Abstract
Milk thistle cold press oil (MTO) is an herbal remedy derived from Silybum marianum which contains a low level of silymarin and mixture of polyphenols and flavonoids. The effect of MTO on the cardiovascular and metabolic complications of obesity was studied in mice that were fed a high-fat diet (HFD) for 20 weeks and treated with MTO for the final 8 weeks of the diet. MTO treatment attenuated HFD-induced obesity, fasting hyperglycemia, hypertension, and induced markers of mitochondrial fusion and browning of white adipose. Markers of inflammation were also attenuated in both adipose and the liver of MTO-treated mice. In addition, MTO resulted in the improvement of liver fibrosis. These results demonstrate that MTO has beneficial actions to attenuate dietary obesity-induced weight gain, hyperglycemia, hypertension, inflammation, and suggest that MTO supplementation may prove beneficial to patients exhibiting symptoms of metabolic syndrome.
Keywords: heme oxygenase, metabolism, mitochondria, type II diabetes
1 |. INTRODUCTION
According to the World Health Organization, annually, over two million people die worldwide from the complications of excessive body fat. Altered adipose tissue function is characterized by an impaired lipid buffering capacity and subsequently by a systemic lipid overflow resulting in ectopic lipid accumulation in several insulin-sensitive peripheral tissues such as skeletal muscle, liver, pancreas, heart, and kidneys (Clichici et al., 2015; Ni & Wang, 2016). This obesity trend is observed in both men and women, having a similar pattern, being around 40%–45% obese in middle age around 35% obese when younger (Zhu et al., 2018). The ectopic deposition of triglycerides triggers a series of cardiometabolic perturbations that lead to the diagnosis of metabolic syndrome (MetS) (Reviewed in (Drummond et al., 2019). This disorder is not only associated with a higher risk of type 2 diabetes and cardiovascular events but also impacts the liver (Meddeb et al., 2017). Recent data suggest that nonalcoholic fatty liver disease (NAFLD), considered the hepatic manifestation of the MetS and precedes the development of MetS (Michel et al., 1990; Raffaele et al., 2016). NAFLD is associated with several metabolic diseases, including diabetes mellitus, obesity, and hypertension. In a 5-year retrospective review, individuals with NAFLD had a higher incidence of impaired fasting glucose and type-2 diabetes mellitus (T2DM) compared with NAFLD-free controls (da Silva et al., 2008). In the last few decades, a higher frequency of obesity, T2DM, and MetS have occurred as a result of various dietary changes (Reviewed in (Abraham & Kappas, 2008) (do Carmo et al., 2012; Sacerdoti et al., 2018). Furthermore, individuals with NAFLD have a higher probability of liver failure and, eventually, cirrhosis (Brestoff et al., 2015; Lizcano, 2019; Warner & Mittag, 2016). Low grade inflammation and mitochondrial dysfunction contribute to the progression of NAFLD to NASH (Nelson et al., 2007) as a result of adipocyte-mediated inflammatory adipokines (Kowdley et al., 2012) and insulin resistance (Vatner et al., 2015). In this regard, beneficial effects have been reported for curcumin and (Rodella et al., 2008; Scapagnini et al., 2011) which increase the antioxidant gene and heme oxygenase-1 (HO-1). Resveratrol administration increases antioxidant genes HO-1 expression, NAD(P)H, and Quinone oxidoreductase 1. Resveratrol, as well as other HO-1 inducers, prevent CVD (Lagouge et al., 2006; Rodella et al., 2008). The antioxidant gene HO-1 is suppressed by hyperglycemia, while glucose deprivation induces HO-1 gene expression (Abraham et al., 2003; Chang et al., 2002, 2003; Moini et al., 1999). Obesity and hyperglycemia downregulate the HO-1 expression and increase the metabolic disease status (Berglund et al., 2009; Singh et al., 2019; Singh et al., 2020; Zhu et al., 2018).
Currently, there is increasing interest in examining the natural antioxidants found in dietary plants including fruits and vegetables that may have beneficial effects on metabolic disease. A Mediterranean diet protects against cardiovascular disease due to its antioxidant effect as a result of elevated levels of the presence of β-carotene, polyphenols, anthocyanins, and ellagic acids (Pittala et al., 2018). Ellagic acid that is found in raspberries and walnuts, as well as phenolic compounds, are shown to inhibit human prostate cancer cells and the promotion of human health (Vanella, Barbagallo, et al., 2013; Vanella, Di, et al., 2013). Certain traditional medicines such as Turmeric (curcumin) and other dietary factor intervention ameliorate metabolic disease in obese mice models (Cao et al., 2012; Son et al., 2013).
Milk thistle oil (MTO) is an herbal remedy derived from the milk thistle plant which is processed using a wide variety of methods, including ethanol extraction and cold pressing. Milk thistle extracted with ethanol contains high levels of silymarin. Silymarin treatment protects the liver against dietary-induced hepatic steatosis and oxidative stress as well as chemical-induced liver injury (Clichici et al., 2015; Ni & Wang, 2016). In contrast, milk thistle extracted as a cold press oil contains very little silymarin. However, it still has beneficial effects due to its content of α-tocopherol as well as polyphenols such as vanillic acid, p-coumaric, polyphenol, and silybin (Meddeb et al., 2017; Pittala et al., 2018) and other free fatty acids which all can react synergistically. Although much is known regarding the beneficial metabolic actions of silymarin, little is known regarding the cardiovascular and metabolic effects of MTO. Thus, we designed the present study to determine the role of cold pressed MTO on the development of diet-induced obesity.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Eight-week-old C57BL6 male mice were fed a HFD (Western diet, containing 60% kcal from fat, 21.3% carbohydrate, and 18.4% protein with total calories of 5.1 kcal/g (Cat no TD.06414, Harlan, Teklad Lab Animal Diets, Indianapolis, IN) for 20 weeks. Control mice were fed a standard laboratory diet containing 14.3% kcal from protein, 4% kcal from fat with total calories of 2.9 kcal/g. Mice were randomly divided into three treatment groups of five animals each as follows: group (1) Control (Ctrl), group (2) HFD, group, and (3) HFD treated for the last 8 weeks with MTO at a dose of 0.1% oil/50 gm body weight/day. HFD mice were treated with MTO at a dose of 0.1% oil/50 gm body weight/day, which is equal to 2% MTO/Kg/day. We calculate the daily consumption of the oils based on a 4g daily food intake in each mouse. Therefore, the dosage give to each mouse corresponds to 80 mg kg−1 day−1 intake. Formulation of MTO used is as follows; p-Cymene, 1.24%, Carvacrol 0.08%, Free fatty acid (FFA) 1,29%, Oleic acid 21.53%, Palmitic acid 11.31%, Linoleic acid 57.44%, and other fatty acid 1.98%, TriNutra Ltd, Nes Ziona, Israel). MTO was mixed into the HFD food which was then packed into chunks and food intake was 4.5–5.0 gm/day. At the end of the experiment, mice were euthanized, assessed for total body weight and fat content. All animal experiments were performed at and followed the NYMC IACUC institutionally approved protocol (22-2-0415H) in accordance with NIH guidelines. Euthanasia was performed by injection of ketamine (100 mg/kg)/xylazine (10 mg/kg) followed by cervical dislocation and tissue collection.
2.2 |. Fasting blood glucose and glucose tolerance testing
Fasting blood glucose was measured from tail blood following a 6-hr fast. Blood pressure was measured by tail-cuff plethysmography using the CODA tail-cuff System (Kent Scientific, CT, Torrington) as previously described. (Raffaele et al., 2019; Schragenheim et al., 2018; Singh et al., 2020).
2.3 |. Determination of oxygen consumption
Mice were allowed to adapt to oxygen consumption chambers over a 3-week period via 2-hr increments, three times a week. Oxygen consumption (VO2) was measured with an Oxylet gas analyzer and airflow unit (Oxylet, Panlab-Bioseb, Vitrolles, France) in individually housed mice. Respiratory quotient (RQ) was calculated as VCO2/VO2. The data for VO2 were expressed as the consumed oxygen per Kilogram body weight per minute (ml/kg/min) (Raffaele et al., 2019; Schragenheim et al., 2018; Singh et al., 2020).
2.4 |. Western blot analysis
Western blots of proteins in the adipose and liver were performed as previously described (Berg & Scherer, 2005; Collins et al., 2016; Hotamisligil, 2006). Primary antibodies for anti-NOV/CCN3, anti-IL-6, anti-pP65, anti-P65, anti-MMP9, anti-MMP2, anti-MT1-MMP, anti-TIMP1, anti-TIMP2, anti-PRDM16, anti-PGC-1α, anti-SOD1, anti-TWIST1, anti-SIRT1, anti-Mfn1, anti-FGF21, anti-CREG1, anti-UCP1, anti-pAKT, anti-AKT, and anti-β-actin were purchased from Cell Signaling Technology, Danvers, MA, USA. The anti-pIR tyr972 antibody was purchased from Millipore, Bedford, MA, USA. The anti-HO-1 antibody was purchased from Enzo Life Sciences, Farmingdale, NY, USA. The secondary antibodies labeled with either IRDye 680 or IRDye 800 were purchased from LICOR Biosciences, Lincoln, NE. Immunoreactivity was visualized and quantified by infrared scanning in the Odyssey system (LICOR Biosciences, Lincoln, NE) and quantified after normalization with β-actin and expressed as arbitrary units (AU).
2.5 |. Statistics
Statistical analysis was performed with Prism 7 (GraphPad Software, San Diego, CA). Data are presented as the means ± standard errors of the mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Newman–Keuls post hoc multiple comparison method. p values of .05 or lower were considered statistically significant.
3 |. RESULTS
3.1 |. Milk thistle seed oil (MTO) treatment attenuates body weight, improves fasting blood glucose, decreases blood pressure, and increases oxygen consumption in HFD fed mice
The 8-week MTO treatment in mice fed a HFD resulted in a significant (p < .05) decrease in body weight (Figure 1a). MTO also decreased HFD-induced fasting blood glucose level and blood pressure (Figure 1b,c). MTO increased the oxygen consumption in mice fed a HFD to levels observed in control mice fed a standard chow diet (Figure 1d).
FIGURE 1.
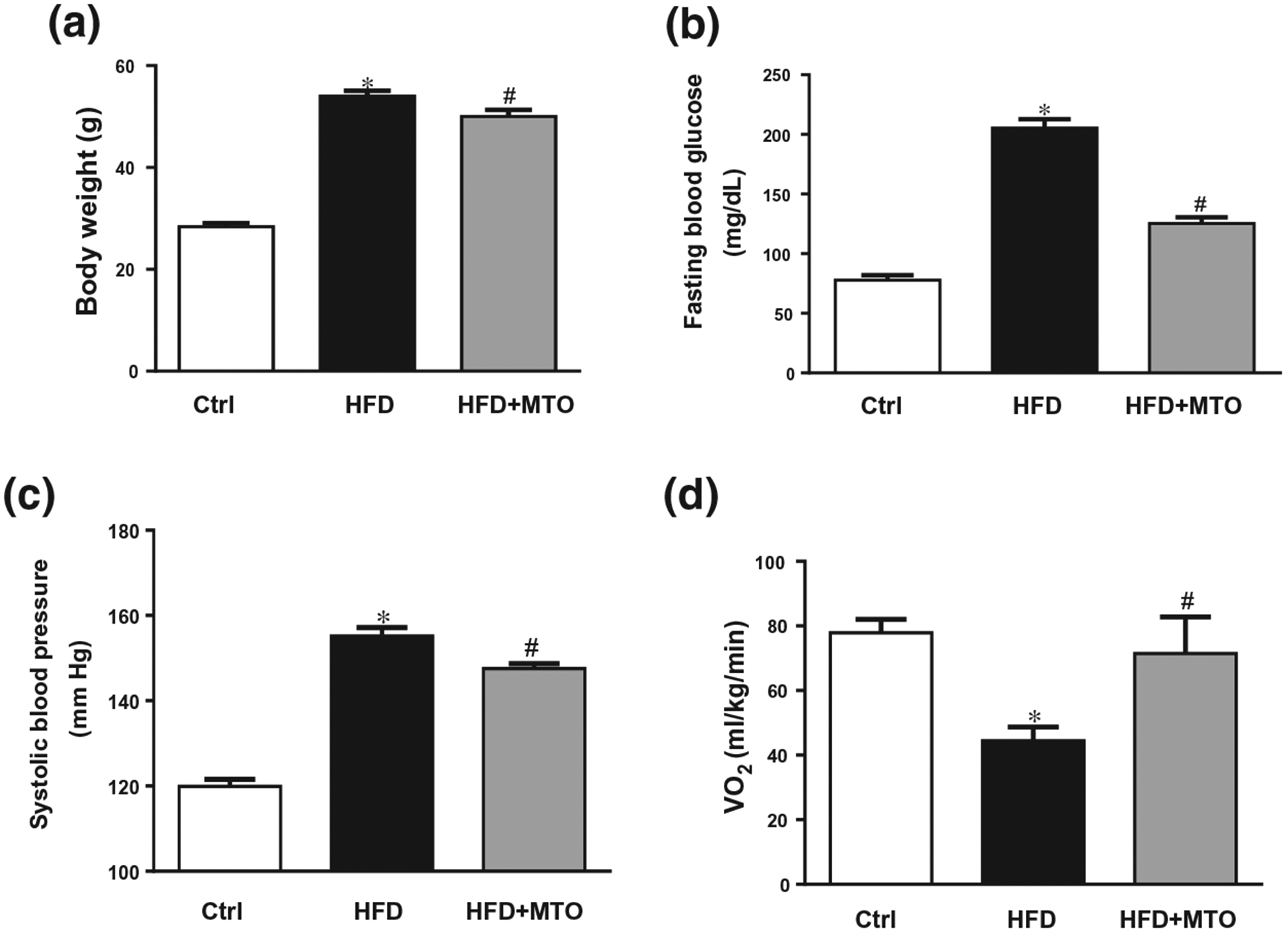
Effect of milk thistle seed oil (MTO) on (a) Body Weight, (b) Fasting blood glucose, (c) Systolic Blood Pressure, and (d) Oxygen consumption (VO2). *p < .05 as compared to the control (Ctrl). #p < .05 as compared to HFD. n = 6/group
3.2 |. MTO decreases HFD-induced inflammation in the liver
Obesity is associated with increased levels of white adipose inflammation. To determine the effect of MTO on adipose inflammation, we measured the levels of several markers of adipocyte inflammation. NOV/CCN3 is a protein whose circulating levels correlate with expression in adipose tissue and contributes to obesity-related inflammation (Martinerie et al., 2016). The IKKβ/NF-κB signaling pathway promotes the inflammatory response in obesity via p65 which is the subunit that contains the activation domain and determines the transcriptional activity of NF-κB. HFD increased the levels of several inflammatory markers in the liver, notably NOV/CCN3, interleukin-6 (IL-6), and phosphorylated p65 (pP65) as compared to mice fed a control diet (Figure 2a–e). MTO significantly (p < .05) reduced all of the markers of hepatic inflammation in mice fed a HFD (Figure 2a–e).
FIGURE 2.
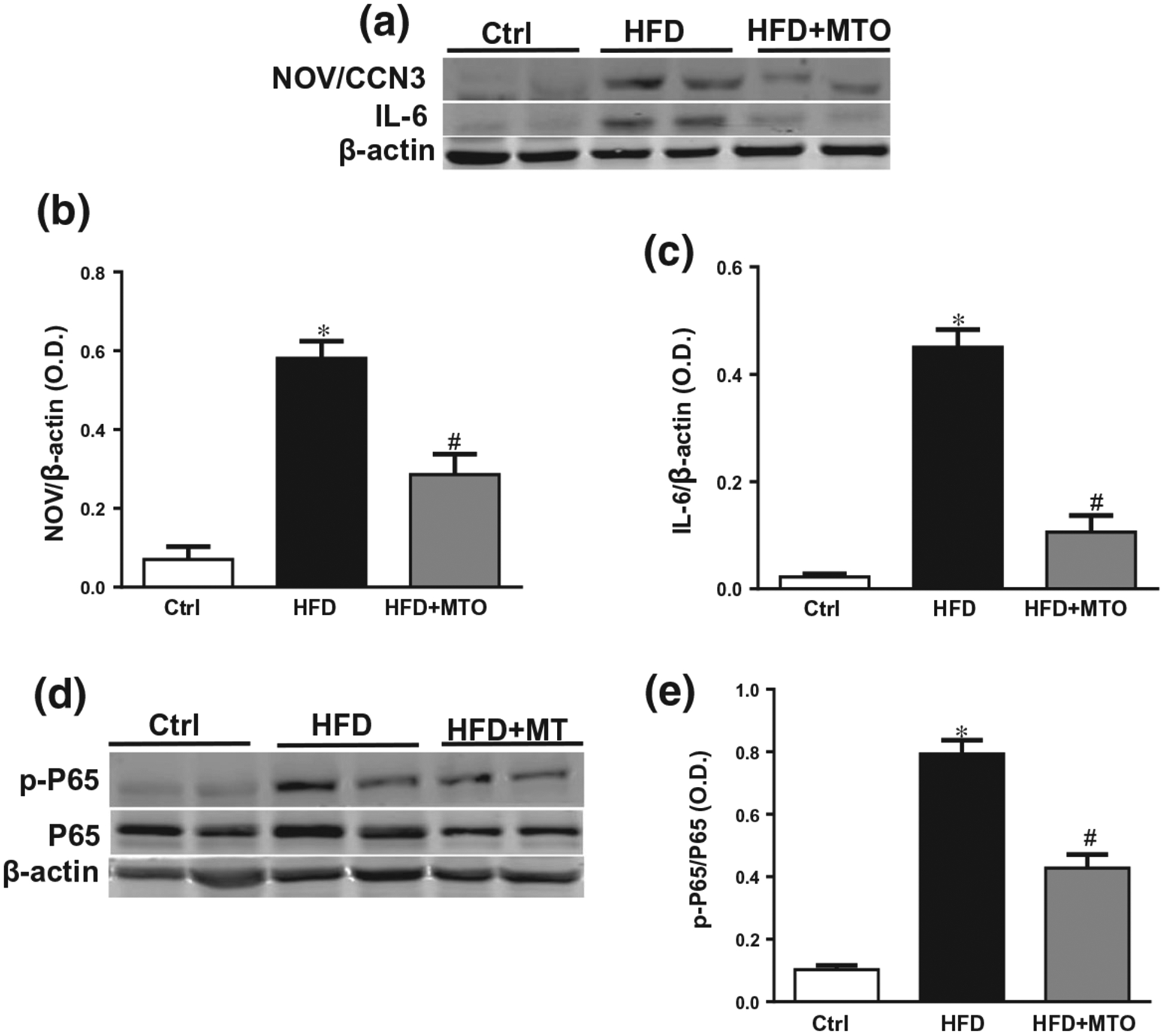
Hepatic levels of NOV/CCN3, interleukin-6 (IL-6), P65, and phosphorylated P65 normalized to β-actin. (a) Representative Western blots, (b) Hepatic levels of NOV/CCN3, (c) Hepatic Levels of IL-6, (d) Representative Western blots, (e) Levels of pP65/P65. *p < .05 as compared to the control (Ctrl). #p < .05 as compared to HFD. n = 6/group
3.3 |. MTO improves HFD-induced hepatic fibrosis in mice fed a HFD
Matrix metalloproteinase 2 and 9 (MMP2 and MMP9) are gelatinases implicated in extracellular matrix degradation and play a pivotal role in pathological progression of hepatic fibrosis. HFD mice showed significant increases in the expression of MMP2 and MMP9 in the liver as compared to the control mice (Figure 3a–c). MTO reduced the hepatic MMP2 and MMP9 expressions (p < .05) in HFD mice (Figure 3a–c). Membrane type 1-matrix metalloproteinase (MT1-MMP/MMP14) is expressed by infiltrated macrophages in hepatic inflammation and facilitates the proteolytic activation of MMP2 and MMP9. MTO diminished the hepatic MT1-MMP levels which were upregulated in HFD mice (Figure 3d,e). Tissue inhibitors of metalloproteinases (TIMPs) are endogenous MMPs inhibitors capable of regulating proteolytic activities of MMPs. Our results show that HFD mice exhibited significantly lower levels of TIMP1 and TIMP2 in the liver as compared to the control mice (Figure 3d,f,g). MTO increased (p < .05) the hepatic TIMP1 and TIMP2 levels in HFD mice (Figure 3d,f,g).
FIGURE 3.
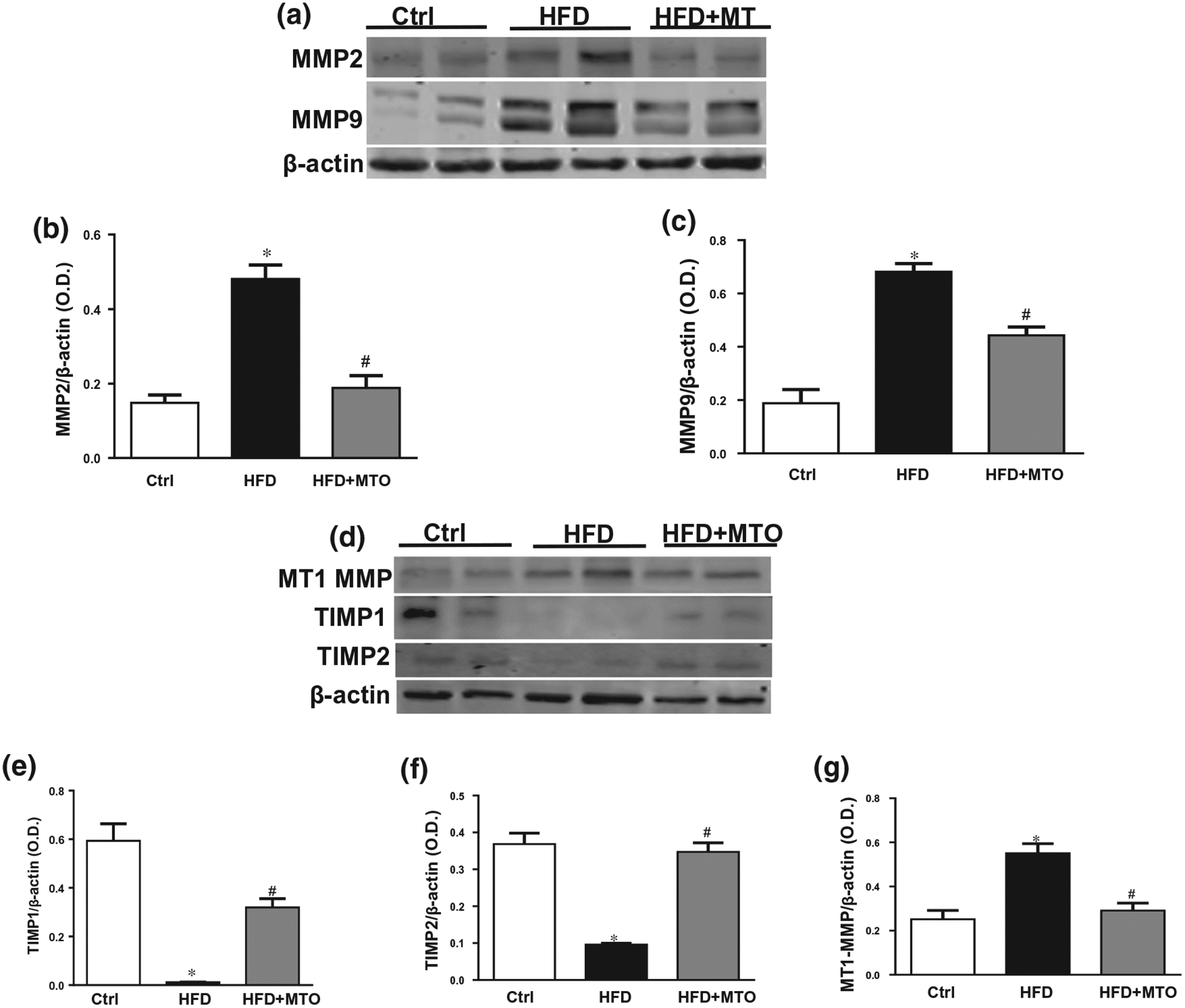
Hepatic levels of matrix metalloproteinase (MMP) 2, MMP9, membrane type 1-MMP, tissue inhibitor of MMP (TIMP)1 and TIMP2 normalized to β-actin. (a) Representative Western blots, (b) Hepatic levels of MMP2, (c) Hepatic levels of MMP9, (d) Representative Western blots, (e) Hepatic levels of MT1-MMP, (f) Hepatic levels of TIMP1, (g) Hepatic levels of TIMP2. *p < .05 as compared to the control (Ctrl) #p < .05 as compared to HFD. n = 6/group
3.4 |. MTO alters antioxidant and fatty acid metabolism and improves hepatic insulin signaling in the liver of mice fed a HFD
We examined the effect of MTO on hepatic antioxidant capacity, fatty acid metabolism, and fibrosis by Western blot. Superoxide dismutase-1 (SOD1) regulates the formation of mitochondrial reactive oxygen species. MTO increased the hepatic SOD1 levels which had decreased in HFD mice (Figure 4a,b). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a transcriptional coactivator that regulates the genes involved in energy metabolism. It is also the master regulator of mitochondrial bio-genesis. HFD decreased the levels of hepatic PGC-1α as compared to the control mice (Figure 4a,b). MTO resulted in a significant increase (p < .05) in hepatic PGC-1α levels as compared to HFD alone (Figure 4a,b).
FIGURE 4.
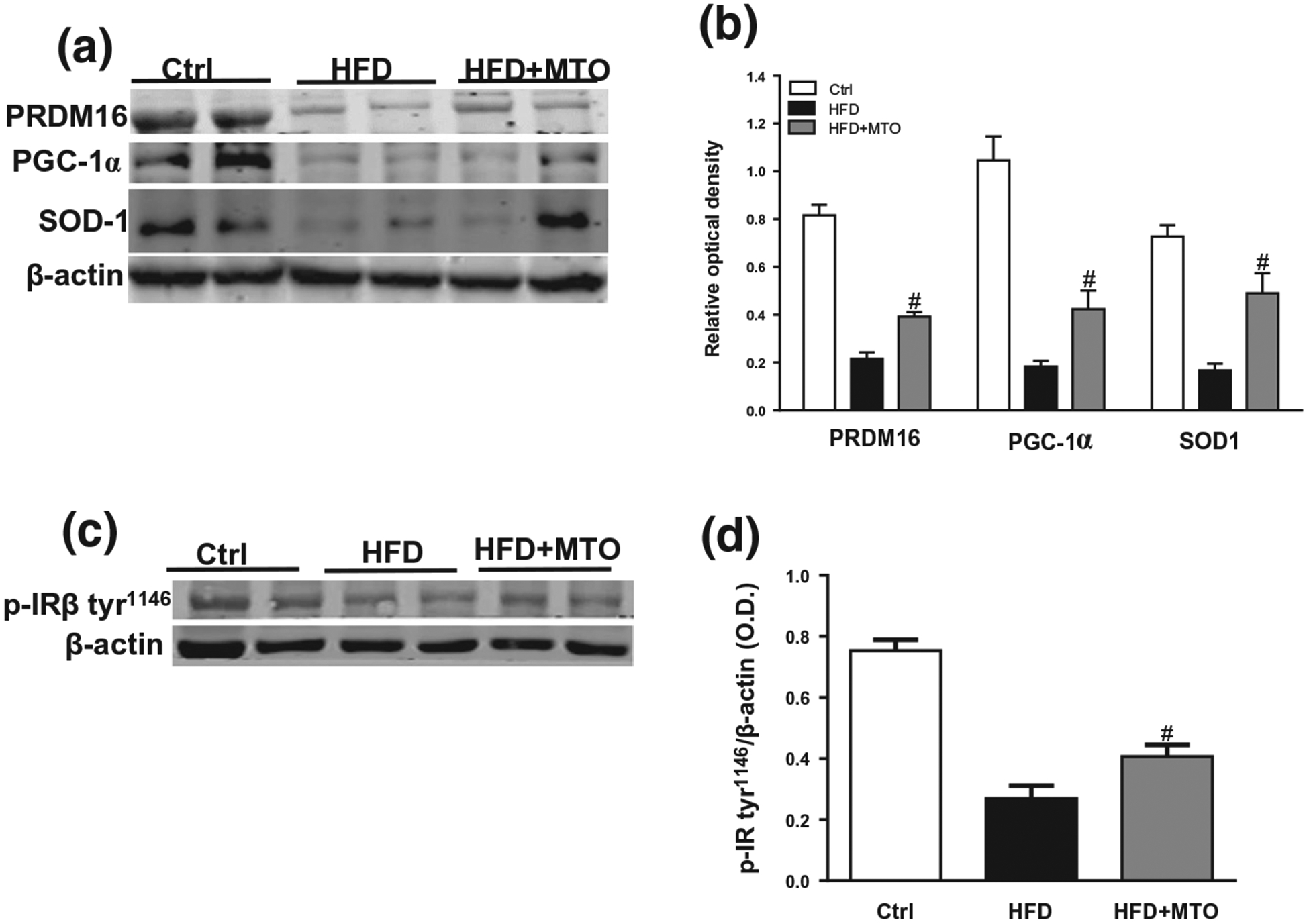
Hepatic levels of superoxide dismutase 1 (SOD1), peroxisome proliferator-activated receptor-gamma coactivator 1-α (PGC-1α), PR domain containing 16 (PRDM16), and phosphorylated insulin receptor β tyrosine 1,146 (p-IRβ Tyr1146) normalized to β-actin. (a) Representative Western blots, (b) Hepatic levels of SOD1, PGC-1α, and PRDM16. (c) Representative Western blots, (d) Hepatic levels of p-IRβ Tyr1146. *p < .05 as compared to the control (Ctrl). #= p < .05 as compared to HFD. n = 6/group
To determine the effects of MTO on obesity-induced hepatic fibrosis, we examined the levels of several proteins critical to its development. PRDM16 is a protein that represses fibrogenesis. HFD feeding induced a significant decrease in hepatic levels of PRMD16 as compared to the control mice (Figure 4a,b). MTO increased (p < .05) the levels of PRMD16 in the liver as compared to HFD feeding alone (Figure 4a,b).
Activation of the β subunit of the insulin receptor (IR) plays an initial role in signal transmission of insulin in the liver. HFD feeding resulted in a significant (p < .05) decrease in the levels of tyrosine 1,146 (tyr1146) phosphorylation of IRβ which was partially restored by MTO (Figure 4c,d).
3.5 |. MTO decreases HFD-induced inflammation in adipose tissue
As seen in the liver, HFD increased the NOV/CCN3 levels in the adipose tissue of mice and these levels were significantly (p < .05) reduced by MTO (Figure 5a,b). TWIST-1 is selectively expressed in adipose tissue, interacts with PGC-1α, and is recruited to the promoters of PGC-1α’s target genes to suppress mitochondrial metabolism and uncoupling. TWIST-1 levels were upregulated in adipose of mice fed a HFD and significantly (p < .05) decreased with MTO (Figure 5a,c). Mice fed a HFD exhibited increased levels of phosphorylated p65 (p-p65) in the adipose tissue and this decrease was significantly attenuated in adipose tissue of MTO-treated mice (Figure 5d,e).
FIGURE 5.
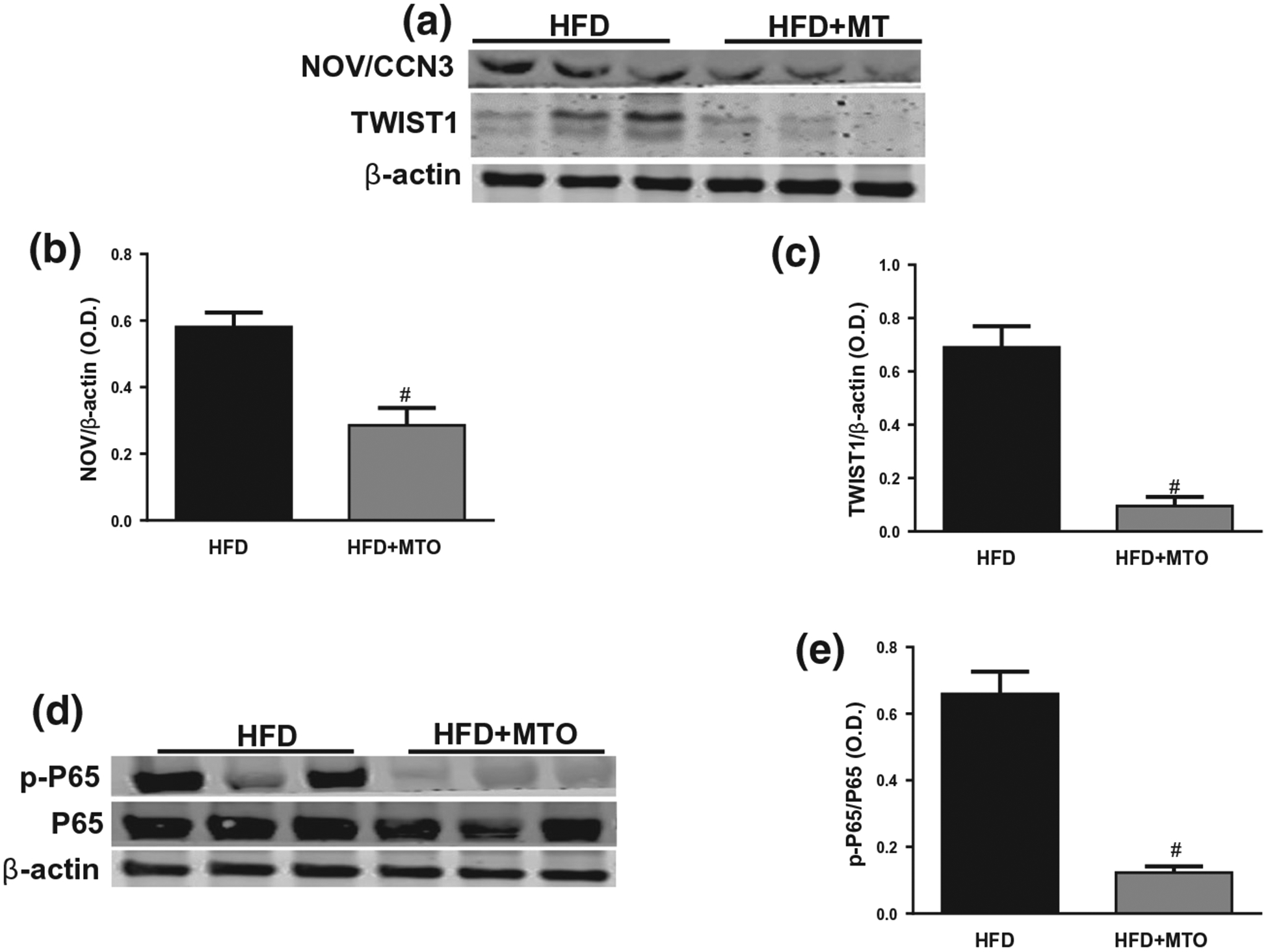
Adipose tissue levels of NOV/CCR3, TWIST1, P65, and phosphorylated P65 normalized to β-actin. (a) Representative Western blots, (b) Adipose levels of NOV/CCR3, (c) Adipose levels of TWIST1, (d) Representative Western blots, (e) Levels of pP65/P65. #p < .05 as compared to HFD. n = 6/group
3.6 |. MTO induces heme oxygenase-1 (HO-1), increases mitochondrial stability, and stimulates brown adipogenesis in white adipose of mice fed a HFD
Adipose tissue expansion in obesity results in the downregulation of HO-1 (Singh et al., 2020). MTO resulted in a significant (p < .05) increase in HO-1 protein levels in white adipose tissue (Figure 6a,b). MTO significantly (p < .05) increased the Mfn1 levels in adipose tissue of mice fed a HFD (Figure 6a,c) which may play a role in the attenuation of HFD-induced hyperglycemia in the MTO-treated mice. MTO increased the SIRT1 levels in adipose tissue (Figure 6a,d), further contributing to the improved insulin function.
FIGURE 6.
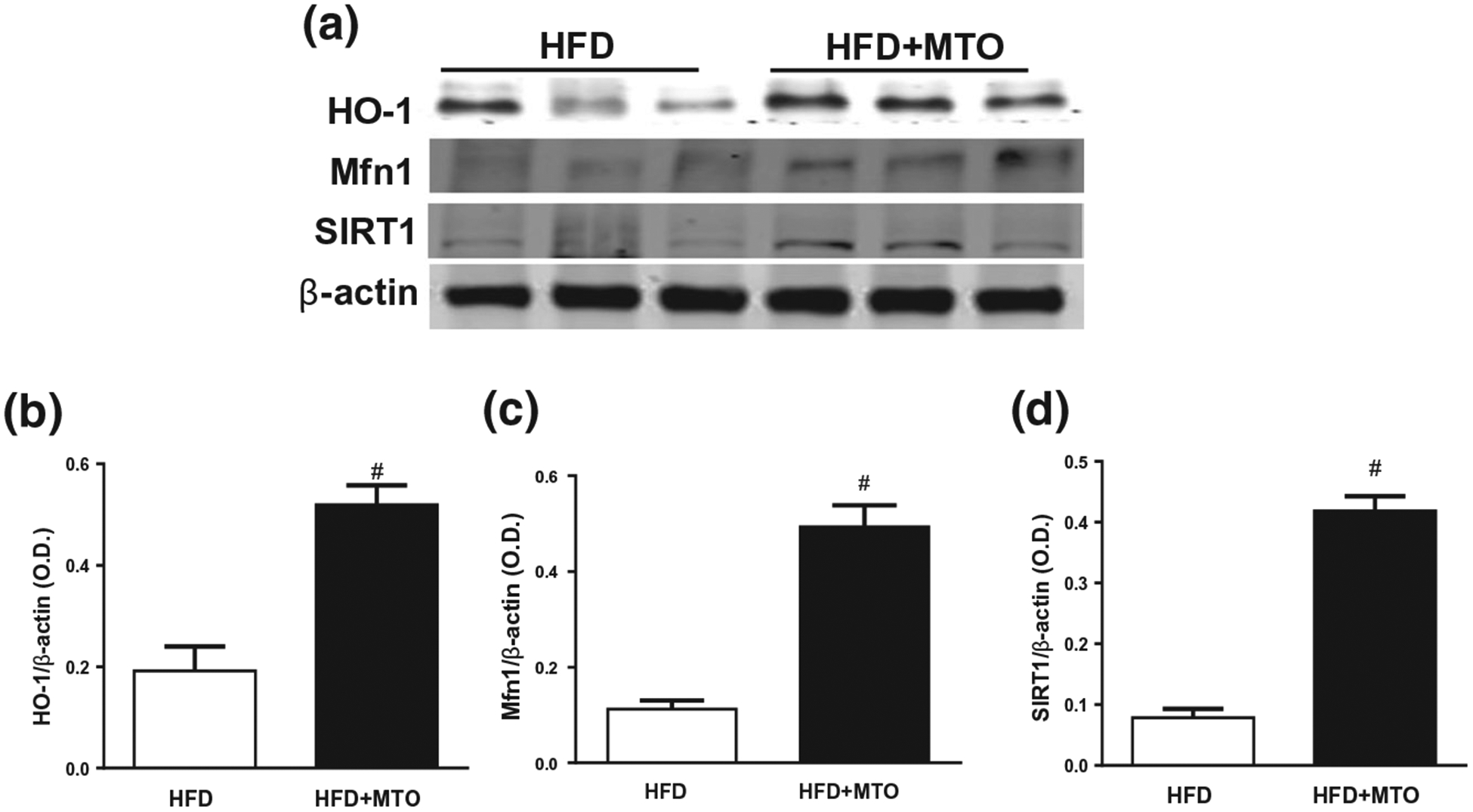
Adipose tissue levels of heme oxygenase-1 (HO-1), mitofusin-1 (Mfn1), and sirtuin1 (SIRT1) normalized to β-actin. (a) Representative Western blots, (b) Adipose levels of HO-1, (c) Adipose levels of Mfn1, (d) Adipose levels of SIRT1. #p < .05 as compared to HFD. n = 6/group
Mitochondria play an essential role in the physiology of adipose tissue. Mitochondrial fusion and fission regulate mitochondrial number and increase of mitochondrial fatty acid oxidation resulting in the formation of brown and beige adipocytes which boosts energy utilization (Pisani et al., 2018). Fibroblast growth factor-21 (FGF21) plays an essential role in glucose uptake in adipocytes (Cuevas-Ramos et al., 2019). MTO significantly (p < .05) increased the levels of FGF21 in adipocytes (Figure 7a,b). MTO also increased (p < .05) the levels of mitochondrial proteins CREG1 and UCP1, which are both involved in the “browning” of white adipose tissue (Figure 7a,c,d).
FIGURE 7.
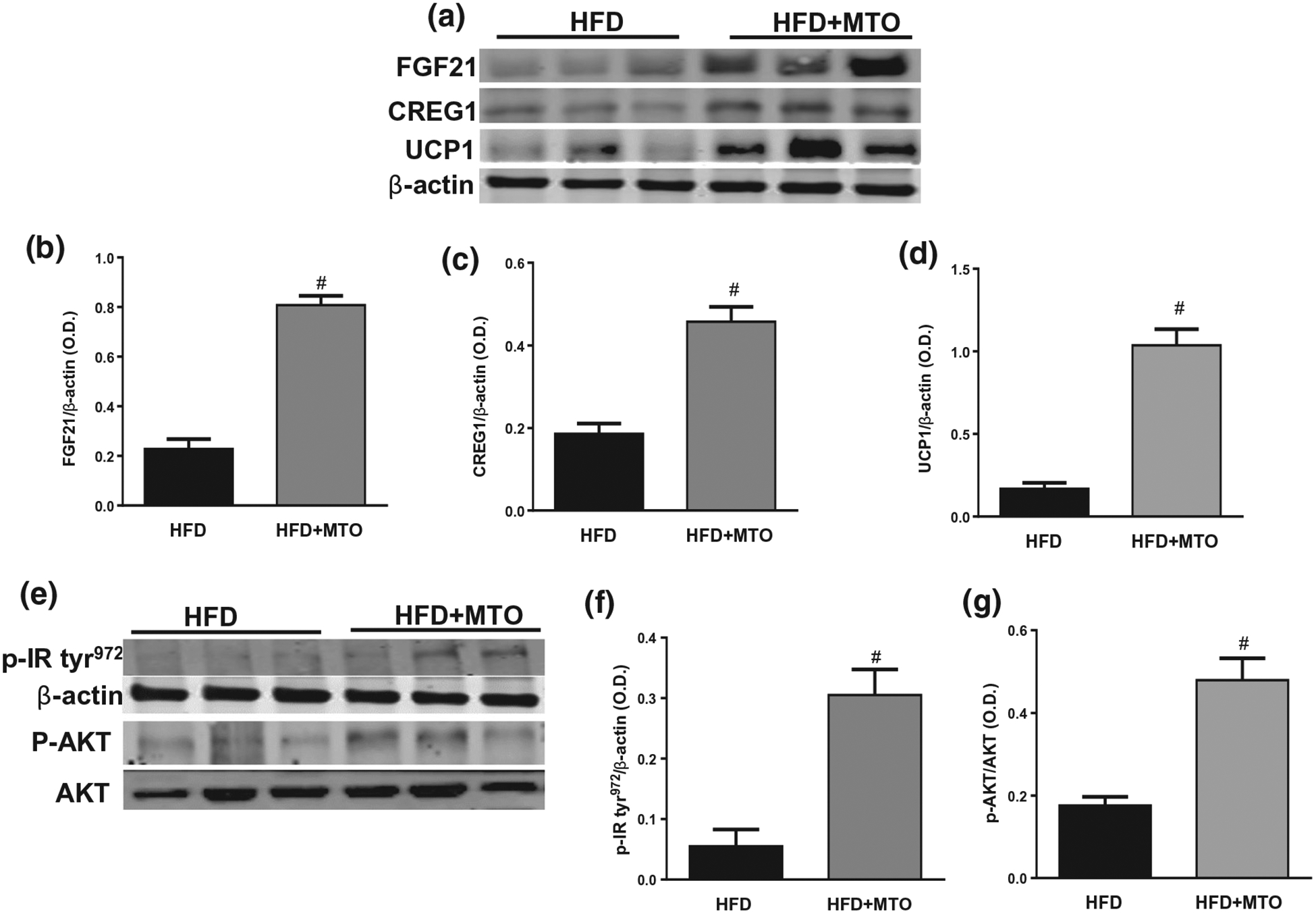
Adipose tissue levels of fibroblast growth factor-21 (FGF21), Cellular Repressor of E1A-stimulated Genes 1 (CREG1), uncoupling protein-1 (UCP1), and phosphorylated tyrosine 972 insulin receptor (p-IRTyr−972) normalized to β-actin and levels of phosphorylated and total AKT. (a) Representative Western blots, (b) Adipose levels of FGF21 (c) Adipose levels of CREG1, (d) Adipose levels of UCP1, (e) Representative Western blots, (f) Adipose levels of p-IRTyr−972, (g) Adipose levels of pAKT/AKT. #p < .05 as compared to HFD. n = 6/group
3.7 |. Improved insulin signaling in adipose tissue of MTO-treated mice on a HFD
MTO significantly (p < .05) increased the phosphorylation of the IR at tyrosine 972 (p-IRtyr972) in adipose tissue as compared to mice fed a HFD (Figure 7e,f). The PI3K/AKT pathway is critical for insulin signaling; hence, any defect in AKT/PKB pathway along with the downstream molecules could lead to insulin resistance. We measured the levels of phosphorylated AKT (pAKT), and total AKT in adipose tissue of control- and MTO-treated mice. MTO increased (p < .05) the levels of pAKT in adipose tissue as compared to the control mice (Figure 7e,g).
4 |. DISCUSSION
In this study, we examined the effectiveness of MTO on the metabolic, cardiovascular, and inflammatory responses to dietary-induced obesity. MTO attenuated HFD-induced weight gain and lowered blood pressure in response to dietary-induced obesity. The ability of MTO to reduce body weight and lower blood pressure concurrently is clinically significant given that drugs that target pathways such as adrenergic receptors or melanocortin-4 receptors lower the body weight but increase the blood pressure (da Silva et al., 2008; do Carmo et al., 2012; Michel et al., 1990). The adverse cardiovascular actions of these weight-loss drugs make them unattractive choices for use in humans. The fact that MTO lowers blood pressure despite increasing metabolism, as indicated by increased oxygen consumption, makes it an attractive therapeutic candidate to treat obese individuals for weight loss as hypertension is often a concurrent condition in these patients. While MTO had a significant effect on body weight, blood pressure, and fasting blood glucose, one limitation of the current study is the lack of sequential measurements of these parameters.
The ability of MTO to increase oxygen consumption, as demonstrated in the present study is related to its actions on mitochondria. MTO increased several markers of mitochondrial fusion, and “browning” of white adipose in mice fed a HFD. Browning of white adipose tissue increases mitochondrial burning of fatty acids and is an emerging focus in the treatment of obesity (Brestoff et al., 2015; Lizcano, 2019; Warner & Mittag, 2016). MTO may affect the mitochondrial function through several potential mechanisms. MTO induces HO-1 in adipose tissue, increasing the production of bilirubin and carbon monoxide (CO). Increased levels of HO-1 increases oxygen consumption and promotes weight loss in obese melanocortin-4 receptor-deficient mice (Csongradi et al., 2012). Moreover, the heme degradation product, CO, also increases oxygen consumption through its ability to “brown” white adipose tissue (Hosick et al., 2014, 2016). MTO also contains a mixture of polyphenols and flavonoids that also improve mitochondrial function and increase fatty acid metabolism (Dilberger et al., 2019; Duluc et al., 2013; Moini et al., 1999).
MTO improved dietary obesity-induced insulin resistance via increased insulin signaling in both adipose tissue and liver. One potential mechanism by which MTO enhanced insulin signaling is through increases in FGF21. Fibroblast growth factor 21 (FGF21) is a metabolic regulator that improves glycemic control in several models of insulin resistance (Berglund et al., 2009; Kim et al., 2013) FGF21 lowers the plasma triglycerides in part by increasing lipid metabolism in white adipose tissue (Schlein et al., 2016). The high levels of polyphenols found in MTO most likely results in increased FGF21 levels as previous studies have reported FGF21 induction in response to polyphenols (Song et al., 2016; Tamura et al., 2019). The levels of PGC-1α were also increased in adipose tissue of MTO-treated mice. FGF21 is also a strong regulator of PGC-1α which is an important mechanism in the browning of adipose tissue by FGF21 (Potthoff et al., 2009). Another potential mechanism for increased insulin sensitivity and increases in FGF 21 signaling is the reduction of oxidative stress by MTO. MTO resulted in significant induction of two important antioxidant genes, HO-1 and SOD1, in adipose tissue and liver, which contributes to the enhanced insulin signaling in each of these tissues.
MMP2 and MMP9 are gelatinases responsible for the degradation of extracellular matrix (ECM) proteins which play a key role in the pathogenesis of NAFLD, steatosis, fibrosis, and cirrhosis (Theret et al., 1999). The expression and proteolytic activity of MMP2 and MMP9 are closely regulated by the endogenous activator MT1-MMP and the inhibitors TIMP1 and TIMP2 (Barchuk et al., 2018). Our findings indicated that MTO attenuates HFD-induced hepatic fibrosis by reduction of MMP2, MMP9, and MT1-MMP expressions in the liver. Moreover, MTO-induced dramatic and concurrent upregulation of TIMP1 and TIMP2, which inhibited the proteolytic activities of MM2 and MMP9. The reduced activity of gelatinases by MTO resulted in decreased matrix turnover and ECM remodeling, contributing to the improvement of obesity-associated hepatic fibrosis. These results are in agreement with previous reports that the active ingredient of milk thistle, Silymarin, showed hepatoprotection against hioacet-amide-induced chronic liver inflammation and fibrosis by downregulation of MMPs (Chen et al., 2012).
The link between obesity, adipose tissue expansion, and inflammation has been intensively studied over the past several years. A wealth of experimental evidence has highlighted the role of adipose tissue in the development of systemic inflammation through the release of various adipokines such as IL-6, which promote inflammation (Berg & Scherer, 2005; Hotamisligil, 2006). MTO decreased several critical markers of inflammation in both adipose tissue and in the liver. Levels of NOV/CCN3, as well as the levels of P65 which is a major regulator of IKKβ/NF-κB signaling, were decreased in adipose tissue and liver of dietary obese mice treated with MTO. This profound anti-inflammatory response in dietary-induced obese mice likely contributed to the vast improvement in insulin signaling and decreased blood pressure observed in the present study. The anti-inflammatory actions of MTO is most likely due to the high levels of polyphenols and flavonoids that attenuate inflammatory markers (Carpi et al., 2019; Collins et al., 2016).
MTO is a mixture of flavonolignans, and the active constituents contain silibinin (50%), isosilybin (5%), silychristin (20%), and silydianin (10%) (Tajmohammadi et al., 2018). Silibinin (or silybin) is the major active component of milk thistle and studies have been shown that silibinin exerts potential hepatoprotective capacities due to the antioxidants and anti-inflammatory effects (Feher & Lengyel, 2012). Moreover, silibinin improved dyslipidemia and hyperglycemia in diet-induced obesity through suppression of NF-κB pathway associated with the activation of Farnesyl X receptor (Gu et al., 2016). In this study, MTO prevented the development of dietary obesity-induced weight gain, hyperglycemia, and hypertension. These positive metabolic and cardiovascular effects were likely due to its actions on: mitochondria and metabolism, as well as the improvements in insulin signaling in both adipose tissue and liver, and decreases fibrosis markers, MMP2 and MMP9 (Figure 8). MTO also had profound anti-inflammatory actions in adipose tissue and liver, through the downregulation of several important inflammatory mediators such as, NOV, TWIST, and NFkB. Overall, our data demonstrate that MTO has a beneficial cardiovascular and metabolic effects in a mouse model of the metabolic syndrome.
FIGURE 8.
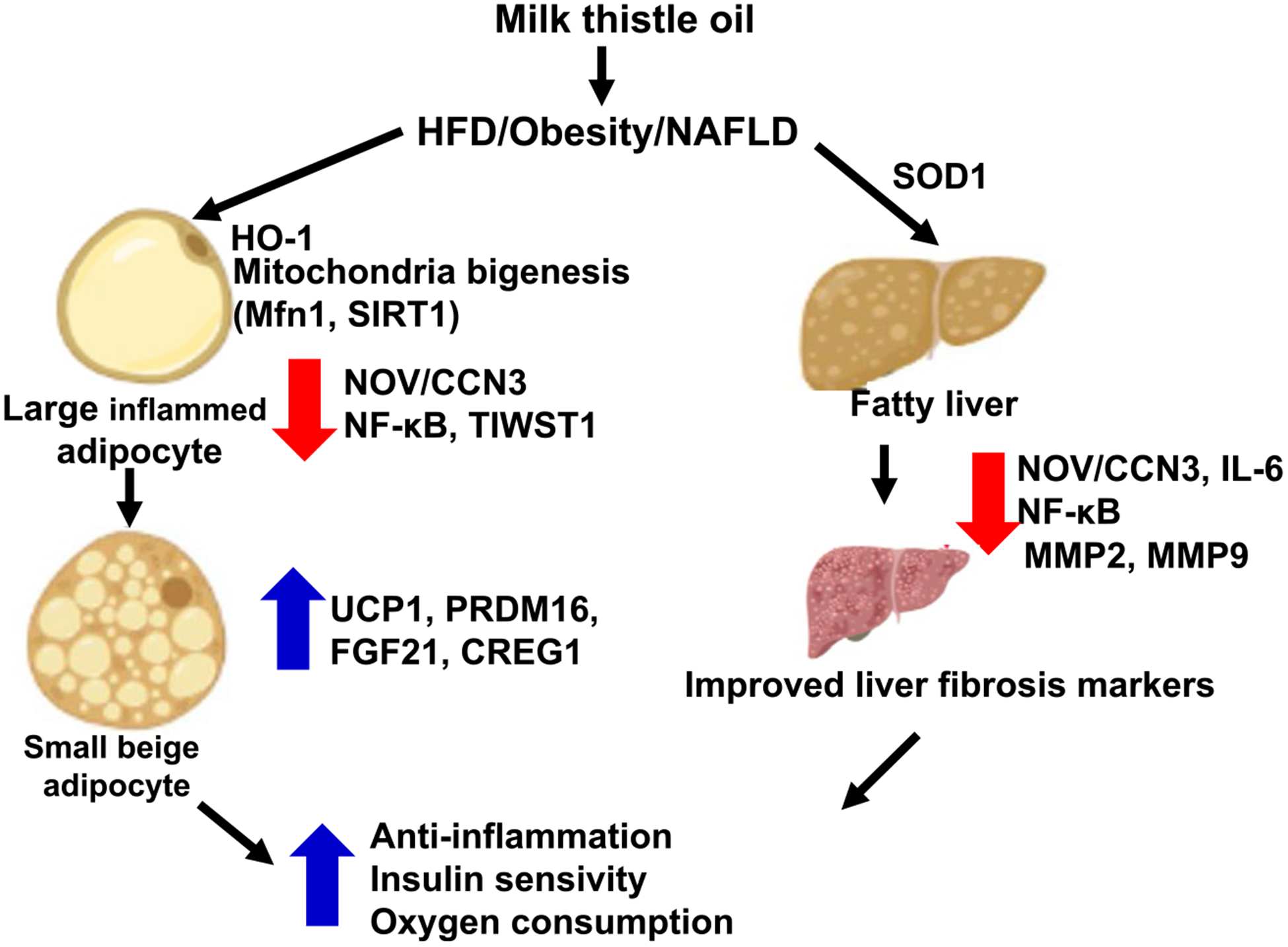
Schematic presentation of postulated mechanisms by which cold pressed milk thistle oil intake to obese mice lead to marked suppression of inflammatory markers including NOV and TWIST and diminished liver fibrosis markers including MMP2 and MMP9. Additionally, cold press MTO reverses these changes, obesity suppress effect, and thermogenic genes such as (UCP1, PRMD16), mitochondrial respiration as displayed by the increase in oxygen consumption and improving insulin receptor phosphorylation. Hence, intake cold press MTO offers a multifunctional clinical approach for the treatment of Metabolic Syndrome
Practical applications.
Natural supplements are increasingly being considered as potential therapies for many chronic cardiovascular and metabolic diseases. Milk thistle cold press oil (MTO) is derived from Silybum marianum which is used as a dietary supplement in different parts of the world. The results of the present study demonstrate that MTO supplementation normalizes several metabolic and cardiovascular complications arising from dietary-induced obesity. MTO supplementation also had anti-inflammatory actions in the adipose as well as the liver. These results suggest that supplementation of MTO into the diet of obese individuals may afford protection against the worsening of cardiovascular and metabolic disease and improve inflammation and liver fibrosis.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health R 56-139561 (NGA), 1R01 DK121748-01A1 (D.E.S.), P01 HL05197-11 (D.E.S.), and the National Institute of General Medical Sciences, P20GM104357-02 (D.E.S.).
Funding information
National Institutes of Health, Grant/Award Number: 56-139561, DK121748-01A1 and HL05197-11; National Institute of General Medical Sciences, Grant/Award Number: P20GM104357-02
Footnotes
CONFLIC T OF INTEREST
The authors declared that they have no conflict of interest.
REFERENCES
- Abraham NG, & Kappas A (2008). Pharmacological and clinical aspects of heme oxygenase. Pharmacological Reviews, 60, 79–127. 10.1124/pr.107.07104 [DOI] [PubMed] [Google Scholar]
- Abraham NG, Kushida T, McClung J, Weiss M, Quan S, Lafaro R, Darzynkiewicz Z, & Wolin M (2003). Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circulation Research, 93(6), 507–514. 10.1161/01.RES.0000091828.36599.34 [DOI] [PubMed] [Google Scholar]
- Barchuk M, Schreier L, Berg G, & Miksztowicz V (2018). Metalloproteinases in nonalcoholic fatty liver disease and their behavior in liver fibrosis. Hormone Molecular Biology and Clinical Investigation, 41(1), 1868–1891. 10.1515/hmbci2018-0037 [DOI] [PubMed] [Google Scholar]
- Berg AH, & Scherer PE (2005). Adipose tissue, inflammation, and cardiovascular disease. Circulation Research, 96(9), 939–949. 96/9/939 [pii]; 10.1161/01.RES.0000163635.62927.34 [DOI] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, & Wasserman DH (2009). Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology, 150(9), 4084–4093. 10.1210/en.2009-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, & Artis D (2015). Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature, 519(7542), 242–246. 10.1038/nature14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Puri N, Sodhi K, Bellner L, Abraham NG, & Kappas A (2012). Apo A1 mimetic rescues the diabetic phenotype of HO-2 knockout mice via an increase in HO-1 adiponectin and LKBI signaling pathway. International Journal of Hypertension, 2012, 628147 10.1155/2012/628147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpi S, Scoditti E, Massaro M, Polini B, Manera C, Digiacomo M, & Nieri P (2019). The extra-virgin olive oil polyphenols oleocanthal and oleacein counteract inflammation-related gene and miRNA expression in adipocytes by attenuating NF-kappaB activation. Nutrients, 11(12), 2855 10.3390/nu11122855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Barbosa-Tessmann I, Chen C, Kilberg MS, & Agarwal A (2002). Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response. Journal of Biological Chemistry, 277(3), 1933–1940. 10.1074/jbc.M108921200 [DOI] [PubMed] [Google Scholar]
- Chang SH, Garcia J, Melendez JA, Kilberg MS, & Agarwal A (2003). Haem oxygenase 1 gene induction by glucose deprivation is mediated by reactive oxygen species via the mitochondrial electron-transport chain. The Biochemical Journal, 371(Pt 3), 877–885. 10.1042/bj20021731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IS, Chen YC, Chou CH, Chuang RF, Sheen LY, & Chiu CH (2012). Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. Journal of the Science of Food and Agriculture, 92(7), 1441–1447. 10.1002/jsfa.4723 [DOI] [PubMed] [Google Scholar]
- Clichici S, Olteanu D, Nagy AL, Oros A, Filip A, & Mircea PA (2015). Silymarin inhibits the progression of fibrosis in the early stages of liver injury in CCl(4)-treated rats. Journal of Medicinal Food, 18(3), 290–298. 10.1089/jmf.2013.0179 [DOI] [PubMed] [Google Scholar]
- Collins B, Hoffman J, Martinez K, Grace M, Lila MA, Cockrell C, Nadimpalli A, Chang E, Chuang C-C, Zhong W, Mackert J, Shen W, Cooney P, Hopkins R, & McIntosh M (2016). A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. Journal of Nutritional Biochemistry, 31, 150–165. 10.1016/j.jnutbio.2015.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csongradi E, Docarmo JM, Dubinion JH, Vera T, & Stec DE (2012). Chronic HO-1 induction with cobalt protoporphyrin (CoPP) treatment increases oxygen consumption, activity, heat production and lowers body weight in obese melanocortin-4 receptor-deficient mice. International Journal of Obesity, 36(2), 244–253. ijo201178 [pii]; 10.1038/ijo.2011.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ramos D, Mehta R, & Aguilar-Salinas CA (2019). Fibroblast growth factor 21 and browning of white adipose tissue. Frontiers in Physiology, 10, 37 10.3389/fphys.2019.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, & Hall JE (2008). Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension, 51(4), 884–890. 10.1161/HYPERTENSIONAHA.107.100636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilberger B, Passon M, Asseburg H, Silaidos CV, Schmitt F, Schmiedl T, Schieber A, & Eckert GP (2019). Polyphenols and metabolites enhance survival in rodents and nematodes-impact of mitochondria. Nutrients, 11(8), 1886 10.3390/nu11081886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, da Silva AA, Rushing JS, & Hall JE (2012). Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 302(5), R561–R567. 10.1152/ajpregu.00392.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GS, Baum J, Greenberg M, Lewis D, & Abraham NG (2019). HO-1 overexpression and underexpression: Clinical implications. clinical implications. Archives of Biochemistry and Biophysics, 2019 September 30;673, 108073 10.1016/j.abb.2019.108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc L, Jacques C, Soleti R, Iacobazzi F, Simard G, & Andriantsitohaina R (2013). Modulation of mitochondrial capacity and angiogenesis by red wine polyphenols via estrogen receptor, NADPH oxidase and nitric oxide synthase pathways. International Journal of Biochemistry & Cell Biology, 45(4), 783–791. 10.1016/j.biocel.2013.01.007 [DOI] [PubMed] [Google Scholar]
- Feher J, & Lengyel G (2012). Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Current Pharmaceutical Biotechnology, 13(1), 210–217. 10.2174/138920112798868818 [DOI] [PubMed] [Google Scholar]
- Gu M, Zhao P, Huang J, Zhao Y, Wang Y, Li Y, Li Y, Fan S, Ma Y-M, Tong Q, Yang LI, Ji G, & Huang C (2016). Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Frontiers in Pharmacology, 7, 345 10.3389/fphar.2016.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosick PA, AlAmodi AA, Hankins M, & Stec D (2016). Chronic treatment with a carbon monoxide releasing molecular revereses dietary induced obesity in mice. Adipocyte, 5(2), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosick PA, AlAmodi AA, Storm MV, Gousset MU, Pruett BE, Gray W, Stout J, & Stec DE (2014). Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet. International Journal of Obesity, 38(1), 132–139. ijo201361 [pii]; 10.1038/ijo.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature, 444(7121), 860–867. nature05485 [pii]; 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, Lee MH, Song HK, Nam DH, Han JY, Han SY, Han KH, Kang YS, & Cha DR (2013). Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology, 154(9), 3366–3376. 10.1210/en.2012-2276 [DOI] [PubMed] [Google Scholar]
- Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, & Nelson JE (2012). Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology, 55(1), 77–85. 10.1002/hep.24706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, & Auwerx J (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell, 127(6), 1109–1122. S0092–8674(06)01428–0 [pii]; 10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Lizcano F (2019). The beige adipocyte as a therapy for metabolic diseases. International Journal of Molecular Sciences, 20(20), 5058 10.3390/ijms20205058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinerie C, Garcia M, Do TTH, Antoine B, Moldes M, Dorothee G, Kazazian C, Auclair M, Buyse M, Ledent T, Marchal P-O, Fesatidou M, Beisseiche A, Koseki H, Hiraoka S, Chadjichristos CE, Blondeau B, Denis RG, Luquet S, & Fève B (2016). NOV/CCN3: A new adipocytokine involved in obesity-associated insulin resistance. Diabetes, 65(9), 2502–2515. 10.2337/db15-0617 [DOI] [PubMed] [Google Scholar]
- Meddeb W, Rezig L, Abderrabba M, Lizard G, & Mejri M (2017). Tunisian milk thistle: An investigation of the chemical composition and the characterization of its cold-pressed seed oils. International Journal of Molecular Sciences, 18(12), 2582 10.3390/ijms18122582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Brodde OE, & Insel PA (1990). Peripheral adrenergic receptors in hypertension. Hypertension, 16(2), 107–120. 10.1161/01.hyp.16.2.107 [DOI] [PubMed] [Google Scholar]
- Moini H, Arroyo A, Vaya J, & Packer L (1999). Bioflavonoid effects on the mitochondrial respiratory electron transport chain and cytochrome c redox state. Redox Report, 4(1–2), 35–41. 10.1179/135100099101534729 [DOI] [PubMed] [Google Scholar]
- Nelson JE, Bhattacharya R, Lindor KD, Chalasani N, Raaka S, Heathcote EJ, Miskovsky E, Shaffer E, Rulyak SJ, & Kowdley KV (2007). HFE C282Y mutations are associated with advanced hepatic fibrosis in Caucasians with nonalcoholic steatohepatitis. Hepatology, 46(3), 723–729. 10.1002/hep.21742 [DOI] [PubMed] [Google Scholar]
- Ni X, & Wang H (2016). Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD). American Journal of Translational Research, 8(2), 1073–1081. [PMC free article] [PubMed] [Google Scholar]
- Pisani DF, Barquissau V, Chambard J-C, Beuzelin D, Ghandour RA, Giroud M, Mairal A, Pagnotta S, Cinti S, Langin D, & Amri E-Z (2018). Mitochondrial fission is associated with UCP1 activity in human brite/beige adipocytes. Molecular Metabolism, 7, 35–44. 10.1016/j.molmet.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittala V, Vanella L, Salerno L, Romeo G, Marrazzo A, Di Giacomo C, & Sorrenti V (2018). Effects of polyphenolic derivatives on heme oxygenase-system in metabolic dysfunctions. Current Medicinal Chemistry, 25(13), 1577–1595. 10.3390/ijms18112268. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, & Burgess SC (2009). FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences USA, 106(26), 10853–10858. 0904187106 [pii]; 10.1073/pnas.0904187106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele M, Bellner L, Singh SP, Favero G, Rezzani R, Rodella LF, & Vanella L (2019). Epoxyeicosatrienoic intervention improves NAFLD in leptin receptor deficient mice by an increase in PGC1alpha-HO-1-PGC1alpha-mitochondrial signaling. Experimental Cell Research, 380(2), 180–187. 10.1016/j.yexcr.2019.04.029 [DOI] [PubMed] [Google Scholar]
- Raffaele M, Li Volti G, Barbagallo IA, & Vanella L (2016). Therapeutic efficacy of stem cells transplantation in diabetes: Role of heme oxygenase. Frontiers in Cell and Developmental Biology, 4, 80 10.3389/fcell.2016.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodella LF, Vanella L, Peterson SJ, Drummond G, Rezzani R, Falck JR, & Abraham NG (2008). Heme oxygenase-derived carbon monoxide restores vascular function in type 1 diabetes. Drug Metabolism Letters, 2(4), 290–300. [DOI] [PubMed] [Google Scholar]
- Sacerdoti D, Singh SP, Schragenheim J, Bellner L, Vanella L, Raffaele M, Meissner A, Grant I, Favero G, Rezzani R, Rodella LF, Bamshad D, Lebovics E, & Abraham NG (2018). Development of NASH in obese mice is confounded by adipose tissue increase in inflammatory NOV and oxidative stress. International Journal of Hepatology, 2018, 3484107 10.1155/2018/3484107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, & Fabio G (2011). Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Molecular Neurobiology, 44(2), 192–201. 10.1007/s12035-011-8181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, Brenner MB, Heeren J, & Scheja L (2016). FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metabolism, 23(3), 441–453. 10.1016/j.cmet.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Schragenheim J, Bellner L, Cao J, Singh SP, Bamshad D, McClung JA, Maayan O, Meissner A, Grant I, Stier CT, & Abraham NG (2018). EET enhances renal function in obese mice resulting in restoration of HO-1-Mfn1/2 signaling, and decrease in hypertension through inhibition of sodium chloride co-transporter. Prostaglandins & Other Lipid Mediators, 137, 30–39. S1098–8823(17)30163–6 [pii]; 10.1016/j.prostaglandins.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Greenberg M, Glick Y, Bellner L, Favero G, Rezzani R, Rodella LF, Agostinucci K, Shapiro JI, & Abraham NG (2020). Adipocyte specific HO-1 gene therapy is effective in antioxidant treatment of insulin resistance and vascular function in an obese mice model. Antioxidants (Basel), 9(1), 40 10.3390/antiox9010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, McClung JA, Thompson E, Glick Y, Greenberg M, Acosta-Baez G, Edris B, Shapiro JI, & Abraham NG (2019). Cardioprotective heme oxygenase-1-PGC-1α signaling in epicardial fat attenuates cardiovascular risk in humans as in obese mice. Obesity (Silver Spring), 27(10), 1634–1643. 10.1002/oby.22608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Lee JH, Chung HT, & Pae HO (2013). Therapeutic roles of heme oxygenase-1 in metabolic diseases: Curcumin and resveratrol analogues as possible inducers of heme oxygenase-1. Oxidative Medicine and Cellular Longevity, 2013, 639541 10.1155/2013/639541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Zheng Z, Wu J, Lai J, Chu Q, & Zheng X (2016). White pitaya (Hylocereus undatus) juice attenuates insulin resistance and hepatic steatosis in diet-induced obese mice. PLoS ONE, 11(2), e0149670 10.1371/journal.pone.0149670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajmohammadi A, Razavi BM, & Hosseinzadeh H (2018). Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phytotherapy Research, 32(10), 1933–1949. 10.1002/ptr.6153 [DOI] [PubMed] [Google Scholar]
- Tamura Y, Tomiya S, Takegaki J, Kouzaki K, Tsutaki A, & Nakazato K (2019). Apple polyphenols induce browning of white adipose tissue. Journal of Nutritional Biochemistry, 77, 108299 10.1016/j.jnutbio.2019.108299 [DOI] [PubMed] [Google Scholar]
- Theret N, Lehti K, Musso O, & Clement B (1999). MMP2 activation by collagen I and concanavalin A in cultured human hepatic stellate cells. Hepatology, 30(2), 462–468. 10.1002/hep.510300236 [DOI] [PubMed] [Google Scholar]
- Vanella L, Barbagallo I, Acquaviva R, Di GC, Cardile V, Abraham NG, & Sorrenti V (2013). Ellagic acid: Cytodifferentiating and antiproliferative effects in human prostatic cancer cell lines. Current Pharmaceutical Design, 19(15), 2728–2736. [DOI] [PubMed] [Google Scholar]
- Vanella L, Di Giacomo C, Acquaviva R, Barbagallo I, Li Volti G, Cardile V, Abraham N, & Sorrenti V (2013). Effects of ellagic Acid on angiogenic factors in prostate cancer cells. Cancers. (basel), 5(2), 726–738. 10.3390/cancers5020726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner DF, Majumdar SK, Kumashiro N, Petersen MC, Rahimi Y, Gattu AK, Bears M, Camporez J-P, Cline GW, Jurczak MJ, Samuel VT, & Shulman GI (2015). Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proceedings of the National Academy of Sciences USA, 112(4), 1143–1148. 10.1073/pnas.1423952112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A, & Mittag J (2016). Breaking BAT: Can browning create a better white? Journal of Endocrinology, 228(1), R19–R29. 10.1530/joe-15-0408 [DOI] [PubMed] [Google Scholar]
- Zhu SY, Jiang N, Yang J, Tu J, Zhou Y, Xiao X, & Dong Y (2018). Silybum marianum oil attenuates hepatic steatosis and oxidative stress in high fat diet-fed mice. Biomedicine & Pharmacotherapy, 100, 191–197. 10.1016/j.biopha.2018.01.144 [DOI] [PubMed] [Google Scholar]


