siRNA) candidates against either APOC3 mRNA or ANGPTL3 mRNA [52]. These therapies trigger the RNA interference mechanism to induce rapid, deep, and durable gene specific silencing while also avoiding off-target effects. Results from the phase 1/2a study of patients taking ARO-APOC3 showed that the mean maximum reduction in plasma apoC-III was up to 94% and for TG it was 95%. It has been suggested that this type of therapy might be particularly well suited for populations with therapy adherence issues since this drug is administered quarterly or at 6 month intervals [53]. Patients given ARO-ANGPTL3 in a Phase 1/2a had a mean maximum decrease of ANGPTL3 of 83% and of TG – 79%. Both candidates had a high level of pharmacologic activity with good safety and tolerability, and the most common adverse events reported were headache, respiratory tract infections, and local injection site reactions [52]. Results from these clinical trials are encouraging and show that siRNA-based treatments may be an effective approach for severe HTG.
Pemafibrate is a highly potent and selective PPARα that significantly reduces TG, apoC-III, and remnant cholesterol while increasing HDL-C [54–56]. Under the name of Parmodia, it received its first global approval on 3 July 2017 for the treatment of hyperlipidemia in Japan [54]. It is being currently investigated for FCS in two randomized, placebo-controlled phase 3 trials [56]. Data from the PROVIDE study showed that pemafibrate treatment significantly reduced TG levels at week 52 by approximately half and was well tolerated in people with type 2 diabetes and HTG [57].
Monocloncal antibodies against ANGPTL3, like evinacumab [58] or against apoC-III, like STT505/STT5058 [59] are other promising approaches being investigated. These antibodies bind to and neutralize the effect of these proteins on the inhibition of lipolysis and thus lower TG levels. Finally, apoC-II-mimetic peptides [11, 60–64] could be a potential new treatment for apoC-II deficiency and other causes of HTG. These peptides have been shown to decrease plasma TG by 80% within a few hours in both apoC-II-deficient mice and hAPOC3-transgenic (Tg) mice [64]. There was also a 80% reduction in plasma apoC-III and 65% reduction in apoB in hAPOC3-Tg mice treated with these peptides [64]. The advantage of this strategy is that apoC-II mimetic peptides both directly activate LPL [60–64] and also antagonize apoC-III by causing its displacement from TRL, leading to increase apoC-III renal clearance [11, 64] and an increase in the hepatic uptake of TRL [62].
Conclusions
HTG is typically a multifactorial disorder; therefore, the clinical management of these patients can be challenging. There is a need for new approaches to improve screening, monitoring and treatment of patients with HTG for not only preventing pancreatitis but also for reducing ASCVD risk. Besides the new omega-3 PUFA therapies that utilize pure EPA that have been shown to lower TG and reduce ASCVD risk when used on top of statins, new drugs are also being actively investigated. More research, however, is needed to better understand the mechanism for the CVD benefit from TG reduction and for identifying which patients would gain the most from such therapies.
Key points.
Patients with HTG are at increased risk for life-threating acute pancreatitis and ACSVD.
Recent USA guidelines for management of HTG stress the importance of identifying patients with elevated plasma TG.
The most effective currently available treatments for HTG are fat-restricted diets, healthy life style schanges, fibrates, and omega-3 PUFA.
Highly purified EPA may be superior to EPA/DHA mixture medicine for ASCVD risk reduction.
Novel HTG therapies based on newly identified targets in TG metabolism are under development and show promise in early stage clinical trials.
Acknowledgments
Financial support
Research was supported by the Intramural Research Program of the NHLBI at the National Institutes of Health. A.W. and A.T.R. have a research grant HL-CR-16-005 with Corvidia Therapeutics, Inc., Waltham, MA, USA. Z.-H.Y. and A.T.R. acknowledge a research grant HL-CR-18-004 with Nippon Suisan Kaisha, Ltd., Tokyo, Japan.
Conflicts of interest
A.W. and A.T.R. have a research grant HL-CR-16-005 with Corvidia Therapeutics, Inc., Waltham, MA, USA and are co-inventors on US patent application PCT/US2018/014532, submitted by Corvidia Therapeutics Inc. that covers composition and use of apoC-II mimetic peptides for the treatment of HTG. A.T.R. is also a co-inventor on US patent #8,936,787 held by the National Institutes of Health that covers composition and use of 18A-CII mimetic peptide. Z.-H.Y. declares no conflict of interest.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
◼ of special interest:
◼◼ of outstanding interest:
- [1].Varbo A, Benn M, Tybjærg-Hansen A et al. Remnant Cholesterol as a Causal Risk Factor for Ischemic Heart Disease. Journal of the American College of Cardiology 2013; 61:427–436. [DOI] [PubMed] [Google Scholar]
- [2].Do R, Willer CJ, Schmidt EM et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013; 45:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jørgensen AB, Frikke-Schmidt R, West AS et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 2013; 34:1826–1833. [DOI] [PubMed] [Google Scholar]
- [4].Ference BA, Kastelein JJP, Ray KK et al. Association of Triglyceride-Lowering LPL Variants and LDL-C–Lowering LDLR Variants With Risk of Coronary Heart Disease. JAMA 2019; 321:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wolska A, Dunbar RL, Freeman LA et al. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017; 267:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosqvist F, McNeil CA, Pramfalk C et al. Fasting hepatic de novo lipogenesis is not reliably assessed using circulating fatty acid markers. The American Journal of Clinical Nutrition 2019; 109:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Olivecrona G Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol 2016; 27:233–241. [DOI] [PubMed] [Google Scholar]
- [8].Shan L, Yu XC, Liu Z et al. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J Biol Chem 2009; 284:1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacobsen AS, Nazir; Blumenthal Roger S; Martin Seth Shay. Hypertriglyceridemia Management According to the 2018 AHA/ACC Guideline. Expert Analysis. American College of Cardiology 2019.A summary of the recent 2018 AHA/ACC guidelines on the management of hypertriglyceridemia.
- [11].Wolska A, Reimund M, Remaley AT. Apolipoprotein C-II: the re-emergence of a forgotten factor. Current opinion in lipidology 2020; 31:147–153. [DOI] [PubMed] [Google Scholar]
- [12].Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. European Heart Journal 2019; 41:99–109c.A detailed clinical review of the hypertriglyceridemia; its epidemiology, causes, and current and emerging treatments.
- [13].Warden BA, Minnier J, Duell PB et al. Chylomicronemia syndrome: Familial or not? Journal of clinical lipidology 2020; 14:201–206. [DOI] [PubMed] [Google Scholar]
- [14].Gaudet D, Blom D, Bruckert E et al. Acute Pancreatitis is Highly Prevalent and Complications can be Fatal in Patients with Familial Chylomicronemia: Results From a Survey of Lipidologist. Journal of Clinical Lipidology 2016; 10:680–681. [Google Scholar]
- [15].Davidson M, Stevenson M, Hsieh A et al. The burden of familial chylomicronemia syndrome: Results from the global IN-FOCUS study. Journal of clinical lipidology 2018; 12:898–907.e892.A report of the IN-FOCUS study on the burden of familial chylomicronemia syndrome and challenges associated with managing this disease.
- [16].Gaudet D, Stevenson M, Komari N et al. The burden of familial chylomicronemia syndrome in Canadian patients. Lipids in health and disease 2020; 19:120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sarwar N, Danesh J, Eiriksdottir G et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007; 115:450–458. [DOI] [PubMed] [Google Scholar]
- [18].Ganda OP, Bhatt DL, Mason RP et al. Unmet Need for Adjunctive Dyslipidemia Therapy in Hypertriglyceridemia Management. J Am Coll Cardiol 2018; 72:330–343. [DOI] [PubMed] [Google Scholar]
- [19].Williams L, Rhodes KS, Karmally W et al. Familial chylomicronemia syndrome: Bringing to life dietary recommendations throughout the life span. Journal of clinical lipidology 2018; 12:908–919. [DOI] [PubMed] [Google Scholar]
- [20].Landray MJ, Haynes R, Hopewell JC et al. Effects of extended-release niacin with laropiprant in high-risk patients. The New England journal of medicine 2014; 371:203–212. [DOI] [PubMed] [Google Scholar]
- [21].Boden WE, Probstfield JL, Anderson T et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. The New England journal of medicine 2011; 365:2255–2267. [DOI] [PubMed] [Google Scholar]
- [22].Mach F, Baigent C, Catapano AL et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). European Heart Journal 2019; 41:111–188. [DOI] [PubMed] [Google Scholar]
- [23].Wang C, Harris WS, Chung M et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 2006; 84:5–17. [DOI] [PubMed] [Google Scholar]
- [24].Balk EM, Lichtenstein AH. Omega-3 Fatty Acids and Cardiovascular Disease: Summary of the 2016 Agency of Healthcare Research and Quality Evidence Review. Nutrients 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Skulas-Ray AC, Kris-Etherton PM, Harris WS et al. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 2011; 93:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leslie MA, Cohen DJ, Liddle DM et al. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis 2015; 14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miyoshi T, Noda Y, Ohno Y et al. Omega-3 fatty acids improve postprandial lipemia and associated endothelial dysfunction in healthy individuals - a randomized cross-over trial. Biomed Pharmacother 2014; 68:1071–1077. [DOI] [PubMed] [Google Scholar]
- [28].Chan DC, Pang J, Barrett PH et al. omega-3 Fatty Acid Ethyl Esters Diminish Postprandial Lipemia in Familial Hypercholesterolemia. J Clin Endocrinol Metab 2016; 101:3732–3739. [DOI] [PubMed] [Google Scholar]
- [29].Yang ZH, Amar M, Sampson M et al. Comparison of Omega-3 Eicosapentaenoic Acid Versus Docosahexaenoic Acid-Rich Fish Oil Supplementation on Plasma Lipids and Lipoproteins in Normolipidemic Adults. Nutrients 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grimsgaard S, Bonaa KH, Hansen JB, Nordoy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr 1997; 66:649–659. [DOI] [PubMed] [Google Scholar]
- [31].Nestel P, Shige H, Pomeroy S et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr 2002; 76:326–330. [DOI] [PubMed] [Google Scholar]
- [32].Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol 2012; 6:5–18. [DOI] [PubMed] [Google Scholar]
- [33].Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr 2013; 33:249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. Journal of lipid research 2003; 44:455–463. [DOI] [PubMed] [Google Scholar]
- [35].Pan M, Maitin V, Parathath S et al. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc Natl Acad Sci U S A 2008; 105:5862–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Skulas-Ray AC, Wilson PWF, Harris WS et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019; 140:e673–e691.AHA science advisory recommendations on the use of omega-3 fatty acids for the management of hypertriglyceridemia.
- [37].Yokoyama M, Origasa H, Matsuzaki M et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007; 369:1090–1098. [DOI] [PubMed] [Google Scholar]
- [38].Watanabe T, Ando K, Daidoji H et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol 2017; 70:537–544. [DOI] [PubMed] [Google Scholar]
- [39].Bhatt DL, Steg PG, Miller M et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019; 380:11–22. [DOI] [PubMed] [Google Scholar]
- [40].Group ASC, Bowman L, Mafham M et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med 2018; 379:1540–1550. [DOI] [PubMed] [Google Scholar]
- [41].Manson JE, Cook NR, Lee IM et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N Engl J Med 2019; 380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].AstraZeneca. Update on Phase III STRENGTH trial for Epanova in mixed dyslipidaemia. 2020. [Google Scholar]
- [43].Chang CH, Tseng PT, Chen NY et al. Safety and tolerability of prescription omega-3 fatty acids: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids 2018; 129:1–12. [DOI] [PubMed] [Google Scholar]
- [44].Allaire J, Vors C, Tremblay AJ et al. High-Dose DHA Has More Profound Effects on LDL-Related Features Than High-Dose EPA: The ComparED Study. J Clin Endocrinol Metab 2018; 103:2909–2917. [DOI] [PubMed] [Google Scholar]
- [45].AkceaTherapeutics. Waylivra: EPAR - product information. In: https://www.ema.europa.eu/en/documents/product-information/waylivra-epar-product-information_en.pdf: 2020. pp. 1–43.
- [46].Witztum JL, Gaudet D, Freedman SD et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. The New England journal of medicine 2019; 381:531–542.Results from the APPROACH study on the efficacy and safety of Volanesorsen in reducing plasma triglycerides in patients with familial chylomicronemia syndrome.
- [47].Gaudet D BA, Tremblay K, Brisson D, Laflamme N, Paquette M, Dufour R, Bergeron J. Natural History (up to 15 years) of Platelet Count in 84 Patients with Familial Hyperchylomicronemia Due to Lipoprotein Lipase Deficiency. Journal of clinical lipidology 2017; 11:797–798. [Google Scholar]
- [48].Larsen LE, Stoekenbroek RM, Kastelein JJP, Holleboom AG. Moving Targets: Recent Advances in Lipid-Lowering Therapies. Arterioscler Thromb Vasc Biol 2019; 39:349–359. [DOI] [PubMed] [Google Scholar]
- [49].Graham MJ, Lee RG, Brandt TA et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. New England Journal of Medicine 2017; 377:222–232. [DOI] [PubMed] [Google Scholar]
- [50].Alexander VJ, Xia S, Hurh E et al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J 2019; 40:2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Crooke ST, Baker BF, Xia S et al. Integrated Assessment of the Clinical Performance of GalNAc(3)-Conjugated 2’-O-Methoxyethyl Chimeric Antisense Oligonucleotides: I. Human Volunteer Experience. Nucleic Acid Ther 2019; 29:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Arrowhead Pharmaceuticals I. Arrowhead Reports Interim Clinical Data on Cardiometabolic Candidates ARO-APOC3 and ARO-ANG3. In: http://ir.arrowheadpharma.com/news-releases/news-release-details/arrowhead-reports-interim-clinical-data-cardiometabolic: 2020. pp. 1–2.
- [53].Schwabe Christian SR, Sullivan David, Baker John,Clifton Peter, Hamilton James, Given Bruce, Melquist Stacey, Knowles Josh, Hegele Robert, Ballantyne Christie. RNA Interference Targeting Apolipoprotein C-III Results in Deep and Prolonged Reductions in Plasma Triglycerides In: AHA Scientific Sessions 2019. Philadelphia, PA, USA: American Heart Association; 2019. [Google Scholar]
- [54].Blair HA. Pemafibrate: First Global Approval. Drugs 2017; 77:1805–1810. [DOI] [PubMed] [Google Scholar]
- [55].Yamashita S, Arai H, Yokote K et al. Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J Clin Lipidol 2018; 12:1267–1279.e1264. [DOI] [PubMed] [Google Scholar]
- [56].Yamashita S, Masuda D, Matsuzawa Y. Clinical Applications of a Novel Selective PPARα Modulator, Pemafibrate, in Dyslipidemia and Metabolic Diseases. J Atheroscler Thromb 2019; 26:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Araki E, Yamashita S, Arai H et al. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52-week data from the PROVIDE study. Diabetes Obes Metab 2019; 21:1737–1744.Results from the PROVIDE study on the efficacy and safety of pemafibrate in reducing plasma triglycerides in patients with hypertriglyceridemia and type 2 diabetes.
- [58].Wang Y, Gusarova V, Banfi S et al. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. Journal of lipid research 2015; 56:1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Khetarpal SA, Zeng X, Millar JS et al. A human APOC3 missense variant and monoclonal antibody accelerate apoC-III clearance and lower triglyceride-rich lipoprotein levels. Nature medicine 2017; 23:1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Amar MJA, Sakurai T, Sakurai-Ikuta A et al. A novel apolipoprotein C-II mimetic peptide that activates lipoprotein lipase and decreases serum triglycerides in apolipoprotein E-knockout mice. J Pharmacol Exp Ther 2015; 352:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sakurai T, Sakurai A, Vaisman BL et al. Creation of Apolipoprotein C-II (ApoC-II) Mutant Mice and Correction of Their Hypertriglyceridemia with an ApoC-II Mimetic Peptide. J Pharmacol Exp Ther 2016; 356:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Komatsu T, Sakurai T, Wolska A et al. Apolipoprotein C-II Mimetic Peptide Promotes the Plasma Clearance of Triglyceride-Rich Lipid Emulsion and the Incorporation of Fatty Acids into Peripheral Tissues of Mice. J Nutr Metab 2019; 2019:7078241–7078241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Reimund M, Wolska A, Risti R et al. Apolipoprotein C-II mimetic peptide is an efficient activator of lipoprotein lipase in human plasma as studied by a calorimetric approach. Biochem Biophys Res Commun 2019; 519:67–72. [DOI] [PubMed] [Google Scholar]
- [64].Wolska A, Lo L, Sviridov DO et al. A dual apolipoprotein C-II mimetic-apolipoprotein C-III antagonist peptide lowers plasma triglycerides. Sci Transl Med 2020; 12.Pre-clinical results from a research on a novel dual apoC-II mimetic-apoC-III antagonist peptide tested in reducing plasma TG in several mouse models for hypertriglyceridemia.
- [65].Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 2007; 176:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tremblay AJ, Lamarche B, Hogue JC, Couture P. Effects of ezetimibe and simvastatin on apolipoprotein B metabolism in males with mixed hyperlipidemia. Journal of lipid research 2009; 50:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Staels B, Dallongeville J, Auwerx J et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998; 98:2088–2093. [DOI] [PubMed] [Google Scholar]
- [68].Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol 2008; 101:20b–26b. [DOI] [PubMed] [Google Scholar]
- [69].CorvidiaTherapeutics. Corvidia Therapeutics Announces Publication in Science Translational Medicine of Strategy for Lowering Triglycerides Using a Mimetic Peptide. In: https://corvidiatx.com/media/: 2020. pp. 1–2.