Abstract
Ischemic stroke is one of the main causes of mortality and disability worldwide. However, efficient therapeutic strategies are still lacking. Stem/progenitor cell-based therapy, with its vigorous advantages, has emerged as a promising tool for the treatment of ischemic stroke. The mechanisms involve new neural cells and neuronal circuitry formation, antioxidation, inflammation alleviation, angiogenesis, and neurogenesis promotion. In the past decades, in-depth studies have suggested that cell therapy could promote vascular stabilization and decrease blood-brain barrier (BBB) leakage after ischemic stroke. However, the effects and underlying mechanisms on BBB integrity induced by the engrafted cells in ischemic stroke have not been reviewed yet. Herein, we will update the progress in research on the effects of cell therapy on BBB integrity after ischemic stroke and review the underlying mechanisms. First, we will present an overview of BBB dysfunction under the ischemic condition and cells engraftment for ischemic treatment. Then, we will summarize and discuss the current knowledge about the effects and underlying mechanisms of cell therapy on BBB integrity after ischemic stroke. In particular, we will review the most recent studies in regard to the relationship between cell therapy and BBB in tissue plasminogen activator (t-PA)-mediated therapy and diabetic stroke.
Keywords: Ischemic stroke, stem cells, blood-brain barrier, tissue plasminogen activator, neurogenesis
1. INTRODUCTION
Ischemic stroke is regarded as the most important cause of death and long-term disability in the modern world. Recombinant tissue plasminogen activator (rt-PA) is the only one treatment approved by the US Food and Drug Administration (FDA). Rt-PA mediated thrombolysis is clinically effective after acute ischemic stroke. However, the narrow therapeutic window and the risk of hemorrhagic transformation (HT) limit its clinical application. In the past decades, stem/progenitor cell-based therapy has emerged as a potential treatment for ischemic stroke. In-depth studies have revealed that cell transplantation can improve stroke outcomes through new neural cells and neuronal circuitry formation, inflammation alleviation, angiogenesis and neurogenesis promotion [1, 2]. Furthermore, there are accumulating evidence suggesting that cell therapy can promote vascular stabilization and decrease blood-brain barrier (BBB) disruption--two factors, which are critical to the success of therapy after ischemic stroke [3-5]. However, the effects and underlying mechanisms on BBB by the engrafted cells in ischemic stroke have not been reviewed yet.
Here, we will provide an overview of current studies on the application of stem/progenitor cells transplantation after ischemic stroke. We will focus on the effects on BBB integrity induced by different sources of cells and discuss the molecular mechanisms underlying these effects. We hope that our review will advance our knowledge of the clinical application of cell therapy after ischemic stroke.
2. BLOOD-BRAIN BARRIER DISFUNCTION IN ISCHEMIC STROKE
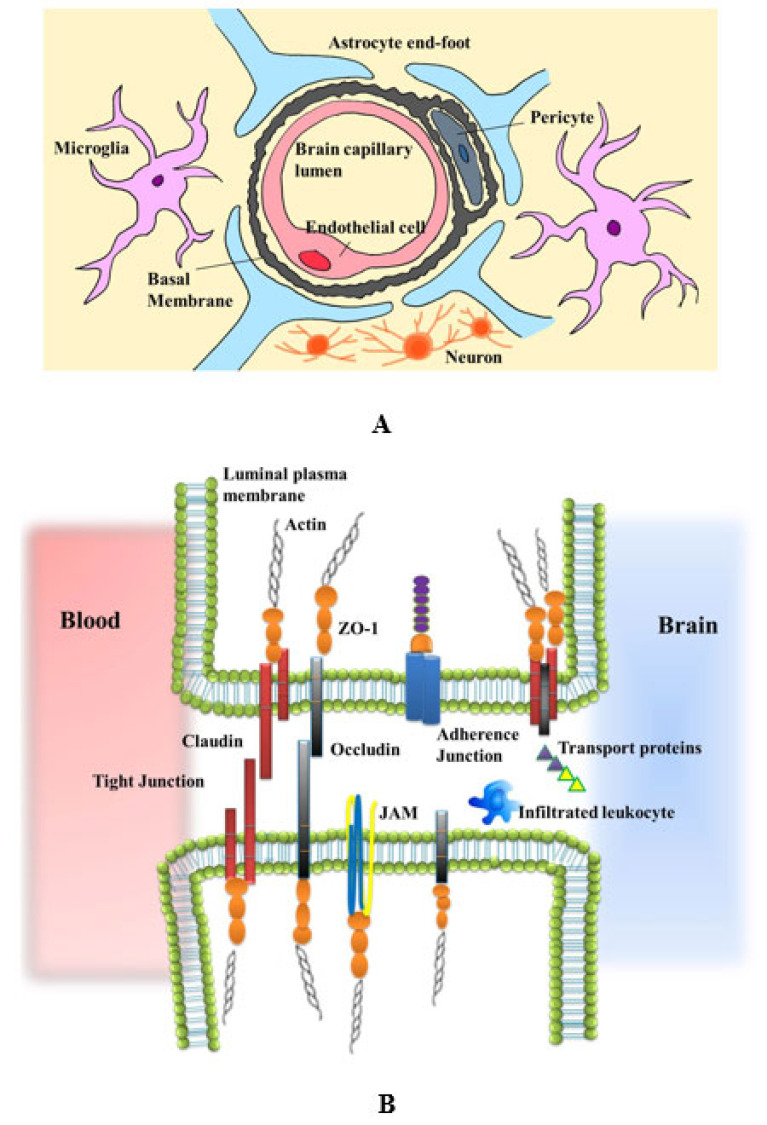
The BBB is a physical and biochemical barrier that separates the central nervous system (CNS) from the systemic circulation. As the gatekeeper of CNS, it mediates the exchange of substances between the brain parenchyma and blood, restricts the influx of potentially toxic metabolites, and effectively maintains CNS homeostasis. The primary structure of BBB consists of microvascular endothelial cells (ECs), tight junctions (TJs), pericytes, astrocytic endfeet and extracellular matrix (ECM) components [6]. The ECs act as the primary unit of vessel walls, pericytes are perivascular multipotent cells embedded in the vascular basement membrane and astrocytic processes almost completely ensheath brain capillaries [7]. Unlike the ECs in other tissues, the ECs in CNS comprise a multiplicity of BBB-specific transporters and channels that control the exchange of substances across cells (transcellular transport) and high electrical resistance TJs to limit movement among adjacent cells (paracellular transport) [8]. The endothelial TJs are large, multiprotein complexes that include interacting transmembrane proteins such as claudins (in particular, claudin-5 and claudin-12), occludins, and junctional adhesion molecules (JAMs), as well as auxiliary cytoplasmic proteins such as zonula occludens (ZO) proteins. The TJs connect extracellular stimuli with intracellular signaling in the ECs to restrain the paracellular diffusion of metabolites across the BBB [9]. The CNS pericytes have a significantly higher density with an endothelial-to-pericyte ratio estimated to be 1:1-3:1 [10, 11]. The vast array of pericyte function displays, that pericytes have some properties of stem cells. Furthermore, it is known that pericytes regulate the BBB integrity, regulate CBF and control inflammation, angio- and neurogenesis [12]. These functions are dependent on appropriate interactions and signaling between pericytes and other cells at the BBB, especially ECs and astrocytes. The astrocytes are the most abundant glial cells with distinctive morphological and functional characteristics that vary within different areas of the brain [13]. Astrocytes maintain BBB properties and homeostatic balance of the neural microenvironment through modulating synaptic transmission and regulating immune reaction [14]. Due to these functional integrations, the concept of BBB is broadened to a new structure, the neurovascular unit (NVU). The NVU comprises BBB-endowed ECs and a perivascular milieu composed of cells including pericytes, smooth muscle cells, astrocytes, neurons/neuronal endings, perivascular macrophages/microglia and ECM (Fig. 1). The NVU mediates neurovascular coupling, modulating vessel tone [15, 16]. These intimately and reciprocally linked cells and matrix generate a complex structure that regulates metabolites exchange between the blood and brain.
Fig (1).
The schematic diagram of the neurovascular unit and tight junctions. (A) The NVU comprises endothelial cells, pericytes, astrocytes that can interact with neurons, perivascular macrophages/microglia and other brain components to impart specific properties on the BBB. (B) The endothelial TJs include interacting transmembrane proteins Claudins, Occludins, and JAMs, as well as auxiliary cytoplasmic proteins ZO proteins. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
BBB is disrupted soon after the onset of ischemia [17-19]. The experimental study on rats showed that BBB permeability began to increase at 3 h of reperfusion after middle cerebral artery occlusion (MCAO), reached a maximum at 48 h, and decreased from 4 days after stroke [18]. In patients after acute ischemic stroke, the damage of BBB could be identified during the first 3 hours after symptom onset by magnetic resonance imaging (MRI) and associated with the development of vasogenic edema [19]. Furthermore, there are indications that reperfusion may cause a biphasic opening of the BBB and initiate secondary injury to the brain [20]. The initial stage of BBB opening is reversible and occurs within several hours post-reperfusion. The second phase of BBB opening can be seen 24 h to 72 h post-reperfusion and is irreversible [21, 22]. While the initial opening is associated with disruption of TJs; the second BBB opening may be related to inflammatory response and contribute significantly to neural cell death.
After BBB disruption, chemicals and fluids extravasate into brain parenchyma across the impaired BBB and lead to cerebral edema. The circulating leukocytes will be recruited, roll along and adhere to the endothelial wall, transmigrate across the BBB, and enter the brain. These infiltrating leukocytes not only release enzymes that degrade the basement membrane and further increase vascular permeability, but also aggravate cerebral inflammatory responses, which can exacerbate brain injury and induce worse clinical prognosis [23, 24]. The BBB disruption after ischemic stroke is regulated by matrix metalloproteinases (MMPs), inflammation, oxidative pathways, vesicular trafficking, etc [25]. MMPs are a family of zinc-binding proteolytic enzymes that can break the TJs and basal lamina protein, aggravate BBB disruption and in turn facilitate toxic substances transportation into the ischemic tissue [26, 27]. MMP-2 and -9 are two prominent proteins that cause BBB disruption in many conditions. In ischemic stroke patients, there is a correlation between the biphasic opening of the BBB and MMPs levels. High MMP-2 levels were increased during the early BBB opening while the increased MMP-9 was associated with the severe and late opening of the BBB [28]. Given that the BBB is functionally important to protect against neural damage and maintain CNS homeostasis, preservation of BBB integrity is an attractive therapeutic strategy for ischemic stroke.
3. THE OVERVIEW OF CELL THERAPY IN ISCHEMIC STROKE
A number of studies have demonstrated that neurogenesis occurs throughout life in localized brain regions such as the subventricular zone (SVZ) of the lateral ventricles, and the subgranular zone (SGZ) of the dentate gyrus [29-31]. After an ischemic injury, the neurogenesis can be activated and promote neural repair [32, 33]. It has been evidenced that the stem cell proliferation starts between 2 to 5 days after stroke and lasts for about 30 days, with a peak on day 7-8 post-ischemia in rats [34, 35]. The post-stroke neurogenesis was also found in the SVZ of the adult macacque monkeys after global ischemia [36]. Consistently, the increased neurogenesis was proved in human brains by immunostaining on the brain specimens of stroke patients [37, 38]. However, this endogenous restorative process is generally insufficient and thus unable to ameliorate ischemic damage and promote functional recovery. Supported by solid experimental and preclinical data, the transplantation of exogenous stem cells has emerged as a promising tool for the treatment of ischemic stroke.
Stem cells are defined as clonogenic cells that own the capacity to self-renew and differentiate into multiple cell lineages [39]. In the past decades, several types of cells such as embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), neural stem cells (NSCs), induced pluripotent stem cells (iPSCs), endothelial progenitor cells (EPCs) and some neural stem cell lines, have been assessed as potential cells therapy for ischemic stroke. The results obtained from these studies, although conflicting or controversial in some aspects, are encouraging. One of the potential mechanisms of cell therapy against ischemic stroke is to replace the dead or damaged cells and rebuild the new neuronal circuitry. On the other side, there are indications that these cells work through bystander effects, such as providing trophic support to the injured tissues, fostering both neurogenesis and angiogenesis to protect brain cells and enhance neuronal regeneration [2, 5]. It means that the engrafted cells can either release growth and neurotrophic factors by themselves or stimulate host cells to upregulate expression of these factors, such as transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), nerve growth factor, and epidermal growth factor [1, 40-42]. Indeed, the bystander effect may be equally or more effective at improving neurological outcome following ischemic insult.
Till now, different routes of cell administration have been used in experimental stroke models and preclinical studies. The local implantation includes intracerebroventricular (I.C.V) or intracerebral (cortex or hippocampus) delivery routes, whereby direct administration of stem/progenitor cells in the infarct areas achieves more vigorous neuroprotective effects. However, these invasive operations may inevitably damage normal brain tissues and difficult to translate into clinical applications [43]. Indirect cell administration, via systemically intra-arterial or intravenous routes, also provides positive effects. Intra-arterial administration induces less injury to the patients than intracerebral implantation, but it is invasive as well. Intravenous administration is a minimally invasive way and easy to be conducted, but the injected cells can be trapped in other organs so that only a small number of cells can reach the brain [44]. As already mentioned, the optimal route of cell delivery remains unresolved. Considering the invasiveness and convenience, systemic infusion of stem/progenitor cells is the most widely used method in the ongoing clinical trials. However, the systemically infused cells have to migrate through the BBB and engraft at ischemic sites to exert their therapeutic effects. Some prior studies demonstrated that interactions of chemokines and chemokine receptors partly mediated the migration of stem/progenitor cells to the lesion site in the brain [45]. The stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor-4 (CXCR4) system assists in the integration of cells into impaired tissue by promoting the adhesion of CXCR4-positive cells onto vascular endothelium [46]. The tropism and migration of stem cells towards the brain, the interactions of stem cells, and BBB in CNS have been reviewed before [24]. After transplantation, the engrafted cells are suggested to have the potential to decrease BBB leakage and stabilize BBB integrity during ischemic stroke, which will lessen neurovascular damage and contribute to their therapeutic efficacy. However, the effects and underlying mechanisms of cell therapy on the BBB permeability in ischemic stroke have not been extensively illustrated.
4. EFFECTS OF CELL THERAPY ON BBB INTEGRITY AFTER ISCHEMIC STROKE
4.1. Embryonic Stem Cells
ESCs are pluripotent cells having the ability to differentiate into a range of cell types [47], which makes them a compelling source in cell transplantation therapies. The previous studies have addressed that ESCs graft reduced the infarct size and improved neurological function in the experimental stroke models. Human ESCs can survive, migrate, differentiate, and increase endogenous nestin expression and accelerate the remodeling process in adult rat cortical peri-infarction zone [48]. However, the ethical concerns and risk of tumorgenicity impedes its clinical use and are therefore investigated to a lesser extent. In this regard, there is little direct data on the effects of ESCs engraftment and BBB integrity after ischemic stroke. Some studies suggested that intravenous murine ESCs could increase the number of microvessels at the border of the lesion site, restored histological and behavioral deficits, and reduced infarct size even 15 days after ischemic stroke, which provided indirect evidence that systemic administration of ESCs might cross BBB and exert neuroprotective effects [49, 50].
4.2. Mesenchymal Stem Cells
MSCs, of the phenotype SH2+, SH3+, SH4+, CD90+, CD44+, CD14−, CD34−, and CD45−, can be harvested from a variety of tissues, such as bone marrow, adipose tissue, umbilical cord blood, and peripheral blood [51]. The profile of the phenotype is often used to distinguish MSCs from hematopoietic cells and allows rapid identification of a cell population. MSCs could also be used both in autologous and allogeneic transplantation for experimental and preclinical assays, which are shown to facilitate the restoration of cerebral CBF and BBB and produce functional benefits following ischemic stroke.
4.2.1. Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs)
BMSCs are the most frequently used MSCs in ischemic stroke research. They support the crosslinking of peripheral cells, astrocytes, and endothelial cells, maintain the integrity of the BBB [52], form a microenvironment supporting neurogenesis, and promote the recovery of neurological function [53]. Infusion of BMSCs in the acute phase of stroke has been shown to improve BBB integrity and improve therapeutic efficacy in animal studies. Borlonga et al. demonstrated that intrastriatal transplantation of BMSCs expedited the return of CBF and BBB to near-normal levels in a dose-dependent manner in MCAO rats, indicating the early restoration of CBF and BBB following BMSCs engraftment may mediate the functional outcomes of stroke animals [3]. Zacharek et al. reported that intravenous administration of BMSCs 24 h post-stroke increased angiopoietin-1 (Ang1), tyrosine kinase Tie2, and occludin expressions in the ischemic border compared to the control MCAO rats [4]. Besides, the authors performed an in vitro study in which MSCs were cocultured with astrocytes or mouse brain endothelial cells using a transwell chamber coculture model. They found that BMSCs delivery might promote vascular stabilization and decrease BBB leakage, by increasing Ang1/Tie2 and VEGF/VEGF receptor 2 expressions, and both together enhanced angiogenesis and vascular maturation after stroke. Ang-1, produced by pericytes [54], had been proved to signal through the Tie2 family to promote vascular stabilization and control BBB permeability after stroke [55]. Tang et al. showed intracranial transplantation of BMSCs within 20 minutes after reperfusion downregulated aquaporin-4 (AQP4) expression, which consequently attenuated astrocyte apoptosis, BBB disruption, and brain edema. Additionally, the upregulation of AQP4 was through p38 but not extracellular signal-regulated kinase 1/2 (ERK1/2) or c-Jun N-terminal kinase (JNK) signaling pathways in response to inflammatory cytokines [56]. AQP4, a water channel protein expressed on the end-feet of astrocytes, has been widely studied as an inflammatory mediator and a key target in the induction of BBB disruption [57]. Furthermore, Cheng et al. discovered intracranial transplantation of BMSCs within 15 min after reperfusion decreased the expressions of MMP-9, IL-1β, IL-6, and TNF-α, alleviated neutrophil infiltration, and attenuated BBB breakdown in MCAO mice [58]. These effects were potentially mediated via an adenosine monophosphate-activated protein kinase (AMPK)-dependent intercellular adhesion molecule-1 (ICAM-1) downregulation. Recently, Namioka et al. provided evidence that intravenous infusion of BMSCs at 8 weeks after MCAO stabilized the BBB, reduced microvascular leakage and improved behavior function, which indicated that BMSCs could exert functional benefits even in the chronic phase of ischemic stroke [59].
To increase stem cell homing capacity, genetic modifications are often used and various agents are co-administrated with stem cells. That could facilitate cell entrance of the brain. Huang et al. genetically changed the corresponding CC chemokine receptor 2 (CCR2) to the MSCs (referred to as MSCCCR2) and delivered modified cells to rats after MCAO via the caudal vein. They discovered that MSCCCR2 dramatically mitigated TJ proteins degradation, increased CD31 expression, and pericyte density in the NVU. MSCCCR2 engraftment reduced inflammation infiltration and ROS generation, exhibited significantly enhanced migration to the ischemic lesions, and improved the neurological outcomes, which was attributed to the peroxiredoxin4-mediated BBB preservation. Similar results were also confirmed using the in vitro BBB model [60]. Administration of hypoxia preconditioning (HP)-treated BMSCs to neonatal stroke of postnatal day 7 rat pups, increased angiogenesis and neurogenesis, improved BBB functions, and CBF in the cerebral cortex, and recovered sensory-motor and olfactory functions [61]. To this respect, BMSCs are also considered as a regenerative therapy for neonatal ischemic stroke. Recently, Nakazaki et al. revealed that intravenous infusion of BMSCs activated both TGF-β and Ang1 signaling pathways to promote the proliferation and differentiation of both endothelial cells and pericytes in the stroke-prone spontaneously hypertensive rats (SHRSP), which was used as a cerebral small vessel disease (CSVD) model [62]. Through remodeling of microvasculature and restoration of the BBB, BMSCs inhibited amyloid-beta (Aβ) accumulation, ameliorated progressive brain atrophy, and improved cognitive dysfunction, implicating BMSCs may represent a novel therapy for CSVD.
Some previous studies indicated that the neurotrophic factors help to stabilize the BBB integrity and improve functional recovery in ischemic stroke. Yoo et al. found that intracranial transplantation of BMSCs on day 3 after stroke decreased the production of monocyte chemoattractant protein-1 (MCP-1) and blocked the infiltration of additional immune cells through the impaired BBB. They also demonstrated that this effect was depended on the TGF-β secreted by BMSCs [63]. MCP-1 is expressed in neurons, astrocytes, and endothelial cells, in cells that regulate cells tropism and migration towards the brain [64]. It is considered as a major player in the regulation of the BBB breakdown and subsequent secondary brain damage [65]. Interestingly, Borlogan et al. reported the increased expression of specific neurotrophic factors such as GDNF, activin A, TGF-β1, TGF-β2 assisted in restoring CBF and BBB in the transplanted stroke brain. They provided a new notion that neuroprotective effect of stem cell therapy did not require the engrafted cells to physically pass the BBB and enter the brain [66]. Some studies hypothesized that the combination pharmacological treatment with stem cell and trophic factors exhibit stronger protective effects on ischemic injury compared with stem cell alone. Huang et al. revealed intravenous transplantation of BMSCs with BDNF 48 h after stroke significantly mitigated BBB dysfunction, activated the neuron-specific enolase (NSE) activity, and inhibited neuronal apoptosis in the ischemic boundary zone on day 14 in MCAO rats [67].
4.2.2. Adipose Tissue-Derived Mesenchymal Stem Cells (ADMSCs)
ADMSCs are derived from adipose tissue and thus are abundant, accessible, and easy to obtain, and readily cultured to a sufficient number for autologous transplantation without ethical issues. Leu et al. discovered that ADMSCs therapy increased the expressions of SDF-1/CXCR4 system, enhanced angiogenesis and neurogenesis, attenuated inflammatory response and apoptosis on day 21 after ischemic stroke, contributing to the recovery of neurological functions [68]. Chi et al. showed that intravenous administration of ADMSCs upregulated the distribution of ZO-1 and Claudin-5, alleviated the impairment of BBB in MCAO rats, which was ascribed to the suppression of ischemia-induced endoplasmic reticulum stress [69]. A recent research further confirmed the beneficial effects of ADMSCs on BBB integrity in ischemic stroke [70]. In addition, the authors suggested that the protective effects of ADMSCs were exerted by suppressing miR-21-3p expression, which then upregulated methionine adenosyltransferase 2B (MAT2B) and subsequently inhibited apoptosis and inflammation in brain tissue. The miR-21-3p/MAT2B signaling plays a critical role in cell apoptosis and inflammation, which has been proved to regulate BBB permeability after traumatic brain injury as well [71].
4.2.3. Umbilical Cord-Derived Mesenchymal cells (UCMSCs)
The human umbilical cord is a promising source of MSCs. Since their collection is non-invasive, painless, and does not evoke the ethical concerns, umbilical cord blood cells (UCBCs) have become an attractive option for ischemic stroke. There are evidence that human UCBCs (HUCBCs) can potentially attenuate the dysfunction of BBB, regulate inflammatory and immune responses, and facilitate the behavioral recovery after ischemic stroke [72, 73]. Lind et al. found that UCBCs administration to stroke animals could restore CBF and BBB integrity, and positively correlate with behavioral recovery [74]. Zhao et al. discovered intranasal administration of human UCMSCs condition medium (HUCMSC-CM) starting 24 h post-stroke exhibited significantly increased expressions of ZO-1, occludin, and desmin, enhanced BBB integrity and promoted functional outcome but did not decrease lesion volume in MCAO rats. Moreover, HUCMSCs-CM significantly decreased the levels of angiopoietin-2 (Ang2) and increased the levels of both Ang1 and Tie2 in the ischemic brain [75]. Ang2, an antagonist for Ang1, can inhibit Ang1-promoted Tie2 signaling and decrease blood vessel maturation and stabilization [76]. Cui et al. demonstrated that the combination of sub-therapeutic doses of simvastatin with HUCBCs treatment increased Ang1/Tie2 and occludin expression in the ischemic brain, which enhances vascular remodeling and preserves BBB integrity [77]. In 2019, Shiao et al. conducted RNA sequencing to study the brain transcriptome change after the systemic administration of HUCBCs in rats after MCAO [78]. They addressed HUCBCs were able to reduce the upregulation of transcripts associated with BBB permeability, thereby reducing infiltration and activation of immune cells, which might be responsible for the downregulation of apoptotic related genes.
4.2.4. Other Sources of Mesenchymal Stem Cells
Being immunoprivileged and having the immunomodulatory ability, the placenta and amniotic fluid are noticeably practical sources of MSCs, which are reported to be associated with BBB permeability and integrity in ischemic stroke. Kholodenko et al. performed a comparative analysis of the efficiency of crossing BBB by intravenous infusion of placental MSC in two MCAO models differing by the severity of the injury. They indicated that the BBB penetration and placental MSCs migration into CNS largely depend on the degree and nature of the injury to the brain [79]. Faezi et al. revealed that I.C.V. administration of human amniotic mesenchymal stem cells condition medium (HAMSC-CM) 30 minutes after reperfusion reduced infarct volume, brain edema and BBB leakage by targeting apoptosis [80]. The data from Nazarinia et al. further support the above foundings. They discovered intravenous injection of HAMSC-CM immediately after reperfusion improved BBB integrity and gave rise to neuroprotection against ischemic injury by suppressing autophagy in a mammalian target of rapamycin (mTOR) dependent mechanism [81].
4.3. Neural Stem/Progenitor Cells (NSCs/NPCs)
Endogenous NSCs mainly reside in the SVZ of the lateral ventricles and SGZ of the dentate gyrus [32]. The NSCs move from the SVZ into the rostral migratory stream and thence to the olfactory bulb where they differentiate into interneurons in rodents. Several studies indicated that circumventricular organs (CVO) also comprise a midline series of adult stem cell niches along the third ventricles and fourth ventricles [82]. The NSCs have the functional properties of self-renewal and multipotency to differentiate into neurons in a regional and developmental stage-appropriate manner throughout life. After ischemic stroke, the NSCs can engraft into ischemic brains survived, migrated to the ischemic lesion, maturing into neurons to replenish the damaged cells and promote neural repair [33, 83, 84]. Up to now, transplantation of exogenous NSCs to aid endogenous neural progenitors has been widely studied.
Under ischemic conditions, BBB leakage is increased in all brain stem cell niches including SVZ, SGZ, CVO, the third ventricles, and fourth ventricles, which may potentially increase access to systemic stroke-related factors and induce endogenous neurogenesis in these niche sites [82]. The effects of exogenous transplanted NSCs on BBB permeability following ischemic stroke were further explored. Huang et al. showed that early transplantation of NSCs helped to protect against damage to the BBB after ischemic stroke. Intracranially injected human NSCs within 24 h post-stroke significantly inhibited the extravasation of the blood-borne substance IgG, suppressed MMP-9 activity and prevented ZO-1 degradation. Moreover, transplantation of human NSC decreased microglia activation as well as downregulated expressions of inflammatory factors and adhesion molecules in mice after ischemic stroke [85]. Similarly, hippocampal transplanted human iPSC-NSCs could rapidly migrate into the site of stroke injury, ameliorate BBB damage and inflammatory response, thus improving neurological and pathophysiological functions in a rodent MCAO model [86]. In another study, Zhang et al. showed that pretreatment of NSCs with adjudin could enhance the viability of NSCs after their transplantation into the stroke-induced infarct area. Transplantation of adjudin-preconditioned mouse embryonic NSCs into the ipsilateral striatum at 24 h after MCAO resulted in decreased infarct volume and amelioration of BBB disruption in adult mice [87]. Adjudin, a small molecular derivative of indazole, has been reported to protect against ischemic injury by inhibition of neuroinflammation and BBB disruption [88]. Furthermore, Doeppner et al. found that intravenous transplantation of adult NPCs at 6 h after stroke has the unique advantage of stabilizing the BBB via mechanisms involving a reduction of MMP9 expression and ROS when compared to intracerebral transplantation. They suggested long-term neuroprotection of NPC in ischemic stroke was rather a consequence of acute neuroprotection and BBB remodeling than a consequence of post-stroke neuroregeneration [89, 90]. In addition, they analyzed the effects of cell delivery timing for intravenous transplantation of NPCs and their underlying mechanisms in MCAO mice [91]. The NPCs were intravenously grafted on day 0, on day 1 or on day 28 after stroke, followed by an observation period of 3 months post-stroke. The results revealed NPC-induced neuroprotection after acute cell delivery was due to the stabilization of the BBB, reduction in microglial activation and modulation of immune responses. On the other hand, post-acute NPC transplantation stimulated post-ischemic regeneration via enhanced angioneurogenesis and increased axonal plasticity. They concluded post-ischemic functional recovery is independent of NPC delivery timing, which offered a broad therapeutic time window for stroke treatment.
4.4. Endothelial Progenitor Cells (EPCs)
EPCs are precursors for the mature endothelium that lines the vascular system, which expresses the surface markers involving CD34 or CD133 and the marker protein VEGF receptor 2 [92]. They are mainly harvested from the mononuclear cell fraction of peripheral blood, leukapheresis products, umbilical cord blood and bone marrow [93]. EPCs administration can enhance BBB integrity and improve neurological function after ischemic stroke [94, 95]. By electron microscopy, Garbuzova-Davis et al. reported intravenous transplantation of human bone marrow EPCs into rats at 48 h after MCAO successfully enhanced mitochondrial function and exerted pinocytotic activity, which might have mediated the restoration of the BBB after ischemic stroke [96]. This BBB repair was observed in the early phase of the stroke. By bioluminescence imaging and MRI dual-mode imaging, Ding et al. described that I.C.V. administration of human umbilical cord blood EPCs reduced the permeability of BBB, promoted myelin recovery, and enhanced the neurogenesis in a photothrombotic stroke model, which was consistent to the histopathological detections [97]. Recently, Sargento-Freitas et al. conducted a clinical study that delivered EPCs into the ischemic patients and assessed the permeability of BBB by MRI at day 0 and 7 after stroke. They demonstrated EPCs improved clinical outcome at 3 months in patients, which was associated with subacute permeability of BBB and miRNAs related to adherens junction pathway [98].
4.5. Other Types of Stem Cells
In the last few years, it has been demonstrated that some novel sources of stem cells such as dental tissue, menstrual blood, breast milk are able to promote functional recovery in ischemic stroke [99-101]. The derived stem cells maintain potency to differentiate into various types of cells forming nervous tissue histo-architecture and display highly proliferative capabilities. They exert neuroprotective effects through secreting neurotrophic factors, modulating the inflammatory response, and enhancing neurogenesis and angiogenesis. Sowa et al. provided evidence that intravenous dental pulp stem cells (DPSC) immediately after MCAO suppressed proinflammatory cytokine levels and ameliorated the disruption of BBB by inhibiting the decline of TJ proteins, which further reduced the level of subsequent ischemia-induced neuronal damage and promoted angiogenesis after ischemic injury [102]. Current data on the relationship between breast milk or menstrual blood-derived stem cells and BBB permeability after ischemic stroke is scarce. In the future, the abundance, frequency, and expansion potential of these stem cells may provide a good choice for ischemic stroke therapy.
5. EFFECTS OF STEM CELLS ON BBB INTEGRITY IN RT-PA-MEDIATED THERAPY
Disruption of BBB, damage to microvessels, and the toxic and non-thrombolytic actions of tPA are the mechanisms underlying delayed rt-PA-induced complications, especially HT [103]. Cells engraftment is implicated to suppress hemorrhagic events after rt-PA therapy in the acute phase of ischemic stroke models. Nakazaki et al. demonstrated that BMSCs decreased the incidence of hemorrhagic events after rt-PA therapy in MCAO rats [104]. They found that intravenous infusion of BMSCs 30 minutes post-reperfusion suppressed the activity of MMP-9, inhibited vascular endothelial dysfunction and mitigated BBB disruption. The combination therapy of BMSCs and rt-PA also facilitated early behavioral recovery in rats. Yang et al. revealed intravenous administration of bone marrow mononuclear cells 2 h after t-PA therapy did not alter the incidence of HT but decreased the severity of HT and reduced BBB permeability after stroke. One possible mechanism could be through the inhibition of MMP3 released by astrocytes via Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway [105].
Liu et al. discovered that intracerebral transplanted BMSC reduced MMP activation, survived and differentiated into microglia and astrocyte at 14 days, alleviating the damage to NVU caused by tPA after ischemic stroke. The MMP activation and BMSC effects were detectable with in vivo and ex vivo optical imaging [106]. Recently, Boese et al. demonstrated that transplantation of NSCs into the hippocampus 24 h post-stroke protected the BBB and reduced infarct volume in aged brains that receive delayed tPA administration. NSCs could preserve BBB integrity through the downregulation of pro-inflammatory factors, MMP-9, and reducing proteolytic cleavage of ZO-1 [107]. Therefore, the aforementioned data supports the notion that extending the therapeutic time window and efficacy for rt-PA might be achieved by combining neuroprotection with stem cells in ischemic stroke.
6. EFFECTS OF STEM CELLS ON BBB IN DIABETIC STROKE
Diabetes mellitus (DM) is a significant risk factor for ischemic stroke. Diabetic animals are more prone to vascular injury, arteriosclerosis and have decreased TJ proteins expression, increased BBB disruption and poor functional outcomes compared to wild-type ischemic animals [108]. Some studies explored the effects of stem cell engraftment on BBB permeability after ischemic stroke in diabetic stroke models. In 2011, Chen et al. found that BMSCs therapy in type 1 diabetic (T1DM) rats failed to improve neurological function, adversely increased BBB leakage and intracranial hemorrhage 2 weeks after stroke, which was ascribed to the increased expression of angiogenin and ED1 (a marker for macrophages) positive macrophages [109]. However, Yan et al. discovered that when combining BMSCs with Niaspan, a drug used to prolong the release of niacin in DM patients, and attenuated the above adverse side-effects of BMSC in T1DM rats [110]. The combination treatment substantially downregulated angiogenin, MMP9, and ED1 expressions and mitigated BBB leakage and cerebral arteriosclerosis-like changes in the ischemic brain. In 2006, Hu et al. reported that intravenous administration of BMSCs starting 24 h after MCAO in type 2 diabetic (T2DM) rats significantly reduced the levels of high-mobility group box 1 (HMGB1) and receptor for advanced glycation endproducts (RAGE), increased ZO-1 expression, decreased BBB leakage and facilitated the functional recovery [111]. However, the treatment failed to decrease the lesion volume. Complementary in vitro data further supported that BMSCs transwell culture treatment significantly reduced the HMGB1 and RAGE expression in microglia after oxygen-glucose deprivation (OGD) exposure. HMGB1 acts as a danger-associated molecular pattern, which can mediate cerebral inflammation and participate in the pathogenesis of ischemic stroke [112]. By interplaying with its receptor RAGE, HMGB1 may increase vascular permeability, accelerate BBB breakdown, and amplify the brain injury after ischemia [113]. Furthermore, they discovered that intravenous injection with BMSCs conditioned medium at 24 h after stroke in T2DM rats also promoted functional outcome, reduced BBB leakage, and increased the levels of Ang1 and Tie2 in the ischemic brain [114]. In another research, Yan et al. revealed the BMSCs administered at 3 days after MCAO decreased BBB leakage and improved functional outcome as well. Delayed BMSCs promoted neurovascular remodeling and anti-inflammatory M2 macrophage polarization, improved NSC migration, and axonal remodeling in the ischemic border zone. Increasing brain platelet-derived growth factor expression may contribute to BMSC-induced neurorestoration [115]. Ding et al. conducted an MRI research and demonstrated that BMSCs transplantation significantly decreased BBB leakage in T2DM rats starting at 1 week after stroke by contrast-enhanced T1-weighted imaging with gadopentetate, and reduced cerebral hemorrhagic spots starting at 3 weeks post-stroke by susceptibility-weighted imaging, although BMSC treatment did not reduce the ischemic lesion volumes as demarcated by T2 maps. These MRI measurements were consistent with histological data [116].
UCBCs transplantation is tested in some diabetic stroke studies as well. Yan et al. showed HUCBCs delivery at 24 h after stroke upregulated Ang-1 and concomitantly reduced RAGE expression in the ischemic brain in T1DM rats. Moreover, HUCBCs increased vascular and arterial density with larger diameters as well as promoted vascular integrity and alleviated BBB leakage [117]. Chen et al. discovered delayed HUCBCs treatment initiated on day 3 in T2DM-MCAO mice that inhibited brain hemorrhage and BBB leakage, increased TJ proteins expression and vascular remodeling as well as white matter remodeling in the ischemic brain. The increasing miR-126 expression may mediate HUCBCs-induced neuroprotective effects [118]. However, Yan et al. found that HUCBCs transplantation did not reduce BBB leakage and lesion volume in T2DM rats, but significantly increased white matter and vascular remodeling as well as decreased neuroinflammatory response and promoted M2 macrophage polarization, which then improved long-term functional outcome and attenuated brain hemorrhage [119]. In addition, Geng et al. reported intravenous administration of EPCs attenuated infarct volume, mitigated BBB leakage, and improved neurological outcomes following MCAO in T1DM mice, which was attributed to the upregulation of hypoxia-inducible factor-1α (HIF-1α) after stroke [120]. HIF-1α is a critical transcription factor in maintaining oxygen homeostasis under physiological conditions and regulating the cellular adaptive reaction under hypoxic conditions [121]. The mechanism by which HIF-1α affects BBB integrity and permeability in acute ischemic stroke warrants further study.
The discrepancy of these data may be related to the inherent heterogeneity of stem cells combined with variations in experimental techniques and models. Cautions should be exercised in translating the therapeutic effects of cell engraftment in nondiabetics to diabetics.
7. CHALLENGES AND CONCERNS FOR CELL THERAPY IN ISCHEMIC STROKE
Despite promising results of preclinical studies, only a few clinical trials were able to confirm the beneficial effects of cell therapy on human patients [122, 123]. Preliminary reports of clinical trials sometimes indicate safety, but not the efficacy of the existing clinical protocols [124]. That clearly indicates the urgent need for further investigation which will be able to close the gap between preclinical and clinical research. In addition to some public concern and ethical problems in respect of the usage of some type of stem cells, there are numerous factors, which need to be further explored. The administration route, cell dosage, optimum timing of treatment, immunogenicity, tumorigenicity, and overall feasibility of use are still in the focus of research. The improvement in pharmacological drugs or genome editing techniques may improve the efficacy of cell therapy by promoting proliferation, migration, differentiation, survival of newborn neuron, and the function connection. Further experimental and clinical studies are necessary to clearly define an effective, feasible, and safe cell-based therapy, which will in turn guide future translational strategies for clinical applications.
CONCLUSION AND PERSPECTIVES
Until now, numerous experimental studies have demonstrated the promising effects of varied types of stem/progenitor cells in treating ischemic stroke in animal models. The results of most studies are encouraging and demonstrated that engrafted cells succeeded in exerting neurofunctional improvements. However, the underlying mechanisms have not been fully elucidated yet. Accumulating evidence suggests that structurally and functionally restoring the BBB by engrafted cells in an acute and sub-acute stroke setting provided therapeutic benefits. The regeneration and the repair of the damaged BBB by these cells is critical to the success of cell therapy after ischemic stroke.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- Aβ
Amyloid Beta
- ADMSCs
Adipose Tissue-Derived Mesenchymal Stem Cells
- AMPK
Adenosine Monophosphate-Activated Protein Kinase
- Ang1
Angiopoietin-1
- Ang2
Angiopoietin-2
- AQP4
Aquaporin-4
- BBB
Blood-Brain Barrier
- BDNF
Brain-Derived Neurotrophic Factor
- BMSCs
Bone Marrow-Derived Mesenchymal Stem Cells
- BMSCs-CM
BMSCs Conditioned Medium
- CBF
cerebral blood flow
- CCR2
CC Chemokine Receptor 2
- CNS
Central Nervous System
- CSVD
Cerebral Small Vessel Disease
- CVO
Circumventricular Organs
- CXCR4
CXC Chemokine Receptor-4
- DM
Diabetes Mellitus
- DPSC
Dental Pulp Stem Cells
- ECM
Extracellular Matrix
- ECs
Endothelial Cells
- EPCs
Endothelial Progenitor Cells
- ERK1/2
Extracellular Signal-Regulated Kinase 1/2
- ESCs
Embryonic Stem Cells
- FDA
Food and Drug Administration
- GDNF
Glial Cell-Derived Neurotrophic Factor
- HAMSC-CM
Human Amniotic Mesenchymal Stem Cells Condition Medium
- HIF-1α
Hypoxia-Inducible Factor-1α
- HMGB1
High-Mobility Group Box 1
- HP
Hypoxia Preconditioning
- HT
Hemorrhagic Transformation
- HUCMSC-CM
Human UCMSCs Condition Medium
- ICAM-1
Intercellular Adhesion Molecule-1
- I.C.V
Intracerebroventricular
- iPSCs
Induced Pluripotent Stem Cells
- JAK
Janus Kinase
- JAMs
Junctional Adhesion Molecules
- JNK
c-Jun N- Terminal Kinase
- MAT2B
Methionine Adenosyltransferase 2B
- MCAO
Middle Cerebral Artery Occlusion
- MCP-1
Monocyte Chemoattractant Protein-1
- MMP
Matrix Metalloproteinase
- MRI
Magnetic Resonance Imaging
- MSCs
Mesenchymal Stem Cells
- mTOR
Mammalian Target of Rapamycin
- NSC/NPC
Neural Stem/Progenitor Cell
- NSE
Neuron-Specific Enolase
- NVU
Neurovascular Unit
- OGD
Oxygen Glucose Deprivation
- RAGE
Receptor for Advanced Glycation Endproducts
- rt-PA
Recombinant Tissue Plasminogen Activator
- SDF-1
Stromal Cell Derived Factor-1
- SGZ
Subgranular Zone
- SHRSP
Stroke Prone Spontaneously Hypertensive Rats
- STAT
Signal Transducer and Activator of Transcription
- SVZ
Subventricular Zone
- T1DM
Type 1 Diabetes Mellitus
- T2DM
Type 2 Diabetes Mellitus
- TGF-β
Transforming Growth Factor-Beta
- TJs
Tight Junctions
- UCBCs
Umbilical Cord Blood Cells
- UCMSCs
Umbilical Cord-Derived Mesenchymal Cells
- VEGF
Vascular Endothelial Growth Factor
- ZO
Zonula Occluden
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the National Natural Science Foundation of China (81801298, 81500916, 81801195), Scientific Foundation of Renji Hospital South Campus (2017PYQA04), and Pujiang Outstanding Youth Project of Renji Hospital South Campus (RJPJYQ2018).
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Marei H.E., Hasan A., Rizzi R., Althani A., Afifi N., Cenciarelli C., Caceci T., Shuaib A. Potential of stem cell-based therapy for ischemic stroke. Front. Neurol. 2018;9:34. doi: 10.3389/fneur.2018.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarmah D., Kaur H., Saraf J., Pravalika K., Goswami A., Kalia K., Borah A., Wang X., Dave K.R., Yavagal D.R., Bhattacharya P. Getting closer to an effective intervention of ischemic stroke: the big promise of stem cell. Transl. Stroke Res. 2018;9(4):356–374. doi: 10.1007/s12975-017-0580-0. [DOI] [PubMed] [Google Scholar]
- 3.Borlongan C.V., Lind J.G., Dillon-Carter O., Yu G., Hadman M., Cheng C., Carroll J., Hess D.C. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010(1-2):108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 4.Zacharek A., Chen J., Cui X., Li A., Li Y., Roberts C., Feng Y., Gao Q., Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow Metab. 2007;27(10):1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalu M.M., Montroy J., Dowlatshahi D., Hutton B., Juneau P., Wesch N., Zhang Y. S.; McGinn, R.; Corbett, D.; Stewart, D.J.A.; A Fergusson, D. From the lab to patients: a systematic review and meta-analysis of mesenchymal stem cell therapy for stroke. Transl. Stroke Res. 2020;11(3):345–364. doi: 10.1007/s12975-019-00736-5. [DOI] [PubMed] [Google Scholar]
- 6.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Saunders N.R., Liddelow S.A., Dziegielewska K.M. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keaney J., Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282(21):4067–4079. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 10.Shepro D., Morel N.M. Pericyte physiology. FASEB J. 1993;7(11):1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 11.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armulik A., Genové G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Siracusa R., Fusco R., Cuzzocrea S. Astrocytes: role and functions in brain pathologies. Front. Pharmacol. 2019;10:1114. doi: 10.3389/fphar.2019.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan S.V., Dave K.R., Perez-Pinzon M.A. Ischemic preconditioning protects astrocytes against oxygen glucose deprivation via the nuclear erythroid 2-related factor 2 pathway. Transl. Stroke Res. 2018;9(2):99–109. doi: 10.1007/s12975-017-0574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mäe M., Armulik A., Betsholtz C. Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr. Pharm. Des. 2011;17(26):2750–2754. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- 16.Muoio V., Persson P.B., Sendeski M.M. The neurovascular unit - concept review. Acta Physiol. (Oxf.) 2014;210(4):790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 17.Steliga A., Kowiański P., Czuba E., Monika W., Moryś J., Lietzau G. Neurovascular unit as a source of ischemic stroke biomarkers-limitations of experimental studies and perspectives for clinical application. Transl. Stroke Res. 2020;11(4):553–579. doi: 10.1007/s12975-019-00744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatashita S., Hoff J.T. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke. 1990;21(4):582–588. doi: 10.1161/01.STR.21.4.582. [DOI] [PubMed] [Google Scholar]
- 19.Giraud M., Cho T.H., Nighoghossian N., Maucort-Boulch D., Deiana G., Østergaard L., Baron J.C., Fiehler J., Pedraza S., Derex L., Berthezène Y. Early blood brain barrier changes in acute ischemic stroke: a sequential MRI study. J. Neuroimaging. 2015;25(6):959–963. doi: 10.1111/jon.12225. [DOI] [PubMed] [Google Scholar]
- 20.Kuroiwa T., Ting P., Martinez H., Klatzo I. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol. 1985;68(2):122–129. doi: 10.1007/BF00688633. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y., Zhang L., Pu H., Mao L., Hu X., Jiang X., Xu N., Stetler R.A., Zhang F., Liu X., Leak R.K., Keep R.F., Ji X., Chen J. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016;7:10523. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y., Rosenberg G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelhardt B. Immune cell entry into the central nervous system: involvement of adhesion molecules and chemokines. J. Neurol. Sci. 2008;274(1-2):23–26. doi: 10.1016/j.jns.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Liu L., Eckert M.A., Riazifar H., Kang D.K., Agalliu D., Zhao W. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013;2013:435093. doi: 10.1155/2013/435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sifat A.E., Vaidya B., Abbruscato T.J. Blood-brain barrier protection as a therapeutic strategy for acute ischemic stroke. AAPS J. 2017;19(4):957–972. doi: 10.1208/s12248-017-0091-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen H., Guan B., Chen X., Chen X., Li C., Qiu J., Yang D., Liu K.J., Qi S., Shen J. Baicalin attenuates blood-brain barrier disruption and hemorrhagic transformation and improves neurological outcome in ischemic stroke rats with delayed t-PA treatment: involvement of ONOO-MMP-9 pathway. Transl. Stroke Res. 2018;9(5):515–529. doi: 10.1007/s12975-017-0598-3. [DOI] [PubMed] [Google Scholar]
- 27.Ayata C., Ropper A.H. Ischaemic brain oedema. J. Clin. Neurosci. 2002;9(2):113–124. doi: 10.1054/jocn.2001.1031. [DOI] [PubMed] [Google Scholar]
- 28.Lucivero V., Prontera M., Mezzapesa D.M., Petruzzellis M., Sancilio M., Tinelli A., Di Noia D., Ruggieri M., Federico F. Different roles of matrix metalloproteinases-2 and -9 after human ischaemic stroke. Neurol. Sci. 2007;28(4):165–170. doi: 10.1007/s10072-007-0814-0. [DOI] [PubMed] [Google Scholar]
- 29.Doetsch F., Caillé I., Lim D.A., García-Verdugo J.M., Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 30.Djavadian R.L. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol. Exp. (Warsz.) 2004;64(2):189–200. doi: 10.55782/ane-2004-1505. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin K., Minami M., Lan J.Q., Mao X.O., Batteur S., Simon R.P., Greenberg D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA. 2001;98(8):4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishibashi S., Sakaguchi M., Kuroiwa T., Yamasaki M., Kanemura Y., Shizuko I., Shimazaki T., Onodera M., Okano H., Mizusawa H. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J. Neurosci. Res. 2004;78(2):215–223. doi: 10.1002/jnr.20246. [DOI] [PubMed] [Google Scholar]
- 34.Yagita Y., Kitagawa K., Ohtsuki T. Takasawa Ki; Miyata, T.; Okano, H.; Hori, M.; Matsumoto, M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32(8):1890–1896. doi: 10.1161/01.STR.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R.L., Zhang Z.G., Zhang L., Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105(1):33–41. doi: 10.1016/S0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 36.Tonchev A.B., Yamashima T., Sawamoto K., Okano H. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. J. Neurosci. Res. 2005;81(6):776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- 37.Jin K., Wang X., Xie L., Mao X.O., Zhu W., Wang Y., Shen J., Mao Y., Banwait S., Greenberg D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA. 2006;103(35):13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama D., Matsuyama T., Ishibashi-Ueda H., Nakagomi T., Kasahara Y., Hirose H., Kikuchi-Taura A., Stern D.M., Mori H., Taguchi A. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur. J. Neurosci. 2010;31(1):90–98. doi: 10.1111/j.1460-9568.2009.07043.x. [DOI] [PubMed] [Google Scholar]
- 39.Weissman I.L., Anderson D.J., Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 40.Hicks C., Stevanato L., Stroemer R.P., Tang E., Richardson S., Sinden J.D. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2013;22(9):1541–1552. doi: 10.3727/096368912X657936. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee S., Williamson D.A., Habib N., Chataway J. The potential benefit of stem cell therapy after stroke: an update. Vasc. Health Risk Manag. 2012;8:569–580. doi: 10.2147/VHRM.S25745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L., Chu L., Ren C., Wang J., Sun S., Li T., Yin Y. Enhanced migration of bone marrow-derived mesenchymal stem cells with tetramethylpyrazine and its synergistic effect on angiogenesis and neurogenesis after cerebral ischemia in rats. Stem Cells Dev. 2019;28(13):871–881. doi: 10.1089/scd.2018.0254. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Frutos B., Otero-Ortega L., Gutiérrez-Fernández M., Fuentes B., Ramos-Cejudo J., Díez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl. Stroke Res. 2016;7(5):378–387. doi: 10.1007/s12975-016-0482-6. [DOI] [PubMed] [Google Scholar]
- 44.Fischer U.M., Harting M.T., Jimenez F., Monzon-Posadas W.O., Xue H., Savitz S.I., Laine G.A., Cox C.S., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponte A.L., Marais E., Gallay N., Langonné A., Delorme B., Hérault O., Charbord P., Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 46.Peled A., Kollet O., Ponomaryov T., Petit I., Franitza S., Grabovsky V., Slav M.M., Nagler A., Lider O., Alon R., Zipori D., Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95(11):3289–3296. doi: 10.1182/blood.V95.11.3289. [DOI] [PubMed] [Google Scholar]
- 47.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P., Li J., Liu Y., Chen X., Kang Q. Transplanted human embryonic neural stem cells survive, migrate, differentiate and increase endogenous nestin expression in adult rat cortical peri-infarction zone. Neuropathology. 2009;29(4):410–421. doi: 10.1111/j.1440-1789.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- 49.Nagai N., Kawao N., Okada K., Okumoto K., Teramura T., Ueshima S., Umemura K., Matsuo O. Systemic transplantation of embryonic stem cells accelerates brain lesion decrease and angiogenesis. Neuroreport. 2010;21(8):575–579. doi: 10.1097/WNR.0b013e32833a7d2c. [DOI] [PubMed] [Google Scholar]
- 50.Tae-Hoon L., Yoon-Seok L. Transplantation of mouse embryonic stem cell after middle cerebral artery occlusion. Acta Cir. Bras. 2012;27(4):333–339. doi: 10.1590/S0102-86502012000400009. [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann S., Voss M., Kaiser S., Kapp U., Waller C.F., Martens U.M. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003;17(6):1146–1149. doi: 10.1038/sj.leu.2402962. [DOI] [PubMed] [Google Scholar]
- 52.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40(3) Suppl.:S13–S15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honmou O., Onodera R., Sasaki M., Waxman S.G., Kocsis J.D. Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol. Med. 2012;18(5):292–297. doi: 10.1016/j.molmed.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Sundberg C., Kowanetz M., Brown L.F., Detmar M., Dvorak H.F. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab. Invest. 2002;82(4):387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z.G., Zhang L., Croll S.D., Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113(3):683–687. doi: 10.1016/S0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 56.Tang G., Liu Y., Zhang Z., Lu Y., Wang Y., Huang J., Li Y., Chen X., Gu X., Wang Y., Yang G.Y. Mesenchymal stem cells maintain blood-brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells. 2014;32(12):3150–3162. doi: 10.1002/stem.1808. [DOI] [PubMed] [Google Scholar]
- 57.Manley G.T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A.W., Chan P., Verkman A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 58.Cheng Z., Wang L., Qu M., Liang H., Li W., Li Y., Deng L., Zhang Z., Yang G.Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J. Neuroinflammation. 2018;15(1):135. doi: 10.1186/s12974-018-1153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Namioka T., Namioka A., Sasaki M., Kataoka-Sasaki Y., Oka S., Nakazaki M., Onodera R., Suzuki J., Sasaki Y., Nagahama H., Kocsis J.D., Honmou O. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a rat model of chronic cerebral infarction. J. Neurosurg. 2018;131:1–8. doi: 10.3171/2018.5.JNS18140. [DOI] [PubMed] [Google Scholar]
- 60.Huang Y., Wang J., Cai J., Qiu Y., Zheng H., Lai X., Sui X., Wang Y., Lu Q., Zhang Y., Yuan M., Gong J., Cai W., Liu X., Shan Y., Deng Z., Shi Y., Shu Y., Zhang L., Qiu W., Peng L., Ren J., Lu Z., Xiang A.P. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics. 2018;8(21):5929–5944. doi: 10.7150/thno.28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Z.Z., Gu X., Ferdinand A., Lee J.H., Ji X., Ji X.M., Yu S.P., Wei L. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transplant. 2015;24(3):391–402. doi: 10.3727/096368915X686887. [DOI] [PubMed] [Google Scholar]
- 62.Nakazaki M., Sasaki M., Kataoka-Sasaki Y., Oka S., Suzuki J., Sasaki Y., Nagahama H., Hashi K., Kocsis J.D., Honmou O. Intravenous infusion of mesenchymal stem cells improves impaired cognitive function in a cerebral small vessel disease model. Neuroscience. 2019;408:361–377. doi: 10.1016/j.neuroscience.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Yoo S.W., Chang D.Y., Lee H.S., Kim G.H., Park J.S., Ryu B.Y., Joe E.H., Lee Y.D., Kim S.S., Suh-Kim H. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol. Dis. 2013;58:249–257. doi: 10.1016/j.nbd.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Che X., Ye W., Panga L., Wu D.C., Yang G.Y. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902(2):171–177. doi: 10.1016/S0006-8993(01)02328-9. [DOI] [PubMed] [Google Scholar]
- 65.Strecker J.K., Minnerup J., Schütte-Nütgen K., Gess B., Schäbitz W.R., Schilling M. Monocyte chemoattractant protein-1-deficiency results in altered blood-brain barrier breakdown after experimental stroke. Stroke. 2013;44(9):2536–2544. doi: 10.1161/STROKEAHA.111.000528. [DOI] [PubMed] [Google Scholar]
- 66.Borlongan C.V., Hadman M., Sanberg C.D., Sanberg P.R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35(10):2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 67.Huang W., Mo X., Qin C., Zheng J., Liang Z., Zhang C. Transplantation of differentiated bone marrow stromal cells promotes motor functional recovery in rats with stroke. Neurol. Res. 2013;35(3):320–328. doi: 10.1179/1743132812Y.0000000151. [DOI] [PubMed] [Google Scholar]
- 68.Leu S., Lin Y.C., Yuen C.M., Yen C.H., Kao Y.H., Sun C.K., Yip H.K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010;8:63. doi: 10.1186/1479-5876-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chi L., Huang Y., Mao Y., Wu K., Zhang L., Nan G. Tail vein infusion of adipose-derived mesenchymal stem cell alleviated inflammatory response and improved blood brain barrier condition by suppressing endoplasmic reticulum stress in a middle cerebral artery occlusion rat model. Med. Sci. Monit. 2018;24:3946–3957. doi: 10.12659/MSM.907096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li C., Fei K., Tian F., Gao C., Yang S. Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating miR-21-3p/MAT2B signaling transduction. Croat. Med. J. 2019;60(5):439–448. doi: 10.3325/cmj.2019.60.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge X., Li W., Huang S., Yin Z., Yang M., Han Z., Han Z., Chen F., Wang H., Lei P., Zhang J. Increased miR-21-3p in Injured brain microvascular endothelial cells after traumatic brain injury aggravates blood-brain barrier damage by promoting cellular apoptosis and inflammation through targeting MAT2B. J. Neurotrauma. 2019;36(8):1291–1305. doi: 10.1089/neu.2018.5728. [DOI] [PubMed] [Google Scholar]
- 72.Chen J., Sanberg P.R., Li Y., Wang L., Lu M., Willing A.E., Sanchez-Ramos J., Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32(11):2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 73.Vendrame M., Cassady J., Newcomb J., Butler T., Pennypacker K.R., Zigova T., Sanberg C.D., Sanberg P.R., Willing A.E. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35(10):2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 74.Lind J., Cheng C., Hadman M., Goodman D., Chopp M., Borlongan C.V. Transplanted stroke animals display normalized cerebral blood flow and BBB permeability during onset of behavioral recovery. IBNS. Abstr; 2003. [Google Scholar]
- 75.Zhao Q., Hu J., Xiang J., Gu Y., Jin P., Hua F., Zhang Z., Liu Y., Zan K., Zhang Z., Zu J., Yang X., Shi H., Zhu J., Xu Y., Cui G., Ye X. Intranasal administration of human umbilical cord mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke. Brain Res. 2015;1624:489–496. doi: 10.1016/j.brainres.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Cui X., Chopp M., Zacharek A., Ye X., Roberts C., Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol. Dis. 2011;43(1):285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui X., Chopp M., Zacharek A., Dai J., Zhang C., Yan T., Ning R., Roberts C., Shehadah A., Kuzmin-Nichols N., Sanberg C.D., Chen J. Combination treatment of stroke with sub-therapeutic doses of Simvastatin and human umbilical cord blood cells enhances vascular remodeling and improves functional outcome. Neuroscience. 2012;227:223–231. doi: 10.1016/j.neuroscience.2012.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shiao M.L., Yuan C., Crane A.T., Voth J.P., Juliano M., Stone L.L.H., Nan Z., Zhang Y., Kuzmin-Nichols N., Sanberg P.R., Grande A.W., Low W.C. Immunomodulation with human umbilical cord blood stem cells ameliorates ischemic brain injury - a brain transcriptome profiling analysis. Cell Transplant. 2019;28(7):864–873. doi: 10.1177/0963689719836763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kholodenko I.V., Yarygin K.N., Gubsky L.V., Konieva A.A., Tairova R.T., Povarova O.V., Kholodenko R.V., Burunova V.V., Yarygin V.N., Skvortsova V.I. Intravenous xenotransplantation of human placental mesenchymal stem cells to rats: comparative analysis of homing in rat brain in two models of experimental ischemic stroke. Bull. Exp. Biol. Med. 2012;154(1):118–123. doi: 10.1007/s10517-012-1890-6. [DOI] [PubMed] [Google Scholar]
- 80.Faezi M., Nasseri Maleki S., Aboutaleb N., Nikougoftar M. The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. J. Chem. Neuroanat. 2018;94:21–31. doi: 10.1016/j.jchemneu.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Nazarinia D., Aboutaleb N., Gholamzadeh R., Nasseri Maleki S., Mokhtari B., Nikougoftar M. Conditioned medium obtained from human amniotic mesenchymal stem cells attenuates focal cerebral ischemia/reperfusion injury in rats by targeting mTOR pathway. J. Chem. Neuroanat. 2019;102:101707. doi: 10.1016/j.jchemneu.2019.101707. [DOI] [PubMed] [Google Scholar]
- 82.Lin R., Cai J., Nathan C., Wei X., Schleidt S., Rosenwasser R., Iacovitti L. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol. Dis. 2015;74:229–239. doi: 10.1016/j.nbd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 83.Darsalia V., Kallur T., Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur. J. Neurosci. 2007;26(3):605–614. doi: 10.1111/j.1460-9568.2007.05702.x. [DOI] [PubMed] [Google Scholar]
- 84.Guzman R., Bliss T., De Los Angeles A., Moseley M., Palmer T., Steinberg G. Neural progenitor cells transplanted into the uninjured brain undergo targeted migration after stroke onset. J. Neurosci. Res. 2008;86(4):873–882. doi: 10.1002/jnr.21542. [DOI] [PubMed] [Google Scholar]
- 85.Huang L., Wong S., Snyder E.Y., Hamblin M.H., Lee J.P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014;5(6):129. doi: 10.1186/scrt519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eckert A., Huang L., Gonzalez R., Kim H.S., Hamblin M.H., Lee J.P. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cells Transl. Med. 2015;4(7):841–851. doi: 10.5966/sctm.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang T., Yang X., Liu T., Shao J., Fu N., Yan A., Geng K., Xia W. Adjudin-preconditioned neural stem cells enhance neuroprotection after ischemia reperfusion in mice. Stem Cell Res. Ther. 2017;8(1):248. doi: 10.1186/s13287-017-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu T., Zhang T., Yu H., Shen H., Xia W. Adjudin protects against cerebral ischemia reperfusion injury by inhibition of neuroinflammation and blood-brain barrier disruption. J. Neuroinflammation. 2014;11:107. doi: 10.1186/1742-2094-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doeppner T.R., Ewert T.A., Tönges L., Herz J., Zechariah A., ElAli A., Ludwig A.K., Giebel B., Nagel F., Dietz G.P., Weise J., Hermann D.M., Bähr M. Transduction of neural precursor cells with TAT-heat shock protein 70 chaperone: therapeutic potential against ischemic stroke after intrastriatal and systemic transplantation. Stem Cells. 2012;30(6):1297–1310. doi: 10.1002/stem.1098. [DOI] [PubMed] [Google Scholar]
- 90.Doeppner T.R., Kaltwasser B., Teli M.K., Sanchez-Mendoza E.H., Kilic E., Bähr M., Hermann D.M. Post-stroke transplantation of adult subventricular zone derived neural progenitor cells--A comprehensive analysis of cell delivery routes and their underlying mechanisms. Exp. Neurol. 2015;273:45–56. doi: 10.1016/j.expneurol.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 91.Doeppner T.R., Kaltwasser B., Teli M.K., Bretschneider E., Bähr M., Hermann D.M. Effects of acute versus post-acute systemic delivery of neural progenitor cells on neurological recovery and brain remodeling after focal cerebral ischemia in mice. Cell Death Dis. 2014;5(8):e1386. doi: 10.1038/cddis.2014.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hristov M., Erl W., Weber P.C. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler. Thromb. Vasc. Biol. 2003;23(7):1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 93.Yip H.K., Chang L.T., Chang W.N., Lu C.H., Liou C.W., Lan M.Y., Liu J.S., Youssef A.A., Chang H.W. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39(1):69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 94.Fan Y., Shen F., Frenzel T., Zhu W., Ye J., Liu J., Chen Y., Su H., Young W.L., Yang G.Y. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann. Neurol. 2010;67(4):488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iskander A., Knight R.A., Zhang Z.G., Ewing J.R., Shankar A., Varma N.R., Bagher-Ebadian H., Ali M.M., Arbab A.S., Janic B. Intravenous administration of human umbilical cord blood-derived AC133+ endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem Cells Transl. Med. 2013;2(9):703–714. doi: 10.5966/sctm.2013-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garbuzova-Davis S., Haller E., Lin R., Borlongan C.V. Intravenously transplanted human bone marrow endothelial progenitor cells engraft within brain capillaries, preserve mitochondrial morphology, and display pinocytotic activity toward blood-brain barrier repair in ischemic stroke rats. Stem Cells. 2017;35(5):1246–1258. doi: 10.1002/stem.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ding J., Zhang Y., Wang C.X., Li P.C., Zhao Z., Wang C., Teng G.J. Dual-modality imaging of endothelial progenitor cells transplanted after ischaemic photothrombotic stroke. Life Sci. 2019;239:116774. doi: 10.1016/j.lfs.2019.116774. [DOI] [PubMed] [Google Scholar]
- 98.Sargento-Freitas J., Aday S., Nunes C., Cordeiro M., Gouveia A., Silva F., Machado C., Rodrigues B., Santo G.C., Ferreira C., Amorim A., Sousa S., Gomes A.C., Castelo-Branco M., Ferreira L., Cunha L. Endothelial progenitor cells enhance blood-brain barrier permeability in subacute stroke. Neurology. 2018;90(2):e127–e134. doi: 10.1212/WNL.0000000000004801. [DOI] [PubMed] [Google Scholar]
- 99.Leong W.K., Henshall T.L., Arthur A., Kremer K.L., Lewis M.D., Helps S.C., Field J., Hamilton-Bruce M.A., Warming S., Manavis J., Vink R., Gronthos S., Koblar S.A. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl. Med. 2012;1(3):177–187. doi: 10.5966/sctm.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Borlongan C.V., Kaneko Y., Maki M., Yu S.J., Ali M., Allickson J.G., Sanberg C.D., Kuzmin-Nichols N., Sanberg P.R. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19(4):439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hassiotou F., Beltran A., Chetwynd E., Stuebe A.M., Twigger A.J., Metzger P., Trengove N., Lai C.T., Filgueira L., Blancafort P., Hartmann P.E. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30(10):2164–2174. doi: 10.1002/stem.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sowa K., Nito C., Nakajima M., Suda S., Nishiyama Y., Sakamoto Y., Nitahara-Kasahara Y., Nakamura-Takahashi A., Ueda M., Kimura K., Okada T. Impact of dental pulp stem cells overexpressing hepatocyte growth factor after cerebral ischemia/reperfusion in rats. Mol. Ther. Methods Clin. Dev. 2018;10:281–290. doi: 10.1016/j.omtm.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bosche B., Mergenthaler P., Doeppner T.R., Hescheler J., Molcanyi M. Complex clearance mechanisms after intraventricular hemorrhage and rt-PA treatment-a review on clinical trials. Transl. Stroke Res. 2020;11(3):337–344. doi: 10.1007/s12975-019-00735-6. [DOI] [PubMed] [Google Scholar]
- 104.Nakazaki M., Sasaki M., Kataoka-Sasaki Y., Oka S., Namioka T., Namioka A., Onodera R., Suzuki J., Sasaki Y., Nagahama H., Mikami T., Wanibuchi M., Kocsis J.D., Honmou O. Intravenous infusion of mesenchymal stem cells inhibits intracranial hemorrhage after recombinant tissue plasminogen activator therapy for transient middle cerebral artery occlusion in rats. J. Neurosurg. 2017;127(4):917–926. doi: 10.3171/2016.8.JNS16240. [DOI] [PubMed] [Google Scholar]
- 105.Yang B., Li W., Satani N., Nghiem D.M., Xi X., Aronowski J., Savitz S.I. Protective effects of autologous bone marrow mononuclear cells after administering t-PA in an embolic stroke model. Transl. Stroke Res. 2018;9(2):135–145. doi: 10.1007/s12975-017-0563-1. [DOI] [PubMed] [Google Scholar]
- 106.Liu N., Deguchi K., Yamashita T., Liu W., Ikeda Y., Abe K. Intracerebral transplantation of bone marrow stromal cells ameliorates tissue plasminogen activator-induced brain damage after cerebral ischemia in mice detected by in vivo and ex vivo optical imaging. J. Neurosci. Res. 2012;90(11):2086–2093. doi: 10.1002/jnr.23104. [DOI] [PubMed] [Google Scholar]
- 107.Boese A.C., Eckert A., Hamblin M.H., Lee J.P. Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains. Exp. Neurol. 2020;329:113275. doi: 10.1016/j.expneurol.2020.113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J., Cui X., Zacharek A., Cui Y., Roberts C., Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42(2):445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen J., Ye X., Yan T., Zhang C., Yang X.P., Cui X., Cui Y., Zacharek A., Roberts C., Liu X., Dai X., Lu M., Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42(12):3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan T., Ye X., Chopp M., Zacharek A., Ning R., Venkat P., Roberts C., Lu M., Chen J. Niaspan attenuates the adverse effects of bone marrow stromal cell treatment of stroke in type one diabetic rats. PLoS One. 2013;8(11):e81199. doi: 10.1371/journal.pone.0081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu J., Liu B., Zhao Q., Jin P., Hua F., Zhang Z., Liu Y., Zan K., Cui G., Ye X. Bone marrow stromal cells inhibits HMGB1-mediated inflammation after stroke in type 2 diabetic rats. Neuroscience. 2016;324:11–19. doi: 10.1016/j.neuroscience.2016.02.058. [DOI] [PubMed] [Google Scholar]
- 112.Kim J.B., Sig Choi J., Yu Y.M., Nam K., Piao C.S., Kim S.W., Lee M.H., Han P.L., Park J.S., Lee J.K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006;26(24):6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang J., Takahashi H.K., Liu K., Wake H., Liu R., Maruo T., Date I., Yoshino T., Ohtsuka A., Mori S., Nishibori M. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42(5):1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- 114.Xiang J., Hu J., Shen T., Liu B., Hua F., Zan K., Zu J., Cui G., Ye X. Bone marrow mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke in type 2 diabetic rats. Neurosci. Lett. 2017;644:62–66. doi: 10.1016/j.neulet.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 115.Yan T., Venkat P., Chopp M., Zacharek A., Ning R., Roberts C., Zhang Y., Lu M., Chen J. Neurorestorative responses to delayed human mesenchymal stromal cells treatment of stroke in type 2 diabetic rats. Stroke. 2016;47(11):2850–2858. doi: 10.1161/STROKEAHA.116.014686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ding G., Chen J., Chopp M., Li L., Yan T., Li Q., Cui C., Davarani S.P., Jiang Q. Cell treatment for stroke in type two diabetic rats improves vascular permeability measured by MRI. PLoS One. 2016;11(2):e0149147. doi: 10.1371/journal.pone.0149147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan T., Venkat P., Ye X., Chopp M., Zacharek A., Ning R., Cui Y., Roberts C., Kuzmin-Nichols N., Sanberg C.D., Chen J. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci. Ther. 2014;20(10):935–944. doi: 10.1111/cns.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen J., Ning R., Zacharek A., Cui C., Cui X., Yan T., Venkat P., Zhang Y., Chopp M. MiR-126 contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 diabetic mice. Stem Cells. 2016;34(1):102–113. doi: 10.1002/stem.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan T., Venkat P., Chopp M., Zacharek A., Ning R., Cui Y., Roberts C., Kuzmin-Nichols N., Sanberg C.D., Chen J. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke. 2015;46(9):2599–2606. doi: 10.1161/STROKEAHA.115.009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geng J., Wang L., Qu M., Song Y., Lin X., Chen Y., Mamtilahun M., Chen S., Zhang Z., Wang Y., Yang G.Y. Endothelial progenitor cells transplantation attenuated blood-brain barrier damage after ischemia in diabetic mice via HIF-1α. Stem Cell Res. Ther. 2017;8(1):163. doi: 10.1186/s13287-017-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boncoraglio G.B., Bersano A., Candelise L., Reynolds B.A., Parati E.A. Stem cell transplantation for ischemic stroke. Cochrance. Database. Syst. Rev. 2010;9:CD007231. doi: 10.1002/14651858.CD007231.pub2. [DOI] [PubMed] [Google Scholar]
- 123.Kondziolka D., Steinberg G.K., Wechsler L., Meltzer C.C., Elder E., Gebel J., Decesare S., Jovin T., Zafonte R., Lebowitz J., Flickinger J.C., Tong D., Marks M.P., Jamieson C., Luu D., Bell-Stephens T., Teraoka J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J. Neurosurg. 2005;103(1):38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 124.Chang Y.S., Ahn S.Y., Yoo H.S., Sung S.I., Choi S.J., Oh W.I., Park W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 2014;164(5):966–972.e6. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]