Abstract
Introduction:
The specific patterns of revascularization of allograft nerves after addition of vascularization remain unknown. The aim of this study was to determine the revascularization patterns of optimized processed allografts (OPA) after surgically induced angiogenesis to the wound bed in a rat sciatic nerve model.
Materials and methods:
In 51 Lewis rats, sciatic nerve gaps were repaired with (i) autografts, (ii) OPA and (iii) OPA wrapped in a pedicled superficial inferior epigastric artery fascia flap (SIEF) to provide vascularization to the wound bed. At two, 12 and 16 weeks, the vascular volume and vascular surface area in nerve samples were measured using micro CT and photography. Cross-sectional images were obtained and the number of vessels was quantified in the proximal, mid and distal sections of the nerve samples.
Results:
At two weeks, the vascular volume of SIEF nerves was comparable to control (P=0.1). The vascular surface area in SIEF nerves was superior to other groups (P<0.05). At 12 weeks, vascularity in SIEF nerves was significantly higher than allografts (P<0.05) and superior compared to all other groups (P<0.0001) at 16 weeks. SIEF nerves had a significantly increased number of vessels compared to allografts alone in the proximal (P<0.05) and mid-section of the graft (P<0.05).
Conclusions:
Addition of surgical angiogenesis to the wound bed greatly improves revascularization. It was demonstrated that revascularization occurs primarily from proximal to distal (proximal inosculation) and not from both ends as previously believed and confirms the theory of centripetal revascularization.
Keywords: Nerve injury, peripheral nerve repair, nerve regeneration, processed nerve allograft, vascularized nerve allograft, angiogenesis, revascularization patterns
Introduction
The outcome of tissue transplantation critically depends on the revascularization process and consequently regeneration of nerve is similarly dependent on this process1–5. Neovascularization precedes neural regeneration and stimulates injured axons and non-neuronal cells to produce a supportive microenvironment6. The nerve vascularization consists of both an extrinsic and intrinsic blood supply7. The intrinsic system is formed by an extensive microvascular network that maintains blood supply within a nerve (epineural, perineural and endoneural vessels)7,8. The extrinsic system consists of vessels that accompany a nerve outside of its epineurium. While it is known that both the intrinsic and extrinsic blood supply systems are interrupted during injury7, little is known about the revascularization patterns after such an injury. In 1945, Tarlov and colleagues demonstrated by roentgenographic studies of transplanted sciatic nerves that an important source of blood supply for grafts is from the surrounding tissue9. Furthermore, they suggested that the vascular pattern between normal nerves and vascularized nerve autografts is similar and revascularization occurs along the preexistent vascular channels by ingrowth of blood vessels from the host stumps (proximal and distal) as well as from the surrounding tissues9. In order to improve clinical outcomes of free autologous nerve grafting, multiple nerve grafts (cable grafts) were applied to increase surface area as it was postulated that this would improve graft revascularization and avoid central necrosis which was observed in larger diameter nerve grafts9,10. To improve outcomes of nerve autografts in severely scarred tissue beds, the application of either vascularized nerve grafts or vascularized flaps around nerve grafts has been suggested11–14. These theories can not be extrapolated to the revascularization of nerve allografts, as in processed nerve allografts, preexistent vascular channels have been removed during the process. Little has been published regarding other strategies to revascularize processed nerve allografts or the patterns that revascularization follows, partly due to the lack of an appropriate model. Thus, there are remaining questions regarding the mechanism and pattern of peripheral nerve allograft revascularization. The purpose of this study was to explore the effect of surgical angiogenesis on processed nerve allografts and to compare the revascularization patterns in nerve autografts, allografts and allografts placed in a vascularized bed to provide insight into neovascularization of nerve grafts.
Materials and methods
Animal experiments were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC A3348–18). For this study, all animals were housed with ad libitum access to food and water, with a twelve-hour light-dark cycle after surgery.
Experimental design
In a total of 51 male Lewis rats, weighing between 250–300 grams (Envigo, USA), unilateral sciatic nerve gaps were repaired with three groups of nerve grafts. The experimental design of this study is depicted in Table 1. In group I (autograft), a unilateral 10-mm sciatic nerve gap was repaired with an ipsilateral reversed autologous graft to create a mismatch in the alignment of the nerve fibers (gold standard). For group II and III, optimized processed allografts (OPA) were used to reconstruct the nerve gap. In group III, these nerve allografts were placed in a vascularized bed using a pedicled superficial inferior epigastric artery fascia (SIEF) flap15. Rats were sacrificed at two weeks (short-term), 12- and 16 weeks (long-term).
Table 1.
Experimental design
| Groups | Surgery | Survival time 2 weeks (N) | Survival time 12 weeks (N) | Survival time 16 weeks (N) |
|---|---|---|---|---|
| I | Autograft | 5 | 6 | 6 |
| II | Optimized processed allograft (OPA) | 5 | 6 | 6 |
| III | OPA + SIEF flap | 5 | 6 | 6 |
Ten-mm sciatic nerve gaps were reconstructed with an autograft (group I, gold standard), an optimized processed allograft (group II, OPA), or an OPA wrapped in a pedicled superficial inferior epigastric fascial (SIEF) flap (group III). Rats were sacrificed at two, 12 and 16 weeks to measure vascularization.
Nerve allograft harvest and processing
Seventeen Sprague-Dawley rats (Envigo, Madison, WI, USA), weighing 250–300 grams, served as donors for harvesting a 15-mm segment of the sciatic nerve bilaterally. The sciatic nerves were cleaned from external debris and processed using a five-day decellularization protocol16. Sprague-Dawley rats were used to obtain a major histocompatibility complex mismatch with the recipient Lewis rats17,18. Briefly, rats were anesthetized in an isoflurane induction chamber and euthanized with an overdose of Pentobarbital Sodium (Fatal Plus, 390 mg/mL, Vortech, Dearborn, MI, USA). The nerves were harvested and collected in RPMI 1640 culture medium. After processing, the nerves were sterilized using γ-irradiation and stored in a Sodium Phosphate Buffer (PBS) at 4°C. All steps were carried out at room temperature with agitation under sterile conditions and in laminar flow hood.
Surgical procedure
Rats were anesthesized in an isoflurane chamber, shaved, prepped and positioned in the nosecone to maintain anesthesia throughout the procedure. Preoperatively, the following were administered subcutaneously: 5 mL of NaCl 0.9% solution (to prevent dehydration), Enrofloxacin (Baytril, Bayer, Germany, 10mg/kg, providing infection profylaxis) and Buprenorphine SR (Buprenorphine SR-LAB, ZooPharm pharmacy, 0.6mg/kg, pain control). During surgery, body temperature was maintained at 37°C with a heating pad.
The sciatic nerve on the left side of each rat was fully exposed proximally from the inferior margin of the piriformis muscle to approximately 5 mm distal to the bifurcation, under an operating microscope (Zeiss OpMi 6, Carl Zeiss Surgica, Oberkochen, Germany). A 10-mm segment of the sciatic nerve was excised by sharp transection with microsurgical scissors. In group I, the nerve segment was reversed and placed as an interposition autograft with six 10–0 nylon (10–0 Ethilon, Ethicon Inc., Sommerville, NJ, USA), epineural interrupted sutures on either side of anastomosis. In group II, the nerve gap was bridged with a 10-mm OPA with use of a similar surgical technique. In group III, the gap was also repaired with a 10-mm OPA, but consecutively a pedicled adipofascial flap was wrapped around the nerve allograft. The superficial inferior epigastric artery fascial (SIEF) flap was harvested as previously described15. Briefly, a 4-cm paramedian abdominal incision on the ipsilateral side of the nerve reconstruction was made. The femoral artery was identified in the groin, whereafter the superficial inferior epigastric (SIE) vessels were exposed. The 4 × 3 cm SIEF flap containing subcutaneous fat, inguinal fat, the femoral vasculature and SIE vessels, was tunneled subcutaneously toward the nerve reconstruction and wrapped around the nerve. Both the proximal and distal nerve anastomoses were covered with the flap.
In all groups, wounds were closed in layers, approximating muscle with two 5–0 absorbable interrupted sutures (5–0 Vicryl Rapide, Ethicon Inc., Sommerville, NJ, USA). The skin was closed subcutaneously, using the same suture. Postoperatively, the rats were kept warm with towels. The rats were observed until completion of the experiment.
Nonsurvival procedure
After completion of the designated survival period, rats were sacrificed and neoangiogenesis was measured using two measures; the vascular surface area and the vascular volume.
Anesthesia
At survival time points (two, 12 and 16 weeks), rats were anesthetized and euthanized with 1 mL intraperitoneal injection of Pentobarbital Sodium.
Vascular preservation
Both thighs as well as the abdomen of the rat were shaved. On both sides, the sciatic nerve was exposed carefully. The vasculature of the lower extremity was preserved by aortic infusion19. A long longitudinal cut was made medially to expose the vena cava and aorta and these were cleaned from debris. These vessels were ligated as proximal as possible using a 5–0 Vicryl suture (Vicryl Rapide, Ethicon Inc., Sommerville, NJ, USA). Distal to the ligation, a catheter was inserted in the aorta. A yellow Microfil® compound (MV 8ml, diluent 15 ml, and curing agent 1.2 ml, Flow Tech, Inc., Carver, MA, USA) was infused into the aorta. After the contrast agents had cured, bilateral sciatic nerves were harvested. Nerves were temporarily stored in PBS and cleared in graded series of ethyl alcohol (25%, 50%, 75%, 95%, 100%) and placed in methyl salicylate. Clearing the nerve tissue while preserving the injected Microfil® allowed for measurement of the vascularity of the nerve segments.
Outcome measurements
Preserved vasculature in the nerve segments was quantified using a SkyScan 1276 micro computed tomography (micro CT, Bruker Corporation, Billerica, MA, USA) to calculate the vascular volume (three dimensional) and a Canon 5D Mark IV camera, (Manual Mode, ISO 200, 1/200th of a sec, f/16), a Canon MP-E 65mm Macro lens and a Canon MT-26-RT Twin Lite Macro strobe light source for calculating the vascular surface area (two dimensional), according to protocol19.
Statistical analysis
The vascular volume and the vascular surface area were analyzed and compared to the non-operated sciatic nerve (control). Analysis of variance (ANOVA) with Bonferroni post-hoc tests were used for comparisons between groups and time points. Results were reported as the mean and standard error or the mean (SEM), and the level of significance was set at α ≤ 0.05.
Cross sectional analysis
To describe the revascularization patterns in various parts of the nerve, cross sectional images from micro CT imaging were obtained for the 12- and 16 week survival periods. The length of the nerve between both anastomoses was divided into three equal sections: proximal (part I), mid (part II) and distal (part III). For each section, four cross sectional images were obtained. Cross-sectional images were divided into three equally concentric rings (central (A), middle (B) and outer (C)). The number of vessels was counted in each ring. Number of vessels in each part of the nerve (proximal, mid or distal) and ring (central, middle or outer) were averaged and compared to other groups of nerve grafts using multivariate analysis of variance (MANOVA) with Bonferroni post-hoc testing. Results were reported as the mean and SEM, and the level of significance was set at α ≤ 0.05.
Results
Macroscopic appearance of the vessels in the nerve samples
All rats were sacrificed at their designated survival periods and successful preservation of vasculature was achieved in all nerve samples (N=51). After clearing had taken place, the nerves were imaged using the micro CT to allow 3D visualization of the vessels (Figure 1). Macroscopic photographs were obtained from these nerve samples for 2D visualization of vessels (Figure 2).
Figure 1. Micro computed tomography (micro CT) images of nerve samples.
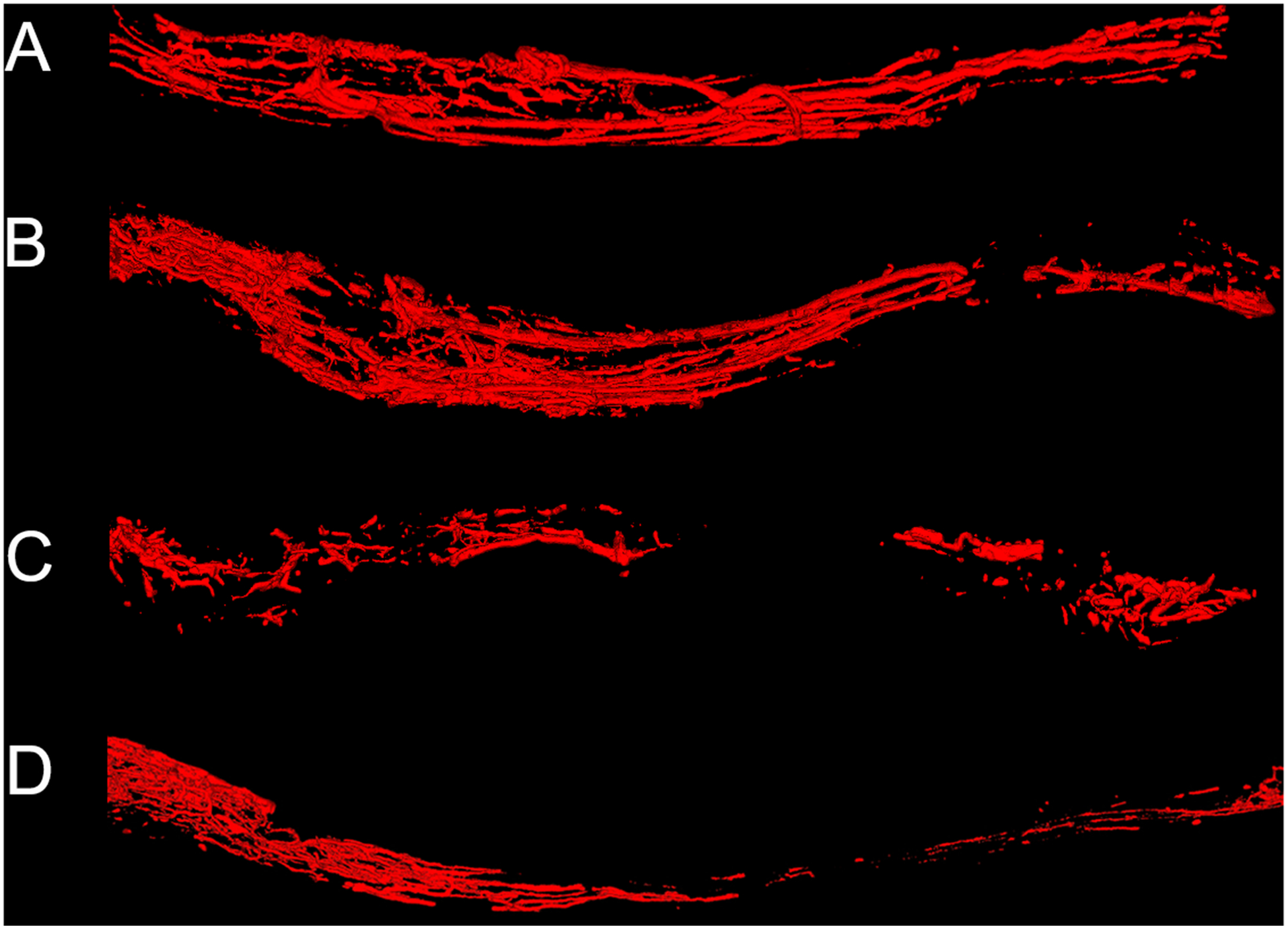
Micro CT images of control nerve (A), autograft (B), allograft (C) and allograft wrapped in a pedicled superficial inferior epigastric fascial (SIEF) flap (D). Images were obtained at 16 weeks. Nerve samples were positioned from proximal to distal (left to right respectively). Scale bar is set at 1 millimeter.
-In the supplementary data videos of these nerve samples were provided to give a 3D representation of the micro CT.
Figure 2. Macroscopic images of nerve samples obtained with conventional digital photography.
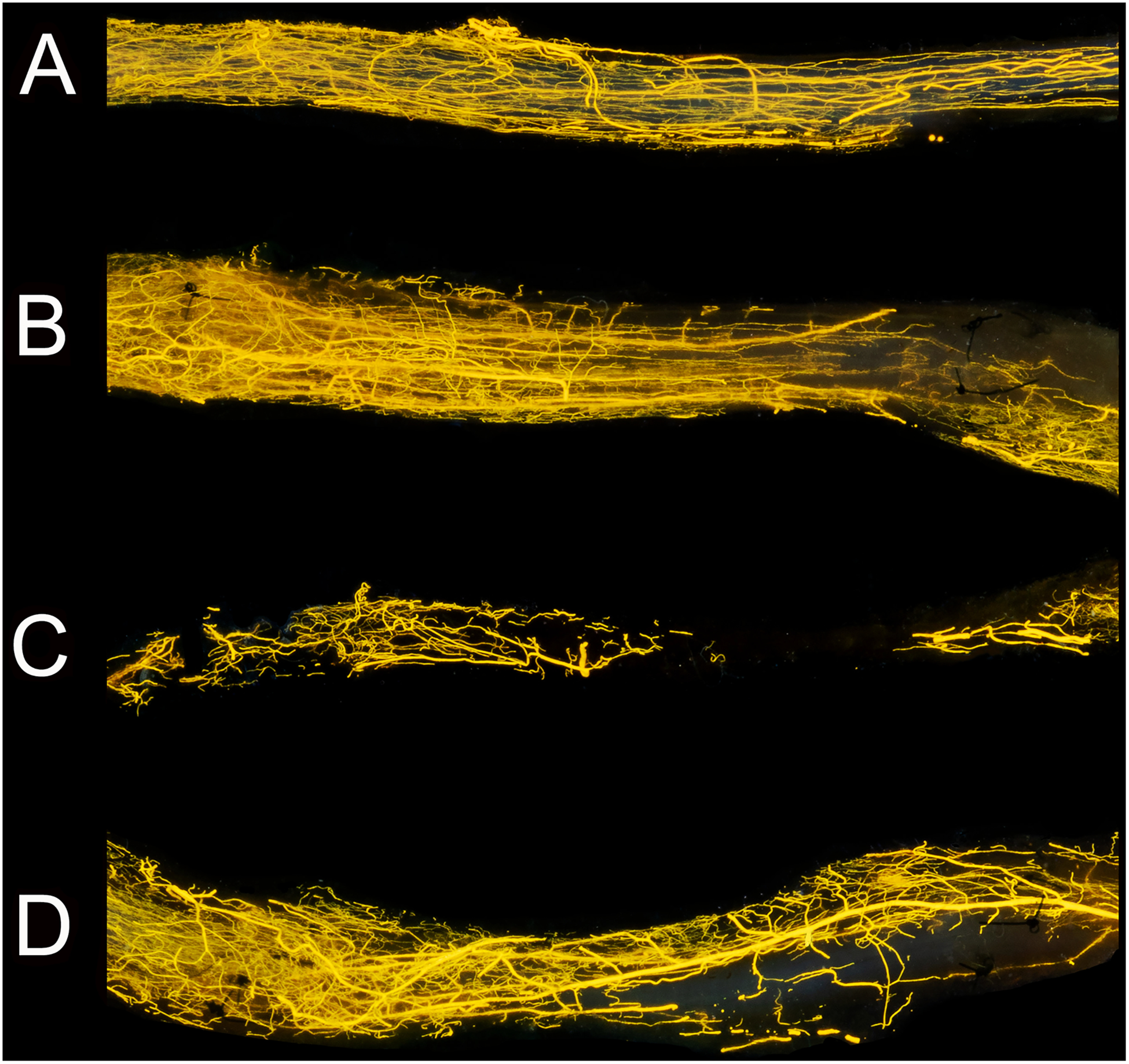
Images of the same samples visualized in Figure 2. Microvessels were clearly seen in the control nerve (A), autograft (B), allograft (C) allograft wrapped in a pedicled superficial inferior epigastric fascial (SIEF) flap (D). These photographs depicted nerve groups at 16 weeks. Sutures that were used to repair the graft were visible in nerve graft groups (B,D) and depicted the border of the analyzed frame. Nerve samples were positioned from proximal to distal (left to right respectively). Scale bar is set at 1 millimeter.
Vascular volume and vascular surface area at two weeks
The vascular volume was successfully measured in the three experimental groups and compared to control. At two weeks, the control nerve samples measured 4.5 ± 0.3% vessel (mean ± SEM), compared to 2.5 ± 0.3% in nerve autografts, 1.4 ± 0.4% in nerve allografts and 3.4 ± 0.6% in the SIEF group. Control samples were superior to autograft (P<0.05) and allograft (P<0.0001), and comparable to the SIEF group (P=0.1, Figure 3A).
Figure 3. Short-term vascularization at two weeks measured by vascular volume (micro CT, 3A) and vascular surface area (conventional digital photography, 3B).
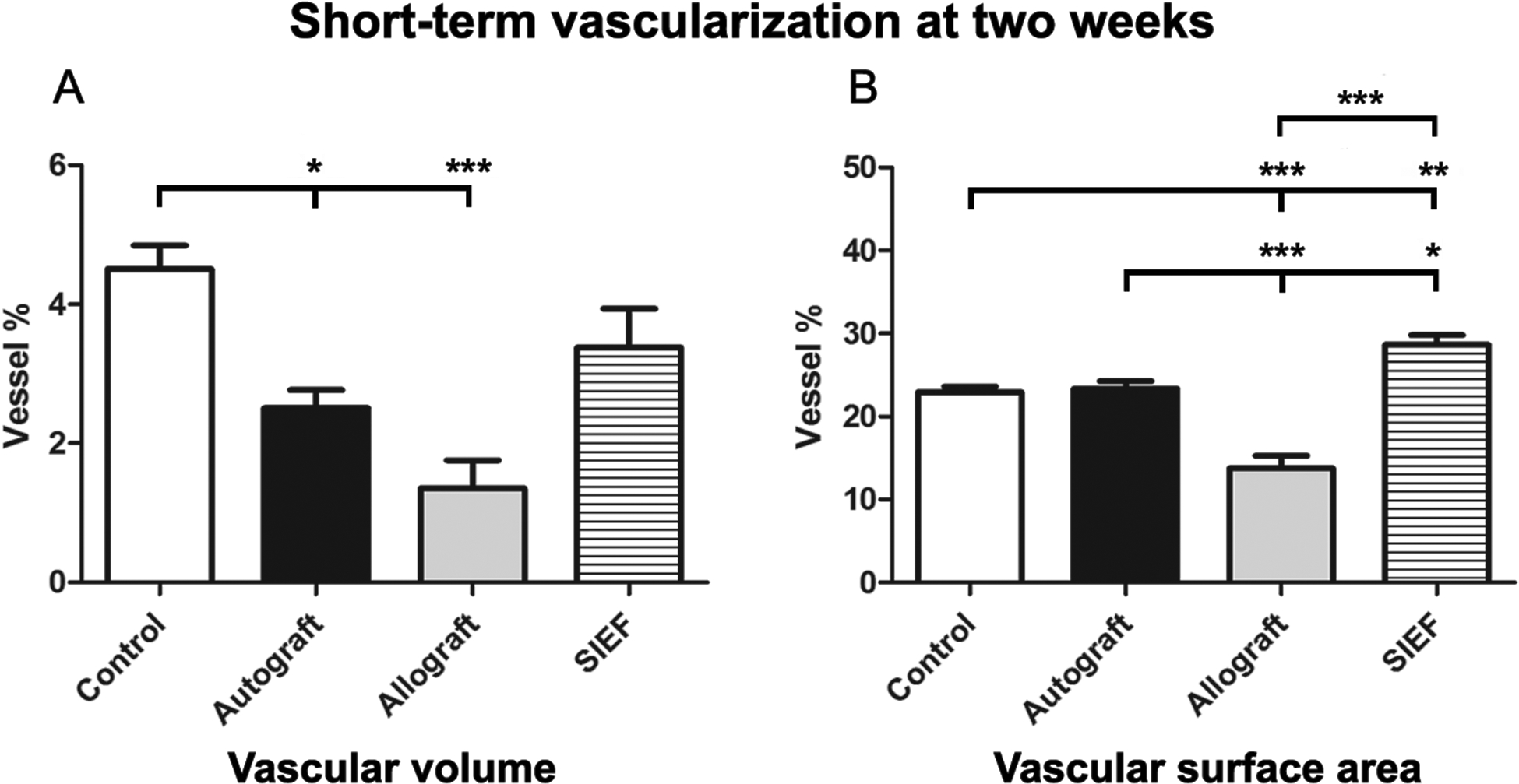
Results of control, autograft, allograft and allograft wrapped in a pedicled superficial inferior epigastric fascial (SIEF) flap expressed as percentage (vessel %) of the total nerve area and were given as the mean ± SEM. Please note that the range of the Y-axes is different. * Indicates significance at P<0.05, ** P<0.01, *** P<0.0001.
SEM = Standard error of the mean
The vascular surface area measured 23.0 ± 0.6% vessel in control samples, 23.4 ± 0.9% in autografts, 13.8 ± 1.5% in allografts and 28.7 ± 1.1% in SIEF nerves. SIEF nerves were superior to all other groups and allografts were inferior to all other groups (P<0.01 compared to control, P<0.05 compared to autograft, P<0.0001 compared to allograft) as shown in Figure 3B.
Long-term outcomes of vascular volume and vascular surface area
Vascularization outcomes obtained using vascular volume and vascular surface area are shown in Table 2 for control, autograft, allograft and SIEF nerve samples at 12- and 16 weeks. Significance is visualized in Figure 4 (vascular volume) and Figure 5 (vascular surface area).
Table 2.
Long-term outcomes of vascular volume and vascular surface area
| Vascular volume | Vascular surface area | |
|---|---|---|
| Control | ||
| 12 wk | 5.0 ± 0.3 | 26.0 ± 1.2 |
| 16 wk | 5.7 ± 0.3 | 30.6 ± 1.1 |
| Autograft | ||
| 12 wk | 3.5 ± 0.4 | 25.0 ± 2.8 |
| 16 wk | 5.0 ± 0.4 | 28.1 ± 3.5 |
| Allograft | ||
| 12 wk | 2.7 ± 0.2 | 18.0 ± 2.2 |
| 16 wk | 3.1 ± 0.2 | 22.1 ± 1.7 |
| SIEF | ||
| 12 wk | 6.1 ± 1.5 | 31.1 ± 2.2 |
| 16 wk | 12.6 ± 2.4 | 37.6 ± 3.4 |
Outcomes of control, autograft, allograft and allograft wrapped in a pedicled superficial inferior epigastric fascial (SIEF) flap were shown in this table. Results were expressed as mean % vessel ± SEM at 12- (first row) and 16 weeks (second row)
SEM = Standard error of the mean
Figure 4. Vascular volume of nerve groups at 12- and 16 weeks using micro CT.
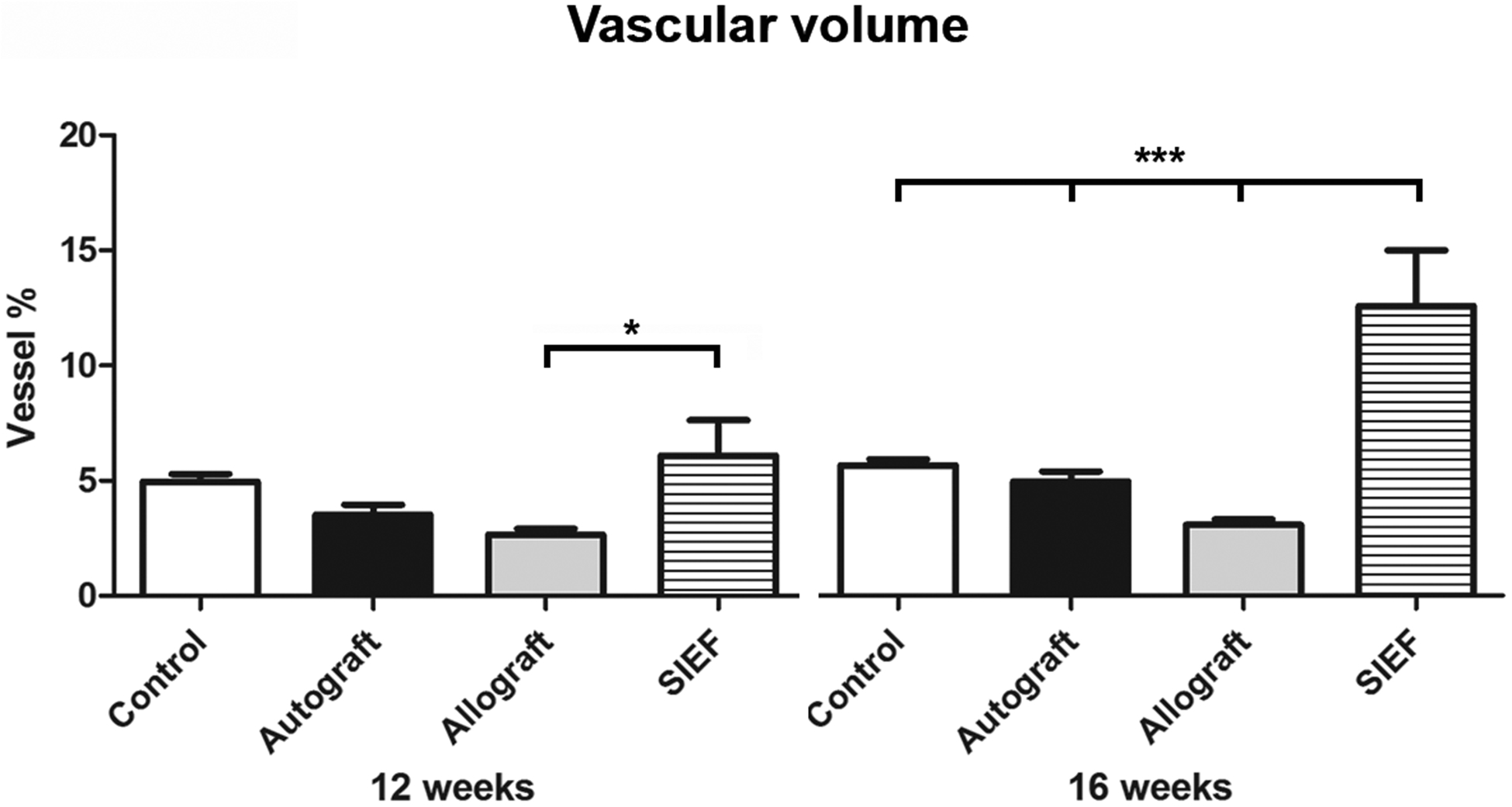
Results of control, autograft, allograft and allograft wrapped in a pedicled superficial inferior epigastric fascial (SIEF) flap were expressed as a percentage (vessel %) of the total nerve area and were given as the mean ± SEM. *Indicates significance at P<0.05, *** P<0.0001.
SEM = Standard error of the mean
Figure 5. Vascular surface area of nerve groups at 12- and 16 weeks using digital photography.
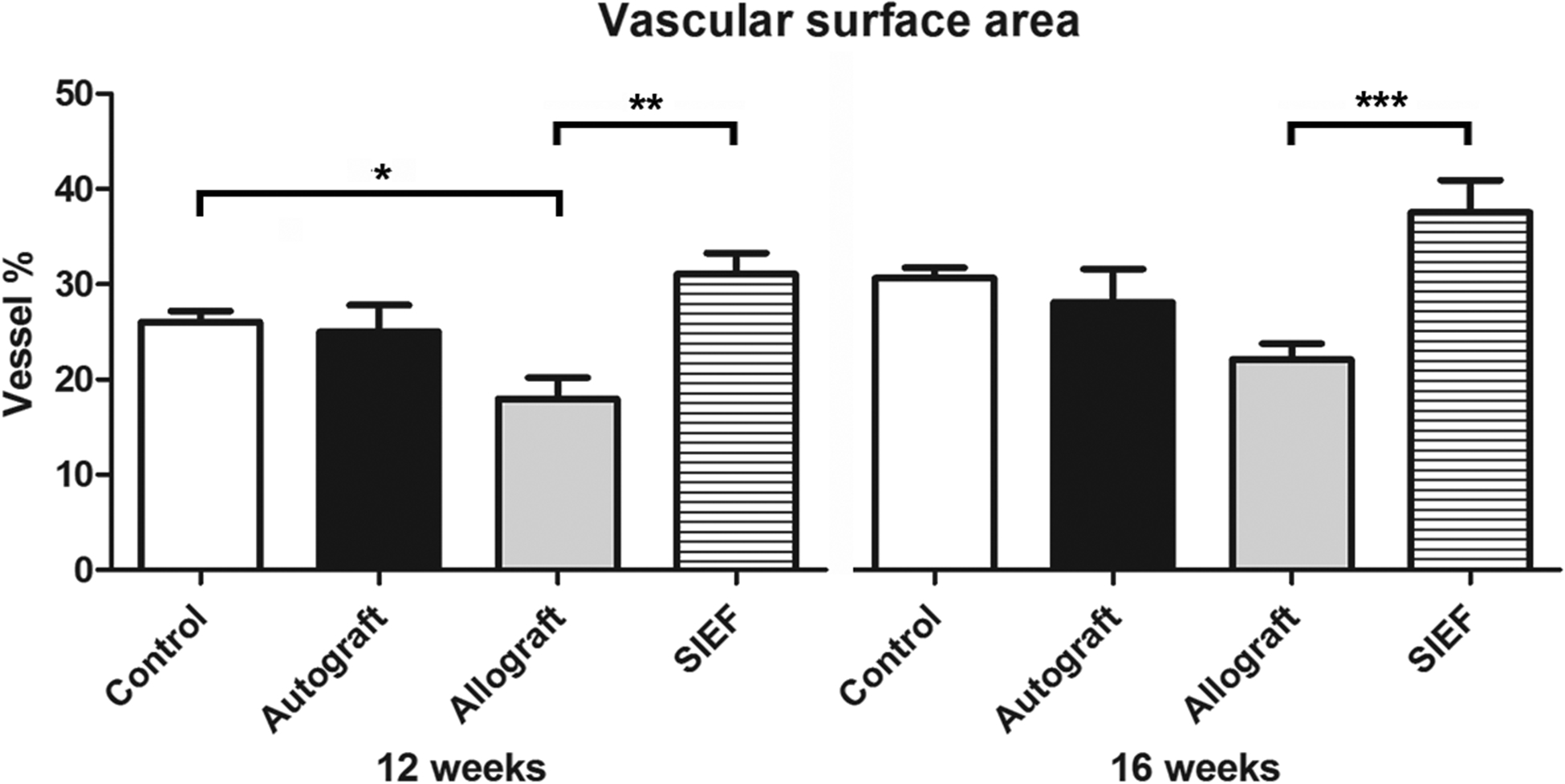
Results of control, autograft, allograft and allograft wrapped in a pedicled superficial inferior epigastric fascial (SIEF) flap were expressed as a percentage (vessel %) of the total nerve area and were given as the mean ± SEM. *Indicates significance at P<0.05, *** P<0.0001.
SEM = Standard error of the mean
Revascularization patterns over time
Starting at two weeks, vascularization consisting of a mesh-network occurred from both host stumps in nerve allograft and SIEF nerve samples, leaving the middle part avascularized. This invasion of microvessels was more evident from the proximal than from the distal end. Over time, these differences became more evident in the 12- and 16 week samples, as the sprouted vessels reached to the middle parts of the nerve. In nerve autografts, longitudinal running vessels were recognized that ran along the entire length of the nerve. These vessels appeared thicker compared to the newly formed vessels in the allograft and SIEF nerve samples.
At 12 weeks, the proximal sections of the nerve samples showed that the addition of vascularization to allograft nerves (SIEF group) resulted in the highest number of vessels in the outer ring of the nerve (P<0.01). The SIEF group had significantly more vessels in the middle and central ring as well, compared to the allograft alone (P<0.05). In the mid-section of the nerve, the number of vessels in the allograft was lowest compared to all other groups in the middle ring (P<0.05). In the central ring, the allograft also measured the least number of vessels and was significantly inferior to SIEF nerves and control nerves (P<0.05). The number of vessels in SIEF nerves and control nerves were similar in the central ring of the mid-section of the nerve. In the distal section of the nerve, a trend towards a higher number of vessels was seen in SIEF nerves, however, this was not significant. Schematic visualization of the number of vessels in different parts of the nerve are depicted in Figure 6.
Figure 6. Nerve vascularization patterns of nerve groups at 12- and 16 weeks.
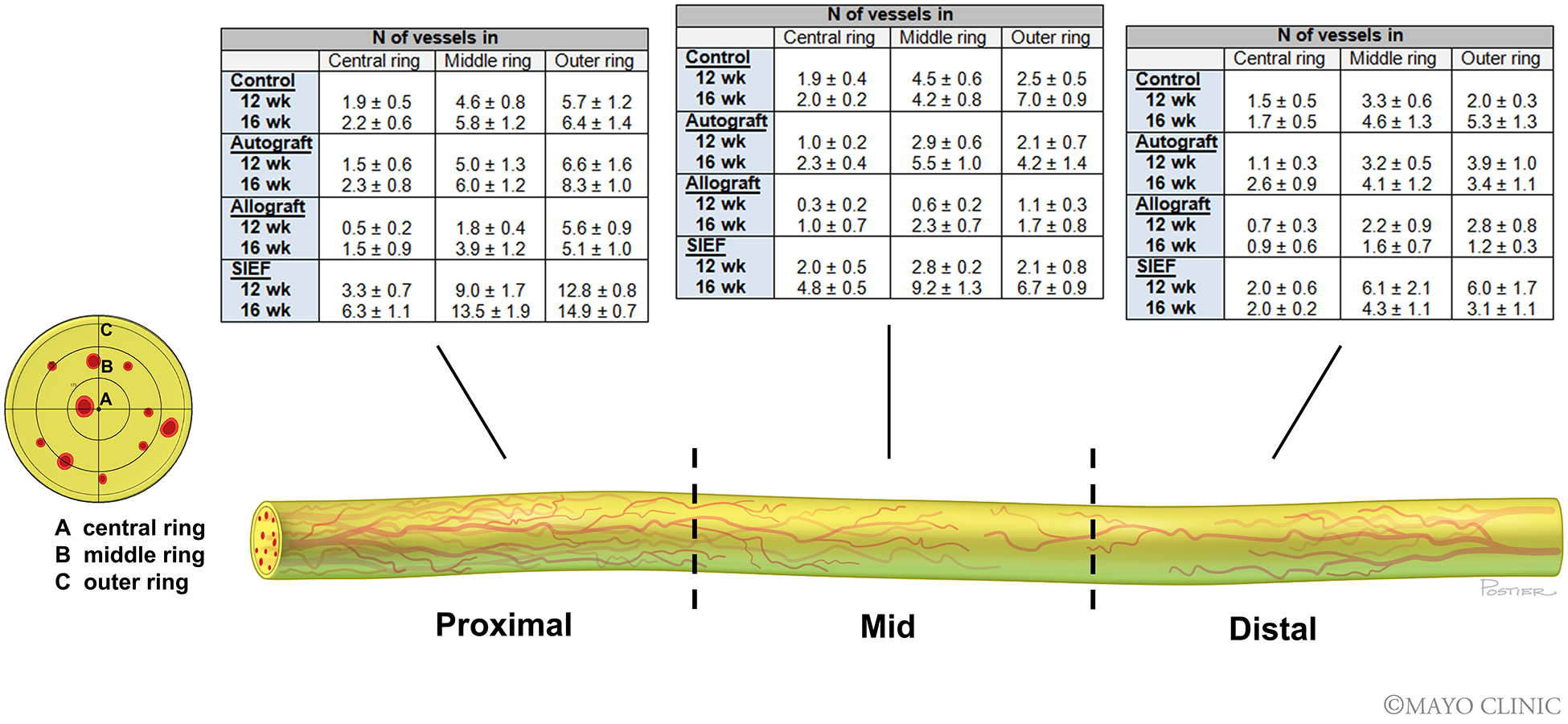
Micro CT cross-sectional images throughout the length of the nerve grafts were obtained. The length of the nerve was divided into three sections: (I) proximal, (II) mid and (III) distal. The cross-sectional images were divided into three rings: (A) central ring, (B) middle ring, (C) outer ring to count the number of vessels. Nerve tissue was depicted in yellow and the vessels were depicted in red. Tables describe the number of vessels in each of the rings per nerve section (proximal, mid and distal) for control, autograft, allograft and allograft wrapped in a superficial inferior epigastric artery fascia (SIEF) flap. The first row denotes the number of vessels (mean ± SEM) at 12 weeks and the second row at 16 weeks.
SEM = Standard error of the mean
At 16 weeks, the proximal section of the nerve showed superiority of the number of vessels in SIEF nerves in all three rings of the cross-sections compared to other groups (P<0.0001 in outer ring, P<0.05 in middle and central ring). In the mid-section of the nerve, the number of vessels in the outer ring was lowest in the allografts and inferior to the SIEF and control nerve samples (P<0.05). In the middle ring, the SIEF group was superior to allograft (P<0.0001) and control nerves (P<0.05) and in the central ring the SIEF group measured the highest number of vessels and was superior to all other groups (P<0.0001). In the distal section of the nerve, no significant differences were found when comparing the groups and different rings (Figure 6).
Discussion
Revascularization of nerve is postulated to occur from (i) extraneural vascular contribution from surrounding beds (centripetal revascularization) and (ii) longitudinal bidirectional inosculation from the proximal and distal ends of the graft9,20,21. Inosculation results in endothelial-lined newly formed blood vessels starting from day three, without formation of vessels in the middle segment of the graft9,22,23. It is believed that the vessels in the mid-section are formed by ingrowth of blood vessels from the surrounding bed, starting by day six to eight22. The larger the grafts, the longer it takes to be completely revascularized, with risk of fibrosis and central necrosis22. Neovascularization is a complex process of critical importance involving endothelial cells, sprouting from the parent vessel, and releasing of growth factors, such as vascular endothelial growth factor (VEGF), a potent angiogenic factor required during tissue repair6,24. The predominant mechanism (centripetal versus bidirectional inosculation) of revascularization remains unknown.
The findings in this study are in line with the theory that inosculation occurs prior to centripetal revascularization. At two weeks, early revascularization in nerve allografts occurred from both nerve stumps, but primarily from proximal to distal. In allografts wrapped in a pedicled flap, this amount of inosculation was greater, suggesting that an improved vascularized bed promotes longitudinal inosculation, in particular proximal inosculation. Favored proximal vascular advancement was also suggested by Chalfoun and colleagues using microvascular blood flow imaging25. This finding may support the fact that success of the nerve graft is partly affected by the length of the nerve graft22 as a longer graft is subject to higher risk of necrosis in the mid to distal sections. Between autografts and control nerves a similar pattern of vascularization was seen, indicating that reestablishment of blood supply occurs along preexisting vascular channels22,26.
In VNGs (vascularized nerve grafts), neovascularization is triggered in the first 72 hours as the blood flow is equal or greater than that in normal nerves suggesting to prevent early ischemia22. This is clearly seen in the increase in vascular volume of the revascularized allografts compared to the allografts at two weeks. The vascular volume represents the actual volume of vessels in the nerve, whereas the vascular surface area is more likely to be an estimation of vessels as a three dimensional structure is converted to a two dimensional structure. In small nerve samples, a high resolution of micro CT is crucial to identify the smallest vessels. When such a micro CT is not available, vascular surface areas can be used to clarify differences between experimental groups as similar trends between the vascular volume and vascular surface areas are seen and these methods are correlated19, however, based on the findings of this study, vascular volume is preferred.
The blood supply of the recipient bed affects the success of a nerve graft substantially, which is believed to be resulting in early revascularization of the graft and ultimately speed of axonal regeneration and degree of restored function of the target muscle22. Central necrosis of thick nerve grafts has been a confirmed problem and commonly described as necrosis of the central ring of the graft (core necrosis)7,27–29. Our data are consistent with previous studies in which not only the core but also the central section of the length of the allograft nerve has been shown to be predisposed to avascularity, with potentially higher risk of necrosis30. A VNG greatly affects vascularization, impeding both types of central necrosis. Due to a decrease in graft ischemic time, VNGs are suggested to lead to faster nerve regeneration than nonvascularized nerve grafts (NVNG)30. However, the rate of axonal regeneration has not found to be different between non-vascularized and conventional nerve grafts30–32. Functional recovery needs to be tested, as suggested to be positively affected by VNGs11.
The theoretical advantages of a VNG or provision of a well-vascularized bed for the nerve graft are well accepted7. Maintaining vascularization after nerve injury may yield several advantages: not only may it restore extrinsic neural blood vessels that were damaged during nerve trauma, intraneural fibrosis secondary to ischemia will also be reduced. This may result in an increase of axonal regeneration particularly in thicker grafts22,33, subsequently preventing target muscle atrophy7. In order to have these potential advantages, surgically provided vascularization to the nerve site needs to be applied accurately. The vascular pedicle should be of large enough diameter to support microvascular anastomoses in the case of free flaps. To overcome this problem a simple pedicled flap could be used. Another condition that influences the outcomes of the peripheral nerve is the fact that blood supply should emanate from a vascular pedicle that travels parallel with the nerve over an adequate distance22,33. The surgical technique that provides the pedicled adipofascial flap has been validated while taken these prerequisite conditions in account15.
The limitation of this study is the large time gap between the short-term and long-term outcomes. To better describe revascularization patterns, time points at four or eight weeks may have provided additional information. Vascular response to nerve injury is composed of two phases: after the early first phase, triggered by Wallerian degeneration in the first week, the second phase comprises from one to six weeks after injury. This phase is characterized by the increase in number of vessels and associated with nerve regeneration and axonal myelination6,34,35. However, differences between these phases, or newly developed vessels could not be distinguished from older vessels using these techniques.
Conclusions
The results of this study highlight the revascularization patterns after interposition nerve grafting in rats over time. The importance of adding a well-vascularized bed for nerve allografts, in means of a pedicled flap wrapped around a nerve, are objectively measured and visualized over the length of the nerve as well as in the various cross sectional rings of the nerve. Based on this study, we can conclude that the blood supply of the recipient bed determines the degree of early revascularization of the nerve graft. Furthermore, revascularization occurs primarily from proximal to distal (proximal inosculation) and not from both ends as previously believed and confirms the theory of centripetal revascularization.
Supplementary Material
Acknowledgments
The authors would like to thank Jim Postier (Rochester, MN) for the artwork of Figure 6.
This study was funded by the NIH RO1, ‘Bridging the gap: angiogenesis and stem cell seeding of processed nerve allograft’. RO1 NS102360.
Study performed at: Mayo Clinic, Rochester, USA
“Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number RO1 NS102360. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health”
Footnotes
Conflicts of interest None declared.
Ethical Approval: Mayo Clinic Institutional Animal Care and Use Committee (IACUC A3348–18)
This study will be presented at the upcoming American Society for Peripheral Nerve (ASPN) meeting 2020.
Supplemental files: Videos of the micro CT imaged nerves in Figure 1.
References
- 1.Ferretti A, Boschi E, Stefani A, et al. Angiogenesis and nerve regeneration in a model of human skin equivalent transplant. Life Sci 2003;73):1985–1994. [DOI] [PubMed] [Google Scholar]
- 2.Gu XH, Terenghi G, Kangesu T, et al. Regeneration pattern of blood vessels and nerves in cultured keratinocyte grafts assessed by confocal laser scanning microscopy. Br J Dermatol 1995;132:376–383. [DOI] [PubMed] [Google Scholar]
- 3.Manek S, Terenghi G, Shurey C, Nishikawa H, Green CJ, Polak JM. Neovascularisation precedes neural changes in the rat groin skin flap following denervation: an immunohistochemical study. Br J Plast Surg 1993;46:48–55. [DOI] [PubMed] [Google Scholar]
- 4.Kangesu T, Manek S, Terenghi G, et al. Nerve and blood vessel growth in response to grafted dermis and cultured keratinocytes. Plast Reconstr Surg 1998;101:1029–1038. [DOI] [PubMed] [Google Scholar]
- 5.Hobson MI, Brown R, Green CJ, Terenghi G. Inter-relationships between angiogenesis and nerve regeneration: a histochemical study. Br J Plast Surg 1997;50:125–131. [DOI] [PubMed] [Google Scholar]
- 6.Caillaud M, Richard L, Vallat JM, Desmouliere A, Billet F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res 2019;14:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Arpa S Vascularized nerve “grafts”: just a graft or a worthwhile procedure? Plast Aesthet Res 2016;2:183–192. [Google Scholar]
- 8.Krames ES. Possible malfunction of electronic medical devices caused by computed tomography scanning. Neuromoduolation 2009;12:5–7. [DOI] [PubMed] [Google Scholar]
- 9.Tarlov IM EJ. Nerve grafts: The importance of an adequate blood supply. J Neurosurg 1945;2:49–71. [Google Scholar]
- 10.Seddon HJ. The use of autogenous grafts for the repair of large gaps in peripheral nerves. Br J Surg 1947;35:151–167. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo Y, Merle M, Michon J, Folliguet B, Barrat E. Free vascularized nerve grafts: an experimental study in the rabbit. Microsurg 1985;6:78–84. [DOI] [PubMed] [Google Scholar]
- 12.Shibata M, Tsai TM, Firrell J, Breidenbach WC. Experimental comparison of vascularized and nonvascularized nerve grafting. J Hand Surg Am 1988;13:358–365. [DOI] [PubMed] [Google Scholar]
- 13.Kanaya F, Firrell J, Tsai TM, Breidenbach WC. Functional results of vascularized versus nonvascularized nerve grafting. Plast Reconstr Surg 1992;89:924–930. [DOI] [PubMed] [Google Scholar]
- 14.Karcher H, Kleinert R. Regeneration in vascularized and free nerve grafts. A comparative morphological study in rats. J Maxillofac Surg 1986;14:341–343. [DOI] [PubMed] [Google Scholar]
- 15.Saffari TM, Bishop AT, Shin AY. The superficial inferior epigastric artery fascia (SIEF) flap in rats. In. Submitted to Journal of Reconstructive Microsurg 2019. [Google Scholar]
- 16.Hundepool CA, Nijhuis TH, Kotsougiani D, Friedrich PF, Bishop AT, Shin AY. Optimizing decellularization techniques to create a new nerve allograft: an in vitro study using rodent nerve segments. Neurosurg Focus 2017;42:E4. [DOI] [PubMed] [Google Scholar]
- 17.Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng 2004;10:1641–1651. [DOI] [PubMed] [Google Scholar]
- 18.Kumta S, Yip K, Roy N, Lee SK, Leung PC. Revascularisation of bone allografts following vascular bundle implantation: an experimental study in rats. Arch Orthop Trauma Surg 1996;115:206–210. [DOI] [PubMed] [Google Scholar]
- 19.Saffari TM, Bishop AT, Shin AY. Novel strategies for objective angiogenesis evaluation of rat nerves using micro CT scanning and conventional photography. In. Submitted to Microsurg 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lind R, Wood MB. Comparison of the pattern of early revascularization of conventional versus vascularized nerve grafts in the canine. J Reconstr Microsurg 1986;2:229–234. [DOI] [PubMed] [Google Scholar]
- 21.Penkert G, Bini W, Samii M. Revascularization of nerve grafts: an experimental study. J Reconstr Microsurg 1988;4:319–325. [DOI] [PubMed] [Google Scholar]
- 22.Terzis JK, Skoulis TG, Soucacos PN. Vascularized nerve grafts. A review. Int Angiol 1995;14:264–277. [PubMed] [Google Scholar]
- 23.Wongtrakul S, Bishop AT, Friedrich PF. Vascular endothelial growth factor promotion of neoangiogenesis in conventional nerve grafts. J Hand Surg Am 2002;27:277–285. [DOI] [PubMed] [Google Scholar]
- 24.Pereira Lopes FR, Lisboa BC, Frattini F, et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol Appl Neurobiol 2011;37:600–612. [DOI] [PubMed] [Google Scholar]
- 25.Chalfoun C, Scholz T, Cole MD, Steward E, Vanderkam V, Evans GR. Primary nerve grafting: A study of revascularization. Microsurg. 2003;23:60–65. [DOI] [PubMed] [Google Scholar]
- 26.Wong D, Dupree J, Terzis JK. Vascularized versus nonvascularized nerve grafts: the controversy persists. 1988.
- 27.Sunderland S Nerves and Nerve Injuries. Vol 2nd edition Edinburgh: Churchill Livingstone; 1978. [Google Scholar]
- 28.Brooks D The place of nerve-grafting in orthopaedic surgery. J Bone Joint Surg Am 1955;37:299–305. [PubMed] [Google Scholar]
- 29.Seddon HJ. Nerve Grafting. J Bone Joint Surg Br 1963;45:447–461. [PubMed] [Google Scholar]
- 30.McCullough CJ, Gagey O, Higginson DW, Sandin BM, Crow JC, Sebille A. Axon regeneration and vascularisation of nerve grafts. An experimental study. J Hand Surg Br 1984;9:323–327. [DOI] [PubMed] [Google Scholar]
- 31.Seckel BR, Ryan SE, Simons JE, Gagne RG, Watkins E, Jr. Vascularized versus nonvascularized nerve grafts: an experimental structural comparison. Plast Reconstr Surg 1986;78:211–220. [DOI] [PubMed] [Google Scholar]
- 32.Pho RW, Lee YS, Rujiwetpongstorn V, Pang M. Histological studies of vascularised nerve graft and conventional nerve graft. J Hand Surg Br 1985;10:45–48. [DOI] [PubMed] [Google Scholar]
- 33.Taylor GI. Nerve grafting with simultaneous microvascular reconstruction. Clin Orthop Relat Res. 1978:56–70. [PubMed] [Google Scholar]
- 34.Podhajsky RJ, Myers RR. The vascular response to nerve crush: relationship to Wallerian degeneration and regeneration. Brain Res 1993;623:117–123. [DOI] [PubMed] [Google Scholar]
- 35.Podhajsky RJ, Myers RR. The vascular response to nerve transection: neovascularization in the silicone nerve regeneration chamber. Brain Res 1994;662:88–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


